Abstract
Vibrio parahaemolyticus and V. alginolyticus, marine foodborne pathogens, were treated with UVC-radiation (240 J/m2) to evaluate alterations in their outer membrane protein profiles. Outer membrane protein patterns of UVC-irradiated bacteria were found altered when analyzed by sodium dodecyl sulphate polyacrylamide gel electrophoresis. Altered proteins were identified by mass spectrometry (MS and MS/MS) and analysis revealed that OmpW, OmpA, Long-chain fatty acid transport protein, Outer membrane receptor protein, Putative uncharacterized protein VP0167, Maltoporin (lamB), Polar flagellin B/D, Agglutination protein Peptidoglycan-associated lipoprotein and MltA-interacting protein MipA were appeared, thereby they can be considered as UVC-stress proteins in some vibrios. In addition, expression of OmpK decreased to non-detectable level. Furthermore, we observed a decrease or an increase in the expression level of other outer membrane proteins.
Keywords: Vibrio, UVC, Alteration, Outer membrane proteins, Mass spectrometry
Introduction
Vibrio, a food-borne pathogen, can sense and respond to changes in their external environment [1]. The ability of Vibrio spp. to sense and respond effectively to changes in the environment is crucial for their survival [2]. Bacteria in nature have cellular physiological systems to protect cells from various environmental stresses such as ultraviolet irradiation [3]. Marine bacteria, such as Vibrio alginolyticus and V. parahaemolyticus, are exposed to solar UV radiation daily. The response of marine bacteria to UVA irradiation is considered important because approximately 95 % of UV radiation is UVA (320–400 nm). On the other hand, bacteria are also exposed to UVC (254 nm) radiation used as a disinfection method to kill bacteria and prevent food poisoning in humans [3].
Ultraviolet-C (UV-C) radiation has been suggested as one of the successful disinfection practices for water treatment. Therefore, UV-sterilization has become a practical solution for safe disinfection of water [4]. The effectiveness of UV radiation in biological inactivation arises primarily from the fact that DNA molecules absorb UV photons between 200 and 300 nm, with peak absorption at 254 nm [5]. This absorption creates damage in the DNA by altering the nucleotide base pairing, thereby creating new linkages between adjacent nucleotides on the same DNA strand [6]. Several investigators have determined the effects of UV radiation on the inhibition of growth [7] and damage of protein, RNA, and DNA for Vibrio [8, 9]. All these effects have been related directly or indirectly to genomic damage arising out of primary photochemical changes in nucleic acids.
In Gram-negative pathogenic bacteria like Vibrio, the outer membrane plays an important role in the infection and pathogenicity to host [10]. In the outer membrane, proteins have a crucial role during many cellular and physiological processes [11]. OMPs play a key role in the adaptation to changes of external environments, due to their location at the outmost area of the cell [12]. Several works have shown that when bacteria are transferred to a new environment, the synthesis of their OMPs change [13]. Several alterations were observed in the OMPs profiles of V. alginolyticus and V. parahaemolyticus under starvation conditions [1]. Furthermore, the altered osmolarity of the culturing medium caused changes in the OMP patterns of V. alginolyticus [12] and V. parahaemolyticus [14]. In Escherichia coli OmpF is highly expressed at low osmolarity and suppressed at high osmolarity, while OmpC is totally the opposite [15]. More and more similar regulations have been found in other bacteria, such as major outer membrane protein and Omp50 in Campylobacter jejuni [16], and OmpK35 and OmpK36 in Klebsiella pneumoniae [17].
The aim of this work was to study the effect of UVC-radiation on the OMPs profiles of V. alginolyticus and V. parahaemolyticus. OMPs pattern were analyzed by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE). To identify the altered OMPs mass spectrometry (MS and MS/MS) was used.
Materials and Methods
Bacterial Strains
Six Vibrio strains were used in this study including three reference strains: Vibrio alginolyticus ATCC 33787 (S1), V. alginolyticus ATCC 17749 (S2), and V. parahaemolyticus ATCC 17802 (S5). In addition, V. parahaemolyticus strain (S6), isolated from the Calich estuary (Alghero, Italy), and two V. alginolyticus strains (S3 and S4) isolated, respectively from the internal organs of aquacultured diseased gilthead sea bream (Sparus aurata) and sea bass (Dicentrarchus labrax), in Tunisian aquaculture farm [18], were included in this work.
UVC Treatment
V. alginolyticus and V. parahaemolyticus were cultivated at 30 °C in Tryptic soy broth 1 % NaCl (TSB 1 %) with shaking (150 rpm). Then, the cultures of Vibrio (OD600 = 0.6) were diluted and spread on Tryptic soy agar 1 % NaCl (TSA 1 %, Pronadisa, Spain) in glass Petri dishes. After 18 h of incubation at 30 °C, bacterial colonies that appeared on the plates were exposed, in triplicate, to UV radiation according to the method described previously [19]. The plates with even bacterial growth were covered with a piece of glass for non-UV treatment (untreated bacteria). The plates (covered and non-covered) were exposed to a 4-W UV lamp with a wavelength of 254 nm. The applied dose was 240 Joules/m2. After exposure, 250 ml Erlenmeyer flasks containing 100 ml of TSB 1 % were inoculated with a loopful of colonies from control and UV treated bacteria. All flasks were kept in at 30 °C for 18 h with a shaking.
OMP Extraction
OMPs of V. alginolyticus and V. parahaemolyticus, before and after irradiation, were prepared according to the method described previously [6]. Briefly, the bacterial cells were harvested by centrifugation at 4,000×g for 15 min at 4 °C. The cells were then washed three times in 40 ml of sterile saline water (0.9 % NaCl) and then resuspended in 5 ml sterile saline water. Cells were disrupted by intermittent sonic oscillation in ice bath (350 W, 10 min × 3). Unbroken cells and cellular debris were removed by centrifugation at 5,000×g for 20 min. Supernatant was collected and was further centrifuged at 100 000×g for 40 min at 4 °C. The pellet was resuspended in 10 ml of 2 % (w/v) sodium lauryl sarcosinate (Sigma, St Louis, MO) and incubated at room temperature for 1 h, followed by centrifugation at 100 000×g for 40 min at 4 °C. The resulting pellet was resuspended in 200 μl of sterile saline water. The concentration of the OMPs in the final preparation was determined using the Bradford Kit (Sigma).
SDS-PAGE
OMPs (3 μg) of UVC irradiated and untreated V. alginolyticus and V. parahaemolyticus were analyzed in triplicate by SDS-PAGE [20] with 15 % acrylamide in the separating gel and 5 % acrylamide in the stacking gel. After separation, the proteins were visualized according to standard procedures by staining with Coomassie brilliant blue G250 (Sigma).
Protein Identification by Mass Spectrometry
After staining with Coomassie brilliant blue G250 (Sigma), 1D gel bands were manually excised from gels and collected in a 96-well plate. Destaining, reduction, alkylation, trypsin digestion of the proteins followed by peptide extraction were carried out with the Progest Investigator (Genomic Solutions, Ann Arbor, MI, USA). After the desalting step (C18-μZipTip, Millipore) peptides were eluted directly using the ProMS Investigator, (Genomic Solutions, Ann Arbor, MI, USA) onto a 96-well stainless steel MALDI target plate (Applied Biosystems/MDS SCIEX, Framingham, MA, USA) with 0.5 μl of CHCA matrix (2.5 mg/ml in 70 % ACN/30 % H2O/0.1 % TFA).
MS and MS/MS Analysis
Raw data for protein identification were obtained on the 4800 MALDI TOF/TOF Analyzer (Applied Biosystems/MDS SCIEX, Framingham, MA, USA) and were analyzed by GPS Explorer 3.6 software (Applied Biosystems/MDS SCIEX, Framingham, MA, USA). For positive-ion reflector mode spectra 3000 laser shots were averaged. For MS calibration, autolysis peaks of trypsin ([M+H]+ = 842.5100 and 2,211.1046) were used as internal calibrators. Monoisotopic peak masses were automatically determined within the mass range 800–4,000 m/z with a signal to noise ratio minimum set to 30. Up to twelve of the most intense ion signals were selected as precursors for MS/MS acquisition excluding common trypsin autolysis peaks and matrix ion signals. In MS/MS positive ion mode, 4000 spectra were averaged, collision energy was 2 kV, collision gas was air and default calibration was set using the Glu1-Fibrino-peptide B ([M+H]+ = 1570.6696) spotted onto fourteen positions of the MALDI target. Combined PMF and MS/MS queries were performed using the MASCOT search engine 2.1 (Matrix Science Ltd., UK) embedded into GPS-Explorer Software 3.6 (Applied Biosystems/MDS SCIEX, Framingham, MA, USA) on the UNIPROT database (downloaded 2009 06 25; 9064751 sequences; 2941541906 residues) with the following parameter settings: 50 ppm peptide mass accuracy, trypsin cleavage, one missed cleavage allowed, carbamidomethylation set as fixed modification, oxidation of methionine was allowed as variable modification, MS/MS fragment tolerance was set to 0.3 Da. Protein hits with MASCOT Protein score ≥82 and a GPS Explorer Protein confidence index = 95 % were used for further manual validation.
Results
Effect of UVC Irradiation on OMPs
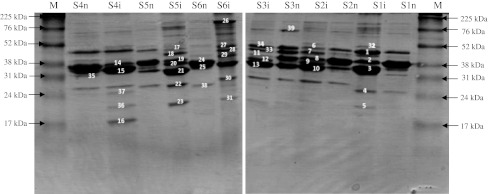
Outer membrane proteins of V. alginolyticus and V. parahaemolyticus cells were analyzed by SDS-PAGE (Fig. 1). Before their treatment with UV-C, each Vibrio strain presents its specific OMPs profile. In addition, almost 15 bands were detected in each profile containing from three to six major OMPs. After UV-C irradiation, several OMPs were to be found altered. These alterations were manifested by the appearance or disappearance of proteins as well as by a modification in the expression level of certain proteins. Indeed, as shown in Fig. 1, we observed alterations of 5 bands in V. alginolyticus (S2), 6 bands in V. alginolyticus (S1, S3 and S4), 7 bands in V. parahaemolyticus (S5) and 9 bands in V. parahaemolyticus (S6).
Fig. 1.
Outer membrane proteins of V. alginolyticus and V. parahaemolyticus cells exposed to UVC radiation. M: high-range rainbow (Amersham, Little Chalfont, Buckinghamshire, UK); S1, S2, S3 and S4: V. alginolyticus strains; S5 and S6: V. parahaemolyticus strains; n: strain non irradiated; i: strain irradiated
OMPs Identification
Altered OMPs of UV-C irradiated bacteria were identified using mass spectrometry (Table 1). Our results showed that OmpU was induced in V. alginolyticus S1 and S2, whereas its expression level was decreased in V. alginolyticus (S3 and S4) and V. parahaemolyticus (S5). For V. parahaemolyticus (S6), we noted that OmpU was appeared while outer membrane protein (ompU) became less abundant. OmpW was appeared in V. alginolyticus (S1 and S4) and V. parahaemolyticus (S5 and S6). The expression level of OmpK was increased in V. alginolyticus (S4) and V. parahaemolyticus (S5), whereas this protein became non detectable in V. parahaemolyticus (S6). After irradiation, we observed that OmpA was appeared in V. alginolyticus (S2 and S3), however this protein persist in V. alginolyticus S4 but with a lower molecular weight compared to non-treated strain. In addition to the modification already cited, we also noted that Outer membrane channel protein (TolC) was appeared in V. parahaemolyticus (S6), whereas its abundance was decreased in V. alginolyticus (S2 and S3) and was increased in V. alginolyticus (S1). Further, Outer membrane protein was induced in irradiated in V. alginolyticus (S2, S3 and S4), Putative outer membrane protein became more abundant in V. alginolyticus (S1) and V. parahaemolyticus (S5). Porin putative was appeared in V. parahaemolyticus (S6), whereas its expression level was decreased in V. alginolyticus (S3) and was increased in V. alginolyticus (S2). In irradiated V. parahaemolyticus (S6) we noted that Long-chain fatty acid transport protein, Outer membrane receptor protein, Putative uncharacterized protein VP0167 and Maltoporin (lamB) were appeared while Putative outer membrane protein OmpA became more abundant. Similar results have been observed in irradiated V. parahaemolyticus ATCC 17802 (S5), which respond to UV radiation by the appearance of Polar flagellin B/D and by increasing the expression of Putative outer membrane porin protein. For V. alginolyticus (S1) we observed that Agglutination protein and Peptidoglycan-associated lipoprotein were appeared after irradiation, while MltA-interacting protein MipA was appeared in V. alginolyticus (S4). Furthermore, Vitamin B12 receptor and Maltoporin (lamB) were decreased in V. alginolyticus (S1) and V. parahaemolyticus (S6), respectively.
Table 1.
List of altered OMPs of UVC-irradiated V. alginolyticus and V. parahaemolyticus identified using MS–MS/MS
| Strain | Band number | Identified protein | Accession number (Uniprot) | Mascot score | Molecular mass (Da) | Expression level |
|---|---|---|---|---|---|---|
| S1 | 01 | Outer membrane channel protein TolC | Q1V4T2|Q1V4T2_VIBAL | 94 | 47,846.3 | + |
| 02 | Outer membrane protein OmpU | Q1V395|Q1V395_VIBAL | 360 | 37,067.4 | − | |
| 03 | Putative outer membrane protein | Q1V592|Q1V592_VIBAL | 331 | 37,991.1 | + | |
| 04 | Outer membrane protein OmpK | Q1VF49|Q1VF49_VIBAL | 493 | 29,310 | + | |
| 05 | Peptidoglycan-associated lipoprotein | Q1V5X7|Q1V5X7_VIBAL | 180 | 18,740.4 | + | |
| Outer membrane protein W (fragment) | P83151|OMPW_VIBAL | 149 | 2,095.1 | + | ||
| 32 | Agglutination protein | Q1V422|Q1V422_VIBAL | 709 | 47,190.7 | + | |
| S2 | 06 | Outer membrane channel protein TolC | Q1V4T2|Q1V4T2_VIBAL | 384 | 47,846.3 | − |
| 07 | Porin, putative | Q1VA77|Q1VA77_VIBAL | 522 | 39,676 | + | |
| 08 | Outer membrane protein OmpU | Q1V395|Q1V395_VIBAL | 360 | 37,067.4 | − | |
| 09 | Outer membrane protein OmpA | Q1VAZ6|Q1VAZ6_VIBAL | 260 | 36,182.2 | + | |
| 10 | Outer membrane protein | A7JZ07|A7JZ07_9VIBR | 198 | 36,074.2 | + | |
| S3 | 11 | Outer membrane protein OmpU | Q1V395|Q1V395_VIBAL | 213 | 37,067.4 | − |
| 12 | Outer membrane protein OmpA | Q1VAZ6|Q1VAZ6_VIBAL | 908 | 36,182.2 | + | |
| 13 | Outer membrane protein | A7JZ07|A7JZ07_9VIBR | 231 | 36,074.2 | + | |
| 33 | Porin, putative | Q1VA77|Q1VA77_VIBAL | 615 | 39,676 | − | |
| 34 | Outer membrane channel protein TolC | Q1V4T2|Q1V4T2_VIBAL | 227 | 47,846.3 | − | |
| 39 | Vitamin B12 receptor | Q1V4E1|Q1V4E1_VIBAL | 1,080 | 68,061.3 | − | |
| S4 | 14 | Outer membrane protein OmpU | Q1V395|Q1V395_VIBAL | 237 | 37,067.4 | − |
| 15 | Outer membrane protein | A7JZ07|A7JZ07_9 VIBR | 406 | 36,074.2 | + | |
| 16 | Outer membrane protein OmpA | Q1VAZ6|Q1VAZ6_VIBAL | 260 | 36,182.2 | + | |
| 35 | Outer membrane protein OmpA | A7K5D9|A7K5D9_9VIBR | 551 | 34,468 | − | |
| 36 | Outer membrane protein W (Fragment) | P83151|OMPW_VBAL | 162 | 2,095.1 | + | |
| 37 | MltA-interacting protein MipA | A7JZF9|A7JZF9_9VIBR | 131 | 31,026.2 | + | |
| S5 | 17 | Maltoporin=lamB | C3W4N2|C3W4N2_VIBPA | 437 | 46,349.8 | − |
| 18 | Polar flagellin B/D | Q56702|FLAB_VIBPA | 905 | 40,149 | + | |
| 19 | Outer membrane protein (ompU) | Q4ZIM7|Q4ZIM7_VIBPA | 324 | 36,189.9 | − | |
| 20 | Putative outer membrane porin protein | Q87QZ0|Q87QZ0_VIBPA | 782 | 39,489 | + | |
| 21 | Putative outer membrane protein | Q87JT2|Q87JT2_VIBPA | 144 | 38,007.2 | + | |
| 22 | Outer membrane protein K=OmpK | B6DVI5|B6DVI5_VIBPA | 668 | 30,280.5 | + | |
| 23 | Outer membrane protein W=OmpW | A6B1W5|A6B1W5_VIBPA | 246 | 23,509.8 | + | |
| S6 | 24 | Outer membrane protein (ompU) | Q4ZIM7|Q4ZIM7_VIBPA | 516 | 36,189.9 | − |
| 25 | Putative outer membrane protein OmpA | Q87JK1|Q87JK1_VIBPA | 156 | 35,645.2 | + | |
| 26 | Outer membrane receptor protein | Q1VAM1|Q1VAM1_VIBL | 1,380 | 93,865.3 | + | |
| 27 | Maltoporin=lamB | C3W4N2|C3W4N2_VIBPA | 313 | 46,349.8 | + | |
| Outer membrane protein TolC | Q87SJ8|Q87SJ8_VIBPA | 148 | 47,954.4 | + | ||
| 28 | Porin, putative | Q1VA77|Q1VA77_VIBAL | 448 | 39,676 | + | |
| Long-chain fatty acid transport protein | Q1V8C7|Q1V8C7_VIBAL | 94 | 45,059.6 | |||
| 29 | Outer membrane protein OmpU | Q1VCV6|Q1VCV6_VIBAL | 186 | 35,837.3 | + | |
| 30 | Outer membrane protein=ompK | Q4PNS4|Q4PNS4_VIBAL | 431 | 31,311.9 | + | |
| Putative uncharacterized protein VP0167 | Q87TA3|Q87TA3_VIBPA | 224 | 28,406.5 | + | ||
| 31 | Outer membrane protein W | A6B1W5|A6B1W5_VIBPA | 145 | 2,095.1 | + | |
| 38 | Outer membrane protein ompK | P51002|OMPK2_VIBPA | 235 | 29,448.1 | − |
S1: V. alginolyticus ATCC 33787, S2: V. alginolyticus ATCC 17749, S3: V. alginolyticus (Sparus aurata), S4: V. alginolyticus (Dicentrarchus labrax), S5: V. parahaemolyticus ATCC 17802, S6: V. parahaemolyticus (Calich estuary). + up-regulaed; − down-regulated
Discussion
V. alginolyticus and V. parahaemolyticus respond to environmental stress by modifying the rate of synthesis of certain proteins [1]. Alterations observed in the OMPs profiles of UV-C irradiated V. alginolyticus and V. parahaemolyticus were manifested by the appearance of proteins as well as by modifications in the expression level of certain proteins. After the beginning of an adverse effect, such as irradiation, the synthetic of proteins are inhibited and cells division is interrupted. In parallel, the expression of several proteins increases; these are the so-called stress proteins [21]. Furthermore, in this work OmpW, OmpA, Long-chain fatty acid transport protein, Outer membrane receptor protein, Putative uncharacterized protein VP0167, Maltoporin (lamB), Polar flagellin B/D, Agglutination protein Peptidoglycan-associated lipoprotein and MltA-interacting protein MipA were appeared, thereby they can be considered as UVC-stress proteins in some vibrios species. OMPs have been produced, probably, to stimulate the bacterial cells to enter an exponential growth phase in a shorter time and grow at a faster rate, this mechanism is important for bacterial population to survive under UV radiation. In addition, Outer membrane protein, Putative outer membrane protein, Putative outer membrane porin protein and Putative outer membrane protein OmpA can be regarded as UV-C inducible proteins since these proteins became more abundant in some tested Vibrio strains. The new and induced OMPs can protect V. alginolyticus and V. parahaemolyticus against UV-C radiation. These results are in accordance with those reported by Nicholson et al. [22] who demonstrate that UV radiation induces the synthesis of several proteins in Phormidium Zaminosum. Bockrath and Hanawalt [23] showed that RecA protein was induced in UV irradiated E. coli. However, Vitamin B12 receptor and Maltoporin (lamb) are down-regulated by UV-C radiation. Additionally, the expression levels of OmpU, OmpK, Outer membrane channel protein (TolC) and Porin putative are variable under UV-C radiation. According to Miller et al. [24] UV radiation can cause indirect damage to DNA by generating reactive oxygen species that then damage bases, break strands, and cross-link DNA and proteins. Such degradation of proteins observed in this work could be due to any of the mechanisms proposed for protein inactivation by UV radiation [25]. These include (i) splitting of disulfide bonds of cystine or photooxidation of tyrosine, thereby changing the tertiary structure of proteins, or (ii) ring cleavage in the aromatic.
Generally, environmental changes influence the expression of OMPs and a rise in temperature may induce significant changes in the OMP expression of E. coli [26] and Borrelia burgdorferi [27]. Acidic pH induced the expression of new proteins on the surface of Yersiniapestis [28] and OMP expression in B. burgdorferi was also altered at different pH values [29]. The alterations observed in the OMP patterns of UV-irradiated V. alginolyticus and V. parahaemolyticus may testify the existence of certain modification of resistance toward some antibiotics. Indeed, Mushtaq et al. [30] described the loss of OprD protein from the OMPs of P. aeruginosa strains, which causes increased resistance against carbapenems. Chen and Livermore [31] demonstrated the resistance to β-lactam antibiotics owing to the lack of OmpC and OmpF in the OMPs of E. coli and Klebsiella pneumoniae.
Many vibrios are pathogenic for humans and/or marine vertebrates and invertebrates [32]. OMPs, whose production is often regulated by environmental cues, play important roles in bacterial pathogenesis by enhancing the adaptability of the pathogens to various environments. Thus, OMPs contribute to the virulence of bacteria and play essential roles in bacterial adaptation to host niches, which are usually hostile to invading pathogens [33]. The various alterations observed in the OMP profiles of UV-C irradiated Vibrio reflect the stability of these virulence factors under stress conditions. In addition, alterations observed between examined vibrios cells are variable. This can be in relation to the genomic plasticity of the tested strains.
In summary, this work showed for the first time alterations in the OMPs profiles of V. alginolyticus and V. parahaemolyticus cells under UV-C radiation. These modifications were manifested by the appearance of proteins as well as by a decrease and/or an increase in the expression level of certain proteins. In addition one OMP becomes non detectable while others were appeared. This allows us to define UVC stress proteins capable to protect these bacteria against UV radiation.
Footnotes
Fethi Ben Abdallah and Rihab Lagha contributed equally to this work.
References
- 1.Ben Abdallah F, Kallel H, Bakhrouf A. Enzymatic, outer membrane proteins and plasmid alterations of starved Vibrio parahaemolyticus and Vibrio alginolyticus cells in seawater. Arch Microbiol. 2009;191:493–500. doi: 10.1007/s00203-009-0477-8. [DOI] [PubMed] [Google Scholar]
- 2.Morita RY. Bacteria in oligotrophic environments: starvation survival lifestyle. New York: Chapman & Hall; 1997. [Google Scholar]
- 3.Friedberg EC. A brief history of the DNA repair field. Cell Res. 2008;18:3–7. doi: 10.1038/cr.2007.113. [DOI] [PubMed] [Google Scholar]
- 4.Ben Said M, Masahiro O, Hassen A. Detection of viable but non cultivable Escherichia coli after UV irradiation using a lytic Qβ phage. Ann Microbiol. 2010;60:121–127. doi: 10.1007/s13213-010-0017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeffrey JB, Huai-Shu X, Colwell RR. Viable but nonculturable bacteria in drinking water. Appl Environ Microbiol. 1990;57:875–878. doi: 10.1128/aem.57.3.875-878.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sabri MY, Zamri-Saad M, Mutalib AR, Israf DA, Muniandy N. Efficacy of an outer membrane protein of Pasteurella haemolytica A2, A7 or A9-enriched vaccine against intratracheal challenge exposure in sheep. Vet Microbiol. 2000;73:13–23. doi: 10.1016/S0378-1135(99)00205-9. [DOI] [PubMed] [Google Scholar]
- 7.Zabala B, García K, Espejo RT. Enhancement of UV light sensitivity of a Vibrio parahaemolyticus O3:K6 pandemic strain due to natural lysogenization by a telomeric phage. Appl Environ Microbiol. 2009;6:1697–1702. doi: 10.1128/AEM.01995-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Booth MG, Jeffrey WH, Miller RV. RecA expression in response to solar UVR in the marine bacterium Vibrio natriegens. Microb Ecol. 2001;42:531–539. doi: 10.1007/s00248-001-1009-5. [DOI] [PubMed] [Google Scholar]
- 9.Hamamoto A, Bandou C, Masayuki N, Mawatari K, Lian X, Yamato M, Harada N, Akutagawa M, Kinouchi Y, Nakaya Y, Takahashi A. Differences in stress response after UVC or UVA irradiation in Vibrio parahaemolyticus. Environ Microbiol Rep. 2010;2:660–666. doi: 10.1111/j.1758-2229.2010.00154.x. [DOI] [PubMed] [Google Scholar]
- 10.Tsolis RM. Comparative genome analysis of the alpha-proteobacteria: relationships between plant and animal pathogens and host specificity. Proc Natl Acad Sci USA. 2002;99:12503–12505. doi: 10.1073/pnas.212508599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qian R, Xiao Z, Zhang C, Chu W, Mao Z, Yu L. Expression and purification of two major outer membrane proteins from Vibrio alginolyticus. World J Microbiol Biotechnol. 2008;24:245–251. doi: 10.1007/s11274-007-9463-y. [DOI] [Google Scholar]
- 12.Xu C, Wang S, Ren H, Lin X, Wu L, Peng X. Proteomic analysis on the expression of outer membrane proteins of Vibrio alginolyticus at different sodium concentrations. Proteomics. 2005;5:3142–3152. doi: 10.1002/pmic.200401128. [DOI] [PubMed] [Google Scholar]
- 13.Wibbenmeyer JA, Provenzano D, Landry CF, Klose KE, Delcour AH. Vibrio cholerae OmpU and OmpT porins are differentially affected by bile. Infect Immun. 2002;70:121–126. doi: 10.1128/IAI.70.1.121-126.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu C, Ren H, Wang S, Peng X. Proteomic analysis of salt-sensitive outer membrane proteins of Vibrio parahaemolyticus. Res Microbiol. 2004;155:835–842. doi: 10.1016/j.resmic.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Cai SJ, Inouye M. EnvZ-OmpR interaction and osmoregulation in Escherichia coli. J Biol Chem. 2002;277:24155–24161. doi: 10.1074/jbc.M110715200. [DOI] [PubMed] [Google Scholar]
- 16.Dedieu L, Pages JM, Bolla JM. Environmental regulation of Campylobacter jejuni major outer membrane protein porin expression in Escherichia coli monitored by using green fluorescent protein. Appl Environ Microbiol. 2002;68:4209–4215. doi: 10.1128/AEM.68.9.4209-4215.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernandez-Alles S, Alberti S, Alvarez D, et al. Porin expression in clinical isolates of Klebsiella pneumoniae. Microbiology. 1999;145:673–679. doi: 10.1099/13500872-145-3-673. [DOI] [PubMed] [Google Scholar]
- 18.Ben Abdallah F, Chaieb K, Kallel H, Bakhrouf A. RT-PCR assays for in vivo expression of Vibrio alginolyticus virulence genes in cultured gilthead Dicentrarchus labrax and Sparus aurata. Ann Microbiol. 2009;59:1–5. [Google Scholar]
- 19.Wang Y, Leung PC, Qian P, Gu JD. Environment effects of UV, H2O2 and Fe3+ on the growth of four environmental isolates of Aeromonas and Vibrio species from a Mangrove. Microbes Environ. 2004;19:163–171. doi: 10.1264/jsme2.19.163. [DOI] [Google Scholar]
- 20.Laemmli UK. Cleavage of structural proteins for the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Kustos I, Kocsis B, Kilar F. Bacterial outer membrane protein analysis by electrophoresis and microchip technology. Expert Rev Proteomics. 2007;4:91–106. doi: 10.1586/14789450.4.1.91. [DOI] [PubMed] [Google Scholar]
- 22.Nicholson P, Osborn RW, Christopher J. How induction of protein synthesis in light, nalidixic acid and heat shock in the cyanobacterium Phormidium laminosum. FEB. 1987;221:110–114. doi: 10.1016/0014-5793(87)80362-9. [DOI] [Google Scholar]
- 23.Bockrath RC, Hanawalt PC. Ultraviolet light induction of recA protein in a recB uvrB mutant of Escherichia coli. J Bacteriol. 1980;143:1025–1028. doi: 10.1128/jb.143.2.1025-1028.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller RV, Jeffrey W, Mitchell D, et al. Bacterial responses to ultraviolet light. ASM News. 1999;65:535–541. [Google Scholar]
- 25.Mody R, Mody B, Dave P. Damage to the plasma membrane in Escherichia coli K-12 induced by far-ultraviolet radiation and its repair. Radiat Res. 1991;127:156–163. doi: 10.2307/3577960. [DOI] [PubMed] [Google Scholar]
- 26.Molloy MP, Herbert BR, Slade MB, Rabilloud T, Nouwens AS, Williams KL, Gooley AA. Proteomic analysis of the Escherichia coli outer membrane. Eur J Biochem. 2000;267:2871–2881. doi: 10.1046/j.1432-1327.2000.01296.x. [DOI] [PubMed] [Google Scholar]
- 27.Obonyo M, Munderloh UG, Sam TN, Kurtti TJ. Cultivation at 37 degrees C enhances Borrelia burgdorferi sensu stricto infectivity for hamsters. Med Microbiol Immunol. 2002;191:33–39. doi: 10.1007/s00430-002-0116-3. [DOI] [PubMed] [Google Scholar]
- 28.Feodorova VA, Devdariani ZL. Expression of acid-stable proteins and modified lipopolysaccharide of Yersinia pestis in acidic growth medium. J Med Microbiol. 2001;50:979–985. doi: 10.1099/0022-1317-50-11-979. [DOI] [PubMed] [Google Scholar]
- 29.Carroll JA, Garon CF, Schwan TG. Effects of environmental pH on membrane proteins in Borrelia burgdorferi. Infect Immun. 1999;67:3181–3187. doi: 10.1128/iai.67.7.3181-3187.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mushtaq S, Ge Y, Livermore DM. Doripenem versus Pseudomonas aeruginosa in vitro: activity against characterized isolates, mutants, and transconjugants and resistance selection potential. Antimicrob Agents Chemother. 2004;8:3086–3092. doi: 10.1128/AAC.48.8.3086-3092.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen HY, Livermore DM (1993) Activity of cefepime and other betalactam antibiotics against permeability mutants of Escherichia coli and Klebsiella pneumoniae. J Antimicrob Chemother (Suppl B):63–74 [DOI] [PubMed]
- 32.Zhang XH, Austin B. Haemolysins in Vibrio species. J Appl Microbiol. 2005;98:1011–1019. doi: 10.1111/j.1365-2672.2005.02583.x. [DOI] [PubMed] [Google Scholar]
- 33.Lin J, Huang S, Zhang Q. Outer membrane proteins: key players for bacterial adaptation in host niches. Microbes Infect. 2002;4:325–331. doi: 10.1016/S1286-4579(02)01545-9. [DOI] [PubMed] [Google Scholar]