Abstract
A xylanase gene (xynZF-2) from the Aspergillus niger XZ-3S was cloned and expressed in Escherichia coli. The coding region of the gene was separated by only one intron with the 68 bp in length. It encoded 225 amino acid residues of a protein with a calculated molecular weight of 24.04 kDa plus a signal peptide of 18 amino acids. The amino acid sequence of the xynZF-2 gene had a high similarity with those of family 11 of glycosyl hydrolases reported from other microorganisms. The mature peptide encoding cDNA was subcloned into pET-28a(+) expression vector. The resultant recombinant plasmid pET-28a-xynZF-2 was transformed into E. coli BL21(DE3), and finally the recombinant strain BL21/xynZF-2 was obtained. A maximum activity of 42.33 U/mg was gained from cellular of E. coli BL21/xynZF-2 induced by IPTG. The optimum temperature and pH for recombinant enzyme which has a good stability in alkaline conditions were 40 °C and 5.0, respectively. Fe3+ had an active effect on the enzyme obviously.
Keywords: Aspergillus niger, Xylanase, Cloning, Expression, Enzymatic properties
Introduction
The largest renewable carbon source on earth is the cell wall of plants, which consist primarily of cellulose, hemicellulose, pectin and lignin [1]. Among these components, hemicellulose is the second-most abundant fraction after cellulose [2]. Xylan which is the main component of plant hemicellulose has a very complex structure. Complete breakdown of this complex polymer requires the action of several enzymes [3]. Among them, the most important one is the β-1,4-xylanase (EC 3.2.1.8), which cleaves internal glycosidic bonds at random or specific positions of the xylan backbone and thus hydrolyzes xylan into xylooligosaccharide and xylose [4]. Applications of xylanases began in the 1980s in the preparation of animal feed, and later developed to the pulp and paper, food, brewing and textile industries. Since then the demands for xylanases have increased dramatically, covering a wide range of industrial fields [5–7].
Based on amino acid sequence homologies and hydrophobic cluster analysis, xylanases have been grouped mainly into two families of glycosyl hydrolases: family GH10 and family GH11 [8, 9]. And the catalysis area of xylanase has the high homology in the same family. Xylanases of family GH11 are mostly single domains with a relatively low molecular weight (MW) (19–25 kDa), the optimum temperature 50–60 °C and a β-jelly roll structure compared to the family F/10 xylanases that are more domains, not only the catalytic domain, but also cellulose binding domain, with a high MW (>30 kDa), the optimum temperature 60–80 °C and a (αβ)8 barrel structure [4, 10]. The major enzymes comprising the GH10 family are endo-1,4-β-xylanases and a small number of endo-1,3-β-xylanases (EC 3.2.1.32). By contrast, the GH11 family consists solely of endo-1,4-β-xylanases and usually gives larger end-products than the GH10 family members [11].
Since 1983, Bernier et al have isolated xylanase gene from Bacillus subtilis PAP II 5 for the first time. Many kinds of xylanases have been cloned [12–17], and expressed in heterologous hosts [13–19]. Although many xylanase genes have been described from diverse microorganisms, relatively little is known about xylanase gene from Aspergillus niger XZ-3S.
Aspergillus niger XZ-3S is a high yield xylanase strains preserved in this laboratory. Here, we reported the sequence, analysis, cloning and expression of xynZF-2 gene from A. niger XZ-3S in Escherichia coli BL21(DE3). To our knowledge, this is the first report describing a xylanase gene from A. niger XZ-3S.
Materials and Methods
Strains and Plasmids
Aspergillus niger XZ-3S, isolated from soil in China as reported previously [20], was used as a source of xylanase and as a RNA and DNA donor strain. E. coli JM109 and BL21(DE3) (Novagen, USA) were used as host cells for gene cloning and expression, respectively. pUCm-T vector for cloning of PCR products was purchased from Shanghai Sangon Co., Ltd. Expression vector pET-28a(+) was obtained from Novagen (Madison, WI).
Reagents
TRIzol reagent, saturated phenol, Ampicillin (Amp), Kanamycin (Kan), and UNIQ-10 Column DNA Gel Extraction Kit were purchased from Sangon (Shanghai, China) Co. Ltd. RNA PCR Kit (AMV) Ver. 3.0, T4 DNA ligase, Taq polymerase, IPTG, X-gal and restriction enzymes were purchased from TaKaRa Biotechnology (Dalian, China) Co. Ltd. Tryptone, yeast extract, and agarose were obtained from BBI (Markham, Canada). The HiTrap affinity column for Ni2+-chelating chromatography was from Amersham Pharmacia Biotech (Uoosala, Sweden). All other chemicals were of analytical grade.
Medium
Luria–Bertani (LB) medium (per liter: 10 g tryptone, 5 g yeast extract and 10 g NaCl, pH 7.4) was used for culturing E. coli. LB solid supplemented with 20 g/l agarose. A. niger liquid fermentation medium (per liter: 10 g tryptone, 5 g yeast extract, 10 g glucose and 200 ml corn cob extract which was made of corn core and prepared by alkaline hydrolysis (pH 9.0) for 2 h at 80 °C, natural pH) was used for culturing A. niger XZ-3S strains for 36 h at 28 °C on a rotary shaker (110 rpm), and then collecting mycelia was used for the XZ-3S total RNA, total genomic DNA extraction.
Total RNA and Genomic DNA Isolation
The mycelia were collected through filtration and washed several times with deionized water. The total RNA was extracted from the mycelia in a one-step method TRIzol (Sangon, China). Extracted total RNA had a ratio of A260–A280 of 1.96. The 18S and 28S rRNA bands, on formaldehyde denatured agarose gel electrophoresis were fungi specific (data not shown). Indicating that the total RNA had high purity and was not decomposed. Extraction of the genomic DNA from A. niger XZ-3S was performed according to the articles [21].
Primers for PCR Amplification
After aligning the sequences of GHF 11 xylanases from the fungi, Aspergillus usamii (Genbank accession number: ABC26493), A. sulphureus (Genbank accession number: AAZ95432), A. tubingensis (Genbank accession number: CAA02442), and A. nidulans (Genbank accession number: CBF75776), we found that there is a peptide fragment, MLTKNLL located in the signal peptide region, with the highest identity. A degenerate primer P1 [5′-ATGCT(G/C)ACCAAGAACCT(G/C/T)CT(G/C)-3′] was designed using the CODEHOP method (http://bioinformatics.weizmann.ac.il/blocks/codehop.html). Primer M13 (original name, M13 Primer M4) [GTTTTCCCAGTCACGAC] and primer OT (original name, Oligo-dT-Adaptor Primer) [GTTTTCCCAGTCACGAC-Oligo dT] were provided by the TaKaRa RNA PCR Kit (AMV) Ver.3.0. For expression of xynZF-2 cDNA on pET-28a in E. coli, forward primer BC [5′-CCGGAATTCGTTCCCCACGACTCTGTCG-3′, with the EcoRΙ site] and reverse primer BZ [5′-CCCAAGCTTTTACTGAACAGTGATGGACG-3′, with the HindIII site] were used for the introduction of restriction sites.
Amplification of the Target Gene
The 3′-end region of xynZF-2 cDNA, originating from the starting codon ATG, was amplified by using RNA PCR Kit (TaKaRa, China). Primer OT was used as the primer for reverse transcription of the first-strand cDNA. Using the resulting first-strand cDNA as the template, PCR amplification was performed with primers P1 and M13 as following conditions: a denaturation at 94 °C for 2 min; 30 cycles of at 94 °C for 30 s, 52 °C for 30 s, 72 °C for 1 min; an elongation at 72 °C for 10 min.
According to the cDNA sequence of the xynZF-2, a reverse primer P2 [TTACTGAACAGTGATGGACGAAGA] was designed corresponding to the transcription terminal region. The conditions of PCR amplification were: a denaturation at 95 °C for 10 min; 30 cycles of at 94 °C for 30 s, 52 °C for 45 s, 72 °C for 1 min; an elongation at 72 °C for 10 min. Using genomic DNA from A. niger XZ-3S as the template, the complete DNA sequence was directly obtained by PCR amplification with primers P1 and P2.
DNA Manipulations and E. coli Transformation
Digestion of DNA with restriction endonucleases, separation of fragments by agarose gel electrophoresis, ligation of DNA fragments, transformation of E. coli with plasmidic DNA and extraction of recombinant DNA were all performed according to the standard method [22].
Nucleotide Sequence Accession Number and Sequence Analysis
The GenBank accession number of xynZF-2 cDNA sequence is JQ700382.
Nucleotide and deduced amino acid sequences were analyzed using the ExPASy Proteomics tools (http://www.expasy.org/tools/). The homology of the sequence was processed with DNAMAN 5.0. Signal peptide was analyzed by SignalP 4.0 server (http://www.cbs.dtu.dk/services/SignalP/). The theoretical isoelectric (pI) and molecular weight (MW) were calculated by Compute pI/MW tool (http://web.expasy.org/compute_pi/). Secondary structure and homology modeling of three-dimensional structure were predicted using CFSSP (Chou & Fasman Secondary Structure Prediction Server) (http://www.biogem.org/tool/chou-fasman/) and SWISS-MODEL (http://swissmodel.expasy.org/workspace/index.php?func=modelling_simple1).
XynZF-2 Expression and Purification
After the complete xynZF-2 gene sequence was obtained, the cDNA fragment encoding the mature peptide of xylanase was amplified with forward primer BC and reverse primer BZ. The DNA fragment was ligated into pET-28a(+) with restriction sites of EcoRΙ and HindШ to generate construct pET-28a-xynZF-2. The expression construct was used to transform E. coli BL21(DE3) for expression with the plasmid pET-28a(+) for control. The transformants were screened on LB broth supplemented with 100 μg/ml kanamycin and cultured with shaking at 37 °C overnight. Twenty microliters of seed culture were transferred into fresh medium and cultured until A600 reached 0.6, and IPTG (final concentration 2 mM) was then added for induction. The bacterial cells were cultured for another 6 h at 29 °C before collection by centrifuge. Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) was applied for conformation of the expressed product.
For xylanase purification, all operations were performed at 4 °C unless otherwise mentioned. After growth in liquid medium, cells were harvested by centrifugation, washed with cold saline, and suspended in 10 ml of cold sodium citrate buffer, pH 4.6. Cells were homogenized by ultrasonic treatment. Supernatant was obtained by centrifugation at 12,000 rpm for 10 min at 4 °C. The crude extract of enzyme was purified by Ni2+ affinity chromatography according to the instructions provided by the manufacturer. The homogeneity of the purified enzyme was monitored by SDS-PAGE. SDS-PAGE was performed in a 12 % (w/v) polyacrylamide gel according to the method of Laemmli [23]. Protein bands were visualized by Coomassie brilliant blue R-250 staining. Protein was determined by the Bradford assay [24] using bovine serum albumin as a standard.
Xylanase Activity Assays
Xylanase activity was measured using 0.5 % (w/v) birchwood xylan (Sigma, USA) as a substrate in 50 mM Na2HPO4–citric acid buffer, pH 4.6, at 40 °C for 15 min. The liberation of reducing sugars was estimated by the dinitrosalicylic acid method [25] using xylose as a standard. Reducing sugars were determined by measuring the absorption at 540 nm relative to a d-xylose standard. One unit of enzyme activity was defined as the quantity of enzyme required to liberate 1 μmol of xylose equivalent per minute at 40 °C, and specific activity was defined as units per mg protein. The results were means of duplicate determination on triple independent measurements.
Properties of Purified XynZF
The temperature optimum was measured by performing the xylanase activity assay for 15 min at temperatures ranging from 35 to 60 °C under pH 4.6 (Na2HPO4–citric acid buffer). The thermostability of xylanase was investigated at temperatures 35, 40, 45, and 50 °C after incubation of the enzyme solutions in absence of substrate for 5, 10, 15, 20, 40 or 60 min, respectively. Residual activities were determined under xylanase activity assay conditions.
The effect of pH on xylanase activity was evaluated at the optimal temperature over a pH range of 3.0–9.0, using appropriate buffers (50 mM): Na2HPO4–citric acid buffer (pH 3.0–5.0), Na2HPO4–NaH2PO4 buffer (pH 6.0–7.0), Tris–HCl buffer (pH 8.0) and glycin–NaOH buffer (pH 9.0) under xylanase activity assay conditions. Further study on the pH stability of the recombinant xylanase was carried out at 40 °C by pre-incubation of the enzyme solutions in the aforementioned buffer systems in the absence of substrate at 40 °C for 1 h. The pH values of various reaction solutions were adjusted to pH 4.6. Then they were subjected to xylanase activity assay.
Metal ions were generally considered as important factors affecting microbial enzyme activity. Each metal ion and EDTA (5 mM) was added in 5 ml of diluted enzyme to a final concentration of 1 mM. The effects of these additives were investigated after 1 h of incubation. The system without any additive was used as a control.
Results and Discussion
Gene Cloning and Sequence Analysis
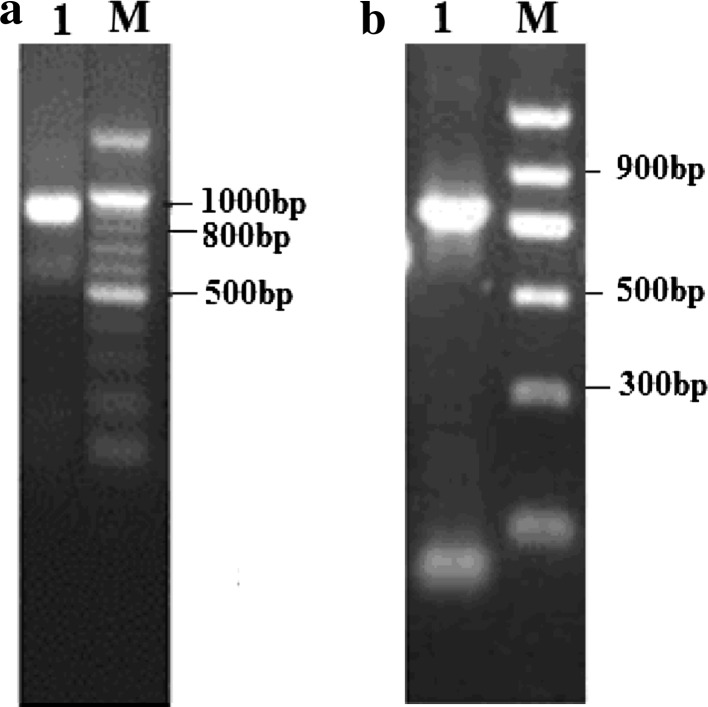
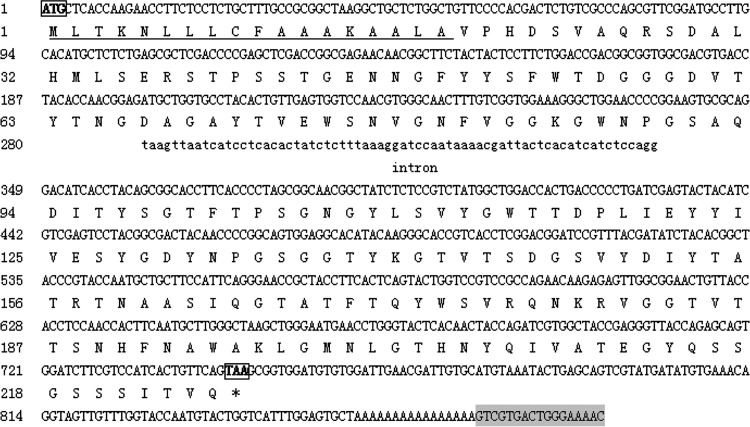
A 818 bp xylanase cDNA sequence was amplified by RT-PCR from the total RNA of A. niger XZ-3S (Fig. 1a), as well as a 746 bp xylanase DNA sequence was amplified by PCR from the genomic DNA (Fig. 2b). The sequencing results showed that the full-length of RT-PCR product was the xynZF-2 cDNA, which was 785 bp in length [not including the poly(A)] harboring 3′-non-encoding regions, as well as an ORF of 678 bp that encoded a 18-aa signal peptide and a 207-aa mature peptide with a calculated MW of 24.04 kDa, isoelectric point (pI) of 5.20 and the formula C1065H1577N283O349S4. The sequence of 746 bp DNA fragment contained the complete nucleotide of the cDNA with one short intervening intron as indicated in Fig. 2.
Fig. 1.
Cloning of the xynZF-2 gene from A. niger XZ-3S. a Amplification of a cDNA fragment encoding the mature peptide. Lane 1 cDNA transcripted with OT was amplified with degenerative primer P1 together with primer M13; lane M DNA marker. b Amplification of the DNA fragment encoding the mature peptide. Lane 1 a 746 bp fragment was obtained by PCR amplification with primers P1 and P2; lane M DNA marker
Fig. 2.
Nucleotide sequence of the xynZF-2 gene from A. niger XZ-3S and its deduced amino acid sequence DNA sequence of intron is shown in lowercase letters. The signal peptide is underlined. The bold letters in boxes, ATG and TAA, indicate the start codon and stop codon. The reverse complementary sequence of M13 primer (5′-GTTTTCCCAGTCACGAC-3′) is showed as the shadow region
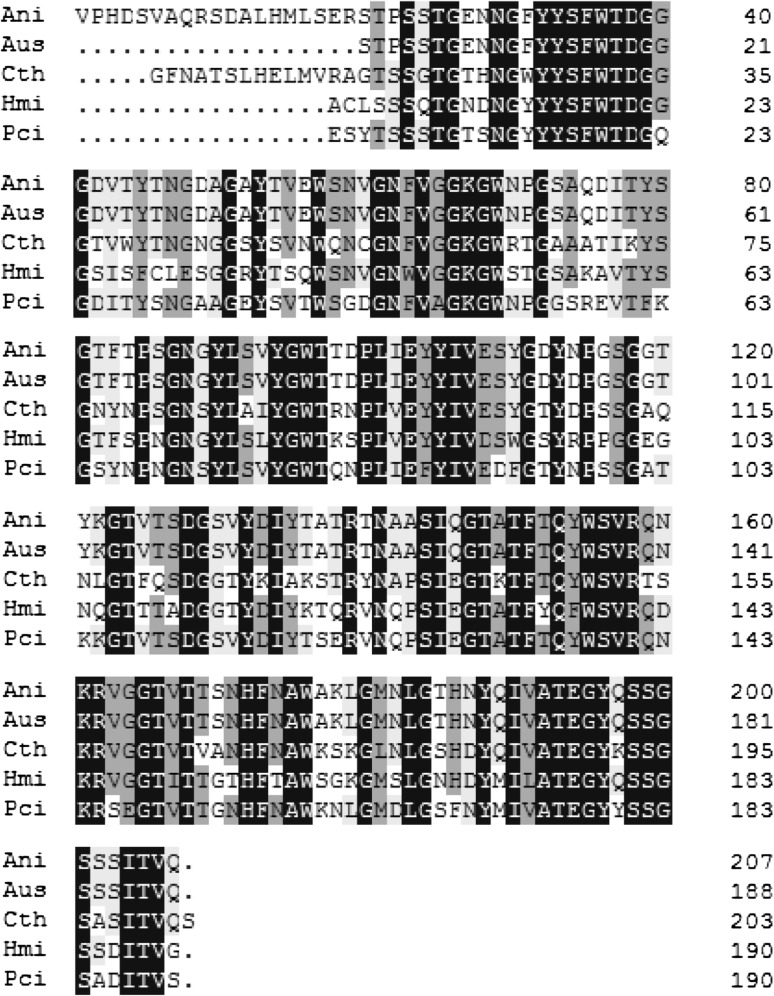
Amino acid homology alignment of the predicted xynZF-2 with other four xylanases from A. usamii (Genbank accession number: AEJ87263), Chaetomium thermophilum (Genbank accession number: CAD48750), Holomastigotoides mirabile (Genbank accession number: BAH56520), and Penicillium citrinum (Genbank accession number: BAE71133) was carried out (Fig. 3). The similarities between A. niger with A. usamii, C. thermophilum, H. mirabile and P. citrinum were 89.47, 60.77, 56.94, and 64.11 %, respectively.
Fig. 3.
Comparison of xynZF-2 to other microbial xylanases. AniAspergillus niger XZ-3S xylanase (207aa), AusAspergillus usamii xylanase (188aa), CthChaetomium thermophilum xylanase (199aa), HmiHolomastigotoides mirabile xylanase (190aa), PciPenicillium citrinum xylanase (190aa)
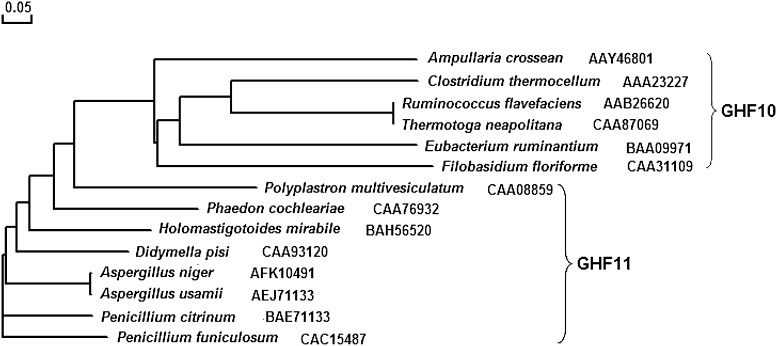
With the sequence of amino acids between xynZF-2 mature peptide and selected 13 kinds of xylanase from Genbank randomly phylogenetic tree analysis, we found that the topology of the evolutionary tree of the xynZF-2 was quite similar to that of the other GHF 11 xylanases and that the xynZF-2 should be classified into the family 11 glycosyl hydrolases (Fig. 4) [4, 10]. Through the CFSSP (Chou & Fasman Secondary Structure Prediction Server) method analysis, we found that the xynZF-2 was consist of α-helix, β-sheet and a small amount of coner with the percentage 32.4, 46.7 and 16.0 %, respectively. Three-dimensional structure modeling showed that xynZF-2 had a typical β-jelly coil structure of GH11 xylanase (Fig. 5).
Fig. 4.
Phylogenetic tree of xylanase gene based on amino acid sequence analysis
Fig. 5.

Three-dimensional structure of xynZF-2 predicted by SWISS-MODEL
Expression of xynZF-2 Gene in E. coli
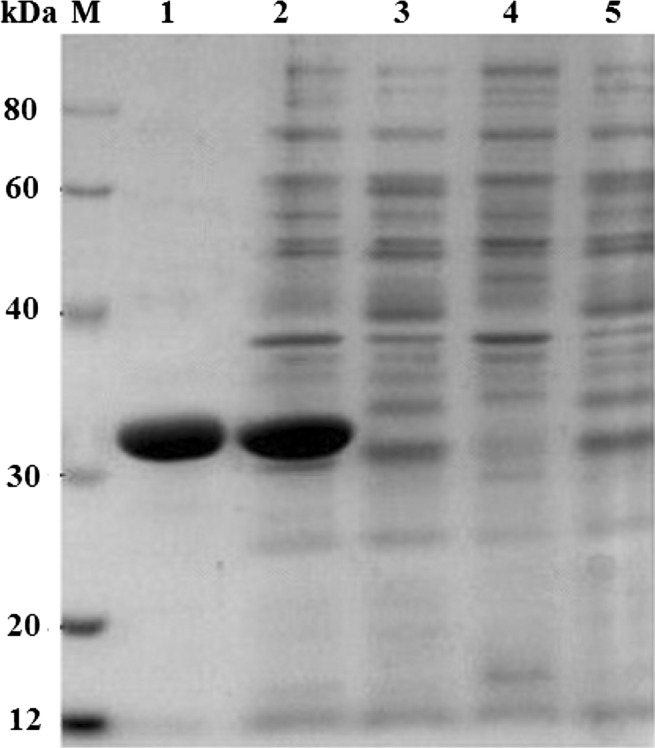
The gene fragment without the signal peptide-coding sequence of the xynZF-2 within the pET-28a-xynZF-2 vector was introduced into E. coli BL21(DE3) cells. After inducing the cells with 2 mM IPTG, the N-terminal His6-tagged xylanase was produced intracellularly. No xylanase was detected in the non-induced cells harboring the pET-28a-xynZF-2 (Fig. 6). SDS-PAGE analysis revealed a band of approximately 31 kDa (Fig. 6, lanes 1, 2), which was higher than the calculated molecular weight (24.04 kDa). Maybe because of the presence of the His-tag fusion peptide in expression vector pET-28a(+), the recombinant protein had 36 extra amino acids.
Fig. 6.
SDS-PAGE of different protein samples. Lane 1 xynZF-2 purified by Ni2+-chelating chromatography, lane 2E. coli pET-28a-xynZF-2 with IPTG induction, lane 3E. coli pET-28a-xynZF-2, lane 4E. coli pET-28a(+) with IPTG induction, lane 5E. coli pET-28a(+), lane M protein markers with the following molecular weight standards: 12, 20, 30, 40, 60, 80 kDa
Properties of XynZF-2
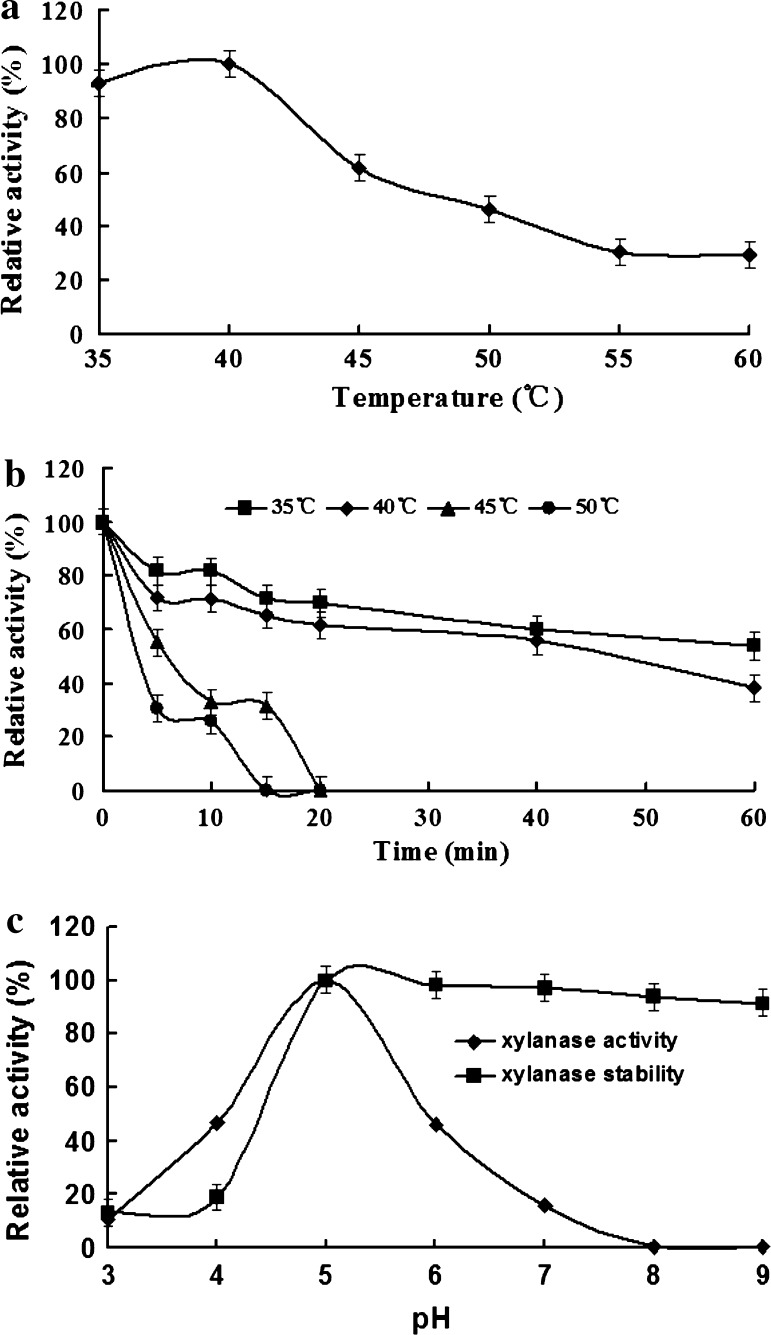
Purified xylanase xynZF-2 was used to evaluate its biochemical properties. The optimum temperature and pH of recombinant enzyme were 40 °C and pH 5.0, respectively (Fig. 7a, c). From the Fig. 7b, we can see that the enzyme activity was relatively stable when the temperature is below 40 °C, but when the temperature is above 40 °C, enzyme activity decreased rapidly. With the enzyme stored at 50 °C for 15 min or so, the activity has been basically completely lost. XynZF-2 is not thermotolerant, which prevents it from being applied in industrial process where high temperature is required, such as pulp and paper industry. Considering the rapid accumulation of DNA, cDNA or amino acid sequences of xylanases from different sources, the sequence-function analysis will be performed to gain a better understanding of xylanase, which will lead to the improvement in enzymatic properties of XynZF-2 by means of genetic engineering technology, such as N-terminus replacement or directed evolution.
Fig. 7.
The effect of pH and temperature on the activity and stability of the recombinant xylanase. a The effect of temperature on xylanase activity. b The thermostability of the xylanase. c The effect of pH on xylanase activity and stability
Recombinant xynZF-2 was stable over a broad pH range, retaining more than 90 % of the activity after incubation at pH 5.0–9.0 for 1 h at 40 °C (Fig. 7c). This result is important because it increases the possibility for using this enzyme as an additive in biotechnological processes that involves alkali conditions.
The activity of the purified recombinant enzyme in the presence of different metal ions or chemical reagents was determined (Table 1). The presence of Fe3+, Mn2+, EDTA, Ni2+, Zn2+ accelerated the activity of the recombinant xynZF-2, and the enzyme was strongly inhibited by Li+. Addition of other reagents, such as Ca2+, Fe2+, Cu2+, Mg2+ and Pb2+, had little or no effect on the activity. The decrease in enzyme activity may be due to involvement of cations/additives with the structure and conformation of the enzyme. Fe3+ and Zn2+ enhanced the xylanase activity as that of B. subtilis AMX-4 [26]. Cu2+ slightly inhibited the activity of xylanase of A. niger XZ-3S as reported in various xylanases from Staphylococcus sp. SG-13 and Plectosphaerella cucumerina [27, 28].
Table 1.
Effects of metal ions and other compounds on xylanase activity
| Metal ions | Relative active (%)a |
|---|---|
| Fe2+ | 92.08 ± 3.18 |
| Cu2+ | 96.16 ± 1.38 |
| Zn2+ | 102.64 ± 2.18 |
| Fe3+ | 117.05 ± 4.67 |
| Mg2+ | 97.36 ± 2.67 |
| Mn2+ | 106.24 ± 3.89 |
| Pb2+ | 95.82 ± 2.14 |
| Li+ | 88.72 ± 4.07 |
| Ca2+ | 93.28 ± 1.19 |
| Ni2+ | 103.12 ± 2.32 |
| EDTA | 105.76 ± 3.21 |
| Control | 100 ± 1.86 |
aMean of three independent readings, SD within 10 %
Acknowledgments
This study was financially supported by the Foundation of He’nan Educational Committee (No. 2011A180026), the Foundation of He’nan Science and Technology Agency of China (No. 112102210299) and the Foundation of He’nan Province of China for Key Young Teacher in University (No. 2011GGJS-125).
References
- 1.Thomson JA. Molecular biology of xylan degradation. FEMS Microbiol Lett. 1993;104:65–82. doi: 10.1111/j.1574-6968.1993.tb05864.x. [DOI] [PubMed] [Google Scholar]
- 2.André RLD, Tony MS, Fausto BRA, Fábio MS, Daniela AR, Adriana FP, Fernando S, Rolf AP, João AJ, Hector FT, Maria LTMP. Heterologous expression of an Aspergillus niveus xylanase GH11 in Aspergillus nidulans and its characterization and application. Process Biochem. 2011;46:1236–1242. doi: 10.1016/j.procbio.2011.01.027. [DOI] [Google Scholar]
- 3.Biely P. Microbial xylanolytic systems. Trends Biotechnol. 1985;3:286–290. doi: 10.1016/0167-7799(85)90004-6. [DOI] [Google Scholar]
- 4.Ahmed S, Riaz S, Jamil A. Molecular cloning of fungal xylanase: an overview. Appl Microbiol Biotechnol. 2009;84:19–35. doi: 10.1007/s00253-009-2079-4. [DOI] [PubMed] [Google Scholar]
- 5.Suchita N, Ramesh CK. Bleaching of wheat straw-rich soda pulp with xylanase from a thermoalkalophilic Streptomyces cyaneus SN32. Bioresour Technol. 2006;97:2291–2295. doi: 10.1016/j.biortech.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 6.Rakhee K, Narayan BB. Application of thermoalkalophilic xylanase from Arthrobacter sp. MTTC 5214 in biobleaching of kraft pulp. Bioresour Technol. 2007;98:897–903. doi: 10.1016/j.biortech.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 7.Kiddinamoorthy J, Anceno AJ, Haki GD, Rakshit SK. Production, purification and characterization of Bacillus sp. GRE7 xylanase and its application in eucalyptus Kraft pulp biobleaching. World J Microbiol Biotechnol. 2008;24:605–612. doi: 10.1007/s11274-007-9516-2. [DOI] [Google Scholar]
- 8.Henrissat B, Bairoch A. Updating the sequence-based classification of glycosyl hydrolases. Biochem J. 1996;316:695–696. doi: 10.1042/bj3160695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang G, Luo H, Meng K, Wang Y, Huang H, Shi P, Pan X, Yang P, Diao Q, Zhang H, Yao B. High genetic diversity and different distributions of glycosyl hydrolase family 10 and 11 xylanases in the goat rumen. PLoS One. 2011;6(2):el6731. doi: 10.1371/journal.pone.0016731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wakiyama M, Yoshihara K, Hayashi S, Ohta K. An extracellular endo-1,4-β-xylanase from Aspergillus japonic properties, and characterization of the encoding gene. J Biosci Bioeng. 2010;109(3):227–229. doi: 10.1016/j.jbiosc.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Collins T, Gerday C, Feller G. Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiol Rev. 2005;29:3–23. doi: 10.1016/j.femsre.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Wang JQ, Zhang HM, Wu MC, Tang CD. Cloning and sequence analysis of a novel xylanase gene, Auxyn10A, from Aspergillus usamii. Biotechnol Lett. 2011;33:1029–1038. doi: 10.1007/s10529-011-0524-9. [DOI] [PubMed] [Google Scholar]
- 13.Yasinok AE, Biran S, Kocabas A, Bakir U. Xylanase from a soil isolate, Bacillus pumilus: gene isolation, enzyme production, purification, characterization and one-step separation by aqueous-two-phase system. World J Microbiol Biotechnol. 2010;26:1641–1652. doi: 10.1007/s11274-010-0340-8. [DOI] [Google Scholar]
- 14.Thayat S, Peechapack S, Kenji M, Fusako K, Kosum C. Cloning of a thermostable xylanase from Actinomadura sp. S14 and its expression in Escherichia coli and Pichia pastoris. J Biosci Bioeng. 2011;111:528–536. doi: 10.1016/j.jbiosc.2010.12.024. [DOI] [PubMed] [Google Scholar]
- 15.Zhao Y, Meng K, Luo H, Yang P, Shi P, Huang H, Bai Y, Yao B. Cloning, expression, and characterization of a new xylanase from alkalophilic Paenibacillus sp. 12-11. J Microbiol Biotechnol. 2011;21:861–868. doi: 10.4014/jmb.1102.02024. [DOI] [PubMed] [Google Scholar]
- 16.Subramaniyan S. Isolation, purification and characterization of low molecular weight xylanase from Bacillus pumilus SSP-34. Appl Biochem Biotechnol. 2012;166:1831–1842. doi: 10.1007/s12010-012-9600-4. [DOI] [PubMed] [Google Scholar]
- 17.Driss D, Bhiri F, Ghorbel R, Chaabouni SE. Cloning and constitutive expression of His-tagged xylanase GH11 from Penicillium occitanis Pol6 in Pichia pastoris X33: purification and characterization. Protein Expr Purif. 2012;83:8–14. doi: 10.1016/j.pep.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 18.Abdul G, Sher AK, Zahid M, Muhammad IR, Farooq L. Heterologous expression of a gene for thermostable xylanase from Chaetomium thermophilum in Pichia pastoris GS115. Mol Biol Rep. 2011;38:3227–3233. doi: 10.1007/s11033-010-9996-2. [DOI] [PubMed] [Google Scholar]
- 19.Huy ND, Kim SW, Park SM. Heterologous expression of endo-1,4-β-xylanase C from Phanerochaete chrysosporium in Pichia pastoris. J Biosci Bioeng. 2011;111:654–657. doi: 10.1016/j.jbiosc.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 20.Zhou CY, Zhang JH, Ma XT, Zhou F, Zhou KN, Feng HG. Optimization of culture conditions for xylanase production by the solid-state fermentation with Aspergillus niger. J Anhui Agric Sci. 2010;38(21):11052–11054. [Google Scholar]
- 21.Zhou CY, Bai JY, Deng SS, Wang J, Zhu J, Wu MC, Wang W. Cloning of a xylanase gene from Aspergillus usamii and its expression in Escherichia coli. Bioresour Technol. 2008;99:831–838. doi: 10.1016/j.biortech.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 22.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 23.Laemmli UK. Cleavage of structural proteins during the assembly of the head of Bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 24.Bradford MM. A rapid sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 25.Bailey MJ, Biely P, Poutanen K. Interlaboratory testing of methods for assay of xylanase activity. J Biotechnol. 1992;23:257–270. doi: 10.1016/0168-1656(92)90074-J. [DOI] [Google Scholar]
- 26.Hong Y. Cloning of the Bacillus subtilis AMX-4 xylanase gene and characterization of the gene product. J Microbiol Biotechnol. 2009;19(12):1514–1519. doi: 10.4014/jmb.0907.07004. [DOI] [PubMed] [Google Scholar]
- 27.Zhang GM, Huang J, Huang GR, Ma LX, Zhang XE. Molecular cloning and heterologous expression of a new xylanase gene from Plectosphaerella cucumerina. Appl Microbiol Biotechnol. 2007;74:339–346. doi: 10.1007/s00253-006-0648-3. [DOI] [PubMed] [Google Scholar]
- 28.Gupta S, Bhushan B, Hoondal GS. Isolation, purification and characterization of xylanase from Staphylococcus sp. SG-13 and its application in biobleaching of kraft pulp. J Appl Microbiol. 2000;88:325–334. doi: 10.1046/j.1365-2672.2000.00974.x. [DOI] [PubMed] [Google Scholar]