Summary
In India, substantial efforts have been made to increase awareness about HIV/AIDS among female sex workers (FSWs). We assessed the impact of awareness regarding safe sex in a cohort of FSWs by studying trends in HIV prevalence, sexually transmitted diseases (STDs), and risk behaviors measured from 1993 to 2002 in Pune, India. A total of 1359 FSWs attending 3 STD clinics were screened for HIV infection, and data on demographics, sexual behaviors, and past and current STDs were obtained. The overall HIV prevalence among FSWs was 54%. Not being married (adjusted odds ratio [AOR] = 1.74, 95% confidence interval [CI]: 1.17 to 2.59), being widowed (AOR = 2.10, 95% CI: 1.16 to 3.80), inconsistent condom use (AOR = 1.60, 95% CI: 1.02 to 2.50), clinical presence of genital ulcer disease (GUD; AOR = 1.66, 95% CI: 1.07 to 2.56), and genital warts (AOR = 4.70, 95% CI: 1.57 to 14.08) were independently associated with HIV infection among FSWs. The prevalence of HIV remained stable over 10 years (46% in 1993 and 50% in 2002; P = 0.80). The prevalence of GUD decreased over time (P < 0.001), whereas that of observed genital discharge remained stable. Reported consistent condom use as well as the proportion of FSWs who refused sexual contact without condoms increased over time (P < 0.001). These data collectively suggest that safe sex interventions have had a positive impact on FSWs in Pune, India.
Keywords: condom, HIV, India, female sex workers, safer sex, sexually transmitted disease
The National AIDS Control Organization (NACO) of India estimated that nearly 5.1 million people were infected with HIV by the end of 2003.1 The major mode of HIV transmission reported in India is by heterosexual contact (85%),2 and female sex workers (FSWs) constitute a core group for transmission of HIV and other sexually transmitted diseases (STDs).3–5 The reported number of HIV-positive FSWs in India is 71,000.1
Maharashtra is a high HIV prevalence state in India, with a reported HIV prevalence of up to 58.7% among FSWs.6 In Mumbai, the largest metropolitan city in Maharashtra, a marked rise in HIV prevalence among FSWs was observed, from 10% in 1986 to 32% in 1991,7 and has further increased to 54% by 2003.8 In Pune, an industrial city in Maharashtra with a population of 3 million, there is an estimated population of 4000 FSWs “residing in brothels” and an additional 2000 floating FSWs who are “not residing in brothels.” Both of these categories of FSWs have an average client turnover of 3 to 4 per day.9
In India, the presence of a large sex industry and a high HIV prevalence among FSWs has led to the introduction of targeted interventions focused on creating awareness in this community and social marketing of condoms by governmental agencies like the NACO and state public health departments as well as various nongovernmental organizations (NGOs) working in the red light area.10
We initiated the first large-scale cohort study in a high-risk population of STD clinic attendees in Pune City and have previously reported HIV prevalence and incidence in male STD patients, FSWs, and other women attending STD clinics.11 The prevalence of HIV infection reported in our various previous reports ranged between 47% and 49%, with an incidence of 20.5% per year.11,12 All these studies reported data for shorter time intervals. We now have data for almost a decade spanning from 1993 to 2002 on these cohorts. The objective of this study was to find out the correlates of HIV infection among FSWs attending STD clinics in Pune, India over 10 years and also to study trends of prevalence of STDs and HIV and risk behaviors in this population.
METHODS
Study Population
Through collaboration between the National AIDS Research Institute and The Johns Hopkins University, a cohort study was initiated in STD clinic attendees in Pune, India to estimate HIV prevalence and incidence and to study associated risk factors. Various studies like the Preparation for AIDS Vaccine Evaluation (PAVE; 1993–1994), HIV Network for Prevention Trials (HIVNET; 1995–1998), and Study of Acute Pathogenesis of HIV Infection (1999–2002) supported the cohort study. Ethical clearance was obtained from The Johns Hopkins University Joint Committee on Clinical Investigation and the Ethical Committee of the National AIDS Research Institute in Pune for this study. The present study population comprises FSWs enrolled in these studies between May 1993 and August 2002. Of 2376 women attending our 3 STD clinics who were screened for HIV infection, we included the women who reported a history of commercial sex work (N = 1359) for this analysis. Most women in this study were seen at 2 clinics, an outpatient STD clinic of a municipal corporation dispensary near the red light area of Pune City and a comprehensive health care clinic run by National AIDS Research Institute located in the red light area.
Study Instruments and Procedure
Information on demographics, sexual history, and risk behavior was collected using structured questionnaires after obtaining written informed consent. FSWs attending our study clinics were screened for HIV and STDs. A pelvic examination was performed, and the clinical diagnosis of genital ulcer, discharge, or warts was based on the physical examination done by the clinician. Specimens were collected from the genital lesions for Gram stains and cultures for Neisseria gonorrhoeae in the case of symptomatic women. Blood was tested for syphilis using a rapid plasma reagent test (RPR), and results were confirmed by the Treponema pallidum hemaglutination test (TPHA). The clinical and laboratory criteria used for the diagnosis of STDs have been described previously.11 Treatment for STDs was given based on the guidelines of the Centers for Disease Control and Prevention (CDC).13
Additional information on sexual behavior was collected from 1998 through 2002. This included information on types of regular partners (having a minimum of 3 or more sexual contacts in the past 6 months) as well as more detailed information on condom use and anal and oral sexual intercourse.
HIV Serology
Serum samples were screened initially using a commercially available enzyme-linked immunosorbent assay (ELISA) kit for detection of HIV-1 and HIV-2 antibodies (HIV EIA, LABSYSTEMS OY, Helsinki, Finland; Detect-HIV, BioChem ImmunoSystems, Montreal, Quebec, Canada; or Immunotest HIV-1/HIV-2 Ab sp, Innogenetics NV, Zwijndercht, Belgium). Specimens found to be reactive by ELISA were confirmed using the Rapid Test Device (Immunocomb II, HIV 1 and 2 BiSpot, Orgenics, Yavne, Israel; HIV Tri-DOT, J Mitra & Company, New Delhi, India; or Capillus HIV-1/HIV-2, Cambridge Diagnostics, Galway, Ireland). Specimens that were discrepant by these assays were confirmed using a third ELISA or commercially available HIV-1/HIV-2 Western blot (INNO-LIA HIV-1/HIV-2 Ab; Innogenetics NVor HIV BLOT 2.2 Western blot assay, Genelabs Diagnostics SA, Geneva, Switzerland).
Statistical Analysis
The statistical analysis was performed using SPSS (version 10.0) and Stata (version 8.0) software. Univariate analysis was performed to determine correlates of HIV infection using logistic regression. Factors found to be significantly associated in the univariate analysis (P < 0.05) and other potential confounders that failed to show a significant association were used in a multiple logistic regression model in a forward stepwise fashion. A χ2 test for linear trend was performed to assess changes in the prevalence of HIV, STDs, and risk behaviors among FSWs over the 10-year study period. We have calculated probability values using the likelihood ratio test.
In the instrument, individuals were asked whether they used condoms, and the structured response categories included “never,” “sometimes,” and “always.” For the purposes of analysis, those responding as always were considered to be “consistent condom users” and those responding as sometimes were considered to be “inconsistent condom users.” It is expected that inconsistent condom users had some risk of HIV exposure.
RESULTS
Profile of Female Sex Workers
Overall, 1359 FSWs were screened for HIV infection between May 1993 and July 2002. The mean age of the participants was 28 years (standard deviation [SD] = 6.7 years). Most FSWs were illiterate (83%). At the time of screening, 16% of FSWs reported being currently married, whereas 39% were unmarried, 9% were widowed, and 36% were divorced or separated. The mean age at initiation of sex was 16 years (SD = 8.28 years). Consistent condom use was reported by only 34% of the FSWs, whereas 11% reported that they had never used condoms. Nearly 84% of FSWs reported more than 1000 lifetime sexual partners.
A history of genital discharge was reported by 42%, and genital ulcer disease (GUD) was reported by 34%, whereas fewer reported a history of genital warts (4%). Twenty percent of the FSWs denied a history of any STD. Based on physical examination at the time of the interview, 9% presented with GUD, 40% had genital discharge, and 3% had genital warts. On examination and laboratory tests, 36% did not have any clinical findings.
Correlates of HIV Infection
The overall HIV prevalence among FSWs was 54% (732 of 1359 women) and ranged from 46% in 1993 to 50% in 2002 during the study period. In univariate analysis, HIV prevalence was significantly higher among women who were unmarried (odds ratio [OR] = 1.86, 95% confidence interval [CI]: 1.3 to 2.6), widowed (OR = 2.15, 95% CI: 1.3 to 3.4), or divorced or separated (OR = 1.47, 95% CI: 1.0 to 2.0) and had initiated sex after 16 years of age (OR = 1.27, 95% CI: 1.0 to 1.6). The HIV prevalence was high among those who reported inconsistent (OR = 1.85, 95% CI: 1.3 to 2.7) or consistent (OR = 1.58, 95% CI: 1.1 to 2.4) condom use compared with those who never used condoms (Table 1).
TABLE 1.
Univariate Analysis: Demographics and Sexual Behavior With HIV Infection Among FSWs in Pune, India
| Characteristics | Total (N = 1359) | Prevalence of HIV (%) |
OR (95% CI) | P |
|---|---|---|---|---|
| Current age (y) | ||||
| <20 | 76 | 48.7 | 1 (Ref) | |
| 20–30 | 909 | 57 | 1.40 (0.88 to 2.24) | 0.16 |
| >30 | 374 | 47.3 | 0.95 (0.58 to 1.55) | 0.83 |
| Marital status | n = 1261 | |||
| Married | 204 | 42.2 | 1 (Ref) | |
| Never married | 496 | 57.5 | 1.86 (1.34 to 2.59) | <0.001* |
| Widowed | 113 | 61.1 | 2.15 (1.34 to 3.44) | 0.001* |
| Divorcee/separated | 448 | 51.8 | 1.47 (1.05 to 2.05) | 0.02* |
| Education | n = 1354 | |||
| None | 1104 | 54.6 | 1 (Ref) | |
| Primary and high school | 202 | 49 | 0.80 (0.59 to 1.07) | 0.14 |
| College or higher | 48 | 13.7 | 1.12 (0.62 to 2.01) | 0.71 |
| Age at initiation of sex | n = 1359 | |||
| Less than 16 y | 584 | 50.5 | 1 (Ref) | |
| More than 16 y | 775 | 56.4 | 1.27 (1.02 to 1.58) | 0.03* |
| Condom use in lifetime | n = 990 | |||
| Never | 143 | 42 | 1 (Ref) | |
| Inconsistent | 510 | 57.1 | 1.85 (1.27 to 2.69) | 0.001* |
| Consistent | 337 | 53.4 | 1.58 (1.07 to 2.35) | 0.02* |
| Lifetime sexual partners | ||||
| 1–100 | 110 | 51.8 | 1 (Ref) | |
| 101–1000 | 103 | 40.8 | 0.64 (0.37 to 1.10) | 0.1 |
| > 1000 | 1146 | 55.2 | 1.14 (0.77 to 1.67) | 0.49 |
| History of genital ulcers | n = 1328 | |||
| No | 875 | 52.7 | 1 (Ref) | |
| Yes | 453 | 55.6 | 1.13 (0.94 to 1.35) | 0.19 |
| History of genital discharge | n = 1331 | |||
| No | 764 | 51.4 | 1 (Ref) | |
| Yes | 567 | 56.8 | 1.20 (1.00 to 1.45) | 0.05* |
| History of genital warts | n = 1328 | |||
| No | 1275 | 52.9 | 1 (Ref) | |
| Yes | 53 | 71.7 | 1.36 (0.94 to 1.96) | 0.1 |
| Diagnosis of genital ulcer | n = 922 | |||
| No | 801 | 51.2 | 1 (Ref) | |
| Yes | 121 | 64.5 | 1.70 (1.14 to 2.53) | <0.001* |
| Diagnosis of genital discharge | n = 871 | |||
| No | 325 | 49.8 | 1 (Ref) | |
| Yes | 546 | 54.9 | 1.23 (0.94 to 1.62) | 0.14 |
| Diagnosis of genital warts | n = 920 | |||
| No | 887 | 52 | 1 (Ref) | |
| Yes | 33 | 78.8 | 3.42 (1.47 to 7.97) | 0.004* |
| Diagnosis of gonorrhea | n = 361 | |||
| No | 320 | 58.1 | 1 (Ref) | |
| Yes | 41 | 58.5 | 1.00 (0.52 to 1.95) | 0.98 |
| Diagnosis of syphilis | n = 871 | |||
| No | 828 | 51.7 | 1 (Ref) | |
| Yes | 43 | 62.8 | 1.57 (0.83 to 2.96) | 0.16 |
| Period | ||||
| 1993–1996 | 738 | 53.3 | 1 (Ref) | |
| 1997–1999 | 351 | 58.7 | 1.25 (0.96 to 1.61) | 0.09 |
| 2000–2002 | 270 | 49.3 | 0.86 (0.65 to 1.13) | 0.28 |
Indicates P values are significant by binary logistics regression analysis.
Ref indicates reference case.
HIV prevalence was also significantly higher among women who had a history of genital discharge (OR = 1.20, 95% CI: 1.0 to 1.5) and a clinical diagnosis of GUD (OR = 1.70, 95% CI: 1.1 to 2.5) or genital warts (OR = 3.42, 95% CI: 1.5 to 8.0) (see Table 1).
Other factors such as age, education, and number of lifetime sexual partners were not associated with HIV infection (see Table 1).
In multivariate analysis, being “never married” (adjusted odds ratio [AOR] = 1.74, 95% CI: 1.1 to 2.6) and “widowed” (AOR = 2.06, 95% CI: 1.1 to 3.8) were associated with a higher prevalence of HIV infection when compared with those who were married. Associations between inconsistent condom use and HIV infection also persisted in the multivariate analysis (AOR = 1.58, 95% CI: 1.0 to 2.5); however, consistent condom use was not associated with high HIV prevalence as in the univariate analysis. Diagnoses of GUD or genital warts were also independently associated with HIV infection (GUD: AOR = 1.66, 95% CI: 1.0 to 2.6 and genital warts: AOR = 4.75, 95% CI: 1.6 to 14.2 (Table 2).
TABLE 2.
Multivariate Analysis: Demographics and Sexual Behavior With HIV Infection Among FSWs in Pune, India
| Characteristics | Prevalence of HIV (%) |
AOR | 95% CI | P |
|---|---|---|---|---|
| Marital status | ||||
| Married | 42.2 | 1 | ||
| Never married | 57.5 | 1.74 | 1.16 to 2.59 | 0.007* |
| Widowed | 61.1 | 2.06 | 1.13 to 3.76 | 0.018* |
| Divorcee/separated | 51.8 | 1.38 | 0.92 to 2.07 | 0.120 |
| Condom use in lifetime | ||||
| Never | 42 | 1 | ||
| Inconsistent | 57.1 | 1.58 | 1.00 to 2.50 | 0.047* |
| Consistent | 53.4 | 1.29 | 0.79 to 2.10 | 0.310 |
| Diagnosis of genital ulcer | ||||
| No | 51.2 | 1 | ||
| Yes | 64.5 | 1.66 | 1.07 to 2.55 | 0.023* |
| Diagnosis of genital warts | ||||
| No | 52 | 1 | ||
| Yes | 78.8 | 4.75 | 1.59 to 14.22 | 0.005* |
Indicates P values are significant by binary logistics regression analysis.
Trends in Prevalence of HIV and Sexually Transmitted Diseases
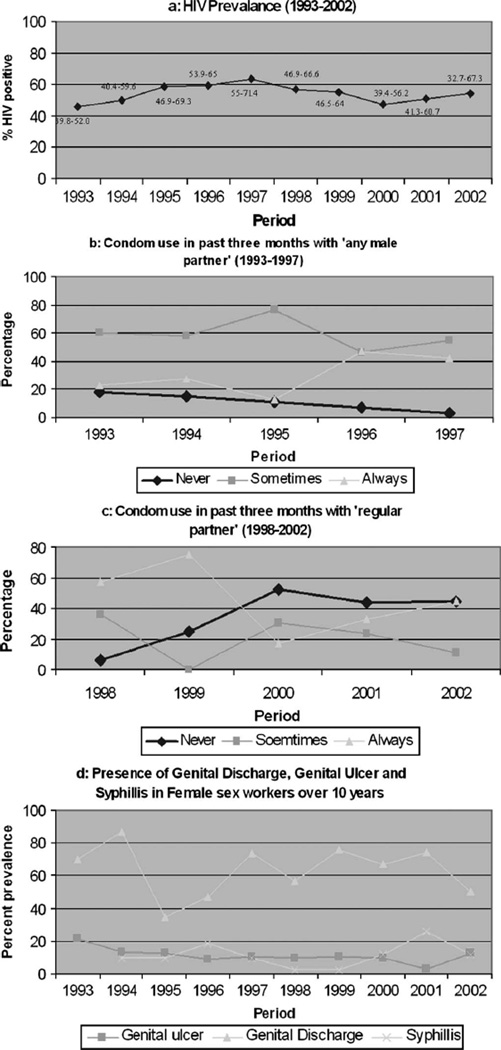
The prevalence of HIV infection remained stable from 1993 to 2002 (Fig. 1A). The age profile of FSWs visiting our clinics also remained stable, with the mean age ranging from 26 to 28 years over the study period (SD of 6–8 years). Over time, the proportion of women who initiated sex at less than 16 years of age decreased (P < 0.001). The mean age at initiation of sex was 16 years in 1993 and 19 years in 2002 (P = 0.06). Between 1993 and 1997, the proportion of women reporting consistent condom use significantly increased from 22% to 42% (P < 0.001) and the proportion reporting never using condoms decreased from 18% to 3% (P < 0.001) (see Fig. 1B). We observed that the proportion of women never using condoms with their regular partners increased from 6% to 44% (P < 0.001) from 1998 to 2002, but we observed no difference in the proportion reporting consistent condom use (see Fig. 1C). The presence of genital ulcers on clinical examination decreased over time (P < 0.001), whereas the prevalence of syphilis increased over time (P = 0.001) (see Fig. 1D). Genital discharge diseases remained stable over the period of 10 years (see Fig. 1D).
FIGURE 1.
HIV, condom use, and STD prevalence among sex workers from 1993 to 2002. (The χ2 test for linear trend is applied to determine the P value.)
Sexual Behavior Among Women Screened Between 1998 and 2002 (data not shown)
Among 485 FSWs enrolled between 1998 and 2002, 49% reported that their first sexual partner was their husband, whereas 9% reported friends and 33% reported unknown persons to be their first sexual partners. More than 60% of these 485 women reported having a regular partner, defined as sexual contact with an individual more than 3 times in the past 6 months. The regular partners were commonly friends (38%) or husbands (24%). Among married FSWs, 87% reported their husband as their regular sexual partner.
Eighteen percent of FSWs reported a history of slippage or breakage of condoms. Thirty-four percent reported customers asking for anal sex, and FSWs agreed in 4% of such instances. Twenty-nine percent of customers asked for oral sex, and 3% of FSWs complied. Ignoring the consistency of the practice, women who refused to have sex with clients without condoms increased from 0% in 1998 to 54% in 2002 (P < 0.001).
DISCUSSION
We initiated the cohort study nearly 7 years after the initial report of HIV infection in an FSW in India in 1986.14 FSWs are known to act as an important core population in sexual transmission of HIV. Their clients act as a bridge population; they acquire HIV or STDs from FSWs and transmit these to their spouses, who have no self-risk behavior but are at risk from their husbands through regular sexual intercourse, primarily without condoms.15 This makes FSWs an especially important target population for HIV prevention efforts. Early prevention and intervention efforts of the NACO (Government of India), NGOs, and community-based organizations were focused on FSWs or their male clients. Despite these efforts, reports from different states in India indicate that HIV prevalence rates among FSWs remained high, especially among high-prevalence states, including the State of Maharasthra, where the present study was carried out.16
The present study was carried out among FSWs attending our study clinics and has limitations in generalizability to the community of sex workers in Pune City, generally because we have not captured the data on other sex workers who were asymptomatic or healthy and on FSWs who were not seeking treatment at government hospitals like ours.
We have presented data on HIV prevalence among FSWs that covers a time span of more than 10 years. It is expected that the true impact of targeted interventions would become visible through temporally collected HIV prevalence and incidence data stretched over the study duration. Our observation that the prevalence of HIV among FSWs remained stable over time reflects some indication of stabilization of the HIV epidemic in this target group. This could be attributed to gradual exhaustion of the susceptible pool or to safe sex practices being adopted by many FSWs. This could therefore also indirectly reflect the efforts by governmental agencies and NGOs to create awareness in the vulnerable population of sex workers.
In our analysis, HIV prevalence was observed to be lower among FSWs who were married compared with those who were unmarried or widowed. Data from 1998 through 2002 suggest that 87% of the married FSWs reported their husbands as their regular partners; this might be indicative of a network of restricted regular sexual partners and limited irregular partners in the case of married sex workers.
Interestingly, we observed higher HIV prevalence among those who reported any condom use compared with those who reported never using condoms. Conventionally, we would expect the opposite effect, and consistent condom use has been reported to be protective against HIV.17–19 Another Indian study has failed to detect such an association, however.20 It is possible that women are more likely to consistently use condoms if they already know they have HIV; thus, FSWs who are already infected with HIV and have the highest risk behavior are also likely to be the ones using condoms consistently. Moreover, the protective effect of condom use might have been nullified by instances of slippage and breakage. It is also possible that in this community with a high level of sexual activity and partner turnover, a reliable history of condom use may not be captured consistently. More detailed qualitative exploration to identify the reasons and patterns of condom use are needed to understand this fully.
In this population, we also observed an increase in reported condom use over time and, more importantly, a marked increase in the proportion of women who refused sex with a customer who would not use a condom. Similar findings were seen in a study of sex workers in Nigeria.21 In one of the most successful Indian HIV prevention programs stressing “no condom, no sex” among FSWs in West Bengal, a decline in HIV prevalence has been reported.22,23 Although condom use with clients seems to have increased, it is a concern that condom use with “regular sexual partners” has not significantly changed; in fact, the proportion of women who reported never using condoms with their regular partners significantly increased over time. Similar observations have been made by others24,25 and indicate that regular partners are possibly perceived as “safe” by these FSWs. These observations highlight a need to continue emphasis on awareness about condom use in FSWs and to educate them to use condoms even with their regular partners.
Previous studies have reported a relation between STDs and HIV infection.4,10,26 Even in the present study, we observed an association between a clinical diagnosis of GUD or genital warts and higher HIV prevalence in FSWs. It is possible that the diagnosis of genital warts is more likely to be a consequence of HIV rather than a direct cause.27
It was encouraging to observe that HIV prevalence among FSWs remained stable at 54% over a period of 10 years. Because our analysis was cross-sectional, it is possible that the observed stability in the HIV prevalence was attributable to a shift in the FSW population attending these STD clinics. The population of FSWs is generally unstable and migratory. It is possible that older, and hence probably HIV-infected, FSWs have moved away from Pune over time. This has been demonstrated by the constant mean age of FSWs over the period of 10 years in our data. The stable prevalence could also be a function of change in health-seeking behavior of FSWs. With a higher level of awareness in the recent times, FSWs have possibly started reporting to the clinics with early symptoms, with suspicion of a disease, or merely for periodic HIV testing rather than waiting until advanced disease occurs. It is also possible that HIV-infected FSWs, being more likely to be symptomatic, are being diverted to newly introduced NGOs offering services and programs in the red light area of Pune.
FSWs are more likely to practice safe sex, and this might have resulted in the concurrent decline in GUD seen in this population over time. Despite this observation, an increasing trend in serologically diagnosed syphilis was observed. We were not able to study trends of other GUDs like genital herpes, chancroid, primary syphilis, or lymphogranuloma because we had used a syndromic clinical diagnosis of GUDs without further classifying them by their cause.
The stable HIV prevalence among FSWs might also be attributed to the impact of awareness programs and promotion of safe sex practices by various governmental agencies (eg, NACO) and NGOs. The parallel decline in GUD might also be a result of these programs. The increase in the rates of refusal of sex without condoms and reluctance to comply with requests for anal or oral sex can be interpreted as “behavioral changes” resulting from efforts of ongoing awareness programs. The success of any intervention can be judged primarily by documenting the decrease in HIV incidence, however.
Stabilization of the HIV epidemic among FSWs has also been observed in other parts of Asia, including Japan and Thailand, primarily because of successful targeted interventions.28,29 The surveillance program in Thailand and efforts in public awareness coupled with a 100% condom use program might have been responsible for the decline in HIV prevalence to 22% in 2003 from its peak (33%) several years ago.29 Other countries such as China, Vietnam, and Nigeria that have not implemented similar programs have observed an increase in HIV seroprevalence among FSWs.30–32 Thus, it seems that continued efforts focusing on imparting education and creating awareness about HIV prevention among FSWs and their male clients might help in stabilizing or reversing HIV trends. There is no room for complacency, and it is important to continue efforts to find a suitable intervention model that is socioculturally acceptable and economically feasible for FSWs and their clients.
ACKNOWLEDGMENTS
The authors acknowledge the support given by the authorities of the collaborating hospitals and Pune Municipal Corporation. The authors thank PAVE/HIVNET/RO1 study staff from the National AIDS Research Institute for excellent counseling, meticulous data collection, clinical care, and laboratory work. The authors thank the NGOs in the red light area and our study participants. They extend special thanks to Lidia Propper from The Johns Hopkins University for providing help in data management of the study.
Supported by the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH) grants R01-A141369, R21-A133879, N01-A135173; Family Health International (FHI); Fogarty AIDS International Training & Research Program (AITRP) TW00010; and Indian Council of Medical Research.
REFERENCES
- 1.National AIDS Control Organization. HIV Estimates 2003. Facts and figures, 2003. Available at: http://www.nacoonline.org/facts_overview.htm.
- 2.National AIDS Control Organization. Monthly updates on AIDS. Facts and figures, September 30, 2004. Available at: http://www.nacoonline.org/facts_reportsept.htm.
- 3.Weniger BG, Limpakarnjanarat K, Ungchusak K, et al. The epidemiology of HIV infection and AIDS in Thailand. AIDS. 1991;5(Suppl):S71–S85. doi: 10.1097/00002030-199101001-00011. [DOI] [PubMed] [Google Scholar]
- 4.Rodrigues JJ, Mehendale SM, Shepherd ME, et al. Risk factors for HIV infection in people attending clinics for sexually transmitted diseases in India. BMJ. 1995;311:283–286. doi: 10.1136/bmj.311.7000.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thuy NT, Lindan CP, Phong TH, et al. Predictors of visits to commercial sex workers by male attendees at sexually transmitted diseases clinics in southern Vietnam. AIDS. 1999;13:719–725. doi: 10.1097/00002030-199904160-00013. [DOI] [PubMed] [Google Scholar]
- 6.Family Health International. Mapping of commercial sex access point and relevant service outlets in Maharashtra. 2001 Available at: http://www.fhi.org/en/HIVAIDS/pub/survreports/Mappavertprepstudyindia.htm.
- 7.Lal S. Current status of AIDS and HIV infection in India. J Indian Med Assoc. 1994;92:3–4. [PubMed] [Google Scholar]
- 8.National AIDS Control Organization. Statewise HIV prevalence. Facts and figures (1998–2003) Available at: http://www.nacoonline.org/facts_statewise.htm.
- 9.Pune Municipal Corporation. Commercial sex workers and AIDS. Available at: http://www.aarogya.com/conditions/communicable/aids/articles.asp.
- 10.National AIDS Control Organization. National baseline high risk and bridge population behavioural surveillance survey, 2001, part 1 (female sex workers and their clients) Available at: http://www.nacoonline.org/publication/41.pdf.
- 11.Mehendale SM, Shepherd ME, Divekar AD, et al. Evidence of high prevalence and rapid transmission of HIV among individuals attending STD clinics in Pune, India. Indian J Med Res. 1996;104:327–335. [PubMed] [Google Scholar]
- 12.Mawar N, Mehendale S, Thilakavathi S, et al. Awareness and knowledge of AIDS and HIV risk among women attending STD clinics in Pune, India. Indian J Med Res. 1997;106:212–222. [PubMed] [Google Scholar]
- 13.Center for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines. MMWR Morb Mortal Wkly Rep. 1993;12:19–82. [Google Scholar]
- 14.Simoes EA, Babu PG, John TJ, et al. Evidence for HTLV-III infection in prostitutes in Tamil Nadu (India) Indian J Med Res. 1987;85:335–338. [PubMed] [Google Scholar]
- 15.Gangakhedkar RR, Bentley ME, Divekar AD, et al. Spread of infection in married monogamous women in India. JAMA. 1997;278:2090–2092. [PubMed] [Google Scholar]
- 16.National AIDS Control Organization. An overview of the spread and prevalence of HIV/AIDS infection in India: facts and figures. Available at: http://www.nacoonline.org/facts_overview.htm.
- 17.Rajanapitayakorn W. “100 percent” condom use seeks to slow HIV spread. Network. 1993;13:30–32. [PubMed] [Google Scholar]
- 18.Morison L, Weiss HA, Buve A, et al. Commercial sex and the spread of HIV in four cities in sub-Saharan Africa. AIDS. 2001;4(Suppl):S61–S69. doi: 10.1097/00002030-200108004-00007. [DOI] [PubMed] [Google Scholar]
- 19.Morisky DE, Ang A, Sneed CD. Validating the effects of social desirability of self-reported condom use behaviour among commercial sex workers. AIDS Educ Prev. 2002;14:351–360. doi: 10.1521/aeap.14.6.351.24078. [DOI] [PubMed] [Google Scholar]
- 20.Desai VK, Kosambiya JK, Thakor HG. Prevalence of sexually transmitted infections and performance of STI syndromes against aetiological diagnosis in female sex workers of red light area in Surat, India. Sex Transm Infect. 2003;79:111–115. doi: 10.1136/sti.79.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Umar US, Adekunle AO, Bakare RA. Pattern of condom use among commercial sex workers in Ibadan, Nigeria. Afr J Med Sci. 2001;30:285–290. [PubMed] [Google Scholar]
- 22.Kumar S. Model for sexual health found in India’s West Bengal. Lancet. 1998;351:46. doi: 10.1016/S0140-6736(05)78070-3. [DOI] [PubMed] [Google Scholar]
- 23.Jana S, Bandyopadhyay N, Mukharjee S, et al. STD/HIV intervention with sex workers in West Bengal, India. AIDS. 1998;12(Suppl B):S101–S108. [PubMed] [Google Scholar]
- 24.Chan MK, Ho KM, Lo KK. A behavioural sentinel surveillance for female sex workers in the social hygiene service in Hong Kong (1999–2000) Int J STD AIDS. 2002;13:815–820. doi: 10.1258/095646202321020071. [DOI] [PubMed] [Google Scholar]
- 25.Spina M, Mancuso S, Sinicco A, et al. Human immunodeficiency virus seroprevalence and condom use among female sex workers in Italy. Sex Transm Dis. 1998;25:451–454. doi: 10.1097/00007435-199810000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Joesoef MR, Cheluget B, Marum LH, et al. Differential of HIV prevalence in women and men who attended sexually transmitted disease clinics at HIV sentinel surveillance sites in Kenya, 1990–2001. Int J STD AIDS. 2003;14:193–196. doi: 10.1258/095646203762869214. [DOI] [PubMed] [Google Scholar]
- 27.Massad LS, Silverberg MJ, Springer G, et al. Effect of antiretroviral therapy on the incidence of genital warts and vulvar neoplasia among women with the human immunodeficiency virus. Am J Obstet Gynecol. 2004;190:1241–1248. doi: 10.1016/j.ajog.2003.12.037. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka M, Nakayama H, Sakumoto M, et al. Reduced chlamydial infection and gonorrhea among commercial sex workers in Fukuoka City, Japan. Int J Urol. 1998;5:471–475. doi: 10.1111/j.1442-2042.1998.tb00390.x. [DOI] [PubMed] [Google Scholar]
- 29.Saengwonloey O, Jiraphongsa C, Foy H. Thailand report: HIV/AIDS surveillance 1998. J Acquir Immune Defic Syndr. 2003;32(Suppl):S63–S67. doi: 10.1097/00126334-200302011-00010. [DOI] [PubMed] [Google Scholar]
- 30.UNAIDS. HIV prevalence among sex workers in selected provinces in China. Monitoring AIDS, 2001. Available at: http://www.unaids.org/EN/other/functionalities/Search.asp?StartRow=0. [Google Scholar]
- 31.Quan VM, Chung A, Long HT, et al. HIV in Vietnam: the evolving epidemic and the prevention response, 1996 through 1999. J Acquir Immune Defic Syndr. 2000;25:360–369. doi: 10.1097/00042560-200012010-00011. [DOI] [PubMed] [Google Scholar]
- 32.Esu-Williams E, Mulanga-Kabeya C, Takena H, et al. Seroprevalence of HIV-1, HIV-2 and HIV-1 group O in Nigeria: evidence for growing increase in HIV infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:204–210. doi: 10.1097/00042560-199711010-00010. [DOI] [PubMed] [Google Scholar]