Background: The molecular mechanisms of soy isoflavones in metastatic cancer remain to be elucidated.
Results: Equol, a daidzein metabolite, regulates eIF4G-mediated cap-independent protein synthesis initiation of proteins relevant for cancer malignancy.
Conclusion: Equol is a potent regulator of the cancer promoting effects of dietary daidzein.
Significance: Consumption of soy may not be advisable for patients with aggressive breast cancer.
Keywords: Cancer Biology, Eif4e, Metastasis, Myc, Protein Synthesis, Eif4G, Equol, IRES-dependent Protein Synthesis
Abstract
Dietary soy is thought to be cancer-preventive; however, the beneficial effects of soy on established breast cancer is controversial. We recently demonstrated that dietary daidzein or combined soy isoflavones (genistein, daidzein, and glycitein) increased primary mammary tumor growth and metastasis. Cancer-promoting molecules, including eukaryotic protein synthesis initiation factors (eIF) eIF4G and eIF4E, were up-regulated in mammary tumors from mice that received dietary daidzein. Herein, we show that increased eIF expression in tumor extracts of mice after daidzein diets is associated with protein expression of mRNAs with internal ribosome entry sites (IRES) that are sensitive to eIF4E and eIF4G levels. Results with metastatic cancer cell lines show that some of the effects of daidzein in vivo can be recapitulated by the daidzein metabolite equol. In vitro, equol, but not daidzein, up-regulated eIF4G without affecting eIF4E or its regulator, 4E-binding protein (4E-BP), levels. Equol also increased metastatic cancer cell viability. Equol specifically increased the protein expression of IRES containing cell survival and proliferation-promoting molecules and up-regulated gene and protein expression of the transcription factor c-Myc. Moreover, equol increased the polysomal association of mRNAs for p 120 catenin and eIF4G. The elevated eIF4G in response to equol was not associated with eIF4E or 4E-binding protein in 5′ cap co-capture assays or co-immunoprecipitations. In dual luciferase assays, IRES-dependent protein synthesis was increased by equol. Therefore, up-regulation of eIF4G by equol may result in increased translation of pro-cancer mRNAs with IRESs and, thus, promote cancer malignancy.
Introduction
Isoflavones found primarily in legumes and particularly in soy are a major class of phytoestrogens that are structurally and/or functionally similar to 17β-estradiol (1). These compounds have received increasing attention for their potential estrogenic or antiestrogenic effects, leading to concerns surrounding the use of phytoestrogen supplements in breast cancer patients who may overexpress estrogen receptors in the tumor tissue (2). Because soy foods have anticancer effects at early stages of carcinogenesis, most studies have focused on breast cancer prevention by soy isoflavones (3). However, the benefits of soy foods as chemopreventives for established breast cancer or as substitutes for hormone replacement therapies remain controversial (3–5).
The second most prominent isoflavone found in soybeans and soy products is the aglycone form daidzein. Intestinal bacteria are central to the absorption and metabolism of isoflavones. After oral ingestion, glucosidases metabolize the β-glycosidic isoflavone daidzin into the bioavailable aglycone daidzein (6). Daidzein can be further metabolized to equol; before final absorption, the intestinal microflora converts daidzein to equol or O-desmethylangolensin (Fig. 1). Rodents are efficient producers of equol. However, not all humans have the gut microflora necessary to convert daidzein to equol, and ∼30–50% of humans are equol producers. The proportion of equol producers also vary with demographic, lifestyle factors, and ethnicity, and certain populations (e.g. Chinese) have been shown to be high equol producers (5, 7). This variation in equol production may explain the discrepancies found in epidemiological studies on the risks or benefits of dietary soy (5, 6, 8–10).
FIGURE 1.
Daidzein is metabolized to equol by intestinal bacteria.
Unlike the metabolite O-desmethylangolensin, which has low biological activity (6, 11), equol is structurally similar to estrogen with 80 times more estrogen receptor-β (ERβ)2 affinity than its precursor daidzein (11–13). Equol has been implicated with decreased prostate cancer cell proliferation and prostate cancer risk by acting as an antagonist for dihydrotesterone (14, 15). In ER (+) T47D and MCF-7 human breast cancer cells, equol increased estrogenic activity and cell proliferation, but dietary equol did not affect tumor growth in nude mice (16–19). Dietary daidzein also failed to reduce chemically induced mammary tumor growth in rats that demonstrated ∼1 μm equol in the serum (20). Others have shown that equol inhibited growth and invasion of ERα (−) ERβ (+) human breast cancer cells and induced cell cycle arrest and apoptosis (14, 21–23). However, caution must be exercised when interpreting in vitro studies because the inhibitory effects of equol in breast cancer cells were observed at concentrations ranging from 50 to 100 μm (14, 22, 23), whereas low concentrations of equol (≤1 μm) increased breast cancer cell proliferation (17, 24). Moreover, dietary soy, where genistein, daidzein, and equol were detected in serum samples, increased mammary epithelial cell proliferation of human subjects (25). Therefore, the association between equol production and cancer risk in humans remains to be adequately characterized (8, 26, 27). Overall, benefits from soy intake are associated with ER (+) breast cancer, and the effect of equol or soy isoflavones on ER (−) breast cancers or established aggressive breast cancers are not well understood (5, 28, 29).
Our recent data using ER (−) highly metastatic MDA-MB-435 human cancer cells reported that dietary daidzein and soy isoflavones (daidzein:genistein:glycitein, 5:4:1) increased mammary tumor growth and metastasis in nude mice (30). PCR analysis of mammary tumors demonstrated that dietary daidzein up-regulated the expression of a number of genes that regulate cell proliferation and survival including CCND1, CTNNB1 (catenin (cadherin-associated protein) β1), GRB2 (growth factor receptor-bound protein 2), JUN (Jun oncogene), MAPK1 (mitogen-activated protein kinase 1), and IRS1 (insulin receptor substrate 1). Of note was the significant up-regulation of eukaryotic initiation factor 4G (EIF4G1) and increased eIF4G and eIF4E protein levels in tumors after daidzein diets (30). Increased levels of eIF4F family members such as eIF4E, -G, and -B have been implicated with specific translation of tumor survival and malignancy-promoting proteins that have mRNAs with long structured 5′-untranslated regions (UTR) and/or internal ribosome entry sites (IRES) (31–33).
The present study was initiated to test the hypothesis that dietary daidzein promotes cancer progression via increased synthesis of cancer promoting molecules. We show that the isoflavone daidzein may promote cancer through the metabolite equol. Equol-mediated eIF4G up-regulation can contribute to non-canonical, eIF4E-independent and, thus, 5′-7-methyl-guanosine (M7G) cap-independent protein synthesis via IRES sites (33, 34). Therefore, equol may specifically direct the synthesis of IRES-containing mRNAs that induce cell survival and cell proliferation and promote cancer malignancy.
EXPERIMENTAL PROCEDURES
Cell Culture
Metastatic variant of MDA-MB-435 (ER−) (gift of Dr. Danny Welch, The University of Kansas Cancer Center) and MDA-MB-231 (ERα-, ERβ+) metastatic human breast cancer cells (American Type Culture Collection, Manassas, VA) were maintained in complete culture medium: Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen) at 37 °C in 5% CO2.
Cell Treatment
Quiescent metastatic cancer cells were treated with 0 (vehicle, 0.1% DMSO), 1, 5, 10, 25, or 50 μm isoflavone daidzein (LC Laboratories, Woburn, MA) or metabolite (R,S) Equol (LC Laboratories, Woburn, MA) in DMEM and 5% FBS media for 24 or 48 h.
Tumor Model
The tumors were derived from our previous study (30). Briefly, female athymic nu/nu mice, 5 weeks old (Charles River Laboratories, Wilmington, MA), were inoculated at the mammary fat pad with green fluorescent protein (GFP)-tagged-MDA-MB-435 cells. After 1 week of tumor inoculation, vehicle (10% ethanol, 90% corn oil), 10 mg/kg body weight (BW) of daidzein or combined soy isoflavones 10 mg/kg BW genistein, 9 mg/kg BW daidzein, and 1 mg/kg BW glycitein were administered 3 times a week by oral gavage for 11 weeks. After necropsy, mammary tumors were excised and stored snap-frozen in liquid nitrogen.
Western Blotting
Cells and tumors were lysed and Western blotted as described in Ref. 30. Primary antibodies to eIF4E, phospho (P)-eIF4ESer-209, eIF4G, p-eIF4GSer-1108, 4E-BP1, p4E-BP1Thr-37/46, c-Myc, p120 catenin, p-p120Thr-916, β-catenin, survivin, Bcl-XL, Bcl2, vascular endothelial growth factor (VEGF), cyclin D, Jun B, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and β actin (Epitomics, Burlingame, CA, Cell Signaling, Danvers, MA; Sigma) were used. Data from mouse mammary tumors were normalized to GFP expression to ensure quantification of proteins from GFP-MDA-MB-435 cells using anti-GFP antibody (Abcam, Cambridge, MA). The integrated density of positive bands was quantified using Image J software, as described in Ref. 30.
Cell Viability Assay
Cell viability was determined by the CellTiter 96 Non-Radioactive Cell Proliferation kit according to the manufacturer's instructions (Promega, Madison, WI). Briefly, quiescent 1 × 105 MDA-MB-435 cells were added to the wells of a 96-well plate and treated for 24 h with vehicle, 1, 5, 10, 25, or 50 μm equol. After equilibration, 15 μl/well MTT (3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide) reagent was added, and the plates were incubated at 37 °C for 4 h. Stop solution (100 μl) was added to each well, and the plates were incubated to facilitate solubilization of newly formed formazan salts. The absorbance at 570 nm was measured using an ELISA plate reader.
Quantitative Real-time Reverse Transcriptase Polymerase Reaction (qRT-PCR) Analysis
As described in Ref. 35, qRT-PCR analysis was performed from cells treated with vehicle or equol for 24 h. Total RNA was extracted using the Qiagen RNeasy Kit (Qiagen, Valencia, CA). RNA concentration was detected using a NanoDrop (Thermo Scientific, Wilmington, DE). RNA (0.5 μg) was used to synthesize cDNA using iScript cDNA synthesis kit (Bio-Rad). Real-time PCR primers were as follows. MYC, forward (5′-TTCTCAGAGGCTTGGCGGGAAA-3′) and reverse (5′-TGCCTCTCGCTGGAATTACTACA-3′); β2 microglobulin (B2M) forward (5′-GGCTATCCAGCGTACTCCAAA-3′) and reverse (5′-CGGCAGGCATACTCATCTTTTT-3′); GAPDH (forward (5′-TTGCCATCAATGACCCCTTCA-3′) and reverse, (5′-CGCCCCACTTGATTTTGGA-3′); CCND1 (forward (5′-TGGTGAACAAGCTCAAGTGGA-3′) and reverse (5′-TGATCTGTTTGTTCTCCTCCGCCT-3′); VEGFA forward, 5′-AGGCGAGGCAGCTTGAGTTAAA-3′ and reverse (5′-TTCTGTCGATGGTGATGGTGTGGT-3′); EIF4G forward (5′-TTGTGGATGATGGTGGCT-3′ and reverse (5′-TTATCTGTGCTTTCTGTGGGT-3′); CTNND1 forward (5′-TCCAGCAAACGATACAGTGG-3′) and reverse (5′-GAACCACCTCTGGCTGAAAT-3′). Real-time reactions were performed using iQ SYBR-Green PCR Master Mix (Bio-Rad). The amplification reaction was performed for 40 cycles (10 s at 95 °C, 30 s at 59 °C, and 30 s at 72 °C). B2M mRNA was used as an internal control. The -fold change was determined by the 2ΔΔCT method as described in Refs. 30 and 35.
Polysomal Fractionation
Sucrose density gradient centrifugation was used to separate the subpolysomal and polysomal fractions as in Refs. 32 and 36. MDA-MB-435 cells were treated with vehicle or equol (25 μm) for 24 h. Five minutes before harvest, 100 μg/ml cycloheximide was added to the culture medium. Cells were washed in ice-cold phosphate-buffered saline supplemented with 100 μg/ml cycloheximide and harvested in polysome lysis buffer (10 mm Tris-HCl at pH 7.4, 40 mm KCl, 3 mm MgCl2, 5% glycerol, 0.2% Nonidet P-40, 150 μg/ml cycloheximide, 1 mm PMSF, 20 mm DTT, 200 μg/ml heparin). Samples were incubated on ice for 10 min and centrifuged at 12,000 × g for 10 min at 4 °C. The resulting supernatant was layered onto 10–50% sucrose density gradients and centrifuged in a Beckman SW41 rotor at 35,000 rpm for 3 h at 4 °C. The A260 of sucrose density gradient fractions (200 μl) was determined through the fractions collected from top to bottom. Consecutive fractions were pooled, generating a total of nine fractions. Sucrose density gradient fractions were resuspended in guanidine thiocyanate buffer containing 10% mercaptoethanol (RLT buffer, RNeasy Mini Kit, Qiagen). RNA was extracted using the RNeasy Mini Kit for isolation of total RNA (Qiagen) following the manufacturer's instructions. RNA preparations from each fraction were subjected to qRT-PCR for CTNND1 (p120-catenin), GAPDH, and EIF4G as described above.
Cap Affinity Chromatography
Cell lysates, after vehicle or 25 μm equol treatment for 24 h, were incubated with 7-methyl-GTP (m7GTP) or control Sepharose 4B beads (Amersham Biosciences) for 1 h at 4 °C as described in Ref. 37–39). Total lysates, washed beads after m7GTP co-capture, and the supernatants were Western-blotted for eIF4E, 4E-BP, or eIF4G.
eIF4G Immunoprecipitation
MDA-MB-435 cells were treated with vehicle or 25 μm equol for 24 h. Cells were lysed in radioimmune precipitation assay buffer (50 mm HEPES, pH 7.0, 2 mm EDTA, 250 mm NaCl, 50 mm NaF, 25 mm Na4O7P2, 2 mm Na3VO4, 1 mm PMSF, 0.1 mm DTT, and 0.5% IGEPAL), and 500 μg each of total protein extracts were incubated with anti-eIF4G antibody (Cell Signaling) (1:50) or control antibody (1:50) for 2 h at 4 °C followed by incubation with protein A Sepharose (cell signaling) for an additional hour. Immunoprecipitates were washed and processed for SDS-PAGE and Western blotting as described in Ref. 40. Immunoprecipitates of eIF4G antibody or control monoclonal antibody, supernatants, and total lysates were immunoblotted with anti-eIF4G (top half of gel) or anti-eIF4E (bottom half of same gel) to visualize eIF4G as a 220-kDa band and eIF4E as a 25-kDa band.
Luciferase Reporter Assays
MDA-MB-435 cells were transfected with a bicistronic reporter system (a kind gift of Dr. Robert Schneider, New York University Langone Medical Center) or control plasmid containing the luciferase constructs without IRES using Lipofectamine 2000 (Invitrogen) as the per manufacturer's directions. As described in Ref. 32, this plasmid contains a cap-dependent Renilla luciferase followed by a 5′-UTR containing the p120 catenin IRES-mediated firefly luciferase. 24 h after transfection, cells were treated with equol for an additional 24 h. The relative IRES activity was analyzed as 570-nm firefly luciferase/480-nm Renilla luciferase in a luminometer using a dual luciferase assay kit (Promega) according to the manufacturer's instructions.
Statistical Analysis
Data were analyzed and reported as the mean ± S.E. in triplicate. Statistical analyses were done using Microsoft Excel and GraphPad Prism. Differences between means were determined using Student's t test, and p ≤ 0.05 was considered significant.
RESULTS
Dietary Daidzein Up-regulates Expression of eIF4G and eIF4E and Increased Protein Levels of mRNAs with IRES Sites in Vivo but Not in Vitro
We recently reported that daidzein increased mammary tumor growth and metastasis in nude mice with mammary tumors established from the ER (−) highly metastatic human cancer cell line MDA-MB-435. Mammary tumors from mice treated with daidzein diets demonstrated a significant 2–3-fold up-regulation of EIF4G1 gene and protein expression and a ∼7.0-fold increase in eIF4E protein levels compared with vehicle controls. Combined soy treatment resulted in a 1.8-fold increase in EIF4E gene and a 2.5-fold increase in protein expression (30).
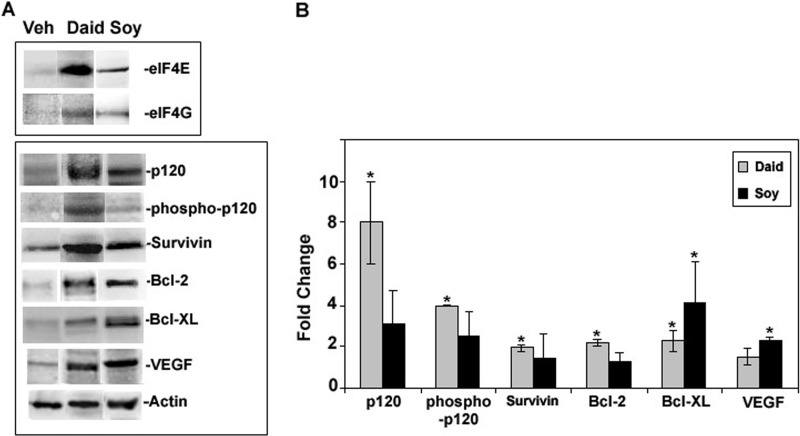
High levels of eukaryotic initiation factors, specifically eIF4G1, have been correlated with increased cap independent translation of specific mRNAs that contain IRESs and long-structured 5′-UTRs (32, 33, 41). To investigate the effect of the overexpressed eIF4F complex on translation of mRNAs sensitive to elevated eIF4F initiation factors, we analyzed protein expression levels of pro-survival, -angiogenesis, and -proliferation molecules known to have mRNAs with long UTRs and/or IRESs (32, 33, 42) from primary tumors of mice after daidzein or combined soy (genistein:daidzein:glycitein, 5:4:1) diets. As shown in Fig. 2, the pro-survival proteins survivin (2-fold), Bcl2 (2.2-fold), and Bcl-XL (2.3-fold) and total and active phospho-p120 catenin (8- and 4-fold, respectively) were significantly increased in tumors after daidzein diets. Combined soy also demonstrated significant increases in expression of Bcl-XL (4-fold) and VEGF (2.3-fold). However, the expression of β-actin, a constitutively expressed mRNA with a short 5′-UTR was not affected by dietary soy isoflavones. Both actin and GFP expression were used as standards for the analysis of -fold differences of IRES containing molecules compared with vehicle controls to ensure analysis of GFP-MDA-MB-435 cells.
FIGURE 2.
Effect of soy isoflavones on protein expression in mammary fat pad tumors from mice treated with vehicle (Veh), genistein, daidzein (daid), or soy isoflavones (genitein:daidzein:glycitein (5:4:1). Mammary fat pad tumors established from GFP-MDA-MB-435 cells from the study described in Ref. 30 were lysed, and the proteins were extracted. A, shown are representative Western blots from tumor extracts immunostained for cancer promoting molecules. These bands are representative of n = 3–4, derived from the same gel for all treatments. B, shown are -fold changes of protein expression compared with vehicle as calculated from the integrated density of positive bands from Western blots and normalized with actin and GFP expression. Values show the mean ± S.E. (n = 3). An asterisk indicates statistical significance of p ≤ 0.05.
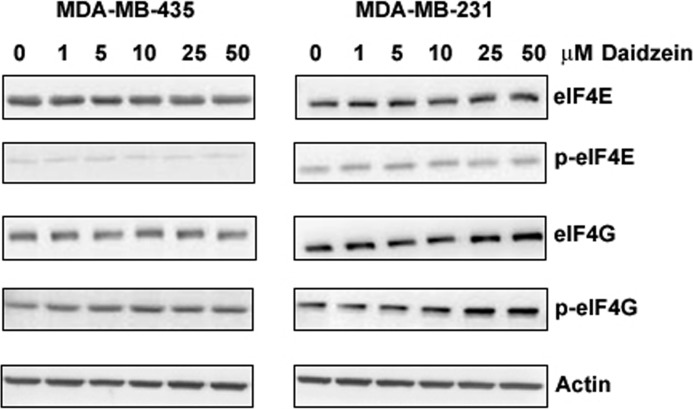
This data implicate dietary daidzein in eIF4F-controlled translation of proteins that regulate cancer progression. Therefore, the molecular mechanisms of daidzein action were further investigated in vitro using the same cell line from the in vivo study, ER (−) MDA-MB-435 cells as well as the ERβ (+) metastatic breast cancer cell line MDA-MB-231. Cells were treated with vehicle or daidzein at 0–50 μm. These concentrations fall within the range of 1–10 μm that has been shown to accumulate in the circulation after consumption of soy products (43). However, we did not detect any significant changes in eIF4E, p-eIF4E, eIF4G, or p-eIF4G in both cell lines after 24 or 48 h treatment of daidzein at all concentrations tested. There were slight increases in eIF4G and p-eIF4G in MDA-MB-231 cells treated with 25 and 50 μm daidzein; nevertheless, these increases were not statistically significant (Fig. 3).
FIGURE 3.
Effect of daidzein on total and phospho (p) eIF4E and eIF4G expression in MDA-MB 435 and MDA-MB-231 cells. Quiescent cells were treated with vehicle or daidzein (0–50 μm) in 5% serum for 24 h, lysed, and Western-blotted with mono-specific antibodies. Left, representative Western blots of MDA-MB 435 cell lysates (n = 3) are shown. Right, representative Western blots of MDA-MB-231 cell lysates (n = 3) are shown.
The Daidzein Metabolite Equol Up-regulates Gene and Protein Expression of eIF4G and c-Myc and Protein Expression of mRNAs with IRESs
Daidzein can be further metabolized to equol (70%) and O-desmethylangolensin (5–20%) (Fig. 1). In rodents, equol is the major circulating metabolite, and all rodents are equol producers (6, 11). Therefore, we reasoned that the daidzein effects on MDA-MB-435 metastatic cell lines in vivo may be due to the metabolite equol. MDA-MB-435 and MDA-MB-231 cells were treated with (R,S)-equol at different concentrations (0–50 μm) and tested for eIF4E and eIF4G expression by Western blotting.
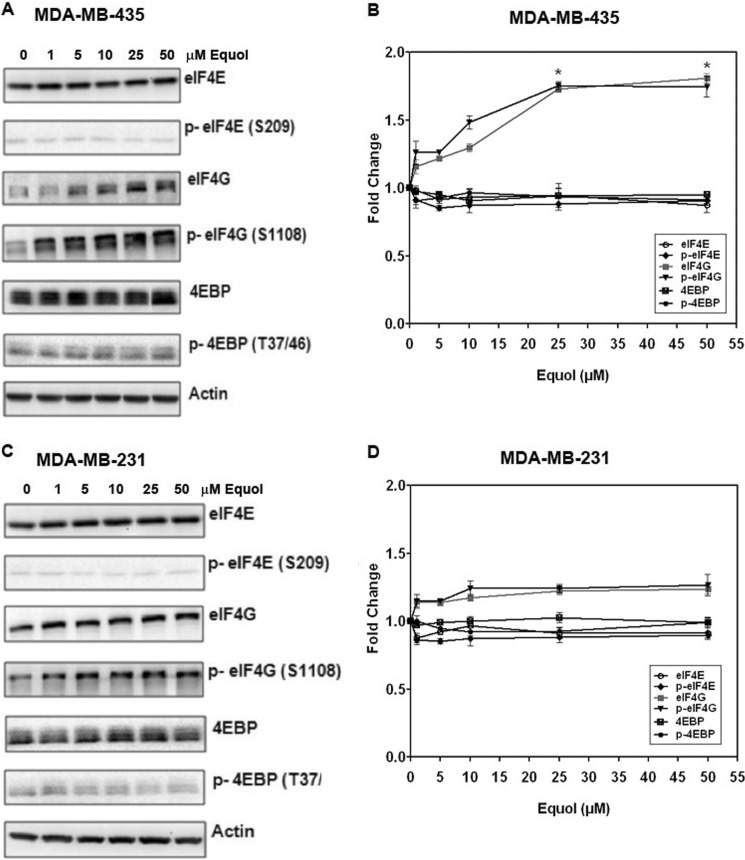
Fig. 4 demonstrates that similar to dietary daidzein in mice, equol increased the expression of total and p-eIF4G in a concentration-dependent manner. Elevation of eIF4G preceded the p-eIF4G levels. Therefore, equol may not specifically affect phosphorylation of eIF4G, but only eIF4G expression. In MDA-MB-435 cells, equol at 25 and 50 μm significantly increased eIF4G protein levels by ∼1.8-fold (p ≤ 0.05) compared with vehicle controls. The increase in eIF4G in the MDA-MB-231 cell line was more modest (∼1.3-fold) but consistent at similar concentrations (>10 μm). It is possible that the presence of ERβ in the MDA-MB-231 cell line may exert a differential effect on equol-mediated eIF4G expression. The protein levels of eIF4E and its inhibitory protein 4E-BP remained unchanged at all concentrations of equol tested in both cell lines, indicating a specific effect on eIF4G expression and not eIF4E expression or regulation.
FIGURE 4.
Effect of equol on total and phospho (p) eIF4E, eIF4G, and 4E-BP expression in MDA-MB 435 and MDA-MB-231 cells. Quiescent cells were treated with vehicle or equol (0–50 μm) for 24 h, lysed, and Western-blotted with mono-specific antibodies. A and B, shown are representative Western blots and -fold changes relative to vehicle, as quantified from Image J analysis of integrated density of positive bands of MDA-MB 435 cell extracts. C and D, shown are representative Western blots and -fold changes relative to vehicle, as quantified from Image J analysis of integrated density of positive bands of MDA-MB-231 cell extracts. Values show the mean ± S.E. (n = 3). An asterisk indicates statistical significance of p < 0.05.
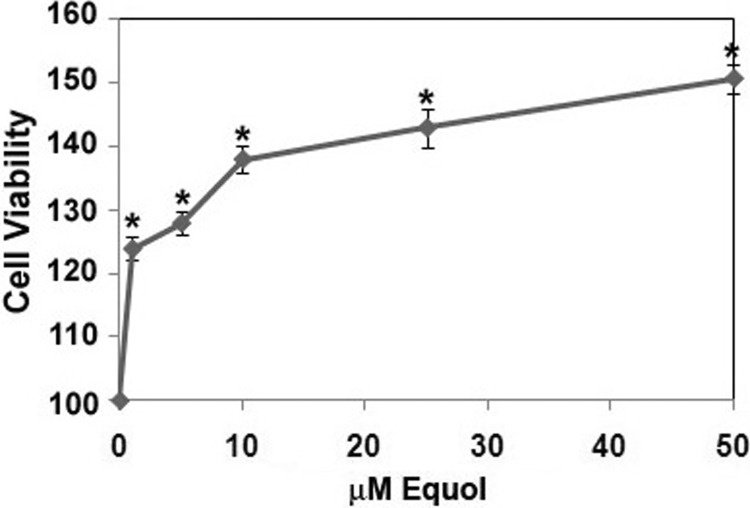
Similar to the effect of dietary daidzein on mammary tumor growth in nude mice, we found that equol enhanced cancer cell viability. Treatment of equol (0–50 μm) to MDA-MB-435 cells increased cell viability starting at 1 μm in a concentration-dependent and statistically significant manner (Fig. 5). This increase in cell number reflects the effect of equol on gene and protein expression of eIF4G.
FIGURE 5.
Effect of equol on cell viability. Quiescent MDA-MB-435 cells were treated with 1–50 μm equol or vehicle for 24 h. Cells were lysed and subjected to an MTT ((3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide)) assay. Results are shown relative to vehicle (100%). n = 3 for all experiments. An asterisk indicates statistical significance of p ≤ 0.05.
A recent study from ER (−) inflammatory breast cancer cells demonstrated that overexpression of eIF4G with no changes in the cap-binding protein eIF4E and its negative regulator 4E-BP1 promotes cap-independent protein synthesis of IRES-containing mRNAs (32). The protein products of these mRNAs have been shown to regulate cancer cell survival and proliferation (44–48). To determine whether increased eIF4G in response to equol may drive IRES-dependent protein synthesis, we tested the protein and gene expression of mRNAs with or without IRESs.
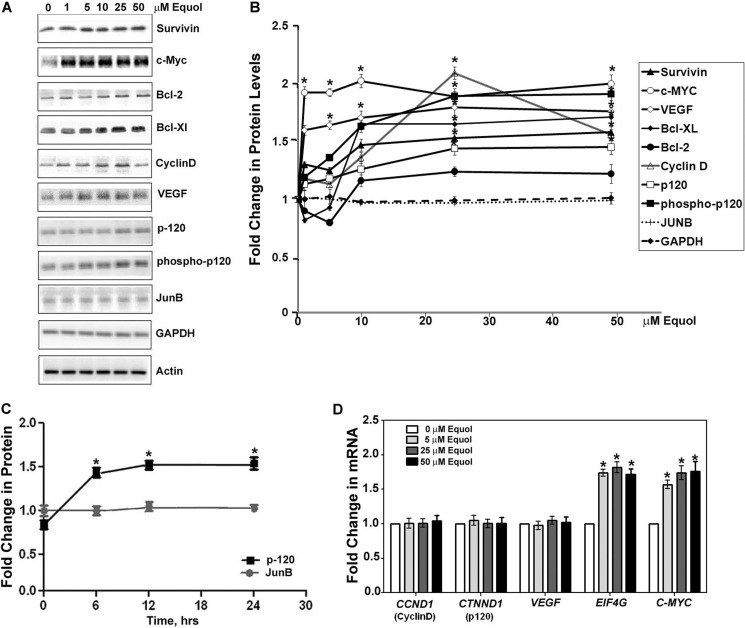
Similar to the effect of dietary daidzein in MDA-MB-435 tumors, equol at 10, 25, and 50 μm up-regulated protein expression of IRES-containing mRNAs: survivin, c-Myc, Bcl-2, Bcl-XL, cyclin D, VEGF, and total and active p120 catenin in the MDA-MB-435 cells by ∼1.3–2.0-fold compared with vehicle. Unlike the other proteins that demonstrated up-regulated expression after 5–50 μm equol, c-Myc protein expression was increased by ∼1.8-fold at all concentrations of equol tested (1–50 μm). Equol treatment at 25 μm resulted in a 2.0-fold significant increase in protein expression of Cyclin D, but this increase dropped off to ∼1.4-fold at 50 μm equol (Fig. 6, A and B).
FIGURE 6.
Expression of cancer promoting molecules after equol treatment. Quiescent MDA-MB-435 cells were treated with vehicle or 1–50 μm equol for 24 h, lysed, and Western-blotted with specific antibodies to the indicated proteins. A, representative Western blots are shown. B, shown are -fold changes relative to actin as calculated by Image J analysis of positive bands from equol treatments (1–50 μm) compared with vehicle controls. n = 3. An asterisk indicates statistical significance of p < 0.05. C, shown is -fold change in protein expression as a function of time in equol. Quiescent MDA-MB-435 cells were treated with vehicle or 25 μm equol for various times as indicated and subjected to lysis and Western blotting for p120 catenin or JunB. -Fold changes relative to actin were calculated by Image J analysis of positive bands from equol treatments compared with vehicle controls (n = 3). An asterisk indicates statistical significance of p < 0.05. D, shown is the effect of equol on gene expression of cancer-promoting molecules. Quiescent MDA-MB-435 cells were treated for 24 h with 5–50 μm equol, and CCND1, CTNND1, VEGF, EIF4G, or MYC expression was quantified by qRT-PCR. -Fold changes in gene expression from cells treated with equol are compared with vehicle (n = 3). An asterisk indicates statistical significance of p < 0.05.
Proteins with mRNAs with short 5′-UTRs that do not contain IRESs (JunB, GAPDH, and actin (49, 50)) were not increased in response to all concentrations of equol tested (0–50 μm) (Fig. 6, A and B). In contrast to JunB, equol increased protein expression of p120-catenin, a molecule with an IRES containing mRNA, by 1.5-fold 6 h after treatment, which remained constant up to 24 h in equol (Fig. 6C).
To determine whether the increased protein expression in response to equol was due to an increase in gene expression, the expression of representative IRES-positive mRNAs was determined by qRT-PCR of cell lysates after vehicle or equol treatment. The IRES containing mRNAs CCND1 (cyclin D), CTNND1 (p120 catenin), and VEGF did not change in response to equol. However, 5–50 μm equol up-regulated gene expression of eIF4G and c-Myc (Fig. 6D).
Because the effect of equol on increased protein levels of IRES-containing mRNAs saturated at 25 μm equol for 24 h, we selected these conditions for the subsequent in vitro assays. This concentration of equol has been found in the urine of humans after consumption of soy foods (51).
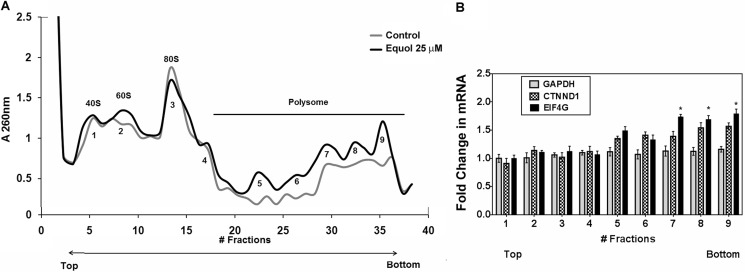
To determine whether the up-regulated eIF4G levels in response to equol resulted in increased protein synthesis initiation, we performed polysomal fractionations of cell lysates after vehicle or equol. The ribosomal and polysomal fractions were isolated from sucrose gradients, and potential association of mRNAs was determined by A260 measurements. Compared with vehicle treatments, equol increased the total mRNA associated with the polysomal fractions (Fig. 7A). qRT-PCR analyses for GAPDH, CTNND1, and EIF4G (with B2M as the control) demonstrated that the IRES containing mRNAs CTNND1 and EIF4G, but not GAPDH, were associated with the heavier polysomal fractions from equol-treated cells. CTNND1 mRNA was increased by 1.5-fold, and EIF4G was significantly increased by 1.7-fold in heavier polysomal fractions from equol-treated cells compared with vehicle controls (Fig. 7B). Similar to the results on protein expression, association of the IRES-negative GAPDH mRNA was not changed by equol treatment. Therefore, the observed equol-mediated up-regulation of p120-catenin protein expression without changes in CTNND1 gene expression (Fig. 6) may indicate preferential synthesis of IRES containing mRNAs in equol-treated cells. The enhanced affinity of EIF4G for the polysome fractions indicates that the EIF4G mRNA elevated in response to equol is translated into protein, thus accounting for the elevated eIF4G protein levels.
FIGURE 7.
Analysis of polysome profiles. Equivalent amounts of total cell lysate from vehicle control or 25 μm equol-treated MDA-MB-435 cells were loaded onto10–50% sucrose gradients. The UV absorbance of pooled sequential gradient fractions (numbered consecutively) was measured at 260 nm. A, shown is the average A260 of fractions from the sucrose gradient for control or equol-treated cells. The 40 S, 60 S, and 80 S fractions were classified as non-polysome fractions. All subsequent fractions were classified as polysome fractions (4–9). n = 3. B, shown is mRNA associated with polysome fractions. These fractions were used to detect GAPDH, CTNND1, and EIF4G mRNA by qRT-PCR. B2M was used as an internal control. Results are shown as the -fold changes in equol-treated cells relative to vehicle controls (n = 3). An asterisk indicates statistical significance of p < 0.05.
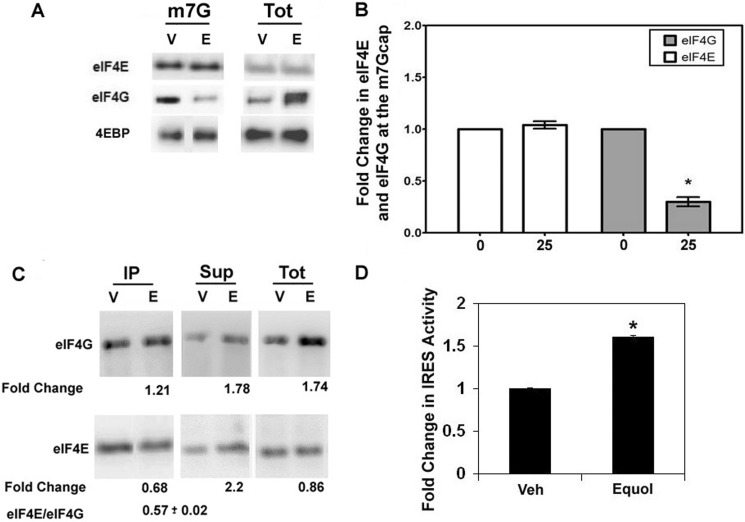
To determine if the increased eIF4G in response to equol was associated at the 5′ cap with eIF4E, synthetic m7GTP co-capture assays were performed from MDA-MB-435 cell lysates treated with vehicle or equol. Fig. 8A demonstrates that total, m7GTP-bound, or free eIF4E or 4E-BP levels remain unchanged after equol treatment. This is consistent with our results that showed no effect of equol on phospho or total eIF4E or 4E-BP protein expression (Fig. 4), indicating that equol does not affect eIF4E expression or activity. Intriguingly, equol treatment significantly decreased the amount of eIF4G co-captured with eIF4E in the m7GTP beads by ∼75% compared with vehicle controls. However, there was a 3-fold increase in eIF4G levels recovered in the total cell lysate and the free pool of eIF4G in the m7GTP pulldown assays (supernatants). This result indicates that the equol-mediated elevated eIF4G is not associated with cap-dependent protein synthesis.
FIGURE 8.
Potential regulation of IRES-dependent protein synthesis initiation by equol. A and B, m7G co-capture assays are shown. Quiescent MDA-MB-435 cells were treated with vehicle (V) or 25 μm equol (E) for 24 h, lysed, and incubated with Sepharose 4B-conjugated 7-methyl-GTP. The pulldown assays were washed and analyzed for eIF4G, eIF4E, and 4E-BP associated with the cap, supernatants, or total cell lysates. A, shown is a representative Western blot of m7G-bound proteins and the total protein (Tot) from cell lysates before pulldown assays. B, shown are -fold changes in eIF4G and eIF4E after vehicle (0) or equol treatment at the m7G cap relative to the total levels of eIF4G or eIF4E in cell lysate. n = 3 ± S.E. An asterisk indicates statistical significance of p ≤ 0.05. C, association of eIF4G and eIF4E with anti-eIF4G immunoprecipitates in response to equol is shown. Quiescent MDA-MB-435 cells were treated with vehicle (V) or 25 μm equol (E) and lysed, and lysates with equal protein were immunoprecipitated using an anti-eIF4G. Representative Western blots stained with eIF4G or eIF4E are shown for immunoprecipitates (IP), supernatants (Sup), and total protein lysates (Tot). The average -fold changes (n = 3) from integrated densities of positive bands from equol-treated cells relative to vehicle are shown. D, relative IRES-dependent protein synthesis after equol treatment is shown. MDA-MB-435 cells expressing a plasmid with a cap-dependent Renilla luciferase followed by a 5′-UTR containing the p120 catenin IRES driving a firefly luciferase or control plasmid without an IRES were treated with vehicle or equol for 24 h. Cells were lysed, and the relative IRES activity analyzed as 570-nm firefly luciferase/480-nm Renilla luciferase. IRES activity was quantified relative to control activity for vehicle or equol-treated cells. Results show -fold change in IRES activity compared with vehicle for n = 3 ± S.E. An asterisk indicates statistical significance of p ≤ 0.05.
Fig. 8C shows the association of eIF4E and eIF4G from anti-eIF4G immunoprecipitates that pulled down equal amounts of eIF4G from vehicle or equol-treated cells. Equol treatment resulted in a statistically significant 32% less eIF4E co-immunoprecipitating with eIF4G. The excess eIF4E was recovered in the supernatants after equol, demonstrating reduced association of eIF4G and eIF4E after equol treatment. The increased eIF4G in response to equol was also recovered in the supernatant.
Dual luciferase assays for cap-dependent and cap-independent, IRES-mediated protein synthesis were performed to determine a function for the excess eIF4G that is not associated with eIF4E. As shown in Fig. 8D, equol treatment of MDA-MB-435 cells, specifically increased IRES-driven firefly luciferase activity by 1.6-fold compared with vehicle (p ≤ 0.01).
DISCUSSION
eIF4F family initiation factors that include eIF4E and eIF4G are overexpressed in advanced cancers and have been shown to be essential for translation of a subset of proteins that regulate cellular bioenergetics, survival, and proliferation (32, 33, 41, 42, 44–48, 52, 53). These pro-cancer mRNAs often contain IRESs and are preferentially translated by elevated eIF4G in malignant cancer cells (32, 33, 54). Therefore, we hypothesized that our previously reported dietary daidzein and combined soy isoflavone-mediated increases in eIF4G and eIF4E expression in mammary tumors (30) may result in enhanced translation of IRES-containing pro-cancer mRNAs.
Results presented herein demonstrate that the effect of daidzein on eIF4G up-regulation could not be recapitulated in vitro with daidzein, but only with equol, the major daidzein metabolite in rodents. We also show that equol increased MDA-MB-435 cell viability similar to the previously reported increase in MDA-MB-435 tumor growth in response to dietary daidzein (30). Therefore, the observed effects of enhanced tumor growth and metastasis by dietary daidzein and soy isoflavones may, at least in part, be due to the metabolite equol.
Dietary daidzein significantly increased eIF4E protein levels and combined soy increased both gene and protein expression of eIF4E (30). However, equol treatment in vitro did not affect eIF4E or its negative regulator 4E-BP expression or activity. Thus, soy consumption may have more profound effects on protein synthesis initiation in cancer cells than the effects of equol described in this report.
Equol has been implicated with daidzein activities in vivo after ingestion of soy foods and has been shown to be more potent than daidzein in vitro (8, 55, 56). Equol is a chiral molecule capable of existing in two enantiomeric forms: R-(+) equol and S-(−) equol; the latter is the natural diasteromer produced by intestinal bacteria (6, 11). Both equol enantiomers show better uptake and have higher bioavailability (65–83%) than the isoflavones daidzein (30–40%) or genistein (7–15%) (57). Both enantiomers bind ERs, with R-equol showing a preference for ERβ (13).
The racemic (R,S)-equol used in this study demonstrated a more pronounced response in up-regulation of eIF4G in ER (−) MDA-MB-435 cells compared with ERβ (+) MDA-MB-231 cells. This result suggests that the effect of equol on eIF4G expression is not ER-dependent or that the presence of ERβ has a protective effect on the cancer promoting action of equol. To our knowledge EIF4G does not have an estrogen response element and, therefore, cannot be directly under the regulation of ER.
A key finding is that Equol up-regulated gene and protein expression of c-Myc at all concentrations tested. The c-Myc transcription factor is one of the most important somatically mutated oncogenes in human cancer and confers a selective advantage to cancer cells by promoting protein synthesis, proliferation, cell survival, differentiation, genetic instability, angiogenesis, hypoxia-mediated cancer progression, and metastasis (33, 33, 47, 62–66). Studies have shown that c-Myc up-regulates both eIF4E and eIF4G gene expression (58). Myc also has an IRES site and thus, in turn, is sensitive to elevated eIF4G and eIF4E levels (48). In MDA-MB-435 cells, c-Myc and eIF4G levels were up-regulated by equol without a concomitant increase in eIF4E. Therefore, similar to a previous report where c-Myc did not affect the eIF4E mRNA or protein levels in a human B cell line (59), it is possible that c-Myc may not regulate eIF4E expression in our system.
MYC has an estrogen response element, and its expression is known to be regulated by estrogen and estrogen mimetics (60) as well as by a plethora of signaling pathways and mechanisms (61). Because the MDA-MB-435 cells are negative for ERα and ERβ but may still express estrogen-related receptors as well as other steroid receptors, equol may activate these receptors to up-regulate MYC expression.
The initial equol-mediated elevation of MYC and EIF4G1 gene expression may result in further synthesis of eIF4G and c-Myc via IRES-driven mechanisms, where eIF4G itself has an IRES site (42, 67). We show that mRNAs for eIF4G are preferentially associated with the polysomal fractions from equol-treated cells, indicating enhanced protein synthesis of the elevated EIF4G mRNA by equol. Moreover, the majority of the equol-mediated up-regulated eIF4G is phosphorylated, thus suggesting it is functional and available for kinase activity in the cytosol. The functional consequence of phosphorylation of eIF4G in translation is not well established. Recent reports have implicated phosphorylation of eIF4G by PAK2 in inhibition of cap-dependent translation but not IRES-driven translation (77), thus implicating elevated p-eIF4G in response to equol in IRES-dependent protein synthesis initiation.
Our results from equol-treated breast cancer cells substantiate the hypothesis that elevated eIF4G by equol increases protein expression of specific mRNAs with IRESs without affecting their gene expression. Of the proteins that were elevated in response to equol, IRES-containing cyclin D1 and c-Myc up-regulation are hallmarks of cancer that have been directly associated with eIF4G up-regulation (32, 33, 42, 68–70). The cell survival genes survivin, Bcl-2, and Bcl-XL and the angiogenesis promoter VEGF are also sensitive to eIF4G levels, have IRES sites, and are elevated in aggressive cancers (71–74).
In MDA-MB-435 cells, p120 catenin protein expression, but not gene expression, was affected by equol. Moreover, p120-catenin mRNAs from equol-treated cells had a higher affinity for the heavy polysomal fractions from sucrose density gradients. p-120 catenin and phosphorylated pThr-916-p120 catenin have been shown to stabilize the E-cadherin axis at cell adhesions and are implicated in regulation of Rho GTPase function leading to increased cancer cell invasion (75). However, the E-cadherin axis is lost in the metastatic cancer cells, used in our study, that have undergone epithelial to mesenchymal transition. Therefore, the elevated p120 catenin in response to equol may contribute to cancer progression via enhanced nuclear transcription regulated by free p120 catenin (76).
We also show that more mRNAs were associated with the polysome fraction from equol-treated cells, indicating enhanced protein synthesis initiation. However, equol treatment disassociated eIF4G from eIF4E in 5′ caps, and the excess eIF4G synthesized in response to equol was not associated with eIF4E. Moreover, equol treatment preferentially increased the expression of IRES-driven luciferase relative to a cap-dependent luciferase. Therefore, taken together, our data suggest that equol-mediated up-regulation of eIF4G directs cap-independent protein synthesis initiation of IRES-containing cell survival and pro-proliferation molecules, whereas eIF4E remains at the 5′ cap, bound to 4E-BP (Fig. 9). Nevertheless, these data do not rule out additional effects of equol on cap-dependent protein synthesis initiation, protein stability, or gene expression.
FIGURE 9.
Potential role of equol in protein synthesis regulation. Increased eIF4G expression by equol is expected to result in enhanced IRES-dependent mRNA translation, whereas eIF4E and 4E-BP remains at the m7G cap. Poly(A)-binding protein (PABP) interacts with the poly(A) tail of the mRNA and eIF4G. eIF4A, eIF4B, and eIF3 interact with eIF4G. eIF3 binds the scaffolding protein eIF4G and the 40 S ribosomal subunit at the IRES.
In conclusion, we have shown that the daidzein metabolite equol may act as a potent regulator of the cancer-promoting effects of dietary daidzein. Therefore, consumption of soy foods may not be advisable for patients with ER (−) breast cancer; however, more research needs to be conducted before definitive dietary recommendations.
Acknowledgments
We thank Drs. Deborah Silvera and Robert Schneider for the dual luciferase assay plasmids containing control or p120 catenin IRES. Dr. Leslie A. Krushel is acknowledged for training with IRES-dependent translation. We also acknowledge the advice and assistance of Zahily Rivera and Dr. José Rodríguez-Medina with the polysomal fractionation assay.
This work was supported, in whole or in part, by National Institutes of Health Grants SC3GM084824 (NIGMS; to S. D.) and G12RR003035 (National Institute of General Medical Sciences to Universidad Central del Caribe) and National Institute of General Medical Sciences Grant G12RR035051 (to the University of Puerto Rico Medical Sciences Campus), This work was also supported by the United States Army/BCRP W81XWH-11-1-0199 and the University of Puerto Rico Medical Sciences Campus Biomedical Graduate Program (to C. D.).
- ER
- estrogen receptor
- eIF
- eukaryotic protein synthesis initiation factor
- IRES
- internal ribosome entry site
- 4E-BP
- 4E-binding protein
- qRT-PCR
- quantitative real-time reverse transcriptase-PCR
- CCND1
- Cyclin D1
- m7GTP
- 7-methyl-GTP
- B2M
- β2 microglobulin.
REFERENCES
- 1. Kuhnle G. G., Dell'Aquila C., Aspinall S. M., Runswick S. A., Mulligan A. A., Bingham S. A. (2008) Phytoestrogen content of foods of animal origin. Dairy products, eggs, meat, fish, and seafood. J. Agric. Food Chem. 56, 10099–10104 [DOI] [PubMed] [Google Scholar]
- 2. Messina M. (2010) A brief historical overview of the past two decades of soy and isoflavone research. J. Nutr. 140, 1350S–1354S [DOI] [PubMed] [Google Scholar]
- 3. Kumar N., Allen K., Riccardi D., Kazi A., Heine J. (2004) Isoflavones in breast cancer chemoprevention. Where do we go from here? Front Biosci. 9, 2927–2934 [DOI] [PubMed] [Google Scholar]
- 4. Rice S., Whitehead S. A. (2006) Phytoestrogens and breast cancer. Promoters or protectors? Endocr. Relat Cancer 13, 995–1015 [DOI] [PubMed] [Google Scholar]
- 5. Messina M., Watanabe S., Setchell K. D. (2009) Report on the 8th International Symposium on the Role of Soy in Health Promotion and Chronic Disease Prevention and Treatment. J. Nutr. 139, 796S–802S [DOI] [PubMed] [Google Scholar]
- 6. Setchell K. D., Clerici C. (2010) Equol. History, chemistry, and formation. J. Nutr. 140, 1355S–1362S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Atkinson C., Newton K. M., Bowles E. J., Yong M., Lampe J. W. (2008) Demographic, anthropometric, and lifestyle factors and dietary intakes in relation to daidzein-metabolizing phenotypes among premenopausal women in the United States. Am. J. Clin. Nutr. 87, 679–687 [DOI] [PubMed] [Google Scholar]
- 8. Magee P. J. (2011) Is equol production beneficial to health? Proc. Nutr. Soc. 70, 10–18 [DOI] [PubMed] [Google Scholar]
- 9. Bandera E. V., King M., Chandran U., Paddock L. E., Rodriguez-Rodriguez L., Olson S. H. (2011) Phytoestrogen consumption from foods and supplements and epithelial ovarian cancer risk. A population-based case control study. BMC. Womens Health 11, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khan S. A., Chatterton R. T., Michel N., Bryk M., Lee O., Ivancic D., Heinz R., Zalles C. M., Helenowski I. B., Jovanovic B. D., Franke A. A., Bosland M. C., Wang J., Hansen N. M., Bethke K. P., Dew A., Coomes M., Bergan R. C. (2012) Soy isoflavone supplementation for breast cancer risk reduction. A randomized phase II trial. Cancer Prev. Res. (Phila) 5, 309–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Setchell K. D., Clerici C. (2010) Equol. Pharmacokinetics and biological actions. J. Nutr. 140, 1363S-1368S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carreau C., Flouriot G., Bennetau-Pelissero C., Potier M. (2009) Respective contribution exerted by AF-1 and AF-2 transactivation functions in estrogen receptor α induced transcriptional activity by isoflavones and equol. Consequence on breast cancer cell proliferation. Mol. Nutr. Food Res. 53, 652–658 [DOI] [PubMed] [Google Scholar]
- 13. Muthyala R. S., Ju Y. H., Sheng S., Williams L. D., Doerge D. R., Katzenellenbogen B. S., Helferich W. G., Katzenellenbogen J. A. (2004) Equol, a natural estrogenic metabolite from soy isoflavones. Convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors α and β. Bioorg. Med. Chem. 12, 1559–1567 [DOI] [PubMed] [Google Scholar]
- 14. Magee P. J., Raschke M., Steiner C., Duffin J. G., Pool-Zobel B. L., Jokela T., Wahala K., Rowland I. R. (2006) Equol. A comparison of the effects of the racemic compound with that of the purified S-enantiomer on the growth, invasion, and DNA integrity of breast and prostate cells in vitro. Nutr. Cancer 54, 232–242 [DOI] [PubMed] [Google Scholar]
- 15. Lund T. D., Blake C., Bu L., Hamaker A. N., Lephart E. D. (2011) Equol an isoflavonoid. Potential for improved prostate health, in vitro and in vivo evidence. Reprod. Biol. Endocrinol. 9, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Welshons W. V., Murphy C. S., Koch R., Calaf G., Jordan V. C. (1987) Stimulation of breast cancer cells in vitro by the environmental estrogen enterolactone and the phytoestrogen equol. Breast Cancer Res. Treat. 10, 169–175 [DOI] [PubMed] [Google Scholar]
- 17. Ju Y. H., Fultz J., Allred K. F., Doerge D. R., Helferich W. G. (2006) Effects of dietary daidzein and its metabolite, equol, at physiological concentrations on the growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in ovariectomized athymic mice. Carcinogenesis 27, 856–863 [DOI] [PubMed] [Google Scholar]
- 18. Tonetti D. A., Zhang Y., Zhao H., Lim S. B., Constantinou A. I. (2007) The effect of the phytoestrogens genistein, daidzein, and equol on the growth of tamoxifen-resistant T47D/PKCα. Nutr. Cancer 58, 222–229 [DOI] [PubMed] [Google Scholar]
- 19. Onoda A., Ueno T., Uchiyama S., Hayashi S., Kato K., Wake N. (2011) Effects of S-equol and natural S-equol supplement (SE5-OH) on the growth of MCF-7 in vitro and as tumors implanted into ovariectomized athymic mice. Food Chem. Toxicol. 49, 2279–2284 [DOI] [PubMed] [Google Scholar]
- 20. Lamartiniere C. A., Wang J., Smith-Johnson M., Eltoum I. E. (2002) Daidzein. Bioavailability, potential for reproductive toxicity, and breast cancer chemoprevention in female rats. Toxicol. Sci. 65, 228–238 [DOI] [PubMed] [Google Scholar]
- 21. Magee P. J., McGlynn H., Rowland I. R. (2004) Differential effects of isoflavones and lignans on invasiveness of MDA-MB-231 breast cancer cells in vitro. Cancer Lett. 208, 35–41 [DOI] [PubMed] [Google Scholar]
- 22. Choi E. J., Kim T. (2008) Equol induced apoptosis via cell cycle arrest in human breast cancer MDA-MB-453 but not MCF-7 cells. Mol. Med. Report 1, 239–244 [PubMed] [Google Scholar]
- 23. Choi E. J., Ahn W. S., Bae S. M. (2009) Equol induces apoptosis through cytochrome c-mediated caspases cascade in human breast cancer MDA-MB-453 cells. Chem. Biol. Interact. 177, 7–11 [DOI] [PubMed] [Google Scholar]
- 24. Liu H., Du J., Hu C., Qi H., Wang X., Wang S., Liu Q., Li Z. (2010) Delayed activation of extracellular-signal-regulated kinase 1/2 is involved in genistein- and equol-induced cell proliferation and estrogen receptor-α-mediated transcription in MCF-7 breast cancer cells. J. Nutr. Biochem. 21, 390–396 [DOI] [PubMed] [Google Scholar]
- 25. McMichael-Phillips D. F., Harding C., Morton M., Roberts S. A., Howell A., Potten C. S., Bundred N. J. (1998) Effects of soy-protein supplementation on epithelial proliferation in the histologically normal human breast. Am. J. Clin. Nutr. 68, 1431S–1435S [DOI] [PubMed] [Google Scholar]
- 26. Jackson R. L., Greiwe J. S., Schwen R. J. (2011) Emerging evidence of the health benefits of S-equol, an estrogen receptor β agonist. Nutr. Rev. 69, 432–448 [DOI] [PubMed] [Google Scholar]
- 27. Lampe J. W. (2010) Emerging research on equol and cancer. J. Nutr. 140, 1369S–1372S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goodman M. T., Shvetsov Y. B., Wilkens L. R., Franke A. A., Le M. L., Kakazu K. K., Nomura A. M., Henderson B. E., Kolonel L. N. (2009) Urinary phytoestrogen excretion and postmenopausal breast cancer risk: the multiethnic cohort study. Cancer Prev. Res. (Phila) 2, 887–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ko K. P., Park S. K., Park B., Yang J. J., Cho L. Y., Kang C., Kim C. S., Gwack J., Shin A., Kim Y., Kim J., Yang H. K., Kang D., Chang S. H., Shin H. R., Yoo K. Y. (2010) Isoflavones from phytoestrogens and gastric cancer risk. A nested case-control study within the Korean Multicenter Cancer Cohort. Cancer Epidemiol. Biomarkers Prev. 19, 1292–1300 [DOI] [PubMed] [Google Scholar]
- 30. Martínez-Montemayor M. M., Otero-Franqui E., Martinez J., De La Mota-Peynado A., Cubano L. A., Dharmawardhane S. (2010) Individual and combined soy isoflavones exert differential effects on metastatic cancer progression. Clin. Exp. Metastasis 27, 465–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sonenberg N., Dever T. E. (2003) Eukaryotic translation initiation factors and regulators. Curr. Opin. Struct. Biol. 13, 56–63 [DOI] [PubMed] [Google Scholar]
- 32. Silvera D., Arju R., Darvishian F., Levine P. H., Zolfaghari L., Goldberg J., Hochman T., Formenti S. C., Schneider R. J. (2009) Essential role for eIF4GI overexpression in the pathogenesis of inflammatory breast cancer. Nat. Cell Biol. 11, 903–908 [DOI] [PubMed] [Google Scholar]
- 33. Silvera D., Formenti S. C., Schneider R. J. (2010) Translational control in cancer. Nat. Rev. Cancer 10, 254–266 [DOI] [PubMed] [Google Scholar]
- 34. Van Der Kelen K., Beyaert R., Inzé D., De Veylder L. (2009) Translational control of eukaryotic gene expression. Crit. Rev. Biochem. Mol. Biol. 44, 143–168 [DOI] [PubMed] [Google Scholar]
- 35. Martínez-Montemayor M. M., Acevedo R. R., Otero-Franqui E., Cubano L. A., Dharmawardhane S. F. (2011) Ganoderma lucidum (Reishi) inhibits cancer cell growth and expression of key molecules in inflammatory breast cancer. Nutr. Cancer 63, 1085–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rivera-Ruiz M. E., Rodríguez-Quiñones J. F., Akamine P., Rodríguez-Medina J. R. (2010) Post-transcriptional regulation in the myo1Δ mutant of Saccharomyces cerevisiae. BMC Genomics 11, 690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang X., Proud C. G. (2007) Methods for studying signal-dependent regulation of translation factor activity. Methods Enzymol. 431, 113–142 [DOI] [PubMed] [Google Scholar]
- 38. Roux P. P., Shahbazian D., Vu H., Holz M. K., Cohen M. S., Taunton J., Sonenberg N., Blenis J. (2007) RAS/ERK signaling promotes site-specific ribosomal protein S6 phosphorylation via RSK and stimulates cap-dependent translation. J. Biol. Chem. 282, 14056–14064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu W., Zhao R., McFarland C., Kieft J., Niedzwiecka A., Jankowska-Anyszka M., Stepinski J., Darzynkiewicz E., Jones D. N., Davis R. E. (2009) Structural insights into parasite eIF4E binding specificity for m7G and m2,2,7G mRNA caps. J. Biol. Chem. 284, 31336–31349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dharmawardhane S., Demma M., Yang F., Condeelis J. (1991) Compartmentalization and actin binding properties of ABP-50. The elongation factor-1 α of Dictyostelium. Cell Motil. Cytoskeleton 20, 279–288 [DOI] [PubMed] [Google Scholar]
- 41. Ramírez-Valle F., Braunstein S., Zavadil J., Formenti S. C., Schneider R. J. (2008) eIF4GI links nutrient sensing by mTOR to cell proliferation and inhibition of autophagy. J. Cell Biol. 181, 293–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hellen C. U., Sarnow P. (2001) Internal ribosome entry sites in eukaryotic mRNA molecules. Genes Dev. 15, 1593–1612 [DOI] [PubMed] [Google Scholar]
- 43. Xu X., Wang H. J., Murphy P. A., Cook L., Hendrich S. (1994) Daidzein is a more bioavailable soymilk isoflavone than is genistein in adult women. J. Nutr. 124, 825–832 [DOI] [PubMed] [Google Scholar]
- 44. Fukuda S., Foster R. G., Porter S. B., Pelus L. M. (2002) The antiapoptosis protein survivin is associated with cell cycle entry of normal cord blood CD34(+) cells and modulates cell cycle and proliferation of mouse hematopoietic progenitor cells. Blood 100, 2463–2471 [DOI] [PubMed] [Google Scholar]
- 45. Yoon A., Peng G., Brandenburger Y., Brandenburg Y., Zollo O., Xu W., Rego E., Ruggero D. (2006) Impaired control of IRES-mediated translation in X-linked dyskeratosis congenita. Science 312, 902–906 [DOI] [PubMed] [Google Scholar]
- 46. Sherrill K. W., Byrd M. P., Van Eden M. E., Lloyd R. E. (2004) BCL-2 translation is mediated via internal ribosome entry during cell stress. J. Biol. Chem. 279, 29066–29074 [DOI] [PubMed] [Google Scholar]
- 47. Shi Y., Sharma A., Wu H., Lichtenstein A., Gera J. (2005) Cyclin D1 and c-myc internal ribosome entry site (IRES)-dependent translation is regulated by AKT activity and enhanced by rapamycin through a p38 MAPK- and ERK-dependent pathway. J. Biol. Chem. 280, 10964–10973 [DOI] [PubMed] [Google Scholar]
- 48. Stoneley M., Chappell S. A., Jopling C. L., Dickens M., MacFarlane M., Willis A. E. (2000) c-Myc protein synthesis is initiated from the internal ribosome entry segment during apoptosis. Mol. Cell. Biol. 20, 1162–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Staber P. B., Vesely P., Haq N., Ott R. G., Funato K., Bambach I., Fuchs C., Schauer S., Linkesch W., Hrzenjak A., Dirks W. G., Sexl V., Bergler H., Kadin M. E., Sternberg D. W., Kenner L., Hoefler G. (2007) The oncoprotein NPM-ALK of anaplastic large-cell lymphoma induces JUNB transcription via ERK1/2 and JunB translation via mTOR signaling. Blood 110, 3374–3383 [DOI] [PubMed] [Google Scholar]
- 50. Lerner R. S., Nicchitta C. V. (2006) mRNA translation is compartmentalized to the endoplasmic reticulum following physiological inhibition of cap-dependent translation. RNA. 12, 775–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Haron H., Ismail A., Shahar S., Azlan A., Peng L. S. (2011) Apparent bioavailability of isoflavones in urinary excretions of postmenopausal Malay women consuming tempeh compared with milk. Int. J. Food Sci. Nutr. 62, 642–650 [DOI] [PubMed] [Google Scholar]
- 52. Kim Y. Y., Von Weymarn L., Larsson O., Fan D., Underwood J. M., Peterson M. S., Hecht S. S., Polunovsky V. A., Bitterman P. B. (2009) Eukaryotic initiation factor 4E binding protein family of proteins. Sentinels at a translational control checkpoint in lung tumor defense. Cancer Res. 69, 8455–8462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Graff J. R., Konicek B. W., Carter J. H., Marcusson E. G. (2008) Targeting the eukaryotic translation initiation factor 4E for cancer therapy. Cancer Res. 68, 631–634 [DOI] [PubMed] [Google Scholar]
- 54. Braunstein S., Karpisheva K., Pola C., Goldberg J., Hochman T., Yee H., Cangiarella J., Arju R., Formenti S. C., Schneider R. J. (2007) A hypoxia-controlled cap-dependent to cap-independent translation switch in breast cancer. Mol. Cell 28, 501–512 [DOI] [PubMed] [Google Scholar]
- 55. Muñoz Y., Garrido A., Valladares L. (2009) Equol is more active than soy isoflavone itself to compete for binding to thromboxane A(2) receptor in human platelets. Thromb. Res. 123, 740–744 [DOI] [PubMed] [Google Scholar]
- 56. Hedlund T. E., Johannes W. U., Miller G. J. (2003) Soy isoflavonoid equol modulates the growth of benign and malignant prostatic epithelial cells in vitro. Prostate 54, 68–78 [DOI] [PubMed] [Google Scholar]
- 57. Setchell K. D., Faughnan M. S., Avades T., Zimmer-Nechemias L., Brown N. M., Wolfe B. E., Brashear W. T., Desai P., Oldfield M. F., Botting N. P., Cassidy A. (2003) Comparing the pharmacokinetics of daidzein and genistein with the use of 13C-labeled tracers in premenopausal women. Am. J. Clin. Nutr. 77, 411–419 [DOI] [PubMed] [Google Scholar]
- 58. Labisso W. L., Wirth M., Stojanovic N., Stauber R. H., Schnieke A., Schmid R. M., Krämer O. H., Saur D., Schneider G. (2012) MYC directs transcription of MCL1 and eIF4E genes to control sensitivity of gastric cancer cells toward HDAC inhibitors. Cell Cycle 11, 1593–1602 [DOI] [PubMed] [Google Scholar]
- 59. Mezquita P., Parghi S. S., Brandvold K. A., Ruddell A. (2005) Myc regulates VEGF production in B cells by stimulating initiation of VEGF mRNA translation. Oncogene 24, 889–901 [DOI] [PubMed] [Google Scholar]
- 60. Dubik D., Shiu R. P. (1992) Mechanism of estrogen activation of c-myc oncogene expression. Oncogene 7, 1587–1594 [PubMed] [Google Scholar]
- 61. Dang C. V. (2010) Enigmatic MYC conducts an unfolding systems biology symphony. Genes Cancer 1, 526–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Stoneley M., Paulin F. E., Le Quesne J. P., Chappell S. A., Willis A. E. (1998) C-Myc 5'-untranslated region contains an internal ribosome entry segment. Oncogene 16, 423–428 [DOI] [PubMed] [Google Scholar]
- 63. McNeil C. M., Sergio C. M., Anderson L. R., Inman C. K., Eggleton S. A., Murphy N. C., Millar E. K., Crea P., Kench J. G., Alles M. C., Gardiner-Garden M., Ormandy C. J., Butt A. J., Henshall S. M., Musgrove E. A., Sutherland R. L. (2006) c-Myc overexpression and endocrine resistance in breast cancer. J. Steroid Biochem. Mol. Biol. 102, 147–155 [DOI] [PubMed] [Google Scholar]
- 64. Wolfer A., Ramaswamy S. (2011) MYC and metastasis. Cancer Res. 71, 2034–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lin C. J., Malina A., Pelletier J. (2009) c-Myc and eIF4F constitute a feed-forward loop that regulates cell growth. Implications for anticancer therapy. Cancer Res. 69, 7491–7494 [DOI] [PubMed] [Google Scholar]
- 66. Doe M. R., Ascano J. M., Kaur M., Cole M. D. (2012) Myc posttranscriptionally induces HIF1 protein and target gene expression in normal and cancer cells. Cancer Res. 72, 949–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Han B., Zhang J. T. (2002) Regulation of gene expression by internal ribosome entry sites or cryptic promoters. The eIF4G story. Mol. Cell. Biol. 22, 7372–7384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jo O. D., Martin J., Bernath A., Masri J., Lichtenstein A., Gera J. (2008) Heterogeneous nuclear ribonucleoprotein A1 regulates cyclin D1 and c-myc internal ribosome entry site function through Akt signaling. J. Biol. Chem. 283, 23274–23287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Spriggs K. A., Cobbold L. C., Jopling C. L., Cooper R. E., Wilson L. A., Stoneley M., Coldwell M. J., Poncet D., Shen Y. C., Morley S. J., Bushell M., Willis A. E. (2009) Canonical initiation factor requirements of the Myc family of internal ribosome entry segments. Mol. Cell. Biol. 29, 1565–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bauer C., Brass N., Diesinger I., Kayser K., Grässer F. A., Meese E. (2002) Overexpression of the eukaryotic translation initiation factor 4G (eIF4G-1) in squamous cell lung carcinoma. Int. J. Cancer 98, 181–185 [DOI] [PubMed] [Google Scholar]
- 71. Graff J. R., Konicek B. W., Vincent T. M., Lynch R. L., Monteith D., Weir S. N., Schwier P., Capen A., Goode R. L., Dowless M. S., Chen Y., Zhang H., Sissons S., Cox K., McNulty A. M., Parsons S. H., Wang T., Sams L., Geeganage S., Douglass L. E., Neubauer B. L., Dean N. M., Blanchard K., Shou J., Stancato L. F., Carter J. H., Marcusson E. G. (2007) Therapeutic suppression of translation initiation factor eIF4E expression reduces tumor growth without toxicity. J. Clin. Invest. 117, 2638–2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Krepela E., Dankova P., Moravcikova E., Krepelova A., Prochazka J., Cermak J., Schützner J., Zatloukal P., Benkova K. (2009) Increased expression of inhibitor of apoptosis proteins, survivin and XIAP, in non-small cell lung carcinoma. Int. J. Oncol. 35, 1449–1462 [DOI] [PubMed] [Google Scholar]
- 73. Chida D., Miura O., Yoshimura A., Miyajima A. (1999) Role of cytokine signaling molecules in erythroid differentiation of mouse fetal liver hematopoietic cells. Functional analysis of signaling molecules by retrovirus-mediated expression. Blood 93, 1567–1578 [PubMed] [Google Scholar]
- 74. Kelly P. N., Strasser A. (2011) The role of Bcl-2 and its pro-survival relatives in tumourigenesis and cancer therapy. Cell Death. Differ. 18, 1414–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Anastasiadis P. Z. (2007) p120-ctn. A nexus for contextual signaling via Rho GTPases. Biochim. Biophys. Acta 1773, 34–46 [DOI] [PubMed] [Google Scholar]
- 76. Pieters T., van Hengel J., van Roy F. (2012) Functions of p120ctn in development and disease. Front Biosci. 17, 760–783 [DOI] [PubMed] [Google Scholar]
- 77. Ling J., Morley S. J., Traugh J. A. (2005) Inhibition of cap-dependent translation via phosphorylation of eIF4G by protein kinase Pak2. EMBO J. 24, 4094–4105 [DOI] [PMC free article] [PubMed] [Google Scholar]