Background: Dysphoric effects of κ-opioid receptor (KOR) agonists require p38 MAPK activation in mice, but sequence differences in human KOR may affect this mechanism.
Results: Differences in p38 activation were observed between human and rodent KOR for pentazocine and butorphanol.
Conclusion: Species differences affect signaling.
Significance: Rodent models may not predict adverse effects of KOR agonists in humans.
Keywords: Arrestin, Cell Signaling, ERK, Opiate Opioid, p38 MAPK, Biased Agonism, Ligand-directed Signaling, Opioid Receptor, p38 MAPK, Signal Transduction
Abstract
KOR activation of Gβγ dependent signaling results in analgesia, whereas the dysphoric effects of KOR agonists are mediated by a different pathway involving G protein receptor kinase and non-visual arrestin. Based on this distinction, a partial KOR agonist that does not efficiently activate arrestin-dependent biased signaling may produce analgesia without dysphoria. No KOR-selective partial agonists are currently available, and preclinical assessment is complicated by sequence differences between rodent (r) and human (h) KOR. In this study, we compared the signaling initiated by the available partial agonists. Pentazocine was significantly more potent at activating p38 MAPK in hKOR than rKOR expressed in HEK293 cells but equally potent at arrestin-independent activation of ERK1/2 in hKOR and rKOR. Similarly, butorphanol increased phospho-p38-ir in hKOR-expressing cells but did not activate p38 in rKOR-HEK293. Like pentazocine, butorphanol was equally efficacious at activating ERK1/2 in rKOR and hKOR. In contrast, levorphanol, nalorphine, and U50,488 did not distinguish between hKOR and rKOR in p38 MAPK activation. Consistent with its low potency at p38 activation, pentazocine did not produce conditioned place aversion in mice. hKOR lacks the Ser-369 phosphorylation site in rKOR required for G protein receptor kinase/arrestin-dependent p38 activation, but mutation of the Ser-358 to asparagine in hKOR blocked p38 activation without affecting the acute arrestin-independent activation of ERK1/2. This study shows that hKOR activates p38 MAPK through a phosphorylation and arrestin-dependent mechanism; however, activation differs between hKOR and rKOR for some ligands. These functional selectivity differences have important implications for preclinical screening of partial KOR agonists.
Introduction
κ-Opioid receptor (KOR)2 agonists are effective analgesics and are thought to have a lower addictive potential than μ-opioid receptor (MOR) agonists (1–3). However, KOR agonists also have dysphoric effects in humans (4) and produce aversion in experimental animals (5–9), unlike MOR agonists, which are euphorigenic. These negative affective responses to available KOR agonists have limited their therapeutic use (10, 11). The cellular and molecular mechanisms of κ-opioid-induced dysphoria (measured operationally as conditioned place aversion in rodents) are not completely clear, but recent results suggest that KOR activation of p38α MAPK in serotonergic neurons innervating the ventral striatum is required (12–14). KOR activation of p38 MAPK follows from G protein receptor kinase 3 (GRK3) phosphorylation of the serine 369 residue within the C-terminal tail of the rodent sequence (rKOR) and subsequent recruitment of nonvisual arrestins that act as a scaffold linking a kinase signaling cascade to p38 MAPK phosphorylation (12, 15). Disruption of this signaling pathway in mice through receptor mutation (KORS369A), GRK3 deletion, or conditional deletion of p38α MAPK blocks the aversive effects of KOR agonists without reducing their analgesic effects (13, 14, 16). These findings have potentially important therapeutic implications, because a selective, partial KOR agonist that does not efficiently activate arrestin-dependent signaling might produce analgesia without significant dysphoria (17). This type of “ligand-directed signaling” or “biased agonism,” where certain ligands preferentially activate a subset of a receptor signaling pathways, has been demonstrated for other receptor systems (18) including MOR; some MOR agonists, such as fentanyl, effectively recruit arrestin, whereas others such as the partial agonist morphine do not; however, both are highly effective analgesics (19).
Development of biased KOR agonists for human treatment is complicated by sequence differences between rKOR and hKOR. Whereas hKOR and rKOR share 94% amino acid sequence homology overall, the amino acid residues involved in GRK/arrestin signaling are not conserved (Fig. 1). In hKOR the residue at 369 is a tyrosine instead of a serine and is not a GRK substrate. The residue responsible for arrestin recruitment to hKOR and desensitization is Ser-358 (20), but residue 358 is an asparagine in rKOR and not a GRK substrate. The significance of these differences in GRK/arrestin-dependent signaling has not been established, and previous studies examining GRK/arrestin-dependent signaling pathways other than desensitization have used rKOR. In this study we asked whether GRK phosphorylation of hKOR(Ser-358) produces equivalent signaling events to phosphorylation of rKOR(S369) by GRK.
FIGURE 1.
Comparison of the amino acid sequences of human and rodent KOR. Transmembrane domains are shaded. Transmembrane domains (TM1–7), intracellular loops (IL1–3), and the intracellular C-terminal tail (CT) are labeled above the domain, and amino acid numbers are indicated on the right. The serine residues in the C-terminal tail implicated in GRK/arrestin-mediated signaling and desensitization in human and rodent KOR and the corresponding amino acid residues in the rodent and human KOR, respectively, are indicated by boxes.
Interestingly, a variant (rs34369022) has been reported for hKOR at the Ser-358 residue (NCBI dbSNP accession number ss43313677; www.ncbi.nlm.nih.gov). In the NCBI dbSNP database, this variant (Gly > Tyr at the cDNA position 1308) is reported in one of the five Celera donors and would result in a serine-to-isoleucine mutation at protein position 358, S358I). If this SNP was not an error in sequencing or a rare, novel individual variant, this would suggest that a subpopulation of individuals may express a variant of KOR that would not be regulated by GRKs and arrestins and may, therefore, alter these individual responses to stress as well as to κ analgesics.
There are no KOR selective partial agonists currently available, but there are non-selective partial KOR agonists used clinically, including butorphanol, nalorphine, and pentazocine. Pentazocine is a mixed acting opioid analgesic with agonist activity at the κ-opioid receptor and δ-receptor and antagonist or partial agonist activity at the μ-opioid receptor (22, 23). The side effect profile of pentazocine in humans includes classic KOR agonist effects such as dysphoria and hallucinations, but individual responses vary, with some individuals reporting euphoric rather than dysphoric effects. The euphorigenic effects are more apparent at lower doses, whereas dysphoria becomes more prominent at higher doses (24–27). Administration of the opioid antagonist naltrexone at doses selective for the MOR effectively block the euphoric response to pentazocine in humans and unveils a stronger aversive dysphoric response that can be blocked by higher doses of naltrexone sufficient to antagonize KOR (28). Because it is not clear how partial agonists produce dysphoric responses in humans, in this study we compared pentazocine-induced signaling through arrestin-dependent and -independent pathways to that of the selective full KOR agonist U50,488.
MATERIALS AND METHODS
Chemicals/Reagents
(−)U50,488 (Tocris), nalorphine (Sigma), butorphanol (Sigma), and levorphanol (Sigma) were dissolved in water. A stock solution of (−)pentazocine (Sigma) was dissolved in a DMSO, then diluted as described below before use. Naloxone (Sigma) and norBNI National Institute on Drug Abuse Drug Supply (NIDA) were dissolved as described below.
Animals
Male C57BL/6 mice (20–30g) were group-housed and kept on a 12-h light/dark cycle with food and water available ad libitum. Animal procedures were approved by the Animal Care and Use Committee of the University of Washington and conform to the guidelines on the care and use of animals promulgated by the National Institutes of Health. Homozygous KOR−/− and MOR−/− mice were generated by homologous recombination as previously described (29, 30).
Conditioned Place Preference/Aversion
C57BL/6 mice were trained in a balanced, unbiased three-chamber conditioning apparatus as described previously (9). Briefly, mice were given a pretest of 30 min in which they were allowed to explore the entire arena. During this time the amount of time spent in each of the three chambers was video-recorded (ZR90, Cannon) and analyzed using Ethovision (v.3.0, Noldus, Wageningen, The Netherlands). Mice were assigned to either vertical- or horizontal-striped chambers based on pretest times. The morning of each of the two training days was paired with vehicle (10 ml/kg intraperitoneal of 15% DMSO, 5% Cremophor and 80% sterile saline), and the afternoon session was paired with drug (pentazocine 10 mg/kg intraperitoneal, pentazocine 10 mg/kg + norBNI 10 mg/kg intraperitoneal, or pentazocine 10 mg/kg + naloxone 5 mg/kg intraperitoneal) with a minimum of 4 h elapsing between training sessions. On the fourth day, mice were allowed to roam freely between all three compartments, and the amount of time spent in each compartment was recorded. Test sessions took place midday. Conditioned place preference or aversion scores were calculated by subtracting the time spent in the drug-paired side during the post-test from time spent in the saline-paired side during the post-test.
Locomotor Activity
Locomotor activity during conditioned place preference training was video-recorded (ZR90, Cannon) and analyzed using Ethovision (v.3.0, Noldus, Wageningen, The Netherlands). Scores represent total distance moved (cm) during the 30-min session.
Antinociceptive Testing
Antinociceptive responses were measured using the warm-water tail-withdrawal assay (8). The response latency for an animal to withdraw its tail after being immersed in 52.5 °C water was measured before treatment with (−)pentazocine (10 mg/kg, intraperitoneal). After drug administration, responses were measured every 10 min for the first 30 min and every 30 min after that for 3 h post drug administration. A 15-s maximal immersion duration was used as a cut-off to prevent tissue damage.
Cell Culture
HEK293 cells expressing rKOR-GFP were generated as described previously (8). HEK293 cells stably expressing FLAG-tagged hKOR or mutant hKOR (S358N) were generated by transfecting HEK293 cells using FuGENE 6 according to the manufacturer's instructions. Plasmids expressing FLAG-tagged human KOR- or FLAG-tagged human KOR (S358N) were previously described (20). HEK293 cells were maintained in Dulbecco's modified medium/F-12 with 10% fetal bovine serum. HEK293 cells expressing KOR were grown in medium supplemented with G418 (200 μg/ml). HEK293 cells expressed comparable levels of receptor: 1.6 and 4.1 pmol of [3H]U69,593 specifically bound per mg of protein, rKOR and hKOR, respectively.
siRNA
HEK293 cells were transfected with siRNA directed against arrestin-2 or arrestin-3 or scrambled siRNA control (Dharmacon Research) using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer's instructions. Transfected cells were passaged onto 6-well plates after 48 h and 24 h later (72 h post transfection) treated as described.
Dominant Positive Arrestin
HEK293 expressing hKOR(S358N) were transiently transfected with phosphorylation independent arrestin-2(R169E) or arrestin-3(R170E) (31, 32) tagged with mCherry at the C terminus as described (33) using FuGENE HD (Promega) according to the manufacturer's instructions. Transfected cells were passaged onto 6-well plates after 48 h, and 24 h later (72 h post-transfection) treated as described.
Radioligand Binding
HEK293 cells were homogenized in ice-cold 50 mm Tris buffer, pH 7.4, followed by centrifugation at 26,000 × g for 30 min at 4 °C. Three 10-cm plates were pooled for each independent replicate. Homogenization of the pelleted membrane fraction and centrifugation were repeated twice. After the final centrifugation step, excess buffer was removed, and the membranes were stored at −20 °C until use. Membranes were resuspended in ice-cold Tris buffer. Protein concentrations were determined by BCA assay. Samples were incubated for 90 min at room temperature with 1 nm KOR ligand [3H]U69,543. GF/B glass fiber filters (Brandel) were preincubated for 90 min at room temperature with Tris buffer, 0.3% polyethyleneimine. After 90 min of incubation at room temperature, samples were placed on ice and collected with the filters with a Brandel 24 well harvester. Filters were washed with 3× with 3 ml of cold Tris buffer and counted in 5 ml of Ecoscint scintillation fluid. Radioligand concentrations were confirmed by scintillation count of free ligand. Ki values were calculated for each experiment; these values were used to calculate Ki and confidence intervals. Statistical significance was calculated by Student's t test using GraphPad Prism.
Immunoblotting
HEK293 cells were serum-starved for 6 h and then treated as described and lysed in lysis buffer (50 mm Tris-HCl, 300 mm NaCl, 1 mm EDTA, 1 mm Na3VO4, 1 mm NaF, 10% glycerol, phosphatase inhibitors, and protease inhibitors). Lysates were sonicated and centrifuged (15,000 × g, 20 min, 4 °C), and the supernatant was stored at −20 °C. Total protein concentration was determined by BCA assay (Pierce) with bovine serum albumin standards before loading 30 μg (phospho-ERK1/2) or 45 μg (phospho-p38 MAPK) onto 10% Bis-Tris precast gels (Invitrogen) and running at 130 V for 1.5–2 h. Blots were transferred to nitrocellulose (Whatman, Middlesex, UK) for 1.5–2 h at 30 V. The nitrocellulose was blocked with 5% BSA-TBS 1 h at room temperature and stained overnight at 4 °C for phospho-ERK1/2-immunoreactivity (ir) or phospho-p38-ir (Cell Signaling, 1:1000) and actin-ir (Abcam, 1:5000) in 5% BSA-TBS. Blots were incubated in IRdye secondary (Li-Cor Biosciences, 1:10,000 for phospho-p38-ir and phospho-ERK1/2-ir or 1:20,000 for actin) in 1:1 Odyssey buffer (Li-Cor) and 5% milk-TBS 1 h at room temperature then scanned on the Odyssey Infrared Imaging System (Li-Cor Biosciences). Band intensity was measured using the Odyssey software and expressed as phospho-ERK1/2-ir or phospho-p38 MAPK-ir band intensity over actin-ir band intensity. Data were normalized to percentage of control sample (basal, 100%) and plotted using GraphPad Prism. Statistical significance (p < 0.05) was determined by Student's t test or analysis of variance followed by Bonferroni's post-hoc test.
Dose-response Curves
Dose-response curves for p38 and ERK1/2 phosphorylation were calculated in GraphPad Prism using a three-parameter least squares nonlinear regression with a 1/Y weighting and a bottom constrained at 100%. Significant differences between EC50 values and Emax were determined by extra sum-of-squares F test. To calculate relative efficacy and confidence interval (C.I.), maximal drug-induced ERK1/2 or p38 phosphorylation was normalized to the increase in phosphorylation stimulated by U50,488 (10 μm) for each experiment. For relative efficacy, statistical significance (p < 0.05) was calculated by Student's t test using GraphPad Prism.
SNP Analysis
To identify common nucleotide sequence polymorphisms, we performed re-sequencing on DNA samples from a discovery panel of 95 individuals from several diverse populations (African American, Caucasian, African, Hispanic, Asian). Polymerase chain reaction (PCR) and sequencing methods for SNP discovery have been published previously (34). For the present study, all of the coding regions of OPRK1 were re-sequenced using M13-tailed PCR amplification of overlapping amplicons. PCR primer sequences are available upon request. All amplicons were purified, diluted, and used in standard Big-Dye terminator sequencing reactions under standard conditions (v3.1 Cycle Sequencing Kit Protocol Manual; Applied Biosystems, Foster City, CA) and run on an ABI 3730XL. Each chromatogram was trimmed of low quality sequence (Phred quality score <25), assembled, and edited in Consed to ensure accuracy. SNPs were identified using the PolyPhred program (Version 5.0) (35). Putative polymorphic sites were flagged by PolyPhred and reviewed for genotype accuracy to remove false positives.
RESULTS
Do hKOR and rKOR Differ in Their Activation of p38 MAPK?
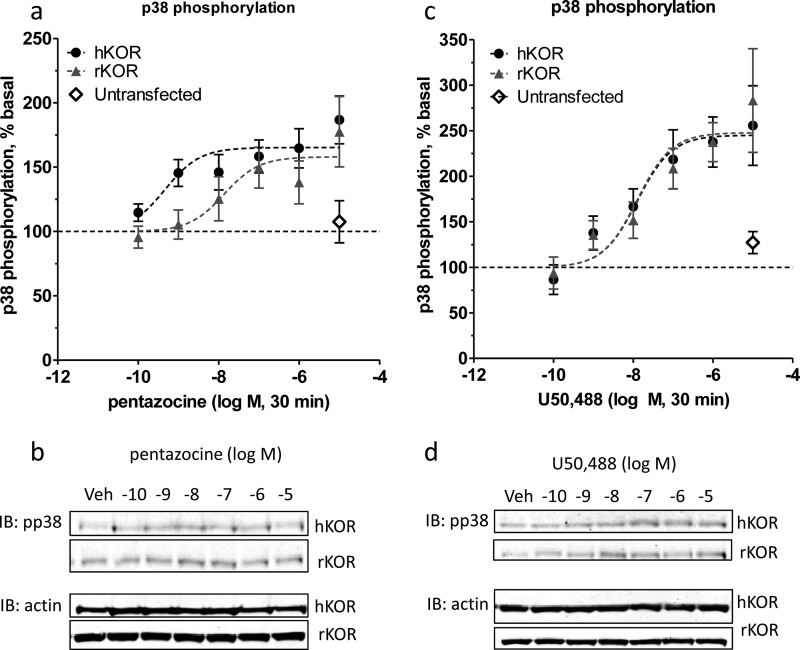
HEK293 cells stably expressing either rKOR or hKOR were treated for 30 min with either U50,488 or pentazocine at concentrations from 100 pm to 10 μm before lysis, and phospho-p38-ir was measured by Western blot (Fig. 2, Table 1). The EC50 values for pentazocine-induced p38 phosphorylation for rKOR and hKOR were 14 nm (C.I. 1.3–140 nm, n = 10) and 0.47 nm (88 pm to 2.5 nm, n = 14), respectively. The 30-fold greater potency of pentazocine for activating p38 MAPK in cells expressing hKOR was statistically significant (p < 0.05). The EC50 values for U50,488-induced p38 phosphorylation for rKOR and hKOR were 21 nm (confidence interval 4.8 nm to 88 nm, n = 9) and 8.6 nm (1.9 nm to 39 nm, n = 12) respectively. In contrast to pentazocine, the potencies of U50,488 stimulation of phospho-p38 for hKOR and rKOR were not significantly different. Neither pentazocine nor U50,488 at the highest concentration tested increased p38 MAPK phosphorylation in untransfected HEK293 (Fig. 2). Consistent with previous in vivo assays (22, 36), pentazocine also appears to be a partial agonist in activating the p38 pathway (having 35–47% of the activity of U50,488 in both hKOR and rKOR). Pentazocine increased phospho-p38-ir to 165 and 158% of basal levels in hKOR and rKOR expressing cells, whereas stimulation with U50,488 resulted in p38 phosphorylation of 240 and 255% over basal. This difference in Emax was significant for both hKOR (p < 0.001) and rKOR (p < 0.001).
FIGURE 2.
Pentazocine is less efficacious than U50,488 in activation of p38. Pentazocine, but not U50,488, is more potent for activation of the p38 pathway in hKOR as compared with rKOR. a and b, HEK293 cells expressing hKOR or rKOR were treated for 30 min with the indicated concentrations of pentazocine (A) or (−)U50,488 (C) before lysis and immunoblotted for phospho-p38-ir (n = 9–14). Dose-response curves are shown. b and d, representative immunoblots (IB) are shown for a and c.
TABLE 1.
Pharmacological data for opioid agonists in hKOR and rKOR expressing HEK293
Shown is a summary of the pharmacological properties of opioid agonists in this study. Shown are EC50 and relative efficacy values and confidence intervals for ERK1/2 phosphorylation after pentazocine and U50,488 treatment and for p38 phosphorylation after all drugs were calculated based on three parameter dose-response curves. Relative efficacy and confidence intervals for p38 MAPK and ERK1/2 phosphorylation after treatment with nalorphine, butorphanol, or levorphanol (30 min) were calculated based on maximal response to each drug normalized to the 10 μm U50,488 response within an experiment. Ki values are an average of Ki values from 4–5 competition binding experiments. Significance differences (p < 0.05) between hKOR and rKOR is indicated (Student's t test for relative efficacy and Ki; extra sum-of-squares F test for EC50). Data rows shown in bold highlight the statistically significant differences noted. n.s., not significant.
| Opioid agonist | hKOR | rKOR | rKOR/hKOR ratio | rKOR vs. hKOR |
|---|---|---|---|---|
| Confidence interval; n | Confidence interval; n | |||
| Butorphanol | ||||
| pp38 relative efficacy | 1.1 (0.65-1.6; 6) | 0.12 (−0.41-0.65; 10)a | 0.1a | p < 0.05 |
| pERK relative efficacy | 0.23 (0.10-0.37; 5) | 0.30 (0.01-0.59; 4) | 1.3 | n.s. |
| pp38/pERK ratio | 4.8 | 0.4a | ||
| pp38 EC50 | 0.11 nm (0.004-3.1 nm; 6) | No significant activationa | ||
| Ki ([3H]U69 binding) | 2.8 nm (0.55-5.0 nm; 5) | 1.9 nm (1.2–2.7 nm; 5) | 0.7 | n.s. |
| Levorphanol | ||||
| pp38 relative efficacy | 1.4 (−0.01-2.8; 6) | 1.3 (−0.33-2.9; 5) | 0.9 | n.s. |
| pERK relative efficacy | 0.11 (0.08-0.15; 4) | 0.33 (0.09-0.58; 4) | 3.0 | p < 0.05 |
| pp38/pERK ratio | 12.6 | 3.9 | ||
| pp38 EC50 | 15 nm (1.5-150 nm; 6) | 16 nm (1.3-160 nm; 5) | 1.1 | n.s. |
| Ki ([3H]U69 binding) | 11 nm (5.4-17 nm; 5) | 26 nm (16-37; 5) | 2.4 | p < 0.01 |
| Nalorphine | ||||
| pp38 relative efficacy | 0.97 (0.77-1.2; 7) | 1.1 (0.43-1.7; 7) | 1.1 | n.s. |
| pERK relative efficacy | 0.23 (0.10-0.35; 5) | 0.56 (0.19-0.93; 4) | 2.4 | p < 0.05 |
| pp38/pERK ratio | 4.2 | 1.9 | ||
| pp38 EC50 | 0.18 nm (0.004-7.6 nm; 7) | 0.16 nm (0.02-1.4 nm; 7) | 0.9 | n.s. |
| Ki ([3H]U69 binding) | 4.1 nm (1.6-6.5 nm; 5) | 9.5 nm (1.3-18 nm M; 5) | 2.1 | n.s. |
| Pentazocine | ||||
| pp38 relative efficacy | 0.47 (0.36-0.56; 14) | 0.35 (0.21-0.51; 10) | 0.7 | n.s. |
| pERK relative efficacy | 0.27 (0.23-0.39; 12) | 0.40 (0.29-0.52; 13) | 1.5 | n.s. |
| pp38/pERK ratio | 1.7 | 0.9 | ||
| pp38 EC50 | 0.47 nm (0.088-2.5 nm; 14) | 14 nm (1.3-140 nm; 10) | 29.8 | p < 0.05 |
| pERK EC50 | 2.3 nm (0.52-10 nm; 12) | 7.2 nm (1.6-32 nm; 13) | 3.1 | n.s. |
| pp38/pERK ratio | 0.2 | 1.9 | ||
| Ki ([3H]U69 binding) | 9.4 (4.1-15 nm; 5) | 15 nm (10-19 nm; 5) | 1.6 | n.s. |
| U50,488 | ||||
| pp38 relative efficacy | 1.0 (0.73-1.3; 12) | 1.0 (0.72-1.3; 9) | 1.0 | n.s. |
| pERK relative efficacy | 1.0 (0.72-1.3; 12) | 1.0 (0.82-1.2; 10) | 1.0 | n.s. |
| pp38/pERK ratio | 1.0 | 1.0 | ||
| pp38 EC50 | 8.6 nm (1.9-39 nm; 12) | 21 nm (4.8-88 nm; 9) | 2.4 | n.s. |
| pERK EC50 | 16 nm (4.4-56 nm; 12) | 16 nm (7.2-35 nm; 10) | 1.0 | n.s. |
| pp38/pERK ratio | 0.5 | 1.3 | ||
| Ki ([3H]U69 binding) | 2.1 nm (1.1-3.0 nm; 4) | 2.4 nm (0.08-4.9 nm; 4) | 1.1 | n.s. |
a p < 0.05.
Are the Differences between hKOR and rKOR Pathway Dependent?
Activation of KOR has been reported to increase phospho-ERK1/2-ir by two separate mechanisms; a G-protein-mediated early phase of ERK1/2 phosphorylation and a slower arrestin-mediated late phase (37). To measure the early phase of ERK1/2 activation through the G-protein pathway, HEK293 expressing rKOR or hKOR were treated for 5 min with pentazocine or U50,488 at concentrations from 100 pm to 10 μm before lysis, and phospho-ERK1/2-ir was measured by Western blot (Fig. 3, Table 1). The EC50 values for pentazocine-induced phospho-ERK1/2-ir for rKOR and hKOR were 7.2 nm (C.I. 1.6–32 nm, n = 13) and 2.3 nm (C.I. 520 pm to 10 nm, n = 12), respectively, with no significant difference. The EC50 values for U50,488-induced ERK1/2 phosphorylation for rKOR and hKOR were 16 nm (C.I. 7.2–35 nm, n = 10) and 16 nm (C.I. 4.4–56 nm, n = 12), respectively, with no significant difference. As was observed for p38 pathway activation, pentazocine appears to be a partial agonist (with 27–40% of U50,488 activity) for stimulation of the early phase of the ERK1/2 pathway. Stimulation of hKOR- and rKOR-expressing cells increased phospho-ERK1/2-ir by 297 and 330% of basal levels, respectively, whereas stimulation with U50,488 increased phospho-ERK1/2-ir to 742 and 670% of basal. This difference in Emax was significant for both hKOR (p < 0.001) and rKOR (p < 0.001).
FIGURE 3.
Pentazocine is less efficacious than U50,488 for activation of ERK1/2. hKOR and rKOR do not differ in potency for the ERK1/2 pathway. a and c, HEK293 cells expressing hKOR or rKOR were treated 5 min with pentazocine (a) or (−)U50,488 (c) before lysis and immunoblotted for phospho-ERK1/2-ir (n = 10–13). Dose-response curves are shown. b and d, representative immunoblots (IB) are shown for a and c.
The difference between pentazocine effects on p38 and ERK activation in hKOR and rKOR is striking and suggests that the sequence differences between hKOR and rKOR more strongly affect p38 activation than the early phase of the ERK1/2 signaling cascade. Furthermore, because U50,488 affected hKOR and rKOR similarly and there were no differences between hKOR and rKOR in pentazocine affinity (Table 1), the results suggest that the differences in pathway activation were a consequence of pentazocine-KOR interaction differences rather than differences in expression or drug affinity.
What Is the Relevance of in Vitro Pharmacological Data for Pentazocine to Behavioral Effects?
The relatively weak p38 activation in rKOR by pentazocine suggests that it may not produce aversion in mice through KOR activation. To assess this, mice were trained in a balanced, unbiased three chamber conditioning apparatus, receiving saline, 10 mg/kg pentazocine or pentazocine in combination with 10 mg/kg norBNI and/or 5 mg/kg naloxone. Mice showed a 200-s place preference for the pentazocine-paired chamber that was not affected by pretreatment with the KOR-selective antagonist norBNI. Pretreatment with naloxone reversed the preference for the pentazocine-paired chamber and unveiled an aversion that was not blocked by pretreatment with norBNI (Fig. 4a). These results suggest that pentazocine did not produce a KOR-mediated aversion in mice, in contrast to reports of KOR-mediated aversive effects in humans.
FIGURE 4.
a, C57BL/6 mice were trained in a balanced, unbiased three-chamber conditioning apparatus. The morning of each of the two training days was paired with vehicle (10 ml/kg intraperitoneal of 15% DMSO, 5% Cremophor, and 80% sterile saline), and the afternoon session was paired with drugs (pentazocine, 10 mg/kg intraperitoneal, pentazocine + norBNI 10 mg/kg intraperitoneal, pentazocine 10 mg/kg + naloxone 5 mg/kg intraperitoneal, or pentazocine 10 mg/kg + norBNI 10 mg/kg + naloxone 5 mg/kg). On the test day mice were allowed to roam freely between all three compartments, and the amount of time spent in each compartment was recorded. Scores were calculated by subtracting the time spent in the drug-paired side of the posttest from time spent in the saline-paired side during the post-test. Significant effect of drug treatment was based on one-way ANOVA (p < 0.0001, n = 4–10), with the Bonferroni-Dunn post-hoc test with correction for multiple comparisons. b, wild type, KOR−/−, and MOR−/− C57/Bl6 mice were injected with 10 mg/kg pentazocine intraperitoneally, and tail flick withdrawal latency was recorded over 180 min post-injection. Two-way repeated measures ANOVA revealed a significant effect of interaction (p < 0.05), time (p < 0.0001), genotype (p < 0.05), and subject matching (p < 0.0001) (n = 13–19). Significance according to the Bonferroni-Dunn post-hoc test of the differences as compared with wild type or KOR−/− is indicated by * or #, respectively (p < 0.05 for one mark, p < 0.01 for two marks). c, total distance traveled during training sessions for conditioned place preference testing in a was recorded. Significant effect of the specific drug treatment (p < 0.01) and drug versus vehicle (p < 0.01) based on two-way repeated measures ANOVA is shown. Significance according to Bonferroni-Dunn post-hoc test of the differences for drug versus vehicle (p < 0.05) is indicated by the *.
To assess the receptor selectivity of pentazocine in mice, wild type, KOR−/−, and MOR−/− mice were injected with pentazocine 10 mg/kg intraperitoneal, and tail flick withdrawal latency was measured over 180 min (Fig. 4b). There was a significant time-dependent (p < 0.0001) increase in tail withdrawal latency and a specific effect of subject pairing (p < 0.0001), genotype (p < 0.05), and interaction (p < 0.05). This antinociceptive response was not significantly different between wild type and KOR−/− mice, whereas MOR−/− had significantly reduced analgesia as compared with wild type and KOR−/− mice. Furthermore, pretreatment with norBNI produced no reduction in pentazocine-induced analgesia in MOR−/− mice (data not shown).
To determine the locomotor effects of pentazocine, total distance traveled was measured during drug training sessions for conditioned place preference. Pentazocine produced neither hypolocomoter effects (typical of κ agonists) nor hyperlocomotor effects (typical of μ agonists) (Fig. 4c).
Do Other Partial KOR Agonists Differ in p38 Activation by hKOR and rKOR?
To determine whether other partial KOR agonists differ in activation of p38 by hKOR and rKOR, HEK293 cells stably expressing rKOR or hKOR were treated 30 min with nalorphine, levorphanol, or butorphanol at concentrations from 100 pm to 10 μm before lysis, and phospho-p38-ir was measured by Western blot. To determine relative efficacy, maximal drug-induced p38 phosphorylation in each experiment was normalized to the increase in phospho-p38-ir stimulated by U50,488 (10 μm) (Table 1). Nalorphine treatment stimulated p38 activation in rKOR and hKOR with relative efficacies of 1.1 (C.I. 0.43–1.7, n = 7) and 0.97 (C.I. 0.77–1.2, n = 7) and EC50 values of 0.16 nm (C.I. 0.02–1.4 nm) and 0.18 nm (C.I. 0.004–7.6 nm), respectively. The relative efficacies of levorphanol for p38 activation by rKOR and hKOR relative to U50,488 were 1.3 (C.I. −0.33–2.9, n = 5) and 0.38 (C.I. −0.01–2.8, n = 6), with EC50 values of 16 nm (C.I. 1.3–160 nm) and 15 nm (C.I. 1.5–150 nm), respectively. No significant difference was observed between hKOR and rKOR in efficacy or potency of nalorphine- or levorphanol-stimulated p38 phosphorylation. Butorphanol increased phospho-p38-ir in hKOR expressing HEK293 cells with an EC50 of 0.11 nm (C.I. 0.004–3.1 nm, n = 6) and an efficacy of 1.1 relative to U50,488 (C.I. 0.65–1.6). In contrast, butorphanol was significantly less efficacious (∼10-fold) for stimulation of p38 phosphorylation in rKOR-expressing cells as compared with hKOR express cells (Student's t test, p < 0.05), with a relative efficacy of 0.12 (C.I. −0.41–0.65, n = 10) and did not stimulate a significant increase in p38 phosphorylation as compared with untransfected HEK293 cells. No increase in p38 phosphorylation was observed in untransfected HEK293 treated with 10 μm nalorphine, levorphanol, or butorphanol (data not shown).
To determine if nalorphine, levorphanol, or butorphanol differ in the acute, G-protein-dependent phase of ERK1/2 activation by hKOR and rKOR, HEK293 cells expressing rKOR or hKOR were treated for 5 min with 10 μm nalorphine, butorphanol, levorphanol, or U50,488 before lysis, and phospho-ERK1/2-ir was measured by Western blot (Table 1). No significant differences were observed between hKOR and rKOR in the relative efficacy of ERK1/2 phosphorylation stimulated by butorphanol. The relative efficacies of levorphanol and nalorphine for ERK1/2 activation were significantly higher in rKOR as compared with hKOR (3.0- and 2.4-fold, respectively). Competition radioligand binding with [3H]U69,593 showed no significant differences between hKOR and rKOR in binding affinity for nalorphine or butorphanol. Levorphanol had a significant 2.4-fold lower affinity for rKOR as compared with hKOR.
Is the Ser-358 Site of hKOR Required for hKOR Activation of p38 upon U50,488 Stimulation?
Because hKOR and rKOR differ in p38 activation and in GRK phosphorylation sites, we next asked if the mechanism for hKOR activation of p38 requires phosphorylation of hKOR by GRK, similar to rKOR. To determine if activation of hKOR induced p38 phosphorylation requires the Ser-358 residue, HEK293 cells expressing hKOR, rKOR, or hKOR(S358N) were treated with 10 μm U50,488 for 5–120 min at 37 °C. This resulted in a significant increase in phospho-p38-ir by 30 min in both hKOR- and rKOR-expressing HEK293, whereas no increases in p38 phosphorylation were detected in HEK293 expressing hKOR(S358N) or in untransfected HEK293 cells (Fig. 5). These data suggest that hKOR activation promotes p38 phosphorylation in a receptor phosphorylation-dependent manner as has previously been reported for rKOR (12).
FIGURE 5.
The Ser-358 residue is required for hKOR activation of p38 MAPK. a, HEK293 cells expressing hKOR, hKOR(S358N), rKOR, or untransfected wild type HEK293 were treated 5–120 min with U50,488 (10 μm) before cell lysis and immunoblotted for phospho-p38-ir. Values represent the mean ± S.E. Significant effect of cell type (p < 0.05) and U50,488 treatment (p < 0.01) based on two-way ANOVA (n = 8–16) is shown. Significance according to the Bonferroni-Dunn post-hoc test, as compared with hKOR(S358N), untransfected, or vehicle is indicated by *, &, or #, respectively (p < 0.05 for single mark or p < 0.01 for double mark). b, representative immunoblots (IB) for a are shown.
Is Arrestin Required for hKOR to Activate p38?
Phosphorylation of p38 in rKOR requires recruitment of arrestin-3 after receptor phosphorylation by GRK3 (12, 16). To determine if hKOR-stimulated p38 phosphorylation also requires arrestin, we transiently transfected hKOR expressing HEK293 with siRNA against arrestin-2, arrestin-3, or a scrambled siRNA control. 72 h after transfection, cells were treated with vehicle or 10 μm U50,488 for 30 min, before lysis and immunoblotting for phospho-p38-ir. To confirm the selectivity of arrestin knockdown, lysates were immunoblotted against arrestin-2 or arrestin-3, and arrestin expression was compared with expression in cells transfected with scrambled siRNA control. Arrestin-2-ir levels were reduced 43% by arrestin-2 siRNA but were unchanged by arrestin-3 siRNA. Arrestin-3-ir levels were reduced 53% by arrestin-3 siRNA but were unchanged by arrestin-2 siRNA (Fig. 6, a–c). U50,488 treatment resulted in a significant increase in phospho-p38-ir in cells transfected with scrambled siRNA (one sample t test, p < 0.05). However, a reduction in the U50,488-stimulated increase in phospho-38-ir was observed compared with cells not exposed to siRNA and transfection reagent. Knockdown of arrestin-3 but not arrestin-2 prevented the U50,488-stimulated increase in p38 phosphorylation (Fig. 6d).
FIGURE 6.
Arrestin-3 is required for hKOR induced p38 MAPK phosphorylation. a, HEK293 cells expressing hKOR were transiently transfected with siRNA against arrestin-2, arrestin-3, or scrambled siRNA control. After 72 h, cells were treated with U50,488 (10 μm) 30 min before cell lysis and immunoblotted for arrestin-2-ir. Significant decrease in arrestin-2 expression as compared with scrambled siRNA controls is indicated by *; one sample t test (p < 0.001, n = 9). b, cells were treated as in a but immunoblotted for arrestin-3-ir. Significant decrease in arrestin-3 expression as compared with scrambled siRNA controls is indicated by *; one sample t test (p < 0.01, n = 5). c, representative immunoblots are shown for a and b. d, cells were treated as in a but immunoblotted against phopho-p38-ir. Significant decrease of U50,488-induced p38 phosphorylation as compared with scrambled siRNA controls is indicated by *; Student's t test (p < 0.05, n = 14–16). e, HEK293 cells expressing hKOR(S358N) were transiently transfected with arrestin-2(R169E), arrestin-3(R170E), or no DNA (transfection reagent control). After 72 h, cells were treated with U50,488 (10 μm) 30 min before cell lysis and immunoblotted for phospho-p38-ir. Significant increase in p38 phosphorylation relative to vehicle-treated transfection reagent control hKOR(S358N) cells is indicated by *; one sample t test (p < 0.05 for single mark or p < 0.01 for double mark, n = 21–23). Significant increase in p38 phosphorylation relative to U50,488 treated transfection reagent control or vehicle treatment for same transfection is indicated by & or #, respectively, Student's t test (p < 0.05, n = 21–23).
To confirm that the absence of p38 phosphorylation in hKOR(S358N) was due to an inability to recruit arrestin, hKOR(S358N)-expressing HEK293 cells were transiently transfected with constructs expressing arrestin-2(R169E) or arrestin-3(R170E). These mutations allow the arrestin molecule to bind to active-state G protein coupled receptors in the absence of receptor phosphorylation, resulting in a dominant-positive phosphorylation-independent arrestin (31, 32). Cells were treated for 30 min with 10 μm U50,488 or vehicle 72 h after transfection before lysis for immunoblot analysis (Fig. 6e). Phosphorylated p38 was measured by immunoblot, and the amount of phospho-p38-ir was normalized to the level in transfection reagent control (no DNA) cells treated with vehicle. Treatment with U50,488 had no significant effect on phospho-p38-ir in transfection reagent control hKOR(S358N) cells. A basal increase in p38 phosphorylation was observed after transfection with arrestin-2(R169E) with no significant further increase in p38 phosphorylation after U50,488. No basal increase was observed in vehicle-treated hKOR(S358N) cells transfected with arrestin-3(R170E), but a significant increase in phospho-p38-ir was observed after U50,488. These data show that expression of arrestin-3(R170E), which does not require receptor phosphorylation to be recruited to the receptor, was sufficient to allow agonist-induced p38 phosphorylation by hKOR (S358N).
Is the Ser-358 Site of hKOR Required for ERK1/2 Activation?
Rapid agonist-induced, GRK/arrestin-independent ERK1/2 activation has been previously reported for both hKOR and rKOR, and a slower GRK/arrestin-dependent phase of phosphorylation after rKOR stimulation has been reported in some cell lines (37). We, therefore, sought to assess the time course of ERK1/2 phosphorylation after hKOR stimulation and to determine if the Ser-358 site plays a role in ERK1/2 phosphorylation after hKOR activation. ERK1/2 phosphorylation levels were analyzed by Western blot after treatment with 10 μm U50,488 for 5–120 min at 37 °C. This treatment resulted in a rapid and significant increase in phospho-ERK1/2-ir at 5 min in HEK293 cells expressing rKOR, hKOR, or hKOR(S358N) but not in untransfected HEK293 cells (Fig. 7). Although there was no significant difference in phospho-ERK1/2-ir between hKOR, rKOR, or hKOR(S358N) at 5 min, ERK1/2 phosphorylation was significantly higher for hKOR compared with hKOR(S358N) at 15 and 30 min after U50,488. Surprisingly, ERK1/2 phosphorylation also remained significantly higher for hKOR as compared with rKOR. ERK1/2 phosphorylation remained significantly elevated in hKOR but not rKOR- or hKOR(S358N)-expressing HEK293 cells up to 60 min after agonist stimulation. This suggests that in addition to the established rapid, receptor phosphorylation-independent phase of ERK1/2 phosphorylation, hKOR has a slower phase of ERK1/2 phosphorylation in HEK293 that requires the Ser-358 site. Furthermore, this late phase of ERK1/2 phosphorylation was more evident in HEK293 cells expressing hKOR than rKOR.
FIGURE 7.
Ser-358 is not required for acute hKOR activation of ERK1/2 but is required for late phase ERK1/2 activation. a, HEK293 cells expressing hKOR, hKOR(S358N), rKOR, and untransfected wild type HEK293 were treated 5–120 min with U50,488 (10 μm) before cell lysis and immunoblotted for phospho-ERK1/2-ir. Significant effect of cell type (p < 0.01), U50,488 treatment (p < 0.01), and interaction (p < 0.05) was revealed by two-way ANOVA (n = 6–11). Significance of the differences according to Bonferroni-Dunn post-hoc test as compared with hKOR(S358N), untransfected, or vehicle is indicated by *, &, or #, respectively (p < 0.05 for single mark, p < .01 for double mark, or p < 0.001 for triple mark). b, representative immunoblots (IB) are shown for a.
Is Arrestin Required for hKOR Activation of Late Phase ERK1/2?
Arrestin recruitment has been implicated in the late phase of ERK1/2 activation by rKOR (37). We hypothesized that arrestin is also required for the late phase of hKOR-stimulated ERK1/2 phosphorylation, which is significantly reduced by mutation of the Ser-358 site. To address this question, we transiently transfected hKOR-expressing HEK293 cells with siRNA against arrestin-2, arrestin-3, or a scrambled siRNA control. 72 h after transfection, cells were treated with vehicle or 10 μm U50,488 for 30 min before lysis and immunoblotting for phospho-ERK1/2-ir. The 30-min time point after agonist stimulation was selected because the greatest difference in ERK phosphorylation between hKOR- and hKOR(S358N)-expressing cells was detected then (Fig. 5a). U50,488 treatment resulted in a significant increase in phospho-ERK1/2-ir in cells transfected with scrambled siRNA (one sample t test, p < 0.0001). However, a reduction in the U50,488-stimulated increase in phospho-ERK1/2-ir was observed compared with cells not exposed to siRNA and transfection reagent. Increased basal phospho-ERK1/2-ir and reduced U50,488 stimulated increase in phospho-ERK1/2-ir were observed as compared cells not exposed to scrambled siRNA and transfection reagent. Knockdown of either arrestin-2 or arrestin-3 significantly reduced the level of ERK1/2 phosphorylation (Fig. 8a). This suggests that both arrestin-2 and arrestin-3 were required for the increased ERK1/2 phosphorylation relative to hKOR(S358N).
FIGURE 8.
Both arrestin-2 and arrestin-3 are required for late phase hKOR activation of ERK1/2. a, HEK293 cells expressing hKOR were transiently transfected with siRNA against arrestin-2, arrestin-3, or scrambled siRNA control. After 72 h cells were treated with U50,488 (10 μm) 30 min before cell lysis and immunoblotted for phospho-ERK1/2-ir. Significant decrease in U50,488 induced ERK1/2 phosphorylation as compared with scrambled siRNA controls is indicated by *, Student's t test (p < 0.01, n = 15–16). b, HEK293 cells expressing hKOR(S358N) were transiently transfected with arrestin-2(R169E), arrestin-3(R170E), or no DNA (transfection reagent control). After 72 h, cells were treated with U50,488 (10 μm) 30 min before cell lysis and immunoblotted for phospho-ERK1/2-ir. Significant increase in p38 phosphorylation relative to vehicle-treated transfection control hKOR(S358N) cells is indicated by *, one sample t test (p < 0.05 for the single mark or p < 0.01 for the double mark, n = 20–22). Significant increase in ERK1/2 phosphorylation relative to vehicle treatment for same transfection is indicated by #, Student's t test (p < 0.05, n = 21–23).
To determine if expression of phosphorylation-independent arrestin-2 or -3 was sufficient to increase the late phase of U50,488-induced ERK1/2 activation in hKOR(S358N)-expressing cells to levels observed in hKOR cells, hKOR(S358N)-expressing HEK293 cells were transiently transfected with constructs expressing mCherry tagged arrestin-2(R169E), arrestin-3(R170E), or a no-DNA transfection reagent control. Cells were treated 72 h after transfection for 30 min with 10 μm U50,488 or vehicle before lysis for immunoblot analysis. Phosphorylated ERK1/2 was measured by immunoblot, and phospho-ERK1/2 was normalized to the level in transfection reagent control (no DNA) cells treated with vehicle. The results obtained showed that ERK1/2 phosphorylation was increased after U50,488 treatment regardless of transfection condition, but transfection with arrestin-2(R169E) or arrestin-3(R170E) alone did not further increase ERK1/2 phosphorylation to the higher ERK1/2 phosphorylation levels observed in cell expressing wild type hKOR at this time point (Fig. 8b). These data show that expression of phosphorylation independent mutants of arrestin-2 or arrestin-3 is not sufficient to recover the late phase of ERK1/2 phosphorylation by hKOR(S358N).
Are Polymorphisms at the Ser-358 Residue Common in the Human Population?
The NCBI Single Nucleotide Polymorphism Database (dbSNP accession number ss43313677; www.ncbi.nlm.nih.gov) reports 10 missense mutations for the OPRK1 gene. This includes a guanine-to-thymine mutation at mRNA position 1308 (rs34369022) that results in mutation of S358I, which would prevent receptor phosphorylation at that residue and subsequent activation of the p38 pathway. To assess if this is a common SNP within the human population, we performed re-sequencing of the OPRK1 gene in 95 individuals from diverse populations (Table 2). Of the 14 SNPs identified, 10 were in coding regions. Three non-synonymous SNPs were found, two of which had been previously reported in dbSNP. The identified missense SNPs were a valine-to-methionine within the second intracellular loop (not previously reported), a lysine-to-glutamate within the second extracellular loop, and an aspartate-to-asparagine in the C tail. No SNPs were identified within codon 358, suggesting that the reported variant is a rare allele (<1/100) or a sequencing mistake. To further explore the frequency of this variant, we accessed the NHLBI Exome Sequencing Project (Exome Variant Server, Seattle, WA; May, 2012), which screened nearly 5500 individuals. A search of this database reveals 23 non-synonymous SNPs within the coding region of the OPRK1 gene but no report of a variant at amino acid position 358, also suggesting that previously reported mutation was extremely rare (<1/10,000) or a false report.
TABLE 2.
Polymorphisms identified in the human κ-opioid receptor gene
Polymorphisms identified in KOR in human population. Data rows shown in bold highlight variants resulting in missense mutations.
| Chromosome 8 position | cDNA position | SNP | Amino acid position | Effect | Minor allele frequency | Variant ID |
|---|---|---|---|---|---|---|
| 54163669 | intron | Cys>Thr | 0.038 | rs9282806 | ||
| 54163562 | 271 | Gly > Thr | 12 | Synonymous | 0.108 | rs1051660 |
| 54147470 | 694 | Cys > Thr | 153 | Synonymous | 0.053 | rs7815824 |
| 54147451 | 713 | Gly > Ala | 160 | Val > Met | 0.005 | |
| 54147368 | 796 | Ala > Gly | 187 | Synonymous | 0.005 | |
| 54147331 | 833 | Ala > Gly | 200 | Lys > Glu | 0.005 | rs77476023 |
| 54147250 | Intron | Gly > Ala | 0.005 | |||
| 54147242 | Intron | Gly > Ala | 0.021 | rs16918900 | ||
| 54142157 | 1078 | Ala > Gly | 281 | Synonymous | 0.258 | rs702764 |
| 54142154 | 1081 | Cys > Thr | 282 | Synonymous | 0.047 | rs16918875 |
| 54142148 | 1087 | Cys > Thr | 284 | Synonymous | 0.005 | rs60353047 |
| 54142052 | 1183 | Cys > Thr | 316 | Synonymous | 0.016 | rs78517449 |
| 54141880 | 1355 | Gly > Ala | 374 | Asp > Asn | 0.016 | rs9282808 |
| 54141824 | 1411 | Gly > Ala | 3′-UTR | 0.245 |
DISCUSSION
The primary findings of this study were that the basic mechanism of p38 MAPK activation is conserved between rKOR and hKOR but that the relative abilities of different agonists to activate specific pathways are not consistent between rKOR and hKOR. This has important implications for the development of biased KOR agonists for therapeutic use, including a limitation on the ability to assess ligand-directed signaling in people based on rodent models.
We found that the nonselective KOR agonist pentazocine is 30-fold more potent for p38 activation in hKOR as compared with rKOR. In contrast, pentazocine was equally potent for arrestin-independent activation of ERK1/2 in hKOR and rKOR, and there was no difference in potency for U50,488 activation of ERK1/2 or p38. Consistent with other studies examining the efficacy of KOR agonists in GTPγS and cAMP inhibition assays (22, 36), we found pentazocine to be a partial agonist for both hKOR and rKOR in both pathways studied.
The effects of pentazocine in mice were complex and not consistent with the model of pentazocine functioning primarily as a KOR agonist. We found that pentazocine produces conditioned place preference and thermal analgesia. Both of these effects were mediated by MOR and unaffected by the disruption of KOR through genetic knock-out or administration of the KOR-selective antagonist norBNI. The opioid receptor mediating pentazocine analgesia in mice is not consistent across studies and seems to be dependent on multiple factors including mouse strain and pain modality (38, 42–44). Similar to other studies (42, 44), we found that in C57Bl/6 mice pentazocine thermal analgesia is mediated through the partial agonism at MOR rather than KOR. Although it has been assumed that pentazocine analgesia is mediated through KOR in humans, few studies have tested this (21), and it is not clear to what extent the mechanisms of pentazocine analgesia are similar between mice and humans.
In the presence of naloxone, mice demonstrated a conditioned place aversion to pentazocine, but this aversion was not blocked by norBNI, showing that it was not KOR-mediated. The mechanism of this aversion is not clear but may be a result of pentazocine activation of δ-receptors (40, 41). These findings in mice are distinctly different from human studies on pentazocine, in which pentazocine produced KOR-dependent dysphoric effects that were enhanced by blocking MOR (28). These results may reflect differences in measurements and assays between humans and mice, although other KOR agonists have been shown to cause KOR-dependent conditioned place aversion in mice. It is possible that the differences between the KOR-dependent aversive properties of pentazocine in humans and mice are partially due to the sequence differences between hKOR and rKOR, as hKOR and rKOR differ in pentazocine potency of p38 activation, which has been shown to play a role in the aversive properties of KOR agonists. Interpretation is limited, however, by the lack of KOR-selectivity, and more selective KOR partial agonists lacking activity at the μ-opioid and δ-receptors will be valuable in understanding the in vivo consequences of these sequence differences.
Differential p38 phosphorylation by hKOR and rKOR was found not to be unique to pentazocine. Butorphanol was similarly efficacious for acute ERK1/2 phosphorylation in hKOR and rKOR expressing HEK293 but did not induce any significant p38 phosphorylation in rKOR-expressing HEK293. In contrast, butorphanol was a potent agonist for p38 activation in hKOR-expressing HEK293, with an efficacy similar to U50,488. Although nalorphine and levorphanol were found to have similar efficacies and potencies for p38 phosphorylation in hKOR- and rKOR-expressing HEK293, these drugs were 2–3-fold more efficacious in rKOR activation of ERK1/2.
Based on these results, there appear to be ligand-specific differences between rKOR and hKOR in activation of MAPKs and of p38 in particular. This may be a result of the difference in the GRK phosphorylation site between hKOR and rKOR and the resulting difference in the molecular environment of the phosphorylated residue. Arrestin binding has previously been shown to be affected by the distribution of charged amino acids and other phosphorylatable residues adjacent to a GRK phosphorylation site (39) that are altered by the differences in location of the GRK site within the KOR C tail. We conclude from this that mice, rats, and rKOR expression systems are not appropriate models for screening for biased KOR ligands for human therapeutics.
Although the ligand-directed signaling properties of KOR agonists are not consistent between hKOR and rKOR, the signaling mechanisms are conserved. p38 activation through hKOR requires the GRK phosphorylation site on the C tail and arrestin-3, whereas the early phase of ERK1/2 phosphorylation is independent of either. There is also evidence that hKOR has a second, slower phase of ERK1/2 phosphorylation that is receptor phosphorylation- and arrestin-dependent, as has previously been shown in some cell lines with rKOR (37). Interestingly, our data show that this late phase of ERK1/2 requires both arrestin-2 and arrestin-3, although how arrestin-2 and -3 cooperate in promoting ERK1/2 signaling is not clear.
Although we cannot extrapolate biased signaling properties of individual agonists from mice and rats to humans, these results show that our understanding of KOR signaling mechanisms in these models likely applies to humans as well. It will be important not to draw conclusions about the signaling properties of particular KOR agonists in humans based purely on rKOR models, but the approach of identifying partial KOR agonists with lower potency for arrestin-mediated pathways than G-protein-mediated pathways holds promise for developing analgesics that lack the abuse potential of MOR agonists and the aversive properties of classic KOR agonists. It is worth noting that the guinea pig KOR is closer in homology to hKOR, containing the Ser-358 residue similar to hKOR. Further studies are needed, but guinea pigs may be a better model system than rats or mice for in vivo screening of biased KOR ligands with human therapeutic potential.
Acknowledgments
We thank Dr. John Pintar for providing the original MOR−/− and KOR−/− mice. We thank Dan Messinger for mouse genotyping.
This work was supported, in whole or in part, by National Institutes of Health Grants T32DA07278 (to H. S., J. R. L., and S. S. S.), KO5 DA020570 (to C. C.), and RO1DA030074 (to C. C.).
- KOR
- κ-opioid receptor
- MOR
- μ-opioid receptor
- ir
- immunoreactivity
- C.I.
- confidence interval
- SNP
- single nucleotide polymorphism
- GRK
- G protein receptor kinase 3
- h-
- human
- r-
- rodent
- Bis-Tris
- Bis-Tris
- ANOVA
- analysis of variance
- norBNI
- norbinaltorphimine.
REFERENCES
- 1. Fraser H. F., Rosenberg D. E. (1964) Studies on the human addiction liability of 2′-hydroxy-5–9-dimethyl-2-(3,3-dimethylally)-6,7-benzomorphan (WIN 20,228). A weak narcotic antagonist. J. Pharmacol. Exp. Ther. 143, 149–156 [PubMed] [Google Scholar]
- 2. Martin W. R., Fraser H. F., Gorodetzky C. W., Rosenberg D. E. (1965) Studies of the dependence-producing potential of the narcotic antagonist 2-cyclopropylmethyl-2′-hydroxy-5,9-dimethyl-6,7-benzomorphan (cyclazocine, WIN-20,740, ARC II-c-3). J. Pharmacol. Exp. Ther. 150, 426–436 [PubMed] [Google Scholar]
- 3. Martin W. R. (1983) Pharmacology of opioids. Pharmacol. Rev. 35, 283–323 [PubMed] [Google Scholar]
- 4. Pfeiffer A., Brantl V., Herz A., Emrich H. M. (1986) Psychotomimesis mediated by κ opiate receptors. Science 233, 774–776 [DOI] [PubMed] [Google Scholar]
- 5. Mucha R. F., Herz A. (1985) Motivational properties of κ and μ-opioid receptor agonists studied with place and taste preference conditioning. Psychopharmacology 86, 274–280 [DOI] [PubMed] [Google Scholar]
- 6. Carlezon W. A., Jr., Thome J., Olson V. G., Lane-Ladd S. B., Brodkin E. S., Hiroi N., Duman R. S., Neve R. L., Nestler E. J. (1998) Regulation of cocaine reward by CREB. Science 282, 2272–2275 [DOI] [PubMed] [Google Scholar]
- 7. Mague S. D., Pliakas A. M., Todtenkopf M. S., Tomasiewicz H. C., Zhang Y., Stevens W. C., Jr., Jones R. M., Portoghese P. S., Carlezon W. A., Jr. (2003) Antidepressant-like effects of κ-opioid receptor antagonists in the forced swim test in rats. J. Pharmacol. Exp. Ther. 305, 323–330 [DOI] [PubMed] [Google Scholar]
- 8. McLaughlin J. P., Marton-Popovici M., Chavkin C. (2003) κ opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci. 23, 5674–5683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Land B. B., Bruchas M. R., Lemos J. C., Xu M., Melief E. J., Chavkin C. (2008) The dysphoric component of stress is encoded by activation of the dynorphin κ-opioid system. J Neurosci. 28, 407–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rosow C. E. (1987) The clinical usefulness of agonist-antagonist analgesics in acute pain. Drug Alcohol Depend. 20, 329–337 [DOI] [PubMed] [Google Scholar]
- 11. Wadenberg ML. (2003) A review of the properties of spiradoline. A potent and selective κ-opioid receptor agonist. CNS Drug Rev. 9, 187–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bruchas M. R., Macey T. A., Lowe J. D., Chavkin C. (2006) κ opioid receptor activation of p38 MAPK is GRK3- and arrestin-dependent in neurons and astrocytes. J. Biol. Chem. 281, 18081–18089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Land B. B., Bruchas M. R., Schattauer S., Giardino W. J., Aita M., Messinger D., Hnasko T. S., Palmiter R. D., Chavkin C. (2009) Activation of the κ opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proc. Natl. Acad. Sci. U.S.A. 106, 19168–19173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bruchas M. R., Schindler A. G., Shankar H., Messinger D. I., Miyatake M., Land B. B., Lemos J. C., Hagan C. E., Neumaier J. F., Quintana A., Palmiter R. D., Chavkin C. (2011) Selective p38α MAPK deletion in serotonergic neurons produces stress resilience in models of depression and addiction. Neuron. 71, 498–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McLaughlin J. P., Xu M., Mackie K., Chavkin C. (2003) Phosphorylation of a carboxyl-terminal serine within the κ-opioid receptor produces desensitization and internalization. J. Biol. Chem. 278, 34631–34640 [DOI] [PubMed] [Google Scholar]
- 16. Bruchas M. R., Land B. B., Aita M., Xu M., Barot S. K., Li S., Chavkin C. (2007) Stress-induced p38 mitogen-activated protein kinase activation mediates κ-opioid-dependent dysphoria. J. Neurosci. 27, 11614–11623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chavkin C. (2011) The therapeutic potential of κ-opioids for treatment of pain and addiction. Neuropsychopharmacology. 36, 369–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Urban J. D., Clarke W. P., von Zastrow M., Nichols D. E., Kobilka B., Weinstein H., Javitch J. A., Roth B. L., Christopoulos A., Sexton P. M., Miller K. J., Spedding M., Mailman R. B. (2007) Functional selectivity and classical concepts of quantitative pharmacology. J. Pharmacol. Exp. Ther. 320, 1–13 [DOI] [PubMed] [Google Scholar]
- 19. von Zastrow M., Svingos A., Haberstock-Debic H., Evans C. (2003) Regulated endocytosis of opioid receptors. Cellular mechanisms and proposed roles in physiological adaptation to opiate drugs. Curr. Opin. Neurobiol. 13, 348–353 [DOI] [PubMed] [Google Scholar]
- 20. Li J., Li J. G., Chen C., Zhang F., Liu-Chen L. Y. (2002) Molecular basis of differences in (−)(trans)-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidiny)-cyclohexyl]benzeneacetamide-induced desensitization and phosphorylation between human and rat κ-opioid receptors expressed in Chinese hamster ovary cells. Mol. Pharmacol. 61, 73–84 [DOI] [PubMed] [Google Scholar]
- 21. Levine J. D., Gordon N. C., Taiwo Y. O., Coderre T. J. (1988) Potentiation of pentazocine analgesia by low-dose naloxone. J. Clin. Invest. 82, 1574–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhu J., Luo L. Y., Li J. G., Chen C., Liu-Chen L. Y. (1997) Activation of the cloned human κ opioid receptor by agonists enhances [35S]GTPγS binding to membranes. Determination of potencies and efficacies of ligands. J. Pharmacol. Exp. Ther. 282, 676–684 [PubMed] [Google Scholar]
- 23. Emmerson P. J., Clark M. J., Mansour A., Akil H., Woods J. H., Medzihradsky F. (1996) Characterization of opioid agonist efficacy in a C6 glioma cell line expressing the μ-opioid receptor. J. Pharmacol. Exp. Ther. 278, 1121–1127 [PubMed] [Google Scholar]
- 24. Beaver W. T., Wallenstein S. L., Houde R. W., Rogers A. (1966) A comparison of the analgesic effects of pentazocine and morphine in patients with cancer. Clin. Pharmacol. Ther. 7, 740–751 [DOI] [PubMed] [Google Scholar]
- 25. Hamilton R. C., Dundee J. W., Clarke R. S., Loan W. B., Morrison J. D. (1967) Studies of drugs given before anaesthesia. 13. Pentazocine and other opiate antagonists. Br. J. Anaesth. 39, 647–656 [DOI] [PubMed] [Google Scholar]
- 26. Jasinski D. R., Martin W. R., Hoeldtke R. D. (1970) Effects of short- and long-term administration of pentazocine in man. Clin. Pharmacol. Ther. 11, 385–403 [DOI] [PubMed] [Google Scholar]
- 27. Zacny J. P., Hill J. L., Black M. L., Sadeghi P. (1998) Comparing the subjective, psychomotor, and physiological effects of intravenous pentazocine and morphine in normal volunteers. J. Pharmacol. Exp. Ther. 286, 1197–1207 [PubMed] [Google Scholar]
- 28. Preston K. L., Bigelow G. E. (1993) Differential naltrexone antagonism of hydromorphone and pentazocine effects in human volunteers. J. Pharmacol. Exp. Ther. 264, 813–823 [PubMed] [Google Scholar]
- 29. Clarke S., Czyzyk T., Ansonoff M., Nitsche J. F., Hsu M. S., Nilsson L., Larsson K., Borsodi A., Toth G., Hill R., Kitchen I., Pintar J. E. (2002) Autoradiography of opioid and ORL1 ligands in opioid receptor triple knockout mice. Eur. J. Neurosci. 16, 1705–1712 [DOI] [PubMed] [Google Scholar]
- 30. Schuller A. G., King M. A., Zhang J., Bolan E., Pan Y. X., Morgan D. J., Chang A., Czick M. E., Unterwald E. M., Pasternak G. W., Pintar J. E. (1999) Retention of heroin and morphine-6 β-glucuronide analgesia in a new line of mice lacking exon 1 of MOR-1. Nat Neurosci. 2, 151–156 [DOI] [PubMed] [Google Scholar]
- 31. Kovoor A., Celver J., Abdryashitov R. I., Chavkin C., Gurevich V. V. (1999) Targeted construction of phosphorylation-independent β-arrestin mutants with constitutive activity in cells. J. Biol. Chem. 274, 6831–6834 [DOI] [PubMed] [Google Scholar]
- 32. Celver J., Vishnivetskiy S. A., Chavkin C., Gurevich V. V. (2002) Conservation of the phosphate-sensitive elements in the arrestin family of proteins. J. Biol. Chem. 277, 9043–9048 [DOI] [PubMed] [Google Scholar]
- 33. Walther C., Nagel S., Gimenez L. E., Mörl K., Gurevich V. V., Beck-Sickinger A. G. (2010) Ligand induced internalization and recycling of the human neuropeptide Y2 receptor is regulated by its C-terminal tail. J. Biol. Chem. 285, 41578–41590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Livingston R. J., von Niederhausern A., Jegga A. G., Crawford D. C., Carlson C. S., Rieder M. J., Gowrisankar S., Aronow B. J., Weiss R. B., Nickerson D. A. (2004) Pattern of sequence variation across 213 environmental response genes. Genome Res. 14, 1821–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stephens M., Sloan J. S., Robertson P. D., Scheet P., Nickerson D. A. (2006) Automating sequence-based detection and genotyping of SNPs from diploid samples. Nat. Genet. 38, 375–381 [DOI] [PubMed] [Google Scholar]
- 36. Remmers A. E., Clark M. J., Mansour A., Akil H., Woods J. H., Medzihradsky F. (1999) Opioid efficacy in a C6 glioma cell line stably expressing the human κ opioid receptor. J. Pharmacol. Exp. Ther. 288, 827–833 [PubMed] [Google Scholar]
- 37. McLennan G. P., Kiss A., Miyatake M., Belcheva M. M., Chambers K. T., Pozek J. J., Mohabbat Y., Moyer R. A., Bohn L. M., Coscia C. J. (2008) κ opioids promote the proliferation of astrocytes via Gβγ and β-arrestin 2-dependent MAPK-mediated pathways. J. Neurochem. 107, 1753–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Suzuki T., Narita M., Misawa M., Nagase H. (1991) Pentazocine-induced biphasic analgesia in mice. Life Sci. 48, 1827–1835 [DOI] [PubMed] [Google Scholar]
- 39. Gurevich V. V., Gurevich E. V. (2006) The structural basis of arrestin-mediated regulation of G-protein-coupled receptors. Pharmacol. Ther. 110, 465–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hiramatsu M., Hoshino T. (2005) Improvement of memory impairment by (+)- and (−)-pentazocine via δ but not κ opioid receptors. Brain Res. 1057, 72–80 [DOI] [PubMed] [Google Scholar]
- 41. Mori T., Yoshizawa K., Nomura M., Isotani K., Torigoe K., Tsukiyama Y., Narita M., Suzuki T. (2012) δ1 receptor function is critical for both the discriminative stimulus and aversive effects of the κ-opioid receptor agonist U-50488H. Addict Biol. 17, 717–724 [DOI] [PubMed] [Google Scholar]
- 42. Ide S., Minami M., Uhl G. R., Satoh M., Sora I., Ikeda K. (2011) (−)-Pentazocine induces visceral chemical antinociception, but not thermal, mechanical, or somatic chemical antinociception, in μ-opioid receptor knockout mice. Mol. Pain 7, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chien C. C., Pasternak G. W. (1995) (−)-Pentazocine analgesia in mice. Interactions with a δ receptor system. Eur. J. Pharmacol. 294, 303–308 [DOI] [PubMed] [Google Scholar]
- 44. Shu H., Hayashida M., Arita H., Huang W., Zhang H., An K., Wu G., Hanaoka K. (2011) Pentazocine-induced antinociception is mediated mainly by μ-opioid receptors and compromised by κ-opioid receptors in mice. J. Pharmacol. Exp. Ther. 338, 579–587 [DOI] [PubMed] [Google Scholar]