Abstract
Background:
Three quarter of endometrial carcinomas are treated at early stage. Still, 15 to 20% of these patients experience recurrence, with little effect from systemic therapies. Homo sapiens v-Ki-ras2 Kirsten rat sarcoma viral oncogenes homologue (KRAS) mutations have been reported to have an important role in tumorigenesis for human cancers, but there is limited knowledge regarding clinical relevance of KRAS status in endometrial carcinomas.
Methods:
We have performed a comprehensive and integrated characterisation of genome-wide expression related to KRAS mutations and copy-number alterations in primary- and metastatic endometrial carcinoma lesions in relation to clinical and histopathological data. A primary investigation set and clinical validation set was applied, consisting of 414 primary tumours and 61 metastatic lesions totally.
Results:
Amplification and gain of KRAS present in 3% of the primary lesions and 18% of metastatic lesions correlated significantly with poor outcome, high International Federation of Gynaecology and Obstetrics stage, non-endometrioid subtype, high grade, aneuploidy, receptor loss and high KRAS mRNA levels, also found to be associated with aggressive phenotype. In contrast, KRAS mutations were present in 14.7% of primary lesions with no increase in metastatic lesions, and did not influence outcome, but was significantly associated with endometrioid subtype, low grade and obesity.
Conclusion:
These results support that KRAS amplification and KRAS mRNA expression, both increasing from primary to metastatic lesions, are relevant for endometrial carcinoma disease progression.
Keywords: endometrial cancer, prognosis, KRAS , amplification, mutation
Endometrial cancer is the most common pelvic gynaecological malignancy in industrialised countries. Although 75% are treated at an early stage, 15 to 20% recur. There is a need for more effective systemic therapies, as no new targeted therapies are yet available in standard clinical care, and response to conventional systemic therapy is limited (Dedes et al, 2011). Several prognostic markers exist, and recent studies have indicated promising new targets to develop novel strategies for systemic therapies in endometrial cancer (Salvesen et al, 2009). Still, no markers are available to predict response to such therapy.
Traditionally, endometrial cancer has been divided into two subgroups, type I and type II carcinomas, to assess the risk of recurrent disease. Type I endometrial carcinoma is associated with good prognosis, low grade, endometrioid morphology and rarely metastasise to regional and distant sites (Fujimoto et al, 2009). Type II endometrial carcinoma is associated with poor prognosis, non-endometrioid histology and high grade. Still, there is considerable overlap, and as tool to predict prognosis this classification may be improved, as 20% of type I cancers recur and 50% of type II cancers do not. Although the molecular alterations reported for type I and type II cancers are overlapping, type I cancers are significantly more often Homo sapiens v-Ki-ras2 Kirsten rat sarcoma viral oncogene (KRAS) and PTEN mutated, microsatellite instable, diploid and expressing oestrogen- and progesterone receptors (ER, PR) (Lax et al, 1998). Type II cancers, in contrast are more often aneuploid and with altered expression of p53, p16 and with hormone receptor loss. These differences are of prognostic value; nevertheless, the molecular characteristics distinguishing Type I and Type II cancers have so far had limited impact for tailoring systemic therapies.
Homo sapiens v-Ki-ras2 Kirsten rat sarcoma viral oncogenes homologue is a small GTPase and a member of the RAS superfamily of proteins linked to the carcinogenic process in preclinical models. Knock-down of KRAS in pancreatic cancer cell lines leads to decreased motility and proliferation and a decreased expression of pERK1/2, SNAIL and Nf-kB, all factors related to epithelial to mesenchymal transition (Rachagani et al, 2011). In colorectal cancer, KRAS has been reported to induce VEGF and inhibit apoptosis through Akt phosphorylation under hypoxic conditions (Zeng et al, 2010).
Activating KRAS mutations have been detected in precursor lesions for colorectal and endometrial cancers indicating that these are early events (Dobrzycka et al, 2009). Mutations have been found to have a prognostic impact in lung-, pancreatic- and colorectal cancers (Gautschi et al, 2007; Ling et al, 2012). In endometrial cancer, findings have been inconsistent, with reported different prognostic impact for different age groups (Ito et al, 1996; Semczuk et al, 1998). Furthermore, KRAS mutations have been found to predict lack of response to EGFR inhibition in lung- and colorectal cancers (Cepero et al, 2010). In endometrial cancer trials of EGFR inhibitors have been limited, although some studies reported partial response (Oza et al, 2008; Dedes et al, 2011).
Other measures for KRAS alterations, although less studied than mutations, have supported a relevance of KRAS status for clinical phenotype in cancer (Wagner et al, 2011). In lung cancer patients with KRAS amplification and KRAS mutations, the latter only associated with poor survival, but with no independent prediction of response to therapy (Sasaki et al, 2011). Lung cancers with KRAS amplification have been reported to have increased expression levels of p21 (CDK1), suggesting an impact on cell cycle regulation (Wagner et al, 2009). In endometrial cancers, one previous study demonstrated a poor prognostic impact of amplifications of the 12p12.1 region harbouring KRAS (Salvesen et al, 2009). On this background, we have investigated several aspects of KRAS alterations including mutation, amplification and mRNA levels in relation to transcriptional alterations and clinical phenotype. To study this, we have applied a unique sample set of freshly frozen primary- and metastatic endometrial carcinoma lesions as primary investigation set for a global and comprehensive characterisation of molecular changes, and an independent, large and extensively annotated patient series for validation of findings. In particular, we wanted to investigate which KRAS alterations in endometrial carcinoma that link to aggressive disease.
Materials and Methods
Patient series
From May 2001 through 2009, freshly frozen and formalin-fixed paraffin-embedded (FFPE) tissues have been prospectively collected from primary- and metastatic endometrial carcinoma lesions from patients treated at the Department of Gynaecology and Obstetrics, Haukeland University Hospital, Bergen, Norway, after collection of informed consent. In total 461 patients where included for the various analyses in this study. Formalin-fixed paraffin-embedded tumour tissue from hysterectomy specimens from 414 primary tumours and 61 metastatic lesions were mounted in tissue micro arrays (TMA) for amplification studies of KRAS using fluorescence in situ hybridisation (FISH). DNA and RNA were extracted from freshly frozen tissue from 264 primary- and 22 metastatic lesions. Two hundred fifteen of these primary tumours and all metastases were included in the FISH series. Extracted DNA was used for SNP array- (74 patients) and mutation analyses (264 patients), RNA were applied for micro-array analysis (122 patients) and 161 patients were used for the qPCR validation series. Primary tumour tissue in TMAs were analysed by immunohistochemistry for ER, PR and p53 protein expressions (461, 461 and 390 patients, respectively). The research has been approved by the Norwegian Data Inspectorate (961478-2), Norwegian Social Sciences Data Services (15501) and the Local ethical committee (REKIII nr. 052.01). Women gave informed consent.
Clinico-pathological data including age at diagnosis, International Federation of Gynaecology and Obstetrics (FIGO) stage according to the 2009 criteria (FIGO IFoGaO, 1989; Mikuta, 1993), histological subtype and grade, treatment and follow-up information were available for all cases and were investigated in relation to KRAS alterations.
Follow-up data regarding recurrence and survival were collected from patient records and correspondence with physicians responsible for outpatient care. Data were crosschecked with data registered at the Norwegian Cancer Registry and Register for Causes of deaths, Statistics, Norway. Date of last follow-up was April 1st 2010. The median follow-up for survivors was 39 months (range 2–90), 48 (12%) patients died because of endometrial cancer during follow-up.
The therapy consisted of hysterectomy and bilateral salpingo-oophorectomy unless surgery was contraindicated owing to co-morbidity. Pelvic lymphadenectomy as part of surgical staging was conducted after an overall assessment of the patients’ condition by the responsible surgeon as previously reported (Trovik et al, 2011). Adjuvant therapy was recommended for patients with FIGO stage ⩾II and high-risk FIGO stage I patients, defined as non-endometrioid tumours or deeply infiltrating endometrioid grade 3 tumours. Of the 461 patients included in our analysis 122 (26.4%) patients were given adjuvant treatment. External radiation was given to 58 (12.6%), internal radiation to 2 (0.4%), chemotherapy to 54 (11.7%), anti-hormonal treatment to 5 (1.1%) and chemotherapy combined with radiation to 3 (0.7%) patients.
Tissue micro-array construction
Haematoxylin and eosin-stained slides from individual tumour specimens were evaluated to identify the area of highest tumour purity. Tissue cylinders of diameter 0.6 mm were punched out from the selected areas for each corresponding paraffin block and mounted into a recipient block using a custom-made precision instrument (Beecher Instruments, Silver Spring, MD, USA). This method has been described and its usefulness validated in several earlier publications (Kononen et al, 1998; Engelsen et al, 2006). The recipient blocks were treated at 40 °C for 20 min and stored at 4 °C before 5 μm microtome sectioning for FISH analyses. Representative tumour tissue was available in TMAs for FISH from 414 hysterectomy specimens and from 61 corresponding metastatic lesions of these patients.
Immunohistochemistry
Tissue micro array sections were dewaxed in xylene and rehydrated in ethanol before microwave antibody retrieval. The sections were incubated for 60 min with p53 antibody (Dako M7001, Copenhagen, Denmark), diluted 1:1000 and for ER and PR as previously reported (Engelsen et al, 2008; Krakstad et al, 2012). The staining was recorded as previously described (Salvesen et al, 2000). In short, a semi-quantitative and subjective grading system was used, and a staining index was calculated as a product of staining intensity (0–3) and area of positive tumour cells (1⩽10%, 2=10–50% and 3⩾50%).
Copy-number assessment
For FISH analysis, TMA sections were incubated at 56 °C overnight and treated according to the Paraffin Pre-treatment Protocol (Abbot molecular, Wiesbaden, Germany). Hybridisation was performed according to protocol from Abbot molecular. Briefly the sections were dewaxed in xylene, dehydrated in 100% ethanol, air-dried and treated with proteases for 12 min, denatured and hybridised overnight at 37 °C with KRAS/centromere enumeration probe (KRAS/CEP12q; Abbot Molecular). Slides were washed with post hybridisation buffer at 72 °C, counterstained with 40,60-diamidino-2-phenylindole (DAPI), mounted, and stored in the dark before signal enumeration. For FISH analysis, the slides were examined by Zeiss fluorescence microscope (Göttingen, Germany) equipped with a × 63 oil immersion objective. Each slide was scanned at low power with a DAPI filter to recognise the TMA map. Areas of optimal tissue digestion and no overlapping nuclei were selected in each core for counting. In each case, signals for probe and control were counted in 40–60 cells. Amplification of KRAS was defined as a final ratio obtained for KRAS/CEP12q probes ⩾2.0; KRAS gain was defined as KRAS/CEP12q ratio>1.0 but <2.0. Micrographs were taken from each amplified spot, using the Zeiss Axiovision software. Copy-number alterations assessed by SNP array were available for a subset of 74 patients from previous studies, and these data were applied for analysis of KRAS copy-number alterations in relation to mRNA expression levels in microarrays.
Oligonucelotide DNA microarray
The RNA was extracted using the RNeasy Mini Kit (Qiagen, Hilden, Germany) and hybridised to Agilent Whole Human Genome Microarrays 44 k (Cat. no. G4112F) according to the manufacturers instruction (www.agilent.com), and as previously described (Krakstad et al, 2012). Microarray data have been deposited in the ArrayExpress Archive database, http://www.ebi.ac.uk/arrayexpress/ (ArrayExpress accession: E-MTAB-1007). mRNA expression data of KRAS were obtained from DNA-microarrays and a significance analysis of microarray (SAM) was performed to investigate genes significantly differentially expressed (FDR <0.05) in KRAS amplified compared with non-KRAS-amplified tumours based on SNP-array data. The subset of patients harbouring KRAS amplifications in FISH analyses with available microarray data was to small to allow meaningful statistical analysis (n=3).
PCR and DNA sequencing
Genomic DNA was extracted from freshly frozen tissue samples and investigated for point mutations in exon 2 codon 12 and 13 of KRAS and exon 9 and 20 of phosphatidylinositol-4,5-bisphosphate 3-kinase PIK3CA, primers and conditions listed in Supplementary Section 1. cDNA was synthesised from 1 μg RNA by the High capacity RNA to cDNA kit (Applied Biosystems, Foster City, CA, USA). Gene expression of KRAS was determined using the TaqMan gene expression assay KRAS-Hs00932330_m1 (Applied Biosystems). All samples were run on micro fluidic cards with GAPDH-Hs99999905_m1 as endogenous control according to manufacturers instruction, and as previously described (Krakstad et al, 2012).
Statistical analysis
Statistics were performed using the statistical programme SPSS 18.0 (Quarry Bay, Hong Kong). Associations between categorical variables were evaluated by Pearson’s χ2-test. Mann–Whitney U-test was used for analysis of continuous variables between categories. P-values represent two-sided tests and are considered of statistical significance when P<0.05. Univariate analyses of time to recurrence (recurrence-free survival) and death because of endometrial carcinoma (disease-specific survival) were performed using the Kaplan–Meyer method. Differences in survival were estimated by the Mantel–Cox log-rank test.
In the statistical analysis, cutoff values were based on quartiles, also considering the frequency distribution for each marker, the size of subgroups and number of events in each category. Groups with similar survival were merged.
Results
Amplification of KRAS is associated with aggressive disease and metastasis
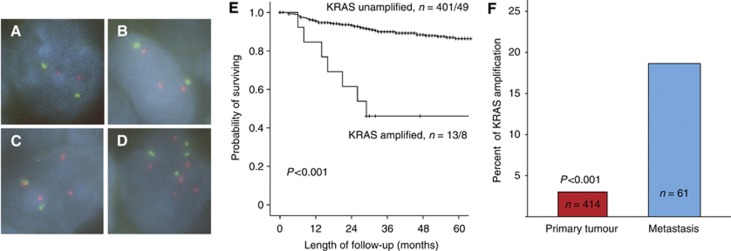
Analysis of KRAS copy-number changes by FISH analysis showed a large variation in KRAS/CEP12q gene probe ratio from 2 : 2 to <20 : 2. Defining cases with a ratio ⩾1 and<2 as gain; and ⩾2 as amplified in the FISH analysis, KRAS gain or amplification was detected in 3% (13 of 414) of the primary endometrial carcinomas investigated (Figure 1A–D). There was no significant difference between gain and amplification of KRAS gene copy number in terms of prognosis. Presence of KRAS gain or amplification were highly significantly associated with traditional markers for aggressive phenotype including high age, FIGO stage, non-endometrioid histology, high grade, presence of lymph node metastasis, aneuploidy and loss of hormone receptors (Table 1). Gain or amplification of KRAS was also highly significantly associated with poor prognosis with a 46% 5-year survival compared with 87% for patients with unamplified status (P<0.001) (Figure 1B). Amplification of KRAS maintains its independent prognostic impact in Cox multivariate analysis when adjusted for age, histological subtype, grade and FIGO stage (HR=2.6, 95% CI: 1.2–5.8, P=0.02). When adjusting for adjuvant treatment, in addition to these clinico-pathological variables, KRAS amplification also maintained its independent prognostic impact in Cox’ multivariate analyses (HR=2.7, 95% CI: 1.2–6.1, P=0.014). When comparing primary and metastatic endometrial carcinoma lesions, we find a significant increase in the proportion of samples with KRAS gain or amplification from 3% in 414 primary lesions investigated to 18% in 61 metastatic lesions studied (P<0.001) (Figure 1C). There was no significant correlation between KRAS amplification and PIK3CA mutations (Table 1). However, KRAS amplification was highly significantly correlated to pathological p53 expression estimated by immunohistochemistry (Table 1). In analysis of differentially expressed genes in tumours harbouring KRAS amplifications (n=10) compared with tumours without KRAS amplifications (n=64), we find seven genes to be significantly differentially expressed. Two genes were upregulated, whereas five genes were downregulated as listed in Table 4.
Figure 1.
Fluorescence in situ hybridisation (FISH) for KRAS copy numbers showing no KRAS amplification with CEP12 (green)/KRAS probe (red) ratio 2:2 (A); KRAS gain with KRAS/CEP12 gene probe ratio 2:3 (B); KRAS amplification with KRAS/CEP12 gene probe ratio 2:4 (C); KRAS polysomy with KRAS/CEP12 ratio 4:6 (D) and impact of copy numbers on disease-specific survival in endometrial carcinoma (E). Survival curves are estimated by the Kaplan–Meier method with numbers of cases (events) given for cases with amplification/gain compared with unamplified cases. Proportion of cases with KRAS gene amplification/gain increased significantly from primary (13 of 414) to metastatic (11 of 61) lesions (P<0.001, FE test) (F).
Table 1. Clinico-pathological variables related to KRAS gene amplification analysed by FISH for 414 patients.
| Variable | Amplified, n (%) | Not amplified, n (%) | P -value a |
|---|---|---|---|
| Age | |||
| ⩽66 | 3 (1.3) | 235 (98.7) | 0.01 |
| >66 | 10 (5.7) | 166 (94.3) | |
| BMI b | |||
| ⩽25 | 3 (2.2) | 135 (97.8) | 0.2 |
| >25 | 10 (4.5) | 212 (95.5) | |
| FIGO stage c | |||
| I–II | 7 (2.0) | 341 (98) | 0.009 |
| III–IV | 6 (9.1) | 60 (90.9) | |
| Histological type d | |||
| Endometroid | 6 (1.8) | 335 (98.2) | 0.003 |
| Non-endometroid | 7 (9.6) | 66 (90.4) | |
| Grade e | |||
| Low-medium | 3 (1.1) | 281 (98.9) | 0.002 |
| High | 10 (7.9) | 117 (92.1) | |
| Lymph node f | |||
| Negative | 5 (1.7) | 298 (98.3) | 0.001 |
| Postitive | 5 (13.5) | 32 (86.5) | |
| Ploidy g | |||
| Diploid | 4 (1.7) | 231 (98.3) | 0.006 |
| Aneuploid | 5 (9.6) | 47 (90.4) | |
| ERα h | |||
| Positive | 5 (1.6) | 306 (98.4) | 0.003 |
| Negative | 8 (8.4) | 87 (91.6) | |
| PR i | |||
| Positive | 3 (1) | 301 (99) | <0.001 |
| Negative | 9 (8.6) | 96 (91.4) | |
| PIK3CA mut j | |||
| N.m.d | 7 (3.8) | 177 (96.2) | 0.33 |
| Mutated | 0 (0) | 31 (100) | |
| P53 k | |||
| High | 9 (100) | 0 (0) | <0.001 |
| Low | 71 (21.9) | 253 (78.1) | |
Abbreviations: BMI=body mass index; ER=oestrogen receptor; FIGO=International Federation of Gynaecology and Obstetrics; FISH=fluorescence in situ hybridisation; KRAS=Homo sapiens v-Ki-ras2 Kirsten rat sarcoma viral oncogenes homologue; N.m.d=no mutations detected; PR=progesterone receptor.
Data missing: b54, d8, e3, f74, g127, h4, i7, j199, k81.
Fisher’s exact test.
FIGO 2009 Criteria.
High KRAS mRNA level reflects aggressive phenotype
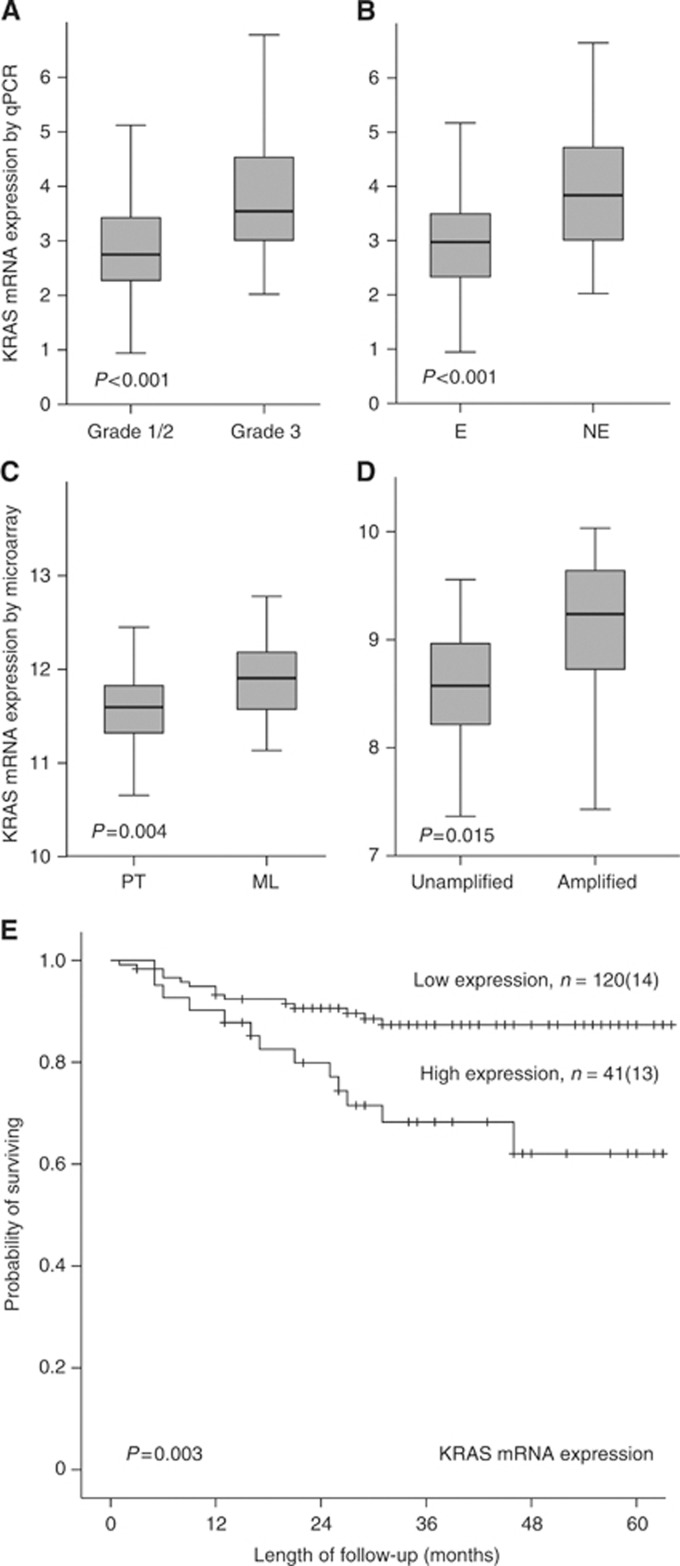
To further investigate the effect of KRAS amplification on mRNA levels, we used mRNA microarrays from 122 primary and 19 metastatic lesions, and a validation cohort analysing mRNA expression of KRAS by qPCR in additionally 161 freshly frozen primary endometrial carcinoma lesions. High KRAS mRNA expression was significantly associated with high FIGO stage, non-endometrioid histology, high grade, lymph node metastasis, aneuploidy and hormone receptor loss (Table 2, Figure 2A and B). The KRAS mRNA levels increased significantly from primary to metastatic lesions (Figure 2C) and in amplified compared to unamplified samples (estimated by SNP array, Figure 2D). In line with this, high KRAS mRNA level was associated with poor prognosis in the validation cohort (n=161; Figure 2E, P=0.003), and with a similar trend for the slightly smaller micro-array cohort (n=122; P=0.08). In Cox multivariate analysis KRAS mRNA expression did not maintain its prognostic significance (HR=1.32, 95% CI: 0.9–2.0, P=0.17) adjusted for age, histological type, grade and FIGO stage. Presence of mutations in the PIK3CA gene was not correlated with increased KRAS mRNA expression (Table 2). High KRAS mRNA expression was significantly correlated to pathological p53 expression by immunohistochemistry (Table 2).
Table 2. Clinico-pathological variables related to KRAS gene expression analysed by qPCR for 161 patients.
| Variable | High expression, n (%) | Low expression, n (%) | P -value |
|---|---|---|---|
| Age | |||
| ⩽66 | 20 (21.5) | 74 (78.5) | 0.2 |
| >66 | 21 (31.3) | 46 (68.7) | |
| BMI a | |||
| ⩽25 | 14 (25.5) | 41 (74.5) | 0.7 |
| >25 | 27 (28.1) | 69 (71.9) | |
| FIGO stage b | |||
| I–II | 28 (21.9) | 100 (78.1) | 0.04 |
| III–IV | 13 (39.4) | 20 (60.6) | |
| Histological type | |||
| Endometroid | 25 (18.9) | 107 (81.1) | <0.001 |
| Non-endometroid | 16 (55.2) | 13 (44.8) | |
| Grade c | |||
| Low-medium | 17 (16) | 89 (84) | <0.001 |
| High | 24 (44.4) | 30 (55.6) | |
| Lymph node d | |||
| Negative | 28 (22.2) | 98 (78.8) | 0.02 |
| Positive | 10 (52.6) | 9 (47.4) | |
| Ploidy e | |||
| Diploid | 19 (19) | 80 (81) | 0.001 |
| Aneuploid | 18 (50) | 18 (50) | |
| ERα f | |||
| Positive | 21 (17) | 101 (83) | <0.001 |
| Negative | 19 (53) | 17 (47) | |
| PR g | |||
| Positive | 20 (16) | 104 (84) | <0.001 |
| Negative | 19 (56) | 15 (44) | |
| PIK3CA mut h | |||
| N.m.d | 33 (25.6) | 96 (74.4) | 0.47 |
| Mutated | 6 (28.6) | 15 (78.4) | |
| P53 i | |||
| High | 20 (57.1) | 15 (42.9) | <0.001 |
| Low | 17 (16.5) | 86 (83.5) | |
Abbreviations: BMI=body mass index; ER=oestrogen receptor; FIGO=International Federation of Gynaecology and Obstetrics; KRAS=Homo sapiens v-Ki-ras2 Kirsten rat sarcoma viral oncogenes homologue; N.m.d=no mutations detected; PR=progesterone receptor.
The P-value was based on the χ2-test or Fisher’s exact test as indicated.
Data missing: a10, c1, d16, e26, f3, g3, h11, i23.
FIGO 2009 criteria.
Figure 2.
Box-plots showing KRAS mRNA expression levels in relation to histological grade (A), endometrioid (E) and non-endometrioid (NE) histological subtypes (B), primary tumours (PT) vs metastatic lesions (ML) (C) and KRAS amplified vs unamplified status (SNP array (Salvesen et al, 2009)) (D). Estimated disease-specific survival according to expression levels of KRAS mRNA (qPCR) according to upper quartile with number of cases (events) given for each category.
Mutations of KRAS
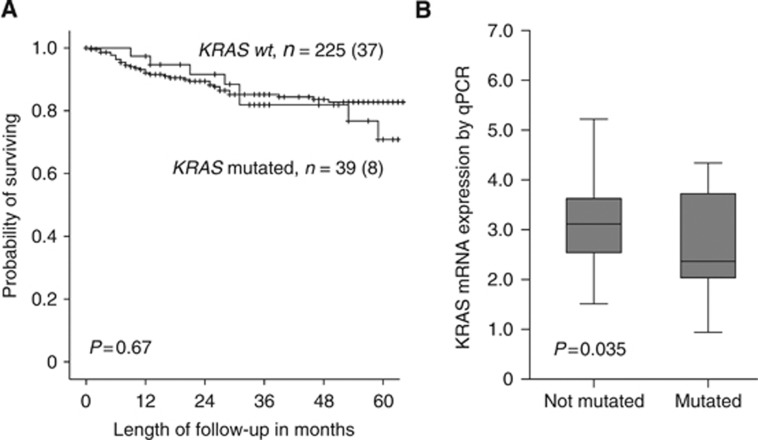
As KRAS mutations have been linked to response to targeted therapy in other cancer types, and its relation to prognosis in endometrial cancer is unsettled, we further investigated primary and metastatic lesions for presence of KRAS mutations in DNA extracted from freshly frozen 264 primary- and 22 metastatic lesions. We found that 14.7% of primary tumours harboured mutations in exon 2 of the KRAS gene. Mutations of KRAS were significantly more often present in grade 1 and 2 tumours, the endometrioid subtype and among obese patients, but no other significant associations were seen between mutational status for KRAS and any of the other variables investigated (Table 3). In line with this, KRAS mutations did not influence prognosis (P=0.67) (Figure 3A). When comparing primary to metastatic endometrial carcinoma lesions, we find no increase in the proportion of samples with KRAS mutations. Contrasting the findings for KRAS amplifications, patients with KRAS mutations have lower KRAS mRNA expression than patients without KRAS mutations in exon 2 (P=0.035) (Figure 3B). Although not significantly anti-correlated, only one of the 39 KRAS-mutated samples was KRAS amplified. Interestingly, we find that only 3 of 36 patients harbouring PIK3CA mutations had overlapping KRAS mutations (Table 3). Mutation of KRAS was not correlated to p53 expression.
Table 3. Clinico-pathological variables correlated to status for KRAS mutation for 264 patients (Sanger sequencing).
| Variable | KRASmutated, n (%) | KRAS wt, n (%) | P -value a |
|---|---|---|---|
| Age | |||
| ⩽66 | 23 (16.1) | 120 (83.9) | 0.3 |
| >66 | 16 (13.2) | 105 (86.8) | |
| BMI b | |||
| ⩽25 | 8 (9.8) | 74 (90.2) | 0.02 |
| >25 | 29 (20.7) | 111 (79.3) | |
| FIGO stage c | |||
| I–II | 30 (13.6) | 185 (86.4) | 0.4 |
| III–IV | 9 (18.4) | 40 (81.6) | |
| Histological type | |||
| Endometroid | 37 (17.1) | 179 (82.9) | 0.01 |
| Non-endometroid | 2 (4.2) | 46 (95.8) | |
| Grade d | |||
| Low-medium | 31 (18.5) | 137 (81.9) | 0.003 |
| High | 8 (7.5) | 86 (92.5) | |
| Lymph node e | |||
| Negative | 27 (14.4) | 161 (85.6) | 0.45 |
| Positive | 6 (21.4) | 22 (78.6) | |
| Ploidy f | |||
| Diploid | 25 (14.8) | 144 (85.2) | 0.5 |
| Aneuploid | 7 (13.5) | 45 (86.5) | |
| ERα g | |||
| Positive | 28 (15.5) | 153 (84.5) | 0.4 |
| Negative | 10 (17.5) | 47 (82.5) | |
| PR h | |||
| Positive | 30 (16.9) | 148 (83.1) | 0.3 |
| Negative | 8 (15.8) | 55 (87.3) | |
| PIK3CA mut i | |||
| N.m.d | 31 (14.6) | 181 (85.4) | 0.2 |
| Mutated | 3 (8.3) | 33 (91.7) | |
| P53 j | |||
| High | 7 (12.7) | 48 (87.3) | 0.3 |
| Low | 26 (17.3) | 124 (82.7) | |
Abbreviations: BMI=body mass index; ER=oestrogen receptor; FIGO=International Federation of Gynaecology and Obstetrics; KRAS=Homo sapiens v-Ki-ras2 Kirsten rat sarcoma viral oncogenes homologue; N.m.d=no mutations detected; PR=progesterone receptor.
Data missing: c42, d2, e48, f25, g26, h23, i16, j59.
χ2-test.
FIGO 2009 criteria.
Figure 3.
Estimated disease-specific survival according to KRAS mutation status in endometrial carcinoma primary tumours with numbers of cases (events) for each category (A). Box-plots showing KRAS mRNA expression levels by qPCR in relation to KRAS mutation status (Mann–Whitney U-test) (B).
Table 4. Genes significantly differentially expressed in patients with amplified KRAS compared with non-amplified patients (FDR ⩽0.05).
| Gene name | Description | Fold change |
|---|---|---|
| Upregulated >1.5 fold | ||
| C6orf117 | Chromosome 6 open reading frame 117 | 3.3 |
| ETS2 | V-ets erythroblastosis virus E26 oncogene homologue 2 | 2.2 |
| Downregulated >1.5 fold | ||
| LMO1 | LIM domain only 1 | −3.8 |
| CRTAC1 | Cartilage acidic protein 1 | −2.5 |
| SOX11 | (Sex determining region Y)-box 11 | −1.8 |
| UGT2A3 | UDP glucuronosyltransferase 2 family, polypeptide A3 | −1.6 |
| FABP1 | Fatty acid-binding protein 1 | −1.5 |
Discussion
Alterations of KRAS are considered to be an important biological factor in several cancer types (Pylayeva-Gupta et al, 2011). Over the last decade, the main focus has been on KRAS mutation as a predictive marker for response to EGFR inhibition (Pao et al, 2005; Lievre et al, 2006). In colorectal- and non-small-cell lung cancers, KRAS mutations have been reported to be associated with poor prognosis (Rosell et al, 1993; Span et al, 1996; Fukuyama et al, 1997). Mutation of KRAS has also been linked to polypoid growth in colorectal cancer (Chiang et al, 1998).
In endometrial cancer, it is mainly KRAS mutations that previously have been studied in relation to clinical phenotype (Mizuuchi et al, 1992; Ito et al, 1996; Esteller et al, 1997; Jones et al, 1997; Semczuk et al, 1998). Several studies have shown that KRAS mutations may be present in endometrial hyperplasia’s with atypia, presumed to be precursor lesions, suggesting mutations as an early event in the endometrial carcinogenesis (Mutter et al, 1999). The prognostic importance of KRAS mutational status in endometrial carcinomas has been inconsistent. Two studies reported a 14% mutation rate with no prognostic impact (Esteller et al, 1997; Semczuk et al, 1998), apparently in line with our data. In contrast, Ito et al (1996) showed that 18% of 221 studied endometrial cancer patients had KRAS mutations associated with lymph-node metastasis and poor survival among patients above 60 years of age (Ito et al, 1996). Their reported mutation rate is in line with our findings, but our higher frequency amongst endometrioid grade 1 and 2 tumours, and the same frequency of mutations detected in primary and metastatic lesions in the present study is in contrast to their findings but more in line with earlier studies linking KRAS mutations to early steps in endometrial carcinogenesis (Pappa et al, 2006).
Interestingly, in the present and to date most comprehensive study of KRAS alterations in primary and metastatic lesions from endometrial carcinoma patients, we find that high KRAS mRNA expression and KRAS amplification, in contrast to KRAS mutation, are associated with a large range of surrogate markers for unfavourable outcome and poor disease-specific survival. Apparently in line with this, we find a trend towards lower KRAS mRNA expression among KRAS-mutated cases, while samples with KRAS amplifications have significantly higher levels of KRAS mRNA expression and aggressive phenotype. Also the fact that mRNA expression levels and KRAS amplification increased significantly from primary- to metastatic lesions suggests an importance of theses alterations later in the carcinogenic process compared with KRAS mutations.
Of the differentially expressed genes in patients harbouring KRAS amplifications it is interesting that upregulation of Ets2 has been associated with poor prognosis in both pancreatic and breast cancer (Zhang et al, 2011; McBryan et al, 2012), and downregulation of SOX11 have been associated with poor prognosis in ovarian cancer (Sernbo et al, 2011). However, more research needs to be done to elucidate KRAS-dependent gene expression regulation in endometrial cancer, which eventually could lead to new KRAS-targeted therapies.
To date, comprehensive genetic profiling of primary lesions searching for potential targets for new therapeutics, have led to only a few biomarker restricted clinical trials, of which some with promising results (Janku et al, 2012). Still, in a setting with systemic disease, molecular alterations in metastatic lesions may be even more important, although so far basically unexplored for KRAS status in endometrial cancers. Our findings support that KRAS amplification and overexpression are more prevalent in metastatic compared with primary lesions, and may be of particular relevance for targeting therapies in a metastatic setting.
Our data clearly suggest that KRAS alterations are linked to clinical phenotypes in endometrial carcinomas with increase in copy-number and mRNA expression levels from primary to metastatic lesions.
Acknowledgments
We thank Britt Edvardsen, Mari Kyllesø Halle, Bendik Nordanger, Antje Krohn, Stian Knappskog and Hua My Hoang for technical assistance. This study was supported by Helse Vest, the University of Bergen, the Norwegian Cancer Society (Harald Andersen grant), and the Research Council of Norway.
Footnotes
Supplementary Information accompanies the paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Cepero V, Sierra JR, Corso S, Ghiso E, Casorzo L, Perera T, Comoglio PM, Giordano S (2010) MET and KRAS gene amplification mediates acquired resistance to MET tyrosine kinase inhibitors. Cancer Res 70(19): 7580–7590 [DOI] [PubMed] [Google Scholar]
- Chiang JM, Chou YH, Chou TB (1998) K-ras codon 12 mutation determines the polypoid growth of colorectral cancer. Cancer Res 58(15): 3289–3293 [PubMed] [Google Scholar]
- Dedes KJ, Wetterskog D, Ashworth A, Kaye SB, Reis-Filho JS (2011) Emerging therapeutic targets in endometrial cancer. Nat Rev Clin Oncol 8(5): 261–271 [DOI] [PubMed] [Google Scholar]
- Dobrzycka B, Terlikowski SJ, Mazurek A, Kowalczuk O, Niklinska W, Chyczewski L, Kulikowski M (2009) Mutations of the KRAS oncogene in endometrial hyperplasia and carcinoma. Folia Histochem Cytobiol 47(1): 65–68 [DOI] [PubMed] [Google Scholar]
- Engelsen IB, Stefansson I, Akslen LA, Salvesen HB (2006) Pathologic expression of p53 or p16 in preoperative curettage specimens identifies high-risk endometrial carcinomas. Am J Obstet Gynecol 195(4): 979–986 [DOI] [PubMed] [Google Scholar]
- Engelsen IB, Stefansson IM, Akslen LA, Salvesen HB (2008) GATA3 expression in estrogen receptor alpha-negative endometrial carcinomas identifies aggressive tumors with high proliferation and poor patient survival. Am J Obstet Gynecol 199(5): 543 e1–543 e7 [DOI] [PubMed] [Google Scholar]
- Esteller M, Garcia A, Martinez-Palones JM, Xercavins J, Reventos J (1997) The clinicopathological significance of K-RAS point mutation and gene amplification in endometrial cancer. Eur J Cancer 33(10): 1572–1577 [DOI] [PubMed] [Google Scholar]
- FIGO IFoGaO (1989) Corpus cancer staging. Int J Gynecol Obstet 28: 189–190 [Google Scholar]
- Fujimoto T, Nanjyo H, Fukuda J, Nakamura A, Mizunuma H, Yaegashi N, Sugiyama T, Kurachi H, Sato A, Tanaka T (2009) Endometrioid uterine cancer: histopathological risk factors of local and distant recurrence. Gynecol Oncol 112(2): 342–347 [DOI] [PubMed] [Google Scholar]
- Fukuyama Y, Mitsudomi T, Sugio K, Ishida T, Akazawa K, Sugimachi K (1997) K-ras and p53 mutations are an independent unfavourable prognostic indicator in patients with non-small-cell lung cancer. Br J Cancer 75(8): 1125–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautschi O, Huegli B, Ziegler A, Gugger M, Heighway J, Ratschiller D, Mack PC, Gumerlock PH, Kung HJ, Stahel RA, Gandara DR, Betticher DC (2007) Origin and prognostic value of circulating KRAS mutations in lung cancer patients. Cancer Lett 254(2): 265–273 [DOI] [PubMed] [Google Scholar]
- Ito K, Watanabe K, Nasim S, Sasano H, Sato S, Yajima A, Silverberg SG, Garrett CT (1996) K-ras point mutations in endometrial carcinoma: effect on outcome is dependent on age of patient. Gynecol Oncol 63(2): 238–246 [DOI] [PubMed] [Google Scholar]
- Janku F, Wheler JJ, Westin SN, Moulder SL, Naing A, Tsimberidou AM, Fu S, Falchook GS, Hong DS, Garrido-Laguna I, Luthra R, Lee JJ, Lu KH, Kurzrock R (2012) PI3K/AKT/mTOR inhibitors in patients with breast and gynecologic malignancies harboring PIK3CA mutations. J Clin Oncol 30(8): 777–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MW, Kounelis S, Hsu C, Papadaki H, Bakker A, Swalsky PA, Finkelstein SD (1997) Prognostic value of p53 and K-ras-2 topographic genotyping in endometrial carcinoma: a clinicopathologic and molecular comparison. Int J Gynecol Pathol 16(4): 354–360 [DOI] [PubMed] [Google Scholar]
- Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP (1998) Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 4(7): 844–847 [DOI] [PubMed] [Google Scholar]
- Krakstad C, Trovik J, Wik E, Engelsen IB, Werner HM, Birkeland E, Raeder MB, Oyan AM, Stefansson IM, Kalland KH, Akslen LA, Salvesen HB (2012) Loss of GPER identifies new targets for therapy among a subgroup of ERalpha-positive endometrial cancer patients with poor outcome. Br J Cancer 106(10): 1682–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lax SF, Pizer ES, Ronnett BM, Kurman RJ (1998) Clear cell carcinoma of the endometrium is characterized by a distinctive profile of p53, Ki-67, estrogen, and progesterone receptor expression. Hum Pathol 29(6): 551–558 [DOI] [PubMed] [Google Scholar]
- Lievre A, Bachet JB, Le Corre D, Boige V, Landi B, Emile JF, Cote JF, Tomasic G, Penna C, Ducreux M, Rougier P, Penault-Llorca F, Laurent-Puig P (2006) KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res 66(8): 3992–3995 [DOI] [PubMed] [Google Scholar]
- Ling J, Kang Y, Zhao R, Xia Q, Lee DF, Chang Z, Li J, Peng B, Fleming JB, Wang H, Liu J, Lemischka IR, Hung MC, Chiao PJ (2012) KrasG12D-induced IKK2/beta/NF-kappaB activation by IL-1alpha and p62 feedforward loops is required for development of pancreatic ductal adenocarcinoma. Cancer Cell 21(1): 105–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBryan J, Theissen SM, Byrne C, Hughes E, Cocchiglia S, Sande S, O'Hara J, Tibbitts P, Hill AD, Young LS (2012) Metastatic progression with resistance to aromatase inhibitors is driven by the steroid receptor coactivator SRC-1. Cancer Res 72(2): 548–559 [DOI] [PubMed] [Google Scholar]
- Mikuta JJ (1993) International Federation of Gynecology and Obstetrics staging of endometrial cancer 1988. Cancer 71(4 Suppl): 1460–1463 [DOI] [PubMed] [Google Scholar]
- Mizuuchi H, Nasim S, Kudo R, Silverberg SG, Greenhouse S, Garrett CT (1992) Clinical implications of K-ras mutations in malignant epithelial tumors of the endometrium. Cancer Res 52(10): 2777–2781 [PubMed] [Google Scholar]
- Mutter GL, Wada H, Faquin WC, Enomoto T (1999) K-ras mutations appear in the premalignant phase of both microsatellite stable and unstable endometrial carcinogenesis. Mol Pathol 52(5): 257–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oza AM, Eisenhauer EA, Elit L, Cutz JC, Sakurada A, Tsao MS, Hoskins PJ, Biagi J, Ghatage P, Mazurka J, Provencher D, Dore N, Dancey J, Fyles A (2008) Phase II study of erlotinib in recurrent or metastatic endometrial cancer: NCIC IND-148. J Clin Oncol 26(26): 4319–4325 [DOI] [PubMed] [Google Scholar]
- Pao W, Wang TY, Riely GJ, Miller VA, Pan Q, Ladanyi M, Zakowski MF, Heelan RT, Kris MG, Varmus HE (2005) KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med 2(1): e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappa KI, Choleza M, Markaki S, Giannikaki E, Kyroudi A, Vlachos G, Voulgaris Z, Anagnou NP (2006) Consistent absence of BRAF mutations in cervical and endometrial cancer despite KRAS mutation status. Gynecol Oncol 100(3): 596–600 [DOI] [PubMed] [Google Scholar]
- Pylayeva-Gupta Y, Grabocka E, Bar-Sagi D (2011) RAS oncogenes: weaving a tumorigenic web. Nat Rev Cancer 11(11): 761–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachagani S, Senapati S, Chakraborty S, Ponnusamy MP, Kumar S, Smith LM, Jain M, Batra SK (2011) Activated Kras(G12D) is associated with invasion and metastasis of pancreatic cancer cells through inhibition of E-cadherin. Br J Cancer 104(6): 1038–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosell R, Li S, Skacel Z, Mate JL, Maestre J, Canela M, Tolosa E, Armengol P, Barnadas A, Ariza A (1993) Prognostic impact of mutated K-ras gene in surgically resected non-small cell lung cancer patients. Oncogene 8(9): 2407–2412 [PubMed] [Google Scholar]
- Salvesen HB, Carter SL, Mannelqvist M, Dutt A, Getz G, Stefansson IM, Raeder MB, Sos ML, Engelsen IB, Trovik J, Wik E, Greulich H, Bo TH, Jonassen I, Thomas RK, Zander T, Garraway LA, Oyan AM, Sellers WR, Kalland KH, Meyerson M, Akslen LA, Beroukhim R (2009) Integrated genomic profiling of endometrial carcinoma associates aggressive tumors with indicators of PI3 kinase activation. Proc Natl Acad Sci USA 106(12): 4834–4839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvesen HB, Das S, Akslen LA (2000) Loss of nuclear p16 protein expression is not associated with promoter methylation but defines a subgroup of aggressive endometrial carcinomas with poor prognosis. Clin Cancer Res 6(1): 153–159 [PubMed] [Google Scholar]
- Sasaki H, Hikosaka Y, Kawano O, Moriyama S, Yano M, Fujii Y (2011) Evaluation of Kras gene mutation and copy number gain in non-small cell lung cancer. J Thorac Oncol 6(1): 15–20 [DOI] [PubMed] [Google Scholar]
- Semczuk A, Berbec H, Kostuch M, Cybulski M, Wojcierowski J, Baranowski W (1998) K-ras gene point mutations in human endometrial carcinomas: correlation with clinicopathological features and patients’ outcome. J Cancer Res Clin Oncol 124(12): 695–700 [DOI] [PubMed] [Google Scholar]
- Sernbo S, Gustavsson E, Brennan DJ, Gallagher WM, Rexhepaj E, Rydnert F, Jirstrom K, Borrebaeck CA, Ek S (2011) The tumour suppressor SOX11 is associated with improved survival among high grade epithelial ovarian cancers and is regulated by reversible promoter methylation. BMC Cancer 11: 405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Span M, Moerkerk PT, De Goeij AF, Arends JW (1996) A detailed analysis of K-ras point mutations in relation to tumor progression and survival in colorectal cancer patients. Int J Cancer 69(3): 241–245 [DOI] [PubMed] [Google Scholar]
- Trovik J, Wik E, Stefansson IM, Marcickiewicz J, Tingulstad S, Staff AC, Njolstad TS, Vandenput I, Amant F, Akslen LA, Salvesen HB (2011) Stathmin overexpression identifies high-risk patients and lymph node metastasis in endometrial cancer. Clin Cancer Res 17(10): 3368–3377 [DOI] [PubMed] [Google Scholar]
- Wagner PL, Perner S, Rickman DS, LaFargue CJ, Kitabayashi N, Johnstone SF, Weir BA, Meyerson M, Altorki NK, Rubin MA (2009) In situ evidence of KRAS amplification and association with increased p21 levels in non-small cell lung carcinoma. Am J Clin Pathol 132(4): 500–505 [DOI] [PubMed] [Google Scholar]
- Wagner PL, Stiedl AC, Wilbertz T, Petersen K, Scheble V, Menon R, Reischl M, Mikut R, Rubin MA, Fend F, Moch H, Soltermann A, Weder W, Altorki NK, Perner S (2011) Frequency and clinicopathologic correlates of KRAS amplification in non-small cell lung carcinoma. Lung Cancer 74(1): 118–123 [DOI] [PubMed] [Google Scholar]
- Zeng M, Kikuchi H, Pino MS, Chung DC (2010) Hypoxia activates the K-ras proto-oncogene to stimulate angiogenesis and inhibit apoptosis in colon cancer cells. PLoS One 5(6): e10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JS, Koenig A, Harrison A, Ugolkov AV, Fernandez-Zapico ME, Couch FJ, Billadeau DD (2011) Mutant K-Ras increases GSK-3beta gene expression via an ETS-p300 transcriptional complex in pancreatic cancer. Oncogene 30(34): 3705–3715 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.