Abstract
T cell Ig and mucin domain 3 (Tim3) is an inhibitory molecule involved in immune tolerance, autoimmune responses, and antiviral immune evasion. However, we recently demonstrated that Tim3 and Galectin-9 (Gal9) interaction induces a program of macrophage activation that results in killing of Mycobacterium tuberculosis in the mouse model of infection. In this study, we sought to determine whether the Tim3–Gal9 pathway plays a similar role in human pulmonary TB. We identified that pulmonary TB patients have reduced expression of Tim3 on CD14+ monocytes in vivo. By blocking Tim3 and Gal9 interaction in vitro, we show that these molecules contribute to the control of intracellular bacterial replication in human macrophages. The antimicrobial effect was partially dependent on the production of IL-1β. Our results establish that Tim3–Gal9 interaction activates human M. tuberculosis –infected macrophages and leads to the control of bacterial growth through the production of the proinflammatory cytokine IL-1β. Data presented in this study suggest that one of the potential pathways activated by Tim3/Gal9 is the secretion of IL-1β, which plays a crucial role in antimicrobial immunity by modulating innate inflammatory networks.
Introduction
Mycobacterium tuberculosis is one of the most successful human pathogens around the world. Resolution of mycobacterial infection depends on the interaction between the bacteria and the host’s innate and adaptive immune systems. Consequently, T cells and macrophages are the crucial components of this protective response to mycobacterial infection (1). Macrophages play a critical role in the pathogenesis of tuberculosis (TB) by providing an intracellular niche for M. tuberculosis and, when appropriately activated, by killing the bacteria directly.
T cell Ig and mucin domain 3 (Tim3) is a negative regulatory molecule that is important for T cell tolerance and has a crucial role in autoimmunity and T cell exhaustion during chronic viral infection (2–6). Tim3 is expressed on T cells, monocytes, macrophages, and dendritic cells (DC) (7–10). Galectin-9 (Gal9) and phosphatidylserine are the two known ligands for Tim3 (4, 11). Macrophages and DC recognize use Tim3 as a receptor for phosphatidylserine,to recognize and engulf apoptotic cells, which can promote Ag cross-presentation and T cell activation (11). Gal9 interaction with the oligosaccharide chains on the Tim3–IgV domain expressed by activated Th1 cells inhibits T cell expansion by transducing an inhibitory signal that leads to apoptosis, T cell exhaustion, or immune tolerance (2–4, 7, 12–14).
In addition to its well-described role in regulating T cells, we have previously shown that Tim3–Gal9 interaction activates M. tuberculosis–infected macrophages, resulting in IL-1β secretion and pathogen clearance (15). Our data suggested that the Tim3–Gal9 pathway acts as a bidirectional pathway, suggesting that it may have evolved to limit tissue inflammation caused by activated T cells while at the same time can stimulate innate immunity to inhibit growth of intracellular pathogens such as M. tuberculosis (15).
In this study, we explored the question of whether the Tim3–Gal9 interaction activates antimicrobial pathways in human cells from patients with pulmonary TB (PTB). Toward this, we characterized the expression profile of Tim3 and Gal9 on human PBMC from patients with PTB infection and elucidated whether Tim3 and Gal9 participate in controlling intracellular bacterial replication in human macrophages. We report in this study that PTB patients have a lower frequency of CD14+Tim3+ monocytes in peripheral blood compared with healthy donors (HD) and that M. tuberculosis infection downregulates cell-surface expression of Tim3 and Gal9 in human macrophages. We further demonstrate that IL-1β is involved in the antimicrobial pathways activated by Tim3 and Gal9 in human macrophages. Blocking Tim3–Gal9 pathway with inhibitory Abs limits the secretion of IL-1β. Finally, we consider whether M. tuberculosis manipulates the Tim3–Gal9 pathway to evade host defenses. Our data provide evidence that Tim3/Gal9 interact to promote microbial immunity in people with PTB.
Materials and Methods
Study population
PTB patients (n = 20) from the National Institute of Respiratory Diseases (INER) of Mexico City, Mexico, were studied. Written informed consent was obtained from all subjects included, and the institutional ethics committee at INER approved this study. All included patients had a negative HIV screening test. PTB diagnosis was based on clinical history, physical examination, chest x-rays, and positive Ziehl–Neelsen test in sputum. In all cases, the diagnosis was confirmed by M. tuberculosis growth in sputum culture. All patients were classified as having TB class 3 category I disease, according to the American Thoracic Society (16). All participating PTB patients had received <1 wk of the directly observed treatment strategy. HD (n = 20) were also recruited at the INER. HD had no clinical or radiographic evidence of respiratory diseases and no known concurrent or past contact with PTB patients. Ten HD were tuberculin skin test (TST) negative (<5 mm), and eight HD had TST in duration of >10 mm. TST-positive and TST-negative HD are presented as a unique group. Differences identified in our study between HD and PTB patients were independent of the TST status of the HD. All participants had received bacillus Calmette–Guérin (BCG) vaccination at birth. Written informed consent was obtained from all participants.
Cells
PBMCs were isolated from 16 ml venous blood in BD vacutainer CPT tubes (BD Biosciences). After centrifugation, plasma was recovered and frozen for future cytokine analysis. Cells were collected and counted to determine their viability by the trypan blue dye exclusion method.
Enrichment of monocytes
Monocytes were enriched by using positive selection via magnetic microbeads coated with Abs to CD14 (Miltenyi Biotec). Monocytes were analyzed by flow cytometry to determine the purity of the cells. The purified cells were routinely 90–95% of the intended cell type.
Enrichment of T cells
T cells were enriched by using negative selection via magnetic microbeads coated with Abs to CD14, CD15, CD16, CD19, CD34, CD36, CD56, CD123, and CD235a (glycophorin A) (Miltenyi Biotec). T cells were analyzed by flow cytometry to determine the purity of the cells. The purified cells were routinely 93–97% of the intended cell type.
Cell differentiation
CD14+-enriched monocytes were plated at 1 × 105 cells/well in 96-well plates (Costar, Ontario, Canada) RPMI 1640 medium (Life Technologies BRL) supplemented with l-glutamine (2 mM; Sigma-Aldrich), streptomycin, penicillin, and 10% human serum. After a 7-d incubation, viable cells were considered monocyte-derived macrophages (MDMs) based on their expression profile of CD14, CD68, and mannose receptor (data not shown). MDM were used in the different experiments.
Multiparametric flow cytometric analysis
To measure Tim3 and Gal9 expression by different cell subsets, 106 PBMC were stained and analyzed by flow cytometry. Briefly, cells were stained for 20 min at 4°C with fluorochrome-conjugated mAb to CD3, CD4, CD8, CD14, CD16, CD33, CD56, CD68, Tim3 (BioLegend), and Gal9 (MBL International). Blocking anti-human Tim3 Abs (clone 1G5 and 4A4) were kindly provided by Dr. Vijay Kuchroo (Brigham and Women’s Hospital, Boston, MA). For intracellular Gal9 staining, cells were incubated with 250 μl Cytofix/Cytoperm (BD Pharmingen) solution for 20 min at 4°C. After permeabilization, cells were washed with Perm/Wash buffer (BD Pharmingen), supernatant was carefully removed, and 50 μl fluorescein anti-human Gal9 mAb was added and incubated at 4°C for 30 min in the dark. After incubation, the cells were washed with Perm/Wash buffer and resuspended in staining buffer prior to flow cytometry. Data were collected using an FACSAria flow cytometer (BD Biosciences, San Jose, CA) using FACS Diva software (V.6.1) and analyzed with FlowJo (Tree Star). Typically, 100,000 events were acquired.
Bacteria
M. tuberculosis-H37Ra and M. tuberculosis-H37Rv were grown to log phase in Middlebrook 7H9 broth (Difco, Detroit, MI) supplemented with 1% glycerol and 10% Middlebrook albumin dextrose catalase enrichment (Difco). Bacteria were harvested and frozen at −80°C in RPMI 1640, 10% FBS, and 6% glycerol. Bacterial CFU were determined by plating serial 10-fold dilutions on 7H10 agar and counting colonies after incubation for 3 wk.
Fluorescein labeling of M. tuberculosis
To label M. tuberculosis with fluorescein, 109 bacteria were pelleted, resuspended in 1 ml PBS (pH 9.1), and mixed with 7.5 μl 20 mg/ml FLUOS (Boehringer, Mannheim, Germany) in DMSO for 10 min at room temperature. Labeled M. tuberculosis bacteria were washed twice and declumped prior to use.
In vitro infections and cocultures
MDM were infected with M. tuberculosis-H37Ra or M. tuberculosis-H37Rv at a multiplicity of infection (MOI) of 10 as described previously (17). In brief, M. tuberculosis were opsonized for 5 min using RPMI 1640 medium supplemented with 2% human serum, 10% FBS, and 0.05% Tween 80 and washed twice with complete medium without antibiotics. Bacteria were passed through a 5-μm syringe filter (Millipore), counted in a Petroff-Hausser chamber, and added to MDM at the indicated MOI. The length of infection was 2 h for all experiments. At days 1 and 4 postinfection, cells were lysed with 0.1% solution of saponin for 5 min and bacteria enumerated by plating serial dilutions of cell lysates on Middlebrook 7H10 agar plates (Difco). Colonies were counted after 21 d. Autologous T cells (105/well) were enriched by using positive selection via magnetic microbeads coated with Abs to CD3 (Miltenyi Biotec) and added to infected MDM after 2 h of infection. The reduction of CFU was calculated as follows: percent reduction = 100 × ([CFU recovered from MDM alone on day 4] − [CFU recovered from MDM cocultured with T cells])/([CFU recovered from MDM alone on day 4] − [CFU recovered from MDM alone on day 1]).
NO production
Supernatants were harvested 24 h postinfection of MDM, and NO production was measured using the Griess reaction. Briefly, 100-μl culture supernatant was incubated with an equal volume of commercial Griess reagent (Sigma-Aldrich) for 5 min at room temperature, and the absorbance was measured at 490 nm. Serially diluted NaNO2 of known concentration was used as a standard to calculate the amount of NO2 measured in the supernatant (18)
Blocking studies
Tim3–Gal9 pathway was blocked by addition of anti-human Tim3 or anti-human Gal9 mAbs. Autologous T cells were preincubated with anti-human Tim3 mAb (clone 4A4; 10 μg/ml; Dr. Vijay Kuchroo [Brigham and Women’s Hospital, Boston, MA]) before addition to M. tuberculosis–infected MDM, or M. tuberculosis–infected MDM were preincubated with anti-human Gal9 mAb (clone 9M1-3; 10 μg/ml; BioLegend) or anti-human Tim3 mAb before addition of T cells. Isotype-matched control mAbs were used. Tim3–Gal9 interaction was also blocked by using increasing concentrations of lactose (25, 50, and 100 mM) that was added to the M. tuberculosis–infected MDM 1 h before addition of the T cells.
Western blot to detect inducible NO synthase and IL-1β
A total of 106 MDM was infected with M. tuberculosis-H37Rv (MOI 10:1; triplicate wells) for 24 h at 37°C in a humidified atmosphere containing 5% CO2. Postinfection, cells were washed with PBS and lysed in Laemmli buffer. Equal amounts of protein were subjected to SDS-PAGE and transferred to a 0.2-μm pore size Immun-Blot polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA). Western blot was performed using an IL-1β (clone 2805; dilution used 1:1000; R&D Systems, Minneapolis, MN) and inducible NO synthase (iNOS; dilution used 1:200; Santa Cruz Biotechnology, Santa Cruz, CA) Abs. The protein band intensities were analyzed using the online ImageJ 1.39c software provided by the National Institutes of Health (http://rsb.info.nih.gov/ij/index.html) as described by Luke Miller (http://www.lukemiller.org/journal/2007/08/quantifying-western-blots-without.html). Background intensity was subtracted from each sample and normalized to GAPDH. Fold change was determined as follows: (treated sample band intensity)/(untreated sample band intensity).
Cytokine analysis
Culture supernatants from M. tuberculosis–infected MDM were filtered through a 0.2-μM filter to remove any bacteria. Supernatants or plasma samples were assayed for cytokines by a sandwich ELISA, which was conducted in accordance with the manufacturer’s instructions, with absorbance being recorded at 405 nm on SoftMax Pro ELISA analysis software (Molecular Devices). IFN-γ and TNF-α were quantified by comparison with the appropriate recombinant standard (purchased from R&D Systems).
Statistical analysis
Data are shown as mean ± SD or median (interquartile range [IQR]). Unpaired Student test or Mann–Whitney U test were used to compare two groups and Kruskal-Wallis test with Dunnett posttest used when more than two groups were compared. The p values < 0.05 were considered to be statistically significant (GraphPad Software, San Diego, CA).
Results
Patients and HD
After approval by the ethics committee and appropriate informed consent, 20 PTB patients (11 men, 9 women) and 20 HD (10 men, 10 women) were recruited. The clinical and demographic characteristics of the groups are described in Table I.
Table I. Baseline characteristics of the study populations.
| PTB Patients (n = 20) | HD (n = 20) | p Value | |
|---|---|---|---|
| Age (y)a | 48.1 ± 17.7 | 40.7 ± 12.4 | 0.16 |
| Male [n (%)] | 11 (55) | 10 (50) | 0.75 |
| Current smokers [n (%)] | 12 (60) | 8 (40) | 0.20 |
| Total leukocytes (× 103/mm3) | 7.5 ± 1.2 | 8.8 ± 1.2 | 0.08 |
| Hemoglobin (g/dl)a | 12.4 ± 2.1 | 13.9 ± 1.3 | 0.13 |
| LDH (mU/ml)a | 388 ± 200 | 303 ± 87.8 | 0.47 |
| Albumin (g/dl)a | 3.1 ± 0.67 | 3.08 ± 0.4 | 1.0 |
Mean ± SD.
LDH, Lactate dehydrogenase.
Tim3 and Gal9 expression on PBMC
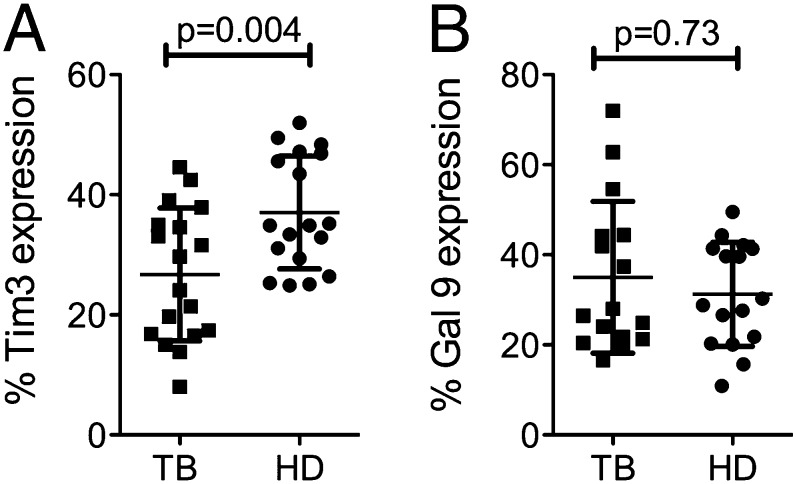
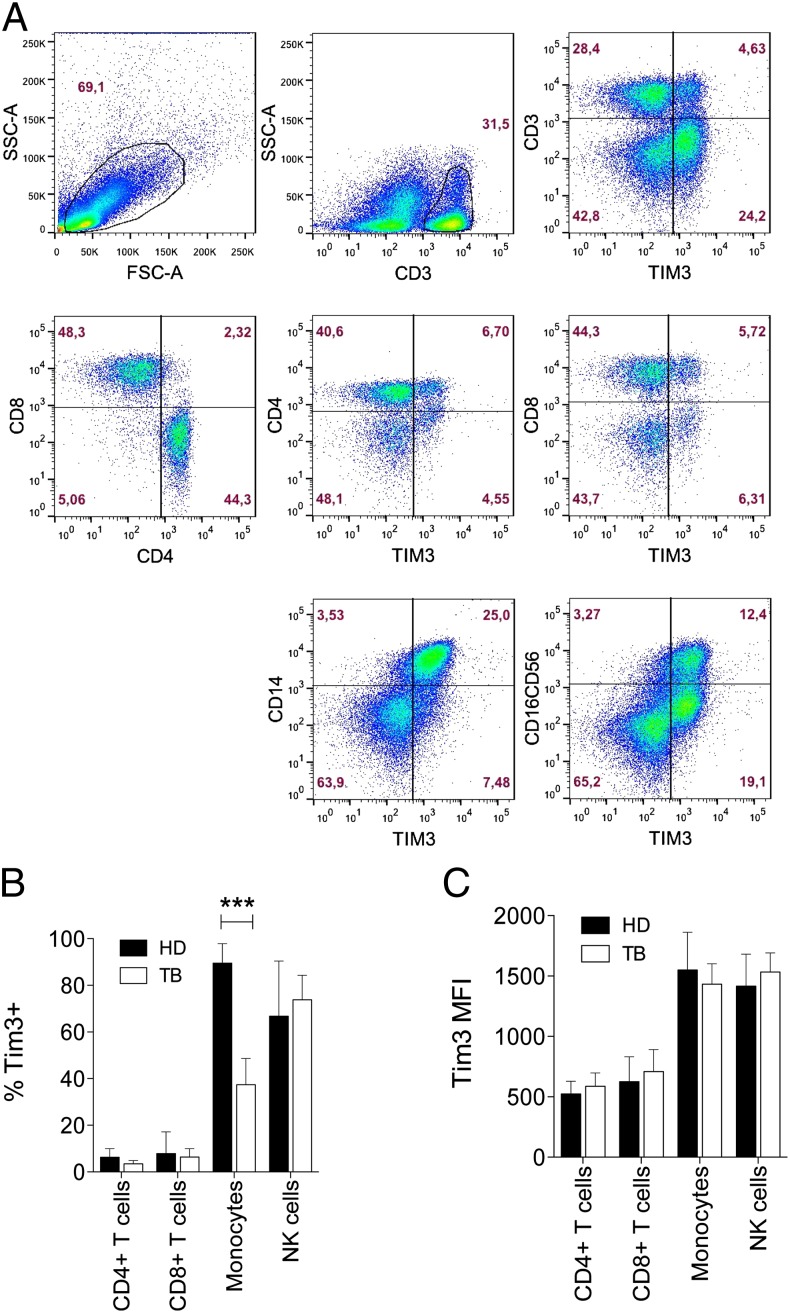
We measured Tim3 and Gal9 expression on PBMC from PTB patients and HD by multiparametric flow cytometry. A lower frequency of Tim3+ cells was detected from PTB patients compared with HD (mean 26.7 ± SD 11 versus 36.6 ± 9.2%, respectively) (p = 0.004) (Fig. 1A). Differences in Gal9 expression on PBMC between PTB and HD were not observed (p = 0.73) (Fig. 1B). To determine which cell types in the peripheral blood of PTB patients expressed lower levels of Tim3, we measured Tim3 expression on CD4+ T cells (CD3+CD4+), CD8+ T cells (CD3+CD8+), monocytes (CD14+), and NK cells (CD16+/CD56+) (Fig. 2A). A lower frequency of CD14+ monocytes expressing Tim3 was detected in PTB patients compared with HD (p < 0.001) (Fig. 2B, 2C). However, Tim3 expression on a per-cell basis (Tim3 mean fluorescence intensity [MFI]) was comparable between PTB and HD.
FIGURE 1.
Tim3 expression is downregulated on total PBMC during TB infection. The percentages of total Tim3+ (A) and Gal9+ (B) cells are indicated for PTB patients (TB) and HD. Horizontal bars represent mean values ± SD. Statistical analyses was performed using the Mann–Whitney U test (n = 20).
FIGURE 2.
Identification of PBMC expressing Tim3. Total mononuclear cells from TB patients and HD were analyzed. The pseudo–color density plots show the gates used to evaluate the expression of Tim3 on CD3+, CD4+, CD8+, CD14+, and CD16/CD56+ cells by flow cytometry. (A) The pseudo–color plots show the strategy used to identify the Tim3 expression on gated live cells. Representative plots are shown from one HD. Numbers indicate the percentage of positive cells in each gate. (B and C) The frequency and MFI of Tim3+ cells in PTB patients and HD were analyzed as indicated. Bars show median values and IQR. ***p < 0.001; statistical analyses were performed using the Mann–Whitney U test (n = 20).
Plasma concentration of proinflammatory cytokines
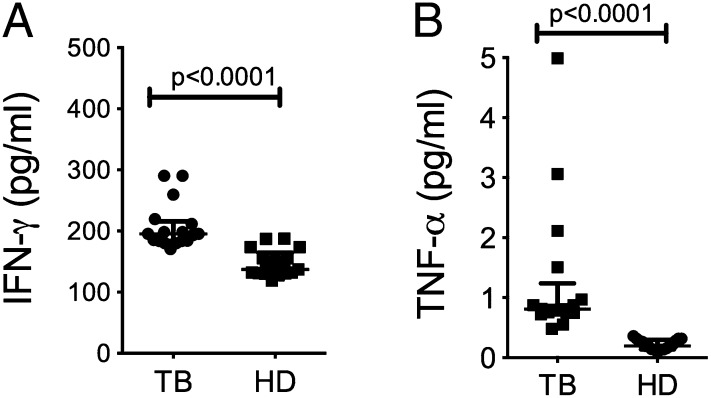
IFN-γ and TNF-α are cytokines produced by Th1 cells that activate macrophages to control intracellular bacterial replication and may regulate Tim3 and Gal9 expression (9, 19–21). To determine if there is an association between levels of Tim3 expression and the plasma concentration of proinflammatory cytokines, we measured IFN-γ and TNF-α by ELISA. PTB patients had higher plasma concentrations of IFN-γ and TNF-α (p < 0.001 for both molecules) (Fig. 3A, 3B). Although PTB patients had higher plasma IFN-γ and TNF-α levels, they had lower frequencies of CD14+Tim3+ monocytes compared with HD (Fig. 1A). These findings suggest that during M. tuberculosis infection, Tim3 and Gal9 expression are regulated by signals other than IFN-γ and TNF-α.
FIGURE 3.
Plasma concentrations of proinflammatory cytokines. Concentrations of IFN-γ (A) and TNF-α (B) were measured in plasma samples from PTB patients and HD by ELISA. Horizontal bars represent median values and IQR. Statistical analyses were performed using the Mann–Whitney U test. (n = 20).
M. tuberculosis downregulates Tim3 expression and limits macrophage activation
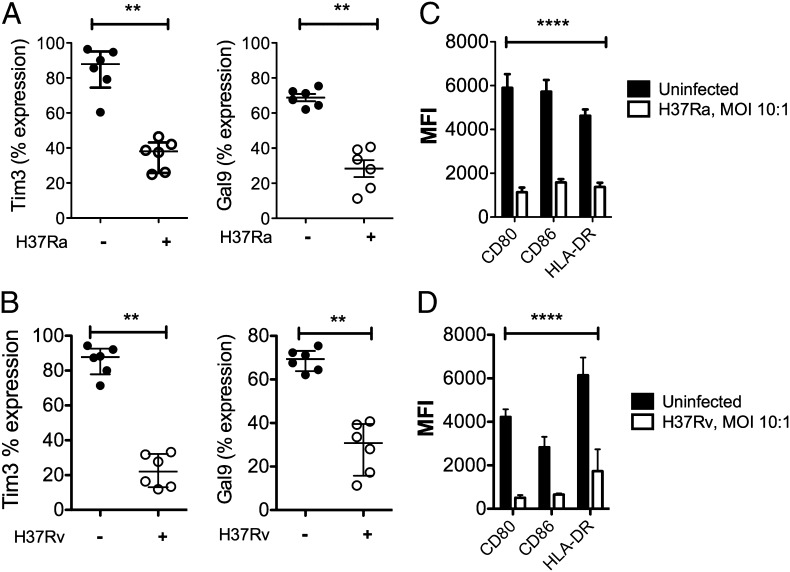
To address whether M. tuberculosis modulates expression of Tim3 and Gal9, we established an in vitro infection model. Peripheral monocytes from HD were differentiated into macrophages (MDM). The surface expression of Tim3 and Gal9 was evaluated before and after in vitro infection with an avirulent or virulent M. tuberculosis strain (H37Ra and H37Rv, respectively). We first measured the phagocytic capacity of MDM by infecting them with fluos-M. tuberculosis-H37Ra and fluos-M. tuberculosis-H37Rv (MOI 10:1) for 2 h. Nearly 59.7 ± 3.2 and 67.8 ± 4.1% of the MDM phagocytosed H37Ra and H37Rv, respectively (n = 5; data not shown). Both bacterial strains led to downregulation of cell-surface Tim3 expression on M. tuberculosis–infected MDM compared with uninfected macrophages (M. tuberculosis-H37Rv–infected MDM, 74.9%; M. tuberculosis-H37Ra–infected MDM, 56.7%) (p < 0.001) (Fig. 4A, 4B). Gal9 expression was also downregulated by M. tuberculosis (p < 0.001). These data parallel our observation that Tim3 expression is downregulated on CD14+ monocytes from PTB patients.
FIGURE 4.
Infection with M. tuberculosis decreases Tim3, Gal9, and costimulatory molecule expression. MDM (106 cells) were infected with M. tuberculosis-H37Ra (A) or M. tuberculosis-H37Rv (B) for 2 h; MOI 10:1. Twenty-four hours later, MDM were washed, removed from plates, and stained with Abs for flow cytometry. The frequency of Tim3+ and Gal9+ MDM between the uninfected and infected MDM was analyzed. **p < 0.001, Mann–Whitney U test. (C and D) MFI of CD80, CD86, and HLA-DR from uninfected and M. tuberculosis–infected MDM was analyzed. Horizontal bars represent median values and IQR. ****p < 0.0001; statistical analyses were performed using the Wilcoxon matched-pairs signed-rank test (n = 6).
Macrophage activation is required to limit intracellular bacterial growth, and, in the absence of activation, the immune response against the bacilli is ineffective. Therefore, we investigated whether expression of other costimulatory molecules was modulated following M. tuberculosis infection. Infection with H37Ra and H37Rv inhibited the expression of CD80, CD86, and HLA-DR (Fig. 4C, 4D). These results were consistent with previous reports showing that M. tuberculosis downregulates the synthesis of MHC class II molecules by murine macrophages as well as by MDM from HD (22–24). All molecules including Tim3 and Gal9 were downregulated to comparable levels.
Impaired secretion of IL-1β and NO after M. tuberculosis infection
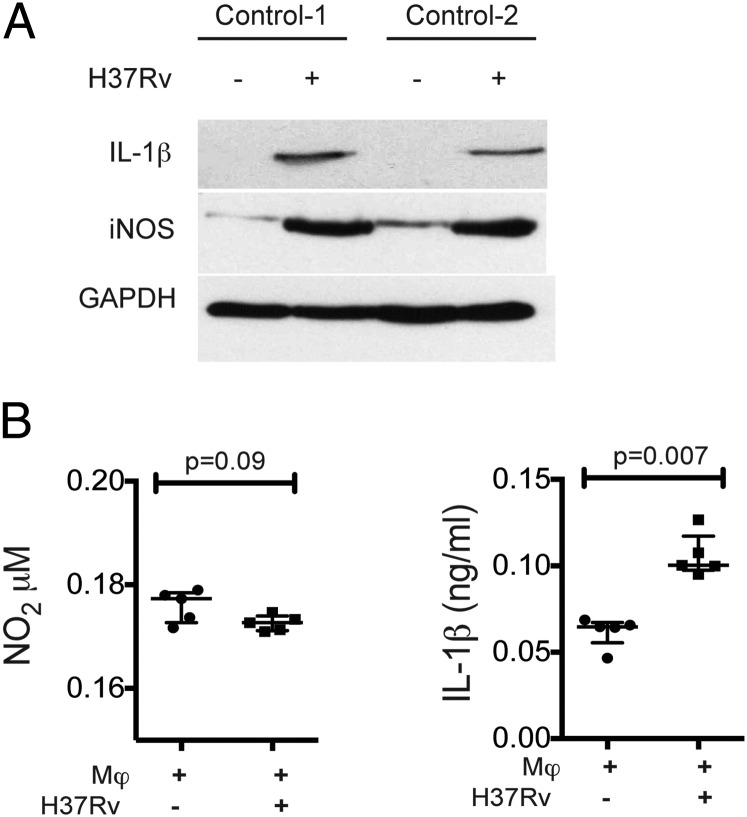
To identify if M. tuberculosis infection alters macrophage activation and secretion of IL-1β and NO, two molecules associated with control of intracellular bacterial growth in murine macrophages, Western blots of the uninfected and M. tuberculosis-H37Rv–infected MDM were performed. Two hours after M. tuberculosis infection, the mature form of IL-1β was detected intracellularly (Fig. 5A), and there was an increase in IL-1β secretion by M. tuberculosis–infected MDM (p = 0.007) (Fig. 5B). In contrast, although iNOS was detected (Fig. 5A), NO was not detected in culture supernatants.
FIGURE 5.
IL-1β and NO2 levels in M. tuberculosis–infected MDM. (A) Detection of mature IL-1β and iNOS in cell lysates from M. tuberculosis–infected MDM by Western blot. Data in (A) are from two independent HD. (B) IL-1β measured by ELISA and NO2 by the Griess reaction in supernatant from uninfected and M. tuberculosis–infected MDM. Horizontal bars represent median values and IQR. Statistical analyses were performed using the Mann–Whitney U test (n = 5).
Tim3–Gal9 interaction suppresses mycobacterial replication
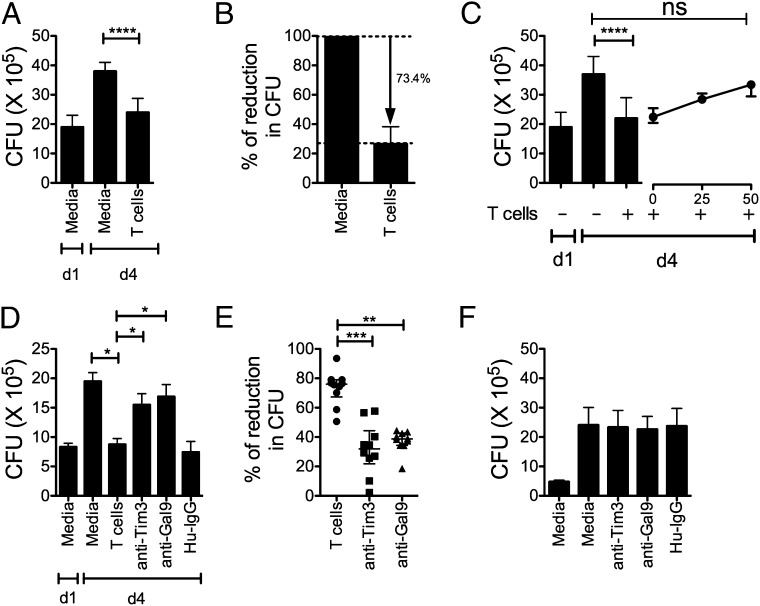
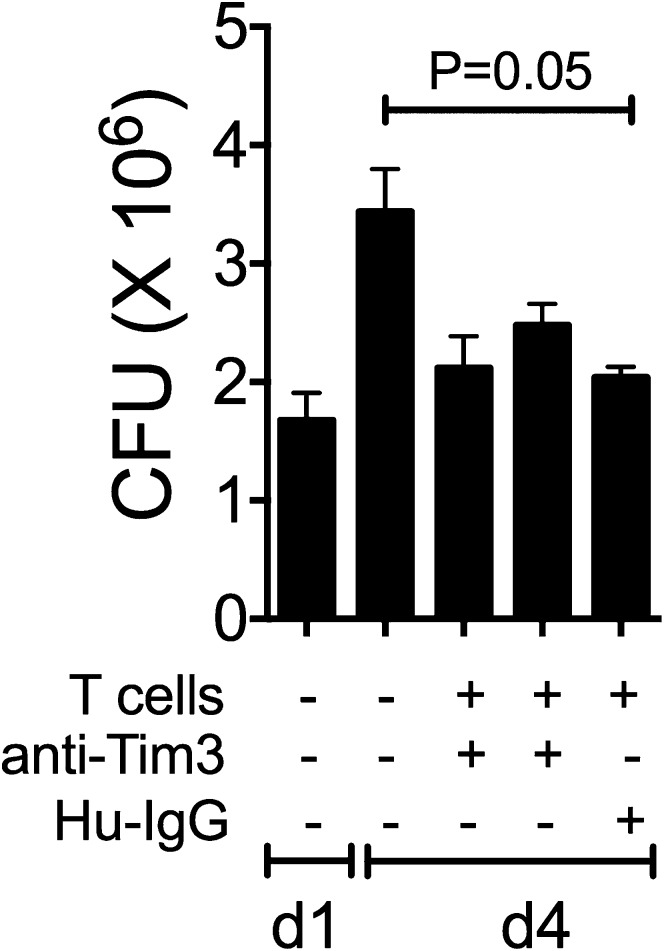
We previously demonstrated that macrophage treatment with the Tim3 fusion protein promotes antimycobacterial activity (15). To delineate how the Tim3–Gal9 pathway participates in antimicrobial immunity in people, we adapted our in vitro infection model for use with human cells. Although a minor proportion of T cells (7.2 of CD4+ and 12.4% of CD8+ T cells) from HD express Tim3, we found that total T cells were able to restrict bacterial replication within 4 d postinfection (Fig. 6A). By normalizing the capacity of T cells to suppress bacterial growth to the amount of bacterial growth in the absence of T cells (100%), we found that T cells (n = 10 unique donors) led to a 73.4% reduction in bacterial growth (Fig. 6B). Because Gal9 binds the oligosaccharide chains on the Tim3–IgV domain, the Tim3–Gal9 interaction can be blocked using lactose. M. tuberculosis–infected MDM were cultured with autologous T cells alone or in presence of increasing concentrations of lactose. Lactose reduced the antimicrobial activity of the macrophage in a dose-dependent manner (Fig. 6C). To more specifically assess the participation of the Tim3–Gal9 interaction in macrophage activation, we blocked Tim3 on autologous T cells or Gal9 on M. tuberculosis–infected macrophages during the coculture. Blockade of both Tim3 and Gal9 with anti-human Tim3 or Gal9 Abs led to a reduction in T cell-dependent antibacterial activity of macrophages compared with isotype control mAb (Fig. 6D). The average reduction of bacterial growth with anti-Tim3 or anti-Gal9 mAbs was 34.6 and 34.2%, respectively (n = 10 donors; Fig. 6E). To exclude the possibility that blocking Abs per se could have a directly activate MDM to restrict M. tuberculosis, we added anti-Tim3, anti-Gal9, or isotype control mAb to the M. tuberculosis–infected MDM in the absence of autologous T cells. No effect on bacterial growth on day 4 postinfection was observed (Fig. 6F). This indicates that Tim3/Gal9 blockade reduces the antibacterial activity mediated by autologous T cells by ∼50%. These results confirm the participation of the Tim3–Gal9 pathway in antimicrobial immunity during TB infection. To analyze if the expression of Tim3 by MDM could have any effect on intracellular bacterial growth, MDM were preincubated with anti-human Tim3 mAb before addition of autologous T cells. From three independent experiments, we identified that Tim3 expression by the macrophage was not mandatory to control bacterial growth (Fig. 7).
FIGURE 6.
Tim3–Gal9 interaction controls bacterial replication. (A) M. tuberculosis–infected MDM were cultured alone or with T cells. Day 1 (d1) represents the CFU in infected MDM alone 24 h postinfection, whereas day 4 (d4) is the CFU recovered 4 d postinfection in the absence of any treatment. Data are representative of 10 independent experiments. Horizontal bars represent median values and IQR from four replicate cultures. ****p < 0.0001, one-way ANOVA compared with day 4 macrophages alone. (B) Autologous T cells reproducibly suppress intracellular M. tuberculosis replication. Data are representative of 10 independent experiments. (C) M. tuberculosis–infected MDM were cultured alone or with T cells in the presence of increasing amounts of lactose. Data are representative of three independent experiments. Horizontal bars represent median values and IQR from four replicate cultures. ****p < 0.0001, Kruskal-Wallis test with Dunnett posttest compared with T cells. (D) M. tuberculosis–infected MDM were cultured alone or with T cells in the presence of 10 μg/ml of anti-human Tim3, anti-human Gal9 mAb, or Hu-IgG (control). Horizontal bars indicate median and IQR from four replicate cultures. Data are representative of one experiment. *p < 0.05, one-way ANOVA compared with T cells. (E) Tim3–Gal9 interaction mediates protection against M. tuberculosis. M. tuberculosis–infected MDM were cultured alone or with T cells in the presence of 10 μg/ml of anti-human Tim3 or anti-human Gal9 mAb. Horizontal bars represent median values and IQR from 10 independent experiments. **p < 0.05, ***p < 0.001, Kruskal-Wallis test compared with T cells. (F) In the absence of T cells, blocking mAbs did not have an effect on bacterial growth. Horizontal bars represent median values and IQR from five independent experiments.
FIGURE 7.
Tim3 expression by the macrophage is not required. M. tuberculosis–infected MDM were cultured alone and preincubated with 10 μg/ml of anti-human Tim3 mAb or with Hu-IgG. Day 1 (d1) represents the CFU in infected MDM alone 24 h postinfection, whereas day 4 (d4) is the CFU recovered 4 d postinfection in the absence of any treatment. Horizontal bars represent median values and IQR from four replicate cultures. Data are representative of three independent experiments. Kruskal-Wallis test compared with day 4 macrophages alone or T cells.
Blockade of Tim3–Gal9 interaction reduces IL-1β cytokine production
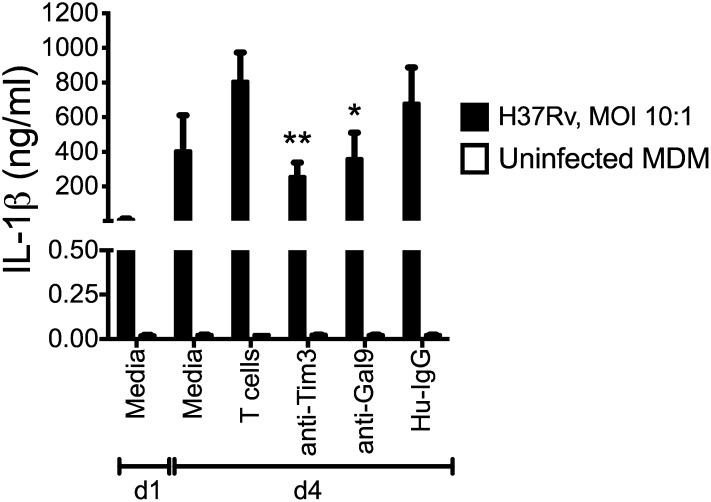
We previously demonstrated that caspase-1–dependent IL-1β secretion promotes antimycobacterial immunity by M. tuberculosis–infected macrophages (15). To identify whether a similar mechanism inhibits intracellular M. tuberculosis growth in human MDM, we measured IL-1β production during coculture of autologous T cells with M. tuberculosis–infected MDM. In 10 independent experiments, the secretion of IL-1β was significantly diminished in the presence of anti-human Tim3 or anti-human Gal9 mAb (Fig. 8). Autologous T cells or blocking mAb did not induce IL-1β secretion in uninfected MDM. These data show that Tim3–Gal9 interaction participates in the antimicrobial response against M. tuberculosis by inducing secretion of IL-1β. Expression of innate cytokines such as IL-1β can activate M. tuberculosis–infected macrophages, promoting control of intracellular bacterial growth by inducing secretion of other proinflammatory cytokines that play a role during PTB. Our results demonstrate that Tim3–Gal9 signaling induced the production of IL-1β, which plays a crucial role in the innate control of M. tuberculosis growth.
FIGURE 8.
Blockade of Tim3–Gal9 interaction reduces production of IL-1β. Supernatants from uninfected and M. tuberculosis–infected MDM cultured alone or with T cells in the presence of 10 μg/ml anti-human Tim3, anti-human Gal9, or Hu-IgG mAbs were collected 24 h postinfection and tested for IL-1β by ELISA. Open bars depict uninfected MDM. Horizontal bars represent median values and IQR from five independent experiments. *p < 0.05, **p < 0.01, Kruskal-Wallis test compared with T cells.
Discussion
We previously reported that the interaction of murine Tim3 with Gal9 expressed by M. tuberculosis–infected macrophages led to macrophage activation, caspase-1–dependent IL-1β secretion, and control of intracellular bacterial growth (15); however, its role in human immune response to M. tuberculosis infection has not been analyzed. In this study, we explored whether the innate antimicrobial pathways activated by Tim3 and Gal9 in murine macrophages are operational in human macrophages and PTB patients. This is the first study, to our knowledge, to demonstrate that: 1) PTB patients and HD have comparable levels of Tim3 on T cells; 2) PTB patients have a reduced cell-surface expression of Tim3 on CD14+ monocytes compared with HD; 3) M. tuberculosis infection inhibits Tim3 and Gal9 expression on MDM; 4) autologous T cells control intracellular bacterial growth in a Tim3- and Gal9-dependent manner; and 5) blocking Tim3 on the T cell and Gal9 on the infected macrophages limits secretion of IL-1β.
It is intriguing that Tim3 expression on T cells is not different between PTB and HD. This observation is in contrast to what has been observed for HIV and hepatitis C virus patients as well as in a cohort of TB patients in China (6, 12, 25). In all of these studies, Tim3 expression was associated with T cell exhaustion with functional inability to produce cytokines and proliferate. It is possible that Tim3 expression on T cells is modulated differently in our cohort, either because of geographical differences in the bacterial strain distribution or underlying differences in the host population. We also observed that fewer Tim3+CD14+ monocytes in the blood of PTB patients compared with HD. Feasible CD14+Tim3+ monocytes may be depleted from the blood and recruited to infected tissues, where they differentiate into macrophages or DC. However, it remains to be determined whether these findings are simply a nonspecific feature of any illness/infection or a particular feature of the TB infection. Tim3 expression has been clearly identified on innate immune cells, especially APCs (7). Many studies have demonstrated that Tim3 might function as an inhibitory molecule on CD4+Th1 T cells; however, its role in myeloid cells (monocytes and macrophages) is still being analyzed. What we currently do know is that Tim3 and Gal-9 have been reported to induce maturation of human monocyte-derived DCs and promote phagocytosis of apoptotic cells and cross-presentation of Ags to T cells (9, 26, 27). Additionally, it has been also demonstrated by Zhang et al. (28) that Tim3 might suppresses monocyte/macrophage function by crosstalk with other negative immune modulators, including programmed cell death-1 and suppressor of cytokine signaling-1 and through altering the JAK/STAT signaling pathway. In our study, we speculate that these mechanisms may be participating and that a low frequency of CD14+Tim3+ monocytes can potentially impact the total frequency of macrophages able to phagocyte the bacilli or diminish the cross presentation and activation of T cells.
It is also unknown for us whether the expression profile of Tim3 would change after treatment of TB. IFN-γ and TNF-α are proinflammatory cytokines critical during the immune response against M. tuberculosis and together with IL-2, IL-7, IL-15, and IL-21 might increase cell-surface expression of Tim3 (19, 25, 29–31). We observed an apparent paradox in that expression of Tim3 on CD14+ monocytes did not correlate with the higher plasma concentrations of IFN-γ and TNF-α examined ex vivo in PTB patients. More studies will be needed to identify the molecular mechanisms controlling Tim3 expression on monocytes from PTB patients.
M. tuberculosis evades innate immune responses in macrophages by arresting phagosome maturation to avoid a potentially bactericidal environment (32) and modulating TLR-induced reduction of costimulatory (CD80, CD86) and Ag-presenting molecule (HLA-DR) expression (23, 24, 33). Our finding that in vitro infection of MDM with either virulent or avirulent M. tuberculosis strains (H37Rv and H37Rv, respectively) decreased cell-surface expression of Tim3 and Gal9 is in agreement with the observation that Tim3 levels are reduced on monocytes from PTB patients and suggest that M. tuberculosis can directly modulate the expression of Tim3. It remains to be determined whether chemotherapy restores Tim3 expression on monocytes in treated PTB patients. If restoration of Tim3 expression parallels reduced bacterial burden, Tim3 expression could monitor treatment success and failures.
Our study has some limitations. First, we conducted this study using peripheral blood samples; these cannot accurately reflect what is happening in the lung tissue of PTB patients or in granulomas. Second, all study participants had been BCG vaccinated at birth, which is an immunization policy in Mexico. We recognize that pre-exposure to M. tuberculosis Ags might modify the expression profile of Tim3 and Gal9 on PBMC. However, this was true for both PTB and HD groups, and, despite the pre-exposure to mycobacterial Ags, we were able to identify differences between PTB patients and HD with respect to the frequency of CD14+Tim3+ monocytes. Third, other control groups such as HD, not vaccinated with BCG with or without latent TB infection, would have allowed us to determine whether BCG vaccination has any effect on Tim3 expression.
In summary, we have shown that Tim3–Gal9 interaction directly participates in the adaptive immune responses against M. tuberculosis infection in humans. We speculate that Tim3 expression by T cells and Gal9 expression by monocytes/macrophages is tightly regulated to modulate macrophage activation and IL-1β secretion. Dysregulation of these molecules could have detrimental consequences for the host and in the control of M. tuberculosis growth. Understanding how the Tim3–Gal9 pathway functions during PTB will provide insight into how the host responds to chronic stimulation by human pathogens and how such responses might be related to the immunopathogenesis of chronic infectious diseases.
Acknowledgments
We thank Lilia Vazquez for technical assistance. We also thank Dr. Vijay Kuchroo (Brigham and Women’s Hospital, Boston, MA) for providing the anti-human Tim3 Abs.
This study was supported by a Consejo Nacional de Ciencia y Tecnología grant (104003 to I.S.-O.) and the Creative and Novel Ideas in HIV Research Award from the National Institutes of Health–funded Centers for AIDS Research grant (5P30A1027767-22 to I.S.-O.).
- BCG
- bacillus Calmette–Guérin
- DC
- dendritic cell
- Gal9
- Galectin 9
- HD
- healthy donor
- INER
- National Institute of Respiratory Diseases
- iNOS
- inducible NO synthase
- IQR
- interquartile range
- MDM
- monocyte-derived macrophage
- MOI
- multiplicity of infection
- PTB
- pulmonary tuberculosis
- TB
- tuberculosis
- Tim3
- T cell Ig and mucin domain 3
- TST
- tuberculin skin test.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Flynn J. L., Chan J. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19: 93–129 [DOI] [PubMed] [Google Scholar]
- 2.Sabatos C. A., Chakravarti S., Cha E., Schubart A., Sánchez-Fueyo A., Zheng X. X., Coyle A. J., Strom T. B., Freeman G. J., Kuchroo V. K. 2003. Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat. Immunol. 4: 1102–1110 [DOI] [PubMed] [Google Scholar]
- 3.Sánchez-Fueyo A., Tian J., Picarella D., Domenig C., Zheng X. X., Sabatos C. A., Manlongat N., Bender O., Kamradt T., Kuchroo V. K., et al. 2003. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat. Immunol. 4: 1093–1101 [DOI] [PubMed] [Google Scholar]
- 4.Zhu C., Anderson A. C., Schubart A., Xiong H., Imitola J., Khoury S. J., Zheng X. X., Strom T. B., Kuchroo V. K. 2005. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 6: 1245–1252 [DOI] [PubMed] [Google Scholar]
- 5.Hafler D. A., Kuchroo V. 2008. TIMs: central regulators of immune responses. J. Exp. Med. 205: 2699–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golden-Mason L., Palmer B. E., Kassam N., Townshend-Bulson L., Livingston S., McMahon B. J., Castelblanco N., Kuchroo V., Gretch D. R., Rosen H. R. 2009. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J. Virol. 83: 9122–9130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monney L., Sabatos C. A., Gaglia J. L., Ryu A., Waldner H., Chernova T., Manning S., Greenfield E. A., Coyle A. J., Sobel R. A., et al. 2002. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 415: 536–541 [DOI] [PubMed] [Google Scholar]
- 8.Hastings W. D., Anderson D. E., Kassam N., Koguchi K., Greenfield E. A., Kent S. C., Zheng X. X., Strom T. B., Hafler D. A., Kuchroo V. K. 2009. TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines. Eur. J. Immunol. 39: 2492–2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson A. C., Anderson D. E., Bregoli L., Hastings W. D., Kassam N., Lei C., Chandwaskar R., Karman J., Su E. W., Hirashima M., et al. 2007. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science 318: 1141–1143 [DOI] [PubMed] [Google Scholar]
- 10.Khademi M., Illés Z., Gielen A. W., Marta M., Takazawa N., Baecher-Allan C., Brundin L., Hannerz J., Martin C., Harris R. A., et al. 2004. T Cell Ig- and mucin-domain-containing molecule-3 (TIM-3) and TIM-1 molecules are differentially expressed on human Th1 and Th2 cells and in cerebrospinal fluid-derived mononuclear cells in multiple sclerosis. J. Immunol. 172: 7169–7176 [DOI] [PubMed] [Google Scholar]
- 11.DeKruyff R. H., Bu X., Ballesteros A., Santiago C., Chim Y. L., Lee H. H., Karisola P., Pichavant M., Kaplan G. G., Umetsu D. T., et al. 2010. T cell/transmembrane, Ig, and mucin-3 allelic variants differentially recognize phosphatidylserine and mediate phagocytosis of apoptotic cells. J. Immunol. 184: 1918–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones R. B., Ndhlovu L. C., Barbour J. D., Sheth P. M., Jha A. R., Long B. R., Wong J. C., Satkunarajah M., Schweneker M., Chapman J. M., et al. 2008. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J. Exp. Med. 205: 2763–2779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koguchi K., Anderson D. E., Yang L., O’Connor K. C., Kuchroo V. K., Hafler D. A. 2006. Dysregulated T cell expression of TIM3 in multiple sclerosis. J. Exp. Med. 203: 1413–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez-Manzanet R., DeKruyff R., Kuchroo V. K., Umetsu D. T. 2009. The costimulatory role of TIM molecules. Immunol. Rev. 229: 259–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jayaraman P., Sada-Ovalle I., Beladi S., Anderson A. C., Dardalhon V., Hotta C., Kuchroo V. K., Behar S. M. 2010. Tim3 binding to galectin-9 stimulates antimicrobial immunity. J. Exp. Med. 207: 2343–2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.2000. Diagnostic Standards and Classification of Tuberculosis in Adults and Children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am. J. Respir. Crit. Care Med. 161: 1376–1395 [DOI] [PubMed] [Google Scholar]
- 17.Sada-Ovalle I., Chiba A., Gonzales A., Brenner M. B., Behar S. M. 2008. Innate invariant NKT cells recognize Mycobacterium tuberculosis-infected macrophages, produce interferon-gamma, and kill intracellular bacteria. PLoS Pathog. 4: e1000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. 1982. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 126: 131–138 [DOI] [PubMed] [Google Scholar]
- 19.Cooper A. M., Dalton D. K., Stewart T. A., Griffin J. P., Russell D. G., Orme I. M. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 178: 2243–2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flynn J. L., Chan J., Triebold K. J., Dalton D. K., Stewart T. A., Bloom B. R. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178: 2249–2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson A. C., Lord G. M., Dardalhon V., Lee D. H., Sabatos-Peyton C. A., Glimcher L. H., Kuchroo V. K. 2010. T-bet, a Th1 transcription factor regulates the expression of Tim-3. Eur. J. Immunol. 40: 859–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noss E. H., Harding C. V., Boom W. H. 2000. Mycobacterium tuberculosis inhibits MHC class II antigen processing in murine bone marrow macrophages. Cell. Immunol. 201: 63–74 [DOI] [PubMed] [Google Scholar]
- 23.Torres M., Ramachandra L., Rojas R. E., Bobadilla K., Thomas J., Canaday D. H., Harding C. V., Boom W. H. 2006. Role of phagosomes and major histocompatibility complex class II (MHC-II) compartment in MHC-II antigen processing of Mycobacterium tuberculosis in human macrophages. Infect. Immun. 74: 1621–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noss E. H., Pai R. K., Sellati T. J., Radolf J. D., Belisle J., Golenbock D. T., Boom W. H., Harding C. V. 2001. Toll-like receptor 2-dependent inhibition of macrophage class II MHC expression and antigen processing by 19-kDa lipoprotein of Mycobacterium tuberculosis. J. Immunol. 167: 910–918 [DOI] [PubMed] [Google Scholar]
- 25.Wang X., Cao Z., Jiang J., Li Y., Dong M., Ostrowski M., Cheng X. 2011. Elevated expression of Tim-3 on CD8 T cells correlates with disease severity of pulmonary tuberculosis. J. Infect. 62: 292–300 [DOI] [PubMed] [Google Scholar]
- 26.Dai S. Y., Nakagawa R., Itoh A., Murakami H., Kashio Y., Abe H., Katoh S., Kontani K., Kihara M., Zhang S. L., et al. 2005. Galectin-9 induces maturation of human monocyte-derived dendritic cells. J. Immunol. 175: 2974–2981 [DOI] [PubMed] [Google Scholar]
- 27.Nakayama M., Akiba H., Takeda K., Kojima Y., Hashiguchi M., Azuma M., Yagita H., Okumura K. 2009. Tim-3 mediates phagocytosis of apoptotic cells and cross-presentation. Blood 113: 3821–3830 [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y., Ma C. J., Wang J. M., Ji X. J., Wu X. Y., Jia Z. S., Moorman J. P., Yao Z. Q. 2011. Tim-3 negatively regulates IL-12 expression by monocytes in HCV infection. PLoS ONE 6: e19664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muñoz-Elías E. J., McKinney J. D. 2005. Mycobacterium tuberculosis isocitrate lyases 1 and 2 are jointly required for in vivo growth and virulence. Nat. Med. 11: 638–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Juarez E., Nuñez C., Sada E., Ellner J. J., Schwander S. K., Torres M. 2010. Differential expression of Toll-like receptors on human alveolar macrophages and autologous peripheral monocytes. Respir. Res. 11: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mujib S., Jones R. B., Lo C., Aidarus N., Clayton K., Sakhdari A., Benko E., Kovacs C., Ostrowski M. A. 2012. Antigen-independent induction of Tim-3 expression on human T cells by the common γ-chain cytokines IL-2, IL-7, IL-15, and IL-21 is associated with proliferation and is dependent on the phosphoinositide 3-kinase pathway. J. Immunol. 188: 3745–3756 [DOI] [PubMed] [Google Scholar]
- 32.Clemens D. L., Horwitz M. A. 1995. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J. Exp. Med. 181: 257–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drage M. G., Pecora N. D., Hise A. G., Febbraio M., Silverstein R. L., Golenbock D. T., Boom W. H., Harding C. V. 2009. TLR2 and its co-receptors determine responses of macrophages and dendritic cells to lipoproteins of Mycobacterium tuberculosis. Cell. Immunol. 258: 29–37 [DOI] [PMC free article] [PubMed] [Google Scholar]