Abstract
Invasion of crops with Aspergillus flavus may result in contamination of food and feed with carcinogenic mycotoxins such as aflatoxins (AF) and cyclopiazonic acid (CPA). In the present study, distribution and toxigenicity of Aspergillus flavus and A. parasiticus in soils of five peanut fields located in Guilan province, Northern Iran was investigated. From a total of 30 soil samples, 53 strains were isolated which all of them were finally identified as A. flavus by a combination of colony morphology, microscopic criteria and mycotoxin profiles. Chromatographic analysis of fungal cultures on yeast extract sucrose broth by tip culture method showed that 45 of the 53 A. flavus isolates (84.9 %) were able to produce either CPA or AFB1, while eight of the isolates (15.1 %) were non-toxigenic. The amounts of CPA and AFB1 produced by the isolates were reported in the range of 18.2–403.8 μg/g and 53.3–7446.3 μg/g fungal dry weights, respectively. Chemotype classification of A. flavus isolates based on the ability for producing mycotoxins and sclerotia showed that 43.4 % were producers of CPA, AFB1 and sclerotia (group I), 13.2 % of CPA and AFB1 (group II), 9.4 % of AFB1 and sclerotia (group III), 13.2 % of AFB1 (group IV), 5.7 % of CPA and sclerotia (group V) and 15.1 % were non-toxigenic with no sclerotia (group VI). No strain was found as producer of only CPA or sclerotia. These results indicate different populations of mycotoxigenic A. flavus strains enable to produce hazardous amounts of AFB1 and CPA are present in peanuts field soils which can be quite important regard to their potential to contaminate peanuts as a main crop consumed in human and animal nutrition.
Keywords: Aspergillus flavus, Cyclopiazonic acid, Aflatoxin, Chemotype diversity, Soil, Peanuts
Introduction
Aflatoxins (AF) are fungal secondary metabolites which, if ingested, can evoke a wide range of toxic responses and disease conditions in higher vertebrates [1]. Members of the genus Aspergillus as the most important AF producers can infect commodities such as corn, peanuts, cotton, tree nuts, sorghum, and other oil seeds under suitable conditions of temperature and relative humidity [2]. Aspergillus section Flavi members such as A. flavus, A. parasiticus and A. nomius can produce AF, but only A. flavus is able to produce cyclopiazonic acid (CPA). Besides A. flavus as the main producer, CPA is also produced by many other Aspergillus and Penicillium species [3]. Recently, Chang et al. showed that CPA is produced by A. flavus in a close relationship with AF and the gene cluster of CPA is in the adjacent of AF gene cluster.
CPA is an indole tetramic acid that is toxic to animals and humans [4–6]. From several evidences, it has been shown that CPA can also be considered as a natural contaminant of food, feed and agricultural crops [7–10]. AF and CPA contamination can occur together in the same food and feed due to the presence of producing fungi from A. flavus populations [4, 7, 11–13]. In general, the ability of A. flavus isolates to produce CPA varies greatly depending on geographic conditions and strains isolated. The incidence of toxigenic A. flavus strains in agricultural soil as the main reservoir of the fungus is important not only from the view point of mycological research, but also for their potential to contaminate susceptible crops and agricultural commodities.
Peanut (Arachis hypogaea) is a very susceptible crop to contamination with AF and closely related mycotoxins. It is a main legume cultivated in different parts of Iran as a nutrient consumed in human and animal nutrition. It is grown mainly in Northern parts of Iran specially Guilan province (around 90 % of peanut cultivation). The main objective of this study was to examine distribution and toxigenicity of A. flavus strains in peanut field soils of Astaneh Ashrafieh, Guilan province as a main center of peanut production in Iran with special attention to their chemotype classification based on the types and amounts of mycotoxins produced.
Materials and Methods
Sampling, Isolation and Identification of Fungi
Thirty soil samples were collected from the 5 cm top of the surface soil at random locations within five peanut fields (A–E, each six soil samples) of Astaneh Ashrafieh, the main site of peanut cultivation in Iran (Guilan province, Northern Iran) during June 2009. Each soil comprised from ten subsamples each of approximately 100 cm3 which were obtained using a sterile trowel at 10 m intervals from the 5 cm top of the surface soil and then mixed thoroughly in a Nylon bag. For isolation of A.flavus and A. parasiticus, the dried soil samples were passed through testing sieves (1 mm mesh) [14]. 2 g of each sieved soil was diluted with 6 ml distilled water in a test tube, mixed vigorously for 20 min., and serially diluted to 10−5 in sterile distilled water. One tenths ml of each dilution was spread onto 9.0 cm petri-dishes containing isolation culture media i.e., Dichloran rosebengal chloramphenicol agar (DRBC) and Aspergillus flavus/parasiticus agar (AFPA) [15, 16]. The cultures were incubated at 28 °C for DRBC and 30 °C for AFPA and checked periodically for colonies with brilliant orange-yellow reverse coloration on AFPA (during 48–72 h) and yellowish-green colonies on DRBC. The putative Aspergillus colonies were purified after sub-culturing on potato dextrose agar plates. Aspergillus species were identified based on microscopic morphology and macroscopic criteria on czapek dox agar (CZ) and czapek yeast extract agar (CYA) plates for 7 days at 25 °C [2, 17].
Detection of CPA and AFB1 Production by TLC
For assessment of CPA and AFB1, all the isolates were cultured in triplicate on 1 ml tips containing 250 μl yeast extract 2 %, sucrose 20 % (YES) broth at 28 °C for 4 days by the tip culture method [18]. After separating the culture medium from the tips using centrifugation, the mycelia weight was calculated. CPA was extracted from the culture broth and assessed by TLC using toluene–ethyl acetate–formic acid (5:4:1, vol/vol/vol) as mobile phase [16, 19]. For detecting AFB1, spotted TLC silica gel 60 F254 plates were developed by chloroform–ethyl acetate–formic acid 90 % (6:3:1, vol/vol/vol) as mobile phase. AFB1 were visualized under UV light (365 nm) and photographed with a TLC scanner CAMAG Reprostar 3 (CAMAG, Switzerland).
Quantitation of CPA and AFB1 by HPLC
For quantitation of AFB1 and CPA, chloroformic extract of the culture broth was injected into a HPLC (KNAUER D-14163 UV–VIS system, Germany) according to Razzaghi-Abyaneh et al. [20]. 50 μl of each chloroformic extract was injected into the HPLC column (TSKgel ODS-80TS; 4.6 mm ID × 15.0 cm, TOSOH BIOSCIENCE, Japan) and eluted at a flow rate of 1 ml/min by methanol/water (70:30) contained 300 mg ZnSO4·7H2O for CPA and by water/acetonitrile/methanol (60:25:15, v/v/v) for AFB1. The amounts of CPA and AFB1 were measured at wavelengths of 284 and 365 nm, respectively. The elution time of the samples was compared with pure AFB1 and CPA standards and quantified on the basis of the ratio of the peak area of samples to those of the standards.
Statistical Analysis
The results were analyzed by SPSS version 16 programme for Windows (SPSS Inc., Chicago, IL). Analyses of variance (ANOVA) and Tukey’s multiple comparison tests at 5 % significance level were used to compare the means of fungal incidence and toxin production data. P < 0.05 was considered significant.
Results
Isolation of A. flavus and A. parasiticus
All the 53 Aspergillus isolates produced yellow-green colonies on CZ, and CYA and showed yellow-orange reverse coloration on AFPA. Microscopically, uni- and biseriated conidial heads, long conidiophores and globose conidia with smooth to finely echinulated walls in variable sizes were observed indicating that all strains were A. flavus. No isolate of A. parasiticus were found in analyzed samples. Table 1 shows detailed information about 53 A. flavus strains isolated. A total of 9, 5, 20, 16 and 3 A. flavus strains were isolated from peanut fields A, B, C, D and E, respectively. All the soils in fields A, C and D, 5/6 soils of field B and 2/6 soils of field E were contaminated with A. flavus strains (Table 1).
Table 1.
Distribution and toxigenicity of A. flavus strains in peanuts field soils
| Peanut field | A. flavus strain | Fungal dry weight (μg) | CPA (μg/g DW) | AFB1 (μg/g DW) |
|---|---|---|---|---|
| A | PICC-AF59 | 41.0 ± 4.2 | 203.9 ± 33.2 | 808.9 ± 83.1 |
| PICC-AF60 | 43.8 ± 8.0 | 71.6 ± 10.2 | 905.8 ± 106.6 | |
| PICC-AF61 | 34.5 ± 3.7 | 34.6 ± 6.0 | 1478.3 ± 173.2 | |
| PICC-AF62 | 39.1 ± 3.5 | 39.5 ± 3.2 | 558.1 ± 62.2 | |
| PICC-AF63 | 35.6 ± 7.2 | ND | 90.4 ± 13.5 | |
| PICC-AF64 | 53.3 ± 9.9 | ND | ND | |
| PICC-AF65 | 33.4 ± 5.5 | ND | ND | |
| PICC-AF66 | 52.0 ± 7.6 | 58.5 ± 8.3 | 943.2 ± 99.1 | |
| PICC-AF67 | 35.1 ± 6.3 | ND | 615.4 ± 47.3 | |
| B | PICC-AF68 | 30.6 ± 4.4 | 149.0 ± 17.7 | ND |
| PICC-AF69 | 46.9 ± 4.8 | 403.8 ± 55.3 | ND | |
| PICC-AF70 | 46.3 ± 6.5 | ND | 444.3 ± 60.0 | |
| PICC-AF71 | 52.8 ± 8.1 | 18.2 ± 3.0 | 291.1 ± 30.2 | |
| PICC-AF72 | 42.4 ± 7.1 | 255.9 ± 29.2 | 1900.1 ± 131.7 | |
| C | PICC-AF73 | 44.8 ± 9.7 | 339.4 ± 41.1 | 7446.3 ± 869.5 |
| PICC-AF74 | 43.5 ± 5.1 | 103.6 ± 12.4 | 234.5 ± 26.6 | |
| PICC-AF75 | 45.6 ± 8.0 | 21.0 ± 3.6 | 480.5 ± 35.2 | |
| PICC-AF76 | 52.0 ± 9.2 | ND | 244.7 ± 18.1 | |
| PICC-AF77 | 50.8 ± 10.5 | 83.8 ± 10.7 | 2677.2 ± 358.1 | |
| PICC-AF78 | 46.6 ± 7.5 | ND | 607.3 ± 50.3 | |
| PICC-AF79 | 49.0 ± 6.0 | ND | 53.3 ± 10.1 | |
| PICC-AF80 | 47.1 ± 8.4 | 300.1 ± 23.5 | 1017.2 ± 99.1 | |
| PICC-AF81 | 34.5 ± 5.5 | 176.6 ± 19.4 | 397.8 ± 42.5 | |
| PICC-AF82 | 54.4 ± 6.8 | 150.8 ± 14.3 | 658.3 ± 61.0 | |
| PICC-AF83 | 44.9 ± 4.0 | 241.6 ± 30.1 | 1582.6 ± 190.8 | |
| PICC-AF84 | 45.9 ± 7.1 | ND | 185.5 ± 20.2 | |
| PICC-AF85 | 52.6 ± 5.6 | ND | ND | |
| PICC-AF86 | 50.4 ± 6.8 | 173.1 ± 14.2 | 126.4 ± 18.6 | |
| PICC-AF87 | 46.0 ± 8.2 | 99.9 ± 13.4 | 2047.4 ± 255.2 | |
| PICC-AF88 | 38.5 ± 2.7 | 234.1 ± 27.9 | 2127.0 ± 343.3 | |
| PICC-AF89 | 41.7 ± 3.3 | 313.6 ± 40.1 | 1043.5 ± 102.7 | |
| PICC-AF90 | 43.5 ± 4.8 | 88.6 ± 12.0 | 306.7 ± 35.2 | |
| PICC-AF91 | 45.0 ± 6.3 | 60.5 ± 9.1 | 670.8 ± 59.0 | |
| PICC-AF92 | 38.8 ± 7.0 | 79.3 ± 11.1 | 143.1 ± 43.2 | |
| D | PICC-AF93 | 50.0 ± 8.8 | 22.2 ± 5.0 | 2913.3 ± 200.8 |
| PICC-AF94 | 34.2 ± 5.5 | 177.0 ± 15.7 | 1360.8 ± 155.1 | |
| PICC-AF95 | 43.5 ± 5.6 | 231.2 ± 39.5 | 143.5 ± 43.5 | |
| PICC-AF96 | 45.8 ± 3.7 | 311.9 ± 40.8 | 774.3 ± 50.7 | |
| PICC-AF97 | 37.6 ± 4.3 | 55.1 ± 9.8 | 1183.9 ± 165.4 | |
| PICC-AF98 | 44.5 ± 6.6 | ND | 2024.8 ± 179.3 | |
| PICC-AF99 | 35.0 ± 4.7 | ND | 930.1 ± 101.8 | |
| PICC-AF100 | 39.1 ± 2.2 | ND | ND | |
| PICC-AF101 | 41.1 ± 6.9 | ND | ND | |
| PICC-AF102 | 36.0 ± 4.4 | ND | 1606.1 ± 195.2 | |
| PICC-AF103 | 36.6 ± 3.6 | 119.4 ± 18.2 | 455.8 ± 61.5 | |
| PICC-AF104 | 45.3 ± 5.2 | 143.1 ± 23.8 | ND | |
| PICC-AF105 | 36.5 ± 4.5 | ND | ND | |
| PICC-AF106 | 42.9 ± 3.9 | ND | ND | |
| PICC-AF107 | 37.0 ± 5.1 | ND | 505.8 ± 45.6 | |
| PICC-AF108 | 51.5 ± 8.0 | 250.2 ± 20.6 | 887.3 ± 66.0 | |
| E | PICC-AF109 | 54.1 ± 7.5 | 65.0 ± 10.6 | 1615.9 ± 150.3 |
| PICC-AF110 | 55.0 ± 4.8 | ND | ND | |
| PICC-AF111 | 41.5 ± 6.2 | ND | 613.1 ± 15.2 | |
| Standard strains | CMI102566 | 39.9 ± 3.3 | 377.5 ± 29.7 | 9450.6 ± 892.4 |
| CMI93803 | 35.6 ± 6.0 | ND | ND |
CPA cyclopiazonic acid, AFB1 aflatoxin B1, DW fungal dry weight, ND not detected
Sclerotia Production
The presence of sclerotia was observed macroscopically as an identifiable criterion for A. flavus isolates. Sclerotia size was evaluated by a measuring reticule with a Nikon Microscope at 40× magnification. Based on the results obtained, 30 of 53 A. flavus strains (56.6 %) were capable to produce sclerotia all from L-type on CZA medium at 30 °C (Table 2). They were classified in chemotypes I, III and V.
Table 2.
Chemotype patterns of A. flavus strains based on sclerotia, aflatoxins, and CPA production
| Chemotype | No. of strains | CPA | AFB1 | AFB2 | Sclerotia |
|---|---|---|---|---|---|
| I | 23 | + | + | − | + |
| II | 7 | + | + | − | − |
| III | 5 | − | + | − | + |
| IV | 7 | − | + | − | − |
| V | 3 | + | − | − | + |
| VI | 8 | − | − | − | − |
CPA-Producing Ability
As shown in Fig. 1, 33 of 53 A. flavus strains (62.3 %) were capable to produce CPA. The amounts of CPA produced by the strains were reported in the range of 18.2–403.8 μg/g fungal dry weight. The strains were classified in five classes including class 1 (non-CPA producers; 20 strains), 2 (<100 μg/g; 14 strains), 3 (100–200 μg/g; 8 strains), 4 (200–300 μg/g; 6 strains) and 5 (>300 μg/g; 5 strains).
Fig. 1.
Classification of A. flavus strains from peanut field soils in relation to CPA production on YES broth
AF-Producing Ability
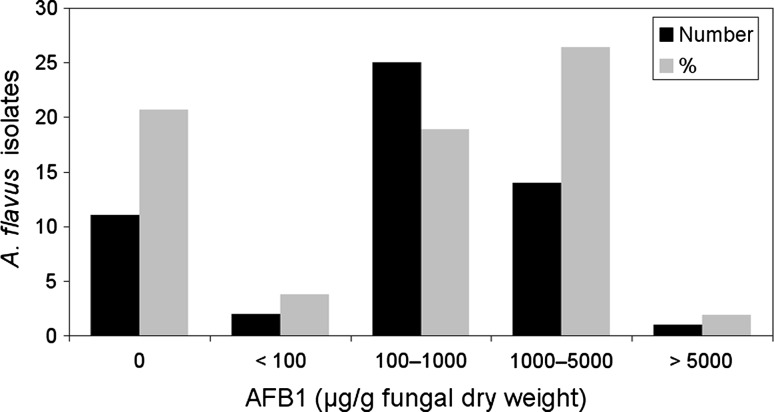
As indicated in Fig. 2, a total of 42 of 53 A. flavus strains (79.2 %) were capable to produce AFB1. None of the strains produced AFB2 or AFG series. The amounts of AFB1 produced by the strains were reported in the range of 53.3–7446.3 μg/g fungal dry weight. In relation to the amounts of AFB1 produced, the strains were classified in five classes including class 1 (non-producers; 11 strains), 2 (<100 μg/g; 2 strains), 3 (100–1,000 μg/g; 25 strains), 4 (1,000–5,000 μg/g; 14 strains) and 5 (>5,000 μg/g; 1 strain).
Fig. 2.
Classification of A. flavus strains from peanut field soils in relation to AFB1 production on YES broth
Chemotype Classification of A. flavus Strains
As shown in Table 2, A. flavus strains were classified into six chemotypes based on the ability for producing mycotoxins and sclerotia. The strains capable to produce CPA, AFB1 and sclerotia (23/53) were classified as chemotype I. They comprised the most prevalent chemotype with an incidence of 43.4 %. Other 30 strains were distributed in chemotypes II–VI including CPA and AFB1 producers (chemotype II; 13.2 %), AFB1 and sclerotia producers (chemotype III; 9.4 %), AFB1 producers (chemotype IV; 13.2 %), CPA and sclerotia producers (chemotype V; 5.7 %) and 15.1 % non-producers of mycotoxins with no sclerotia (chemotype VI).
Discussion
In the present study, different communities of toxigenic and non-toxigenic A. flavus strains isolated from peanuts field soils were identified that were capable of producing various amounts of CPA and/or AFB1. Co-contamination of agricultural commodities with AF and CPA has been reported by different researchers [7, 9, 12, 21, 22]. Although CPA mycotoxicoses has not been reported in the field, its association with “kodo poisoning” after consumption of CPA-contaminated kodo millet has been proposed in India [23, 24]. It has been shown that the ability of A. flavus isolates to produce CPA and AF vary greatly which affects by several parameters such as genetics of isolates, the source of isolation and geographic conditions [3, 10]. So, different results have been reported for the isolates from various regions of the world [9, 13, 16, 21, 25]. Although toxigenic Aspergillus strains have been reported from various crops and agricultural commodities, agricultural soil serves as the main reservoir of these fungi all over the world [26–28]. In the present study, A. flavus was isolated as sole species of Aspergillus section Flavi from peanut field soils. In total, 45 of the 53 peanuts field soil isolates of A. flavus (84.9 %) were able to produce either CPA or AFB1. In a study on the occurrence of Aspergillus section Flavi in 300 soil samples from three peanut-growing regions of Argentina, both A. flavus and A. parasiticus were isolated [29]. In a similar study by these authors on soil samples of peanut-growing region in Argentina during 1998–2001, it was shown that 80 % of 369 A. flavus isolates produced both AF and CPA [30]. Examination of peanut seeds in five regions of Egypt in the year 2010 showed that among a total of 88 Aspergillus section Flavi isolates, 95 % were A. flavus as the only AF-producing species [31]. 28/32 A. flavus (87 %) isolated in the year 2008 and 51/56 (91 %) isolated in the year 2007 produced AFB1 in the range of 12.8–75,843 and 10.9–248,460 ng/g respectively. All aflatoxigenic isolates produced AFB1 and AFB2, but not AFG series. Analysis of soil samples from four peanuts production regions in the State of São Paulo, Brazil in the year 2012 showed that A. flavus was the most frequent species of the genus Aspergillus (13.4 %) [32]. Nesci et al. (2011) showed that among the Aspergillus populations recovered from peanut seeds in Argentina, A. flavus was the most frequently isolated (79 %), and A. parasiticus (5.9 %) was the third prevalent species [33]. Totally, 79 A. flavus and 2 A. parasiticus were isolated. AFB1 produced by 52 strains out of 79 of A. flavus ranging from <0.002 to 225 μg/ml and 2 strains of A. parasiticus ranging from <0.002 to 25 μg/ml. From a total number of 25 peanut samples and 11 farm soils tested in Vietnam, A. flavus was found in 9 (36.0 %) and 3 (27.3 %) samples, respectively. Totally, 36 A. flavus were isolated from contaminated soils (7 isolates) and peanuts (29 isolates) of which 2 (28.6 %) and 11 (37.9 %) produced AF [21]. From the study of Razzaghi-Abyaneh et al. [16] on corn field soils, it was shown that 25 of 58 A. flavus isolates (43.1 %) produced CPA; while 16 isolates (27.6 %) were aflatoxigenic and only 4 isolates (6.9 %) produced sclerotia. The authors classified the isolates in 5 chemotypes based on their ability to produce CPA, AF and sclerotia. Rodrigues et al. [34] showed that among 13 strains of A. flavus isolated from almonds, 10 (77.0 %) were atoxigenic and only 2 (15.0%) were capable to produce CPA and AFB1. Vaamonde et al. [9] reported that A. flavus strains from various crops may be different in their aflatoxin producing ability. They showed that 29 % of the total A. flavus isolates were able to produce aflatoxin. Horn and Dorner [13] showed that the majority of A. flavus isolates (more than 80.0 %) from soils of USA produced both CPA and AF. Different results of aflatoxigenicity among Aspergillus section Flavi population may be attributed to differences in prevailing climatic conditions in the regions analyzed, the cultivars grown, local agricultural practices, the incidence of insect damage and seasonal variations in affecting factors.
Sclerotia are compacted bodies of aggregated hyphae, which resist unfavorable environmental conditions due to their resistance to chemical and biological degradation [35]. Production of sclerotia by A. flavus isolates in relation to mycotoxin production has been a matter of controversy for many years. According to the description of Horn et al., sclerotia size is a phenotypic character within A. flavus strains that can be used to create two different groups: the large strains (L) having sclerotia >400 μm in diameter and the small strains (S) with sclerotia <400 μm. In the present study, all 30 sclerotia positive A. flavus strains produced CPA (3 strains), AFB1 (5 strains) or both (23 strains) [36]. This is in accordance to the data from other researchers implying a positive correlation between CPA, AF and sclerotia production [9, 13, 37]. All sclerotia producing strains examined in our study were classified as L strains because of producing sclerotia >400 μm in diameter. We classified A. flavus isolates into six chemotypes (I–VI) based on CPA, AF and sclerotia production patterns. The group I (CPA and AFB1 positive) was the most prominent chemotype which comprised nearly 40 % of A. flavus strains. In our previous study on A. flavus strains isolated from corn field soils, the group of non-producers of both AF and CPA was the most abundant chemotype reported [16].
Overall, the data from present study clearly show that diverse populations of CPA- and AF-producing A. flavus strains are present in peanut field soils as potential threats for agriculture and public health. These fungi have considerable variation in the types and amounts of mycotoxins produced. So, studying of their genetic diversity and community structures can help us to make a better understanding about population dynamics of the fungus in relation to prevent mycotoxin contamination of crops and agricultural commodities in practice.
Acknowledgments
This study was supported financially by a grant from Research Deputy of Faculty of Medical Sciences at Tarbiat Modares University. The authors wish to thank Mrs. Razeghi and Ms. Jamali for their helpful assistance.
References
- 1.Bennett JW, Klich M. Mycotoxins. Clin Microbiol Rev. 2003;16:497–516. doi: 10.1128/CMR.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varga J, Frisvad JC, Samson RA. Two new aflatoxin producing species and an overview of Aspergillus section Flavi. Stud Mycol. 2011;69:57–80. doi: 10.3114/sim.2011.69.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang PK, Horn BW, Dorner JW. Clustered genes involved in cyclopiazonic acid production are next to the aflatoxin biosynthesis gene cluster in Aspergillus flavus. Fungal Genet Biol. 2009;46:176–182. doi: 10.1016/j.fgb.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Purchase IF. The acute toxicity of the mycotoxin cyclopiazonic acid to rats. Toxicol Appl Pharmacol. 1971;18:114–123. doi: 10.1016/0041-008X(71)90320-6. [DOI] [PubMed] [Google Scholar]
- 5.Norred WP, Porter JK, Dorner JW, Cole RJ. Occurrence of the mycotoxin cyclopiazonic acid in meat after oral administration to chickens. J Agric Food Chem. 1988;36:113–116. doi: 10.1021/jf00079a028. [DOI] [Google Scholar]
- 6.Riley RT, Goeger DE, Yoo H, Showker JL. Comparison of three tetramic acids and their ability to alter membrane function in cultured skeletal muscle cells and sarcoplasmic reticulum vesicles. Toxicol Appl Pharmacol. 1992;114:261–267. doi: 10.1016/0041-008X(92)90076-5. [DOI] [PubMed] [Google Scholar]
- 7.Lansden JA, Davidson JI. Occurrence of cyclopiazonic acid in peanuts. Appl Environ Microbiol. 1983;45:766–769. doi: 10.1128/aem.45.3.766-769.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martins ML, Martins HM. Natural and in vitro coproduction of cyclopiazonic acid and aflatoxins. J Food Prot. 1999;62:292–294. doi: 10.4315/0362-028x-62.3.292. [DOI] [PubMed] [Google Scholar]
- 9.Vaamonde G, Patriarca A, Fernandez Pinto V, Comerio R, Degrossi C. Variability of aflatoxin and cyclopiazonic acid production by Aspergillus section Flavi from different substrates in Argentina. Int J Food Microbiol. 2003;88:79–84. doi: 10.1016/S0168-1605(03)00101-6. [DOI] [PubMed] [Google Scholar]
- 10.Astoreca AL, Dalsero AM, Fernandez Pinto V, Vaamonde G. A survey on distribution and toxigenicity of Aspergillus section Flavi in poultry feeds. Int J Food Microbiol. 2011;146:38–43. doi: 10.1016/j.ijfoodmicro.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 11.Dorner JW, Cole RJ, Lomax LG, Gosser HS, Diener UL. Cyclopiazonic acid production by Aspergillus flavus and its effects on broiler chickens. Appl Environ Microbiol. 1983;46:698–703. doi: 10.1128/aem.46.3.698-703.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Urano T, Trucksess MW, Beaver RW, Wilson DM, Dorner JW, Dowell FE. Co-occurrence of cyclopiazonic acid and aflatoxins in corn and peanuts. J AOAC Int. 1992;75:838–841. [Google Scholar]
- 13.Horn BW, Dorner JW. Regional differences in production of aflatoxin B1 and cyclopiazonic acid by soil isolates of Aspergillus flavus along a transect within the United States. Appl Environ Microbiol. 1999;65:1444–1449. doi: 10.1128/aem.65.4.1444-1449.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donner M, Atehnkeng J, Sikora RA, Bandyopadhyay R, Cotty PJ. Distribution of Aspergillus section Flavi in soils of maize fields in three agroecological zones of Nigeria. Soil Biol Biochem. 2009;41:37–44. doi: 10.1016/j.soilbio.2008.09.013. [DOI] [Google Scholar]
- 15.Pitt JI, Hocking AD, Glenn DR. An improved medium for the detection of Aspergillus flavus and A. parasiticus. J Appl Bacteriol. 1983;54:109–114. doi: 10.1111/j.1365-2672.1983.tb01307.x. [DOI] [PubMed] [Google Scholar]
- 16.Razzaghi-Abyaneh M, Shams-Ghahfarokhi M, Allameh A, Kazeroon-Shiri A, Ranjbar-Bahadori S, Mirzahoseini H, Rezaee MB. A survey on distribution of Aspergillus section Flavi in corn field soils in Iran: population patterns based on aflatoxins, cyclopiazonic acid and sclerotia production. Mycopathologia. 2006;161:183–192. doi: 10.1007/s11046-005-0242-8. [DOI] [PubMed] [Google Scholar]
- 17.Klich MA, Pitt JI. A laboratory guide to the common Aspergillus species and their teleomorphs. Sydney: Commonwealth Scientific and Industrial Research Organization, Division of Food Processing; 1988. [Google Scholar]
- 18.Yabe K, Nakamura H, Ando Y, Terakudo N, Nakajima H, Hamasaki T. Isolation and characterization of Aspergillus parasiticus mutants with impaired aflatoxin production by novel tip culture method. Appl Environ Microbiol. 1988;54:2096–2100. doi: 10.1128/aem.54.8.2096-2100.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dorner JW. Production of cyclopiazonic acid by Aspergillus tamarii Kita. Appl Environ Microbiol. 1983;46:1435–1437. doi: 10.1128/aem.46.6.1435-1437.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Razzaghi-Abyaneh M, Yoshinari T, Shams-Ghahfarokhi M, Rezaee MB, Nagasawa H, Sakuda S. Dillapiol and apiol as specific inhibitors for the biosynthesis of aflatoxin G1 in Aspergillus parasiticus. Biosci Biotechnol Biochem. 2007;71:2329–2332. doi: 10.1271/bbb.70264. [DOI] [PubMed] [Google Scholar]
- 21.Tran-Dinh N, Kennedy I, Bui T, Carter D. Survey of Vietnamese peanuts, corn and soil for the presence of Aspergillus flavus and Aspergillus parasiticus. Mycopathologia. 2009;168:257–268. doi: 10.1007/s11046-009-9221-9. [DOI] [PubMed] [Google Scholar]
- 22.Lomax LG, Cole RJ, Dorner JW. The toxicity of cyclopiazonic acid in weaned pigs. Vet Pathol. 1984;21:418–424. doi: 10.1177/030098588402100408. [DOI] [PubMed] [Google Scholar]
- 23.Rao LB, Husain A. Presence of cyclopiazonic acid in kodo millet (Paspalum scrobiculatum) causing ‘kodua poisoning’ in man and its production by associated fungi. Mycopathologia. 1985;89:177–180. doi: 10.1007/BF00447028. [DOI] [PubMed] [Google Scholar]
- 24.Antony M, Shukla Y, Janardhanan KK. Potential risk of acute hepatotoxicity of kodo poisoning due to exposure to cyclopiazonic acid. J Ethnopharmacol. 2003;87:211–214. doi: 10.1016/S0378-8741(03)00146-6. [DOI] [PubMed] [Google Scholar]
- 25.Giorni P, Magan N, Pietri A, Bertuzzi T, Battilani P. Studies on Aspergillus section Flavi isolated from maize in northern Italy. Int J Food Microbiol. 2007;113:330–338. doi: 10.1016/j.ijfoodmicro.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Manabe M, Tsuruta O, Tanaka K, Matsuura S. Distribution of aflatoxin-producing fungi in soil in Japan. Trans Mycol Soc Jpn. 1976;17:436–444. [Google Scholar]
- 27.Lillehoj EB, McMillian WW, Guthrie WD, Barry D. Aflatoxin-producing fungi in preharvest corn: inoculums source in insects and soils. J Environ Qual. 1980;9:691–694. doi: 10.2134/jeq1980.00472425000900040030x. [DOI] [Google Scholar]
- 28.Horn BW, Greene RL, Dorner JW. Effect of corn and peanut cultivation on soil populations of Aspergillus flavus and A. parasiticus in southwestern Georgia. Appl Environ Microbiol. 1995;61:2472–2475. doi: 10.1128/aem.61.7.2472-2475.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barros G, Torres A, Palacio G, Chulze S. Aspergillus species from section Flavi isolated from soil at planting and harvest time in peanut-growing regions of Argentina. J Sci Food Agric. 2003;83:1303–1307. doi: 10.1002/jsfa.1539. [DOI] [Google Scholar]
- 30.Barros G, Torres A, Chulze S. Aspergillus flavus population isolated from soil of Argentina’s peanut-growing region. Sclerotia production and toxigenic profile. J Sci Food Agric. 2005;85:2349–2353. doi: 10.1002/jsfa.2257. [DOI] [Google Scholar]
- 31.Sultan Y, Magan N. Mycotoxigenic fungi in peanuts from different geographic regions of Egypt. Mycotoxin Res. 2010;26:133–140. doi: 10.1007/s12550-010-0048-5. [DOI] [PubMed] [Google Scholar]
- 32.Atayde DD, Reis TA, Godoy IJ, Zorzete P, Reis GM, Corrêa B. Mycobiota and aflatoxins in a peanut variety grown in different regions in the state of São Paulo, Brazil. Crop Prot. 2012;33:7–12. doi: 10.1016/j.cropro.2011.11.013. [DOI] [Google Scholar]
- 33.Nesci A, Montemarani A, Etcheverry M. Assessment of mycoflora and infestation of insects, vector of Aspergillus section Flavi, in stored peanut from Argentina. Mycotoxin Res. 2011;27:5–12. doi: 10.1007/s12550-010-0069-0. [DOI] [PubMed] [Google Scholar]
- 34.Rodrigues P, Venancio A, Kozakiewicz Z, Lima N. A polyphasic approach to the identification of aflatoxigenic and non-aflatoxigenic strains of Aspergillus section Flavi isolated from Portuguese almonds. Int J Food Microbiol. 2008;129:187–193. doi: 10.1016/j.ijfoodmicro.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 35.Willetts HJ, Bullock S. Developmental biology of sclerotia. Mycol Res. 1992;96:801–816. doi: 10.1016/S0953-7562(09)81027-7. [DOI] [Google Scholar]
- 36.Horn BW, Greene R, Sobolev VS, Dorner JW, Powell JH, Layton RC. Association of morphology and mycotoxin production with vegetative compatibility groups in A. flavus, A. parasiticus, and A. tamarii. Mycologia. 1996;88:574–587. doi: 10.2307/3761151. [DOI] [Google Scholar]
- 37.Cotty PJ, Bayman P, Egel DS, Elis KS. Agriculture, aflatoxins and Aspergillus. In: Powell KA, Renwick A, Peberdy JF, editors. The genus Aspergillus: from taxonomy and genetics to industrial applications. New York: Plenum Press; 1994. pp. 1–27. [Google Scholar]