Abstract
The gut–brain axis refers to the bidirectional communication between the gut and the brain. Four information carriers (vagal and spinal afferent neurons, immune mediators such as cytokines, gut hormones and gut microbiota-derived signalling molecules) transmit information from the gut to the brain, while autonomic neurons and neuroendocrine factors carry outputs from the brain to the gut. The members of the neuropeptide Y (NPY) family of biologically active peptides, NPY, peptide YY (PYY) and pancreatic polypeptide (PP), are expressed by cell systems at distinct levels of the gut–brain axis. PYY and PP are exclusively expressed by endocrine cells of the digestive system, whereas NPY is found at all levels of the gut–brain and brain–gut axis. The major systems expressing NPY comprise enteric neurons, primary afferent neurons, several neuronal pathways throughout the brain and sympathetic neurons. In the digestive tract, NPY and PYY inhibit gastrointestinal motility and electrolyte secretion and in this way modify the input to the brain. PYY is also influenced by the intestinal microbiota, and NPY exerts, via stimulation of Y1 receptors, a proinflammatory action. Furthermore, the NPY system protects against distinct behavioural disturbances caused by peripheral immune challenge, ameliorating the acute sickness response and preventing long-term depression. At the level of the afferent system, NPY inhibits nociceptive input from the periphery to the spinal cord and brainstem. In the brain, NPY and its receptors (Y1, Y2, Y4, Y5) play important roles in regulating food intake, energy homeostasis, anxiety, mood and stress resilience. In addition, PP and PYY signal to the brain to attenuate food intake, anxiety and depression-related behaviour. These findings underscore the important role of the NPY-Y receptor system at several levels of the gut–brain axis in which NPY, PYY and PP operate both as neural and endocrine messengers.
Keywords: Anxiety, Cytokines, Depression, Food intake, Gut–brain axis, Gut hormones, Immune system, Inflammation, Neuropeptide Y, Pain, Peptide YY, Pancreatic polypeptide, Satiety, Stress resilience, Visceral hyperalgesia
1. The gut–brain axis involves neural, immune and endocrine signalling pathways
The terms “gut–brain axis” and “brain–gut axis” denote the bidirectional communication between the gut and the brain (Fig. 1). Apart from the autonomic regulation of digestion by the central, parasympathetic, sympathetic and enteric nervous systems as well as by neuroendocrine factors (derived from the adrenal medulla and cortex), there is ongoing communication from the gut to the brain in health and disease. Normally, most information that is transmitted from the digestive system to the central nervous system does not reach the level of consciousness. However, visceral information is continuously fed into subcortical regions of the brain including the limbic system and the autonomic and neuroendocrine centres in the hypothalamus and brainstem. Under pathological conditions, the input from the gut can reach the cortex, giving rise to the sensation of nausea, discomfort or pain. This afferent part of the gut–brain–gut axis has recently been in the focus of investigation in order to understand why gastrointestinal disease is associated with pain and a number of psychiatric disturbances including anxiety, neuroticism and depression.
Fig. 1.
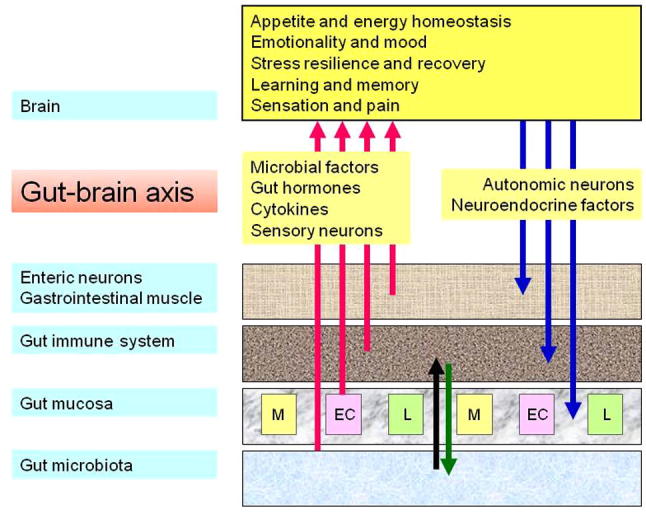
The bidirectional gut–brain axis. Four communication pathways (sensory neurons, cytokines, gut hormones and microbial factors) signal from the gut to the brain where they can modify cerebral function and behaviour. Two pathways (autonomic and neuroendocrine outputs) signal from the brain to the gut. EC, M and L denote different populations of endocrine cells in the gastrointestinal mucosa.
In a traditional view, the gut–brain axis uses 3 major information carriers for the communication between the gut and the brain (Fig. 1): neural messages carried by vagal and spinal afferent neurons, immune messages carried by cytokines, and endocrine messages carried by gut hormones. Each of these communication systems is abundantly present in the gastrointestinal tract which for this reason has been designated as a major neurological (Holzer et al., 2001), immune (Wittig and Zeitz, 2003) and endocrine (Ahlman and Nilsson, 2001; Murphy and Bloom, 2006) organ. The gastrointestinal immune system is important in defending the body against foreign antigens and pathogens that enter with the food and in maintaining homeostasis with the extensive microbial community living in the intestine. The gut microbiota and the gastrointestinal immune system interact with each other via the gastrointestinal mucosa, and this interaction has important functional implications within and outside the gastrointestinal tract.
It is increasingly emerging that the gut microbiota uses a fourth information pathway to signal to distant organs including the brain: apart from modulating the generation of cytokines in the intestinal immune system they also release signalling molecules such as lipopolysaccharide (LPS) and peptidoglycan components (Clarke et al., 2010) that can directly act on the central nervous system (Fig. 1). Thus, the physiological roles of the symbiotic gut microflora relate not only to the regulation of digestion, nutrition, mucosal function and intestinal immunity, but also to metabolic homeostasis, systemic immunity and brain function (emotion, mood, cognition). With more than 20 hormones produced, the gut is also a major endocrine organ (Murphy and Bloom, 2006). Formed in specialised endocrine cells of the gastrointestinal mucosa, gut hormones are involved in the coordination of digestion, the signalling of hunger and satiety and the regulation of energy homeostasis, but also in the control of emotion and mood. Importantly, the 4 communication pathways between the gut and the brain do not operate in isolation but are closely interrelated with each other. For instance, cytokines and gut hormones can act on afferent neurons in the vagus nerve and in this way send messages to the brain. Understanding the operation of the gut–brain axis in health and disease is thus not only relevant to gastroenterology but also to neurology and psychiatry and is likely to reveal important targets for novel therapeutic strategies.
2. Neuropeptide Y, peptide YY and pancreatic polypeptide are expressed at distinct levels along the gut–brain axis
The members of the neuropeptide Y (NPY) family of biologically active peptides, NPY, peptide YY (PYY) and pancreatic polypeptide (PP), are expressed by cell systems at distinct levels of the gut–brain axis. Consisting of 36 amino acids, NPY, PYY and PP share the PP fold (hairpin fold) tertiary structural motif. The N-terminus of NPY and PYY is readily truncated by dipeptidyl peptidase 4 (EC 3.4.14.5) and other enzymes (e.g., aminopeptidase-P, endopeptidase-24.11), yielding the fragments NPY3–36 and PYY3–36 (Mentlein et al., 1993; Medeiros and Turner, 1994). The functional implication of the NPY family of peptides in the gut–brain communication is corroborated by the occurrence of 5 NPY receptor types, termed Y1, Y2, Y4, Y5 and y6 (a human pseudogene), along the gut–brain signalling pathways. Coupled to pertussis toxin-sensitive Gi/o protein transduction mechanisms (Redrobe et al., 2004a; Alexander et al., 2011), the Y receptor subtypes display characteristic affinities for the different members of the peptide family and their fragments [Cox, 2007a; Alexander et al., 2011). While NPY and PYY do not grossly differ in their affinities for the Y1, Y2 and Y5 receptors subtypes, it is particularly worth noting that PYY3–36 is a preferred agonist at Y2 receptors while PP binds preferentially to Y4 receptors (McGowan and Bloom, 2004; Cox, 2007a; Alexander et al., 2011).
2.1. Pancreatic polypeptide
PYY and PP are almost exclusively expressed at the level of the digestive system, whereas NPY is found at all levels of the gut–brain and brain–gut axis (Fig. 2). PP, as its name implies, is postprandially secreted from the pancreas in which it is synthesised by endocrine F cells of the pancreatic islets (Ekblad and Sundler, 2002). In addition, PP is expressed in a sparse number of endocrine cells in the small and large intestine, these cells being distinct from PYY-positive cells (Cox, 2007a). Both the release of PP and the biological actions of PP on digestion and food intake, which are preferentially mediated by Y4 receptors, require signalling in the parasympathetic vagus nerve (Small and Bloom, 2005; Field et al., 2010). Although Y4 receptors are expressed in the brain, and Y4 receptor knockout is associated with reduced anxiety- and depression-related behaviour (Painsipp et al., 2008b; Tasan et al., 2009), there is a lack of evidence that PP is produced in the brain, given that the immunoreactivity which was once believed to reflect PP turned out to be NPY (Allen et al., 1983; DiMaggio et al., 1985).
Fig. 2.
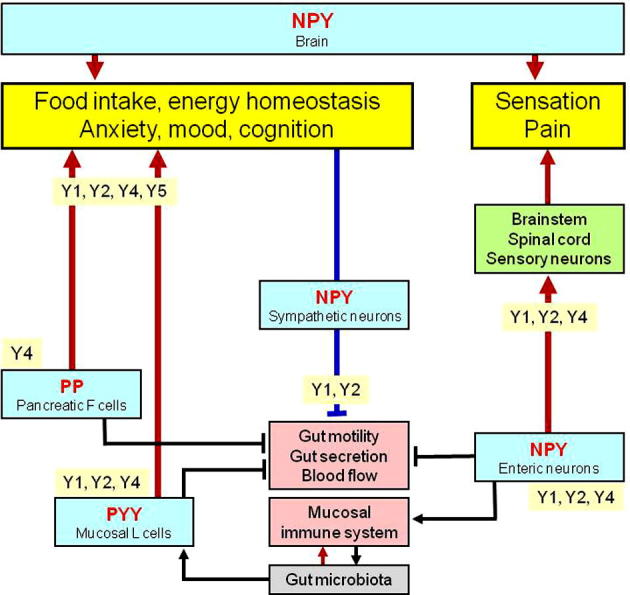
The NPY-Y receptor sytem in the gut–brain axis. The graph shows the major sources of NPY, PYY and PP along the gut–brain axis and the Y receptor subtypes which mediate the effects of these peptides at the different levels of the gut–brain axis. The arrow symbols denote stimulation, the tack symbols denote inhibition.
2.2. Peptide YY
The major source of PYY in the gut is the endocrine L cell which occurs most abundantly in the lower gastrointestinal tract (Ekblad and Sundler, 2002; McGowan and Bloom, 2004; Cox, 2007a,b; Ueno et al., 2008). In addition, PYY-positive L cells contain glicentin, a proglucagon-derived peptide, glucagon-like peptide-1 and glucagon-like peptide-2 (Ekblad and Sundler, 2002; Cox, 2007a). Given that they likewise express gustducin, a taste-related G protein, as well as bitter and sweet taste receptors, the PYY-containing L cells are thought to be important chemosensors in the intestine (Rozengurt et al., 2006). Other, but minor sources of PYY in the digestive system are enteric neurons of the stomach (Böttcher et al., 1993) and pancreatic endocrine cells (Cox, 2007b). Since PYY is postprandially released from intestinal L cells, the peptide is thought to be a satiety signal, which fits with its effect to reduce food intake (McGowan and Bloom, 2004). Once released, PYY is in part cleaved to PYY3–36 which, unlike the parent peptide, is a preferential agonist at Y2 receptors (Small and Bloom, 2005; Cox, 2007b). Evidence for the expression of PYY in the brain is comparatively sparse. Morimoto et al. (2008) used radioimmunoassay, immunocytochemistry and reverse transcription polymerase chain reaction to find expression of PYY in the human brain and pituitary gland. In the rodent brain, PYY-positive neurons have been localised to the hypothalamus, pons, medulla and spinal cord (Ekblad and Sundler, 2002).
2.3. Neuropeptide Y
In contrast to PP and PYY, NPY is expressed at all levels of the gut–brain and brain–gut axis, the major systems expressing this peptide being enteric neurons, primary afferent neurons, several neuronal pathways throughout the brain and sympathetic neurons (Fig. 2). Within the gut, the major source of NPY is constituted by enteric neurons. NPY occurs in the two major enteric nerve plexuses and has been localised to distinct interneurons and descending inhibitory motoneurons of the myenteric plexus as well as to noncholinergic secretomotor neurons of the submucosal plexus (Cox, 2007a). In the inhibitory motor neurons, NPY is frequently colocalised with vasoactive intestinal polypeptide and nitric oxide synthase (Schicho et al., 2003; Cox, 2007a). In addition to enteric neurons, NPY is also expressed in postganglionic sympathetic neurons in which it is colocalised with noradrenaline and adenosine triphosphate (Cox, 2007a; Holzer, 2012). NPY is preferentially found in sympathetic neurons supplying the vascular system but is largely absent from sympathetic neurons innervating the gastrointestinal mucosa (Lomax et al., 2010).
A further but very minor source of NPY in the gut is provided by primary afferent neurons originating in the dorsal root ganglia (Brumovsky et al., 2007). Under physiological conditions the NPY content of these neurons is very low, but under conditions of nerve injury the expression of NPY can be markedly upregulated, particularly in medium- and large-sized sensory neurons (Brumovsky et al., 2007). Nerve injury and inflammation also has an impact on the presence of Y1 and Y2 receptors in primary afferent neurons, these receptors being primarily expressed by small, presumably nociceptive sensory neurons (Brumovsky et al., 2007). Within the spinal cord there is an abundant NPY-containing neuropil which is made of at least 3 different neuron populations: inhibitory interneurons also containing γ-aminobutyric acid (GABA), descending noradrenergic neurons originating in the locus coeruleus, and primary afferent nerve endings (Brumovsky et al., 2007). The abundance of NPY in the spinal cord is matched by an extensive distribution of Y1 receptors which in the rat and mouse have been localised to 7 distinct types of neurons within the dorsal horn of the spinal cord (Brumovsky et al., 2007). By contrast, Y2 receptors appear to occur exclusively on the central terminals of primary afferent neurons in the superficial laminae of the spinal cord (Brumovsky et al., 2007).
Within the brain, NPY is the most abundant neuropeptide, being expressed by a multitude of neuronal systems in regions spanning from the medullary brainstem to the cerebral cortex. In the context of the gut–brain axis it is particularly worth noting that NPY occurs in the nucleus of the solitary tract and ventrolateral medulla, periaqueductal grey and locus coeruleus, paraventricular nucleus of the thalamus, hypothalamus (arcuate nucleus, paraventricular nucleus and other regions), septum, hippocampus, amygdala, basal ganglia, nucleus accumbens and cerebral cortex (Wettstein et al., 1995; Kask et al., 2002; Eaton et al., 2007). Several important pathways utilising NPY as a neurotransmitter have been identified. These include noradrenergic neurons originating in the locus coeruleus of the brainstem and issuing both ascending and descending projections in the central nervous system, neurons expressing both NPY and agouti-related protein (AgRP) originating in the arcuate nucleus of the hypothalamus, and distinct pathways operating in the limbic system (Wettstein et al., 1995; Kask et al., 2002; Eaton et al., 2007). The major receptor subtypes which NPY acts on in the brain are the Y1 and Y2 receptors, the localization of which are discussed when the functional implications of NPY in the cerebral part of the gut–brain axis are considered. While Y1 and Y2 receptors are widely distributed in the central nervous system, the localization of Y4 and Y5 receptors is restricted to particular regions of the brain such as nucleus of the solitary tract, area postrema, nucleus ambiguus, hypothalamus, thalamus and amygdala (Kask et al., 2002; McGowan and Bloom, 2004; Eaton et al., 2007; Tasan et al., 2009).
3. NPY, PYY and PP play specific roles at distinct levels of the gut–brain axis
3.1. NPY, PYY and PP inhibit gastrointestinal motility and secretion and thereby alter the input to the brain
The gut hormones PYY and PP and the enteric neuropeptide NPY subserve many functions in the regulation of digestion, which have been reviewed elsewhere (Fujimiya and Inui, 2000; Cox, 2007a,b). Thus, only a brief summary of their functional implications within the digestive system is given here. These implications cannot go unnoticed, however, because any change in gastrointestinal function will also issue messages sent to the brain (Fig. 2), either via a neural route or via the blood stream (gut hormones, cytokines). It is also obvious that the functional implications of NPY, PYY and PP depend critically on the expression of different Y receptor subtypes by the control and effector systems of digestion.
PP is released from the endocrine pancreas following a meal and acts preferentially via Y4 and Y5 receptors. It inhibits gastric emptying through an action that involves the vagus nerve; this effect may in part account for its ability to reduce appetite, an effect that also depends on intact vagal signalling (Murphy and Bloom, 2006; Field et al., 2010). By targeting Y4 receptors on the intestinal epithelium, PP can also inhibit intestinal electrolyte and water secretion (Tough et al., 2006). In addition, PP has an inhibitory action on intestinal motor activity and peristalsis (Fujimiya and Inui, 2000), an effect that in the guinea-pig small intestine is mediated by enteric neurons (Holzer et al., 1986).
PYY is released from intestinal L cells following a meal in proportion to energy intake, particularly by fat and protein (Field et al., 2010). The meal-evoked release of PYY occurs long before the nutrients have reached the lower gut where the PYY-expressing L cells are most abundant, which indicates that a neural mechanism including the vagus nerve is involved (Fu-Cheng et al., 1997; Cox, 2007b). Gastric acid secretion, cholecystokinin, bile acids and free fatty acids of a particular length (C12) also stimulate the release of PYY (McGowan and Bloom, 2004; Feltrin et al., 2006) as do short chain fatty acids (SCFAs), particularly butyrate, which are produced through fermentation of indigestible carbohydrates by the colonic microbiota (Cherbut et al., 1998). Once released, part of PYY is enzymatically cleaved to PYY3–36, the major circulating form of PYY (Mentlein et al., 1993). This transformation is paralleled by a shift in the Y receptor subtypes stimulated by the peptide: while the full length peptide activates Y1, Y2 and Y5 receptors, the effects of PYY3–36 are preferentially mediated by Y2 and, to some extent, Y5 receptors (Mentlein et al., 1993; McGowan and Bloom, 2004; Cox, 2007b).
PYY and PYY3–36 have profound effects on gastrointestinal motility and secretion. The inhibitory action of PYY and PYY3–36 on gut function is mediated by 3 major Y receptor subtypes: Y1 receptors on enterocytes, myenteric and submucosal neurons and endothelial cells, Y2 receptors on myenteric and submucosal neurons, extrinsic primary afferent nerve fibres, and Y4 receptors on enterocytes (Cox, 2007a,b; Wang et al., 2010). Plasma concentrations roughly equivalent to postprandial levels inhibit gastric acid secretion, gastric emptying, the cephalic phase of gallbladder contraction and mouth-to-caecum transit time (Field et al., 2010). The inhibition of gastric acid secretion involves Y1 and Y2 receptors and is mediated both by an action in the brainstem and stomach (Yang, 2002), while the effect of PYY3–36 to increase gastric pressure via stimulation of Y2 receptors does not involve the vagus nerve (Janssen et al., 2012). PYY released by SCFAs (administered exogenously or produced by the intestinal microbiota) inhibits gastrointestinal motility and electrolyte and water secretion by neural and non-neural mechanisms (Cox, 2007b). In addition, PYY promotes mucosal cell differentiation (Halldén and Aponte, 1997).
In physiological terms, PYY is thought to be a major mediator of ileal and colonic brakes which are set into operation when fat reaches the lower gut: the brakes slow gastric emptying and intestinal transit, which in conjunction with the antisecretory action of PYY facilitates nutrient absorption (Pironi et al., 1993; Lin et al., 1996; Nightingale et al., 1996; Van Citters and Lin, 2006; Cox, 2007b). The effect of PYY and PYY3–36 to inhibit colonic transit in the mouse is mediated by Y2 receptors on the enteric nervous system (Wang et al., 2010; Tough et al., 2011). There is also growing evidence that PYY and NPY exert a tonic influence on gastrointestinal motor and secretory activity. While Y2 receptor antagonists per se do not alter gastric motor tone (Janssen et al., 2012), Y1 and Y2 receptor antagonism as well as PYY and NPY knockout modify colonic ion transport and motility (Tough et al., 2011). The pertinent findings indicate that, both in the human and mouse colonic mucosa, PYY and NPY exert a tonic antisecretory effect mediated by epithelial Y1 and neural Y2 receptors. Colonic transit is tonically accelerated by Y1 receptor stimulation and tonically inhibited by Y2 receptor stimulation (Tough et al., 2011).
Emerging evidence also suggests that PYY shares some of the incretin-like activity of glucagon-like peptide-1, another gut hormone derived from enteroendocrine L cells (Cox et al., 2010). These cells use G protein-coupled receptors (GPRs) such as GPR119 to respond to luminal nutrients and to release glucagon-like peptide-1 as well as PYY. The effect of GPR119 agonism to improve glucose tolerance in association with enhanced glucose-induced circulating insulin concentrations is mediated by PYY (Cox et al., 2010). This newly discovered role in glucose homeostasis makes PYY a potentially useful target for the treatment of diabetes and obesity.
The gastrointestinal Y receptor subtypes are prominently targeted by NPY which in the gut is expressed by inhibitory enteric motor pathways, secretomotor neurons and sympathetic neurons. NPY inhibits gastrointestinal motility as well as electrolyte and water secretion, and its inhibitory action on intestinal Cl− secretion is seen in all regions of the intestine (Saria and Beubler, 1985; Hubel and Renquist, 1986; Holzer-Petsche et al., 1991; Cox, 2007a). Although Y2 receptors play a major role in the antisecretory/proabsorptive effect of NPY and PYY, Y1 receptors on secretomotor neurons and epithelial cells, and Y4 receptors on enterocytes are also likely to contribute (Cox and Tough, 2002; Tough et al., 2006; Cox, 2007a). The prominent location of Y receptor subtypes on enteric neurons is also responsible for the inhibitory effect of NPY on gastrointestinal motility (Fujimiya and Inui, 2000). Peristalsis in the guinea-pig small intestine is depressed by NPY through interruption of excitatory pathways in the enteric nervous system (Holzer et al., 1987).
3.2. NPY interacts with the immune system and promotes gastrointestinal inflammation
There is ample evidence that NPY is among the neuropeptides which have a distinct impact on immune function, within and outside the gastrointestinal tract (Bedoui et al., 2007; Wheway et al., 2007a; Dimitrijević and Stanojević, in press). This is particularly true for NPY released from the sympathetic nerve fibres which in lymphoid tissues form close contacts with immune cells. Following release, NPY acts on Y receptors (notably of the Y1, Y2, Y4 and Y5 subtype) expressed by distinct classes of immune cells (e.g., dendritic cells, mononuclear cells, macrophages, granulocytes, T and B lymphocytes) to modify their activity (Wheway et al., 2005, 2007b; Mitić et al., 2011; Dimitrijević and Stanojević, in press). However, NPY acts not only as a neuroimmune transmitter but also as a paracrine or autocrine immune mediator, given that immune cells (e.g., B and T lymphocytes, macrophages) themselves are capable of producing and releasing NPY (Wheway et al., 2007a,b). In this way NPY can affect both innate and adaptive immunity, leading to either immune activation or suppression depending on its concentration, the Y receptors activated and cell types involved (Bedoui et al., 2007; Wheway et al., 2007a; Dimitrijević and Stanojević, in press). The effects of NPY include modulation of immune cell trafficking, T helper cell differentiation, cytokine secretion, natural killer cell activity, phagocytosis and production of reactive oxygen species (Hernanz et al., 1996; Bedoui et al., 2007; Wheway et al., 2007a; Dimitrijević and Stanojević, in press).
In the gastrointestinal tract, NPY plays an important role in regulating inflammatory processes, given that NPY-containing nerve fibres are in close contact with immune cells such as IgA-producing lymphocytes in the mouse ileum lamina propria (Shibata et al., 2008). The proliferation of lymphocytes from the human colonic lamina propria is also stimulated by NPY (Elitsur et al., 1994). The ability of NPY to promote colonic inflammation is supported by several lines of evidence. Firstly, NPY knockout mice are largely resistant to the induction of dextran sulfate sodium (DSS)-induced colitis (Chandrasekharan et al., 2008; Painsipp et al., 2011a). Secondly, the result of NPY deletion is reproduced by treatment with a NPY antisense oligodeoxynucleotide (Pang et al., 2010) and by knockout or antagonism of Y1 receptors (Hassani et al., 2005), which demonstrates that the Y1 receptor plays a pivotal role in the proinflammatory effect of NPY. The antiinflammatory phenotype of Y1 receptor knockout mice results from a defect in antigen-presenting cell function, a reduction of TNF-α and IL-12 production by macrophages, and a decrease in the number of effector T cells (Wheway et al., 2005). Thirdly, experimentally induced colitis is associated with an increase in the colonic synthesis of NPY (Chandrasekharan et al., 2008; Pang et al., 2010; Baticic et al., 2011), a reduction of colonic Y1 receptor expression and a loss of the antisecretory action of NPY in the colon (Klompus et al., 2010). In contrast, the colonic levels of PYY are decreased in rats with DSS-induced colitis (Hirotani et al., 2008). These experimental data are in line with a decrease of colonic PYY levels in patients with inflammatory bowel disease (Tari et al., 1988; El-Salhy et al., 1997; Schmidt et al., 2005), while circulating levels of PYY and NPY are enhanced (Adrian et al., 1986; Straub et al., 2002).
Given that NPY is an activator of antigen-presenting cell function and a negative regulator of T cell function (Wheway et al., 2005, 2007a,b; Dimitrijević and Stanojević, in press), the impact of NPY on gut inflammation is complex and involves a variety of down-stream mechanisms. For instance, NPY enhances the expression of neuronal nitric oxide synthase which is a determinant of oxidative stress and subsequent inflammation (Chandrasekharan et al., 2008). It is conceivable that PYY and PP also influence the intestinal immune system, acting via the same Y receptors as NPY. The proinflammatory effect of NPY could in part be counter-regulated by the vasoconstrictor effect of the peptide, given that the sympathetic constriction of splanchnic resistance vessels is co-mediated by the sympathetic triad adenosine triphosphate (ATP), noradrenaline and NPY (Holzer, 2012). In addition, NPY is able to potentiate the constrictor effect of noradrenaline and ATP. Both the vasoconstrictor response to NPY and its action to augment noradrenaline- and ATP-induced mesenteric vasoconstriction are mediated by postjunctional Y1 receptors (Holzer, 2012).
3.3. The impact of the gut microbiota on the gut–brain axis may involve NPY and PYY
Endocrine cells in the gut mucosa are in direct contact with the intestinal microbiota, and there is emerging evidence that an interaction between these systems has an impact on the satietogenic and metabolic effects of gut hormones including PYY (Cani et al., 2009; Delzenne et al., 2011). In order to comprehend the implications of this relationship a brief survey of the emerging roles of the gut microbiota in the gut–brain axis is in place. The intestinal microflora is relevant to nutrition, digestion, metabolic homeostasis, local and systemic immune function, and neurobiological homeostasis (Melgar and Shanahan, 2010; Cani and Delzenne, 2011; Grenham et al., 2011; DuPont and DuPont, 2011; Bercik et al., 2012). It has even been shown that, within a critical window of time, the presence of commensal gut microbiota is required for normal brain development and synaptogenesis (Heijtz et al., 2011). A change in the composition of the gut microbiota is an important factor leading to gastrointestinal immune activation (Shanahan, 2011). Likewise, dysbiosis of the intestinal microflora (Krogius-Kurikka et al., 2009; Tana et al., 2010; Jeffery et al., 2012) and upregulation of mucosal Toll-like receptors (McKernan et al., 2011) have been implicated in the aetiology of irritable bowel syndrome (IBS). Analysis of the faecal microbiota reveals subgroups of IBS patients who either have a normal composition of gut microbiota or show substantial microbiota-wide changes characterised by an increased Firmicutes:Bacteroidetes ratio (Jeffery et al., 2012). A number of clinical variables, including rectal pain threshold and depression, are associated with distinct microbial signatures, which may have a bearing on the pathophysiology and diagnosis of this functional gastrointestinal disorder (Jeffery et al., 2012).
The contention that intestinal dysbiosis, as has been found to occur in patients with IBS (Krogius-Kurikka et al., 2009; Tana et al., 2010; Jeffery et al., 2012), contributes to the psychiatric comorbidity seen in these patients is backed by three lines of experimental investigation: experimental changes in the intestinal microbiota composition, neurobiological and behavioural phenotyping of germ-free mice, and analysis of the systemic effects of probiotics. Antibiotic-induced dysbiosis of the intestinal microflora increases the expression of brain-derived neurotrophic factor in the hippocampus and enhances exploratory behaviour (Bercik et al., 2011a). Memory is impaired in germ-free mice, and exposure to stress combined with Citrobacter rodentium infection causes prolonged memory dysfunction (Gareau et al., 2011). Long-term treatment of mice with the probiotic Lactobacillus rhamnosus has a significant impact on brain neurochemistry, reduces anxiety- and depression-related behaviour and improves stress coping (Bravo et al., 2011). These behavioural effects are mediated by the vagus nerve and are associated with region-dependent alterations in the cerebral GABA receptor expression (Bravo et al., 2011).
The impact of intestinal dysbiosis on brain function takes place independently of inflammation and does not depend on the autonomic nervous system (Bercik et al., 2011a). In addressing the question as to how gastrointestinal microbiota signal to the brain, several possibilities come to mind: constituents of the microbiota themselves (Clarke et al., 2010), sensory neurons, circulating cytokines, and gut hormones. Administration of Escherichia coli LPS into the rat jejunum causes vagal afferent input to the nucleus of the solitary tract (Gakis et al., 2009) as does intraperitoneal injection of interleukin-1β or tumour necrosis factor-α (Holzer et al., 2004). It is worth noting that the anxiolytic and antidepressant effects of probiotics are blocked by vagotomy (Bercik et al., 2011b; Bravo et al., 2011).
There is some evidence that the NPY–PYY system has an impact on the composition and function of the gut microbiota and its relevance to the gut–brain axis. For instance, NPY has been found to exhibit a direct antimicrobial effect against various gut bacteria including E. coli, Enterococcus faecalis, and Lactobacillus acidophilus (El Karim et al., 2008). A relationship between the gut microbiota and the enteroendocrine PYY system can be envisaged if the role of gut microbiota in the fermentation of nondigestible carbohydrates is considered. While the human gut has a limited repertoire of glycoside hydrolases, the gut microbiota synthesises a large arsenal of these enzymes which process complex dietary carbohydrates to SCFAs, principally acetate, propionate, and butyrate (Samuel et al., 2008). Fermentation of prebiotic fibre also promotes satiety and lowers hunger and energy intake, an effect that is associated with enhanced release of glucagon-like peptide-1 and PYY from the gut (Cani et al., 2009; Cani and Delzenne, 2011; Delzenne et al., 2011). Since PYY is a satiety factor, it is conceivable that it contributes to the prebiotic-induced reduction of food intake. The effect of PYY on energy homeostasis, along with that of PP and NPY, is dealt with in a separate section of this review.
The mechanisms of the interaction between microbiota, SCFAs and PYY-releasing L cells are still little understood. On the one hand, prebiotic supplementation may lead to a long-term change in gut microbiota composition. On the other hand, SCFAs which have many effects on gut mucosal physiology (Guilloteau et al., 2010; Macia et al., 2012) may directly interact with the PYY-producing cells since Gpr41, a G protein-coupled receptor for SCFAs, is expressed by a subset of enteroendocrine cells in the gut epithelium (Samuel et al., 2008). Colonisation of the mouse colon with a fermentative human microbial community increases the plasma level of PYY, an effect that is blunted by knockout of Gpr41. Gpr41 deficiency is associated with a reduced expression of PYY, an increase in intestinal transit rate and an attenuation of energy harvest (Samuel et al., 2008). Microbiota-fermented SCFAs acting via Gpr41 on PYY-expressing L cells could thus be an important link in microbial–host communication.
3.4. The NPY system protects against distinct behavioural disturbances caused by peripheral immune challenge
Infection and inflammation are increasingly recognised to contribute to the pathogenesis of mood disorders including major depression (Raison et al., 2006; Dantzer et al., 2008; Maes et al., 2012). Peripheral induction of cytokines by infection or cancer often induces a sequence of behavioural alterations comprising sickness followed by depression, these effects being abrogated by cytokine antagonists or cytokine synthesis blockers (Yirmiya, 1996; Anisman et al., 2005; Dunn et al., 2005; Raison et al., 2006; Dantzer et al., 2008; Pyter et al., 2009). Emerging clinical evidence suggests that activation of the intestinal immune system by constituents of the intestinal microbiota can give rise to a similar syndrome of behavioural alterations (Maes et al., 2009). Bacterial cell wall components such as LPS may penetrate the intestinal mucosal barrier once it has become leaky (Maes et al., 2012) as a result of stress, low-grade intestinal inflammation or a dysbalance between the intestinal microbiota and the intestinal immune system. As a consequence, translocation of bacterial cell wall constituents or intestinal bacteria themselves across the intestinal mucosa has been proposed to be a pathogenetic factor in psychiatric diseases such as depression and chronic fatigue syndrome (Maes et al., 2009). In support of this hypothesis, the serum concentrations of immunoglobulin A and M against lipopolysaccharide of gram-negative enterobacteria are increased in patients with major depression (Maes et al., 2012).
Experimentally, the impact of peripheral immune challenge on brain function and behaviour can be modelled by systemic administration of LPS or Bacille Calmette-Guérin (BCG). These interventions induce the formation of cytokines and evoke a behavioural syndrome that follows a distinct time course (Yirmiya, 1996; Dantzer et al., 2008; Moreau et al., 2008). Initially, a response termed “sickness behaviour” is observed, including anorexia, reduction of locomotion and a decrease in social interaction. Once the sickness behaviour in terms of anorexia and sedation is over, behavioural symptoms indicative of depression such as anhedonia and passive stress coping may persist for several weeks (Frenois et al., 2007; Moreau et al., 2008; Painsipp et al., 2011b). The signalling pathways whereby peripheral immune challenge alters brain mechanisms involve proinflammatory cytokines such interleukin-6, tumour necrosis factor-α and interferon-γ, which reach the brain via the bloodstream but also excite vagal afferent neurons and lead to the expression of cytokines by cerebral microglial cells and astrocytes (Anisman et al., 2005; Dunn et al., 2005; Raison et al., 2006; Dantzer et al., 2008).
The impact of peripheral immune challenge on brain function and behaviour involves several brain nuclei (Frenois et al., 2007) that express NPY and various Y receptors. NPY is involved in the regulation of emotional-affective behaviour (Heilig, 2004; Redrobe et al., 2004a; Morales-Medina et al., 2010), and there is indirect evidence that NPY-expressing neurons in the arcuate and paraventricular nuclei of the hypothalamus counteract the behavioural responses to immune stress and infection (McCarthy et al., 1995; Sonti et al., 1996; McMahon et al., 1999). This implication has been borne out in knockout experiments in which the cerebral NPY-Y receptor system has been found to protect against distinct behavioural disturbances in response to peripheral immune challenge. For instance, deletion of NPY as well as NPY plus PYY aggravates the BCG-induced loss of body weight and markedly delays recovery from this weight loss (Painsipp et al., submitted for publication). This finding attests to an important physiological role of NPY and PYY in maintaining energy homeostasis in the face of infection and immune stimulation (Painsipp et al., submitted for publication).
Analogous observations have been made when the acute sickness and delayed depression-like responses to LPS are analysed in Y2 and Y4 knockout mice. Thus, Y2 receptor knockout mice are particularly susceptible to the acute action of LPS to attenuate locomotion and suppress social interaction (Painsipp et al., 2008a). In contrast, the LPS-induced rise of temperature and circulating corticosterone is suppressed by Y2 receptor knockout (Painsipp et al., 2008a). The short-term effect of LPS to enhance anxiety is enhanced in Y2 and Y4 receptor knockout mice (Painsipp et al., 2008a, 2010a). In Y4 receptor knockout mice, the anxiogenic response to LPS persists at least for 4 weeks post-treatment by which time it has waned in WT mice (Painsipp et al., 2010a). Depression-related behaviour is enhanced 1 day post-LPS in control and Y2 receptor knockout mice, but not in Y4 receptor knockout mice. Four weeks post-treatment the depressogenic effect of LPS has waned in wild-type mice, but is maintained in Y2 receptor knockout mice and first observed in Y4 receptor knockout mice (Painsipp et al., 2010a). Thus, knockout of Y2 and/or Y4 receptors unmasks the ability of immune challenge with LPS to cause a delayed and prolonged increase in anxiety- and/or depression-like behaviour. These findings suggest that NPY acting via Y2 and Y4 receptors prevents the development of long-term anxiety- and depression-like behaviour caused by immune challenge (Painsipp et al., 2010a).
3.5. NPY inhibits nociceptive transmission in the spinal cord and brainstem
Abdominal pain arises from various conditions such as ulceration, perforation, muscle spasms, intestinal obstruction and inflammation (e.g., oesophagitis, gastritis, colitis). As in other tissues, inflammation has a major impact on the nociceptive system, and the pain associated with post-infectious IBS is likewise associated with low-grade inflammation and immune activation in the colonic mucosa. The propensity of the gut towards inflammation is related to its task to defend the body against antigens and pathogens, and to the huge microbiota community that inhabits the colon and is balanced by the local immune system. Apart from its adverse impact on bowel function, inflammation causes hyperalgesia and pain, given that inflammatory mediators sensitise nociceptive pathways (Holzer and Holzer-Petsche, 2009; Hughes et al., 2009; Knowles and Aziz, 2009). Hypersensitivity to pain stimuli can develop in all components of the sensory innervation of the gastrointestinal tract, which is unique since 2 major populations of sensory neurons with input to the central nervous system are present: spinal afferent neurons originating from the dorsal root ganglia, and vagal afferent neurons originating primarily from the nodose ganglion. Neurophysiologically distinct populations of these afferent neurons supply the mesentery, serosa, muscularis and mucosa of the alimentary canal and differ in their sensory modalities (Hughes et al., 2009; Knowles and Aziz, 2009).
Spinal afferent neurons, which contain low amounts of NPY, terminate in the spinal cord where interneurons and descending noradrenergic neurons express appreciable amounts of NPY (Brumovsky et al., 2007; Smith et al., 2007). An abundant occurrence of Y1 and Y2 receptors in the spinal cord enables NPY to play an important role in the processing of incoming nociceptive information. Germ-line knockout of Y1 receptors or conditional knockdown of NPY is associated with thermal, chemical and mechanical hyperalgesia (Naveilhan et al., 2001; Shi et al., 2006; Painsipp et al., 2010b; Solway et al., 2011). Inflammation-independent pain evoked by intraperitoneal injection of MgSO4 and inflammation-dependent visceral pain evoked by intraperitoneal injection of acetic acid is likewise exacerbated by Y1 receptor deletion (Naveilhan et al., 2001). Peripheral inflammation leads to an upregulation of Y1 receptors in spinal afferent neurons and in the dorsal horn of the spinal cord (Ji et al., 1994). However, these studies have primarily focused on somatic pain, and it awaits to be explored whether the antinociceptive role of NPY in the spinal cord also holds true for visceral pain. Two major mechanisms whereby NPY controls pain transmission in the spinal cord have been envisaged: inhibition of transmitter release from the terminals of primary afferent neurons, mediated primarily by Y2 receptors, and inhibition of postsynaptic neurons in the dorsal horn, mediated primarily by Y1 receptors (Brumovsky et al., 2007; Smith et al., 2007).
Apart from spinal sensory neurons, vagal afferent neurons have also been established to play a role in visceral nociception, particularly in visceral chemonociception. Thus, vagal afferents signal intragastric acid challenge to the nucleus tractus solitarii (NTS) as visualised by expression of c-Fos, a marker of neuronal activation, and evoke behavioural reactions indicative of pain (Schuligoi et al., 1998; Lamb et al., 2003). Y2 and Y4 receptors are the Y receptor subtypes prevailing in the rat NTS (Gustafson et al., 1997; Larsen and Kristensen, 1997; Dumont et al., 1998; Parker and Herzog, 1999), and gene deletion experiments have revealed that endogenous NPY acting via Y2 and Y4 receptors controls the chemonociceptive input from the stomach to the brainstem (Wultsch et al., 2005). Specifically, the intragastric acid-evoked expression of c-Fos in the NTS is enhanced in Y2 and Y4 receptor knockout mice. Further analysis has shown that NPY acting via Y2 and Y4 receptors depresses the gastric input to the NTS by a central site of action (Wultsch et al., 2005).
NPY may be both a transmitter and modulator of the communication between vagal afferents and their projection neurons in the NTS, since this neuropeptide (Lawrence et al., 1998) as well as Y1, Y2 and Y4 receptors (Ergene et al., 1993; Gustafson et al., 1997; Larsen and Kristensen, 1997; Zhang et al., 1997; Dumont et al., 1998; Parker and Herzog, 1999; Kopp et al., 2002) are expressed by certain vagal afferents and NTS neurons. While the Y2 receptor-mediated effects of NPY arise most likely from a presynaptic site of action on vagal afferents (Ergene et al., 1993; Zhang et al., 1997), the site of Y4 receptors in the control of afferent input to the brainstem remains to be analysed. The role of NPY in the gut–brain axis at the level of the brainstem is not limited to the control of chemonociceptive input but also extends to the reflex control of visceral functions. Thus, stimulation of Y receptors in the brainstem influences gastric motility, acid secretion and mucosal integrity (Chen et al., 1997; Yang et al., 1999; Yang, 2002).
3.6. NPY may protect from the impact of stress on the gut–brain axis
Physical stress such as infection, immune challenge, inflammation and pain as well as psychosocial stress have a marked impact on brain function and behaviour. Chronic abdominal pain, which is a common symptom of inflammatory bowel disease and functional gastrointestinal disorders (Knowles and Aziz, 2009), is frequently accompanied by anxiety and mood disorders (Mawdsley and Rampton, 2005; Graff et al., 2009; Taché and Bernstein, 2009), and psychological stress and depression go often hand in hand with an aggravation of intestinal inflammation (Mittermaier et al., 2004; Ghia et al., 2009). Psychosocial stress is also known to trigger or exacerbate visceral pain, and IBS is frequently associated with stress hypersensitivity and impaired stress coping (Levy et al., 2006; Longstreth et al., 2006; Nicholl et al., 2008; Spiller and Garsed, 2009). Neuroanatomical and functional brain imaging studies indicate that noxious signals from the body are processed in brain areas that are closely interrelated with the neuronal circuitry relevant to anxiety and mood (Mayer et al., 2008). Experimental studies confirm that gastrointestinal inflammation alters emotional-affective behaviour in a gender-dependent manner (Painsipp et al., 2007, 2011a; Goehler et al., 2008). Thus, experimental gastritis is associated with an increase of anxiety in female mice (Painsipp et al., 2007), whereas experimental colitis enhances anxiety-related behaviour in male and depression-like behaviour in female mice (Painsipp et al., 2011a).
Since NPY plays a role in pain, mood and stress coping, it may also be relevant to the impact of stress on the gut–brain axis. This argument takes account of a large body of experimental and clinical evidence that NPY is an important regulator of emotional processing. NPY as well as Y1, Y2 and Y5 receptors are widely expressed in cerebral areas critical to the regulation of anxiety, mood, cognition and stress resilience (Kask et al., 2002; Heilig, 2004; Redrobe et al., 2004a; Eva et al., 2006; Morales-Medina et al., 2010). The expression of NPY in the human brain is related to polymorphisms in the NPY gene, and a low NPY expression genotype is associated with negative emotional processing, diminished stress resilience, a risk for major depression, and a reduced antidepressant treatment response (Zhou et al., 2008; Domschke et al., 2010; Mickey et al., 2011). Exposure of individuals with a low NPY expression genotype to negative stimuli causes an exaggerated activation of the amygdala, medial prefrontal cortex and anterior cingulate cortex (Zhou et al., 2008; Domschke et al., 2010; Mickey et al., 2011). The concentration of NPY in the cerebrospinal fluid and plasma is reduced in patients with post-traumatic stress disorder, while trauma-exposed individuals who do not develop or have recovered from post-traumatic stress disorder have enhanced plasma levels of NPY (Morgan et al., 2003; Yehuda et al., 2006; Sah et al., 2009). It is argued, therefore, that the cerebrospinal and plasma concentration of NPY is a biological correlate of resilience to or recovery from the adverse effects of stress (Yehuda et al., 2006).
Animal experiments confirm that NPY is involved in the emotional processing of stress. The amygdala, which is a key brain region coordinating behavioural stress responses (McEwen, 2007), contains high levels of NPY and Y1, Y2, Y4 and Y5 receptors (Parker and Herzog, 1999; Kask et al., 2002; Kopp et al., 2002; Wolak et al., 2003; Tasan et al., 2009). Intracerebroventricular and intraamygdalar administration as well as amygdalar overexpression of NPY have an anxiolytic effect which is primarily mediated by Y1 receptors, although other receptors (e.g., Y5) may also contribute (Heilig et al., 1989; Karlsson et al., 2005; Sørensen et al., 2004; Primeaux et al., 2005; Fendt et al., 2009). The action of NPY to attenuate anxiety- and depression-related behaviour and improve stress coping is corroborated by the anxiogenic and depressive phenotype of NPY and Y1 receptor knockout mice (Bannon et al., 2000; Karl et al., 2006, 2008; Painsipp et al., 2011a). In contrast, genetic deletion or pharmacological antagonism of Y2 and Y4 receptors attenuates anxiety- and depression-like behaviour (Redrobe et al., 2003; Tschenett et al., 2003; Bacchi et al., 2006; Painsipp et al., 2008b; Tasan et al., 2009, 2010), which indicates that NPY signalling via Y2 and Y4 receptors has a negative impact on emotional-affective behaviour. The opposing effects of Y2 and Y1 receptor activation are due to their differential location at cerebral synapses: NPY-mediated transmission via excitatory postsynaptic Y1 receptors is counter-regulated by presynaptic Y2 receptor activation which inhibits the release of NPY and other transmitters (Heilig, 2004; Chee and Colmers, 2008; Morales-Medina et al., 2010).
Stress-induced changes in the amygdalar expression of NPY and Y1 receptors and a specific anti-stress action of intraamygdalar administration of NPY provide further evidence for an important role of NPY in stress processing. For instance, acute restraint stress decreases NPY expression in the amygdala, whereas intermittent restraint stress over a 10 day period or exposure to a variable stress paradigm for 1 week increases NPY expression within this structure (Thorsell et al., 1998, 1999; McGuire et al., 2011). In contrast, exposure to footshocks for as little as 15 min is able to enhance the amygdalar NPY expression as measured 2 weeks after the intervention (de Lange et al., 2008). Y1 receptor expression in the amygdala is increased by acute restraint stress, but left unaltered by prolonged restraint over a period of 10 days (Mele et al., 2004), whereas a single session of inescapable foot shock reduces amygdalar Y1 receptor expression (Hendriksen et al., 2012). Transgenic NPY overexpression attenuates anxiety-like responses induced by restraint stress (Thorsell et al., 2000) much as repeated infusion of NPY into the basolateral amygdala prevents stress-induced behavioural changes in the social interaction test (Sajdyk et al., 2008). Behavioural disturbances evoked by exposure of mice to predator-scent stress are followed by a marked downregulation of NPY in the hippocampus, periaqueductal grey and amygdala, and intracerebral administration of NPY after stress exposure can reduce the manifestation of stress-induced disruption of behaviour (Cohen et al., 2012).
Given that NPY promotes stress resilience and aids the recovery from stress, it is emerging that the NPY system also influences the impact of stress on the gut–brain axis. Exposure of rats to water avoidance stress lowers the plasma level of PYY, a change that is associated with an increase in gastrointestinal motility (Liang et al., 2012). Trinitrobenzene sulfonic acid-induced colitis increases the NPY concentration in brain and plasma (Baticic et al., 2011) whereas the expression of NPY mRNA in the brain remains unaltered by Helicobacter pylori infection (Bercik et al., 2009). Gastrointestinal inflammation enhances anxiety- and depression-related behaviour in a gender-dependent manner (Painsipp et al., 2007, 2011a; Goehler et al., 2008), these effects being modified by genetic deficiency of PYY and/or NPY. Specifically, the ability of experimental colitis to enhance anxiety-related behaviour in male mice is prevented by genetic deletion of PYY, but not NPY, whereas the increase in depression-like behaviour which DSS causes in female mice is abolished by NPY and PYY knockout (Painsipp et al., 2011a). These observations attest to distinct roles of PYY and NPY in the impact of intestinal inflammation on the gut–brain axis. In addition, the depression-like phenotype of PYY knockout animals (Painsipp et al., 2011a) suggests that alterations in the expression of this gut hormone modify mood and stress coping. The gender-related impact on behaviour may be related to a gender-dependent implication of NPY in the stress-evoked release of corticosterone (Forbes et al., 2012).
NPY is not only a stress mediator in the central nervous system but also in the periphery. The stress-related implications of NPY impact on many physiological systems including the cardiovascular system, the gastrointestinal tract, the immune system, metabolism, and adaptation to stress (Hirsch and Zukowska, 2012). It is obvious that these implications also affect gut–brain and brain–gut communication. For instance, deletion of NPY alters gastrointestinal, feeding and corticosterone responses to restraint stress in a gender-dependent manner (Forbes et al., 2012). Thus, the stress-induced delay of upper gastrointestinal transit is more pronounced in female than in male NPY knockout mice. In addition, deletion of NPY exaggerates stress-induced defaecation and reduces food intake, these effects being gender-independent (Forbes et al., 2012). The stress-evoked release of corticosterone is enhanced by NPY deletion in male mice, and further experiments indicate that NPY inhibits corticosterone release via peripheral Y1 and Y2 receptor activation (Forbes et al., 2012).
3.7. PYY, PP and NPY regulate food intake and energy homeostasis
The gastrointestinal tract is a major endocrine organ that produces and releases a variety of messengers that act either in a paracrine or endocrine manner to coordinate digestion and regulate metabolic homeostasis. The implications of PP, PYY and NPY in gut–brain signalling are particularly well exemplified by their effects on hunger, food intake, satiety and energy balance. Since these roles have been extensively reviewed elsewhere (Murphy and Bloom, 2006; Field et al., 2010; Kirchner et al., 2010; Zhang et al., 2011), only some of the salient features are considered here. PP and PYY function as satiety factors, slowing the gastrointestinal transit of chyme, inhibiting further intake of food and modifying metabolic and energy homeostasis (Field et al., 2010). Since these actions take place, at least in part, in the brain, it should be concluded that PP and PYY are able to signal to the brain.
PP, which is a preferential agonist at Y4 receptors, is released postprandially from endocrine cells in pancreatic islets under vagal cholinergic control (Schwartz, 1983; Murphy and Bloom, 2006; Field et al., 2010). Peripheral administration of PP causes Y4 receptor-dependent neuronal stimulation (c-Fos expression) in the brainstem, hypothalamus and amygdala (Tasan et al., 2009). However, it is not clear whether PP can directly reach these cerebral structures, although there is evidence that PP can enter the brain (Inui et al., 1993; Banks et al., 1995) preferentially via circumventricular organs such as the area postrema (Dumont et al., 2007). PP causes a negative energy balance by decreasing food intake and gastric emptying and by increasing energy expenditure, these effects also involving the vagus nerve (Asakawa et al., 2003; Murphy and Bloom, 2006; Field et al., 2010). Knockout of the Y4 gene leads to an increase in nocturnal feeding (Edelsbrunner et al., 2009b), while transgenic overexpression of PP in mice reduces food intake and body weight gain (Ueno et al., 1999). A reduction of food intake by PP is also seen in lean humans as well as in obese patients with Prader-Willi syndrome (Berntson et al., 1993; Batterham et al., 2003b). In the rodent brain, Y4 receptors have been localised to the medulla, locus coeruleus, hypothalamus, hippocampus, medial and basolateral amygdala, and ventral tegmental area (Dumont et al., 1998; Parker and Herzog, 1999; Campbell et al., 2003; Fetissov et al., 2004). While the role of Y4 receptors in synaptic transmission is little known, Y4 receptors in the hypothalamus are involved in presynaptic inhibition of transmitter release (Acuna-Goycolea et al., 2005).
PYY is released postprandially from intestinal L cells in proportion to energy intake (Field et al., 2010) and in part truncated to PYY3–36 which is the main circulating form of PYY and a relatively selective Y2 receptor agonist. PYY and PYY3–36 inhibit gastric acid secretion, gastrointestinal transit and food intake in rodents and humans (Batterham et al., 2002; Challis et al., 2003; Nonaka et al., 2003; Koda et al., 2005; Field et al., 2010). The anorectic effect of PYY3–36 is seen in both lean and obese subjects and in higher doses is associated with nausea and vomiting (Batterham et al., 2002, 2003a; Field et al., 2010). Food intake is inhibited by PYY3–36 both via a stimulant effect on Y2 receptors on vagal afferent neurons (Koda et al., 2005; Ueno et al., 2008) and an interaction with Y2 receptors in the hypothalamus. This is consistent with the ability of PYY3–36 to gain access to the brain via circumventricular organs such as the area postrema and subfornical organ (Dumont et al., 2007) but also to permeate the blood–brain barrier to a certain extent (Nonaka et al., 2003).
Within the brain, PYY3–36 reduces food intake primarily via activation of Y2 receptors in the arcuate nucleus which is an important centre for integrating peripheral and central signals in the control of appetite and energy homeostasis (Chee and Colmers, 2008). The arcuate nucleus contains at least two populations of neurons that are relevant in this respect: orexigenic neurons expressing NPY and AgRP and anorexigenic neurons expressing pro-opiomelanocortin (POMC). These neurons send projections to various areas of the hypothalamus (including the paraventricular nucleus) and reciprocally inhibit the orexigenic/anorexigenic tone exerted by NPY/AgRP neurons and POMC neurons, respectively (Chee and Colmers, 2008). The anorectic effect of PYY3–36 is mediated primarily by presynaptic Y2 receptors on NPY/AgRP neurons in the arcuate nucleus, inhibiting their orexigenic action and disinhibiting POMC neurons (Batterham et al., 2002; Murphy and Bloom, 2006; Ueno et al., 2008; Field et al., 2010). In addition, peripheral infusion of PYY3–36, at doses mimicking the plasma levels of PYY3–36 in the fed state, modulates neural activity in corticolimbic areas (Batterham et al., 2007).
NPY is one of the most potent orexigenic peptides found in the brain (Chee and Colmers, 2008; Zhang et al., 2011), the NPY/AgRP neurons in the arcuate nucleus taking a central place in this respect. Their orexigenic effect is primarily mediated by Y1 receptors, although Y5 receptors also play a role (Zhang et al., 2011). Selective ablation of NPY/AgRP neurons in the arcuate nucleus of adult mice results in a life-threatening reduction of food intake (Gropp et al., 2005; Luquet et al., 2005). These finding indicates that feeding depends critically on the function of NPY/AgRP neurons. Conversely, pathologies associated with a decrease in food intake such as experimental colitis lead to increased release of NPY from the paraventricular nucleus of the hypothalamus (Ballinger et al., 2001). Experiments with NPY knockout mice attest to a physiological function of endogenous NPY in balancing energy intake with physical activity-related energy expenditure (Edelsbrunner et al., 2009a; Zhang et al., 2011). Besides the hypothalamus, many other neuronal systems in the brain express NPY, and it is likely that some of these NPY neurons (e.g., in the brainstem, nucleus accumbens and corticolimbic system) likewise play a role in the regulation of appetite and food intake.
3.8. PYY, PP and other gut hormones regulate anxiety and mood
Apart from regulating ingestion and energy homeostasis, gut hormones such as ghrelin, PYY, glucagon-like peptide-1 and glucagon-like peptide-2 have also an impact on emotional-affective behaviour. Seen from an evolutionary point of view, co-regulation of appetite and emotional state is an important strategy for survival, given that anxiety would be an adverse condition when there is a need to seek food. Indeed, ghrelin which is released from the upper gastrointestinal tract under conditions of hunger reduces both anxiety-like and depression-related behaviour (Lutter et al., 2008). Under fed conditions, behaviour is changed to a hedonic state as observed when PYY3–36 is administered to reach postprandial plasma concentrations of the peptide (Batterham et al., 2007). Physiologically, however, emotion and mood under fed conditions will be determined by the presence of a variety of gut hormones that are released postprandially. Among these, glucagon-like peptide-1 has been found to enhance anxiety-related behaviour (Möller et al., 2002; Kinzig et al., 2003; Gulec et al., 2010), while glucagon-like peptide-2 attenuates depression-like behaviour (Iwai et al., 2009).
The ability of PYY to promote hedonic behaviour is supported by the finding that knockout of PYY increases depression-like behaviour but does not alter anxiety (Painsipp et al., 2011a). This finding indicates that the gut hormone PYY or its major circulating form, PYY3–36, is able to signal to the central nervous system, either by gaining direct access to the brain (Nonaka et al., 2003; Dumont et al., 2007) or by activating vagal afferent input to the brainstem (Koda et al., 2005; Ueno et al., 2008). Although the biological actions of PYY3–36 are mediated primarily by Y2 receptors, it is not yet understood which Y receptors mediate the antidepressant effect of gut-derived PYY, given that stimulation of Y2 receptors in the brain increases anxiety- and depression-like behaviour (Heilig, 2004). In addition, knockout of Y2 receptors reduces anxiety-related and depression-like behaviour and impairs learning and memory (Tschenett et al., 2003; Redrobe et al., 2003, 2004b; Painsipp et al., 2008b; Tasan et al., 2009, 2010). The anxiolytic and antidepressant action of Y2 receptor knockout has been localised to the deletion of presynaptic Y2 receptors in the central and basolateral amygdala (Tasan et al., 2010).
PP whose biological actions are preferentially mediated by Y4 receptors is also able to influence emotional-affective behaviour. Thus, anxiety- and depression-like behaviour is attenuated and novelty-evoked locomotion increased in Y4 receptor knockout mice (Painsipp et al., 2008b; Tasan et al., 2009). The anxiolytic-like phenotype of Y4 receptor knockout mice is consistent with the anxiogenic phenotype of PP-overexpressing mice (Ueno et al., 2007). Since intracerebroventricular PP fails to alter anxiety-related behaviour (Asakawa et al., 1999) while chronic peripheral administration of PP reduces anxiety (Asakawa et al., 2003), it would appear that PP modifies anxiety- and depression-like behaviour through an action in the periphery or in the area postrema outside the blood–brain barrier (Larsen and Kristensen, 1997; Dumont et al., 2007; Tasan et al., 2009). This possibility is backed by the ability of peripheral PP to induce Y4 receptor-dependent c-Fos expression in brainstem, hypothalamus and amygdala and by the expression of an appreciable number of Y4 receptors in these regions (Tasan et al., 2009). In addition, knockout of the Y4 receptor is associated with an increase in the circulating level of PP (Sainsbury et al., 2002).
4. Conclusions
As outlined in this review, the study of the gut–brain axis has proved to be of major relevance not only to gastroenterology but also to experimental and clinical neuroscience, notably psychiatry. The gut microbiota which is a symbiotic superorganism by itself, the intestinal mucosa and the intestinal immune system issue multiple signals to the brain. These signals are carried by sensory neurons, immune mediators, gut hormones and microbiota-derived signalling molecules. In disease, this input from the gastrointestinal tract gives rise to disturbances of digestion and gastrointestinal function, pain and hyperalgesia, emotional-affective disturbances, defective stress coping and cognitive impairment. In addition, the autonomic and neuroendocrine outputs of the brain to the gut may also be deranged.
The NPY-Y receptor system provides several targets whereby gut–brain axis disorders could potentially be managed. The different members of this peptide family, NPY, PYY and PP, operate both as neural and endocrine messengers at distinct levels of the gut–brain axis (Fig. 2). PYY and PP are exclusively expressed by endocrine cells of the digestive system, whereas NPY is found at all levels of the gut–brain and brain–gut axis. In the gastrointestinal tract, NPY and PYY inhibit gastrointestinal motility and electrolyte secretion and may also interact with the intestinal microbiota. Y1 receptors operated by NPY exert a proinflammatory action in the gut, and the NPY-Y receptor system protects against distinct behavioural disturbances caused by peripheral immune challenge, attenuating the acute sickness response and preventing the development of long-term depression. Similarly, NPY inhibits nociceptive input from the periphery to the spinal cord and brainstem. In the brain, NPY and its receptors (Y1, Y2, Y4, Y5) play important roles in regulating locomotion, exploration, food intake, energy homeostasis, anxiety, mood and stress resilience. This action profile is in part shared by the gut hormones PP and PYY which signal to the brain to attenuate food intake, anxiety and depression-related behaviour.
These findings underscore an important role of the NPY-Y receptor system at several levels of the gut–brain axis and support the hypothesis that Y receptors are worthwhile targets for the therapy of gut–brain axis disorders such as IBS. Unfortunately, the pharmacology of the NPY-Y receptor system is developed only to a limited extent so that many therapeutic opportunities arising from the implications of the NPY-Y receptor system in the gut–brain axis cannot yet be thoroughly evaluated. However, gene knockout and transgenic approaches attest to the validity of the NPY system as a therapeutic target, a conclusion that is increasingly supported by clinical studies. Given that the NPY-Y receptor system operates as a multi-level homeostatic mechanism, pharmacological manipulation of this system may have considerable therapeutic efficacy.
Acknowledgements
This study was supported by the Zukunftsfonds Steiermark (Grant 262), the Austrian Science Funds (FWF Grants L25-B05 and P23097-B18), and the Federal Ministry of Science and Research of the Republic of Austria (Grant GZ 80.104/2-BrGT/2007).
References
- Acuna-Goycolea C., Tamamaki N., Yanagawa Y., Obata K., van den Pol A.N. Mechanisms of neuropeptide Y, peptide YY, and pancreatic polypeptide inhibition of identified green fluorescent protein-expressing GABA neurons in the hypothalamic neuroendocrine arcuate nucleus. J. Neurosci. 2005;25:7406–7419. doi: 10.1523/JNEUROSCI.1008-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian T.E., Savage A.P., Bacarese-Hamilton A.J., Wolfe K., Besterman H.S., Bloom S.R. Peptide YY abnormalities in gastrointestinal diseases. Gastroenterology. 1986;90:379–384. doi: 10.1016/0016-5085(86)90936-4. [DOI] [PubMed] [Google Scholar]
- Ahlman H., Nilsson O. The gut as the largest endocrine organ in the body. Ann. Oncol. 2001;12(Suppl. 2):S63–S68. doi: 10.1093/annonc/12.suppl_2.s63. [DOI] [PubMed] [Google Scholar]
- Alexander S.P.H., Mathie A., Peters J.A. Guide to receptors and channels (GRAC), 5th ed. Br. J. Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen Y.S., Adrian T.E., Allen J.M., Tatemoto K., Crow T.J., Bloom S.R. Neuropeptide Y distribution in the rat brain. Science. 1983;221:877–879. doi: 10.1126/science.6136091. [DOI] [PubMed] [Google Scholar]
- Anisman H., Merali Z., Poulter M.O., Hayley S. Cytokines as a precipitant of depressive illness: Animal and human studies. Curr. Pharm. Des. 2005;11:963–972. doi: 10.2174/1381612053381701. [DOI] [PubMed] [Google Scholar]
- Asakawa A., Inui A., Ueno N., Fujimiya M., Fujino M.A., Kasuga M. Mouse pancreatic polypeptide modulates food intake, while not influencing anxiety in mice. Peptides. 1999;20:1445–1448. doi: 10.1016/s0196-9781(99)00155-2. [DOI] [PubMed] [Google Scholar]
- Asakawa A., Inui A., Yuzuriha H., Ueno N., Katsuura G., Fujimiya M., Fujino M.A., Niijima A., Meguid M.M., Kasuga M. Characterization of the effects of pancreatic polypeptide in the regulation of energy balance. Gastroenterology. 2003;124:1325–1336. doi: 10.1016/s0016-5085(03)00216-6. [DOI] [PubMed] [Google Scholar]
- Bacchi F., Mathé A.A., Jimenez P., Stasi L., Arban R., Gerrard P., Caberlotto L. Anxiolytic-like effect of the selective Neuropeptide Y Y2 receptor antagonist BIIE0246 in the elevated plus-maze. Peptides. 2006;27:3202–3207. doi: 10.1016/j.peptides.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Ballinger A.B., Williams G., Corder R., El-Haj T., Farthing M.J. Role of hypothalamic neuropeptide Y and orexigenic peptides in anorexia associated with experimental colitis in the rat. Clin. Sci. (Lond.) 2001;100:221–229. [PubMed] [Google Scholar]
- Banks W.A., Kastin A.J., Jaspan J.B. Regional variation in transport of pancreatic polypeptide across the blood–brain barrier of mice. Pharmacol. Biochem. Behav. 1995;51:139–147. doi: 10.1016/0091-3057(94)00412-c. [DOI] [PubMed] [Google Scholar]
- Bannon A.W., Seda J., Carmouche M., Francis J.M., Norman M.H., Karbon B., McCaleb M.L. Behavioral characterization of neuropeptide Y knockout mice. Brain Res. 2000;868:79–87. doi: 10.1016/s0006-8993(00)02285-x. [DOI] [PubMed] [Google Scholar]
- Baticic L., Detel D., Kucic N., Buljevic S., Pugel E.P., Varljen J. Neuroimmunomodulative properties of dipeptidyl peptidase IV/CD26 in a TNBS-induced model of colitis in mice. J. Cell. Biochem. 2011;112:3322–3333. doi: 10.1002/jcb.23261. [DOI] [PubMed] [Google Scholar]
- Batterham R.L., Cowley M.A., Small C.J., Herzog H., Cohen M.A., Dakin C.L., Wren A.M., Brynes A.E., Low M.J., Ghatei M.A., Cone R.D., Bloom S.R. Gut hormone PYY3–36 physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- Batterham R.L., Cohen M.A., Ellis S.M., Le Roux C.W., Withers D.J., Frost G.S., Ghatei M.A., Bloom S.R. Inhibition of food intake in obese subjects by peptide YY3–36. N. Engl. J. Med. 2003;349:941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- Batterham R.L., Le Roux C.W., Cohen M.A., Park A.J., Ellis S.M., Patterson M., Frost G.S., Ghatei M.A., Bloom S.R. Pancreatic polypeptide reduces appetite and food intake in humans. J. Clin. Endocrinol. Metab. 2003;88:3989–3992. doi: 10.1210/jc.2003-030630. [DOI] [PubMed] [Google Scholar]
- Batterham R.L., Ffytche D.H., Rosenthal J.M., Zelaya F.O., Barker G.J., Withers D.J., Williams S.C. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature. 2007;450:106–109. doi: 10.1038/nature06212. [DOI] [PubMed] [Google Scholar]
- Bedoui S., von Hörsten S., Gebhardt T. A role for neuropeptide Y (NPY) in phagocytosis: Implications for innate and adaptive immunity. Peptides. 2007;28:373–376. doi: 10.1016/j.peptides.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Bercik P., Verdu E.F., Foster J.A., Lu J., Scharringa A., Kean I., Wang L., Blennerhassett P., Collins S.M. Role of gut–brain axis in persistent abnormal feeding behavior in mice following eradication of Helicobacter pylori infection. Am. J. Physiol. 2009;296:R587–R594. doi: 10.1152/ajpregu.90752.2008. [DOI] [PubMed] [Google Scholar]
- Bercik P., Denou E., Collins J., Jackson W., Lu J., Jury J., Deng Y., Blennerhassett P., Macri J., McCoy K.D., Verdu E.F., Collins S.M. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141:599–609. doi: 10.1053/j.gastro.2011.04.052. [DOI] [PubMed] [Google Scholar]
- Bercik P., Park A.J., Sinclair D., Khoshdel A., Lu J., Huang X., Deng Y., Blennerhassett P.A., Fahnestock M., Moine D., Berger B., Huizinga J.D., Kunze W., McLean P.G., Bergonzelli G.E., Collins S.M., Verdu E.F. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut–brain communication. Neurogastroenterol. Motil. 2011;23:1132–1139. doi: 10.1111/j.1365-2982.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercik P., Collins S.M., Verdu E.F. Microbes and the gut–brain axis. Neurogastroenterol. Motil. 2012;24:405–413. doi: 10.1111/j.1365-2982.2012.01906.x. [DOI] [PubMed] [Google Scholar]
- Berntson G.G., Zipf W.B., O’Dorisio T.M., Hoffman J.A., Chance R.E. Pancreatic polypeptide infusions reduce food intake in Prader-Willi syndrome. Peptides. 1993;14:497–503. doi: 10.1016/0196-9781(93)90138-7. [DOI] [PubMed] [Google Scholar]
- Böttcher G., Ekblad E., Ekman R., Håkanson R., Sundler F. Peptide YY: A neuropeptide in the gut. Immunocytochemical and immunochemical evidence. Neuroscience. 1993;55:281–290. doi: 10.1016/0306-4522(93)90472-r. [DOI] [PubMed] [Google Scholar]
- Bravo J.A., Forsythe P., Chew M.V., Escaravage E., Savignac H.M., Dinan T.G., Bienenstock J., Cryan J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumovsky P., Shi T.S., Landry M., Villar M.J., Hökfelt T. Neuropeptide tyrosine and pain. Trends Pharmacol. Sci. 2007;28:93–102. doi: 10.1016/j.tips.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Campbell R.E., Smith M.S., Allen S.E., Grayson B.E., Ffrench-Mullen J.M., Grove K.L. Orexin neurons express a functional pancreatic polypeptide Y4 receptor. J. Neurosci. 2003;23:1487–1497. doi: 10.1523/JNEUROSCI.23-04-01487.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani P.D., Delzenne N.M. The gut microbiome as therapeutic target. Pharmacol. Ther. 2011;130:202–212. doi: 10.1016/j.pharmthera.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Cani P.D., Lecourt E., Dewulf E.M., Sohet F.M., Pachikian B.D., Naslain D., De Backer F., Neyrinck A.M., Delzenne N.M. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am. J. Clin. Nutr. 2009;90:1236–1243. doi: 10.3945/ajcn.2009.28095. [DOI] [PubMed] [Google Scholar]
- Challis B.G., Pinnock S.B., Coll A.P., Carter R.N., Dickson S.L., O’Rahilly S. Acute effects of PYY3–36 on food intake and hypothalamic neuropeptide expression in the mouse. Biochem. Biophys. Res. Commun. 2003;311:915–919. doi: 10.1016/j.bbrc.2003.10.089. [DOI] [PubMed] [Google Scholar]
- Chandrasekharan B., Bala V., Kolachala V.L., Vijay-Kumar M., Jones D., Gewirtz A.T., Sitaraman S.V., Srinivasan S. Targeted deletion of neuropeptide Y (NPY) modulates experimental colitis. PLoS One. 2008;3:e3304. doi: 10.1371/journal.pone.0003304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee M.J., Colmers W.F. Y eat? Nutrition. 2008;24:869–877. doi: 10.1016/j.nut.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Chen C.H., Stephens R.L., Rogers R.C. PYY and NPY: Control of gastric motility via action on Y1 and Y2 receptors in the DVC. Neurogastroenterol. Motil. 1997;9:109–116. doi: 10.1046/j.1365-2982.1997.d01-26.x. [DOI] [PubMed] [Google Scholar]
- Cherbut C., Ferrier L., Rozé C., Anini Y., Blottière H., Lecannu G., Galmiche J.P. Short-chain fatty acids modify colonic motility through nerves and polypeptide YY release in the rat. Am. J. Physiol. 1998;275:G1415–G1422. doi: 10.1152/ajpgi.1998.275.6.G1415. [DOI] [PubMed] [Google Scholar]
- Clarke T.B., Davis K.M., Lysenko E.S., Zhou A.Y., Yu Y., Weiser J.N. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat. Med. 2010;16:228–231. doi: 10.1038/nm.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H., Liu T., Kozlovsky N., Kaplan Z., Zohar J., Mathé A.A. The neuropeptide Y (NPY)-ergic system is associated with behavioral resilience to stress exposure in an animal model of post-traumatic stress disorder. Neuropsychopharmacology. 2012;37:350–363. doi: 10.1038/npp.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox H.M. Neuropeptide Y receptors; antisecretory control of intestinal epithelial function. Auton. Neurosci. 2007;133:76–85. doi: 10.1016/j.autneu.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Cox H.M. Peptide YY: A neuroendocrine neighbor of note. Peptides. 2007;28:345–351. doi: 10.1016/j.peptides.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Cox H.M., Tough I.R. Neuropeptide Y, Y1, Y2 and Y4 receptors mediate Y agonist responses in isolated human colon mucosa. Br. J. Pharmacol. 2002;135:1505–1512. doi: 10.1038/sj.bjp.0704604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox H.M., Tough I.R., Woolston A.M., Zhang L., Nguyen A.D., Sainsbury A., Herzog H. Peptide YY is critical for acylethanolamine receptor Gpr119-induced activation of gastrointestinal mucosal responses. Cell Metab. 2010;11:532–542. doi: 10.1016/j.cmet.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R., O’Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange R.P., Wiegant V.M., Stam R. Altered neuropeptide Y and neurokinin messenger RNA expression and receptor binding in stress-sensitised rats. Brain Res. 2008;1212:35–47. doi: 10.1016/j.brainres.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Delzenne N.M., Neyrinck A.M., Bäckhed F., Cani P.D. Targeting gut microbiota in obesity: Effects of prebiotics and probiotics. Nat. Rev. Endocrinol. 2011;7:639–646. doi: 10.1038/nrendo.2011.126. [DOI] [PubMed] [Google Scholar]
- DiMaggio D.A., Chronwall B.M., Buchanan K., O’Donohue T.L. Pancreatic polypeptide immunoreactivity in rat brain is actually neuropeptide Y. Neuroscience. 1985;15:1149–1157. doi: 10.1016/0306-4522(85)90259-3. [DOI] [PubMed] [Google Scholar]
- Dimitrijević, M., Stanojević, S., in press. The intriguing mission of neuropeptide Y in the immune system. Amino Acids. [DOI] [PubMed]
- Domschke K., Dannlowski U., Hohoff C., Ohrmann P., Bauer J., Kugel H., Zwanzger P., Heindel W., Deckert J., Arolt V., Suslow T., Baune B.T. Neuropeptide Y (NPY) gene: Impact on emotional processing and treatment response in anxious depression. Eur. Neuropsychopharmacol. 2010;20:301–309. doi: 10.1016/j.euroneuro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Dumont Y., Jacques D., Bouchard P., Quirion R. Species differences in the expression and distribution of the neuropeptide Y Y1, Y2, Y4, and Y5 receptors in rodents, guinea pig, and primates brains. J. Comp. Neurol. 1998;402:372–384. [PubMed] [Google Scholar]
- Dumont Y., Moyse E., Fournier A., Quirion R. Distribution of peripherally injected peptide YY ([125I]PYY3–36) and pancreatic polypeptide ([125I]hPP) in the CNS: Enrichment in the area postrema. J. Mol. Neurosci. 2007;33:294–304. doi: 10.1007/s12031-007-9007-9. [DOI] [PubMed] [Google Scholar]
- Dunn A.J., Swiergiel A.H., de Beaurepaire R. Cytokines as mediators of depression: What can we learn from animal studies? Neurosci. Biobehav. Rev. 2005;29:891–909. doi: 10.1016/j.neubiorev.2005.03.023. [DOI] [PubMed] [Google Scholar]
- DuPont A.W., DuPont H.L. The intestinal microbiota and chronic disorders of the gut. Nat. Rev. Gastroenterol. Hepatol. 2011;8:523–531. doi: 10.1038/nrgastro.2011.133. [DOI] [PubMed] [Google Scholar]
- Eaton K., Sallee F.R., Sah R. Relevance of neuropeptide Y (NPY) in psychiatry. Curr. Top. Med. Chem. 2007;7:1645–1659. doi: 10.2174/156802607782341037. [DOI] [PubMed] [Google Scholar]
- Edelsbrunner M.E., Herzog H., Holzer P. Evidence from knockout mice that peptide YY and neuropeptide Y enforce murine locomotion, exploration and ingestive behaviour in a circadian cycle- and gender-dependent manner. Behav. Brain Res. 2009;203:97–107. doi: 10.1016/j.bbr.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelsbrunner M.E., Painsipp E., Herzog H., Holzer P. Evidence from knockout mice for distinct implications of neuropeptide-Y Y2 and Y4 receptors in the circadian control of locomotion, exploration, water and food intake. Neuropeptides. 2009;43:491–497. doi: 10.1016/j.npep.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblad E., Sundler F. Distribution of pancreatic polypeptide and peptide YY. Peptides. 2002;23:251–261. doi: 10.1016/s0196-9781(01)00601-5. [DOI] [PubMed] [Google Scholar]
- El Karim I.A., Linden G.J., Orr D.F., Lundy F.T. Antimicrobial activity of neuropeptides against a range of micro-organisms from skin, oral, respiratory and gastrointestinal tract sites. J. Neuroimmunol. 2008;200:11–16. doi: 10.1016/j.jneuroim.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Elitsur Y., Luk G.D., Colberg M., Gesell M.S., Dosescu J., Moshier J.A. Neuropeptide Y (NPY) enhances proliferation of human colonic lamina propria lymphocytes. Neuropeptides. 1994;26:289–295. doi: 10.1016/0143-4179(94)90113-9. [DOI] [PubMed] [Google Scholar]
- El-Salhy M., Danielsson A., Stenling R., Grimelius L. Colonic endocrine cells in inflammatory bowel disease. J. Intern. Med. 1997;242:413–419. doi: 10.1046/j.1365-2796.1997.00237.x. [DOI] [PubMed] [Google Scholar]
- Ergene E., Dunbar J.C., Barraco R.A. Visceroendocrine responses elicited by neuropeptide Y in the nucleus tractus solitarius. Brain Res. Bull. 1993;32:461–465. doi: 10.1016/0361-9230(93)90291-i. [DOI] [PubMed] [Google Scholar]
- Eva C., Serra M., Mele P., Panzica G., Oberto A. Physiology and gene regulation of the brain NPY Y1 receptor. Front. Neuroendocrinol. 2006;27:308–339. doi: 10.1016/j.yfrne.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Feltrin K.L., Patterson M., Ghatei M.A., Bloom S.R., Meyer J.H., Horowitz M., Feinle-Bisset C. Effect of fatty acid chain length on suppression of ghrelin and stimulation of PYY, GLP-2 and PP secretion in healthy men. Peptides. 2006;27:1638–1643. doi: 10.1016/j.peptides.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Fendt M., Bürki H., Imobersteg S., Lingenhöhl K., McAllister K.H., Orain D., Uzunov D.P., Chaperon F. Fear-reducing effects of intra-amygdala neuropeptide Y infusion in animal models of conditioned fear: An NPY Y1 receptor independent effect. Psychopharmacology (Berl.) 2009;206:291–301. doi: 10.1007/s00213-009-1610-8. [DOI] [PubMed] [Google Scholar]
- Fetissov S.O., Kopp J., Hökfelt T. Distribution of NPY receptors in the hypothalamus. Neuropeptides. 2004;38:175–188. doi: 10.1016/j.npep.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Field B.C., Chaudhri O.B., Bloom S.R. Bowels control brain: Gut hormones and obesity. Nat. Rev. Endocrinol. 2010;6:444–453. doi: 10.1038/nrendo.2010.93. [DOI] [PubMed] [Google Scholar]
- Forbes S., Herzog H., Cox H. A role for neuropeptide Y in the gender-specific gastrointestinal, corticosterone and feeding responses to stress. Br. J. Pharmacol. 2012;166:2307–2316. doi: 10.1111/j.1476-5381.2012.01939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenois F., Moreau M., O’Connor J., Lawson M., Micon C., Lestage J., Kelley K.W., Dantzer R., Castanon N. Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology. 2007;32:516–531. doi: 10.1016/j.psyneuen.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu-Cheng X., Anini Y., Chariot J., Castex N., Galmiche J.P., Rozé C. Mechanisms of peptide YY release induced by an intraduodenal meal in rats: Neural regulation by proximal gut. Pflügers Arch. 1997;433:571–579. doi: 10.1007/s004240050316. [DOI] [PubMed] [Google Scholar]
- Fujimiya M., Inui A. Peptidergic regulation of gastrointestinal motility in rodents. Peptides. 2000;21:1565–1582. doi: 10.1016/s0196-9781(00)00313-2. [DOI] [PubMed] [Google Scholar]
- Gakis G., Mueller M.H., Hahn J., Glatzle J., Grundy D., Kreis M.E. Neuronal activation in the nucleus of the solitary tract following jejunal lipopolysaccharide in the rat. Auton. Neurosci. 2009;148:63–68. doi: 10.1016/j.autneu.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Gareau M.G., Wine E., Rodrigues D.M., Cho J.H., Whary M.T., Philpott D.J., MacQueen G., Sherman P.M. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60:307–317. doi: 10.1136/gut.2009.202515. [DOI] [PubMed] [Google Scholar]
- Ghia J.E., Blennerhassett P., Deng Y., Verdu E.F., Khan W.I., Collins S.M. Reactivation of inflammatory bowel disease in a mouse model of depression. Gastroenterology. 2009;136:2280–2288. doi: 10.1053/j.gastro.2009.02.069. [DOI] [PubMed] [Google Scholar]
- Goehler L.E., Park S.M., Opitz N., Lyte M., Gaykema R.P.A. Campylobacter jejuni infection increases anxiety-like behavior in the holeboard: Possible anatomical substrates for viscerosensory modulation of exploratory behavior. Brain Behav. Immun. 2008;22:354–366. doi: 10.1016/j.bbi.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff L.A., Walker J.R., Bernstein C.N. Depression and anxiety in inflammatory bowel disease: A review of comorbidity and management. Inflamm. Bowel Dis. 2009;15:1105–1118. doi: 10.1002/ibd.20873. [DOI] [PubMed] [Google Scholar]
- Grenham S., Clarke G., Cryan J.F., Dinan T.G. Brain–gut–microbe communication in health and disease. Front. Physiol. 2011;2:94. doi: 10.3389/fphys.2011.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gropp E., Shanabrough M., Borok E., Xu A.W., Janoschek R., Buch T., Plum L., Balthasar N., Hampel B., Waisman A., Barsh G.S., Horvath T.L., Brüning J.C. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat. Neurosci. 2005;8:1289–1291. doi: 10.1038/nn1548. [DOI] [PubMed] [Google Scholar]
- Guilloteau P., Martin L., Eeckhaut V., Ducatelle R., Zabielski R., Van Immerseel F. From the gut to the peripheral tissues: The multiple effects of butyrate. Nutr. Res. Rev. 2010;23:366–384. doi: 10.1017/S0954422410000247. [DOI] [PubMed] [Google Scholar]
- Gulec G., Isbil-Buyukcoskun N., Kahveci N. Effects of centrally-injected glucagon-like peptide-1 on pilocarpine-induced seizures, anxiety and locomotor and exploratory activity in rat. Neuropeptides. 2010;44:285–291. doi: 10.1016/j.npep.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Gustafson E.L., Smith K.E., Durkin M.M., Walker M.W., Gerald C., Weinshank R., Branchek T.A. Distribution of the neuropeptide Y Y2 receptor mRNA in rat central nervous system. Mol. Brain Res. 1997;46:223–235. doi: 10.1016/s0169-328x(97)00017-x. [DOI] [PubMed] [Google Scholar]
- Halldén G., Aponte G.W. Evidence for a role of the gut hormone PYY in the regulation of intestinal fatty acid-binding protein transcripts in differentiated subpopulations of intestinal epithelial cell hybrids. J. Biol. Chem. 1997;272:12591–12600. doi: 10.1074/jbc.272.19.12591. [DOI] [PubMed] [Google Scholar]
- Hassani H., Lucas G., Rozell B., Ernfors P. Attenuation of acute experimental colitis by preventing NPY Y1 receptor signaling. Am. J. Physiol. 2005;288:G550–G556. doi: 10.1152/ajpgi.00182.2004. [DOI] [PubMed] [Google Scholar]
- Heijtz R.D., Wang S., Anuar F., Qian Y., Björkholm B., Samuelsson A., Hibberd M.L., Forssberg H., Pettersson S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M. The NPY system in stress, anxiety and depression. Neuropeptides. 2004;38:213–224. doi: 10.1016/j.npep.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Heilig M., Söderpalm B., Engel J.A., Widerlöv E. Centrally administered neuropeptide Y (NPY) produces anxiolytic-like effects in animal anxiety models. Psychopharmacology (Berl.) 1989;98:524–529. doi: 10.1007/BF00441953. [DOI] [PubMed] [Google Scholar]
- Hendriksen H., Bink D.I., Daniels E.G., Pandit R., Piriou C., Slieker R., Westphal K.G., Olivier B., Oosting R.S. Re-exposure and environmental enrichment reveal NPY-Y1 as a possible target for post-traumatic stress disorder. Neuropharmacology. 2012;63:733–742. doi: 10.1016/j.neuropharm.2012.05.028. [DOI] [PubMed] [Google Scholar]
- Hernanz A., Tato E., De la Fuente M., de Miguel E., Arnalich F. Differential effects of gastrin-releasing peptide, neuropeptide Y, somatostatin and vasoactive intestinal peptide on interleukin-1 beta, interleukin-6 and tumor necrosis factor-alpha production by whole blood cells from healthy young and old subjects. J. Neuroimmunol. 1996;71:25–30. doi: 10.1016/s0165-5728(96)00118-x. [DOI] [PubMed] [Google Scholar]
- Hirotani Y., Mikajiri K., Ikeda K., Myotoku M., Kurokawa N. Changes of the peptide YY levels in the intestinal tissue of rats with experimental colitis following oral administration of mesalazine and prednisolone. Yakugaku Zasshi. 2008;128:1347–1353. doi: 10.1248/yakushi.128.1347. [DOI] [PubMed] [Google Scholar]
- Hirsch D., Zukowska Z. NPY and stress 30 years later: The peripheral view. Cell. Mol. Neurobiol. 2012;32:645–659. doi: 10.1007/s10571-011-9793-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P. Neural regulation of gastrointestinal blood flow. In: Johnson L.R., editor. Physiology of the Gastrointestinal Tract. 5th ed. Academic Press; Oxford: 2012. pp. 817–845. [Google Scholar]
- Holzer P., Holzer-Petsche U. Pharmacology of inflammatory pain: Local alteration in receptors and mediators. Dig. Dis. 2009;27(Suppl. 1):24–30. doi: 10.1159/000268118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P., Barthó L., Lippe I.T., Petritsch W., Leb G. Effect of pancreatic polypeptide on the motility of the guinea-pig small intestine in vitro. Regul. Pept. 1986;16:305–314. doi: 10.1016/0167-0115(86)90030-3. [DOI] [PubMed] [Google Scholar]
- Holzer P., Lippe I.T., Barthó L., Saria A. Neuropeptide Y inhibits excitatory enteric neurons supplying the circular muscle of the guinea pig small intestine. Gastroenterology. 1987;92:1944–1950. doi: 10.1016/0016-5085(87)90628-7. [DOI] [PubMed] [Google Scholar]
- Holzer P., Schicho R., Holzer-Petsche U., Lippe I.T. The gut as a neurological organ. Wien. Klin. Wochenschr. 2001;113:647–660. [PubMed] [Google Scholar]
- Holzer P., Danzer M., Schicho R., Samberger C., Painsipp E., Lippe I.T. Vagal afferent input from the acid-challenged rat stomach to the brainstem: Enhancement by interleukin-1β. Neuroscience. 2004;129:439–445. doi: 10.1016/j.neuroscience.2004.07.040. [DOI] [PubMed] [Google Scholar]
- Holzer-Petsche U., Petritsch W., Hinterleitner T., Eherer A., Sperk G., Krejs G.J. Effect of neuropeptide Y on jejunal water and ion transport in humans. Gastroenterology. 1991;101:325–330. doi: 10.1016/0016-5085(91)90007-8. [DOI] [PubMed] [Google Scholar]
- Hubel K.A., Renquist K.S. Effect of neuropeptide Y on ion transport by the rabbit ileum. J. Pharmacol. Exp. Ther. 1986;238:167–169. [PubMed] [Google Scholar]
- Hughes P.A., Brierley S.M., Blackshaw L.A. Post-inflammatory modification of colonic afferent mechanosensitivity. Clin. Exp. Pharmacol. Physiol. 2009;36:1034–1040. doi: 10.1111/j.1440-1681.2009.05248.x. [DOI] [PubMed] [Google Scholar]
- Inui A., Okita M., Miura M., Hirosue Y., Mizuno N., Baba S., Kasuga M. Plasma and cerebroventricular fluid levels of pancreatic polypeptide in the dog: Effects of feeding, insulin-induced hypoglycemia, and physical exercise. Endocrinology. 1993;132:1235–1239. doi: 10.1210/endo.132.3.8440184. [DOI] [PubMed] [Google Scholar]
- Iwai T., Hayashi Y., Narita S., Kasuya Y., Jin K., Tsugane M., Oka J. Antidepressant-like effects of glucagon-like peptide-2 in mice occur via monoamine pathways. Behav. Brain Res. 2009;204:235–240. doi: 10.1016/j.bbr.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Janssen P., Verschueren S., Rotondo A., Tack J. Role of Y2 receptors in the regulation of gastric tone in rats. Am. J. Physiol. 2012;302:G732–G739. doi: 10.1152/ajpgi.00404.2011. [DOI] [PubMed] [Google Scholar]
- Jeffery I.B., O’Toole P.W., Ohman L., Claesson M.J., Deane J., Quigley E.M., Simrén M. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut. 2012;61:997–1006. doi: 10.1136/gutjnl-2011-301501. [DOI] [PubMed] [Google Scholar]
- Ji R.R., Zhang X., Wiesenfeld-Hallin Z., Hökfelt T. Expression of neuropeptide Y and neuropeptide Y (Y1) receptor mRNA in rat spinal cord and dorsal root ganglia following peripheral tissue inflammation. J. Neurosci. 1994;14:6423–6434. doi: 10.1523/JNEUROSCI.14-11-06423.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl T., Burne T.H., Herzog H. Effect of Y1 receptor deficiency on motor activity, exploration, and anxiety. Behav. Brain Res. 2006;167:87–93. doi: 10.1016/j.bbr.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Karl T., Duffy L., Herzog H. Behavioural profile of a new mouse model for NPY deficiency. Eur. J. Neurosci. 2008;28:173–180. doi: 10.1111/j.1460-9568.2008.06306.x. [DOI] [PubMed] [Google Scholar]
- Karlsson R.M., Holmes A., Heilig M., Crawley J.N. Anxiolytic-like actions of centrally-administered neuropeptide Y, but not galanin, in C57BL/6J mice. Pharmacol. Biochem. Behav. 2005;80:427–436. doi: 10.1016/j.pbb.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Kask A., Harro J., von Hörsten S., Redrobe J.P., Dumont Y., Quirion R. The neurocircuitry and receptor subtypes mediating anxiolytic-like effects of neuropeptide Y. Neurosci. Biobehav. Rev. 2002;26:259–283. doi: 10.1016/s0149-7634(01)00066-5. [DOI] [PubMed] [Google Scholar]
- Kinzig K.P., D’Alessio D.A., Herman J.P., Sakai R.R., Vahl T.P., Figueiredo H.F., Murphy E.K., Seeley R.J. CNS glucagon-like peptide-1 receptors mediate endocrine and anxiety responses to interoceptive and psychogenic stressors. J. Neurosci. 2003;23:6163–6170. doi: 10.1523/JNEUROSCI.23-15-06163.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner H., Tong J., Tschöp M.H., Pfluger P.T. Ghrelin and PYY in the regulation of energy balance and metabolism: Lessons from mouse mutants. Am. J. Physiol. 2010;298:E909–E919. doi: 10.1152/ajpendo.00191.2009. [DOI] [PubMed] [Google Scholar]
- Klompus M., Ho W., Sharkey K.A., McKay D.M. Antisecretory effects of neuropeptide Y in the mouse colon are region-specific and are lost in DSS-induced colitis. Regul. Pept. 2010;165:138–145. doi: 10.1016/j.regpep.2010.05.014. [DOI] [PubMed] [Google Scholar]
- Knowles C.H., Aziz Q. Basic and clinical aspects of gastrointestinal pain. Pain. 2009;141:191–209. doi: 10.1016/j.pain.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Koda S., Date Y., Murakami N., Shimbara T., Hanada T., Toshinai K., Niijima A., Furuya M., Inomata N., Osuye K., Nakazato M. The role of the vagal nerve in peripheral PYY3–36-induced feeding reduction in rats. Endocrinology. 2005;146:2369–2375. doi: 10.1210/en.2004-1266. [DOI] [PubMed] [Google Scholar]
- Kopp J., Xu Z.Q., Zhang X., Pedrazzini T., Herzog H., Kresse A., Wong H., Walsh J.H., Hökfelt T. Expression of the neuropeptide Y Y1 receptor in the CNS of rat and of wild-type and Y1 receptor knock-out mice. Focus on immunohistochemical localization. Neuroscience. 2002;111:443–532. doi: 10.1016/s0306-4522(01)00463-8. [DOI] [PubMed] [Google Scholar]
- Krogius-Kurikka L., Lyra A., Malinen E., Aarnikunnas J., Tuimala J., Paulin L., Mäkivuokko H., Kajander K., Palva A. Microbial community analysis reveals high level phylogenetic alterations in the overall gastrointestinal microbiota of diarrhoea-predominant irritable bowel syndrome sufferers. BMC Gastroenterol. 2009;9:95. doi: 10.1186/1471-230X-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb K., Kang Y.M., Gebhart G.F., Bielefeldt K. Gastric inflammation triggers hypersensitivity to acid in awake rats. Gastroenterology. 2003;125:1410–1418. doi: 10.1016/j.gastro.2003.07.010. [DOI] [PubMed] [Google Scholar]
- Larsen P.J., Kristensen P. The neuropeptide Y Y4 receptor is highly expressed in neurones of the rat dorsal vagal complex. Mol. Brain Res. 1997;48:1–6. doi: 10.1016/s0169-328x(97)00069-7. [DOI] [PubMed] [Google Scholar]
- Lawrence A.J., Castillo-Melendez M., McLean K.J., Jarrott B. The distribution of nitric oxide synthase-, adenosine deaminase- and neuropeptide Y-immunoreactivity through the entire rat nucleus tractus solitarius: Effect of unilateral nodose ganglionectomy. J. Chem. Neuroanat. 1998;15:27–40. doi: 10.1016/s0891-0618(98)00020-9. [DOI] [PubMed] [Google Scholar]
- Levy R.L., Olden K.W., Naliboff B.D., Bradley L.A., Francisconi C., Drossman D.A., Creed F. Psychosocial aspects of the functional gastrointestinal disorders. Gastroenterology. 2006;130:1447–1458. doi: 10.1053/j.gastro.2005.11.057. [DOI] [PubMed] [Google Scholar]
- Liang C., Luo H., Liu Y., Cao J., Xia H. Plasma hormones facilitated the hypermotility of the colon in a chronic stress rat model. PLoS One. 2012;7:e31774. doi: 10.1371/journal.pone.0031774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H.C., Zhao X.T., Wang L., Wong H. Fat-induced ileal brake in the dog depends on PYY. Gastroenterology. 1996;110:1491–1495. doi: 10.1053/gast.1996.v110.pm8613054. [DOI] [PubMed] [Google Scholar]
- Lomax A.E., Sharkey K.A., Furness J.B. The participation of the sympathetic innervation of the gastrointestinal tract in disease states. Neurogastroenterol. Motil. 2010;22:7–18. doi: 10.1111/j.1365-2982.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- Longstreth G.F., Thompson W.G., Chey W.D., Houghton L.A., Mearin F., Spiller R.C. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- Luquet S., Perez F.A., Hnasko T.S., Palmiter R.D. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- Lutter M., Sakata I., Osborne-Lawrence S., Rovinsky S.A., Anderson J.G., Jung S., Birnbaum S., Yanagisawa M., Elmquist J.K., Nestler E.J., Zigman J.M. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat. Neurosci. 2008;11:752–753. doi: 10.1038/nn.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macia L., Thorburn A.N., Binge L.C., Marino E., Rogers K.E., Maslowski K.M., Vieira A.T., Kranich J., Mackay C.R. Microbial influences on epithelial integrity and immune function as a basis for inflammatory diseases. Immunol. Rev. 2012;245:164–176. doi: 10.1111/j.1600-065X.2011.01080.x. [DOI] [PubMed] [Google Scholar]
- Maes M., Yirmyia R., Noraberg J., Brene S., Hibbeln J., Perini G., Kubera M., Bob P., Lerer B., Maj M. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: Leads for future research and new drug developments in depression. Metab. Brain Dis. 2009;24:27–53. doi: 10.1007/s11011-008-9118-1. [DOI] [PubMed] [Google Scholar]
- Maes M., Kubera M., Leunis J.C., Berk M. Increased IgA and IgM responses against gut commensals in chronic depression: Further evidence for increased bacterial translocation or leaky gut. J. Affect. Disord. 2012;141:55–62. doi: 10.1016/j.jad.2012.02.023. [DOI] [PubMed] [Google Scholar]
- Mawdsley J.E., Rampton D.S. Psychological stress in IBD: New insights into pathogenic and therapeutic implications. Gut. 2005;54:1481–1491. doi: 10.1136/gut.2005.064261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer E.A., Bradesi S., Chang L., Spiegel B.M., Bueller J.A., Naliboff B.D. Functional GI disorders: From animal models to drug development. Gut. 2008;57:384–404. doi: 10.1136/gut.2006.101675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy H.D., Dryden S., Williams G. Interleukin-1β-induced anorexia and pyrexia in rat: Relationship to hypothalamic neuropeptide Y. Am. J. Physiol. 1995;269:E852–E857. doi: 10.1152/ajpendo.1995.269.5.E852. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol. Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McGowan B.M.C., Bloom S.R. Peptide YY and appetite control. Curr. Opin. Pharmacol. 2004;4:583–588. doi: 10.1016/j.coph.2004.06.007. [DOI] [PubMed] [Google Scholar]
- McGuire J.L., Larke L.E., Sallee F.R., Herman J.P., Sah R. Differential regulation of neuropeptide Y in the amygdala and prefrontal cortex during recovery from chronic variable stress. Front. Behav. Neurosci. 2011;5:54. doi: 10.3389/fnbeh.2011.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKernan D.P., Gaszner G., Quigley E.M., Cryan J.F., Dinan T.G. Altered peripheral toll-like receptor responses in the irritable bowel syndrome. Aliment. Pharmacol. Ther. 2011;33:1045–1052. doi: 10.1111/j.1365-2036.2011.04624.x. [DOI] [PubMed] [Google Scholar]
- McMahon C.D., Buxton D.F., Elsasser T.H., Gunter D.R., Sanders L.G., Steele B.P., Sartin J.L. Neuropeptide Y restores appetite and alters concentrations of GH after central administration to endotoxic sheep. J. Endocrinol. 1999;161:333–339. doi: 10.1677/joe.0.1610333. [DOI] [PubMed] [Google Scholar]
- Medeiros M.D., Turner A.J. Processing and metabolism of peptide-YY: Pivotal roles of dipeptidylpeptidase-IV, aminopeptidase-P, and endopeptidase-24.11. Endocrinology. 1994;134:2088–2094. doi: 10.1210/endo.134.5.7908871. [DOI] [PubMed] [Google Scholar]
- Mele P., Oberto A., Serra M., Pisu M.G., Floris I., Biggio G., Eva C. Increased expression of the gene for the Y1 receptor of neuropeptide Y in the amygdala and paraventricular nucleus of Y1R/LacZ transgenic mice in response to restraint stress. J. Neurochem. 2004;89:1471–1478. doi: 10.1111/j.1471-4159.2004.02444.x. [DOI] [PubMed] [Google Scholar]
- Melgar S., Shanahan F. Inflammatory bowel disease – From mechanisms to treatment strategies. Autoimmunity. 2010;43:463–477. doi: 10.3109/08916931003674709. [DOI] [PubMed] [Google Scholar]
- Mentlein R., Dahms P., Grandt D., Krüger R. Proteolytic processing of neuropeptide Y and peptide YY by dipeptidyl peptidase IV. Regul. Pept. 1993;49:133–144. doi: 10.1016/0167-0115(93)90435-b. [DOI] [PubMed] [Google Scholar]
- Mickey B.J., Zhou Z., Heitzeg M.M., Heinz E., Hodgkinson C.A., Hsu D.T., Langenecker S.A., Love T.M., Peciña M., Shafir T., Stohler C.S., Goldman D., Zubieta J.K. Emotion processing, major depression, and functional genetic variation of neuropeptide Y. Arch. Gen. Psychiatry. 2011;68:158–166. doi: 10.1001/archgenpsychiatry.2010.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitić K., Stanojević S., Kuštrimović N., Vujić V., Dimitrijević M. Neuropeptide Y modulates functions of inflammatory cells in the rat: Distinct role for Y1, Y2 and Y5 receptors. Peptides. 2011;32:1626–1633. doi: 10.1016/j.peptides.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Mittermaier C., Dejaco C., Waldhoer T., Oefferlbauer-Ernst A., Miehsler W., Beier M., Tillinger W., Gangl A., Moser G. Impact of depressive mood on relapse in patients with inflammatory bowel disease: A prospective 18-month follow-up study. Psychosom. Med. 2004;66:79–84. doi: 10.1097/01.psy.0000106907.24881.f2. [DOI] [PubMed] [Google Scholar]
- Möller C., Sommer W., Thorsell A., Rimondini R., Heilig M. Anxiogenic-like action of centrally administered glucagon-like peptide-1 in a punished drinking test. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2002;26:119–122. doi: 10.1016/s0278-5846(01)00223-8. [DOI] [PubMed] [Google Scholar]
- Morales-Medina J.C., Dumont Y., Quirion R. A possible role of neuropeptide Y in depression and stress. Brain Res. 2010;1314:194–205. doi: 10.1016/j.brainres.2009.09.077. [DOI] [PubMed] [Google Scholar]
- Moreau M., André C., O’Connor J.C., Dumich S.A., Woods J.A., Kelley K.W., Dantzer R., Lestage J., Castanon N. Inoculation of Bacillus Calmette-Guerin to mice induces an acute episode of sickness behavior followed by chronic depressive-like behavior. Brain Behav. Immun. 2008;22:1087–1095. doi: 10.1016/j.bbi.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C.A., Rasmusson A.M., Winters B., Hauger R.L., Morgan J., Hazlett G., Southwick S. Trauma exposure rather than posttraumatic stress disorder is associated with reduced baseline plasma neuropeptide-Y levels. Biol. Psychiatry. 2003;54:1087–1091. doi: 10.1016/s0006-3223(03)00433-5. [DOI] [PubMed] [Google Scholar]
- Morimoto R., Satoh F., Murakami O., Totsune K., Saruta M., Suzuki T., Sasano H., Ito S., Takahashi K. Expression of peptide YY in human brain and pituitary tissues. Nutrition. 2008;24:878–884. doi: 10.1016/j.nut.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Murphy K.G., Bloom S.R. Gut hormones and the regulation of energy homeostasis. Nature. 2006;444:854–859. doi: 10.1038/nature05484. [DOI] [PubMed] [Google Scholar]
- Naveilhan P., Hassani H., Lucas G., Blakeman K.H., Hao J.X., Xu X.J., Wiesenfeld-Hallin Z., Thorén P., Ernfors P. Reduced antinociception and plasma extravasation in mice lacking a neuropeptide Y receptor. Nature. 2001;409:513–517. doi: 10.1038/35054063. [DOI] [PubMed] [Google Scholar]
- Nicholl B.I., Halder S.L., Macfarlane G.J., Thompson D.G., O’Brien S., Musleh M., McBeth J. Psychosocial risk markers for new onset irritable bowel syndrome – Results of a large prospective population-based study. Pain. 2008;137:147–155. doi: 10.1016/j.pain.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nightingale J.M.D., Kamm M.A., van der Sijp J.R.M., Ghatei M.A., Bloom S.R., Lennard-Jones J.E. Gastrointestinal hormones in short bowel syndrome. PYY may be the colonic brake to gastric emptying. Gut. 1996;39:267–272. doi: 10.1136/gut.39.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka N., Shioda S., Niehoff M.L., Banks W.A. Characterization of blood–brain barrier permeability to PYY3–36 in the mouse. J. Pharmacol. Exp. Ther. 2003;306:948–953. doi: 10.1124/jpet.103.051821. [DOI] [PubMed] [Google Scholar]
- Painsipp E., Wultsch T., Shahbazian A., Edelsbrunner M., Kreissl M.C., Schirbel A., Bock E., Pabst M.A., Thoeringer C.K., Huber H.P., Holzer P. Experimental gastritis in mice enhances anxiety in a gender-related manner. Neuroscience. 2007;150:522–536. doi: 10.1016/j.neuroscience.2007.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painsipp E., Herzog H., Holzer P. Implication of neuropeptide-Y Y2 receptors in the effects of immune stress on emotional, locomotor and social behavior of mice. Neuropharmacology. 2008;55:117–126. doi: 10.1016/j.neuropharm.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painsipp E., Wultsch T., Edelsbrunner M.E., Tasan R.O., Singewald N., Herzog H., Holzer P. Reduced anxiety-like and depression-related behavior in neuropeptide Y Y4 receptor knockout mice. Genes Brain Behav. 2008;7:532–542. doi: 10.1111/j.1601-183X.2008.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painsipp E., Herzog H., Holzer P. Evidence from knockout mice that neuropeptide-Y Y2 and Y4 receptor signalling prevents long-term depression-like behavior caused by immune challenge. J. Psychopharmacol. 2010;24:1551–1560. doi: 10.1177/0269881109348171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painsipp E., Sperk G., Herzog H., Holzer P. Delayed stress-induced differences in locomotor and depression-related behaviour in female neuropeptide-Y Y1 receptor knockout mice. J. Psychopharmacol. 2010;24:1541–1549. doi: 10.1177/0269881109104851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painsipp E., Herzog H., Sperk G., Holzer P. Sex-dependent control of murine emotional-affective behaviour in health and colitis by peptide YY and neuropeptide Y. Br. J. Pharmacol. 2011;163:1302–1314. doi: 10.1111/j.1476-5381.2011.01326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painsipp E., Köfer M.J., Sinner F., Holzer P. Prolonged depression-like behavior caused by immune challenge: Influence of mouse strain and social environment. PLoS One. 2011;6:e20719. doi: 10.1371/journal.pone.0020719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painsipp, E., Köfer, M.J., Dischinger, U.S., Sinner, F., Sperk, G., Herzog, H., Holzer, P., submitted for publication. Neuropeptide Y and peptide YY participate in the sickness and behavioural disturbance caused by Bacille Calmette-Guérin. PLoS One.
- Pang X.H., Li T.K., Xie Q., He F.Q., Cui D.J., Chen Y.Q., Huang X.L., Gan H.T. Amelioration of dextran sulfate sodium-induced colitis by neuropeptide Y antisense oligodeoxynucleotide. Int. J. Colorectal Dis. 2010;25:1047–1053. doi: 10.1007/s00384-010-0964-z. [DOI] [PubMed] [Google Scholar]
- Parker R.M., Herzog H. Regional distribution of Y-receptor subtype mRNAs in rat brain. Eur. J. Neurosci. 1999;11:1431–1448. doi: 10.1046/j.1460-9568.1999.00553.x. [DOI] [PubMed] [Google Scholar]
- Pironi L., Stanghellini V., Miglioli M., Corinaldesi R., De Giorgio R., Ruggeri E., Tosetti C., Poggioli G., Morselli Labate A.M., Monetti N., Gozzetti G., Barbara L., Go V.L.W. Fat-induced ileal brake in humans: A dose-dependent phenomenon correlated to the plasma levels of PYY. Gastroenterology. 1993;105:733–739. doi: 10.1016/0016-5085(93)90890-o. [DOI] [PubMed] [Google Scholar]
- Primeaux S.D., Wilson S.P., Cusick M.C., York D.A., Wilson M.A. Effects of altered amygdalar neuropeptide Y expression on anxiety-related behaviors. Neuropsychopharmacology. 2005;30:1589–1597. doi: 10.1038/sj.npp.1300705. [DOI] [PubMed] [Google Scholar]
- Pyter L.M., Pineros V., Galang J.A., McClintock M.K., Prendergast B.J. Peripheral tumors induce depressive-like behaviors and cytokine production and alter hypothalamic–pituitary–adrenal axis regulation. Proc. Natl. Acad. Sci. USA. 2009;106:9069–9074. doi: 10.1073/pnas.0811949106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison C.L., Capuron L., Miller A.H. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redrobe J.P., Dumont Y., Herzog H., Quirion R. Neuropeptide Y (NPY) Y2 receptors mediate behaviour in two animal models of anxiety: Evidence from Y2 receptor knockout mice. Behav. Brain Res. 2003;141:251–255. doi: 10.1016/s0166-4328(02)00374-1. [DOI] [PubMed] [Google Scholar]
- Redrobe J.P., Carvajal C., Kask A., Dumont Y., Quirion R. Neuropeptide Y and its receptor subtypes in the central nervous system: Emphasis on their role in animal models of psychiatric disorders. Handb. Exp. Pharmacol. 2004;162:101–136. [Google Scholar]
- Redrobe J.P., Dumont Y., Herzog H., Quirion R. Characterization of neuropeptide Y, Y2 receptor knockout mice in two animal models of learning and memory processing. J. Mol. Neurosci. 2004;22:159–166. doi: 10.1385/JMN:22:3:159. [DOI] [PubMed] [Google Scholar]
- Rozengurt N., Wu S.V., Chen M.C., Huang C., Sternini C., Rozengurt E. Co-localisation of the α subunit of gustducin with PYY and GLP-1 in L-cells of human colon. Am. J. Physiol. 2006;291:G792–G802. doi: 10.1152/ajpgi.00074.2006. [DOI] [PubMed] [Google Scholar]
- Sah R., Ekhator N.N., Strawn J.R., Sallee F.R., Baker D.G., Horn P.S., Geracioti T.D. Low cerebrospinal fluid neuropeptide Y concentrations in posttraumatic stress disorder. Biol. Psychiatry. 2009;66:705–707. doi: 10.1016/j.biopsych.2009.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainsbury A., Schwarzer C., Couzens M., Jenkins A., Oakes S.R., Ormandy C.J., Herzog H. Y4 receptor knockout rescues fertility in ob/ob mice. Genes Dev. 2002;16:1077–1088. doi: 10.1101/gad.979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdyk T.J., Johnson P.L., Leitermann R.J., Fitz S.D., Dietrich A., Morin M., Gehlert D.R., Urban J.H., Shekhar A. Neuropeptide Y in the amygdala induces long-term resilience to stress-induced reductions in social responses but not hypothalamic–adrenal–pituitary axis activity or hyperthermia. J. Neurosci. 2008;28:893–903. doi: 10.1523/JNEUROSCI.0659-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel B.S., Shaito A., Motoike T., Rey F.E., Backhed F., Manchester J.K., Hammer R.E., Williams S.C., Crowley J., Yanagisawa M., Gordon J.I. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc. Natl. Acad. Sci. USA. 2008;105:16767–16772. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saria A., Beubler E. Neuropeptide Y (NPY) and peptide YY (PYY) inhibit prostaglandin E2-induced intestinal fluid and electrolyte secretion in the rat jejunum in vivo. Eur. J. Pharmacol. 1985;119:47–52. doi: 10.1016/0014-2999(85)90320-6. [DOI] [PubMed] [Google Scholar]
- Schicho R., Schemann M., Pabst M.A., Holzer P., Lippe I.T. Capsaicin-sensitive extrinsic afferents are involved in acid-induced activation of distinct myenteric neurons in the rat stomach. Neurogastroenterol. Motil. 2003;15:33–44. doi: 10.1046/j.1365-2982.2003.00384.x. [DOI] [PubMed] [Google Scholar]
- Schmidt P.T., Ljung T., Hartmann B., Hare K.J., Holst J.J., Hellström P.M. Tissue levels and post-prandial secretion of the intestinal growth factor, glucagon-like peptide-2, in controls and inflammatory bowel disease: Comparison with peptide YY. Eur. J. Gastroenterol. Hepatol. 2005;17:207–712. doi: 10.1097/00042737-200502000-00012. [DOI] [PubMed] [Google Scholar]
- Schuligoi R., Jocic M., Heinemann A., Schöninkle E., Pabst M.A., Holzer P. Gastric acid-evoked c-fos messenger RNA expression in rat brainstem is signaled by capsaicin-resistant vagal afferents. Gastroenterology. 1998;115:649–660. doi: 10.1016/s0016-5085(98)70144-1. [DOI] [PubMed] [Google Scholar]
- Schwartz T.W. Pancreatic polypeptide: A unique model for vagal control of endocrine systems. J. Auton. Nerv. Syst. 1983;9:99–111. doi: 10.1016/0165-1838(83)90134-0. [DOI] [PubMed] [Google Scholar]
- Shanahan F. The colonic microflora and probiotic therapy in health and disease. Curr. Opin. Gastroenterol. 2011;27:61–65. doi: 10.1097/MOG.0b013e328340076f. [DOI] [PubMed] [Google Scholar]
- Shi T.J., Li J., Dahlström A., Theodorsson E., Ceccatelli S., Decosterd I., Pedrazzini T., Hökfelt T. Deletion of the neuropeptide Y Y1 receptor affects pain sensitivity, neuropeptide transport and expression, and dorsal root ganglion neuron numbers. Neuroscience. 2006;140:293–304. doi: 10.1016/j.neuroscience.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Shibata M., Hisajima T., Nakano M., Goris R.C., Funakoshi K. Morphological relationships between peptidergic nerve fibers and immunoglobulin A-producing lymphocytes in the mouse intestine. Brain Behav. Immun. 2008;22:158–166. doi: 10.1016/j.bbi.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Small C.J., Bloom S.R. The therapeutic potential of gut hormone peptide YY3–36 in the treatment of obesity. Expert Opin. Investig. Drugs. 2005;14:647–653. doi: 10.1517/13543784.14.5.647. [DOI] [PubMed] [Google Scholar]
- Smith P.A., Moran T.D., Abdulla F., Tumber K.K., Taylor B.K. Spinal mechanisms of NPY analgesia. Peptides. 2007;28:464–474. doi: 10.1016/j.peptides.2006.09.029. [DOI] [PubMed] [Google Scholar]
- Solway B., Bose S.C., Corder G., Donahue R.R., Taylor B.K. Tonic inhibition of chronic pain by neuropeptide Y. Proc. Natl. Acad. Sci. USA. 2011;108:7224–7229. doi: 10.1073/pnas.1017719108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonti G., Ilyin S.E., Plata-Salaman C.R. Neuropeptide Y blocks and reverses interleukin-1β-induced anorexia in rats. Peptides. 1996;17:517–520. doi: 10.1016/0196-9781(96)00016-2. [DOI] [PubMed] [Google Scholar]
- Sørensen G., Lindberg C., Wörtwein G., Bolwig T.G., Woldbye D.P. Differential roles for neuropeptide Y Y1 and Y5 receptors in anxiety and sedation. J. Neurosci. Res. 2004;77:723–729. doi: 10.1002/jnr.20200. [DOI] [PubMed] [Google Scholar]
- Spiller R., Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology. 2009;136:1979–1988. doi: 10.1053/j.gastro.2009.02.074. [DOI] [PubMed] [Google Scholar]
- Straub R.H., Herfarth H., Falk W., Andus T., Schölmerich J. Uncoupling of the sympathetic nervous system and the hypothalamic–pituitary–adrenal axis in inflammatory bowel disease? J. Neuroimmunol. 2002;126:116–125. doi: 10.1016/s0165-5728(02)00047-4. [DOI] [PubMed] [Google Scholar]
- Taché Y., Bernstein C.N. Evidence for the role of the brain–gut axis in inflammatory bowel disease: Depression as cause and effect? Gastroenterology. 2009;136:2058–2061. doi: 10.1053/j.gastro.2009.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tana C., Umesaki Y., Imaoka A., Handa T., Kanazawa M., Fukudo S. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol. Motil. 2010;22:512–519. doi: 10.1111/j.1365-2982.2009.01427.x. [DOI] [PubMed] [Google Scholar]
- Tari A., Teshima H., Sumii K., Haruma K., Ohgoshi H., Yoshihara M., Kajiyama G., Miyachi Y. Peptide YY abnormalities in patients with ulcerative colitis. Jpn. J. Med. 1988;27:49–55. doi: 10.2169/internalmedicine1962.27.49. [DOI] [PubMed] [Google Scholar]
- Tasan R.O., Lin S., Hetzenauer A., Singewald N., Herzog H., Sperk G. Increased novelty-induced motor activity and reduced depression-like behavior in neuropeptide Y (NPY)-Y4 receptor knockout mice. Neuroscience. 2009;158:1717–1730. doi: 10.1016/j.neuroscience.2008.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasan R.O., Nguyen N.K., Weger S., Sartori S.B., Singewald N., Heilbronn R., Herzog H., Sperk G. The central and basolateral amygdala are critical sites of neuropeptide Y/Y2 receptor-mediated regulation of anxiety and depression. J. Neurosci. 2010;30:6282–6290. doi: 10.1523/JNEUROSCI.0430-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsell A., Svensson P., Wiklund L., Sommer W., Ekman R., Heilig M. Suppressed neuropeptide Y (NPY) mRNA in rat amygdala following restraint stress. Regul. Pept. 1998;75–76:247–254. doi: 10.1016/s0167-0115(98)00075-5. [DOI] [PubMed] [Google Scholar]
- Thorsell A., Carlsson K., Ekman R., Heilig M. Behavioral and endocrine adaptation, and up-regulation of NPY expression in rat amygdala following repeated restraint stress. Neuroreport. 1999;10:3003–3007. doi: 10.1097/00001756-199909290-00024. [DOI] [PubMed] [Google Scholar]
- Thorsell A., Michalkiewicz M., Dumont Y., Quirion R., Caberlotto L., Rimondini R., Mathé A.A., Heilig M. Behavioral insensitivity to restraint stress, absent fear suppression of behavior and impaired spatial learning in transgenic rats with hippocampal neuropeptide Y overexpression. Proc. Natl. Acad. Sci. USA. 2000;97:12852–12857. doi: 10.1073/pnas.220232997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tough I.R., Holliday N.D., Cox H.M. Y4 receptors mediate the inhibitory responses of pancreatic polypeptide in human and mouse colon mucosa. J. Pharmacol. Exp. Ther. 2006;319:20–30. doi: 10.1124/jpet.106.106500. [DOI] [PubMed] [Google Scholar]
- Tough I.R., Forbes S., Tolhurst R., Ellis M., Herzog H., Bornstein J.C., Cox H.M. Endogenous peptide YY and neuropeptide Y inhibit colonic ion transport, contractility and transit differentially via Y1 and Y2 receptors. Br. J. Pharmacol. 2011;164:471–484. doi: 10.1111/j.1476-5381.2011.01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschenett A., Singewald N., Carli M., Balducci C., Salchner P., Vezzani A., Herzog H., Sperk G. Reduced anxiety and improved stress coping ability in mice lacking NPY-Y2 receptors. Eur. J. Neurosci. 2003;18:143–148. doi: 10.1046/j.1460-9568.2003.02725.x. [DOI] [PubMed] [Google Scholar]
- Ueno N., Inui A., Iwamoto M., Kaga T., Asakawa A., Okita M., Fujimiya M., Nakajima Y., Ohmoto Y., Ohnaka M., Nakaya Y., Miyazaki J.I., Kasuga M. Decreased food intake and body weight in pancreatic polypeptide-overexpressing mice. Gastroenterology. 1999;117:1427–1432. doi: 10.1016/s0016-5085(99)70293-3. [DOI] [PubMed] [Google Scholar]
- Ueno N., Asakawa A., Satoh Y., Inui A. Increased circulating cholecystokinin contributes to anorexia and anxiety behavior in mice overexpressing pancreatic polypeptide. Regul. Pept. 2007;141:8–11. doi: 10.1016/j.regpep.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Ueno H., Yamaguchi H., Mizuta M., Nakazato M. The role of PYY in feeding regulation. Regul. Pept. 2008;145:12–16. doi: 10.1016/j.regpep.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Van Citters G.W., Lin H.C. Ileal brake: Neuropeptidergic control of intestinal transit. Curr. Gastroenterol. Rep. 2006;8:367–373. doi: 10.1007/s11894-006-0021-9. [DOI] [PubMed] [Google Scholar]
- Wang L., Gourcerol G., Yuan P.Q., Wu S.V., Million M., Larauche M., Taché Y. Peripheral peptide YY inhibits propulsive colonic motor function through Y2 receptor in conscious mice. Am. J. Physiol. 2010;298:G45–G56. doi: 10.1152/ajpgi.00349.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettstein J.G., Earley B., Junien J.L. Central nervous system pharmacology of neuropeptide Y. Pharmacol. Ther. 1995;65:397–414. doi: 10.1016/0163-7258(95)98598-k. [DOI] [PubMed] [Google Scholar]
- Wheway J., Mackay C.R., Newton R.A., Sainsbury A., Boey D., Herzog H., Mackay F. A fundamental bimodal role for neuropeptide Y1 receptor in the immune system. J. Exp. Med. 2005;202:1527–1538. doi: 10.1084/jem.20051971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheway J., Herzog H., Mackay F. NPY and receptors in immune and inflammatory diseases. Curr. Top. Med. Chem. 2007;7:1743–1752. doi: 10.2174/156802607782341046. [DOI] [PubMed] [Google Scholar]
- Wheway J., Herzog H., Mackay F. The Y1 receptor for NPY: A key modulator of the adaptive immune system. Peptides. 2007;28:453–458. doi: 10.1016/j.peptides.2006.09.030. [DOI] [PubMed] [Google Scholar]
- Wittig B.M., Zeitz M. The gut as an organ of immunology. Int. J. Colorectal. Dis. 2003;18:181–187. doi: 10.1007/s00384-002-0444-1. [DOI] [PubMed] [Google Scholar]
- Wolak M.L., DeJoseph M.R., Cator A.D., Mokashi A.S., Brownfield M.S., Urban J.H. Comparative distribution of neuropeptide Y Y1 and Y5 receptors in the rat brain by using immunohistochemistry. J. Comp. Neurol. 2003;464:285–311. doi: 10.1002/cne.10823. [DOI] [PubMed] [Google Scholar]
- Wultsch T., Painsipp E., Thoeringer C.K., Herzog H., Sperk G., Holzer P. Endogenous neuropeptide Y depresses the afferent signaling of gastric acid challenge to the mouse brainstem via neuropeptide Y type Y2 and Y4 receptors. Neuroscience. 2005;136:1097–1107. doi: 10.1016/j.neuroscience.2005.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. Central and peripheral regulation of gastric acid secretion by peptide YY. Peptides. 2002;23:349–358. doi: 10.1016/s0196-9781(01)00611-8. [DOI] [PubMed] [Google Scholar]
- Yang H., Kawakubo K., Tache Y. Intracisternal PYY increases gastric mucosal resistance: Role of cholinergic, CGRP, and NO pathways. Am. J. Physiol. 1999;277:G555–G562. doi: 10.1152/ajpgi.1999.277.3.G555. [DOI] [PubMed] [Google Scholar]
- Yehuda R., Brand S., Yang R.K. Plasma neuropeptide Y concentrations in combat exposed veterans: Relationship to trauma exposure, recovery from PTSD, and coping. Biol. Psychiatry. 2006;59:660–663. doi: 10.1016/j.biopsych.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Yirmiya R. Endotoxin produces a depressive-like episode in rats. Brain Res. 1996;711:163–174. doi: 10.1016/0006-8993(95)01415-2. [DOI] [PubMed] [Google Scholar]
- Zhang X., Shi T., Holmberg K., Landry M., Huang W., Xiao H., Ju G., Hökfelt T. Expression and regulation of the neuropeptide Y Y2 receptor in sensory and autonomic ganglia. Proc. Natl. Acad. Sci. USA. 1997;94:729–734. doi: 10.1073/pnas.94.2.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Bijker M.S., Herzog H. The neuropeptide Y system: Pathophysiological and therapeutic implications in obesity and cancer. Pharmacol. Ther. 2011;131:91–113. doi: 10.1016/j.pharmthera.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Zhou Z., Zhu G., Hariri A.R., Enoch M., Scott D., Sinha R., Virkkunen M., Mash D.C., Lipsky R.H., Hu X.Z., Hodgkinson C.A., Xu K., Buzas B., Yuan Q., Shen P.H., Ferrell R.E., Manuck S.B., Brown S.M., Hauger R.L., Stohler C.S., Zubieta J.K., Goldman D. Genetic variation in human NPY expression affects stress response and emotion. Nature. 2008;452:997–1001. doi: 10.1038/nature06858. [DOI] [PMC free article] [PubMed] [Google Scholar]


