Abstract
Background:
Gestational trophoblastic diseases (GTDs) are related to trophoblasts, and human chorionic gonadotropin (hCG) is secreted by GTDs as well as normal placentas. However, the asparagine-linked sugar chains on hCG contain abnormal biantennary structures in invasive mole and choriocarcinoma, but not normal pregnancy or hydatidiform mole. N-acetylglucosaminyltransferase-IV (GnT-IV) catalyses β1,4-N-acetylglucosamine branching on asparagine-linked oligosaccharides, which are consistent with the abnormal sugar chain structures on hCG.
Methods:
We investigated GnT-IVa expression in GTDs and placentas by immunohistochemistry, western blot, and RT–PCR. We assessed the effects of GnT-IVa knockdown in choriocarcinoma cells in vitro and in vivo.
Results:
The GnT-IVa was highly expressed in trophoblasts of invasive mole and choriocarcinoma, and moderately in extravillous trophoblasts during the first trimester, but not in hydatidiform mole or other normal trophoblasts. The GnT-IVa knockdown in choriocarcinoma cells significantly reduced migration and invasive capacities, and suppressed cellular adhesion to extracellular matrix proteins. The extent of β1,4-N-acetylglucosamine branching on β1 integrin was greatly reduced by GnT-IVa knockdown, although the expression of β1 integrin was not changed. In vivo studies further demonstrated that GnT-IVa knockdown suppressed tumour engraftment and growth.
Conclusion:
These findings suggest that GnT-IVa is involved in regulating invasion of choriocarcinoma through modifications of the oligosaccharide chains of β1 integrin.
Keywords: beta1 integrin, choriocarcinoma, human chorionic gonadotropin, invasion, N-acetylglucosaminyltransferase-IV
Gestational trophoblastic diseases (GTDs) are a spectrum of cellular proliferations arising from placental villous trophoblasts and include hydatidiform mole and tumours that arise from trophoblasts, such as invasive mole, choriocarcinoma, and placental site trophoblastic tumour (PSTT; World Health Organization., 1983). Hydatidiform moles are not tumours but abnormal conceptuses caused by genetic fertilisation disorders, and are classified into two entities as follows: complete hydatidiform mole, which is androgenetic in origin, and partial hydatidiform mole, which is mostly a dispermic triploid (Kajii and Ohama, 1977). Invasive mole is a benign tumour, which occurs after 10–20% of complete hydatidiform mole and 1–2% partial hydatidiform mole (Goto et al, 1993). However, chemotherapy is needed for treatment of invasive mole because the tumour arises from myometrial invasion of molar villi, and 15–40% of patients show metastasis to lung or vagina (Soper, 2006; Lurain, 2010). Choriocarcinoma is a malignant epithelial tumour that is associated with all kinds of pregnancies, but tends to develop from hydatidiform mole more than normal delivery and abortion (Lurain, 2010). PSTT is a rare malignant tumour arising from intermediate trophoblasts in placental site (Shih and Kurman, 1998). Although recently all invasive moles can be in primary remission, 1–3% of invasive moles have recurrence and the survival rate of choriocarcinoma and PSTT are about 85% and 70%, respectively (Khan et al, 2003; Schmid et al, 2009). These indicates that invasive mole is a pre-malignant disease, and hydatidiform mole has a greater potential to develop into a malignancy than normal pregnancy, but the mechanism by which trophoblasts become malignant remains unclear.
Human chorionic gonadotropin (hCG) is a glycoprotein hormone that is produced by syncytiotrophoblasts of human placentas as well as GTDs. Human chorionic gonadotropin is a heterodimer composed of the αhCG and βhCG subunits. The αhCG subunit is asparagine-linked (N-linked) glycosylated at Asn-52 and Asn-78, and the βhCG subunit contains two N-linked sugar chains at Asn-13 and Asn-30, and four serine-linked (O-linked) sugar chains at Ser-121, Ser-127, Ser-132, and Ser-138 (Cole et al, 1984; Kobata and Takeuchi, 1999). The N-linked sugar chains of hCG in normal pregnancy and hydatidiform mole contained monoantennary, biantennary, and fucosylated biantennary, but abnormal biantennary sugar chains were added in choriocarcinoma and a triantennary sugar chain added in choriocarcinoma and invasive mole (Mizuochi et al, 1983; Endo et al, 1987).
N-acetylglucosaminyltransferase IV (GnT-IV), which transfers an N-acetylglucosamine (GlcNAc) group to the core α1,3mannose of N-glycans forming a β1-4 linkage, can act on biantennary sugar chains and generate the triantennary sugar chains on hCG in invasive mole and choriocarcinoma. The activities and mRNA expression levels of glycosyltransferases (GnT-I to -V, β1-4galactosyltransferase, and α-mannosidase II) were examined in placentas and choriocarcinoma, and the GnT-IV activities and the GnT-IVa mRNA level in the choriocarcinoma cell lines were significantly higher than in the normal placentas (Takamatsu et al, 1999, 2004). However, no study has examined GnT-IVa protein expression and localisation in GTDs compared with normal human placentas or the function of GnT-IVa in trophoblasts.
In this study, we examined GnT-IVa expression in trophoblastic cells of GTDs and normal placentas, and the role of GnT-IVa in trophoblastic cells, especially in choriocarcinoma.
Materials and methods
Tissue collection and processing
Informed consent was obtained from patients for the use of placental samples and GTD tissue specimens. First-trimester and early second-trimester placentas were obtained from women undergoing elective pregnancy terminations. Full-term placental samples were collected during elective Caesarean sections before the onset of labour. The GTD tissues were obtained from patients who underwent surgical treatment, and the specimens were classified based on their histopathological characteristics. None of the patients had received chemotherapy for the disease before surgery. All tissue samples were fixed in 10% formaldehyde, embedded in paraffin, and routinely stained with haematoxylin and eosin for histological examination. Some of the tissue samples were washed with phosphate-buffered saline (PBS), frozen in liquid nitrogen immediately after removal, and then stored at −80 °C until protein extraction. This study was approved by the ethics committee of Nagoya University Graduate School of Medicine.
Immunohistochemistry
Immunohistochemical staining was performed using the avidin−biotin immunoperoxidase technique. Sections (4-μm-thick) were immunostained as previously described (Yamamoto et al, 2005), using an anti-GnT-IVa Ab (M-71; Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted 1 : 400 and anti-hCG Ab (N1534; DAKO, Carpinteria, CA, USA). To evaluate the expression level of GnT-IVa in GTDs, the specimens of hydatidiform mole (n=11), invasive mole (n=4), choriocarcinoma (n=8), and PSTT (n=3) were used. The GnT-IVa expression levels were classified semiquantitatively, based on the total scores of the per cent positivity of stained tumour cells and the staining intensity. Namely, the per cent positivity was scored as 0 if <5% (negative), 1 if 5–20%, 2 if 20–50%, 3 if 50–70%, and 4 if >70% of cells stained.
Cell lines and culture
Human choriocarcinoma cell lines (Jar, BeWo, and JEG-3) were purchased from the American Type Culture Collection (Manassas, VA, USA). The NaUCC (CC)-1, CC-3, CC-4, and CC-6 are human choriocarcinoma cell lines that were previously established in our laboratory (Ino et al, 1991). The human extravillous trophoblast (EVT) cell line HTR-8/SVneo was kindly provided by Dr Charles H Graham (Graham et al, 1993). All cell lines were grown in RPMI 1640 (Sigma, St Louis, MO, USA), supplemented with 10% FCS, penicillin (100 U ml−1), streptomycin (100 μg ml−1), and 2 mℳ glutamine. Cultures were incubated at 37 °C in 5% CO2.
Western blot analysis
Western blot analysis for GnT-IVa protein was performed as previously described (Yamamoto et al, 2007), with an anti-GnT-IVa mAb (M-71; Santa Cruz Biotechnology) diluted 1 : 1000. Immunoreactive proteins were stained using a chemiluminescence detection system (ECL; Amersham, Arlington Heights, IL, USA). An Ab against β-actin (AC-15; Sigma) was used to standardise the protein loading.
RNA extraction and quantitative RT–PCR
Total RNA extraction and quantitative real-time PCR were performed as previously described (Mano et al, 2009). We used the following primers for GnT-IVa: forward primer, 5′-ACCAAGGGCATACGCTGGAG-3′ reverse primer, 5′-GTTCTTGGTTGCCGCTATGGA-3′, and the following primers for GAPDH: forward primer, 5′-CGGGAAACTGTGGCGTGAT-3′ reverse primer, 5′-ATGCCAGTGAGCTTCCCGT-3'. The PCR profile was an initial incubation at 95 °C for 10 s, followed by 45 cycles of denaturation at 95 °C for 5 s, and annealing and extension at 60 °C for 30 s.
Datura stramonium agglutinin blot analysis
Lectin blot analysis was performed as previously reported (Yamamoto et al, 2009), using HRP-labelled Datura stramonium agglutinin (DSA; Seikagaku, Tokyo, Japan) diluted 1 : 2000, and DSA recognises β1-4GlcNAc branching.
Silencing of GnT-IVa by small interfering RNA and short hairpin RNA transfection
Small interfering RNAs (siRNAs) were designed and synthesised by Nippon EGT (Toyama, Japan) to target GnT-IVa (siRNA1: 5′-AGAUGGCUAUUUCAGAAUATT-3′ and 5′-UAUUUCUGAAAUAGCCAUCUTT-3′ siRNA2: 5′-GAAGAUGGCUAUUUCAGAATT-3′ and 5′-UUCUGAAAUAGCCAUCUUCTT-3′). The non-targeting siRNAs (Nippon EGT) were used as a control (control siRNA: 5′-GGAUUAUUACGCAGUUAAATT-3′ and 5′-UUUAACUGCGUAAUAAUCCTT-3′). Jar cells were grown in 60-mm plates to 60% confluency and then transfected with 60 pmol of siRNAs using Lipofectamine RNAiMAX transfection reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. After treating Jar cells with siRNAs (siRNA1, siRNA2, and control siRNA) for 8 h, the medium containing the siRNAs and transfection reagents were removed and cells were cultured in fresh culture medium for at least 24 h before each experiment. Transfected cells were cultured for 24 h before adhesion assay and immunoprecipitation, and for 48 h before zymography and hCG assay.
To produce stable GnT-IVa knockdown cells for in vivo studies, the oligonucleotide sequences designed by Block-it RNAi Designer (Invitrogen) in the construction of the short hairpin RNA (shRNA) vector were as follows: 5′-CACCGCTATTGTATGAGTCATAATTCGAAAATTATGACTCATACAATAGC-3′ and 5′-AAAAGVTATTGTATGAGTCATAATTTTCGAATTATGACTGATACAATAGC-3′. The shRNA vector was synthesised by Invitrogen using the oligonucleotides and pENTR/H1/TO (Invitrogen). The vector plasmid was transfected into Jar cells using Lipofectamine 2000 reagent (Invitrogen) and selected by adding zeosin. The original pENTR/H1/TO vector was used as a control shRNA.
In vitro cell proliferation assay
Cells (5 × 103) were plated in 100 μl of medium in 96-well plates and incubated for 72 h at 37 °C. Cell viability was determined using the modified tetrazolium salt assay using the Cell Titer 96 Aqueous One Solution Proliferation Assay kit (Promega, Madison, WI, USA) according to the manufacturer’s instructions. The mean values of three independent experiments performed in eight wells are shown.
Transwell migration and invasion assay
The migration and invasion assay was performed as previously reported (Yamamoto et al, 2005). Jar cells were transfected with siRNA 24 h before seeding for the migration and invasion assays, and both assays were performed after 24 h incubation. The number of cells was counted under a microscope at × 200 magnification. Data were obtained from three individual experiments performed in triplicate.
Zymography and cell adhesion assay
Cells (1 × 105) were plated in a 24-well chamber and incubated with serum-free medium for 48 h after siRNA transfection. Zymography was performed as previously reported (Yamamoto et al, 2009).
Cells (4 × 104) were plated in 96-well plates coated with fibronectin, collagen type I or type IV (Becton, Dickinson and Company, Franklin Lakes, NJ, USA), centrifuged at 1500 r.p.m. for 15 s, and allowed to attach to each matrix at 37 °C for 30 min. After washing with PBS, absorbance readings at 492 nm (A492 nm) were performed using a microplate reader (Multiskan Bichromatic; Labsystems, Helsinki, Finland). The rate of cell adherence was calculated as follows: (A492 nm (matrix)−A492 nm (no matrix))/A492 nm (no matrix) (Inamori et al, 2006; Yamamoto et al, 2009). Data were obtained from three individual experiments performed in eight wells.
Lectin blot analysis on immunoprecipitated β hCG
Immunoprecipitation was performed using 1.5 mg of protein that was extracted from cells with an anti-human βhCG mAb (HCG-60; Santa Cruz Biotechnology) or β1 integrin mAb (BV7; Abcam, Cambridge, UK), as previously described (Yamamoto et al, 2007). The anti-βhCG mAb and anti-β1 integrin mAb (MAB2247; Chemicon International, Temecula, CA, USA) were used at a dilution of 1 : 200 and 1 : 1000, respectively, and western blotting and DSA lectin blotting were performed using the above protocol.
Human chorionic gonadotropin assay
Hormone assays were performed on culture supernatant. Cells (5 × 104) were plated in a 24-well chamber and incubated with 1 ml serum-free medium for 48 h after siRNA transfection. The total hCG levels were quantified in triplicate by enzyme immunoassay using an αhCG mAb and a βhCG-CTP mAb (SRL Inc., Tokyo, Japan).
In vivo studies
Female BALB/c slc nu/nu mice (5 weeks old) were purchased from Japan SLC (Nagoya, Japan).The treatment protocol followed the guidelines for animal experimentation adopted by Nagoya University. Cells (5 × 106) per 0.2 ml of PBS/mouse were injected subcutaneously on the right flank to examine implantation and survival analysis by GnT-IVa stable knockdown. Each group consisted of seven mice. The overall survival was defined as the time between the date of inoculation and the date of death due to tumour.
Statistical analysis
The non-parametric Kruskal–Wallis test was performed to compare immunostaining scores among all histological types. Data are expressed as the mean±s.d. For data of in vitro experiments, statistical comparisons among groups were performed using the one-way ANOVA with Bonferroni corrections. Overall survival curves were analysed by the log-rank test. Differences were considered significant when P<0.05.
Results
Immunohistochemical expression of GnT-IVa in GTD and placenta
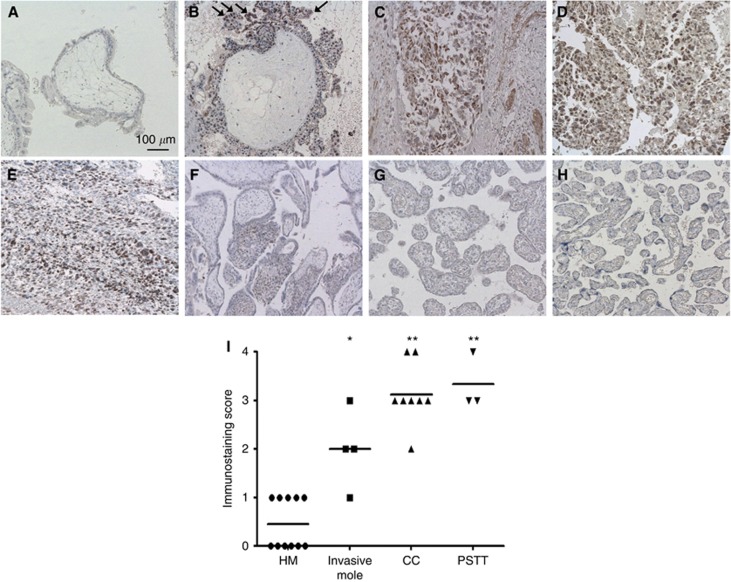
We first examined GnT-IVa protein localisation in GTDs (hydatidiform mole, invasive mole, choriocarcinoma, and PSTT) and placentas by immunohistochemistry. The GnT-IVa was highly expressed in trophoblasts in invasive mole, choriocarcinoma, and PSTT, but not or very weakly in hydatidiform mole (Figures 1A−E). The staining in trophoblasts of invasive mole was stronger at the edge of the invasive site than other parts (arrows in Figure 1B). In placenta, there was no staining in trophoblasts after the second trimester, and only EVTs moderately expressed GnT-IVa in the first trimester (Figures 1F−H). We examined the immunostaining score of 26 GTD patients, and the medians were 0.5 in hydatidiform mole, 2.0 in invasive mole, 3.1 in choriocarcinoma, and 3.0 in PSTT. These results showed that GnT-IVa expression is significantly higher in invasive mole (P=0.004), choriocarcinoma (P<0.001), and PSTT (P<0.001) compared with hydatidiform mole (Figure 1I).
Figure 1.
Immunostaining of GnT-IVa in GTD and placenta. Immunostaining using an anti-GnT-IVa Ab showed (A) weak or no GnT-IVa expression in molar trophoblasts, but GnT-IVa staining was detected in trophoblasts of (B) invasive mole, and was (C) strongly expressed in the invasive site of the invasive mole and (D) choriocarcinoma. (E) Placental site trophoblastic tumour (PSTT) also expressed GnT-IVa. (F) Immunohistochemical staining of GnT-IVa in a first-trimester placental section showed that GnT-IVa was localised in the cytoplasm of EVTs, but not expressed in other trophoblasts (G) in second- and (H) third-trimester placentas. Arrows indicate the trophoblasts at the edge of the invasive site of the invasive mole showing stronger staining than other parts. Magnification, × 100; scale bar=100 μm. (I) The immunostaining score of GnT-IVa expression in all GTDs (n=26), including hydatidiform mole (n=11), invasive mole (n=4), choriocarcinoma (n=8), and PSTT (n=3). Each bar represents the mean score. Statistical analyses were performed by the Kruskal–Wallis test. HM, hydatidiform mole; CC, choriocarcinoma. *P<0.01, **P<0.001.
The GnT-IVa mRNA and protein expression in various trophoblasts
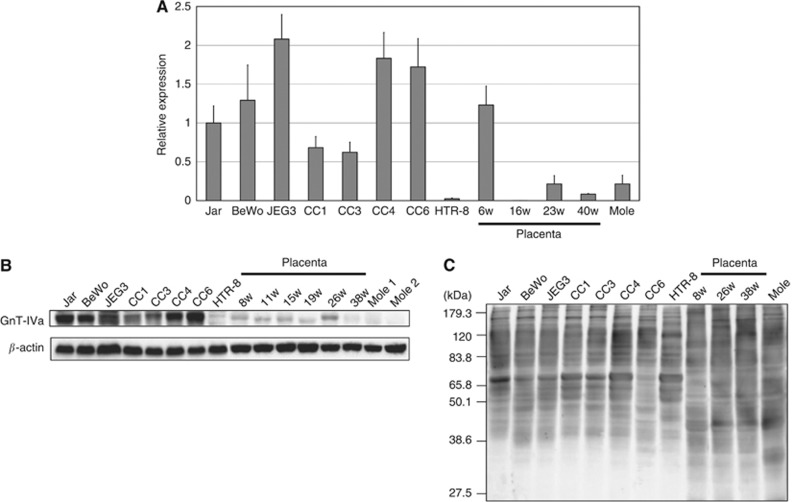
The GnT-IVa mRNA expression was examined in seven choriocarcinoma cell lines, one EVT cell line, placental tissues, and hydatidiform mole tissues by quantitative PCR. The GnT-IVa mRNA was expressed from 0.6- to 2.1-fold higher in choriocarcinoma cell lines compared with Jar cells but 0.02-fold higher in HTR-8/SVneo. In placenta at 6 weeks of gestation, the expression was almost the same in the first trimester as Jar, but the levels in placenta after the second trimester and hydatidiform mole were weak (Figure 2A). In addition, western blot analysis demonstrated that the GnT-IVa protein was detected as a 75-kDa band (Figure 2B) in all choriocarcinoma cell lines but not in hydatidiform mole. HTR-8/SVneo and placental tissues showed very weak expression at the protein level. These results were the same as the results of immunohistochemical analysis.
Figure 2.
The GnT-IVa mRNA and protein expression in various trophoblast cells. (A) Quantitative real-time PCR of GnT-IVa mRNA expression in choriocarcinoma cell lines, HTR-8/SVneo cells, normal placenta, and molar tissues. Each bar represents the mean expression level compared with Jar cells. The data obtained from three separate experiments in triplicate are shown as means±s.d. (B) A representative western blot demonstrating GnT-IVa protein expression in all choriocarcinoma cell lines, and weak or no GnT-IVa expression in HTR-8/SVneo cells, normal placenta, and molar tissues. (C) The DSA lectin blotting, demonstrating the extent of β1-4GlcNAc branching catalysed by GnT-IV. Three independent experiments yielded similar results. HTR8, HTR-8/SVneo.
β 1-4GlcNAc branching in trophoblast cell lines and human placenta
We performed lectin blot analysis on total cellular proteins using DSA to determine the levels of β1-4GlcNAc branching. Glycoproteins from choriocarcinoma cell lines and HTR-8/SVneo stained more strongly with DSA than samples from placenta and hydatidiform mole, and the molecular sizes of the major glycoproteins recognised by DSA were distributed over approximately 40–150 kDa (Figure 2C). This analysis revealed that GnT-IVa target proteins exist in all kinds of trophoblasts, and GnT-IVa produces more β1-4GlcNAc branching in choriocarcinoma and EVT than placenta and hydatidiform mole.
Knockdown of GnT-IVa expression in Jar cells
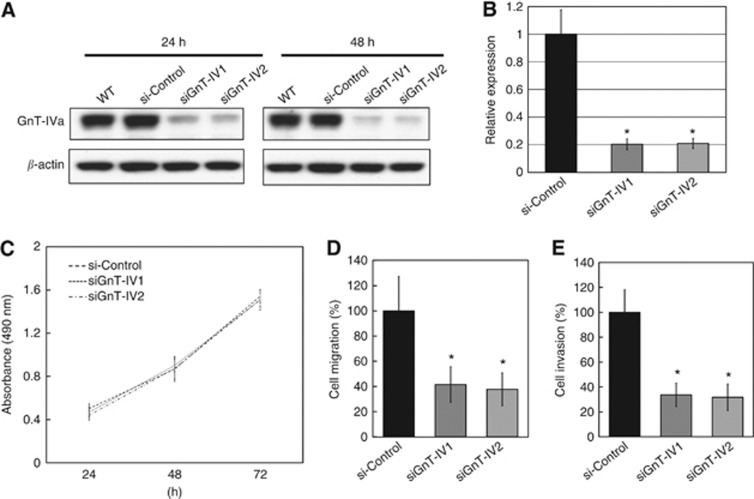
Our results showed that GnT-IVa was expressed strongly in trophoblasts of choriocarcinoma and invasive mole, and moderately in EVTs, and the common characteristic of these cells is invasiveness. To investigate the function of GnT-IVa in trophoblasts, we used siRNA to establish a GnT-IVa knockdown model using Jar cells. The GnT-IVa protein expression in GnT-IVa siRNA transfectants (siGnT-IV1 and siGnT-IV2) was effectively decreased compared with the parental or control siRNA transfectants (si-control) at 24 and 48 h after transfection (Figure 3A). Quantitative RT–PCR analysis also indicated that siGnT-IV1 and siGnT-IV2 suppressed GnT-IVa mRNA expression to 20.3% and 20.9%, respectively, compared with si-control (Figure 3B).
Figure 3.
The siRNA-mediated GnT-IVa knockdown in Jar cells decreased GnT-IVa expression, and migration and invasion abilities. (A) The GnT-IVa protein expression in GnT-IVa siRNA transfectants (siGnT-IV1 and siGnT-IV2) was effectively downregulated compared with parent Jar cells (WT) and control siRNA transfectant (si-control) at 24 h and 48 h after transfection. (B) Quantitative RT–PCR analysis also indicated that siGnT-IV1 and siGnT-IV2 suppressed GnT-IVa mRNA expression compared with si-control. The data obtained from three separate experiments in triplicate are shown as means±s.d. (C) Graphical depiction of the relative absorbance readings after the modified tetrazolium salt (MTS) assays, demonstrating that GnT-IVa knockdown did not affect cell proliferation. The mean values of three independent experiments performed in eight wells are shown. (D) Graphical depiction of data obtained from the migration assays (n=3) and (E) matrigel invasion assays (n=3) after GnT-IVa knockdown, exhibiting the decreases in the relative distances of siGnT-IV1 and siGnT-IV2 compared with si-control. Data were obtained from three individual experiments performed in triplicate. Each bar represents the mean as a percentage of the si-control±s.d. WT, Jar cells; si-Control; control siRNA-transfected cells; siGnT-IV, GnT-IV siRNA-transfected cells. *P<0.01.
Effects of GnT-IVa knockdown on cell proliferation, migration, and invasion
We assessed the effects of GnT-IVa knockdown on cell proliferation by modified tetrazolium salt assay. After GnT-IVa siRNAs treatment, the cell number increased at 72 h of incubation; however, this was not significantly different from the si-control (Figure 3C). The migration assay revealed that decreased GnT-IVa expression reduced the migratory ability in both siGnT-IV1 and siGnT-IV2 cells compared with si-control (41.5±14.0% and 37.7±13.0%, respectively; Figure 3D). In the invasion assay, we confirmed that siGnT-IV1 and siGnT-IV2 had a significantly lower potential to invade compared with the si-control (33.6±9.4% and 31.7±10.6%, respectively; Figure 3E). These results showed that decreased GnT-IVa expression reduced the invasive and migratory capabilities of choriocarcinoma.
Effects of GnT-IVa knockdown on gelatinase activity and cell adhesion to the extracellular matrix
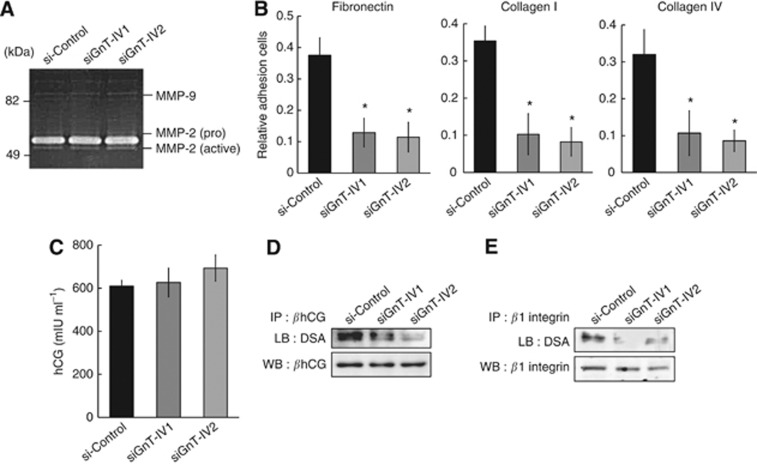
Invasion with trophoblasts and cancer cells is a multistep process involving attachment to a basement membrane or extracellular matrix (ECM) components, followed by degradation and subsequent migration through the degraded components. Type IV collagenases, such as MMP-2 and MMP-9, are thought to be the principal mediators of trophoblast invasion as well as cancer invasion. Hence, we performed gelatin zymography and found that Jar predominantly secrete MMP-2. The GnT-IVa knockdown did not significantly affect the expression of either the pro form (72 kDa) or the active form (62 kDa) of this enzyme (Figure 4A). Next we examined the adherence potential of Jar to ECM proteins after GnT-IVa knockdown, and the rate of cell adherence was calculated as shown in previous reports (Inamori et al, 2006; Yamamoto et al, 2009). The rate of cell attachment to fibronectin, collagen type I, and collagen type IV decreased approximately 0.3-fold by GnT-IVa knockdown (Figure 4B).
Figure 4.
The GnT-IVa knockdown reduces ECM protein adherence and β1-4GlcNAc branching on hCG and β1 integrin in Jar cells. (A) Culture supernatants were separated on a gelatin-embedded 10% polyacrylamide gel. Three independent experiments showed similar results. (B) Graphical depiction of the relative adherence rates of control siRNA (si-control) and GnT-IVa siRNA-transfected cells (siGnT-IV) on fibronectin, collagens type I, and type IV, demonstrating decreased ECM interactions with reduced GnT-IVa expression in siGnT-IV cells. Data were obtained from three individual experiments performed in eight wells. Each bar represents the mean cell adherence rate±s.d. (C) Graphical depiction of the total level of secreted hCG by enzyme immunoassay (EIA), demonstrating that GnT-IVa knockdown did not affect hCG secretion. (D) Representative western blot after βhCG immunoprecipitation from si-control and siGnT-IV cells, followed by assessment of β1-4GlcNAc branching via DSA lectin blotting (upper panel). The membrane was reprobed with specific mAbs to βhCG (lower panel) to verify that equal protein amounts were immunoprecipitated. Three independent experiments showed similar results. (E) Representative western blot after β1 integrin immunoprecipitation from si-control and siGnT-IVa cells, followed by assessment of β1-4GlcNAc branching via DSA lectin blotting (upper panel). The membrane was reprobed with specific mAbs to β1 integrin (lower panel) to verify that equal protein amounts were immunoprecipitated. Three independent experiments showed similar results. Each bar represents the mean distance as a percentage of the si-control±s.d.; si-control, control siRNA transfectant; siGnT-IV, GnT-IVa siRNA transfectants. *P<0.01.
The GnT-IVa knockdown reduced β 1-4GlcNAc branching of β hCG but not hCG secretion
As GnT-IVa is an enzyme that catalyses β1-4GlcNAc addition to hCG sugar chains, we investigated the affect of GnT-IVa knockdown on its abundance in hCG. First we examined the difference in the levels of secreted hCG by enzyme immunoassay, and there were no differences in hCG secretion by Jar cells after GnT-IVa siRNA transfection as well as control siRNA transfection (Figure 4C). Next we examined the level of N-glycan sugar chains by immunoprecipitating with the βhCG antibody followed by lectin blot with DSA, which recognises β1-4GlcNAc branching. The levels of β1-4GlcNAc branching on βhCG in siGnT-IV1 and siGnT-IV2 decreased significantly compared with si-control (Figure 4D, upper panel); however, βhCG expression was not affected by GnT-IVa knockdown (Figure 4D, lower panel).
The GnT-IVa knockdown reduced β 1-4GlcNAc branching of β 1 integrin
To investigate a new target molecule for GnT-IVa, which affected the invasive ability in choriocarcinoma, we examined the glycosylation on β1 integrin because GnT-IVa knockdown decreased the adhesion abilities to ECMs and integrin is a major carrier of N-glycans (Gu et al, 2009). The levels of β1-4GlcNAc branching on β1 integrin were decreased significantly by GnT-IVa knockdown (Figure 4E, upper panel), although β1 integrin expression was the same level in siGnT-IV1 and siGnT-IV2 as si-control (Figure 4E, lower panel). These results suggest that diminished β1-4GlcNAc branching on β1 integrin as a result of GnT-IVa knockdown might be associated with the decreased invasive capacity of choriocarcinoma.
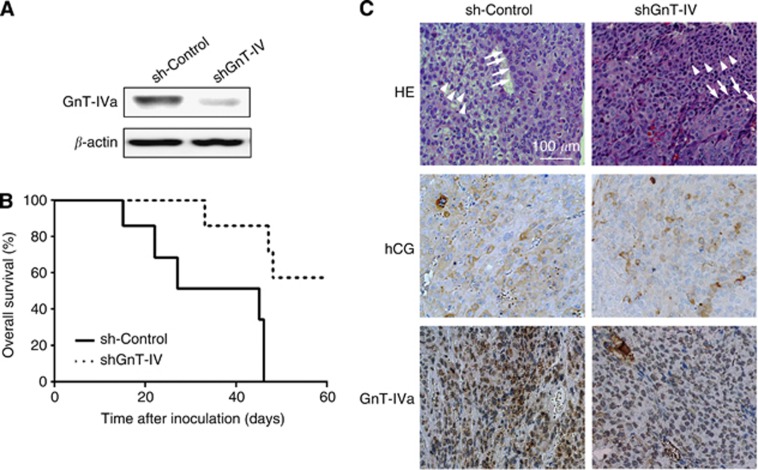
In vivo effects of GnT-IVa knockdown on choriocarcinoma engraftment and growth
To investigate whether the suppression of GnT-IVa expression in Jar could influence the invasive ability in vivo, we established a stable GnT-IVa knockdown model of choriocarcinoma, using an shRNA vector, and analysed the effects on tumorigenicity in nude mice. The GnT-IVa protein expression in GnT-IVa shRNA transfectants (shGnT-IV) was effectively decreased compared with the control shRNA transfectants (sh-control; Figure 5A). We inoculated the cells of sh-control or shGnT-IV subcutaneously into seven nude mice for each group, and sh-control cells developed tumours in all cases. The tumour of sh-control rapidly generated lethally and the mice with sh-control died at 31 days after the inoculation on average. On the other hand, shGnT-IV cells engrafted in only three cases and significantly suppressed growing tumours. The mice with shGnT-IV could survive significantly longer than those with the sh-control (P=0.003, Figure 5B). Histopathological examination revealed that the engraftment tumours contained a two-cell pattern of choriocarcinoma, consisting of syncytiotrophoblastic cells and cytotrophoblastic cells (Figure 5C, upper panels, arrows, and arrowheads), and were positive for hCG in both sh-control and shGnT-IV; however, GnT-IVa staining in tumours of shGnT-IV was weaker than tumours of sh-control (Figure 5C).
Figure 5.
Knockdown of GnT-IVa attenuated the tumorigenic activity of Jar cells. (A) The GnT-IVa protein expression in shGnT-IVa transfectant (shGnT-IV) was effectively downregulated compared with control shRNA transfectant (sh-control). (B) Overall survival curves for the nude mice subcutaneously injected with sh-control cells (n=7) and shGnT-IV cells (n=7), demonstrating that GnT-IVa knockdown promoted survival as compared with the control (P=0.003). (C) Haematoxylin and eosin staining of tumours derived from both sh-control and shGnT-IV cells revealed a two-cell pattern of choriocarcinoma (upper panels), consisting of syncytiotrophoblastic cells (arrows) and cytotrophoblastic cells (arrowheads). Immunohistochemistry revealed that tumours expressed hCG (middle panels) and GnT-IVa (lower panels), and the staining of GnT-IVa in the tumour cells was more weak in shGnT-IV compared with sh-control; sh-control, control shRNA transfectant; shGnT-IV, shGnT-IVa transfectant. Magnification, × 100; scale bar=100 μm.
Discussion
The glycosylation of glycoproteins has a key role in a variety of specific biological interactions (Hakomori, 1989). In particular, branching of N-linked oligosaccharides regulates the metastatic potential of cancer cells (Zhao et al, 2008; Gu et al, 2009). N-acetylglucosaminyltransferase-IV is one of the glycosyltransferases involved in N-glycan biosynthesis and has two isoenzymes, GnT-IVa and GnT-IVb. The GnT-IVb is broadly expressed across various organs at almost the same level and exhibits housekeeping gene-like expression (Yoshida et al, 1999), whereas the GnT-IVa gene is thought to have an essential role in elevated GnT-IV activity. The enzymatic activities and expression levels of GnT-I to -V were examined in normal human placentas and three human choriocarcinoma cell lines, and GnT-IVa mRNA, but not GnT-IVb mRNA, was strongly expressed in all three choriocarcinoma cell lines (Takamatsu et al, 1999). In addition, previous reports have shown that structural alterations in N-glycans of hCG produced by malignant (choriocarcinoma) and pre-malignant trophoblastic disease (invasive mole) could be catalysed by GnT-IV (Mizuochi et al, 1983; Endo et al, 1987). Our results also show that GnT-IVa expresses strongly in malignant and pre-malignant trophoblastic cells, but not in normal or benign (hydatidiform mole) trophoblastic cells. Therefore, we examined the role of GnT-IVa in trophoblastic diseases using choriocarcinoma cells.
Immunoprecipitation with a βhCG antibody and subsequent DSA lectin blot showed that βhCG is a target molecule of GnT-IVa in choriocarcinoma as previous reports suggested. The hCG has many important functions in pregnancy, including the promotion of progesterone production, implantation and decidualisation, angiogenesis, cytotrophoblast differentiation, and immune cell regulation (Norris et al, 2011). Furthermore, there are many hCG variants, and hyperglycosylated-hCG (hCG-H), which is a glycosylation variant of hCG, has been suggested to be involved in regulation of trophoblast invasion (Cole et al, 2006; Cole, 2010). Although regular hCG secreted by syncytiotrophoblasts has monoantennary and biantennary N-linked oligosaccharides, and mostly trisaccharide O-linked oligosaccharides, hCG-H has predominantly larger fucosylated triantennary N-linked oligosaccharides and/or double-sized hexasaccharide O-linked oligosaccharides (Cole, 2010). The N-linked and O-linked hCG-H were detected in the urine of choriocarcinoma patients, but not in normal pregnant women in 1983 and 1985, respectively (Mizuochi et al, 1983; Cole et al, 1985). The function of N-linked hCG-H has not been investigated, whereas it has been reported that O-linked hCG-H is produced in choriocarcinoma and during very early normal pregnancy, and promotes the invasion of choriocarcinoma and EVTs at implantation sites (Cole, 2010). In our study, we showed that suppressing GnT-IVa reduced cell invasion in choriocarcinoma, although it did not affect the secretion of hCG or O-linked hCG-H (data not shown). These results may suggest that both N-linked hCG-H and O-linked hCG-H have the same important function in promoting invasion that differs from the functions of regular hCG.
Our lectin blot experiments demonstrated that numerous proteins in choriocarcinoma exhibit β1-4GlcNAc glycosylation, which were approximately 40–150 kDa. In a study with GnT-IVa knockout mice, glucose transporter 2 (Glut-2) was identified as a target glycoprotein of GnT-IVa in pancreatic β cells, and loss of GnT-IVa attenuated the half-life of Glut-2 cell surface expression and led to a metabolic dysfunction diagnosis of type 2 diabetes (Ohtsubo et al, 2005). Eight members of the Glut family have been described in human placental tissue and trophoblasts, but Glut-2 is not expressed (Baumann et al, 2002). Other target molecules were suggested to be γ-glutamyltranspeptidase and carcinoembryonic antigen because the abnormal biantennary structure by GnT-IV was detected on these proteins when they were purified from hepatocellular carcinoma and colon cancer, respectively (Yamashita et al, 1987, 1989). In mouse hepatocarcinoma, it has been reported recently that GnT-IVa increases migration and metastasis capabilities through glycosylation of CD147, which is also named the ECM metalloproteinase inducer, (Fan et al, 2012). CD147 stimulates the production of MMPs and has been reported to express strongly in choriocarcinoma by immunohistochemistry (Singh et al, 2012). However, GnT-IVa knockdown did not affect MMP activities, but decreased the adherence potential of cells to ECM proteins in choriocarcinoma cells. These results suggest that one of the adhesion molecules is modulated by GnT-IVa and involved in regulation of choriocarcinoma invasion.
The staining levels in DSA lectin blot of choriocarcinoma cell lines and HTR-8/SVneo were not the same as the levels of GnT-IVa expression in western blot. It may be because the β1-4GlcNAc branching on N-glycans can be the common substrate for other N-acetylglucosaminyltransferases, such as GnT-III and GnT-V (Schachter et al, 1989). GnT-V catalyses the formation of β1-6GlcNAc branching structure, which can be recognised by leukoagglutinating phytohemagglutinin (L4-PHA) lectin, and GnT-III reduces the level of β1-6GlcNAc branching by catalysing the addition of β1,4-bisecting-N-acetylglucosamine on N-glycans (Zhao et al, 2008; Gu et al, 2009). The DSA lectin blot after immunoprecipitation for a specific molecule can show the level of β1-4GlcNAc branching on the molecule clearly as DSA lectin blots after immunoprecipitations for Glut-2 by Ohtsubo et al (2005) and for β1 integrin in our study. We performed L4-PHA lectin blot with and without immunoprecipitation for β1 integrin, and there were no differences in the levels of β1-6GlcNAc branching between si-control cells and siGnT-IV cells in both L4-PHA lectin blot (data not shown). Furthermore, suppression of GnT-V in Jar decreased β1-6GlcNAc branching on α5β1 integrin and increased invasion ability and adhesion ability to ECMs (Yamamoto et al, 2009). These results suggest that GnT-V and GnT-III may not affect the functional changes by glycosylation on N-glycans, including β1 integrin, by GnT-IV in Jar cells.
In adhesion molecules, integrin and E-cadherin are major carriers of N-glycans. The changes of biological functions of both molecules by N-glycosylation are considered to be associated with a carcinogenic process and tumourigenesis (Zhao et al, 2008; Gu et al, 2009). E-cadherin mediates cell–cell adhesion, whereas integrins are αβ heterodimers and the N-terminal domain of each subunit contains ECM binding site. Among the integrin superfamily, 12 members containing β1 subunit and β1 integrin complexes, including α5β1 integrin (fibronectin receptor) and α3β1 integrin (laminin receptor), have been reported to change biological functions in cancers by N-glycosylation by GnT-III and GnT-V (Isaji et al, 2004; Zhao et al, 2008; Gu et al, 2009). Our adhesion assay showed that GnT-IVa knockdown reduced adhesion of Jar cells to collagen type I and type IV, as well as fibronectin. Therefore, we investigated the change of β1-4GlcNAc glycosylation level on β1 integrin, and our results suggest that GnT-IVa might be involved in regulating trophoblast invasion through glycosylation of β1 integrin. However, more research is needed to confirm the relationship between invasion and β1-4GlcNAc glycosylation on β1 integrin.
In vivo studies confirmed our findings that GnT-IVa stimulates the abilities of adhesion to ECMs and invasion in choriocarcinoma. The GnT-IVa knockdown significantly reduced the potential for tumourigenesis and immunohistochemistry demonstrated that the cells of the tumour produced by shGnT-IV expressed GnT-IVa significantly weakly compared with the tumour by sh-control, although the morphology and staining for hCG as choriocarcinoma were the same in these two groups. These results suggest that only trophoblastic cells expressing GnT-IVa can adhere to ECMs and migrate by increasing of β1-4GlcNAc glycosylation on β1 integrin, which is consistent with the results that trophoblastic cells of invasive mole, choriocarcinoma, and PSTT expressed GnT-IVa strongly, but not in hydatidiform mole in immunohistochemistry, western blot, and RT–PCR.
We examined GnT-IVa localisation in normal placentas during all trimesters, as well as in trophoblastic disease. In the human placenta, cytotrophoblastic stem cells at the basement of villi differentiate in two distinct directions: syncytiotrophoblasts and EVTs (Damsky et al, 1992). Syncytiotrophoblasts originate from the syncytial layer in floating villi, which primarily manage transport and endocrine functions. On the other hand, EVTs located in anchoring villi develop and invade the maternal decidua, myometrium, and uterine vasculature until the 10th gestational week. As trophoblast cells migrate from the anchoring villus, they downregulate α6β4 integrin and upregulate α5β1 and α1β1 integrin (laminin/collagen receptor; Damsky et al, 1994), and β1-6 branching of α5β1 integrin by GnT-V is decreased (Yamamoto et al, 2009). When examining invasive function, Handschuh et al (2007) reported that invasive EVTs secreted hCG and expressed the LH/CG receptor, and that hCG produced by invasive EVTs induced a 10-fold increase in EVT invasion, although hCG produced by syncytiotrophoblasts had no effect on invasion. They also showed that O-linked hCG-H was located in invasive and endovascular EVTs, but not in syncytiotrophoblasts during the first trimester (Guibourdenche et al, 2010). Our results revealed that only EVTs in the first trimester strongly expressed GnT-IVa in placentas. These results suggest that GnT-IVa may regulate the invasion of EVTs in the first trimester by glycosylation of the N-glycan on hCG and β1 integrin, and that EVTs secrete N-linked hCG-H. Western blot and RT–PCR showed that mRNA and protein expression levels of GnT-IVa in placental tissues were weak, except mRNA expression early in the first trimester. These tissues are villi, which consist of cytotrophoblasts and syncytiotrophoblasts. However, some parts of the tissues might have EVTs, and these cells might affect the results of RT–PCR and western blot, especially in the first trimester. Further studies will be required to reveal the function of GnT-IVa and N-linked hCG-H in implantation in early pregnancy.
In summary, we have provided the first evidence of a functional role for GnT-IVa in choriocarcinoma migration and invasion, and a new target molecule of GnT-IVa to be β1 integrin. Our results show that GnT-IVa is strongly expressed in trophoblastic cells that have invasive potential, including choriocarcinoma, invasive mole, PSTT, and EVTs in the first trimester. These findings suggest that GnT-IVa can be a good marker for malignant/pre-malignant GTDs and is involved in regulating trophoblast invasion.
Acknowledgments
We thank the laboratory of Dr Charles H Graham (Queen’s University, Kingston, ON, Canada) for the generous gift of HTR-8/SVneo cells, and Ms Yuka Sakaguchi for her assistance with experiments. This work was supported by Grants-in-aid number 23592445 (to EY) from the Japanese Ministry of Education, Culture, Sports, Science, and Technology.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Baumann MU, Deborde S, Illsley NP (2002) Placental glucose transfer and fetal growth. Endocrine 19: 13–22 [DOI] [PubMed] [Google Scholar]
- Cole LA (2010) Biological functions of hCG and hCG-related molecules. Reprod Biol Endocrinol 8: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole LA, Birken S, Perini F (1985) The structures of the serine-linked sugar chains on human chorionic gonadotropin. Biochem Biophys Res Commun 126: 333–339 [DOI] [PubMed] [Google Scholar]
- Cole LA, Dai D, Butler SA, Leslie KK, Kohorn EI (2006) Gestational trophoblastic diseases: 1. Pathophysiology of hyperglycosylated hCG. Gynecol Oncol 102: 145–150 [DOI] [PubMed] [Google Scholar]
- Cole LA, Perini F, Birken S, Ruddon RW (1984) An oligosaccharide of the O-linked type distinguishes the free from the combined form of hCG alpha subunit. Biochem Biophys Res Commun 122: 1260–1267 [DOI] [PubMed] [Google Scholar]
- Damsky CH, Fitzgerald ML, Fisher SJ (1992) Distribution patterns of extracellular matrix components and adhesion receptors are intricately modulated during first trimester cytotrophoblast differentiation along the invasive pathway, in vivo. J Clin Invest 89: 210–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsky CH, Librach C, Lim KH, Fitzgerald ML, McMaster MT, Janatpour M, Zhou Y, Logan SK, Fisher SJ (1994) Integrin switching regulates normal trophoblast invasion. Development 120: 3657–3566 [DOI] [PubMed] [Google Scholar]
- Endo T, Nishimura R, Kawano T, Mochizuki M, Kobata A (1987) Structural differences found in the asparagine-linked sugar chains of human chorionic gonadotropins purified from the urine of patients with invasive mole and with choriocarcinoma. Cancer Res 47: 5242–5245 [PubMed] [Google Scholar]
- Fan J, Wang S, Yu S, He J, Zheng W, Zhang J (2012) N-acetylglucosaminyltransferase IVa regulates metastatic potential of mouse hepatocarcinoma cells through glycosylation of CD147. Glycoconj J 29: 323–334 [DOI] [PubMed] [Google Scholar]
- Goto S, Yamada A, Ishizuka T, Tomoda Y (1993) Development of postmolar trophoblastic disease after partial molar pregnancy. Gynecol Oncol 48: 165–170 [DOI] [PubMed] [Google Scholar]
- Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, Lala PK (1993) Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res 206: 204–211 [DOI] [PubMed] [Google Scholar]
- Gu J, Isaji T, Sato Y, Kariya Y, Fukuda T (2009) Importance of N-glycosylation on alpha5beta1 integrin for its biological functions. Biol Pharm Bull 32: 780–785 [DOI] [PubMed] [Google Scholar]
- Guibourdenche J, Handschuh K, Tsatsaris V, Gerbaud P, Leguy MC, Muller F, Brion DE, Fournier T (2010) Hyperglycosylated hCG is a marker of early human trophoblast invasion. J Clin Endocrinol Metab 95: E240–E244 [DOI] [PubMed] [Google Scholar]
- Hakomori S (1989) Aberrant glycosylation in tumors and tumor-associated carbohydrate antigens. Adv Cancer Res 52: 257–331 [DOI] [PubMed] [Google Scholar]
- Handschuh K, Guibourdenche J, Tsatsaris V, Guesnon M, Laurendeau I, Evain-Brion D, Fournier T (2007) Human chorionic gonadotropin produced by the invasive trophoblast but not the villous trophoblast promotes cell invasion and is down-regulated by peroxisome proliferator-activated receptor-gamma. Endocrinology 148: 5011–5019 [DOI] [PubMed] [Google Scholar]
- Inamori K, Gu J, Ohira M, Kawasaki A, Nakamura Y, Nakagawa T, Kondo A, Miyoshi E, Nakagawara A, Taniguchi N (2006) High expression of N-acetylglucosaminyltransferase V in favorable neuroblastomas: involvement of its effect on apoptosis. FEBS Lett 580: 627–632 [DOI] [PubMed] [Google Scholar]
- Ino K, Goto S, Kosaki A, Nomura S, Asada E, Misawa T, Furuhashi Y, Mizutani S, Tomoda Y (1991) Growth inhibitory effect of bestatin on choriocarcinoma cell lines in vitro. Biotherapy 3: 351–357 [DOI] [PubMed] [Google Scholar]
- Isaji T, Gu J, Nishiuchi R, Zhao Y, Takahashi M, Miyoshi E, Honke K, Sekiguchi K, Taniguchi N (2004) Introduction of bisecting GlcNAc into integrin alpha5beta1 reduces ligand binding and down-regulates cell adhesion and cell migration. J Biol Chem 279: 19747–19754 [DOI] [PubMed] [Google Scholar]
- Kajii T, Ohama K (1977) Androgenetic origin of hydatidiform mole. Nature 268: 633–634 [DOI] [PubMed] [Google Scholar]
- Khan F, Everard J, Ahmed S, Coleman RE, Aitken M, Hancock BW (2003) Low-risk persistent gestational trophoblastic disease treated with low-dose methotrexate: efficacy, acute and long-term effects. Br J Cancer 89: 2197–2201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobata A, Takeuchi M (1999) Structure, pathology and function of the N-linked sugar chains of human chorionic gonadotropin. Biochim Biophys Acta 1455: 315–326 [DOI] [PubMed] [Google Scholar]
- Lurain JR (2010) Gestational trophoblastic disease I: epidemiology, pathology, clinical presentation and diagnosis of gestational trophoblastic disease, and management of hydatidiform mole. Am J Obstet Gynecol 203: 531–539 [DOI] [PubMed] [Google Scholar]
- Mano Y, Shibata K, Sumigama S, Hayakawa H, Ino K, Yamamoto E, Kajiyama H, Nawa A, Kikkawa F (2009) Tocilizumab inhibits interleukin-6-mediated matrix metalloproteinase-2 and -9 secretions from human amnion cells in preterm premature rupture of membranes. Gynecol Obstet Invest 68: 145–153 [DOI] [PubMed] [Google Scholar]
- Mizuochi T, Nishimura R, Derappe C, Taniguchi T, Hamamoto T, Mochizuki M, Kobata A (1983) Structures of the asparagine-linked sugar chains of human chorionic gonadotropin produced in choriocarcinoma. Appearance of triantennary sugar chains and unique biantennary sugar chains. J Biol Chem 258: 14126–14129 [PubMed] [Google Scholar]
- Norris W, Nevers T, Sharma S, Kalkunte S (2011) Review: hCG, preeclampsia and regulatory T cells. Placenta 32: S182–S185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsubo K, Takamatsu S, Minowa MT, Yoshida A, Takeuchi M, Marth JD (2005) Dietary and genetic control of glucose transporter 2 glycosylation promotes insulin secretion in suppressing diabetes. Cell 123: 1307–1321 [DOI] [PubMed] [Google Scholar]
- Schachter H, Brockhausen I, Hull E (1989) High-performance liquid chromatography assays for N-acetylglucosaminyltransferases involved in N- and O-glycan synthesis. Methods Enzymol 1989: 351–397 [DOI] [PubMed] [Google Scholar]
- Schmid P, Nagai Y, Agarwal R, Hancock B, Savage PM, Sebire NJ, Lindsay I, Wells M, Fisher RA, Short D, Newlands ES, Wischnewsky MB, Seckl MJ (2009) Prognostic markers and long-term outcome of placental-site trophoblastic tumours: a retrospective observational study. Lancet 374: 48–55 [DOI] [PubMed] [Google Scholar]
- Shih IM, Kurman RJ (1998) Epithelioid trophoblastic tumor: a neoplasm distinct from choriocarcinoma and placental site trophoblastic tumor simulating carcinoma. Am J Surg Pathol 22: 1393–1403 [DOI] [PubMed] [Google Scholar]
- Singh M, Kindelberger D, Nagymanyoki Z, Ng SW, Quick CM, Yamamoto H, Fichorova R, Fulop V, Berkowitz RS (2012) Vascular endothelial growth factors and their receptors and regulators in gestational trophoblastic diseases and normal placenta. J Reprod Med 57: 197–203 [PubMed] [Google Scholar]
- Soper JT (2006) Gestational trophoblastic disease. Obstet Gynecol 108: 176–187 [DOI] [PubMed] [Google Scholar]
- Takamatsu S, Katsumata T, Inoue N, Watanabe T, Fujibayashi Y, Takeuchi M (2004) Abnormal biantennary sugar chains are expressed in human chorionic gonadotropin produced in the choriocarcinoma cell line, JEG-3. Glycoconj J 20: 473–481 [DOI] [PubMed] [Google Scholar]
- Takamatsu S, Oguri S, Minowa MT, Yoshida A, Nakamura K, Takeuchi M, Kobata A (1999) Unusually high expression of N-acetylglucosaminyltransferase-IVa in human choriocarcinoma cell lines: a possible enzymatic basis of the formation of abnormal biantennary sugar chain. Cancer Res 59: 3949–3953 [PubMed] [Google Scholar]
- World Health Organization (1983) Gestational Trophoblastic Diseases: Report of a WHO Scientific Group pp. 7–42. World Health Organization: Geneva [PubMed] [Google Scholar]
- Yamamoto E, Ino K, Miyoshi E, Inamori K, Abe A, Sumigama S, Iwase A, Kajiyama H, Shibata K, Nawa A, Kikkawa F (2009) N-acetylglucosaminyltransferase V regulates extravillous trophoblast invasion through glycosylation of alpha5beta1 integrin. Endocrinology 150: 990–999 [DOI] [PubMed] [Google Scholar]
- Yamamoto E, Ino K, Miyoshi E, Shibata K, Takahashi N, Kajiyama H, Nawa A, Nomura S, Nagasaka T, Kikkawa F (2007) Expression of N-acetylglucosaminyltransferase V in endometrial cancer correlates with poor prognosis. Br J Cancer 97: 1538–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto E, Ito T, Abe A, Sido F, Ino K, Itakura A, Mizutani S, Dovat S, Nomura S, Kikkawa F (2005) Ikaros is expressed in human extravillous trophoblasts and involved in their migration and invasion. Mol Hum Reprod 11: 825–831 [DOI] [PubMed] [Google Scholar]
- Yamashita K, Totani K, Iwaki Y, Takamisawa I, Tateishi N, Higashi T, Sakamoto Y, Kobata A (1989) Comparative study of the sugar chains of gamma-glutamyltranspeptidases purified from human hepatocellular carcinoma and from human liver. J Biochem 105: 728–735 [DOI] [PubMed] [Google Scholar]
- Yamashita K, Totani K, Kuroki M, Matsuoka Y, Ueda I, Kobata A (1987) Structural studies of the carbohydrate moieties of carcinoembryonic antigens. Cancer Res 47: 3451–3459 [PubMed] [Google Scholar]
- Yoshida A, Minowa MT, Takamatsu S, Hara T, Oguri S, Ikenaga H, Takeuchi M (1999) Tissue specific expression and chromosomal mapping of a human UDP-N-acetylglucosamine: alpha1,3-d-mannoside beta1, 4-N-acetylglucosaminyltransferase. Glycobiology 9: 303–310 [DOI] [PubMed] [Google Scholar]
- Zhao YY, Takahashi M, Gu JG, Miyoshi E, Matsumoto A, Kitazume S, Taniguchi N (2008) Functional roles of N-glycans in cell signaling and cell adhesion in cancer. Cancer Sci 99: 1304–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]