Abstract
Cross-presentation is important for initiating CTL responses against tumors. Delivery of exogenous Ags to the cross-presentation pathway in dendritic cells (DCs), using a number of different carriers, has been attempted to further understand the mechanisms underlying cross-presentation and to develop therapeutic tumor vaccines. The present study reports a new antigenic carrier molecule: a single-chain V region fragment (scFv) of a nucleic acid–hydrolyzing Ab, 3D8. A fusion protein comprising 3D8 scFv and the CTL epitope OVA250–264 (chicken OVA aa 250–264) was internalized by DC2.4 DCs and processed via a proteasome-dependent, brefeldin- and cycloheximide-sensitive, chloroquine- and primaquine-insensitive pathway, resulting in loading of the CTL epitope onto H-2Kb. In vivo cross-presentation and cross-priming were efficient, even without adjuvant; injection of mice with 3D8 scFv-OVA250–264 induced cross-presentation of the CTL epitope by draining lymph node CD11c+ B7.1+ MHC class IIhigh DCs, elicited a CTL response, and suppressed the growth of tumors expressing the OVA epitope. This report shows that an anti-nucleic acid Ab is used to deliver exogenous Ag to the cross-presentation pathway and inhibit in vivo tumor growth.
Introduction
Antigens captured from the extracellular environment by APCs are processed and then presented on MHC class I molecules to CD8+ CTLs in a process called “cross-presentation,” resulting in the stimulation of CTLs, or “cross-priming” (1). The most efficient APCs for cross-presentation and cross-priming are dendritic cells (DCs) (1, 2). DCs take up exogenous Ags and process them either via a cytosolic pathway dependent on TAP and proteasomes or via the endosomal pathway (which is independent of TAP and proteasomes) (3). However, the molecular machinery involved in cross-presentation has not been fully defined. For example, the molecules responsible for phagosome–cytosol export have not been identified (3). The physiological significance of cross-presentation is evident during defense against many infectious agents that do not infect APCs, and against tumors that do not originate from APCs; in both cases, cross-presentation is required to generate CTLs that are specific for the causative infectious agents and tumor Ags (2).
Molecules capable of transferring exogenous Ag to the cross-presentation pathway have been examined in a number of studies to better understand the mechanisms underlying cross-presentation and to develop tumor vaccines that enhance CTL responses. For example, heat shock proteins (Hsp) such as Hsp70, Hsp90, and gp96 coupled to tumor cell peptides are internalized by APCs via a number of cellular receptors, including CD91, CD40, TLR2/4, LOX-1, and SR-A, whereupon they initiate tumor-specific CTL responses (4–9). Recent re-evaluation of the role of CD91 in gp96-mediated cross-presentation shows the importance of fluid phase–mediated, rather than receptor-mediated, uptake pathways and highlights the role of heparan sulfate proteoglycans (HSPGs) in surface binding of gp96 (10). As for the cross-presentation pathway, the involvement of TAP-independent endosomal pathways was reported for Hsp90–peptide complexes (9) and for a CTL epitope coupled to penetratin, a cell-penetrating peptide derived from Drosophila antennapedia (11). However, many of the steps involved in cross-presentation are still not fully understood.
Previously, we demonstrated that a 27-kDa recombinant nucleic acid–hydrolyzing single-chain Fv (3D8 scFv) was internalized by HeLa cells via a caveolae/lipid raft endocytosis pathway, and that HSPGs are the putative cell surface receptors that facilitate this (12, 13). 3D8 scFv accumulates in the cytosol and is not translocated into late endosomes/lysosomes, the endoplasmic reticulum (ER), the Golgi, or the nucleus; the scFv finally induces apoptotic cell death via the degradation of cellular RNAs (12, 13). Besides 3D8 scFv, endocytosis of some anti-DNA mAbs has been observed in non-APCs (14–16); however, their delivery of exogenous Ag to the cross-presentation pathway in APCs has not been shown.
The current study examined whether 3D8 scFv was able to access the cross-presentation pathway in murine DCs and cross-prime CTLs. 3D8 scFv efficiently delivered a CTL epitope to the proteasome-dependent cross-presentation pathway in DCs. In addition, Ag delivered by 3D8 scFv induced cross-presentation and cross-priming in vivo. Furthermore, therapeutic vaccination using 3D8 scFv fused to a CTL epitope suppressed the growth of tumors expressing the CTL epitope.
Materials and Methods
Cells
The B16 murine melanoma cell line (H-2Kb) was obtained from Yonsei University (Seoul, Korea). The DC2.4 murine DC line (H-2Kb) (17) and MO5, an OVA-transfected clone derived from a B16 melanoma (H-2Kb) (18), were kindly provided by Dr. K.L. Rock (University of Massachusetts Medical School, Worcester, MA). CD8OVA1.3, a T hybridoma cell line specific for OVA257–264–H-2Kb (19), was a generous gift from Dr. C.V. Harding (Case Western Reverse University, Cleveland, OH). B16 and CD8OVA1.3 cells were cultured in DMEM supplemented with 10% FCS and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin). DC2.4 cells and MO5 cells were grown in RPMI 1640 medium supplemented with 10% FCS, 2 mM l-glutamine, 100 μM nonessential amino acids, 10 mM HEPES, 50 μM 2-ME, and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin). MO5 culture medium was supplemented with G418 (1 mg/ml).
Production of scFv-OVA fusion proteins
The DNA fragments encoding a model H-2Kb epitope, SIINFEKL (OVA257–264) or SGLEQLESIINFEKL (OVA250–264), were inserted into the pIg20-scFv bacterial expression vectors to generate 3D8-OVA257–264, 3D8-OVA250–264, or control HW6 OVA250–264 (Fig. 1A). Recombinant proteins containing a protein-A tag were purified from the supernatants of bacterial cultures, using IgG-Sepharose affinity chromatography (12).
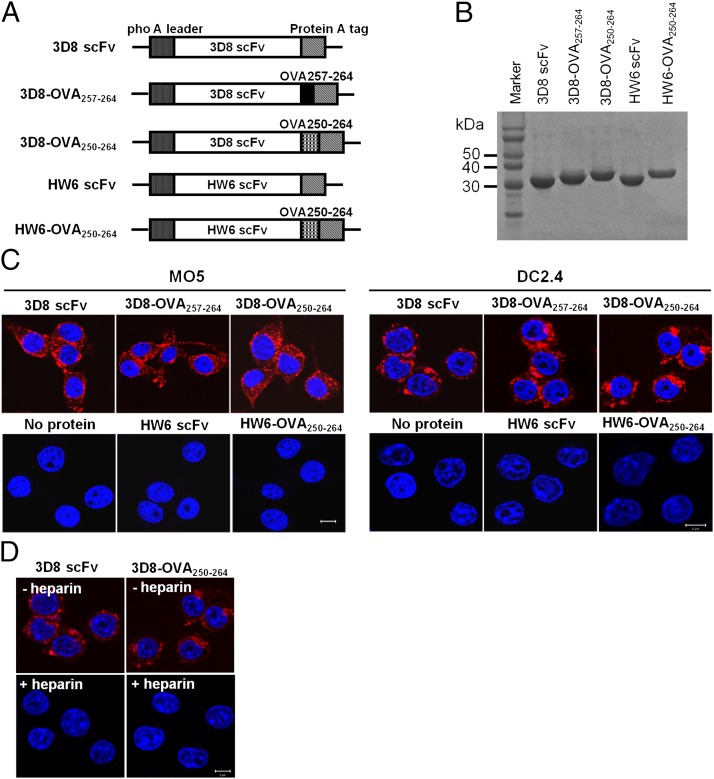
FIGURE 1.
Production of 3D8-OVA fusion proteins and their internalization by MO5 and DC2.4 cells. (A) Schematic diagram showing the expression vectors for the indicated proteins. (B) SDS-PAGE of purified proteins. Proteins (∼20 μg) were separated on 12% SDS-PAGE gels and visualized with Coomassie blue. (C and D) Cell-penetrating activity of proteins. MO5 and DC2.4 cells were incubated with the indicated proteins (10 μM) for 6 h at 37°C in the absence (C) or presence (D) of heparin (50 IU/ml). After washing, fixation, and permeabilization, the cells were incubated with rabbit IgG, followed by TRITC-labeled anti-rabbit IgG Ab (red). Nuclei were stained with Hoechst 33342 prior to analyses under a confocal microscope. Scale bar, 10 μm. Data are representative of three independent experiments.
Isolation of CD11c+ DCs from lymph nodes
CD11c+ dendritic cells were purified from the lymph nodes of C57BL/6 mice, using a MACS system (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s protocol. The purity of CD11c+ cells was >80%, as assessed by flow cytometric analysis.
Cross-presentation assay
As previously described (19), cross-presentation was examined by measuring IL-2 production by DC2.4, lymph node cells, or purified CD11c+ cells cocultured with CD8OVA1.3 T hybridoma cells, which secrete IL-2 when presented with H-2Kb loaded with SIINFEKL. DC2.4 cells (1 × 105 cells per well) or CD11c+ cells (5 × 104 cells per well) were incubated with CD8OVA1.3 T hybridoma cells (0.5 or 1 × 105 cells per well) in the presence of scFv-OVA proteins for 24 h at 37°C. IL-2 levels in the supernatants were measured with an ELISA kit (R&D Systems). In experiments using various inhibitory drugs, cross-presentation assays incorporated a DC2.4 cell fixation step. Briefly, MG-132 (20 μM), lactacystin (10 μM), polymyxin B (20 μg/ml), brefeldin A (5 μg/ml), chloroquine (100 μM), primaquine (50 μM), cycloheximide (5 μg/ml), or heparin (50 IU/ml) was added to the culture of DC2.4 cells for 30 min or 60 min prior to the addition of scFv-OVA proteins (5 μM) or the OVA257–264 peptide (1 μM; Peptron; Daejeon, Korea). After 6 h of incubation at 37°C, DC2.4 cells were washed three times with 2% FCS-PBS and fixed with 1% paraformaldehyde. CD8OVA1.3 T cells were then incubated with the fixed DC2.4 cells for 24 h at 37°C, followed by measurement of IL-2 levels in the supernatant. To demonstrate the processing of scFv-OVA proteins, prefixed DC2.4 cells were incubated with scFv-OVA proteins for 6 h at 37°C and then cocultured with CD8OVA1.3 T cells. All drugs were purchased from Sigma.
Confocal microscopy
To detect endocytosis of scFv-OVA proteins, cells (5 × 104 cells per well in a six-well plate) were pulsed with 5 μM proteins for 2 h at 37°C, washed once with PBS, and further cultured for 2 h at 37°C. Cells were then washed twice, fixed with 2% paraformaldehyde in PBS for 10 min, and permeabilized with PBS containing 0.1% saponin for 10 min. After blocking with 2% BSA-PBS for 1 h, the cells were labeled with 5 μg/ml rabbit IgG (Sigma), followed by TRITC-conjugated anti-rabbit IgG Ab (Sigma). Nuclei were stained with Hoechst 33342 (Invitrogen). When indicated, cells were pretreated with 50 IU/ml of heparin (Sigma) for 1 h at 37°C and then pulsed with 5 μM 3D8-OVA250–264 for 6 h at 37°C in the presence of heparin. Cells were washed with 3% HCl (pH 2.5) for 3 min to remove surface-bound proteins and then fixed. Finally, cells were analyzed by confocal microscopy (Carl Zeiss LSM 710).
To detect the H-2Kb–OVA257–264 complex, cells were pulsed with 5 μM scFv proteins or 1 μM SIINFEKL peptide for 6 h at 37°C and then labeled with biotin–anti-(H-2Kb–OVA257–264) Ab (clone 25-D1.16; eBioscience) followed by FITC-Streptavidin (Vector Laboratories).
To examine the translocation of 3D8-OVA250–264 to the lysosomes, 3D8-OVA250–264 was labeled using a Rhodamine Red-X protein labeling kit according to the manufacturer’s instructions (Invitrogen). Cells were pulsed with Rhodamine-labeled 3D8-OVA250–264 (3 μM) for 2 h at 37°C and then chased for 0.5–6 h at 37°C. LysoTracker Green (0.5 μM; Invitrogen) was added to the cells 30 min before each chase.
Flow cytometry
To detect the H-2Kb–OVA257–264 complex, DC2.4 cells were pulsed with scFv-OVA proteins (5 μM) for 6 h at 37°C, washed, fixed, and labeled with biotin–anti-(H-2Kb–OVA257–264) Ab followed by FITC-Streptavidin. To characterize OVA-presenting cells in mice injected with scFv-OVA, lymph node cells were labeled (after preincubation with an FcγR blocking Ab for 5 min at 4°C) with Abs against CD11c (N418), CD80 (16-10A1), MHC class II (I-A/I-E, M5/114.15.2), and H-2Kb–OVA257–264 (25-D1.16) (all from eBioscience, San Diego, CA) and detected with secondary Abs conjugated with FITC, PE, PerCP, and PE, respectively. Flow cytometric analyses were performed using a FACSCanto flow cytometer (Becton Dickinson, San Diego, CA).
MTT assay
Cell viability was analyzed using the colorimetric MTT assay, as previously described (20). Briefly, cells were seeded at a density of 1 × 104 cells per well in 96-well plates and then cultured in the presence of scFv-OVA protein (10 μM) for 48 h at 37°C. MTT solution (10 μl; 5 mg/ml in PBS) was then added to the wells. After 4 h, the culture medium was removed, and the formazan product formed in the cells was solubilized with 100 μl DMSO. Cell viability was determined by measuring the absorbance at 570 nm, using a microplate reader (Molecular Devices).
Cytosolic fractionation
Cytosolic fractions were prepared as previously described, with slight modifications (21). In brief, cells (1 × 106) were pulsed with 5 μM 3D8-OVA250–264 for 2 h, washed, and incubated for a further 3, 6, 12, or 24 h. Cells were harvested in Tris-EDTA buffer (500 μl per well), and the volume was adjusted to 2 ml per well by adding PBS. The suspended cells were divided into two equal halves (1 ml) to prepare total cell lysates and cytosolic fractions for each time point. The total cell lysate sample was washed and mixed with SDS sample buffer. The other sample was washed, homogenized in 200 μl fractionation buffer [10 mM Tris (hydroxymethyl) aminomethane/acetic acid, pH 7.0; 250 mM sucrose] using a 26-guage needle, and then centrifuged at 4000 × g for 2 min to pellet the debris, plasma membranes, and nuclei. Subsequently, the supernatant was centrifuged at 400,000 × g for 30 min to obtain a highly purified cytosolic fraction. All procedures were carried out at 4°C or on ice.
Western blotting
3D8-OVA250–264 was detected in the total cell lysate and cytosolic fractions by Western blotting with a rabbit anti-3D8 scFv Ab (13), an HRP-conjugated goat anti-rabbit IgG secondary Ab (Zymed Laboratories), and an ECL kit (Amersham Pharmacia Biotech). Poly(ADP-ribose) polymerase PARP cleavage was detected using rabbit anti-PARP (Cell Signaling Technology). Rabbit anti–β-actin (Cell Signaling Technology) was used to normalize protein loading.
Immunization of mice with 3D8-OVA250–264
All animal experiments were performed using a protocol approved by the Ajou University Animal Care and Use Committee. To assess cross-presentation, seven-wk-old male C57BL/6 mice (H-2Kb) from DBL (Chungbuk, Korea) were inoculated s.c. (once in the right flank) with 3D8 scFv, 3D8-OVA250–264, HW6-OVA250–264, or full-length OVA protein at a dose of 50 μg per mouse. After 48 h, the right inguinal and left cervical lymph nodes were removed and subjected to a cross-presentation assay. To examine cross-priming by scFv-OVA, C57BL/6 mice were immunized (s.c. on the flank on days 1 and 7) with the indicated scFv-OVA or full-length OVA proteins at a dose of 25 μg per mouse per injection. On day 15, splenocytes were isolated and used in a 51Cr release assay.
51Cr release assay
The CTL activity of the stimulated splenocyte population was assessed in a 51Cr release assay, as described previously (22). Briefly, splenocytes (1 × 108) were isolated from immunized mice and stimulated in vitro for 5 d by coculture with MO5 cells (1 × 106) treated with mitomycin C (50 μg/ml). The splenocytes were harvested and cocultured with 51Cr-labeled target cells (MO5 or B16) at different effector/target cell ratios for 4 h at 37°C. The release of 51Cr was then measured. The spontaneous release of 51Cr in the absence of effector cells and the maximal release by treatment of cells with 1% Triton X-100 were also evaluated. The percentage lysis of target cells was calculated as follows: cpm of the test sample − cpm of spontaneous release/cpm of maximal release − cpm of spontaneous release.
Tumor growth
C57BL/6 mice were s.c. injected in the flank with either 5 × 104 MO5 or 5 × 104 B16 cells. When tumors reached 40–50 mm3, five mice per group were s.c. injected with 50 μg 3D8 scFv, 3D8-OVA250–264, HW6-OVA250–264, or full-length OVA protein (or with PBS as a negative control) daily in the nape of the neck for 3 consecutive days. Mice were sacrificed when tumor volume reached 4000–5000 mm3. Tumor volume was calculated as the following: longest diameter × shortest diameter × 3/2 × π/6.
Results
3D8-OVA fusion proteins are internalized by DC2.4 DCs
To monitor cross-presentation, 3D8 scFv and a control HW6 scFv specific for human TRAIL receptor 2 were tagged with a CTL epitope (Fig. 1A). 3D8 scFv was tagged with the exact H-2Kb epitope, SIINFEKL (OVA257–264), or with a longer epitope, SGLEQLESIINFEKL (OVA250–264), which contains the linker sequence “SGLEQLE” (preceding SIINFEKL) to augment Ag-processing efficiency (23). The proteins were purified in soluble form from bacterial culture supernatants to avoid the need for cell lysis and refolding. Purity was >95% on SDS-PAGE gels (Fig. 1B). The endotoxin level in these proteins was <0.2 EU/μg, as determined by the Pyrosate test kit (Chester Springs, PA) (data not shown). The nucleic acid–binding and –hydrolyzing activity of 3D8 scFv (12, 13) was retained by the 3D8-OVA fusion proteins (Supplemental Fig. 1). The cell-penetrating activity of the 3D8-OVA fusion proteins and 3D8 scFv were comparable in MO5 and DC2.4 cells (Fig. 1C). HW6 scFv did not internalize in either cell type. Internalization of 3D8-OVA250–264 fusion proteins was inhibited by heparin, which is a representative heparan sulfate (Fig. 1D). These results are compatible with our previous observations in HeLa cells and clearly show that 3D8-OVA proteins are internalized by both DC2.4 and MO5 cells.
3D8-OVA fusion proteins have little effect on the viability of DC2.4 DCs
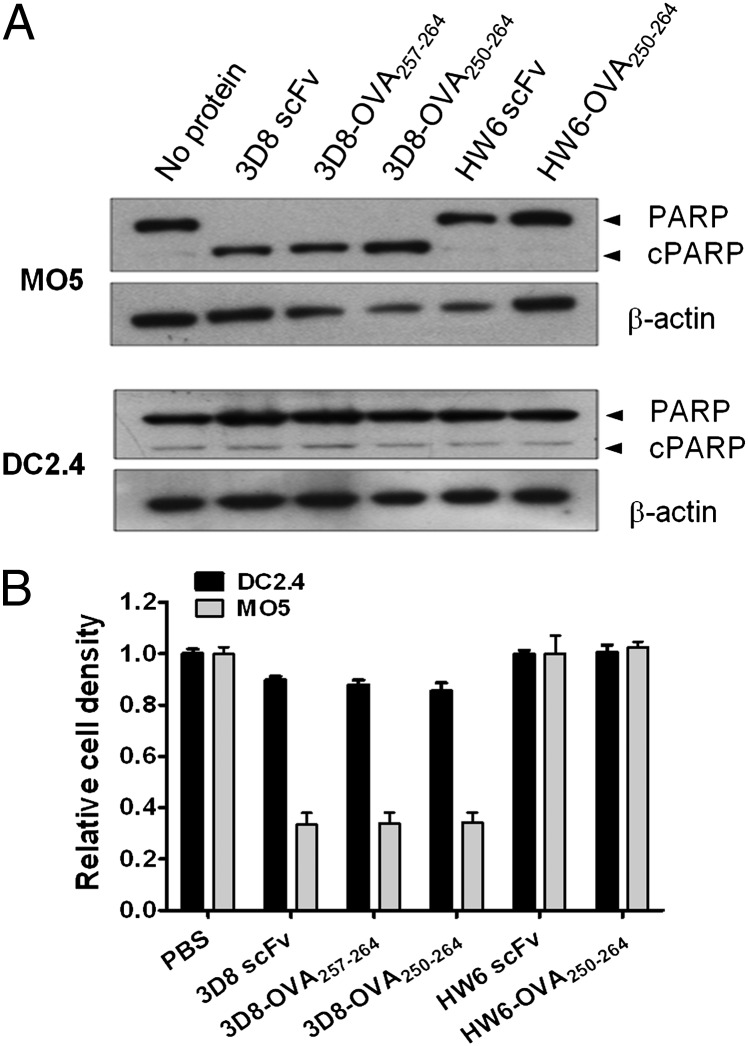
Our previous work showed that 3D8 scFv (10 μM) induced HeLa cell death 48 h after internalization (13). Therefore, to examine whether 3D8-OVA fusion proteins similarly induce cell death in DC2.4 cells, we analyzed PARP cleavage 48 h after treatment with 3D8-OVA fusion proteins, using MO5 cells as a control (Fig. 2A). Increased PARP cleavage was induced in MO5 cells, but not in DC2.4 cells, by 3D8 scFv or 3D8-OVA, implying that cell death in MO5 cells, but not in DC2.4 cells, is induced by the uptake of 3D8 scFv or 3D8-OVA proteins. Concordantly, the MTT assay showed that cell viability decreased by 70% in MO5 cells treated with 3D8 scFv or 3D8-OVA proteins, but by <10% in DC2.4 cells (Fig. 2B). These results indicate that DC2.4 cells are much more tolerant to the cytotoxic activity of 3D8-OVA fusion proteins than are MO5 cells.
FIGURE 2.
The effect of 3D8-OVA fusion proteins on MO5 and DC2.4 cell viability. MO5 and DC2.4 cells were cultured in the presence of the indicated protein (10 μM) for 48 h; PARP cleavage was analyzed by Western blotting (A), and cell viability was determined using an MTT assay (B). The relative viability was calculated as the absorbance of the sample/the absorbance of PBS. Data represent the mean ± SE of triplicate wells and are representative of three independent experiments.
Cytosolic translocation and proteasome-involved degradation of 3D8-OVA fusion protein in DC2.4 DCs
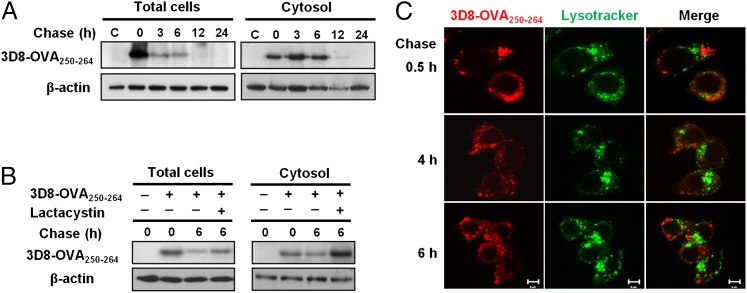
To analyze the stability and cytosolic translocation of the 3D8-OVA fusion protein in DC2.4 cells, we chased 3D8-OVA250–264 in total cell lysates and the cytosolic fractions of cells pulsed for 2 h with 3D8-OVA250–264. 3D8-OVA250–264 was detected in the cytosolic fraction at 0 h chase, indicating the release of 3D8-OVA250–264 (possibly from the endosomal compartment) into the cytosol during 2-h pulse. 3D8-OVA250–264 was detected in the total cell lysates and in the cytosolic fractions during the early chase periods (0–6 h); however, it was difficult to detect 3D8-OVA250–264 after 12 h (Fig. 3A). In the presence of lactacystin, a proteasome inhibitor, the amount of 3D8-OVA250–264 increased in the total cell lysate and cytosolic fraction after 6 h compared with that in the absence of lactacystin (Fig. 3B; Supplemental Fig. 2). These findings indicate proteasome-involved degradation of 3D8-OVA250–264.
FIGURE 3.
Cytosolic translocation and proteasome-involved degradation of 3D8-OVA250–264. (A) DC2.4 cells were pulsed with 5 μM of 3D8-OVA250–264 for 2 h. Then, cells were washed and chased for the indicated times, followed by Western blotting. C, Not pulsed with 3D8-OVA250–264. Data are representative of two independent experiments. (B) DC2.4 cells were pulsed with 5 μM of 3D8-OVA250–264 in the presence or absence of lactacystin (10 μM) for 2 h followed by incubation for 6 h in the presence or absence of lactacystin. 3D8-OVA250–264 was detected by Western blotting. β-actin was used as a loading control. Data are representative of two independent experiments. (C) DC2.4 cells were pulsed with 3 μM of rhodamine-labeled-3D8-OVA250–264 (red) and then chased for the indicated times under confocal microscopy. Late endosomes and lysosomes were labeled with LysoTracker (green). Scale bar, 5 μm. Data are representative of two independent experiments.
Next, we determined whether 3D8-OVA250–264 localized in late endosomes and lysosomes. DC2.4 cells were pulsed for 2 h with rhodamine–3D8-OVA250–264 and then chased by confocal microscopy. 3D8-OVA250–264 did not localize in the late endosomes or lysosomes labeled with LysoTracker Green during the first 6 h of the chase (Fig. 3C), suggesting that the late endosomes and lysosomes were unlikely to be involved in the degradation of 3D8-OVA250–264.
Cross-presentation of 3D8-OVA proteins by DC2.4 DCs occurs via a proteasome-dependent pathway
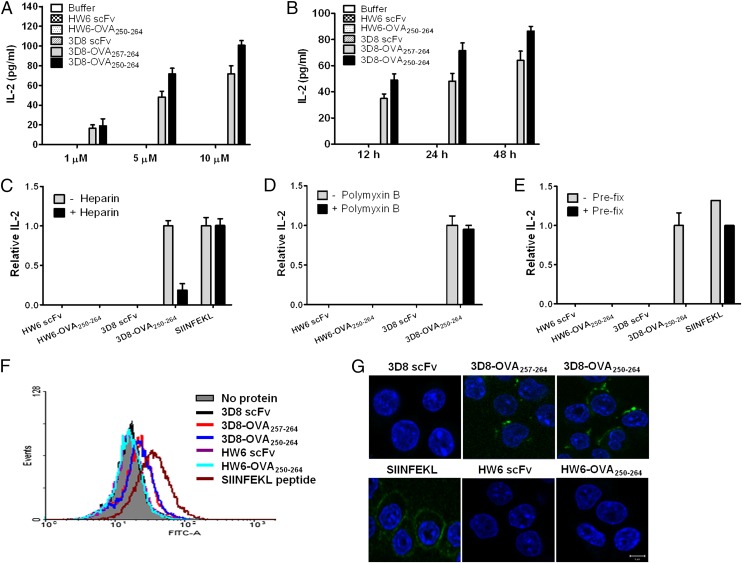
To assess the cross-presentation of 3D8-OVA fusion proteins, we used a coculture system comprising DC2.4 cells expressing H-2Kb and CD8OVA1.3 T hybridoma cells, which produce IL-2 upon recognition of the OVA257–-264–H-2Kb complex (19). DC2.4 cells were cocultured with CD8OVA1.3 T hybridoma cells in the presence of 1–10 μM 3D8-OVA proteins or HW6-OVA proteins (Fig. 4A) for 12–48 h (Fig. 4B). Both 3D8-OVA250–264 and 3D8-OVA257–264 induced IL-2 production in a time- and dose-dependent manner. The control HW6 scFv and HW6-OVA250–264 proteins did not stimulate IL-2 release. Because 3D8-OVA250–264 induced higher IL-2 production than did 3D8-OVA257–264, the following experiments were performed using 3D8-OVA250–264. In the presence of heparin, which blocks internalization of 3D8-OVA250–264, IL-2 production stimulated by 3D8-OVA250–264 was reduced by ∼70% (Fig. 4C; see Supplemental Table I for the actual amount of IL-2). To rule out the effect of possible LPS contamination of the 3D8-OVA250–264 on IL-2 production, polymyxin B (which neutralizes LPS) (24) was added to the coculture. IL-2 production induced by 3D8-OVA250–264 was unaffected (Fig. 4D). To exclude the possible presence of free peptide in the 3D8-OVA250–264, IL-2 production was assessed in cultures stimulated with prefixed DC2.4 cells (Fig. 4E). In contrast to the positive control OVA peptide (SIINFEKL), IL-2 production was not detected in cultures with prefixed DC2.4 cells in the presence of 3D8-OVA250–264. These results indicate that cross-presentation of 3D8-OVA250–264 occurs in DC2.4 cells.
FIGURE 4.
Cross-presentation of 3D8-OVA fusion proteins by DC2.4 cells. (A and B) DC2.4 cells and CD8OVA1.3 T cells were cocultured in the presence of the indicated proteins at the indicated concentrations for 24 h (A) or at 5 μM for the indicated times (B). IL-2 levels in the culture supernatant were then measured. Data represent the mean ± SE of triplicate samples. (C and D) DC2.4 cells were pulsed with the indicated proteins (5 μM) in the presence or absence of either polymyxin B (20 μg/ml) or heparin (50 IU/ml) for 6 h. DC2.4 cells were washed, fixed, and then incubated with CD8OVA1.3 T cells for 24 h at 37°C. (E) Prefixed DC2.4 cells were pulsed with the indicated proteins (5 μM) for 6 h and then incubated with CD8OVA1.3 T cells for 24 h at 37°C. (C–E) IL-2 levels were measured in the culture supernatant and expressed as a ratio against the amount of IL-2 in the absence of heparin, polymyxin B, and prefixation. Data represent the mean ± SE of three independent experiments. (F and G) Flow cytometric analysis (F) and confocal microscopy (G) of DC2.4 cells pulsed with the indicated proteins (5 μM) for 6 h at 37°C and labeled with a biotin-conjugated Ab specific for the H-2Kb–OVA257–264 complex and FITC-Streptavidin. Nuclei were stained with Hoechst 33342. Scale bars, 10 μm. Data are representative of two independent experiments.
To further demonstrate the cross-presentation of 3D8-OVA250–264, the H-2Kb–OVA257–264 complex was detected with an Ab specific for this complex and analyzed using flow cytometry (Fig. 4F) and confocal microscopy (Fig. 4G). H-2Kb–OVA257–264 complexes were detected on the surface of DC2.4 cells pulsed with 3D8-OVA for 6 h or with the SIINFEKL peptide (a positive control). These data demonstrate that 3D8 scFv can deliver Ag to the cross-presentation pathway in DC2.4 cells.
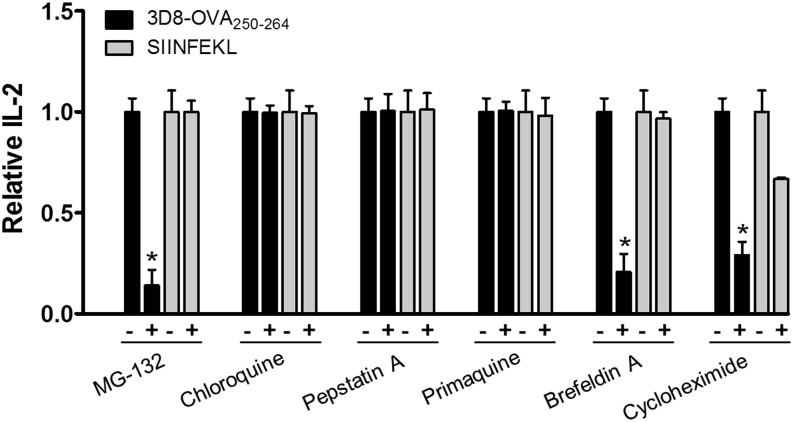
Exogenous Ags can be cross presented via the “cytosolic pathway” (proteasome/TAP dependent) or the “endosomal pathway” (proteasome/TAP independent) (1). Therefore, we examined the cross-presentation pathway for 3D8-OVA250–264 in DC2.4 cells by inhibiting each of the pathways. DC2.4 cells were pulsed with 3D8-OVA250–264, or with SIINFEKL as a control, in the presence of the indicated inhibitors. Cells were then fixed and used to stimulate CD8OVA1.3 T hybridoma cells (Fig. 5; see Supplemental Table I for the actual amounts of IL-2). The proteasome inhibitor MG-132 (25) strongly inhibited IL-2 production under pulse conditions with 3D8-OVA250–264. By contrast, chloroquine, which neutralizes pH in the endosomes, or pepstatin A, an inhibitor of lysosomal proteolysis, did not suppress IL-2 production. These results agree with those presented in Fig. 3 and demonstrate that the 3D8-OVA250–264 protein is mainly processed by proteasomes in the cytoplasm, independently of vacuolar acidification and protease activity. After processing by the proteasome in DCs, peptide loading onto MHC class I molecules takes place within the ER or within early endocytic compartments (1, 26).
FIGURE 5.
3D8-OVA250–264 is cross presented via the classical cytosolic MHC class I pathway. DC2.4 cells were pulsed with 3D8-OVA250–264 (5 μM) or OVA257–264 peptide (1 μM) in the presence or absence of the indicated inhibitors for 6 h at 37°C. DC2.4 cells were washed, fixed, and then incubated with CD8OVA1.3 T hybridoma cells for 24 h at 37°C. IL-2 production is expressed as a ratio against the amount of IL-2 in the absence of each inhibitor. Data represent the mean ± SE from four independent experiments. *p < 0.005, Student t test.
Cross-presentation via peptide loading in the ER is sensitive to brefeldin A, a drug that inhibits the export of proteins from the ER to the Golgi and induces retrograde protein transport from the Golgi to the ER (1). In contrast, cross-presentation via peptide loading within the early endocytic compartment is affected by primaquine, which interferes with the recycling of endosomes to the plasma membrane (24). When DC2.4 cells were pretreated with primaquine and pulsed with 3D8-OVA250–264, IL-2 production was unchanged; however, it was suppressed by treatment with brefeldin A. These results suggest that peptides generated from 3D8-OVA250–264 may be loaded onto MHC class I molecules in the ER in DC2.4 cells and not in early endocytic compartments. Treatment with cycloheximide, an inhibitor of protein biosynthesis, reduced IL-2 production under pulse conditions with 3D8-OVA250–264 to a similar extent as brefeldin A, indicating that newly synthesized MHC class I molecules may be required for cross-presentation of 3D8-OVA250–264. The actual reduction in H-2Kb expression levels by cells treated with primaquine, brefeldin A, and cycloheximide was confirmed by flow cytometry using an anti–H-2Kb Ab (data not shown).
Taken together, these results indicate that 3D8-OVA250–264 may be presented by DC2.4 cells via the canonical cytosolic pathway, in which 3D8-OVA250–264 is degraded by the proteasome and the peptides are loaded onto MHC class I molecules in the ER.
In vivo cross-presentation and cross-priming of 3D8-OVA protein
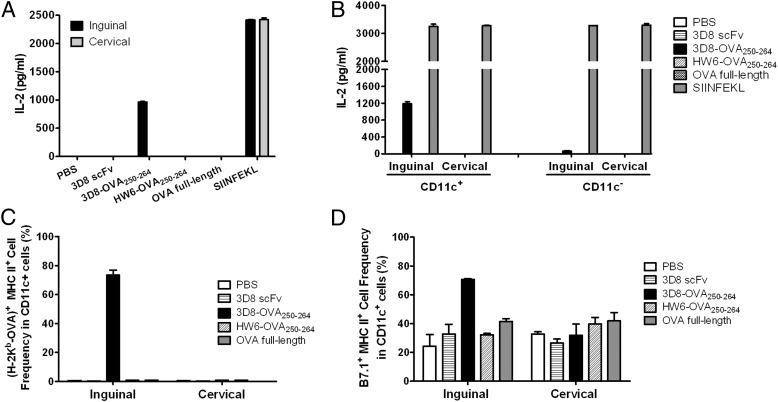
To examine the in vivo cross-presentation of 3D8-OVA250–264, mice were immunized with 3D8-OVA250–264 or a control protein such as 3D8 scFv, HW6-OVA250–264, or full-length OVA protein. After 48 h, cells were isolated from the draining right inguinal lymph nodes and the nondraining cervical lymph nodes and incubated with CD8OVA1.3 T hybridoma cells for 24 h to monitor IL-2 production. When a positive control OVA peptide (SIINFEKL) was added to cocultures of CD8OVA1.3 T hybridoma cells and lymph node cells from PBS-injected mice, IL-2 production (∼2,000 pg/ml) was observed; however, no IL-2 was detected in cocultures with nondraining cervical lymph node cells, regardless of the injected proteins, or in the cocultures with draining inguinal lymph node cells from all mice, except those injected with 3D8 scFv250–264. Significant IL-2 levels (∼700 pg/ml) were detected in the cocultures with draining inguinal lymph node cells from mice injected with 3D8-OVA250–264 (Fig. 6A). These results demonstrate that 3D8-OVA250–264 is cross presented in vivo. To identify cells cross presenting 3D8-OVA, CD11c+ cells and CD11c− cells were isolated from the lymph nodes of mice immunized as described above, and then incubated with CD8OVA1.3 T hybridoma cells to monitor IL-2 production (Fig. 6B). CD11c+ cells from the draining inguinal lymph nodes of mice injected with 3D8-OVA250–264 stimulated high levels of IL-2 production (∼1200 pg/ml), but CD11c− cells stimulated only very low levels (∼60 pg/ml). Concordantly, H-2Kb–OVA257–264 complexes were observed in >70% cells of inguinal lymph node CD11c+ cells from mice immunized with 3D8-OVA250–264, but not from nondraining lymph node CD11c+ cells of all mice. (Fig. 6C). Furthermore, almost all CD11c+ cells expressing H-2Kb–OVA257–264 complexes displayed MHC class II and B7.1 molecules on their surface (Fig. 6C, 6D, Supplemental Fig. 3). Some CD11c− cells from the inguinal lymph nodes of mice immunized with 3D8-OVA250–264 displayed H-2Kb–OVA257–264 complexes and B7.1 (data not shown), which might account for the low level of IL-2 production shown in Fig. 6B. Taken together, these results indicate that 3D8-OVA induces CD11c+ DC maturation in the absence of adjuvant and is cross presented by these cells.
FIGURE 6.
In vivo cross-presentation of 3D8-OVA250–264 by mature CD11c+ DCs. (A and B) C57BL/6 mice (n = 2 per group) were s.c. injected in the right flank with the indicated proteins (50 μg) or PBS. After 48 h, (A) total lymph node cells (5 × 105) and (B) CD11c+ or CD11c− cells (5 × 104) isolated from the right inguinal and left cervical lymph nodes were cocultured with CD8OVA1.3 T hybridoma cells (0.5 or 1 × 105) for 24 h at 37°C. IL-2 levels were then measured. SIINFEKL: IL-2 production in the cocultures of CD8OVA1.3 T hybridoma cells and lymph node cells in vitro pulsed with SIINFEKL peptide (1 μM) for 24 h. Data represent the mean ± SE of two independent experiments performed in triplicate. (C and D) The indicated lymph node cells were isolated from C57BL/6 mice 48 h after s.c. injection in the right flank with the indicated proteins (50 μg) or PBS. Expression of CD11c, H-2Kb–OVA complexes, MHC class II molecules, and B7.1 was then analyzed by flow cytometry. Data represent the mean ± SE of two independent experiments performed in duplicate, with eight mice per group.
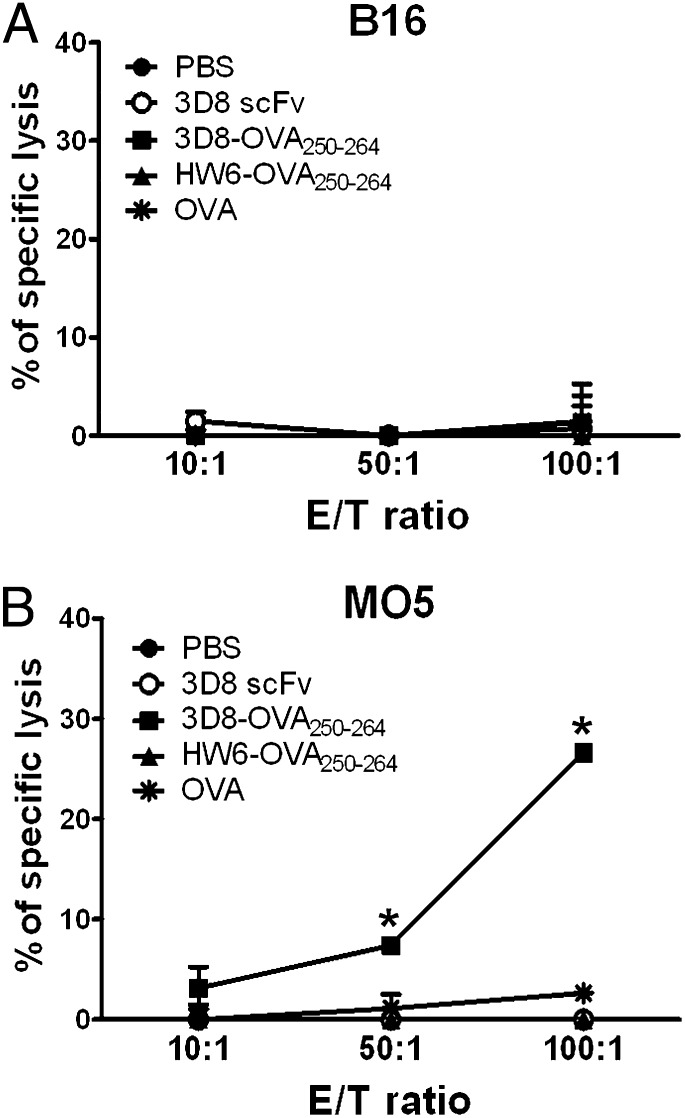
To further support in vivo cross-presentation of 3D8-OVA250–264, C57BL/6 mice were immunized twice with 3D8-OVA250–264 and splenocytes were isolated for the assay of OVA-specific CTL activity 7 d later. CTL activity was evaluated according to the percentage lysis of target cells in a 51Cr release assay using B16 or MO5 cells as target cells. The MO5 cell line is an OVA-transfected subclone of a B16 melanoma. Splenocytes from mice injected with 3D8-OVA250–264 showed a significant level of CTL activity against MO5 cells, but not against B16 cells (Fig. 7). This finding indicates that 3D8-OVA250–264 is cross presented in vivo, resulting in the generation of OVA-specific CTL. Further, it should be noted that cross-priming of 3D8-OVA250–264 also occurred in the absence of adjuvant.
FIGURE 7.
In vivo cytotoxic T cell stimulation of 3D8-OVA250–264. Splenocytes from C57BL/6 mice immunized with the indicated proteins (50 μg) or PBS were subjected to a 51Cr release assay using B16 (A) or MO5 (B) as target cells. Data represent the mean ± SE of two independent experiments performed in triplicate. *p < 0.005, Student t test.
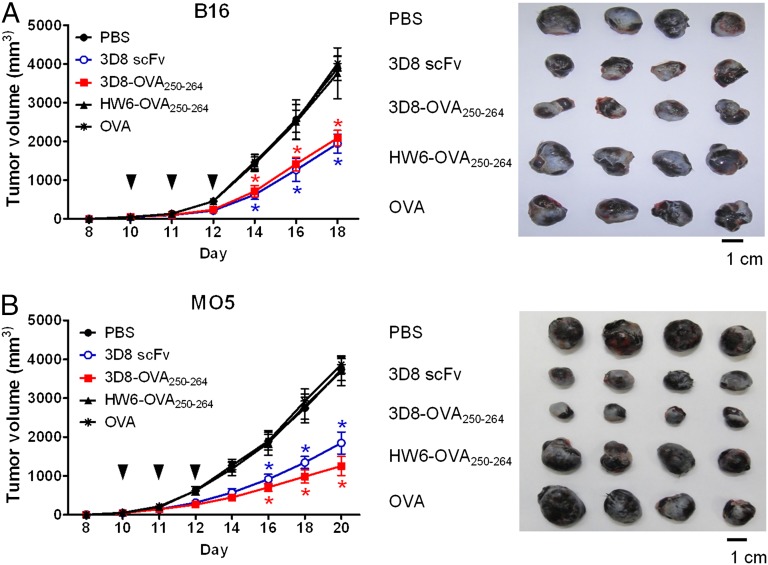
Finally, in vivo cross-presentation and cross-priming of 3D8-OVA250–264 were investigated by analyzing the therapeutic effect of 3D8-OVA250–264 against an established tumor. Because both 3D8-OVA250–264 and 3D8 scFv were cytotoxic for MO5 in vitro (Fig. 2), we reasoned that although both proteins might similarly inhibit B16 tumor growth, 3D8-OVA250–264 might inhibit MO5 tumor growth to a greater extent than 3D8 scFv because only 3D8-OVA250–264 could initiate OVA-specific CTL responses, which would attack MO5 cells expressing OVA, but not B16 cells. To verify this, we injected mice bearing a MO5 or B16 tumors of ∼50 mm3 with 50 μg of 3D8 scFv, 3D8-OVA250–264, HW6-OVA250–264, or full-length OVA protein s.c. daily for 3 consecutive days and analyzed tumor growth (Fig. 8). Tumor growth was not inhibited in the control mice injected with HW6-OVA250–264 or full-length OVA protein compared with that in mice injected with PBS. However, tumor growth was inhibited by >50% in mice injected with 3D8 scFv or 3D8-OVA250–264 compared with that in control mice. As expected, the inhibitory effect of 3D8-OVA250–264 on B16 tumor growth was similar to that of 3D8 scFv; however, its effect on MO5 tumor growth was ∼1.5-fold greater than that of 3D8 scFv. These results indicate that 3D8-OVA250–264 efficiently induces an OVA-specific CTL immune response after cross-presentation.
FIGURE 8.
Inhibitory effects of 3D8-OVA250–264 on tumor growth in mice. C57BL/6 mice were s.c. injected into the flank with B16 (A) or MO5 (B) tumor cells. When tumors reached 40–50 mm3, mice were treated s.c. with 50 μg of the indicated protein or PBS daily for 3 consecutive days (arrowheads). Tumor growth was measured for the indicated days. Four representative tumors removed on day 18 for B16 or day 20 for MO5 are shown in the right panels. All the experiments were performed twice with five mice per group. Data represent the mean ± SE of two independent experiments. *p < 0.001, Student t test.
Discussion
In this study, we showed that 3D8 scFv can deliver Ag to the cross-presentation pathway both in vitro and in vivo. 3D8-OVA250–264 was internalized by DC2.4 DCs and translocated to the cytosol, where OVA peptides were released from 3D8 scFv by the action of proteasomes. The released OVA peptides were loaded onto MHC class I molecules and presented on the surface of DC2.4 cells. Draining lymph node CD11c+ DCs from mice cross presented OVA 48 h after injection of 3D8-OVA250–264. Ag targeting to the cross-presentation pathway in DCs has been studied using Ags coupled to carriers such as cell-penetrating peptides (CPPs) (27–29), endocytosed Hsps (9, 30), and Abs against DC-specific endocytic receptors, including DC-SIGN and DEC205 (26). In particular, Ag targeting to the cross-presentation pathway in both murine and human DCs has been extensively studied by Steinman’s group, using an anti-DEC205 Ab (31, 32). However, cross-presentation of anti–nucleic acid Abs has not been demonstrated, even though their internalization has been observed in a variety of cells, such as kidney cell lines, an ovary cell line, and rat hepatoma cells (15, 16, 33). Thus, our finding that 3D8 scFv is internalized by DCs, followed by cytosolic translocation and delivery of Ag to the cross-presentation pathway, may provide useful insights into the novel functions of anti–nucleic acid Abs.
Our results show that 3D8-OVA250–264 was cross presented via the proteasome/TAP-dependent (sensitive to MG132) and ER peptide-loading (sensitive to brefeldin A) pathways. Whole OVA protein is cross presented via the proteasome/TAP-dependent early endosome peptide-loading pathway when internalized via a mannose receptor (CD206) (24, 34). In this pathway, Ags are exported into the cytosol and processed by cytosolic proteasomes; the Ag-derived peptides are then reimported into the early endosomes via TAP in the endosomal membrane, ready for loading onto MHC class I molecules (this pathway is sensitive to primaquine treatment) (24, 34). In contrast, cross-presentation of Hsp-associated Ags, which are internalized through CD91, CD40, and scavenger receptors, can occur either via a TAP- and proteasome-dependent cytosolic pathway, or a TAP- and proteasome-independent endosomal pathway, depending on the amino acid sequence of the peptide bound to the Hsp (9, 30, 35). It remains to be clarified whether the cross-presentation pathway of 3D8 scFv is affected by the antigenic epitope sequences of its cargo.
The cell surface molecules involved in 3D8-OVA250–264 internalization by DCs were not identified in this study. Strong candidates are HSPGs, which are involved in the endocytosis of 3D8 scFv by HeLa cells (13) and in the endocytosis of CPPs, which contain multiple cationic amino acid residues similar to 3D8 scFv (36). It is reported that clustering of HSPGs triggers cytoskeletal remodeling and internalization pathways, and that HSPGs promote MHC class II-restricted Ag presentation by acting as coreceptors during Fcγ-RII–mediated Ag presentation (37, 38). However, in the current study, endocytosis of 3D8 scFv was not affected by treatment with heparinase III (which cleaves heparan sulfate of HSPGs; data not shown), despite the inhibition of 3D8 scFv endocytosis in the presence of heparin, a competitor of HSPGs. Therefore, identification of the molecules involved in endocytosis of 3D8 scFv by DCs requires further investigation.
We also demonstrated the in vivo antitumor effects of 3D8 scFv. In our previous work, 3D8 scFv caused hydrolysis of cellular RNAs, mainly in HeLa cells, resulting in cell death (13). Concordantly, we observed reduced viability of MO5 cells after uptake of 3D8 scFv. Furthermore, 3D8 scFv inhibited the growth of pre-existing MO5 tumors when injected adjacent to the tumor. It is plausible that 3D8 scFv might be internalized into tumor cells and translocated to the cytosol, where it breaks down cellular RNAs, eventually triggering cell death. In vivo cross-presentation of 3D8-OVA250–264 indirectly supported the in vivo uptake of 3D8 scFv by MO5 cells and its cytosolic translocation. We should note that the cytotoxic effects of 3D8 scFv differed between MO5 and DC2.4 DCs. Further studies are required to evaluate whether tumor cells preferentially internalize 3D8-OVA, and whether they are more susceptible to 3D8 scFv-mediated cytotoxicity than are normal cells.
Fusion of a CTL epitope increases the therapeutic effect of 3D8-scFv against tumors expressing the corresponding CTL epitope. 3D8-OVA250–264 inhibited the growth of pre-established MO5 tumors significantly more than did 3D8 scFv, whereas the inhibitory effect of 3D8-OVA250–264 on B16 tumor growth was similar to that of 3D8 scFv. This finding can be explained by the strong CTL response to OVA elicited by injection of 3D8-OVA250–264. Accumulating evidence shows the efficacy of prophylactic tumor vaccines incorporating Hsp70 (39), CPPs (27, 40), and α2M* (41); however, little evidence exists for the efficacy of therapeutic vaccines. Moreover, CPPs require adjuvant to generate an effective antitumor response. Generally, vaccination with protein Ags in soluble form is not sufficient to induce strong CTL responses, even if Ags are cross presented by DCs. The use of adjuvants is usually necessary to stimulate innate immunity via TLR ligands expressed by DCs and to generate the immunogenic environment required for CTL induction (24, 42, 43). Peptide epitopes incorporated into CPP carriers were able to elicit strong Ag-specific CTL responses when administrated along with adjuvants such as CpG and OK432 (27, 28, 44). However, some protein carriers, such as α2M*, Hsp70, and Hsp90, possess intrinsic innate immunity-stimulating ability. Ags incorporated with the α2M* protein, which is internalized via CD91, were able to elicit antitumor activity in the absence of an adjuvant because α2M* itself can function as an adjuvant to stimulate innate immunity (41). In another study, an Hsp70-coupled peptide cross primed peptide-specific T cells without adjuvant in mice and activated TLR4-deficient DCs to release proinflammatory cytokines (45). Hsp90 efficiently induced peptide-specific CTL responses in mice, which were comparable to those induced by immunization with a peptide emulsion in CFA (9). We found that 3D8-OVA250–264 elicits the maturation of draining lymph node DCs (Fig. 6C, 6D) and Ag-specific CTL responses in vivo in the absence of adjuvant and enhances the antitumor effects of 3D8 scFv. Unexpectedly 3D8 scFv did not induce maturation (B7.1 expression) on DCs. We speculate that 3D8-OVA250–264 may indirectly induce B7.1 expression through interaction with OVA-specific T cells. Our findings raise the question of how 3D8 scFv plays a role as an adjuvant.
In conclusion, this report shows cross-presentation of an anti–nucleic acid scFv (3D8 scFv) and its associated in vivo therapeutic antitumor effects. As it does not require an adjuvant to elicit a CTL response, a 3D8 scFv-based vaccine strategy would be worthy of further investigation for use as a cancer immunotherapeutic.
Supplementary Material
Acknowledgments
We thank Dr. K.L. Rock for kindly providing the DC2.4 and MO5 cells and Dr. C.V. Harding for the CD8OVA1.3 clone.
This work was supported by the Science Research Center Program of Korea Science and Engineering Foundation (Grant 2011-0030829) and the Post-BioGreen 21 Program (Grant PJ008146) of the Rural Development Administration of Korea.
The online version of this article contains supplemental material.
- CPP
- cell-penetrating peptide
- DC
- dendritic cell
- ER
- endoplasmic reticulum
- Hsp
- heat shock protein
- HSPG
- heparan sulfate proteoglycan
- PARP
- poly(ADP-ribose) polymerase
- scFv
- single-chain V region fragment.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Kasturi S. P., Pulendran B. 2008. Cross-presentation: avoiding trafficking chaos? Nat. Immunol. 9: 461–463 [DOI] [PubMed] [Google Scholar]
- 2.Kurts C., Robinson B. W., Knolle P. A. 2010. Cross-priming in health and disease. Nat. Rev. Immunol. 10: 403–414 [DOI] [PubMed] [Google Scholar]
- 3.Amigorena S., Savina A. 2010. Intracellular mechanisms of antigen cross presentation in dendritic cells. Curr. Opin. Immunol. 22: 109–117 [DOI] [PubMed] [Google Scholar]
- 4.Binder R. J., Han D. K., Srivastava P. K. 2000. CD91: a receptor for heat shock protein gp96. Nat. Immunol. 1: 151–155 [DOI] [PubMed] [Google Scholar]
- 5.Becker T., Hartl F. U., Wieland F. 2002. CD40, an extracellular receptor for binding and uptake of Hsp70-peptide complexes. J. Cell Biol. 158: 1277–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vabulas R. M., Ahmad-Nejad P., Ghose S., Kirschning C. J., Issels R. D., Wagner H. 2002. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J. Biol. Chem. 277: 15107–15112 [DOI] [PubMed] [Google Scholar]
- 7.Delneste Y., Magistrelli G., Gauchat J., Haeuw J., Aubry J., Nakamura K., Kawakami-Honda N., Goetsch L., Sawamura T., Bonnefoy J., Jeannin P. 2002. Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity 17: 353–362 [DOI] [PubMed] [Google Scholar]
- 8.Berwin B., Hart J. P., Rice S., Gass C., Pizzo S. V., Post S. R., Nicchitta C. V. 2003. Scavenger receptor-A mediates gp96/GRP94 and calreticulin internalization by antigen-presenting cells. EMBO J. 22: 6127–6136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurotaki T., Tamura Y., Ueda G., Oura J., Kutomi G., Hirohashi Y., Sahara H., Torigoe T., Hiratsuka H., Sunakawa H., et al. 2007. Efficient cross-presentation by heat shock protein 90-peptide complex-loaded dendritic cells via an endosomal pathway. J. Immunol. 179: 1803–1813 [DOI] [PubMed] [Google Scholar]
- 10.Jockheck-Clark A. R., Bowers E. V., Totonchy M. B., Neubauer J., Pizzo S. V., Nicchitta C. V. 2010. Re-examination of CD91 function in GRP94 (glycoprotein 96) surface binding, uptake, and peptide cross-presentation. J. Immunol. 185: 6819–6830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pouniotis D. S., Apostolopoulos V., Pietersz G. A. 2006. Penetratin tandemly linked to a CTL peptide induces anti-tumour T-cell responses via a cross-presentation pathway. Immunology 117: 329–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim Y. R., Kim J. S., Lee S. H., Lee W. R., Sohn J. N., Chung Y. C., Shim H. K., Lee S. C., Kwon M. H., Kim Y. S. 2006. Heavy and light chain variable single domains of an anti-DNA binding antibody hydrolyze both double- and single-stranded DNAs without sequence specificity. J. Biol. Chem. 281: 15287–15295 [DOI] [PubMed] [Google Scholar]
- 13.Jang J. Y., Jeong J. G., Jun H. R., Lee S. C., Kim J. S., Kim Y. S., Kwon M. H. 2009. A nucleic acid-hydrolyzing antibody penetrates into cells via caveolae-mediated endocytosis, localizes in the cytosol and exhibits cytotoxicity. Cell. Mol. Life Sci. 66: 1985–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee E. J., Jang E. J., Lee E., Yu J., Chung H. Y., Jang Y. J. 2007. Cell-penetrating autoantibody induces caspase-mediated apoptosis through catalytic hydrolysis of DNA. Bioorg. Med. Chem. 15: 2016–2023 [DOI] [PubMed] [Google Scholar]
- 15.Yanase K., Smith R. M., Puccetti A., Jarett L., Madaio M. P. 1997. Receptor-mediated cellular entry of nuclear localizing anti-DNA antibodies via myosin 1. J. Clin. Invest. 100: 25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zack D. J., Stempniak M., Wong A. L., Taylor C., Weisbart R. H. 1996. Mechanisms of cellular penetration and nuclear localization of an anti-double strand DNA autoantibody. J. Immunol. 157: 2082–2088 [PubMed] [Google Scholar]
- 17.Shen Z., Reznikoff G., Dranoff G., Rock K. L. 1997. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J. Immunol. 158: 2723–2730 [PubMed] [Google Scholar]
- 18.Falo L. D., Jr., Kovacsovics-Bankowski M., Thompson K., Rock K. L. 1995. Targeting antigen into the phagocytic pathway in vivo induces protective tumour immunity. Nat. Med. 1: 649–653 [DOI] [PubMed] [Google Scholar]
- 19.Harding C. V., Song R. 1994. Phagocytic processing of exogenous particulate antigens by macrophages for presentation by class I MHC molecules. J. Immunol. 153: 4925–4933 [PubMed] [Google Scholar]
- 20.Park K. J., Lee S. H., Kim T. I., Lee H. W., Lee C. H., Kim E. H., Jang J. Y., Choi K. S., Kwon M. H., Kim Y. S. 2007. A human scFv antibody against TRAIL receptor 2 induces autophagic cell death in both TRAIL-sensitive and TRAIL-resistant cancer cells. Cancer Res. 67: 7327–7334 [DOI] [PubMed] [Google Scholar]
- 21.Schröter C. J., Braun M., Englert J., Beck H., Schmid H., Kalbacher H. 1999. A rapid method to separate endosomes from lysosomal contents using differential centrifugation and hypotonic lysis of lysosomes. J. Immunol. Methods 227: 161–168 [DOI] [PubMed] [Google Scholar]
- 22.Xie Y. C., Hwang C., Overwijk W., Zeng Z., Eng M. H., Mulé J. J., Imperiale M. J., Restifo N. P., Sanda M. G. 1999. Induction of tumor antigen-specific immunity in vivo by a novel vaccinia vector encoding safety-modified simian virus 40 T antigen. J. Natl. Cancer Inst. 91: 169–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Craiu A., Akopian T., Goldberg A., Rock K. L. 1997. Two distinct proteolytic processes in the generation of a major histocompatibility complex class I-presented peptide. Proc. Natl. Acad. Sci. USA 94: 10850–10855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burgdorf S., Schölz C., Kautz A., Tampé R., Kurts C. 2008. Spatial and mechanistic separation of cross-presentation and endogenous antigen presentation. Nat. Immunol. 9: 558–566 [DOI] [PubMed] [Google Scholar]
- 25.Rock K. L., Gramm C., Rothstein L., Clark K., Stein R., Dick L., Hwang D., Goldberg A. L. 1994. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell 78: 761–771 [DOI] [PubMed] [Google Scholar]
- 26.Burgdorf S., Kurts C. 2008. Endocytosis mechanisms and the cell biology of antigen presentation. Curr. Opin. Immunol. 20: 89–95 [DOI] [PubMed] [Google Scholar]
- 27.Lu J., Higashimoto Y., Appella E., Celis E. 2004. Multiepitope Trojan antigen peptide vaccines for the induction of antitumor CTL and Th immune responses. J. Immunol. 172: 4575–4582 [DOI] [PubMed] [Google Scholar]
- 28.Brooks N. A., Pouniotis D. S., Sheng K. C., Apostolopoulos V., Pietersz G. A. 2010. A membrane penetrating multiple antigen peptide (MAP) incorporating ovalbumin CD8 epitope induces potent immune responses in mice. Biochim. Biophys. Acta 1798: 2286–2295 [DOI] [PubMed] [Google Scholar]
- 29.Tacken P. J., Joosten B., Reddy A., Wu D., Eek A., Laverman P., Kretz-Rommel A., Adema G. J., Torensma R., Figdor C. G. 2008. No advantage of cell-penetrating peptides over receptor-specific antibodies in targeting antigen to human dendritic cells for cross-presentation. J. Immunol. 180: 7687–7696 [DOI] [PubMed] [Google Scholar]
- 30.Castellino F., Boucher P. E., Eichelberg K., Mayhew M., Rothman J. E., Houghton A. N., Germain R. N. 2000. Receptor-mediated uptake of antigen/heat shock protein complexes results in major histocompatibility complex class I antigen presentation via two distinct processing pathways. J. Exp. Med. 191: 1957–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bozzacco L., Trumpfheller C., Siegal F. P., Mehandru S., Markowitz M., Carrington M., Nussenzweig M. C., Piperno A. G., Steinman R. M. 2007. DEC-205 receptor on dendritic cells mediates presentation of HIV gag protein to CD8+ T cells in a spectrum of human MHC I haplotypes. Proc. Natl. Acad. Sci. USA 104: 1289–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonifaz L. C., Bonnyay D. P., Charalambous A., Darguste D. I., Fujii S., Soares H., Brimnes M. K., Moltedo B., Moran T. M., Steinman R. M. 2004. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J. Exp. Med. 199: 815–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weisbart R. H., Stempniak M., Harris S., Zack D. J., Ferreri K. 1998. An autoantibody is modified for use as a delivery system to target the cell nucleus: therapeutic implications. J. Autoimmun. 11: 539–546 [DOI] [PubMed] [Google Scholar]
- 34.Burgdorf S., Kautz A., Böhnert V., Knolle P. A., Kurts C. 2007. Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science 316: 612–616 [DOI] [PubMed] [Google Scholar]
- 35.Torigoe T., Tamura Y., Sato N. 2009. Heat shock proteins and immunity: application of hyperthermia for immunomodulation. Int. J. Hyperthermia 25: 610–616 [DOI] [PubMed] [Google Scholar]
- 36.Morris M. C., Deshayes S., Heitz F., Divita G. 2008. Cell-penetrating peptides: from molecular mechanisms to therapeutics. Biol. Cell 100: 201–217 [DOI] [PubMed] [Google Scholar]
- 37.Léonetti M., Gadzinski A., Moine G. 2010. Cell surface heparan sulfate proteoglycans influence MHC class II-restricted antigen presentation. J. Immunol. 185: 3847–3856 [DOI] [PubMed] [Google Scholar]
- 38.Dehio C., Freissler E., Lanz C., Gómez-Duarte O. G., David G., Meyer T. F. 1998. Ligation of cell surface heparan sulfate proteoglycans by antibody-coated beads stimulates phagocytic uptake into epithelial cells: a model for cellular invasion by Neisseria gonorrhoeae. Exp. Cell Res. 242: 528–539 [DOI] [PubMed] [Google Scholar]
- 39.Takemoto S., Nishikawa M., Otsuki T., Yamaoka A., Maeda K., Ota A., Takakura Y. 2009. Enhanced generation of cytotoxic T lymphocytes by increased cytosolic delivery of MHC class I epitope fused to mouse heat shock protein 70 via polyhistidine conjugation. J. Control. Release 135: 11–18 [DOI] [PubMed] [Google Scholar]
- 40.Dakappagari N. K., Sundaram R., Rawale S., Liner A., Galloway D. R., Kaumaya P. T. 2005. Intracellular delivery of a novel multiepitope peptide vaccine by an amphipathic peptide carrier enhances cytotoxic T-cell responses in HLA-A*201 mice. J. Pept. Res. 65: 189–199 [DOI] [PubMed] [Google Scholar]
- 41.Bowers E. V., Horvath J. J., Bond J. E., Cianciolo G. J., Pizzo S. V. 2009. Antigen delivery by alpha(2)-macroglobulin enhances the cytotoxic T lymphocyte response. J. Leukoc. Biol. 86: 1259–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hemmi H., Akira S. 2005. TLR signalling and the function of dendritic cells. Chem. Immunol. Allergy 86: 120–135 [DOI] [PubMed] [Google Scholar]
- 43.Ke Y., Li Y., Kapp J. A. 1995. Ovalbumin injected with complete Freund’s adjuvant stimulates cytolytic responses. Eur. J. Immunol. 25: 549–553 [DOI] [PubMed] [Google Scholar]
- 44.Mitsui H., Inozume T., Kitamura R., Shibagaki N., Shimada S. 2006. Polyarginine-mediated protein delivery to dendritic cells presents antigen more efficiently onto MHC class I and class II and elicits superior antitumor immunity. J. Invest. Dermatol. 126: 1804–1812 [DOI] [PubMed] [Google Scholar]
- 45.Moroi Y., Mayhew M., Trcka J., Hoe M. H., Takechi Y., Hartl F. U., Rothman J. E., Houghton A. N. 2000. Induction of cellular immunity by immunization with novel hybrid peptides complexed to heat shock protein 70. Proc. Natl. Acad. Sci. USA 97: 3485–3490 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.