Abstract
Ligninolytic enzyme complexes are involved in lignin degradation. Among them laccases are outstanding because they use molecular oxygen as a co-substrate instead of hydrogen peroxide as used by peroxidases. Bacterial laccase of Bacillus genus was first reported in Claus and Filip (Microbiol Res 152:209–216, 1997), since then more bacterial laccases have been found. In this research, laccase-producing bacteria were screened from pulp and paper industry wastewater, bagass and sugarcane rhizosphere. Nutrient agar medium containing 0.5 mM of guaiacol was used. It was observed that the laccase-producing strains developed brown colour from which 16 strains of Bacillus were identified. One of the isolated strains was identified as Bacillus subtilis WPI based on the results of biochemical tests and 16S rDNA sequence analysis. This strain showed laccase-like activity towards the oxidizing substrates ABTS and guaiacol. In this study guaiacol was used as the substrate of laccase activity assay. For determination of laccase activity of this isolate guaiacol was used as a substrate of assay for the first time in this study. SDS-PAGE and Native-PAGE confirmed the presence of laccase.
Keywords: Laccase, Bacillus subtilis, Pulp and paper wastewater, Isolation, Guaiacol
Introduction
Laccases (benzenediol: oxygen oxidoreductases; EC 1.10.3.2) are copper-containing enzymes that belong to the so-called blue copper oxidases. These enzymes are responsible for the oxidation of a variety of phenolic compounds as well as aromatic amines with the reduction of molecular oxygen to water [2]. In addition, in the presence of small molecular-weight compounds named redox mediators, laccases are able to oxidize non-phenolic structures [3]. This together with the fact that they only use molecular oxygen as a co-substrate instead of hydrogen peroxides as used by peroxides, make laccases very attractive for different biotechnological applications.
Laccase and laccase-producing microorganisms play an important role in bioremediation of aromatic substances from contaminated soils, industrial pollutants and xenobiotics. The most extensively studied applications of laccases include denim finishing [4], pulp delignification, textile dye bleaching, wastewater detoxification and transformation of antibiotics and steroids [5]. Laccases are generally found in plants and fungi [6], but they have also been reported in a few bacteria including Azospirillum lipoferum [7], Bacillus sphaericus [1], Marinomonas mediterranea [8] and Streptomyces griseus [9]. Although fungal laccases have a higher reduction potential of type I copper than bacterial laccases and this makes them more suitable for commercial applications [10] their use present the following drawbacks: slow growth and difficulty in controlling the glycosylation degree [11]. In addition, bacterial laccases are more amenable to genetic manipulation than fungal laccases [12]. Therefore, research and study of bacterial laccases is highly interesting.
In the present study, we isolated laccase-producing bacteria from pulp and paper wastewater, sugarcane rhizosphere and bagasse. From the former, a novel laccase-producer was isolated and identified as Bacillus subtilis WPI.
Materials and Methods
Sample Collection
The samples were obtained from pulp and paper industry wastewater, sugarcane rhizosphere and bagass in Ahvaz, Iran. Soil samples were collected in sterilized plastic bags from a depth of 10–15 cm below the earth’s surface. All the samples were kept at 4 °C until used.
Isolation and Screening of Bacteria Strains
10 g of air-dried soil samples, bagass, degraded tree barks and 10 mL of wastewater were transferred to 250-mL Erlenmeyer flasks containing 100 mL of sterile saline solution (0.9 % w/v NaCl) [13]. The flasks were incubated for 120 min at 40 °C under shaking (130 rpm). Then, 1-mL aliquots were added to 9 mL sterile saline solution and serial dilutions (10−1–10−9) were prepared. About 100 μl of 10−3 and 10−5 dilutions were spread on Petri plates containing nutrient agar supplemented with 2–4 mg/mL of Amphotericin B. Individual bacterial colonies from the plates were screened on Petri plates containing nutrient agar supplemented with 0.5 mM guaiacol to detect laccase activity [14]. The plates were incubated at 25–33 °C for 96 h. The isolation and screening procedures were repeated several times until the isolates were found to be pure. The laccase-producing strains were examined for morphological, physiological and biochemical characteristics with reference to growth conditions and production of laccase. Isolated bacteria were maintained on nutrient agar medium in slants at 4 °C. Pre-culturing medium contained (w/v): nutrient broth (0.8 %), meat extract (1 %), peptone (1 %), tryptone (1 %) and NaCl (0.05 %).The cultures were incubated at 37 °C on a rotary shaker at 180 rpm. After 18 h, 5 % (v/v) of liquid culture was used to inoculate the laccase production medium containing (w/v): yeast extract (0.15 %), tryptone (0.15 %) and NaCl (0.5 %). Cultures were incubated at 37 °C on a rotary shaker at 200 rpm [12].
Laccase Activity Assay
Bacterial cultures were centrifuged (6,000×g) for 20 min at 4 °C to precipitate the cellular debris and obtain clear supernatants. Then, bacterial pellets were washed with phosphate buffer (0.1 M; pH 6.5) containing 10 mM of phenylmethylsulfonyl fluoride (PMSF) to inhibit the protease activity in the supernatant before sonication (5 times, 45 s each time with 30 s between each sonication, 20 MHz). The cell extract was obtained by centrifugation (14,000×g) at 4 °C for 20 min and used as crude intracellular enzyme.
Catalase (1,000 U/mL; Sigma-Aldrich) was added to the assay solution and incubated for 1 h at 37 °C to remove the possible effect of H2O2 produced by the bacteria [15]. Laccase activity was determined spectrophotometrically at 436 nm (ε436 = 29,300 M−1 cm−1) as described by Niku-Paavola et al. [16] with 2,2-azino-di-[3-ethylbenzo-thiazolin-sulphonate] (ABTS) as a substrate. One activity unit (U) was defined as the amount of enzyme that oxidized 1 μmol of ABTS per min at 25 °C and the activities were expressed in U/L. Laccase activity was also determined spectrophotometrically at 465 nm with 2 mM guaiacol as a substrate in a reaction mixture containing 50 mM phosphate buffer (pH 6.5) (ε465 = 48,000 M−1 cm−1). One unit of enzyme activity was defined as the amount of enzyme that increased the absorbance by 0.001 units per min at 55 °C [14]. The activities were expressed in U/L. All spectrophotometric measurements were carried out using a UV–Vis spectrophotometer (SPEKOL 2000, Germany). All assays were carried out in triplicate.
SDS-PAGE and Native-PAGE
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) of the cell extract was performed on a 13 % resolving gel and a 5 % stacking gel [17]. Prior to gel application, the samples were precipitated with trichloroaceticacid (TCA) and then denatured by boiling them for 5 min. Samples were run with pre-stained standard molecular weight marker (protein marker P-7708S, New England Biolabs, USA) in Tris–glycine buffer (pH 9.5) at room temperature and a constant current of 20 mA for 60 min. Protein bands were visualized by Coomassie Brilliant Blue R 230 (Sigma, St. Louis, MO, USA) staining. To detect laccase activity Native-PAGE was used. The procedure used was the cell extract without TCA treatment and without boiling the sample (to preserve laccase activity), no SDS was added and then trying an ABTS staining to detect specifically the band with laccase activity. The gel was rinsed with water and subsequently submerged in 10 mM succinic buffer (pH 5.0) with 5 mM ABTS where the laccase active bands were highlighted in dark green.
Extraction of Genomic DNA, PCR Amplification and 16s rDNA Sequencing
Bacterial DNA was extracted and purified following the instructions of the DNA extraction Kit (Bioneer, Korea). The quality of extracted DNA was determined on 1 % agarose gel electrophoresis.
The 16S rDNA was amplified using Universal 16S rDNA polymerase chain reaction (PCR) forward primer (CCGAATTCGTCGACAACAGAGTTTGATCCTGGCTCAG) and reverse primer (CCCGGGATCCAAGCTTACGGTTACCTTGTTACGACTT) [18]. All PCR amplifications were carried out in a thermal cycler (Biorad, USA). We used a hot-start procedure (4 min, 94 °C) before the enzyme was added to prevent non-specific annealing of the primers. Negative control (PCR mixture without template DNA) was included. DNA was amplified during 30 cycles (94 °C for 1 min, 62 °C for 30 s, 72 °C for 1 min, plus one additional cycle with a final 20 min chain elongation) [19]. Sequencing results of 16S rDNA were analyzed with BLAST from the National Centre for Biotechnology Information (NCBI, http://www.ncbi.nlm.nih.gov/). Multiple sequence alignment was performed by 16S rDNA sequences of 19 type strains. Phylogenetic tree of bacteria were conducted based on 16S rDNA genes using free trial CLC Main Workbench 5.
Biochemical Characterization
Our strains were examined for morphological, physiological and biochemical characteristics with reference to Bergey’s Manual of Systematic Bacteriology. Gram staining, catalase and cythochrom C oxidase were performed according to standard protocol. Gram’s characteristics and cell morphology of the our isolated strain were determined by microscopy. For carbon sources utilization, the pure cultures were inoculated respectively into peptone-water culture medium containing 1 % substrates, and incubated at 37 °C for 48 h. Control tubes contained uninoculated medium and were incubated at the same conditions. Some biochemical metabolic ability was determined by inoculating isolates onto selective media such as casein, urea, gelatin and starch agar, to identify protease, urease, gelatin hydrolyze and amylase producers, respectively. We used a nitrate broth medium to determine if our strain is capable of reducing nitrate (NO3−) to nitrite (NO2−) or other nitrogenous compounds via the action of the enzyme nitratase (also called nitrate reductase). After incubation, these tubes are first inspected for the presence of gas in the Durham tube. This further testing includes the addition of sulfanilic acid (often called nitrate I) and dimethyl-alpha-napthalamine (nitrate II). If nitrite is present in the media, then it will react with nitrate I and nitrate II to form a red compound. If instead, the tube turns red after the addition of Zn, this indicates a negative result. MR/VP test were used to determine which fermentation pathway of is used to utilize glucose. We used the Voges-Proskauer test to detects the presence of acetoin, a precursor of 2,3-butanediol. In order to test this pathway, an aliquot of the MR/VP culture is removed and α-naphthol and KOH were added. They are shaken together vigorously and set aside for about 1 h until the results can be read. We used SIM medium. It tests the ability of our strain to do several things: reduce sulfur, produce Indole and swim through the agar. We used Simmon citrate medium used to determine if our strain can use citrate as its sole carbon source and energy. The pure cultures were inoculated into Simmons citrate agar and SIM, and incubated at 37 °C for 24 h for All above test and other biochemical test were carried out in triplicate.
Results
Isolation and Screening of Bacillus subtilis
WPI from pulp and paper wastewater 96 different colonies were isolated in a first step. Then, they were screened on nutrient agar medium containing 0.5 mM guaiacol and, among them, 16 strains developed brown colour around the margin of the colonies (Fig. 1). These 16 strains were identified as Bacillus by biochemical methods. One of the potential strains having high level of laccase activity was selected for further studies. This strain presented colonies of 1–3 mm in diameter, white colour and was Gram positive. It was motile and capable to hydrolyze gelatine. The strain was urase, catalase, casein protease, cytochorme C oxidase and amylase positive. It was also able to reduce NO3− to NO2−and Voges-Proskauer (VP) tests reaction was positive. Citrate test and Indol production were negative. The strain was facultative anaerobic and was able to produce acid compounds from utilized glucose and sucrose. The strain was not able to use manitol, lactose and xylose (Table 1). PCR amplification and sequencing of the 16S rDNA gene of this strain was done. Product of PCR amplification was about 1,500 bp (Fig. 2). 16S rDNA sequence was edited to a total length 7 of 1,391 bp after direct sequencing. Phylogenetic tree of bacteria based on 16S rDNA genes is shown in (Fig. 3). The morphological and biochemical characteristics of this strain according to Bergey’s Manual of Systematic Bacteriology and the comparative analysis of 16S rDNA sequence showed that it was close to the members of B. subtilis. The obtained 16S rDNA sequence was deposited in Gene Bank under accession No JN082773 for isolate B. subtilis WPI.
Fig. 1.
Screening of isolated strains on Petri plates containing nutrient agar medium. aBacillus subtilis WPI on nutrient agar medium without guaiacol; bB. subtilis WPI on nutrient agar medium containing guaiacol
Table 1.
The morphological and biochemical characteristics of bacterial isolate (Bacillussubtilis WP1)
| Characteristics | Bacillus subtilis WPI |
|---|---|
| Colony diameter | 1–3 mm |
| Colony color | White |
| Cell morphology | Rod |
| Gram staining | + |
| Motility | + |
| Gelatin hydrolysis | + |
| Indol | − |
| Urase | + |
| Oxidase | + |
| Catalase | + |
| Casein protease | + |
| Amylase | + |
| NO3− reduction to NO2− | + |
| VP reaction | + |
| Utilization of: | |
| Manitol | − |
| Lactose | − |
| Glucose | + |
| Citrate | − |
| Xylose | − |
| Sucrose | + |
Fig. 2.

PCR products of 16S rDNA from Bacillus subtilis WPI (1500 bp). Lane L: molecular weight marker 1 KB (Fermentas, UK); Lane S: B. subtilis WPI
Fig. 3.
Phylogenetic tree of 16S rDNA sequence of Bacillus subtilis WPI strain and related taxa
Enzyme Activity
We studied laccase activity of B. subtilis at stationary phase of growth with different methods and substrates. B. subtilis supernatant was able to oxidize ABTS and guaiacol (Fig. 4) shows the enzyme solution with these two substrates and the ability of laccase to oxidize them. The activity of laccase for this strain was higher with ABTS (2.28 U/L) than with guaiacol (1.6 U/L).
Fig. 4.
The assay solution with different substrates. a the assay solution with ABTS (a) before adding ABTS, (b) after adding ABTS. b the assay solution with guaiacol (a) before adding guaiacol, (b) after adding guaiacol
SDS-PAGE and Native-PAGE
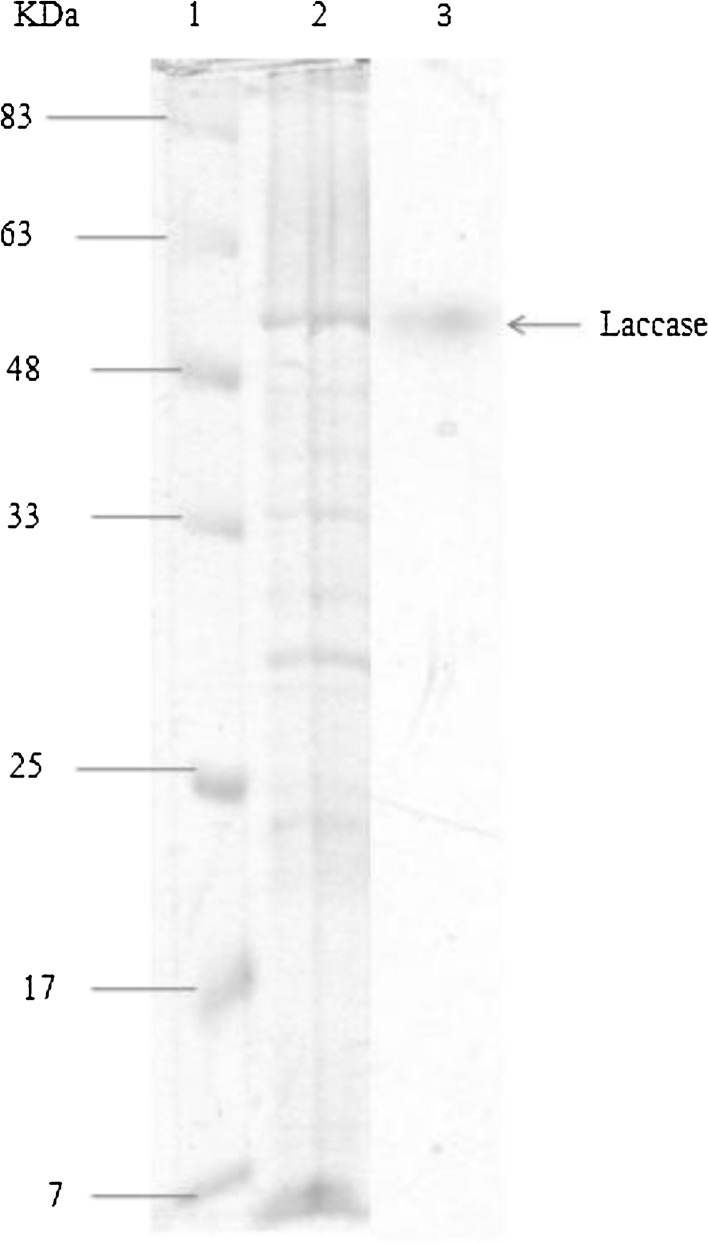
Molecular weight of B. subtilis WPI laccase was determined by SDS-PAGE. Thus, SDS-PAGE showed different protein bands of which the band at 55 kDa corresponded to laccase as revealed by activity staining of Native-PAGE (Fig. 5).
Fig. 5.
SDS-PAGE and Native PAGE of the cell extract of Bacillus subtilis WP1. Lane 1 Pre-stained size marker of protein Lane 2B. subtilis WPI cell extract SDS-PAGE. Lane 3: laccase activity was detected by activity staining with ABTS in succinic buffer (pH 5.0). The loaded samples on the gel were the soluble proteins released after sonication (for more details please see the “Materials and methods” section)
Discussion
In this research, 16 strains belonging to genus Bacillus were screened on nutrient agar medium containing guaiacol. One novel strain with high laccase-producing potential was isolated and identified as B. subtilis WPI by biochemical and molecular methods. This strain was isolated from wastewater of a pulp and paper industry in Iran. The laccase produced by B. subtilisWPI was more sensitive towards ABTS than guaiacol as a substrate. This is in agreement with the results reported by Liers et al. [20] for a purified laccase from the AscomyceteXylariapolymorpha and Li et al. [21] for laccase from the BasidiomyceteTrametes sp. It has been reported that the lower laccase activity towards guaiacol is due to laccase inactivation by reaction products [22]. Laccase in general combine high affinity for ABTS and syringaldazine with high catalytic constant, whereas the oxidation of guaiacol is considerably slower and the respective Km constant higher. In this research the highest laccase activity was obtained with ABTS and enzyme band was detected by activity staining with ABTS. Also in this study Catalysis of guaiacol by a B. subtilis was assayed for the first time.
Fungal laccases have been extensively described by several substrates such as the classes of chemical compounds including (1) o-diphenols (catechol, pyrogallol, guaiacol, and protocatechic), (2) p-diphenoland p-substitute daromatic compounds as typical p-phenol oxidase (p-cresol, p-aminophenol), (3) m-diphenolssuch as resorcinol, orcinol and (4) Other laccase substrates such as syringaldazine, 1-naphthol, ABTS but no data on B. subtilis laccase activity has been reported specially guaiacol (o-diphenols). Whereas guaiacol was more appropriate for screening laccase producer B. subtilis but was not efficient for assaying laccase activity of B. subtilis.
The laccase from B. subtilis WPI had a molecular weight of 55 kDa and was determined by SDS-PAGE (Ruijssenaars and Hartmans [23]. A molecular weight of 56 kDa for a laccase from Bacillus halodurans and 50 kDa of from a Bacillus sp. HR03 has been determined [14]. Niladevi et al. [24] have found that an unusual halotolerant-alkaline laccase from Streptomyces psammoticus has a molecular mass of about 43 kDa and recently Mohammadian et al. [25] determined a molecular weight of 65 kDa for a laccase from Bacillus sp. HR03. More recently, Reiss et al. [26] estimated a molecular weight of 58 kDa for a purified CotA-type laccase from Bacillus pumilus.
Acknowledgments
This research was supported by Biotechnology and Biological Research Center of Shahid Chamran University of Ahvaz (Iran).
References
- 1.Claus H, Filip Z. The evidence of a laccase-like enzyme activity in a Bacillus sphaericus strain. Microbiol Res. 1997;152:209–216. doi: 10.1016/S0944-5013(97)80014-6. [DOI] [Google Scholar]
- 2.Thurston CF. The structure and function of fungal laccases. Microbiology. 1994;140:19–26. doi: 10.1099/13500872-140-1-19. [DOI] [Google Scholar]
- 3.Desai SS, Nityanand C. Microbial laccases and their application. Asian J Biotechnol. 2011;3:98–124. doi: 10.3923/ajbkr.2011.98.124. [DOI] [Google Scholar]
- 4.Pazarlıoglu NK, Sariisik M, Telefoncu A. Laccase: production by Trametes versicolor and application to denim washing. Process Biochem. 2005;40(1):673–1678. [Google Scholar]
- 5.Xu F. Applications of oxidoreductases: recent progress. Ind Biotechol. 2005;1:38–50. doi: 10.1089/ind.2005.1.38. [DOI] [Google Scholar]
- 6.Gardiol AE, Hernandez RJ, Harte BR (1998) Device for detecting oxygen with oxidase, US Pat. 5, 804, 401
- 7.Faure D, Bouillant ML, Bally R. Isolation of Azospirillum lipoferum 4T mutants affected in melanization and laccase activity. Appl Environ Microbiol. 1994;60:3413–3415. doi: 10.1128/aem.60.9.3413-3415.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Endo K, Hosono K, Beppu T, Ueda K. A novel extra cytoplasmatic phenol oxidase of Streptomyces: its possible involvement in the onset of morphogenesis. Microbiology. 2002;148:1767–1776. doi: 10.1099/00221287-148-6-1767. [DOI] [PubMed] [Google Scholar]
- 9.Hullo MF, Moszer I, Danchin A, Martin-Verstraete I. CotA of Bacillus subtilisis a copper-dependent laccase. J Bacteriol. 2001;183:5426–5430. doi: 10.1128/JB.183.18.5426-5430.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Augustine AJ, Kragh ME, Sarangi R, Fujii S, Liboiron BD, Stoj CS, Kosman DJ, Hodgson KO, Hedman B, Solomon EI. Spectroscopic studies of perturbed T1 Cu sites in the multicopper oxidases Saccharmycescerevisiae Fet3p and Rhusvernicifera laccase: allosteric coupling between the T1 and trinuclear Cu sites. Biochemistry. 2008;47:2036–2045. doi: 10.1021/bi7020052. [DOI] [PubMed] [Google Scholar]
- 11.Rodgers CJ, Blanford CF, Giddens SR, Skamnioti P, Armstrong FA, Gurr SJ. Designer laccases: a vogue for high-potential fungal enzymes? Trends Biotechnol. 2010;28:63–72. doi: 10.1016/j.tibtech.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Koschorreck K, Schmid RD, Urlacher VB. Improving the functional expression of a Bacillus licheniformis laccase by random and site-directed mutagenesis. BMC Biotechnol. 2009;9:12–21. doi: 10.1186/1472-6750-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.BadoeiDalfard A, Khajeh K, Soudi MR, Naderi-Manesh H, Ranjbar B, Hassan Sajed R. Isolation and biochemical characterization of laccase and tyrosinase activities in a novel melanogenic soil bacterium. Enzyme Microb Technol. 2006;39:1409–1416. doi: 10.1016/j.enzmictec.2006.03.029. [DOI] [Google Scholar]
- 14.Bains J, Capalash N, Sharma P. Laccase from a non-melanogenic, alkalotolerantγ-proteobacterium JB isolated from industrial wastewater drained soil. Biotechnol Lett. 2003;25(1):155–1159. doi: 10.1023/A:1024569722413. [DOI] [PubMed] [Google Scholar]
- 15.Machado KMG, Matheus DR. “Potential of a ligninolytic enzymatic complex produced by Pleurotusostreatus during growth on solid substrate for the biodegradation of organic pollutants”Brazilian. J Microbiol. 2006;37:468–473. [Google Scholar]
- 16.Niku-Paavola ML, Raaska L, Itvaara M. Detection of white-rot fungi by a nontoxic stain. Mycol Res. 1990;94:27–31. doi: 10.1016/S0953-7562(09)81260-4. [DOI] [Google Scholar]
- 17.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacterio phage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18.Papizadeh M, Roayaei Ardakani M, Ebrahimipour Gh, Motamedi H. Utilization of dibenzothiophene as sulfur source by Microbacterium sp. NISOC-06. World J Microbiol Biotechnol. 2010;26:1195–1200. doi: 10.1007/s11274-009-0288-8. [DOI] [PubMed] [Google Scholar]
- 19.Duarte GF, Rosado AS, Seldin L, Araujo W, Van Elsas JD. Analysis of bacterial community structure in sulfurous-oil containing soils and detection of species carrying dibenzothiophene desulfurization (dsz) genes. Appl Environ Microbiol. 2001;67:1052–1062. doi: 10.1128/AEM.67.3.1052-1062.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liers C, Ullrich R, Pecyna M, Schlosser D, Hofrichter M. Production, purification and partial enzymatic and molecular characterization of a laccase from the wood-rotting ascomycete Xylariapolymorpha. Enzyme Microbial Technol. 2007;41:785–793. doi: 10.1016/j.enzmictec.2007.07.002. [DOI] [Google Scholar]
- 21.Li A, Zhu Y, Xu L, Zhu W, Tian X. Comparative study on the determination of assay for laccase of Trametes sp. Af J Biochem Res. 2008;2:181–183. [Google Scholar]
- 22.Robles A, Lucas R, Cienfuegos GA, Galvez A. Phenol-oxidase (laccase) activity in strains of the hyphomycete Chalaraparadoxa isolated from olive mill wastewater disposal ponds. Enzyme Microb Technol. 2000;26:484–490. doi: 10.1016/S0141-0229(99)00197-0. [DOI] [PubMed] [Google Scholar]
- 23.Ruijssenaars HJ, Hartmans S. A cloned Bacillus halodurans multicopper oxidase exhibiting alkaline laccase activity. Appl Microbiol Biotechnol. 2004;65:177–182. doi: 10.1007/s00253-004-1571-0. [DOI] [PubMed] [Google Scholar]
- 24.Niladevi KN, Jacob N, Prema P. Evidence for a halotolerant-alkaline laccase in Streptomyces psammoticus Purification and characterization. Process Biochem. 2008;43:654–660. doi: 10.1016/j.procbio.2008.02.002. [DOI] [Google Scholar]
- 25.Mohammadian M, Fathi-Roudsari M, Mollania N, Badoei-Dalfard A, Khajeh K. Enhanced expression of a recombinant bacterial laccase at low temperature and microaerobic conditions: purification and biochemical characterization. J Ind Microbiol Biotechnol. 2010;37:863–869. doi: 10.1007/s10295-010-0734-5. [DOI] [PubMed] [Google Scholar]
- 26.Reiss R, Ihssen J, Thony-Meyer L. Bacillus pumilus laccase: a heat stable enzyme with a wide substrate spectrum. BMC Biotechnol. 2011;11:1–11. doi: 10.1186/1472-6750-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]