Abstract
The study evaluated the effect of media based on plant extracts: potato, carrot and barley malt broth, on growth and astaxanthin synthesis by yeast Xanthophyllomyces dendrorhous DSM 5626 and its mutants. The carrot medium promoted carotenogenesis most effectively. In cultures on this medium the highest volumetric and cellular concentrations of astaxanthin were recorded for four out of five tested strains. Also the share of astaxanthin in the total carotenoids produced by the tested strains was the highest.
Keywords: Astaxanthin, Carotenoids, Plant extracts, Xanthophyllomyces dendrorhous
Introduction
Astaxanthin is the most important and the most expensive carotenoid pigment used in aquaculture [1]. Its commercial attractiveness is also related with its antioxidant potential [2–4]. At present chemical synthesis is the primary source of commercially available astaxanthin. However, in recent years the use of chemically synthesized compounds as food and feed additives has been subjected to strict restrictions. This is connected with increased concerns related to the harmful effect of synthetic pigments on human health and on the environment. Also consumers prefer natural products or those containing natural additives, originating from plant or microbial systems.
Among primary astaxanthin producers the interest of the scientific and business community has focused for years on yeast Xanthophyllomyces dendrorhous (perfect state of Phaffia rhodozyma [5]). However, astaxanthin content in wild strains of X. dendrorhous is very low [6]. For this reason alone they may not be used as its practical and economic source. The production of astaxanthin by X. dendrorhous is most frequently enhanced by mutagenization [7–9]. Another trend in research is to develop a new culture medium, facilitating an intensified commercial-scale production of this pigment by yeast X. dendrorhous and its mutants. The greatest hopes are associated with the potential utilization of natural complex media based on plant extracts or wastes produced by the agri-food industry. The primary raw materials in this respect include stillage [10] and lignocellulose biomass [9, 11]. The astaxanthin synthesis by X. dendrorhous and its mutants was also verified on media enriched with natural origin substances. Mollases [12, 13], date juice from Yucca fillifera [14], pineapple juice [15] and grape juice [16] were used in supplementation. Yeast cultures were also run on nutrient-rich coconut milk [17]. A promising concept was also to supplement growth media for X. dendrorhous and its mutants with fungal elicitors [18, 19] and plant extracts, being sources of astaxanthin precursors [8].
This study evaluated the applicability of plant origin culture media, i.e. potato, carrot and diluted malt broth, in the production of astaxanthin using X. dendrorhous DSM 5626 and its three mutants. The plants used in the extract preparation are common crops grown almost all over the world.
Materials and Methods
Strains
Experiments were conducted using yeast X. dendrorhous DSM 5626 (=P. rhodozyma ATCC 24202 and CBS 5905) from Deutche Sammlung von Mikroorganismen and Zellkulturen and its four astaxanthin overproducing mutants from our laboratory. Mutations were performed with the 4-day culture of X. dendrorhous DSM 5626 (OD = 0.3–0.4 at λ = 600 nm). The mutant 26UV was obtained as result of exposure of parental yeast to UV radiation (λ = 254 nm) for 5 min. Mutants 13B and 34B were isolated from medium containing benomyl at 0.015 and 0.1 mg/ml concentration, respectively and 10BE from medium with ethidium bromide at a 1 mg/ml concentration. The strains were maintained on YM slants (1 % glucose, 0.5 % peptone, 0.3 % malt extract, 0.3 % yeast extract and 2 % agar) until use.
Growth Conditions
Strains were cultivated on the following test culture media: 10 % carrot extract, 20 % potato extract (both extracted with boiling water) and diluted malt broth (60 g/l). The extracts from carrot and potatoes were enriched with 1 % glucose. The carrot extract additionally was supplemented with 0.1 % peptone and 0.1 % NaCl. pH of the media was adjusted to 5.0.
Yeast strains were cultivated two times in a liquid medium. Four millilitres of the final culture were brought into 250 ml Erlenmeyer flasks containing 76 ml of an appropriate medium. Cultures were grown for 5 days at 22 °C in orbital shakers (agitation speed, 200 rpm) at a constant illumination of 600 lx.
Carotenoid Extraction and Analysis
Pigments from yeast cells were isolated based on the method described by Sedmak et al. [20], as modified by the author of this study. The amount of 10 ml of culture was centrifuged and then the pellet was washed twice with distilled water and resuspended in 5 ml of DMSO (preheated to 55 °C). The entire volume was vortexed for 30 s and next 5 ml of the hexane fraction from petroleum were added. The volume was again vortexed for 30 s and 20 % NaCl aqueous solution was added in batches at 0.5 ml. The hexane phase with pigments was separated by centrifugation.
The total carotenoid, astaxanthin yields and sugar contents in samples were determined on a Waters Alliance HPLC System 2695. Analysis conditions of pigments were described by Gramza-Michałowska and Stachowiak [4] and sugar analyses were described by Szwengiel et al. [21]. Biomass was measured by DCW (dry cell weight)
Statistical Analysis
All experiments were performed in triplicate. The data were analyzed by one-way analysis of variance (ANOVA). Differences between treatments were examined for the level of significance by Tukey’s range test.
Results and Discussion
Characteristics of Yeast Growth on Test Media
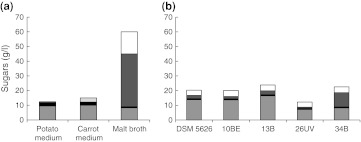
The total sugars and their profile in test media are given on Fig. 1a. All sugars presented in media are metabolized by X. dendrorhous DSM 5626 [22].
Fig. 1.
Sugar content a in test media prior to onset of culture, b in malt broth after 5 days of yeast culture. Column marking:  glucose;
glucose;  fructose;
fructose;  sucrose;
sucrose;  maltose;
maltose;  maltotriose and dextrins
maltotriose and dextrins
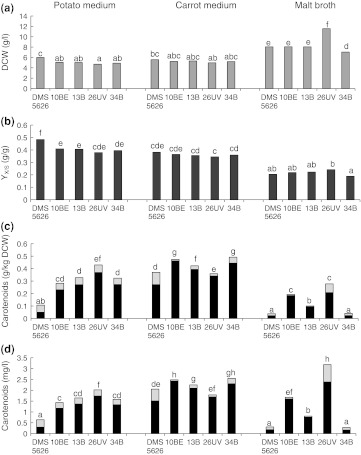
The highest growth yield of yeast strains was obtained in cultures on malt broth (Fig. 2a). For the mutant 26UV, dry cell weight was as much as two-fold higher in comparison to the other strains. A yield of biomass, approx. 5.5 g/l, was obtained in cultures on potato and carrot media irrespective of the strain. After five days of culture all the strains used all sugars found in both media. In contrast, sugar utilization was incomplete in cultures on malt broth (Fig. 1b). Fructose in the postculture supernatants was detected at the initial level. All strains utilized almost all the maltose (92–98 %), maltotriose and other oligosaccharides at 26–72 %, while the concentration of glucose increased. This indicates that yeast DSM 5626 and the mutants while assimilating glucose, which is a priority source of carbon for these yeasts, at the same time hydrolyze maltose and maltotriose to glucose, or that these sugars are degraded prior to the assimilation of glucose. An et al. [12] in cultures of X. dendrorhous mutant 2A2N on the medium with molasses observed that this strain first metabolized saccharose and only after it had been depleted—glucose, and eventually—fructose. Similar observations were made by Fontana et al. [13] for a culture of DSM strain 5626 on diluted cane molasses.
Fig. 2.
The effect of DSM 5626 and its mutants in 5-day cultures: a growth yield (DCW dry cell weight), b medium composition on growth parameters and carotenoid production by Xanthophyllomyces dendrorhous biomass yield to carbohydrates (YX/S), c cellular concentration of carotenoids:  astaxanthin;
astaxanthin;  others carotenoids and d volumetric concentration of carotenoids:
others carotenoids and d volumetric concentration of carotenoids:  astaxanthin;
astaxanthin;  others carotenoids. c–d Statistical differences concern only astaxanthin
others carotenoids. c–d Statistical differences concern only astaxanthin
The content of individual carbon sources in the post culture malt broth, particularly the surprising increase in glucose content, makes it possible to present a hypothesis that the mechanism of assimilation of maltose and higher polymers of glucose may be identical as in case of sucrose.
Figure 2b presents values of ratios of biomass yields to carbohydrates YX/S. They clearly indicate a significant effect of medium composition on the capacity to embed a carbon source into the cell biomass by a given strain. However, statistically significant differences generally were not found between YX/S ratios obtained for the tested strains in cultures on the same medium.
The lowest values of YX/S were recorded in cultures on malt broth despite the fact that on this medium all strains used two times more of the carbon substrate than they did on the others and biomass yield was the highest (Fig. 2a). The low value of YX/S needs to be linked with the basic carbon substrate found in the broth, i.e. maltose, maltotriose and other oligosaccharides, which accounted for 85 % of the total absorbed sugars. Probably the necessity of their degradation requires an additional expenditure of metabolic energy.
On the other hand, X. dendrorhous is Crabtree positive and a change of aerobic metabolism into aerobic fermentation occurs already at a 5 % glucose concentration in the culture medium. This is usually accompanied by a reduced production of biomass and carotenoids [23]. Luna-Flores et al. [14] in cultures of X. dendrorhous DSM 5626 mutant 25-2 observed a reduction of YX/S values from 0.45 to 0.32 g/g with an increasing concentration of the carbon substrate in the medium from 10 to 40 g/l. In this study the total level of the carbon substrate in malt broth at the beginning of the culture was 60 g/l (Fig. 1a). Depending on the strain, values of YX/S recorded on this medium fell within the range of 0.19–0.24 g/g (Fig. 2b) and they were 1.5–2.5 times lower than in case of the other media, in which the content of the carbon substrate was 11–15 g/l (Fig. 1a).
For all strains, the highest values of YX/S were recorded in cultures on the potato medium. In case of the parental strain, YX/S reached 0.48 g/g, while for mutants it was lower by 15–20 %. In cultures on the carrot medium values of YX/S did not differ significantly between strains.
Biosynthesis of Astaxanthin on Test Media
Irrespective of the medium composition, astaxanthin was the basic pigment produced by the tested mutants (Fig. 2c, d). For the parental strain in culture on the carrot medium and the malt broth it was identical. Only in the culture of this strain on the potato medium the share of astaxanthin in total carotenoids was low, amounting to 47 %. Literature data indicate that among all the carotenoids produced by wild strains of X. dendrorhous, astaxanthin constituted 40–100 %, depending on the adopted culture conditions [11, 24].
For all the tested strains the carrot medium proved to be the medium most effectively promoting carotenogenesis (Fig. 2c, d). For the parental strain and mutants: 10BE, 13B and 34B, the highest cellular and volumetric concentrations of astaxanthin were recorded in cultures on this medium. The highest amounts of astaxanthin were produced by 10BE and 34B, amounting to 0.46 g/kg and 2.42 mg/l and 0.44 g/kg and 2.30 mg/l, respectively. In cultures on the carrot medium the highest share of astaxanthin was also recorded in the total carotenoids produced by the tested strains.
The carrot medium may contain some precursors of carotenoid synthesis. Some products of thermal degradation of carotenoids (carrots boiled for 1 h) may include cis-isomers, epoxides and compounds with a cleaved chain (apocarotenoids) [25]. Some of them may be slightly soluble in water, penetrate into yeast cells and stimulate carotenogenesis and astaxanthin synthesis.
Literature sources indicate that such compounds, e.g. mevalonic acid, may intensify the synthesis of astaxanthin by yeasts X. dendrorhous [7]. Echavarri-Erasun and Johnson [18] observed an increased synthesis of astaxanthin under the influence of filtrates obtained from the culture of fungi Epicoccum nigrum, capable of synthesizing isoprenoids (carotenoid precursors), as well as carotenoid pigments, which are found at the earlier stages of the astaxanthin synthesis pathway. Experiments conducted by Wang et al. [19] also showed that the presence of fungal elicitors in the culture medium, particularly from carotenoid-synthesizing yeasts Rhodotorula rubra and R. glutinis, clearly stimulated the synthesis of astaxanthin in P. rhodozyma AS. 2.1557. In turn, Kim et al. [8] showed that an addition of plant extracts from Allium fistulosum (welsh onion) and Perilla frutescens (common perilla) significantly improved the efficiency of carotenoids in cultures of mutant X. dendrorhous G276. The authors suggested that plant juices contain inducers or precursors of astaxanthin synthesis, since an increased efficiency of production in case of this pigment was not connected with an increase in biomass efficiency.
On the other hand, the other components found in the carrot extract may simply promote good condition of yeast cells and in this way intensify their metabolic activity. Carrot, apart from carotenoids (mainly β- and α-carotene), is also a source of vitamins: B1, B2, C, and minerals, mainly potassium, calcium, phosphorus, magnesium [26]. Dominínguez-Bocanegra and Torres-Muñoz [17] were of an opinion that a considerable increase in the efficiency of astaxanthin production both in wild strains of X. dendrorhous and its mutants, recorded in their study in cultures run on coconut milk, is the result of the presence of vitamins, minerals, amino acids, i.e. the so-called growth biostimulants activating cellular metabolism. Also Wang et al. [19] indicated that fungal elicitors intensifying carotenogenesis in P. rhodozyma AS 2.1557 are preparations with a rich composition. They contain proteins, polysaccharides, glycoproteins, peptides, oligosaccharides, lipids, i.e. compounds which may stimulate growth and production of pigments.
On the other hand, the medium prepared on the basis of potato extract, which is a good source of vitamins (C, B1, B3, B6, pathothenic acid, riboflavin) and minerals (potassium, phosphorus, magnesium) [27], should also satisfy the nutrient requirements of X. dendrorhous. In this study it turned out to be a good source of substrates for all the tested yeast strains (Fig. 2a, b). However, in cultures on potato extract the cellular and volumetric concentrations of astaxanthin, as well as the share of astaxanthin in total carotenoids produced by the tested strains were lower in comparison to the carrot medium (Fig. 2c, d).
For all strains the lowest cellular concentration of astaxanthin and total carotenoids were recorded in cultures on malt broth (Fig. 2c). For the parental strain and mutants 13B and 34B, also the volumetric concentration of pigments was lowest (Fig. 2d). As it was mentioned above, astaxanthin synthesis is subjected to substrate repression and is usually reduced at a carbon substrate concentration of as little as 5 %. Ramírez et al. [28], in cultures on the medium supplemented with date juice from Y. fillifera, recorded the maximum productivity and carotenoid concentrations in cells of P. rhodozyma CBS 9505 mutant 25-2 at a total content of the carbon substrate of 22.5 g/l. An increase in the amount of the substrate in the media to over 30 g/l strongly inhibited carotenogenesis, although it had an advantageous effect on the biomass synthesis. In this study the level of the carbon substrate in the malt broth was two times higher (Fig. 1a). But for the mutant 26UV in the malt broth culture, a very high volumetric concentration of total carotenoids was obtained. Literature data show that the composition of the culture medium and culture parameters strongly determine carotenogenesis and the level of synthesized astaxanthin in the wild strains of X. dendrorhous and their mutants [12–17]. Thus they should be selected individually.
Concluding Remarks
Among the media tested within this study we need to focus on the carrot medium, prepared on the basis of carrot extract and enriched with a small amount of peptone, glucose and NaCl. This variant most effectively promoted carotenogenesis. In cultures on this medium the highest cellular and volumetric concentrations of astaxanthin were recorded for four out of five tested strains. Also the share of astaxanthin in total carotenoids produced by the tested strains was highest.
Astaxanthin yields obtained in cultures of mutants on the carrot medium may probably be further improved thanks to the selection of a culture method promoting an increase in biomass yield, and stimulating cellular pigment concentration. Spectacular success in this respect was reported by de la Fuente et al. [29], who obtained 235 mg/l of astaxanthin in the X. dendrorhous VKPM Y2476 at, applying ultraviolet illumination of 1,000 lx. The authors suggested that probably yeast exposure to the light generates active oxygen molecules, which may play a role in the stimulation of the carotenogenesis.
Acknowledgments
The author is grateful to the Ministry of Science and Higher Education, Poland (Grant no. 2 PO6T 024 30) for the financial support of this work.
References
- 1.Breithaupt DE. Modern application of xanthophylls in animal feeding—a review. Trends Food Sci Technol. 2007;18:501–506. doi: 10.1016/j.tifs.2007.04.009. [DOI] [Google Scholar]
- 2.Sontocono M, Zurria M, Berrettini M, Fedeli D, Falcioni G. Lutein, zeaxanthin and astaxanthin protect against DNA damage in SK-N-SH human neuroblastoma cells induced by reactive nitrogen species. J Photochem Photobiol. 2007;88:1–10. doi: 10.1016/j.jphotobiol.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Chan KC, Mong MC, Yin MC. Antioxidative and anti-inflammatory neuroprotective effects of astaxanthin and canthaxanthin in nerve growth factor differentiated PC12 cells. J Food Sci. 2009;74(7):225–231. doi: 10.1111/j.1750-3841.2009.01274.x. [DOI] [PubMed] [Google Scholar]
- 4.Gramza-Michałowska A, Stachowiak B. The antioxidant potential of carotenoid extract from Phaffia rhodozyma. Acta Sci Pol Technol Aliment. 2010;9(2):171–188. [Google Scholar]
- 5.Golubev WI. Perfect state of Rhodomyces dendrorhous (Phaffia rhodozyma) Yeast. 1995;11:101–110. doi: 10.1002/yea.320110202. [DOI] [PubMed] [Google Scholar]
- 6.Flenø B, Christensen I, Larsen R, Johansen SR, Johnson E (1997) Astaxanthin-producing yeast cells, methods for their preparation and their use. US Patent 5,599,711
- 7.Calo P, Velázquez JB, Sieiro C, Blanco P, Longo E, Villa TG. Analysis of astaxanthin and other carotenoids from several Phaffia rhodozyma mutants. J Agric Food Chem. 1995;43:1396–1399. doi: 10.1021/jf00053a049. [DOI] [Google Scholar]
- 8.Kim SK, Lee JH, Lee CH, Yoon YC. Increased carotenoid production in Xanthophyllomyces dendrorhous G276 using plant extracts. J Microbiol. 2007;45(2):128–132. [PubMed] [Google Scholar]
- 9.Montanti JM, Nghiem NP, Johnston D. Production of astaxanthin from cellulosic biomass sugars by mutants of the yeast Phaffia rhodozyma. Appl Biochem Biotechnol. 2011;164:655–665. doi: 10.1007/s12010-011-9165-7. [DOI] [PubMed] [Google Scholar]
- 10.Ananda N, Vadlani PV. Production and optimization of carotenoid-enriched dried distiller’s grains with solubles by Phaffia rhodozyma and Sporobolomyces roseus fermentation of whole stillage. J Ind Microbiol Biotechnol. 2010;37:1183–1192. doi: 10.1007/s10295-010-0765-y. [DOI] [PubMed] [Google Scholar]
- 11.Parajó JC, Santos V, Vázquez M, Cruz JM. Production of carotenoids by Xanthophyllomyces dendrorhous growing on enzymatic hydrolysates of prehydrolysed wood. Food Chem. 1997;60(3):347–355. doi: 10.1016/S0308-8146(96)00341-X. [DOI] [Google Scholar]
- 12.An GH, Jang BG, Cho MH. Cultivation of the carotenoid-hyperproducing mutant 2A2 N of the red yeast Xanthophyllomyces dendrorhous (Phaffia rhodozyma) with molasses. J Biosci Bioeng. 2001;92(2):121–125. doi: 10.1263/jbb.92.121. [DOI] [PubMed] [Google Scholar]
- 13.Fontana JD, Guimrães MF, Martins NT, Fontana CA, Baro NM. Culture of the astaxanthinogenic yeast Phaffia rhodozyma in low-cost media. Appl Biochem Biotechnol. 1996;57(58):413–422. doi: 10.1007/BF02941721. [DOI] [PubMed] [Google Scholar]
- 14.Luna-Flores CH, Ramírez-Cordova JJ, Pelayo-Ortiz C, Herrera-López EJ. Batch and fed-batch modeling of carotenoids production by Xanthophyllomyces dendrorhous using Yucca fillifera date juice as substrate. Biochem Eng J. 2010;53:131–136. doi: 10.1016/j.bej.2010.10.004. [DOI] [Google Scholar]
- 15.Jirasripongpun K, Pewiong W, Kitraksa P, Krudngern C. Carotenoid production by Xanthophyllomyces dendrorhous: use of pineapple juice as a production medium. Lett Appl Microbiol. 2008;47:112–116. doi: 10.1111/j.1472-765X.2008.02396.x. [DOI] [PubMed] [Google Scholar]
- 16.Vázquez M, Martin AM. Optimization of Phaffia rhodozyma continuous culture through response surface methodology. Biotech Bioeng. 1998;57(3):314–320. doi: 10.1002/(SICI)1097-0290(19980205)57:3<314::AID-BIT8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 17.Dominíguez-Bocanegra AR, Torres-Muñoz JA. Astaxanthin hyperproduction by Phaffia rhodozyma (now Xanthophyllomyces dendrorous) with raw milk as sole source of energy. Appl Microbiol Biotechnol. 2004;66:249–252. doi: 10.1007/s00253-004-1686-3. [DOI] [PubMed] [Google Scholar]
- 18.Echavarri-Erasun C, Johnson EA. Stimulation of astaxanthin formation in the yeast Xanthophyllomyces dendrorhous by the fungus Epicoccum nigrum. FEMS Yeast Res. 2004;4:511–519. doi: 10.1016/S1567-1356(03)00177-6. [DOI] [PubMed] [Google Scholar]
- 19.Wang W, Yu L, Zhou P. Effects of different fungal elicitors on growth, total carotenoids and astaxanthin formation by Xanthophyllomyces dendrorhous. Bioresour Technol. 2006;97:26–31. doi: 10.1016/j.biortech.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 20.Sedmak JJ, Weerasinghe DK, Jolly SO. Extraction and quantification of astaxanthin from Phaffia rhodozyma. Biotechnol Tech. 1990;4:107–112. doi: 10.1007/BF00163282. [DOI] [Google Scholar]
- 21.Szwengiel A, Czarnecka M, Czarnecki Z. Levan synthesis during associated action of levansucrase and Candida cacaoi DSM 2226 yeast. Pol J Food Nutr Sci. 2007;57(4):433–440. [Google Scholar]
- 22.Palágyi ZS, Ferenczy L, Vágvölgyi CS. Carbon-source assimilation pattern of astaxanthin-producing yeast Phaffia rhodozyma. World J Microbiol Biotechnol. 2001;17:95–97. doi: 10.1023/A:1016689512718. [DOI] [Google Scholar]
- 23.Reynders MB, Rawling DE, Harrison STI. Demonstration of the Crabtree effect in Phaffia rhodozyma during continuous and fed-batch cultivation. Biotechnol Lett. 1997;19:549–552. doi: 10.1023/A:1018341421122. [DOI] [Google Scholar]
- 24.Parajó JC, Santos V, Vázquez M. Optimization of carotenoid production by Phaffia rhodozyma cells grown on xylose. Process Biochem. 1998;33(2):181–187. doi: 10.1016/S0032-9592(97)00045-9. [DOI] [Google Scholar]
- 25.Zepka LQ, Mercadante AZ. Degradation compounds of carotenoids formed during heating of a stimulated cashew apple juice. Food Chem. 2009;117:28–34. doi: 10.1016/j.foodchem.2009.03.071. [DOI] [PubMed] [Google Scholar]
- 26.Sharma KD, Karki S. Chemical composition, functional properties and processing of carrot—a review. J Food Sci Technol. 2012;49(1):22–32. doi: 10.1007/s13197-011-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murniece I, Karklina D, Galoburda R, Santare D, Skrabule I, Costa HS. Nutritional composition of freshly harvested and stored Latvian potato (Solanum tuberosum L.) varieties depending on traditional cooking methods. J Food Compost Anal. 2011;24:699–710. doi: 10.1016/j.jfca.2010.09.005. [DOI] [Google Scholar]
- 28.Ramírez J, Nuñez ML, Valdivia R. Increased astaxanthin production by a Phaffia rhodozyma mutant grown on date juice from Yucca fillitera. J Ind Microbiol Biotechnol. 2000;24:187–190. doi: 10.1038/sj.jim.2900792. [DOI] [Google Scholar]
- 29.Fuente JL, Rodríguez-Sáiz M, Schleissner C, Díez B, Peiro E, Barredo JL. High-titer production of astaxanthin by the semi-industrial fermentation of Xanthophyllomyces dendrorhous. J Biotechnol. 2010;148:144–146. doi: 10.1016/j.jbiotec.2010.05.004. [DOI] [PubMed] [Google Scholar]