Abstract
Atherosclerosis is a multifactorial chronic inflammatory disease characterized by the presence of T-cells, macrophages, and dendritic cells in the arterial intima. Classical risk factors lead to over-expression of stress proteins, especially heat shock protein 60 (HSP60). HSP60 on the surface of arterial endothelial cells (ECs) then becomes a target for pre-existing adaptive anti-HSP60 immunity resulting in infiltration of the intima by mononuclear cells. In the present study, T-cells derived from early, clinically still inapparent human atherosclerotic lesions were analyzed phenotypically and for their reactivity against HSP60 and HSP60-derived peptides. HSP60 was detected in ECs and CD40- and HLA Class II-positive cells within the intima. Effector memory CD4+ T-cells producing high amounts of interferon-γ and low levels of interleukin-4 were the dominant subpopulation. T-cells derived from late lesions displayed a more restricted T-cell receptor repertoire to HSP60-derived peptides than those isolated from early lesions. Increased levels of soluble HSP60 and circulating anti-human HSP60 autoantibodies were found in donors with late but not early lesions. This is the first functional study of T-cells derived from early human atherosclerotic lesions that supports the previously proposed concept that HSP60-reactive T-cells initiate atherosclerosis by recognition of atherogenic HSP60 epitopes.
Keywords: Atherosclerosis, Inflammation, Early lesions, Heat shock protein 60, T-cell epitopes, Autoantibodies
Highlights
► First analysis of T-cells derived from early human atherosclerotic lesions. ► Proof that HSP60 reactive T-cells precede anti-HSP60 antibodies in atherogenesis. ► Identification of potentially atherogenic HSP60 peptides in human atherosclerosis. ► Interesting future diagnostic and therapeutic potential. ► Support of the “Autoimmune Concept of Atherogenesis”.
1. Introduction
Atherosclerosis is a multifactorial, inflammatory disease characterized by the presence of T-cells, macrophages, and dendritic cells (DCs) in the arterial intima in response to injury caused by classical atherosclerosis risk factors. Adhesion molecules and chemotactic factors mediate migration of cells of the immune system into the arterial wall. Leukocyte adhesion and rolling depend on P- and E-selectins and intercellular and vascular cell adhesion molecules 1 (ICAM-1 and VCAM-1) [1], while monocyte migration is mediated by monocyte chemotactic protein (MCP-1) and its receptor CCR2 [2,3].
Under physiological conditions, the stress protein heat shock protein 60 (HSP60) functions as a chaperone known to assist protein folding and intracellular transport. HSP60 is typically expressed in the mitochondria and is highly conserved with 97% homology between bacterial species and more than 50% homology between microbial and human molecules [4]. HSP60 is a strongly immunogenic microbial antigen able to induce protective humoral and cellular immune responses. Exposed to classical atherosclerotic risk factors, ECs simultaneously express HSP60 and adhesion molecules on their surface [5,6]. HSP60 surface expressions have been shown in stressed human umbilical venous endothelial cells (HUVECs) and aortic endothelial cells (ECs) [7–9] and also in bacterially infected HUVECs [10,11]. HSP60-expressing ECs can then become target cells for pre-existing cellular and humoral immunity against this ubiquitous protein leading to the formation of inflammatory lesions in the intima.
In humans, T-cells isolated from surgically removed advanced (late) atherosclerotic lesions (LL = plaques) which recognize HSP60 have a restricted T-cell receptor (TCR)α/β repertoire and predominantly produce Th1 cytokines [12,13]. It has also been shown that increased levels of anti-HSP60 autoantibodies correlate with the severity of LL [14]. Furthermore, studies performed in animals following immunization with HSP60 have shown excessive plaque formation under normo and hypercholesterolemic conditions [15–17].
These and other data demonstrate the importance of immunity against HSP60 in the development and progression of atherosclerosis. However, the role of HSP60 in the initiation of atherosclerosis is still unclear. To our knowledge, the present study is the first to investigate phenotypic and functional characteristic of T-cells isolated from early, clinically still inapparent human atherosclerotic lesions (EL) and to compare these with data on LL by evaluating their reactivity to human HSP60 (hHSP60) and hHSP60-derived peptides.
2. Material and methods
The study was approved by the Ethics Committee of the Innsbruck Medical University (#UN2670).
2.1. Atherosclerotic lesions
2.1.1. Early atherosclerotic lesions (EL)
Iliac arteries with clinically and macroscopically inapparent early atherosclerotic lesions at the known predilections sites [18] at arterial branching points as well as blood samples from 7 healthy transplant organ donors (5 male, 2 female, age: 54 ± 5.4 years) who died due to cerebrovascular incidents (brain death) were obtained. All clinical data are presented in Table 1. In three cases (EL 2, 4, and 5), pathohistological data were not available. In these instances, macroscopically healthy, very small arterial tissue specimens were taken from the known predilection sites at the branching point of the iliac artery and immediately processed in toto for cell cultures. The higher serum values of C-reactive protein (CRP) in EL donors as compared to LL patients was due to the fact that these brain death individuals were kept under intensive care conditions known to be associated with an “inflammatory storm” [19]. Pathological classification of atherosclerotic lesions (score I–VI, see Table 1) was performed according to the criteria recommended by the American Heart Association [20]. Austria is part of Eurotransplant and complete laboratory values are often not available from donors the organs of whom are transported to the local transplantation unit.
Table 1.
Demographic and clinical characteristics of patients with early and late lesions (plaques).
| Demographic and clinical characteristics | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EL/LL | Age | Sex | BMI | Smoker | DM | Infections | Hypercholesterolemia | CRP | Hypertension | History of CVD | Therapy | Lesion score I–IV |
| EL1 | 65 | F | 36 | ND | No | No | No | 1.45 | Yes | PAE | Antibiotics, anticoagulation, antidiabetic, antihemorrhagic, catecholamine, cardiovascular medication, protone pump inhibitor, thyroidal, ureostatic | II |
| EL2a | 46 | M | 24 | ND | No | Urinary tract infection | Yes | 2.67 | Yes | No | Antibiotics, anticoagulation, antidiabetic, antidiuretic, cardiovascular medication, protone pump inhibitor, steroid, secretolytic | ND |
| EL3 | 48 | M | 28 | Yes | No | No | Yes | 9.8 | Yes | No | Antibiotics, antidiabetic, cardiovascular medication | II |
| EL4a,b | 55 | F | 19 | No | No | No | Yes | 0.5 | No | No | Catecholamine | ND |
| EL5a | 70 | M | 26 | No | No | No | ND | 31.9 | No | No | Antidiuretic | ND |
| EL6 | 65 | M | 25 | ND | No | No | ND | 1.55 | Yes | No | Analgesic, antibiotic, anticoagulation, cardiovascular medication, protone pump inhibitor | II |
| EL7 | 29 | M | 25 | No | No | No | No | 10.6 | No | No | Antibiotic, catecholamines | II |
| LL1 | 70 | M | 29.2 | No | Yes | No | Yes | 0.07 | Yes | No | Anticoagulation, cardiovascular medication, protone pump inhibitor, respiratory tract, steroid | V |
| LL2 | 77 | M | 21 | No | No | Candidiasis | Yes | 0.06 | Yes | CHD, MI, PAOD | Anticoagulation, cardiovascular medication, protone pump inhibitor | V |
| LL3 | 82 | F | 18.2 | Nob | No | No | No | 0.06 | Yes | No | Anticoagulation, cardiovascular medication, neuroleptic medication | V |
| LL4 | 72 | F | 25.5 | No | No | No | Yes | 0.2 | Yes | No | Anticoagulation, cardiovascular medication | V |
| LL5 | 85 | M | 22.6 | No | No | No | Yes | 0.7 | Yes | CHD, PAOD, ACM infarction | Anticoagulation, cardiovascular medication | V |
| LL6 | 76 | M | 20.6 | No | No | No | No | 0.07 | Yes | ACM infarction | Anticoagulation, protone pump inhibitor, ureostatic | V |
| LL7 | 56 | M | 25.5 | Yes | No | No | Yesc | 0.4 | No | CHD, NSTEMI, stroke | Anticoagulation, antidepressivum/antipsychotic/antiepileptic, cardiovascular medication, vitamin | V |
| LL8 | 75 | M | 23.5 | No | No | No | Yes | 0.15 | Yes | CHD, TIA, ACM infarction | Anticoagulation, cardiovascular medication | V |
EL = early lesion, LL = late lesion (plaques), F = female, M = male, BMI = body mass index, ND = not determined, DM = diabetes mellitus, CRP = C-reactive protein, CVD = cardiovascular disease, CHD = coronary heart disease, MI = myocardial infarction, PAE = pulmonary artery embolism, PAOD = peripheral arterial occlusive disease, ACM = arteria cerebri media infarction, NSTEMI = non-ST-elevation myocardial infarction, TIA = transient ischemic attack.
No tissue for immunohistochemistry.
Exsmoker for 10 years.
Normal cholesterol levels but increased triglyceride levels.
2.1.2. Late atherosclerotic lesions (LL)
Internal carotid arteries with advanced atherosclerotic lesions were obtained from 8 patients undergoing surgery either for symptomatic or high-grade asymptomatic stenosis with written informed consent (6 male, 2 female, age: 74.2 ± 3.1 years). For more detailed clinical information see Table 1. Informed consent was obtained from all donors of LL.
2.2. Peripheral blood mononuclear cells (PBMCs) and plasma
PBMCs were obtained by density gradient centrifugation (GE Healthcare Bio-Sciences AB; Sweden) from freshly drawn venous blood. After washing, PBMC were resuspended in RPMI 1640 (Lonza, Belgium) supplemented with 1% penicillin/streptomycin, 1% glutamine, and 10% fetal calf serum (FCS; Serum Supreme, Lonza, Belgium). Plasma samples were obtained from all 7 donors with EL and 8 patients with LL and two healthy age- and sex-matched control groups, one young control group (Control Y; age: 33 ± 4 years; n = 26) and one elderly control group (Control E; age: 74 ± 4 years; n = 14). PBMCs were kept at −80 °C until used for expansion. Plasma samples were stored at −20 °C until use. Informed consent was obtained from all healthy controls.
2.3. Immunohistochemistry
The presence of CD1a, CD3, CD4, CD8, CD40, CD68, tryptase mast cells, CD106 (VCAM-1), and CD62E (E-selectin) molecules were analyzed by immunohistochemistry in formalin-fixed, paraffin-embedded arterial specimens from 4 EL and 8 LL donors (see Supplementary Table 1). Briefly, eight-μm-thick tissue slides were treated with xylene (3× for 3 min), 100% ethanol (2× for 2 min), 96% ethanol (2× for 3 min), 70% ethanol (1× for 3 min), TBS (pH = 7.2) (1× for 3 min), and finally washed in water. Antigen retrieval was achieved either with 1× epitope retrieval solution, pH = 9, (Eubio, Austria) or 0.5 M EDTA for 10 min at 750 W in a microwave oven. Slides were cooled, rinsed with TBS, and blocked either for 6 min with ultra-V block solution (Thermo Scientific, USA) or overnight with blocking buffer (5% in TBS), (Roche Diagnostics, Germany) containing 10% FCS. Following the blocking step, slides were washed (3× for 5 min) with TBS at room temperature (RT). Antibodies and corresponding controls (Supplementary Figure 1) were added to the tissue sections as shown in Supplementary Table 1 and incubated for 1 h at RT. Following 3 further washing steps and addition of primary antibody enhancer (Thermo Scientific, USA) the sections were first incubated for 10 min at RT with the primary antibody enhancer and then treated with AP-polymer (Thermo Scientific, USA) for 15 min at RT. For visualization, either fast red (Sigma–Aldrich, USA) or 3,3′ diaminobenzidine (DAB) (Sigma–Aldrich, USA) was used when AP-anti-mouse or HRP-anti-mouse antibody was applied, respectively. Staining was monitored microscopically and color development was stopped with water. Nuclear staining was performed by incubation of the slides with Mayer's hemalum solution (1:10) for 10 min.
2.4. Immunofluorescence
To identify HSP60-expressing cells in atherosclerotic lesions, triple immunofluorescence staining was performed. Paraffin embedded tissues were processed as indicated before. Following the blocking step, the sections were treated with either monoclonal anti-human antibodies for ECs (vWF), CD40, CD106 or anti-HLA-DR, DP, DQ and corresponding controls (Supplementary Figure 1) incubated for 1 h at RT. Slides were washed (3× for 3 min) in TBS to remove excess antibody. For visualization, goat Alexa 488 labeled anti-mouse IgG (Invitrogen, USA) was applied to the sections and incubated for 1 h in the dark at RT. HSP60 expression, in combination with the markers indicated above, was determined first by incubation with polyclonal rabbit anti-human HSP60 IgG (Santa Cruz Biotechnology, USA) for 1 h and then by incubation with goat Alexa 568-labeled anti-rabbit IgG (Invitrogen, USA) for 1 h at RT in the dark. Nuclear staining was achieved by incubation with Hoechst stain (1:40,000) for 10 min. Microscopic analyses and documentation were carried out at room temperature with a Nikon Eclipse E800 microscope connected to Digital Sight DS-5M with a DS-L1 control unit (Nikon, Japan).
3. Generation of T-cell lines from early atherosclerotic lesions
EL and LL from 7 to 8 donors respectively, were cut into small pieces and cultured in RPMI 1640 medium (Lonza, Belgium) supplemented with 1% penicillin/streptomycin, 1% glutamine (Lonza, Belgium), 7% human serum (Type AB; Lonza, Belgium) and recombinant human interleukin (rhIL)-2 (20 U/mL) for 7 days in a petri dish (Sarstedt, USA). T-cells migrating out of the tissue fragments were harvested and cell suspensions were filtered through a cell strainer (100 μm; Becton Dickinson, USA). The T-cell suspension was then transferred to a 24-well plate (Sarstedt, USA). These T-cells were then expanded by stimulation with 1 μg/mL each of anti-human anti-CD3/anti-CD28 antibodies (eBioscience, Austria) in the presence of autologous irradiated PBMCs and rhIL-2 (10 U/mL) for 7 days. After expansion, intralesional hHSP60-specific T-cell blasts were stimulated with recombinant human HSP60 (rhHSP60) protein (10 μg/mL) together with autologous feeder cells in the presence of rhIL-2 (10 U/mL) once a week for two weeks. Cell culture medium and rhIL-2 (10 U/mL) were replaced every third day. Finally, T-cells were harvested, counted, and used for phenotypic and functional studies.
3.1. Phenotypic characterization
Phenotypic characterization of intralesional hHSP60-specific T-cells from 7 EL and 8 LL donors was performed using monoclonal antibodies specific for CD3, CD4, CD8, CD45RA, CD45RO, CD28, and CD25 (all purchased from BD Pharmingen, USA) labeled with fluorescein isothiocyanate (FITC), phycoerythrin (PE), peridinin chlorophyll protein (PerCP), or allophycocyanin (APC) for 30 min at 4 °C. Subsequently, cells were washed twice with FACS buffer (0.5% BSA in PBS) and measured by four-color flow cytometry. Mouse IgG isotype controls and unstained cells were used to define the level of non-specific background signal (Supplementary Figure 2). All analyses were performed using a FACSCalibur flow cytometer utilizing CELLQuest software (BD Pharmingen, USA).
3.2. Intracellular cytokine staining
Interferon (IFN)γ, IL-4, IL-10 (BD Pharmingen, USA), IL-17 (Peprotech, USA), and transforming growth factor (TGF)β (R&D systems, USA) production was determined in intralesional hHSP60-specific T-cells from 7 EL and 8 LL donors by intracellular staining. T-cells were stimulated with 30 ng/mL phorbol myristate acetate (PMA) and 5 μg/mL ionomycin/mL in the presence of 10 μg of brefeldin A/mL for 4 h at 37 °C (Sigma–Aldrich, USA). After washing with PBS, stimulated T-cells were resuspended in FACS buffer and incubated with monoclonal antibodies for CD4-FITC and CD8-PerCP for 30 min at 4 °C. Following two washing steps, cells were resuspended in Cytofix-Cytoperm (BD Pharmingen, USA) according to the specifications of the manufacturer. Monoclonal antibodies against IFNγ, IL-4, IL-10, IL-17, and TGFβ were added to the stimulated cells resuspended in perm-wash buffer (BD Pharmingen, USA) for 30 min at 4 °C. Intracellular cytokine production was measured by flow cytometry. Mouse IgG isotype controls and unstained cells were used to define the level of non-specific background signal (Supplementary Figure 2). All analyses were performed in a FACSCalibur flow cytometer utilizing CELLQuest software.
3.3. Intralesional T-cell reactivity to hHSP60 protein and hHSP60-derived peptide pools
To span the 573-long amino acid sequence of the hHSP60 protein, 113 15-mer synthetic peptides overlapping 10 amino acids were produced (Peptides and Elephants, Germany). Twelve peptide pools containing equal amounts of 10 overlapping peptides were used in each pool (Supplementary Table 2). T-cells (1 × 104) were co-cultured with 5 × 104 autologous irradiated (30Gy) PBMCs and stimulated with either 10 μg/mL rhHSP60 or peptide pools in which each peptide component was present at 10 μg/mL, in a 96-well plate (Sarstedt, USA). Anti-human anti-CD3/anti-CD28 antibodies (1 μg/mL each) and PHA (5 μg/mL) (Sigma–Aldrich, USA) were used as positive controls. After 3 days of incubation, cells were pulsed for the last 10 h with 1 μCi of 3H-thymidine (ICN Biomedicals Inc., USA). 3H-thymidine incorporation was assessed by scintillation counting. All assays were carried out in triplicates. The response to a given peptide pool was considered positive when the stimulation index was higher than two.
3.4. hHSP60-specific epitope recognition by intralesional T-cells
To identify HSP60 peptide-specific responses, 1 × 104 intralesional atherosclerotic T-cells from 7 EL and 8 LL donors were co-cultured with 5 × 104 autologous irradiated PBMCs and stimulated with 10 individual peptides (10 μg/mL) contained in the corresponding positive responder pool. All 7 EL and 8 LL were used individually in these experiments. As indicated before, monoclonal anti-human anti-CD3/anti-CD28 antibodies and PHA were used as positive controls. A non-related peptide (pepC, AAAAEEEEE, 10 μg/mL; Bachem, Germany) was used as a negative peptide control. After 3 days of incubation, cells were pulsed with 1 μCi of 3H-thymidine. 3H-thymidine incorporation was assessed by scintillation counting. All assays were carried out in triplicates. The response to a given peptide was considered positive when the stimulation index was higher than two.
3.5. Enzyme-linked immunosorbent assay (ELISA)
3.5.1. Soluble HSP60 (sHSP60) protein
The concentration of sHSP60 was assessed in plasma samples from persons with 7 early and 8 late lesions and healthy (young: Control Y, n = 26 and elderly: Control E, n = 14) controls by a sandwich ELISA as described previously [14]. Results are indicated as ng of sHSP60/mL. All assays were carried out in triplicates.
3.5.2. Anti-hHSP60 autoantibodies
The levels of anti-hHSP60 autoantibodies present in plasma/serum from donors with 7 EL and 8 LL and healthy (young: Control Y, n = 26 and elderly: Control E, n = 14) controls were determined by ELISA as described [21]. Results are indicated as anti-hHSP60 autoantibody titers. All assays were carried out in triplicates.
3.6. Statistical analysis
Data were expressed as the mean ± SEM. Statistical analysis was performed using the Student's two-tailed t test. Differences were considered significant at p < 0.05.
4. Results
4.1. T-cells are present in early atherosclerotic lesions
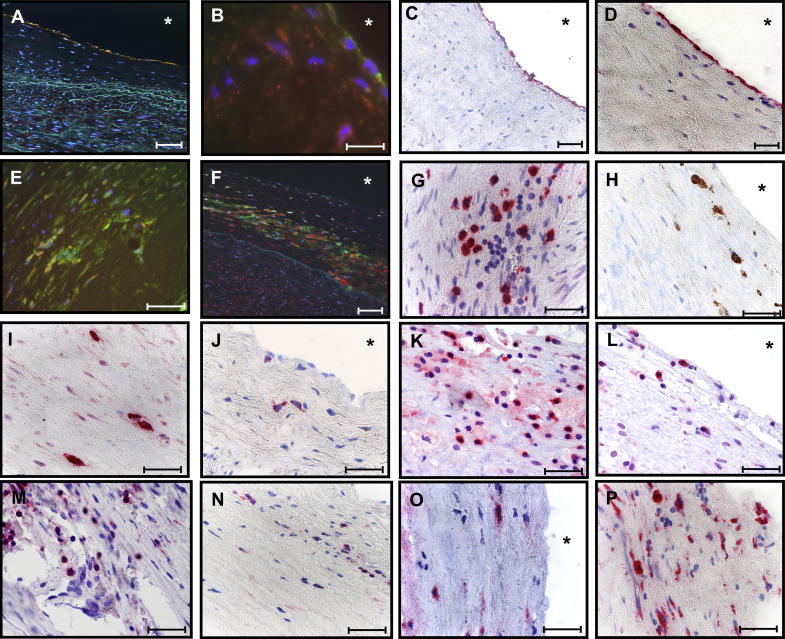
HSP60 was expressed by ECs at arterial branching sites stressed by turbulent blood flow conditions [22] (Fig. 1A); simultaneous expression of adhesion molecules, such as VCAM-1 (CD106) (Fig. 1B, C) and E-selectin (Fig. 1D) was detected at very early stages of the disease. HSP60 expression was also observed in intralesional cells co-expressing CD40+ (Fig. 1E), an inflammatory marker and co-stimulatory molecule, which plays an important role in atherosclerosis [23,24]. HSP60 was also demonstrated in HLA-DR+ intralesional cells (Fig. 1F). Surface expression of adhesion molecules provides the prerequisites for adhesion and subsequent transmigration of circulating T-cells (CD3+) (Fig. 1G), macrophages (CD68+) (Fig. 1H), mast cells (Fig. 1I), and dendritic cells (CD1a+) (Fig. 1J) into the intima. Both, CD4+ (Fig. 1K) and CD8+ (Fig. 1L) T-cells were identified demonstrating that a specific immunologic-inflammatory process was taking place intima. In LL (plaques), CD3+ (Fig. 1M), CD4+ (Fig. 1N), CD8+ (Fig. 1O), and CD68+ (Fig. 1P) cells were present. CD3+ T-cells (CD4+ > CD8+) prevailed over CD68+ macrophages in EL, the opposite was true in LL.
Fig. 1.
Immunohistological analysis of human atherosclerotic lesions. HSP60 expression (red) was determined in (A) ECs at arterial bifurcation areas stressed by turbulent blood flow conditions (vWF+, green, 200×, scale bar 200 μm) with simultaneous expression of adhesion molecules as (B) VCAM-1 (CD106, green, scale bar 200 μm), (C) CD106, and (D) CD62E (40×, scale bar 100 μm). Mononuclear cells in the intima expressed (E) CD40+ (green) and (F) HLA-DR+ (green, 200×, scale bar 200 μm). Subsets of (G) CD3+, (H) macrophages (CD68+, brown), (I) mast-cells (tryptase+), (J) DCs (CD1a+), (K) CD4+ T-cells, and (L) CD8+ T-cells were identified infiltrating the intima. (M) CD3+, (N) CD4+, (O) CD8+ T-cells, and (P) CD68+ were identified in advanced atherosclerotic lesions. Images were acquired using a Nikon Eclipse E800 microscope. Original magnification 600× (scale bar 100 μm). All immunohistological stainings and analysis of these were performed at least three times. A representative immunohistological analysis from 4 EL and 8 LL donors are demonstrated here.
4.2. T-cells from early lesions are mostly CD4 memory effector T-cells
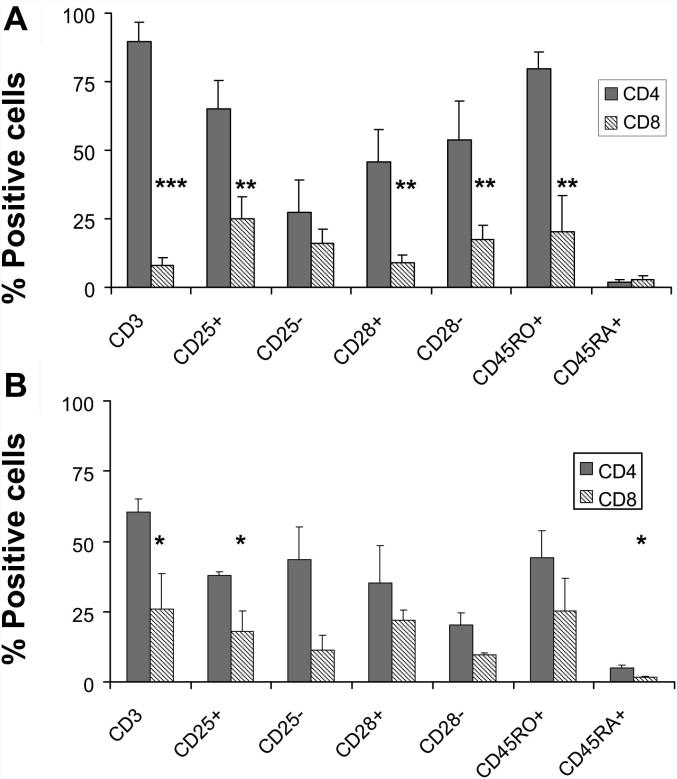
T-cells isolated from EL were mostly CD4+ T-cells with high expression of the memory cell marker CD45RO and almost complete down-regulation of CD45RA (Fig. 2A). A subset of these cells co-expressed CD25 and showed a modest accumulation of cells lacking the expression of the co-stimulatory molecule CD28. Thus, the dominant phenotype of T-cells from EL is the CD4+CD45RO+CD25+CD28− effector memory type (Fig. 2A). Although T-cells isolated from LL also showed an effector memory phenotype (Fig. 2B), there was an increase in the number of CD8+ T-cells at this stage of the disease.
Fig. 2.
Intralesional atherosclerotic T-cells are mostly memory effector T-cells. Surface expression of CD4, CD8, CD45RO, CD45RA, CD25, and CD28 were determined in intralesional CD3+ T-cells isolated from (A) EL (n = 7) and (B) LL (n = 8). CD4+ significantly predominate over CD8+ T-cells in atherosclerotic lesions (EL p < 0.005; LL p < 0.05). Significant differences in the surface expression of phenotypic markers are indicated in EL and LL T-cells (∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.005). Phenotypic characterization was performed by four-color flow cytometry. Bars indicate the percentage of positive cells as mean ± SEM. These experiments were performed once (in triplicates) for each donor.
4.3. Intralesional atherosclerotic T-cells produce high amounts of IFNγ
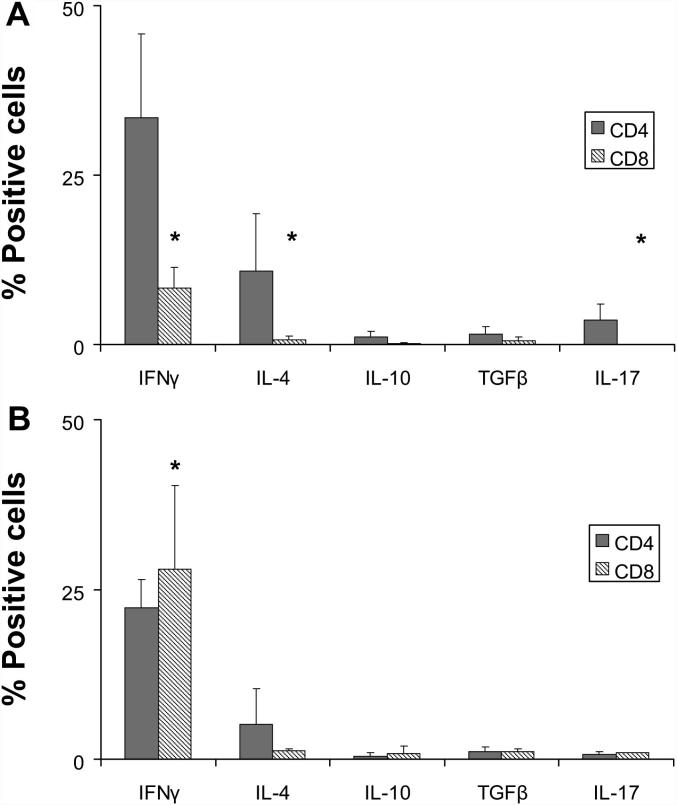
Both CD4+ and CD8+ T-cells produced higher levels of IFNγ than IL-10, IL-17, and TGFβ (Fig. 3). Interestingly, in EL, CD4+ T-cells significantly contributed to the increased levels of IFNγ, IL-4 (p < 0.01), and IL-17 as compared to CD8+ T-cells (p < 0.05) whereas, there was a significant increase (p < 0.05) in the percentage of IFNγ-producing CD8+ T-cells isolated from LL (Fig. 3B) [13]. Furthermore, a Th1-driven cytokine production by LL-derived cells is confirmed by the reduction of IL-4-producing CD4+ T-cells at late stages of the disease (Fig. 3B).
Fig. 3.
T-cells isolated from atherosclerotic lesions produce the proinflammatory cytokine IFNγ. IFNγ, IL-4, IL-10, IL-17, and TGFβ production in CD4+ and CD8+ T-cells were determined in (A) EL (n = 7) and (B) LL (n = 8) donors by intracellular cytokine analysis after unspecific stimulation with PMA ionomycin. IFNγ-, IL-4-(∗∗p < 0.01), and IL-17-producing CD4+ T-cells were predominantly present as compared with the cytokine profile of CD8+ T-cells in EL (∗p < 0.05). No significant differences were observed in the cytokine production profile within LL T-cells. However, a significant increase in the frequency of IFNγ-producing CD8 was observed in LL T-cells as compared with EL T-cells (∗p < 0.05). Bars indicate the percentage (mean ± SEM) of positive cytokine-producing cells by four-color cytometry. These experiments were performed once for each donor.
4.4. Autoreactive T-cells recognizing hHSP60 are present in early atherosclerotic lesions
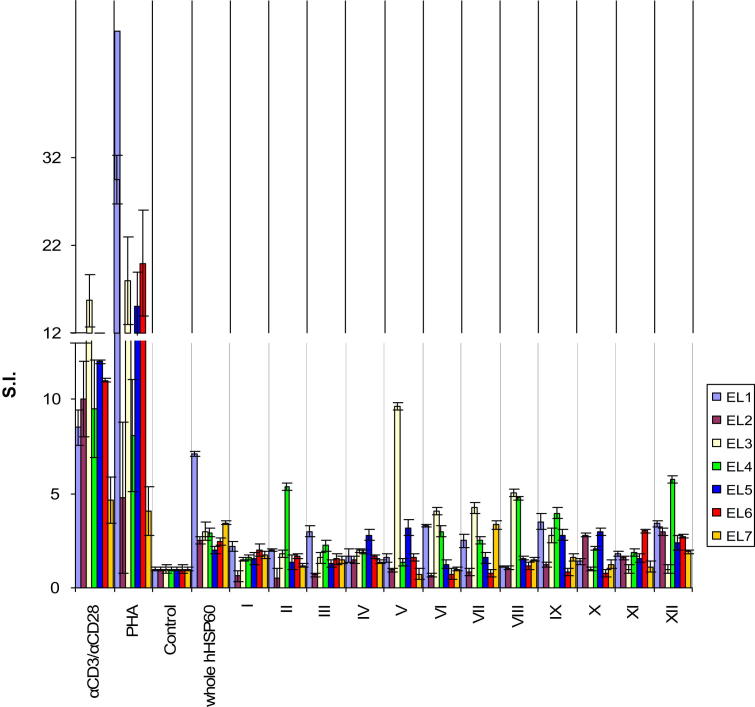
Autoreactive T-cells with high proliferative capacity against the whole hHSP60 protein (S.I. = 3.3 ± 0.6) were identified in EL. Stimulation with specific regions of the protein represented in the different peptide pools resulted in proliferation of these T-cells (Fig. 4, Supplementary Figure 3). Peptide pools III (positive/total donors: 2/7), V (2/7), VI (3/7), VII (3/7), VIII (2/7), IX (4/7), X (2/7), and XII (5/7) (Fig. 4, Table 2) induced proliferative responses by T-cells from EL from multiple donors suggesting the presence of atherogenic HSP60 peptides involved in the initiation of the disease.
Fig. 4.
EL T-cells proliferate in response to whole hHSP60 protein and recognize hHSP60-derived peptide pools. The proliferative cellular response of 1 × 104 intralesional T-cells isolated from 7 EL donors was evaluated upon stimulation with either whole hHSP60 or hHSP60 peptide pools (indicated by Roman numerals, all at 10 μg/mL). PHA and monoclonal anti-CD3/anti-CD28 antibodies (αCD3/αCD28) were used for positive controls. Antigen was omitted in the negative control (Control). Data are shown as stimulation index (S.I. ± SEM). All assays were carried out in triplicates.
Table 2.
Reactivity of T-cells from early atherosclerotic lesions to rhHSP60 protein and hHSP60-derived peptide pools I–XII.
| Donor | S.I. |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| hHSP60 | I | II | III | IV | V | VI | VII | VIII | IX | X | XI | XII | |
| EL1 | 7.1 | 2.0 | 2.0 | 3.0 | 1.7 | 1.6 | 3.3 | 2.0 | 1.1 | 3.5 | 1.4 | 1.8 | 3.4 |
| EL2 | 2.5 | 0.6 | 0.5 | 0.7 | 1.5 | 1.0 | 0.6 | 0.9 | 1.1 | 1.2 | 2.8 | 1.6 | 3.0 |
| EL3 | 3.0 | 1.5 | 1.8 | 1.6 | 2.0 | 9.6 | 4.1 | 4.3 | 5.1 | 2.8 | 1.0 | 1.0 | 1.0 |
| EL4 | 2.9 | 1.6 | 5.4 | 2.2 | 1.9 | 1.3 | 3.0 | 2.5 | 4.8 | 4.0 | 2.0 | 1.9 | 5.7 |
| EL5 | 2.0 | 1.6 | 1.3 | 1.3 | 2.8 | 3.2 | 1.3 | 1.6 | 1.6 | 2.8 | 3.0 | 1.6 | 2.4 |
| EL6 | 2.4 | 2.0 | 1.7 | 1.5 | 1.7 | 1.6 | 0.7 | 0.8 | 1.2 | 0.8 | 0.8 | 3.0 | 2.7 |
| EL7 | 3.5 | 1.8 | 1.2 | 1.5 | 1.4 | 0.7 | 1.0 | 3.3 | 1.5 | 1.6 | 1.3 | 1.1 | 1.9 |
T-cell immune response to whole hHSP60 and twelve peptide pools (Roman numerals) is shown for 7 patients with EL. The response was considered positive when S.I. was higher than two and this is shown in bold.
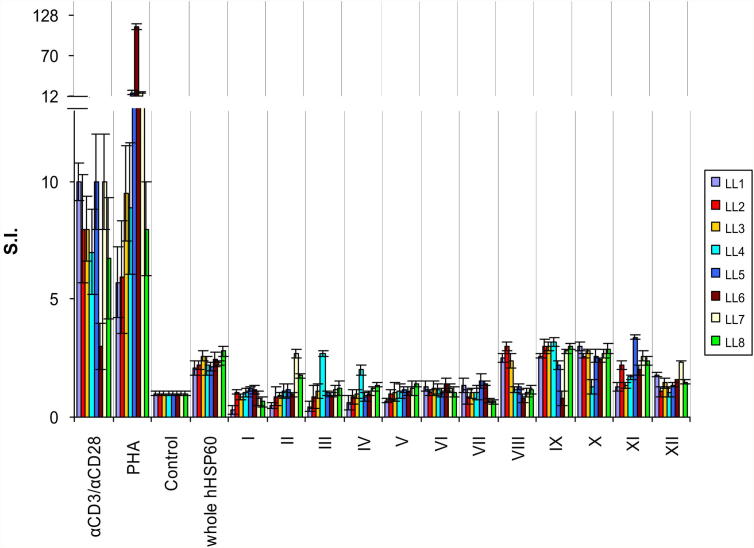
4.5. Autoreactive T-cells from advanced atherosclerotic lesions display restricted peptide recognition
T-cells from LL also showed proliferation in response to stimulation with whole rhHSP60 (S.I. = 2.3 ± 0.1) (Fig. 5, Supplementary Figure 4) which, interestingly, is restricted to a particular region of the protein (amino acids 401–510) represented by pools IX and X (Fig. 5, Supplementary Figure 4). These findings are in agreement with earlier results [13] demonstrating that T-cells from LL display a highly monoclonal and oligoclonal TCRα/β repertoire.
Fig. 5.
LL T-cells display restricted peptide recognition. S.I. of 1 × 104 T-cells isolated from 8 LL donors upon stimulation with either whole hHSP60 protein or hHSP60 peptide pools (indicated by Roman numerals, all at 10 μg/mL). PHA and monoclonal anti-CD3/anti-CD28 antibodies (αCD3/αCD28) were used as positive control. Antigen was omitted in the negative control (Control). Data are shown as stimulation index (S.I. ± SEM). All assays were carried out in triplicates.
4.6. Fine specificity of autoreactive intralesional atherosclerotic T-cells
Autoreactive intralesional atherosclerotic T-cells with a positive proliferative response to hHSP60 peptide pools were then screened against each of the individual overlapping peptides forming the respective pool (Supplementary Table 2). T-cells from EL recognized a total of 24 hHSP60 peptides (Supplementary Figure 4, Supplementary Table 3) eight of which induced proliferation of T-cells (Table 3), suggesting that these peptides may be involved in the initiation of the disease. Peptide 111 (551–565) was recognized by 71.4% of T-cells from EL while peptide 54 (266–280), 57 (281–295), 66 (326–340), and 84 (416–430) were recognized by 42.9% of T-cells. Peptides 50 (246–260), 85 (421–435), and 88 (436–450) were recognized by 28.6% of T-cells from EL (Table 3). In contrast, of the 19 peptides recognized by T-cells from LL, peptides 85 (421–435) and 95 (471–485) induced proliferative response in 75% of T-cells, while overlapping peptides 84 (416–430) and 96 (476–490) were recognized by 50% of T-cells (Table 3, Supplementary Figure 5, Supplementary Table 4). Interestingly, both EL and LL T-cells recognized peptides 84 and 85. No reactivity was observed against a non-related control peptide (pepC). Taken together, these results demonstrate a highly specific reactivity of T-cells from EL to hHSP60 peptides. Additionally, the cellular immune response to the HSP60 epitopes in LL was increasingly restricted.
Table 3.
Atherogenic peptides recognized by T-cells from early atherosclerotic lesions (EL) and from advanced late atherosclerotic lesions (LL).
| Number | Sequence | Location | Frequency |
|---|---|---|---|
| Atherogenic peptides from early atherosclerotic lesions | |||
| 50 | LSEKKISSIQSIVPA | 246–260 | 2/7 |
| 54 | AHRKPLVIIAEDVDG | 266–280 | 3/7 |
| 57 | EALSTLVLNRLKVGL | 281–295 | 3/7 |
| 66 | GEEGLTLNLEDVQPH | 326–340 | 3/7 |
| 84 | EKKDRVTDALNATRA | 416–430 | 3/7 |
| 85 | VTDALNATRAAVEEG | 421–435 | 2/7 |
| 88 | IVLGGGCALLRCIPA | 436–450 | 2/7 |
| 111 | KEEKDPGMGAMGGMG | 551–565 | 5/7 |
| Atherogenic peptides from late atherosclerotic lesions | |||
| 84 | EKKDRVTDALNATRA | 416–430 | 4/8 |
| 85 | VTDALNATRAAVEEG | 421–435 | 6/8 |
| 95 | TLKIPAMTIAKNAGV | 471–485 | 6/8 |
| 96 | AMTIAKNAGVEGSLI | 476–490 | 4/8 |
Eight common HSP60 peptides induced a proliferative response in early atherosclerotic lesion (EL) and four in late atherosclerotic lesions (LL). Overlapping amino acids are represented in bold.
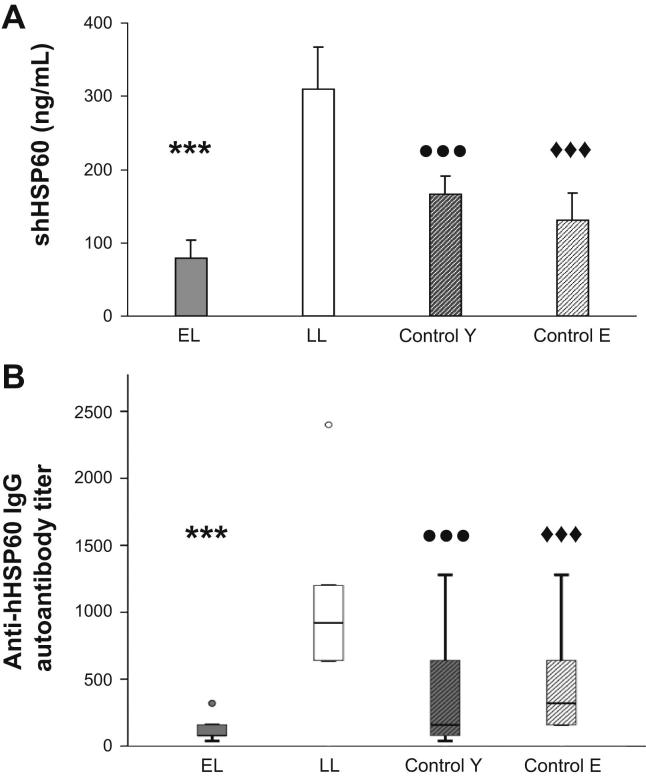
4.7. sHSP60 and anti-HSP60 autoantibody production may be an indicator of atherosclerosis stage
Levels of circulating sHSP60 present in plasma of LL donors (310 ± 57.8) was almost double of that found in plasma of EL donors or in the control groups (Fig. 6A), (EL: 78.6 ± 25.2; Control Y: 167 ± 24; Control E: 131 ± 35.7). However, no significant differences were found between EL and the healthy control groups.
Fig. 6.
Soluble HSP60 (sHSP60) and anti-hHSP60 IgG autoantibody production may be an indicator of the atherosclerosis stage. Levels of circulating (A) sHSP60 (ng/mL) and (B) anti-hHSP60 IgG autoantibodies (titer) were determined in the plasma of EL (n = 7) and LL (n = 8) donors, young controls (control Y, n = 26), and elderly controls (control E, n = 14) by ELISA. Values are indicated as mean ± SEM. Significant differences are shown as follows: (A) sHSP60: EL vs LL (∗∗∗p < 0.005); LL vs Control Y (•••p < 0.005); LL vs control E (♦♦♦p < 0.005), (B) Anti-hHSP60 IgG autoantibodies: EL vs LL (∗∗∗p < 0.005); LL vs Control Y (•••p < 0.005); LL vs control E (♦♦♦p < 0.005). All assays were carried out in triplicates.
The levels of anti-HSP60 autoantibodies (expressed as titer) in the four study groups were determined. Again, significantly higher titers of autoantibodies were detected in LL (1070 ± 213.6) as compared to the EL (134.5 ± 28.2) donors and the control groups (Control Y: 364.8 ± 73; Control E: 416 ± 112.8) (Fig. 6B). These results confirm earlier findings from our group that patients with LL display increased amounts of circulating sHSP60 and anti-HSP60 autoantibodies [14,21]. However, no difference in these humoral atherosclerosis biomarkers emerged in persons with EL compared to healthy controls.
5. Discussion
HSP60 have been shown to play an important role in the generation and progression of atherogenesis. Specific T-cells and antibodies generated against HSP60 following recurrent microbial infections carry the danger of a cross-reaction between pro and eukaryotic (microbial and human) HSP60. Biochemically altered autologous HSP60 can also lead to the formation of bona fide autoreactive HSP60-specific T-cells and autoantibodies [18,25]. In humans, peripheral HSP60-reactive T-cells from young clinically healthy persons correlate with increased intima-media thickness (IMT) at different vascular territories [26,27]. However, anti-hHSP60 reactivity of peripheral T-cells from elderly persons did not correlate with an increased IMT, suggesting that HSP60-specific cellular immunity plays an important role at very early stages of the disease, while anti-hHSP60 antibodies seem to have an accelerating and perpetuating effect [28]. Furthermore, T-cells isolated from LL show reactivity to bacterial and human HSP60, have a restricted T-cell repertoire, and recognize HSP60-derived peptides [12,13,29].
Despite the obviously important role of HSP60 in the progression of atherosclerosis, the immune response of T-cells from human EL, clinically still inapparent atherosclerotic lesions has not been analyzed so far. In the present study, HSP60 expression on ECs from EL and simultaneous up-regulation of adhesion molecules, such as VCAM-1 and E-selectin (Fig. 1), previously shown in LL, were observed [11]. This allows for interaction of HSP60-specific T-cells with stressed ECs under arterial blood flow conditions [30]. Intralesional activated CD40+/HLA-DR+ mononuclear cells also displayed HSP60 expression, suggesting that HSP60 may be implicated in the activation and maturation of DCs as observed in Hodgkin's disease [31]. In fact, HSP60 may act as a “danger signal” for pre-existing adaptive immunity. CD4+ and CD8+ T-cells as well as macrophages and DCs are present within the intima providing the appropriate microenvironment for an initial antigen-driven T-cell-mediated inflammatory process. T-cells from EL are predominantly CD4+ with a memory effector phenotype characterized by the expression of CD25. Here we demonstrate, albeit on morphological evidence, that HSP60-specific CD4+CD25+CD45RO+ T-cells interact with ECs that express HSP60 and adhesion molecules on their surface after being exposed to various risk factors at sites with predilection for later development of atherosclerotic lesions. If such risk factors persist, the inflammatory process progresses within the intima where it is continuously stimulated by intralesional HSP60-expressing cells.
T-cells from early lesions produced high amounts of the pro-inflammatory cytokine IFNγ while the Th2 cytokine IL-4 was detected only at low levels (Fig. 3). It has been demonstrated in several studies that atherosclerosis can be attenuated via Th1 cytokine inhibition [32–34] and/or with stimulation of Th2 cytokine production [35,36]. The accumulation of IFNγ-producing CD8+ T-cells in atherosclerotic plaques may contribute to a general increase in the serum level of the IFNγ, as reflected by high plasma levels of neopterin in patients with complex carotid plaques [37], acute coronary syndrome (ACS) [38–40], and chronic CMV infections [41].
This study provides functional proof for the presence of autoreactive hHSP60-specific T-cells in early stages of the disease. Proliferation of T-cells from in response to hHSP60, and their recognition of hHSP60-derived peptide pools (Fig. 4, Table 2) corroborate the notion of a local antigen-driven process. It is of interest to compare our observations on T-cell lines from both EL and LL with those of Benagiano et al. on T-cell clones from LL only [12]. Furthermore, while we assessed T-cell reactivity exclusively to hHSP60-derived T-cell peptides, Benagiano et al. also investigated cross-reactivity against Chlamydia pneumoniae HSP60 (cHSP60). With respect to LL, we confirmed the data of Benagiano et al. Since all four epitopes of hHSP60 (Table 3) were also identified by these authors, two specific for hHSP60 (471–485 and 476–490) and two cross-reactive between hHSP60 and cHSP60 (416–430 and 421–435) [12]. More importantly, EL, not yet been studied by other authors so far, contain T-cells that show interesting parallels to the findings of Benagiano et al. as well to our own data on LL T-cells. Seven of the eight EL-associated hHSP60 epitopes described by us (Table 3) were also identified by these authors studying LL T-cells for reactivity to either hHSP60 and/or cHSP60 [12]. In the present study, two of the eight EL hHSP60 epitopes were also recognized by LL T-cells (416–430 and 421–435). One EL HSP60 epitope (551–565) recognized by T-cells derived from more than 70% of EL appeared neither in the investigation of LL by Benagiano et al. nor in our present study [12]. Except for the latter epitope, this congruence is a strong indication that these hHSP60 epitopes recognized already by EL T-cells play a pathogenic role throughout atherogenesis and may represent interesting early targets for prevention and therapy. However, taking into account the fact that such an elaborate investigation so far could, of course, only be performed in a limited number of subjects with inapparent lesions, the issue of private vs common, i.e. shared potentially atherogenic, hHSP60 epitopes has to be considered in the context of personalized medicine.
Stressed cells up-regulate mitochondrial production of HSP60 that are then translocated into the cytosol, transported to the cell surface, and released into the microenvironment by mechanisms that are still not well understood [42,43]. LL donors had significantly higher serum levels of sHSP60 than donors with EL or healthy controls (Fig. 6). However, no difference in serum levels of sHSP60 was found between EL donors and healthy controls. These findings corroborate our previous notion that levels of circulating sHSP60 may be an indicator of cardiovascular diseases in general and advanced degree of atherosclerosis in particular [14,44]. In fact, increased levels of circulating sHSP60 may reflect the inflammatory processes occurring in the arterial wall as well as the release from necrotic cells within the plaque [45]. Extracellular HSP60 can interact with endothelial surface receptors such as CD14, CD40 and Toll-like receptors (TLRs), and activate antigen-presenting cells [46,47].
Finally, we determined the levels of anti-hHSP60 autoantibodies in the plasma of individuals with early and late lesions, as well as two age- and sex-matched healthy control groups. The results revealed a significant increase in the levels of anti-hHSP60 autoantibodies in patients with LL in comparison to donors with EL and healthy controls (Fig. 6). Interestingly, the group with early lesions did not show a significant difference in the anti-hHSP60 autoantibody titers compared with age-matched controls. Taken together, both low levels of sHSP60 and low titers of anti-hHSP60 autoantibodies in EL donors suggest that the humoral immune response is not involved in the initiation of the disease, but rather in its progression to advanced stages. This is comparable to what have earlier been described in a large population-based prospective study involving an elderly cohort, which showed a good correlation between serum antibodies to mHSP65 and atherosclerosis [21], while only a weak or no such correlation emerged in two younger groups [26,27]. Furthermore, high levels of antibodies against mHSP65 that cross-react with the hHSP60 are not only a predictor of morbidity but also of mortality from cardiovascular disease [44]. These parameters have been shown by us and other laboratories to be biomarkers for atherosclerosis independent of other classical disease biomarkers [48]. If anti-HSP60 antibodies are injected into mice directly they do of course act as endothelial stressors entailing intimal arterial infiltration mediated by HSP60-reactive T-cells [49].
In conclusion, this is the first report determining the reactivity of T-cells isolated from human early clinically still inapparent atherosclerotic lesions reacting to potentially atherogenic peptides from hHSP60. Furthermore, these T-cells produced abundant levels of the pro-inflammatory cytokine IFNγ and induced activation and differentiation of macrophages, contributing to the thickening of the intima at the known arterial predisposed sites. T-cells initiate the inflammatory processes in the intima, and anti-hHSP60 autoantibodies accelerate and perpetuate the disease. The existence of atherogenic “private” and “common”, i.e. shared hHSP60 peptides makes these epitopes attractive diagnostic and therapeutic targets.
Funding sources
This work is supported by the Austrian Science Fund (FWF; P19881-B05) to GW.
Disclosures
This article has been written, reviewed, and approved by all contributing authors. The authors declare that they have no competing interests as defined by the Journal of Autoimmunity, or other interests that might be perceived to influence the results and discussion in this manuscript.
Acknowledgments
We would like to thank Christina Mayerl, PhD for technical and administrative help; Ruth Pfeilschifter-Resch for help with histological sections; the staff of the Immunology Division, Institute for Biomedical Aging Research, Austrian Academy of Sciences, Innsbruck, for technical help and Marius C. Wick, MD for contributing reagents. Healthy plasma controls were kindly provided by Michael Keller, Institute for Biomedical Aging Research, Innsbruck, Austria. rhIL-2 was kindly provided by Dr. Frank Kalthoff, Novartis, Vienna, Austria.
Footnotes
Supplementary data related to this article can be found online at http://dx.doi.org/10.1016/j.jaut.2012.07.006.
Appendix A. Supplementary data
References
- 1.Collins R.G., Velji R., Guevara N.V., Hicks M.J., Chan L., Beaudet A.L. P-selectin or intercellular adhesion molecule (ICAM)-1 deficiency substantially protects against atherosclerosis in apolipoprotein E-deficient mice. J Exp Med. 2000;191:189–194. doi: 10.1084/jem.191.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boring L., Gosling J., Cleary M., Charo I.F. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–897. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- 3.Gu L., Okada Y., Clinton S.K., Gerard C., Sukhova G.K., Libby P. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275–281. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 4.Young D.B. Heat-shock proteins: immunity and autoimmunity. Curr Opin Immunol. 1992;4:396–400. doi: 10.1016/s0952-7915(06)80029-4. [DOI] [PubMed] [Google Scholar]
- 5.Schett G., Xu Q., Amberger A., Van der Zee R., Recheis H., Willeit J. Autoantibodies against heat shock protein 60 mediate endothelial cytotoxicity. J Clin Invest. 1995;96:2569–2577. doi: 10.1172/JCI118320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amberger A., Maczek C., Jurgens G., Michaelis D., Schett G., Trieb K. Co-expression of ICAM-1, VCAM-1, ELAM-1 and Hsp60 in human arterial and venous endothelial cells in response to cytokines and oxidized low-density lipoproteins. Cell Stress Chaperones. 1997;2:94–103. doi: 10.1379/1466-1268(1997)002<0094:ceoive>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfister G., Stroh C.M., Perschinka H., Kind M., Knoflach M., Hinterdorfer P. Detection of HSP60 on the membrane surface of stressed human endothelial cells by atomic force and confocal microscopy. J Cell Sci. 2005;118:1587–1594. doi: 10.1242/jcs.02292. [DOI] [PubMed] [Google Scholar]
- 8.Soltys B.J., Gupta R.S. Immunoelectron microscopic localization of the 60-kDa heat shock chaperonin protein (Hsp60) in mammalian cells. Exp Cell Res. 1996;222:16–27. doi: 10.1006/excr.1996.0003. [DOI] [PubMed] [Google Scholar]
- 9.Soltys B.J., Gupta R.S. Cell surface localization of the 60 kDa heat shock chaperonin protein (hsp60) in mammalian cells. Cell Biol Int. 1997;21:315–320. doi: 10.1006/cbir.1997.0144. [DOI] [PubMed] [Google Scholar]
- 10.Kol A., Bourcier T., Lichtman A.H., Libby P. Chlamydial and human heat shock protein 60s activate human vascular endothelium, smooth muscle cells, and macrophages. J Clin Invest. 1999;103:571–577. doi: 10.1172/JCI5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Q., Schett G., Seitz C.S., Hu Y., Gupta R.S., Wick G. Surface staining and cytotoxic activity of heat-shock protein 60 antibody in stressed aortic endothelial cells. Circ Res. 1994;75:1078–1085. doi: 10.1161/01.res.75.6.1078. [DOI] [PubMed] [Google Scholar]
- 12.Benagiano M., D'Elios M.M., Amedei A., Azzurri A., van der Zee R., Ciervo A. Human 60-kDa heat shock protein is a target autoantigen of T cells derived from atherosclerotic plaques. J Immunol. 2005;174:6509–6517. doi: 10.4049/jimmunol.174.10.6509. [DOI] [PubMed] [Google Scholar]
- 13.Rossmann A., Henderson B., Heidecker B., Seiler R., Fraedrich G., Sing M. T-cells from advanced atherosclerotic lesions recognize hHSP60 and have a restricted T-cell receptor repertoire. Exp Gerontol. 2008;43:229–237. doi: 10.1016/j.exger.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Xu Q., Schett G., Perschinka H., Mayr M., Egger G., Oberhollenzer F. Serum soluble heat shock protein 60 is elevated in subjects with atherosclerosis in a general population. Circulation. 2000;102:14–20. doi: 10.1161/01.cir.102.1.14. [DOI] [PubMed] [Google Scholar]
- 15.Xu Q., Dietrich H., Steiner H.J., Gown A.M., Schoel B., Mikuz G. Induction of arteriosclerosis in normocholesterolemic rabbits by immunization with heat shock protein 65. Arterioscler Thromb. 1992;12:789–799. doi: 10.1161/01.atv.12.7.789. [DOI] [PubMed] [Google Scholar]
- 16.Xu Q., Kleindienst R., Schett G., Waitz W., Jindal S., Gupta R.S. Regression of arteriosclerotic lesions induced by immunization with heat shock protein 65-containing material in normocholesterolemic, but not hypercholesterolemic, rabbits. Atherosclerosis. 1996;123:145–155. doi: 10.1016/0021-9150(96)05800-5. [DOI] [PubMed] [Google Scholar]
- 17.George J., Shoenfeld Y., Afek A., Gilburd B., Keren P., Shaish A. Enhanced fatty streak formation in C57BL/6J mice by immunization with heat shock protein-65. Arterioscler Thromb Vasc Biol. 1999;19:505–510. doi: 10.1161/01.atv.19.3.505. [DOI] [PubMed] [Google Scholar]
- 18.Wick G., Knoflach M., Xu Q. Autoimmune and inflammatory mechanisms in atherosclerosis. Annu Rev Immunol. 2004;22:361–403. doi: 10.1146/annurev.immunol.22.012703.104644. [DOI] [PubMed] [Google Scholar]
- 19.Apostolakis E., Parissis H., Dougenis D. Brain death and donor heart dysfunction: implications in cardiac transplantation. J Card Surg. 2010;25:98–106. doi: 10.1111/j.1540-8191.2008.00790.x. [DOI] [PubMed] [Google Scholar]
- 20.Stary H.C., Chandler A.B., Dinsmore R.E., Fuster V., Glagov S., Insull W., Jr. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the committee on vascular lesions of the council on arteriosclerosis, American heart association. Circulation. 1995;92:1355–1374. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 21.Xu Q., Willeit J., Marosi M., Kleindienst R., Oberhollenzer F., Kiechl S. Association of serum antibodies to heat-shock protein 65 with carotid atherosclerosis. Lancet. 1993;341:255–259. doi: 10.1016/0140-6736(93)92613-x. [DOI] [PubMed] [Google Scholar]
- 22.Hochleitner B.W., Hochleitner E.O., Obrist P., Eberl T., Amberger A., Xu Q. Fluid shear stress induces heat shock protein 60 expression in endothelial cells in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2000;20:617–623. doi: 10.1161/01.atv.20.3.617. [DOI] [PubMed] [Google Scholar]
- 23.Mach F., Schonbeck U., Sukhova G.K., Atkinson E., Libby P. Reduction of atherosclerosis in mice by inhibition of CD40 signalling. Nature. 1998;394:200–203. doi: 10.1038/28204. [DOI] [PubMed] [Google Scholar]
- 24.Schonbeck U., Sukhova G.K., Shimizu K., Mach F., Libby P. Inhibition of CD40 signaling limits evolution of established atherosclerosis in mice. Proc Natl Acad Sci U S A. 2000;97:7458–7463. doi: 10.1073/pnas.97.13.7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grundtman C., Kreutmayer S.B., Almanzar G. Heat shock protein 60 and immune inflammatory responses in atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31(5):960–968. doi: 10.1161/ATVBAHA.110.217877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knoflach M., Kiechl S., Kind M., Said M., Sief R., Gisinger M. Cardiovascular risk factors and atherosclerosis in young males: ARMY study (atherosclerosis risk-factors in male youngsters) Circulation. 2003;108:1064–1069. doi: 10.1161/01.CIR.0000085996.95532.FF. [DOI] [PubMed] [Google Scholar]
- 27.Knoflach M., Kiechl S., Penz D., Zangerle A., Schmidauer C., Rossmann A. Cardiovascular risk factors and atherosclerosis in young women: atherosclerosis risk factors in female youngsters (ARFY study) Stroke. 2009;40:1063–1069. doi: 10.1161/STROKEAHA.108.525675. [DOI] [PubMed] [Google Scholar]
- 28.Knoflach M., Kiechl S., Mayrl B., Kind M., Gaston J.S., van der Zee R. T-cell reactivity against HSP60 relates to early but not advanced atherosclerosis. Atherosclerosis. 2007;195:333–338. doi: 10.1016/j.atherosclerosis.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 29.Curry A.J., Portig I., Goodall J.C., Kirkpatrick P.J., Gaston J.S. T lymphocyte lines isolated from atheromatous plaque contain cells capable of responding to Chlamydia antigens. Clin Exp Immunol. 2000;121:261–269. doi: 10.1046/j.1365-2249.2000.01294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seitz C.S., Kleindienst R., Xu Q., Wick G. Coexpression of heat-shock protein 60 and intercellular-adhesion molecule-1 is related to increased adhesion of monocytes and T cells to aortic endothelium of rats in response to endotoxin. Lab Invest. 1996;74:241–252. [PubMed] [Google Scholar]
- 31.Hsu P.L., Hsu S.M. Abundance of heat shock proteins (hsp89, hsp60, and hsp27) in malignant cells of Hodgkin's disease. Cancer Res. 1998;58:5507–5513. [PubMed] [Google Scholar]
- 32.Whitman S.C., Ravisankar P., Daugherty A. IFN-gamma deficiency exerts gender-specific effects on atherogenesis in apolipoprotein E−/− mice. J Interferon Cytokine Res. 2002;22:661–670. doi: 10.1089/10799900260100141. [DOI] [PubMed] [Google Scholar]
- 33.Elhage R., Jawien J., Rudling M., Ljunggren H.G., TAkeda K., Akira S. Reduced atherosclerosis in interleukin-18 deficient apolipoprotein E-knockout mice. Cardiovasc Res. 2003;59:234–240. doi: 10.1016/s0008-6363(03)00343-2. [DOI] [PubMed] [Google Scholar]
- 34.Hauer A.D., Uyttenhove C., de Vos P., Stroobant V., Renauld J.C., van Berkel T.J. Blockade of interleukin-12 function by protein vaccination attenuates atherosclerosis. Circulation. 2005;112:1054–1062. doi: 10.1161/CIRCULATIONAHA.104.533463. [DOI] [PubMed] [Google Scholar]
- 35.Von Der Thusen J.H., Kuiper J., Fekkes M.L., De Vos P., Van Berkel T.J., Biessen E.A. Attenuation of atherogenesis by systemic and local adenovirus-mediated gene transfer of interleukin-10 in LDLr−/− mice. FASEB J. 2001;15:2730–2732. doi: 10.1096/fj.01-0483fje. [DOI] [PubMed] [Google Scholar]
- 36.Pinderski L.J., Fischbein M.P., Subbanagounder G., Fishbein M.C., Kubo N., Cheroutre H. Overexpression of interleukin-10 by activated T lymphocytes inhibits atherosclerosis in LDL receptor-deficient mice by altering lymphocyte and macrophage phenotypes. Circ Res. 2002;90:1064–1071. doi: 10.1161/01.res.0000018941.10726.fa. [DOI] [PubMed] [Google Scholar]
- 37.Sugioka K., Naruko T., Hozumi T., Nakagawa M., Kitabayashi C., Ikura Y. Elevated levels of neopterin are associated with carotid plaques with complex morphology in patients with stable angina pectoris. Atherosclerosis. 2010;208:524–530. doi: 10.1016/j.atherosclerosis.2009.07.054. [DOI] [PubMed] [Google Scholar]
- 38.Liuzzo G., Kopecky S.L., Frye R.L., O'Fallon W.M., Maseri A., Goronzy J.J. Perturbation of the T-cell repertoire in patients with unstable angina. Circulation. 1999;100:2135–2139. doi: 10.1161/01.cir.100.21.2135. [DOI] [PubMed] [Google Scholar]
- 39.Methe H., Brunner S., Wiegand D., Nabauer M., Koglin J., Edelman E.R. Enhanced T-helper-1 lymphocyte activation patterns in acute coronary syndromes. J Am Coll Cardiol. 2005;45:1939–1945. doi: 10.1016/j.jacc.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 40.Yamashita H., Shimada K., Seki E., Mokuno H., Daida H. Concentrations of interleukins, interferon, and C-reactive protein in stable and unstable angina pectoris. Am J Cardiol. 2003;91:133–136. doi: 10.1016/s0002-9149(02)03097-7. [DOI] [PubMed] [Google Scholar]
- 41.Almanzar G., Schwaiger S., Jenewein B., Keller M., Herndler-Brandstetter D., Wuerzner R. Long-term cytomegalovirus infection leads to significant changes in the composition of the CD8+ T-cell repertoire, which may be the basis for an imbalance in the cytokine production profile in elderly persons. J Virol. 2005;79:3675–3683. doi: 10.1128/JVI.79.6.3675-3683.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gupta S., Knowlton A.A. HSP60 trafficking in adult cardiac myocytes: role of the exosomal pathway. Am J Physiol Heart Circ Physiol. 2007;292:H3052–H3056. doi: 10.1152/ajpheart.01355.2006. [DOI] [PubMed] [Google Scholar]
- 43.Schett G., Metzler B., Kleindienst R., Amberger A., Recheis H., Xu Q. Myocardial injury leads to a release of heat shock protein (hsp) 60 and a suppression of the anti-hsp65 immune response. Cardiovasc Res. 1999;42:685–695. doi: 10.1016/s0008-6363(99)00012-7. [DOI] [PubMed] [Google Scholar]
- 44.Xiao Q., Mandal K., Schett G., Mayr M., Wick G., Oberhollenzer F. Association of serum-soluble heat shock protein 60 with carotid atherosclerosis: clinical significance determined in a follow-up study. Stroke. 2005;36:2571–2576. doi: 10.1161/01.STR.0000189632.98944.ab. [DOI] [PubMed] [Google Scholar]
- 45.Bennett M.R., Boyle J.J. Apoptosis of vascular smooth muscle cells in atherosclerosis. Atherosclerosis. 1998;138:3–9. doi: 10.1016/s0021-9150(98)00013-6. [DOI] [PubMed] [Google Scholar]
- 46.Binder R.J., Vatner R., Srivastava P. The heat-shock protein receptors: some answers and more questions. Tissue Antigens. 2004;64:442–451. doi: 10.1111/j.1399-0039.2004.00299.x. [DOI] [PubMed] [Google Scholar]
- 47.Pockley A.G., Muthana M., Calderwood S.K. The dual immunoregulatory roles of stress proteins. Trends Biochem Sci. 2008;33:71–79. doi: 10.1016/j.tibs.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 48.Okada T., Ayada K., Usui S., Yokota K., Cui J., Kawahara Y. Antibodies against heat shock protein 60 derived from Helicobacter pylori: diagnostic implications in cardiovascular disease. J Autoimmun. 2007;29:106–115. doi: 10.1016/j.jaut.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 49.Mandal K., Foteinos G., Jahangiri M., Xu Q. Role of antiheat shock protein 60 autoantibodies in atherosclerosis. Lupus. 2005;14:742–746. doi: 10.1191/0961203305lu2212oa. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.