Background: Plants remobilize leaf starch at night to support metabolism and growth.
Results: α-Amylase (AMY3) and two debranching enzymes (ISA3 and LDA) are involved in starch degradation, but the third debranching enzyme (ISA1-ISA2) is not.
Conclusion: Blocking all partially redundant steps of degradation causes massive starch accumulation, depleted sugars, and reduced growth.
Significance: These discoveries could help in the design of improved starch crops.
Keywords: Arabidopsis, Carbohydrate Metabolism, Photosynthesis, Plant Biochemistry, Polysaccharide, alpha-Amylase, Arabidopsis thaliana, Debranching Enzyme, Plant Growth, Starch Metabolism
Abstract
In this study, we investigated which enzymes are involved in debranching amylopectin during transient starch degradation. Previous studies identified two debranching enzymes, isoamylase 3 (ISA3) and limit dextrinase (LDA), involved in this process. However, plants lacking both enzymes still degrade substantial amounts of starch. Thus, other enzymes/mechanisms must contribute to starch breakdown. We show that the chloroplastic α-amylase 3 (AMY3) also participates in starch degradation and provide evidence that all three enzymes can act directly at the starch granule surface. The isa3 mutant has a starch excess phenotype, reflecting impaired starch breakdown. In contrast, removal of AMY3, LDA, or both enzymes together has no impact on starch degradation. However, removal of AMY3 or LDA in addition to ISA3 enhances the starch excess phenotype. In plants lacking all three enzymes, starch breakdown is effectively blocked, and starch accumulates to the highest levels observed so far. This provides indirect evidence that the heteromultimeric debranching enzyme ISA1-ISA2 is not involved in starch breakdown. However, we illustrate that ISA1-ISA2 can hydrolyze small soluble branched glucans that accumulate when ISA3 and LDA are missing, albeit at a slow rate. Starch accumulation in the mutants correlates inversely with plant growth.
Introduction
Starch is the major storage carbohydrate in plants and forms insoluble, semicrystalline granules composed of two glucose polymers: amylopectin and amylose. Amylopectin accounts for 60–90% of the granule weight. It is a branched polymer in which the glucosyl units are linked via α-1,4-glucosidic bonds to form linear chains. These chains are linked via α-1,6-glucosidic bonds (branch points). Pairs of neighboring chains longer than 9 glucose units are able to form double helices (1), which arrange into ordered crystalline arrays. This arrangement is the structural basis of the insoluble nature of the starch granule (2).
Starch is commonly classified as storage or transient starch. Both are used to fuel growth and respiration during times when no photosynthesis is possible. Storage starch is deposited for long periods in amyloplasts of non-photosynthetic organs (seeds, tubers, or roots) to serve as an energy source during germination and sprouting. In contrast, transitory starch is made in the chloroplasts of photosynthetic tissues during the light period and is used during the following night to supply the plant with carbohydrates and energy. Transitory starch can be seen as a carbohydrate reservoir, which buffers the diurnal changes in the supply of photoassimilates (3). Much progress has been made in understanding the pathways and regulation of transitory starch metabolism in Arabidopsis thaliana. Arabidopsis partitions a significant proportion of its photoassimilates (typically 30–40%) into starch during the day and then degrades almost all of it at a nearly constant rate during the night (3).
Starch breakdown requires the concerted activities of enzymes belonging to several classes. Current evidence suggests that the relative importance of each enzyme may vary between plant species and tissue types. For example, in germinating cereals, storage starch breakdown is initiated by α-amylases (hydrolyzing α-1,4-bonds), which are secreted from aleurone cells into the starchy endosperm (4, 5). α-Amylases are endoamylases that liberate a mixture of short linear and branched glucans, which are hydrolyzed by other enzymes, such as β-amylases (exoamylases that liberate maltose) and debranching enzymes (that hydrolyze the α-1,6-branch points). In contrast, the major path of transient starch degradation in chloroplasts seems to depend on β-amylases rather than α-amylases. Arabidopsis mutants lacking the major chloroplastic β-amylases (BAMs)2 show a severe starch excess (sex) phenotype, resulting from a reduced capacity to remobilize the starch at night (6). Arabidopsis plants lacking the sole chloroplastic α-amylase (AMY3) have starch degradation rates similar to wild-type plants and do not have a sex phenotype (7).
Transient starch breakdown is dependent on glucan phosphorylation, mediated by the enzymes glucan water dikinase (GWD1) and phosphoglucan water dikinase (PWD). Glucosyl residues are phosphorylated at the C6 position by GWD1 (8) or at the C3 position by PWD. PWD depends strongly on the prior action of GWD1 (9, 10). Mutants lacking PWD have a moderate sex phenotype, whereas gwd1 mutants have the highest sex phenotype described so far (11). Glucan phosphorylation is also important for storage starch degradation in potatoes, where GWD1 antisense lines show reduced tuber starch breakdown compared with the wild type when stored in the cold (12). Glucan phosphorylation is proposed to disrupt the double helical packing, facilitating the action of β-amylases on linear chain segments (13). The removal of the phosphates by the phosphoglucan phosphatases SEX4 (starch excess 4) and LSF2 (like sex four 2) prevents the phosphate groups themselves from obstructing degradation (β-amylases can neither can hydrolyze nor pass them (14, 15)).
Branch points also limit the progression of β-amylases along glucan chains. Therefore, debranching enzymes (DBEs) are also required for amylopectin degradation. DBEs can be classified into isoamylases (ISA1, ISA2, and ISA3) and limit dextrinase (LDA). Arabidopsis ISA1 and ISA2 contribute to a single enzyme activity required for normal starch synthesis (16–18). The isa1 and isa2 knock-out mutants lack the same multimeric isoamylase activity, and starch is largely replaced by the branched, water-soluble polysaccharide, phytoglycogen. In contrast, ISA3 and LDA are involved in starch breakdown (18–20). The isa3 knock-out mutant has a sex phenotype, whereas lda mutants have normal starch content. However, the isa3lda double mutant has a more severe sex phenotype than isa3 plants (19), suggesting an overlap in the functions of the two enzymes. All three DBEs hydrolyze α-1,6-branch points but differ in their substrate specificities. LDA and ISA3 have a preference for substrates with short external branches, such as β-limit dextrins, whereas ISA1-ISA2 prefers substrates with longer chains, as found in amylopectin (21–23). This might reflect their different roles in starch metabolism.
Despite their strong sex phenotype, isa3lda plants still can mobilize some of their starch at night (19). This cannot be explained by hydrolytic attack on the outer chains and must involve the removal of α-1,6-bonds from the granule surface. Consistent with this, isa3lda mutants accumulate small soluble branched glucans not usually observed in wild-type plants. These branched glucans were suggested to be starch degradation products liberated by AMY3 (19). Therefore, we tested whether another enzyme(s) can contribute to the liberation of α-1,6-bonds from the starch granule using genetics. We show that AMY3 is the source of the small branched glucans and that its removal from the isa3 or isa3lda backgrounds reduces the capacity of the plants to degrade starch. In plants lacking ISA3, LDA and AMY3, starch breakdown is completely blocked. Our results also indicate that all three enzymes can act directly on starch granules independently of each other. We also investigated whether ISA1-ISA2 contributes to starch breakdown but conclude that under normal conditions it does not. Our data allow us to refine the emerging model for starch degradation in leaves.
EXPERIMENTAL PROCEDURES
Plants and Growth Conditions
A. thaliana plants were grown in Percival AR95 climate chambers at 20 °C, 60% relative humidity, a 12-h photoperiod, and a light intensity of 150 μmol of photons/m2/s. Sown seeds were stratified for 48 h at 4 °C and transferred to the growth chambers. Two weeks after germination, seedlings were transferred into 200-ml pots and harvested 2–3 weeks later. Single mutants used here were described earlier: amy3-2, SAIL_613D12 (7); isa3-2, GABI_280G10 (19); lda-2, SALK_060765 (19); isa1-1, SALK_042704 (16); isa2–1, dbe1-1 (24); pwd, SALK_110814 (10); and gwd1-3, sex1-3 (11). Multiple mutants that were described earlier include isa3-2/lda-2 (19), isa1-1/isa2-1/isa3-2/lda-2 (18), and amy3-2/isa1-1/isa2-1/isa3-2/lda-2 (18). New mutant combinations (amy3-2/lda-2, amy3-2/isa3-2, and amy3-2/isa3-2/lda-2) were obtained by crossing single or double mutants and identifying plants homozygous for the desired mutant alleles by PCR as described by Streb et al. (18). Further, all mutant combinations were tested for the presence or absence of AMY3, ISA3, and LDA protein and/or activity (supplemental Fig. S1), either by immunoblotting (for AMY3 and ISA3) or red pullulan-containing native gels (for LDA).
Extraction and Measurement of Metabolites
Plants were harvested, weighed, frozen in liquid N2, and stored at −80 °C until use. Extraction was performed using perchloric acid as described by Delatte et al. (16). Glucose polymers (starch, phytoglycogen, and small branched glucans) were quantified by enzymatic digestion with α-amylase (pig pancreas; Roche Applied Science) and amyloglucosidase (Aspergillus niger; Roche Applied Science), and released glucose was measured spectrophotometrically (25). Neutral sugars were quantified using high performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD) according to Egli et al. (26).
Structural Analysis of Starch and Small Branched Glucans
Soluble and insoluble fractions from the perchloric acid-extracted plant material were used for glucan structural analysis. Equal quantities of glucans from four individually extracted plants were analyzed separately. Starch samples (100 μg) were boiled in 200 μl of water for 10 min. Debranching with Pseudomonas amyloderamosa isoamylase (Sigma-Aldrich) and Klebsiella planticola (Megazyme) in 10 mm sodium acetate (pH 4.8) was carried out for 2 h at 37 °C. The resulting linear glucans chains were analyzed by HPAEC-PAD as described previously (18). For analysis of small branched glucans, soluble fractions (corresponding to 5 mg of plant fresh weight (FW)) were analyzed as for the neutral sugars (26). Purification of different species of small branched glucan was performed starting with 100 μg of glucan separated with a Dionex PA-100 semipreparative column (9 × 250 mm) using the following conditions: eluent A, 100 mm NaOH; eluent B, 150 mm NaOH and 500 mm sodium acetate. The flow rate was 2.0 ml/min. The gradient was 0–7 min, 100% A; 7–29.5 min, linear gradient to 60% A and 40% B; 29.5–45 min, linear gradient to 45% A and 55% B; 45–54 min, linear gradient to 15% A and 85% B; 54–70 min, step to 100% A (column reequilibration). Fractions were collected manually. The resulting glucan fractions were spiked with 5 nmol of cellobiose as an internal standard. The collected material was reanalyzed by HPAEC-PAD either with or without debranching (as described above for starch).
Debranching Enzyme Activity
The assay was performed as described by Delatte et al. (19). Soluble proteins were extracted from 150 mg of fresh leaf material in 1 ml of extraction medium (100 mm MOPS, pH 7.2, 1 mm EDTA, 5 mm DTT, 1 mm CaCl2). Extracts were desalted by passage through NAP-10 columns pre-equilibrated with extraction medium. The assay substrate β-limit dextrin was obtained by digesting potato amylopectin to completion with β-amylase (from barley; Megazyme) in 10 mm sodium acetate, pH 6.0. The β-limit dextrin was purified free of maltose by three successive precipitations with 75% (v/v) methanol. Protein extracts (50 μl, from 7.5 mg of plant material) were incubated with 0.5 mg of β-limit dextrin and 20 units of β-amylase in a total volume of 100 μl at 30 °C for 60 min. Each sample replicate was assayed in duplicate. Control reactions lacking the β-amylase, the β-limit dextrin, or the plant extracts were used to obtain background values, which were subtracted. After incubation, samples were added to 5 nmol of cellobiose, heated at 95 °C for 10 min, and passed through Dowex columns as described above. Maltose and maltotriose were quantified and normalized to the cellobiose standard.
Size Exclusion Chromatography (SEC)
Soluble proteins for SEC were extracted from leaves (800 mg) of 5-week-old plants in 1 ml of extraction medium containing 100 mm Tris (pH 7.5), 300 mm KCl, 5 mm DTT, 1× Complete Protease Inhibitor Mixture (Roche Applied Science), using glass homogenizers. Homogenates were subject to centrifugation (16,000 × g, 10 min, 4 °C). Supernatants were passed through a 0.2-μm filter and concentrated with Amicon® Ultra-4 10-kDa centrifugal filter devices (Millipore). Soluble protein extracts (500 μl, 2.35 mg of total protein) were immediately subjected to SEC using an Åkta Explorer100 and a HiLoad 16/600 Superdex 200-pg column (GE Healthcare) in 100 mm Tris (pH 7.5), 300 mm KCl, 1 mm DTT (flow rate 0.5 ml/min). Fractions (2 ml) were collected and stored at −80 °C. Column calibration was performed using the Gel Filtration HMW calibration kit (GE Healthcare).
SDS-PAGE Blotting and Native PAGE
Proteins in 30 μl of the 2-ml SEC fractions were separated by SDS-PAGE, transferred to PVDF membranes, and probed with antibodies against AMY3 or ISA3 (19). Signals were developed using Immun-StarTM HRP (Bio-Rad). Alternatively, proteins were separated by native PAGE in gels containing 6% (w/v) polyacrylamide and 1% (w/v) red pullulan (Megazyme). After electrophoresis, gels were incubated at 37 °C in medium containing 100 mm Tris, pH 7.2, 1 mm MgCl2, 1 mm CaCl2, and 5 mm DTT until a clear band of LDA activity appeared.
RESULTS
Generation of Mutant Combinations Lacking ISA3, LDA, and AMY3
To investigate the function of AMY3 in isa3, lda, or isa3lda mutant backgrounds, we crossed the previously described double mutant isa3-2/lda-2 (16) with the amy3-2 single mutant (7). The F2 population was screened for new combinations, resulting in amy3lda, amy3isa3, and amy3isa3lda.
All mutant combinations and the Col-0 wild type were grown in a 12-h photoperiod. The different genotypes varied greatly in size. The lda and amy3 single mutants and the amy3lda double mutant were similar in size to the wild type, whereas the isa3 and isa3lda mutants were smaller. Loss of AMY3 in isa3 and isa3lda mutants led to an even more pronounced growth retardation (Fig. 1).
FIGURE 1.
Growth phenotype of wild type and different mutant combinations of plants lacking AMY3, ISA3, and LDA. Plants were grown under 12-h light/12-h dark conditions and photographed after 5 weeks. Representative plants were selected to illustrate the effect on growth of the different mutant combinations.
We investigated the rates of starch synthesis and breakdown in all genotypes. Plants were harvested at different time points throughout the diurnal cycle. In wild-type plants, starch is synthesized during the light phase with a nearly linear rate and subsequently degraded during the night almost to completion (Fig. 2A). As shown previously, the starch content of amy3 and lda single mutants displayed a similar pattern to the wild type (lda (19) and amy3 (7)). The amy3lda double mutant phenotype was indistinguishable from the single mutants (Fig. 2A) and from the wild type, showing that starch degradation can proceed at a normal rate in the absence of both proteins. The situation was different in mutants lacking ISA3. The starch content was elevated in isa3 mutants, compared with the wild type, and even higher in isa3lda double mutants, as described previously (19). Interestingly, amy3isa3 mutants had starch contents similar to isa3lda plants throughout the diurnal cycle. This illustrates that both AMY3 and LDA are involved in starch breakdown if ISA3 is missing. Delatte et al. (19) hypothesized earlier that LDA acts exclusively in the stroma hydrolyzing the small branched glucans liberated by AMY3. If so, the loss of AMY3 would be epistatic to the loss of LDA (i.e. the loss of LDA should make no difference to the amy3isa3 phenotype). However, starch content was drastically increased in amy3isa3lda triple mutants (Fig. 2B) compared with the double mutants. These data indicate that LDA does not work exclusively downstream of AMY3. Instead they suggest that all three enzymes are able to work directly on the starch granule surface. The lower starch content of amy3isa3 and isa3lda compared with the triple mutant is presumably due to the fact that each double mutant still has some enzymatic activity left to degrade starch during the night (i.e. LDA and AMY3, respectively).
FIGURE 2.
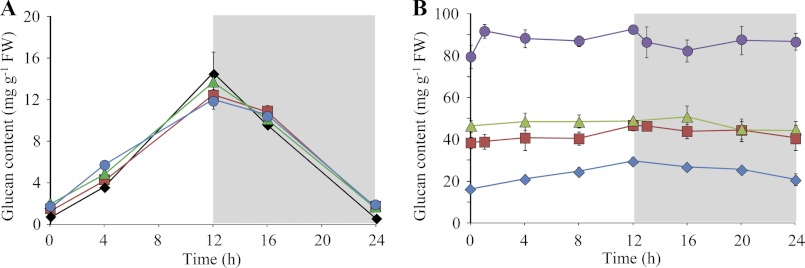
Altered starch content in mutant plants lacking combinations of AMY3, ISA3, and LDA. Plants were grown for 28 days (A) or 35 days (B) in a 12-h light/12-h dark regime and harvested at the indicated time points. Samples comprising all of the leaves from a single plant were extracted using perchloric acid. Starch in the insoluble fraction was measured after enzymatic hydrolysis to glucose. Each point is the mean ± S.E. (error bars) (n = 4). A, Starch in wild-type (black diamonds), amy3 (red squares), lda (green triangles), and amy3lda plants (blue circles). B, starch in isa3 (blue diamonds), isa3lda (red squares), amy3isa3 (green triangles), and amy3isa3lda plants (purple circles). All genotypes have elevated starch compared with the wild type (A).
Because starch is the major source for sugars during the night, we determined the soluble sugar content at the end of the night in genotypes with elevated starch content (Table 1). As expected, plants with most severe sex phenotypes (the amy3isa3lda triple mutant) had the lowest sugar levels at the end of the night. Plants with a milder phenotype (isa3) had sugar levels comparable with the wild type. The phenotypes of the double mutants amy3isa3 and isa3lda were intermediate between them. Overall, there was a good inverse correlation between the severity of the sex phenotype and sugar content and between the severity of the sex phenotype and plant size.
TABLE 1.
Sugar content at the end of the night
Plants were grown, harvested at the end of the 12-h night, and extracted as described in the legend to Fig. 2. Glucose, fructose, sucrose, and maltose in the soluble fraction were analyzed by HPAEC-PAD. Each value is the mean ± S.E. (n = 4). Footnotes indicate values that differ significantly from the wild-type values (Student's t test).
| Genotype | Glucose | Fructose | Sucrose | Maltose |
|---|---|---|---|---|
| μg/g FW | μg/g FW | μg/g FW | μg/g FW | |
| Wild type | 102.7 ± 6.1 | 57.7 ± 4.9 | 304.8 ± 17.1 | 19.7 ± 2.5 |
| isa3 | 111.8 ±16.7 | 64.6 ± 12.3 | 409.5 ± 32.3a | 32.0 ± 7.7 |
| isa3lda | 120.1 ±22.0 | 52.9 ± 7.5 | 257.6 ± 12.8 | 8.7 ± 2.5a |
| amy3isa3 | 85.1 ±13.0 | 39.7 ± 3.8a | 196.8 ± 13.2b | 5.0 ± 1.0b |
| amy3isa3lda | 61.8 ± 5.4b | 26.1 ± 4.3b | 147.0 ± 11.2c | 4.2 ± 1.8b |
| pwd | 95.6 ± 12.9 | 50.9 ± 6.6 | 357.4 ± 38.5 | 23.4 ± 6.7 |
| gwd1 | 59.8 ± 5.4b | 27.1 ± 4.5b | 128.6 ± 14.7c | 6.0 ± 0.9b |
a p ≤ 0.05.
b p ≤ 0.01.
c p ≤ 0.001.
Loss of ISA3, LDA, and AMY3 Prevents Starch Breakdown
It is difficult to determine the exact rates of starch breakdown in sex mutants. This is partly because the severity of the phenotype changes between different batches of plants (depending on age and exact growth conditions) but primarily due to the fact that the decrease in starch content during a 12-h night is small relative to the overall starch levels. Therefore, we performed an extended night experiment in which plants were subjected to an additional 48 h of darkness after the normal 12-h night. Both double mutants (amy3isa3 and isa3lda) degraded a substantial amount of their starch. Interestingly, amy3isa3lda triple mutants did not mobilize any of their starch, suggesting a complete block in starch breakdown (Table 2).
TABLE 2.
Starch content during an extended night period
Plants were grown for 35 days in a 12-h light/12-h dark regime. After a normal night, plants were subjected to a night phase extended by 48 h. Samples comprising all leaves from a single plant were harvested at time points during the extended night and extracted using perchloric acid. Starch in the insoluble fraction was measured after enzymatic hydrolysis to glucose. Each value is the mean ± S.E. (n = 4). Footnotes indicate values that differ significantly from the normal end-of-night values of the corresponding genotype (Student's t test). NA, not analyzed.
| Genotype | Normal night (12-h dark) starch | Extended night starch |
|
|---|---|---|---|
| 36-h dark | 60-h dark | ||
| mg/g FW | mg/g FW | mg/g FW | |
| Wild type | 0.25 ± 0.08 | NA | NA |
| isa3lda | 19.76 ± 0.27 | 12.52 ± 0.85a | 10.13 ± 1.61a |
| amy3isa3 | 37.52 ± 2.97 | 25.34 ± 2.29b | 24.87 ± 1.22a |
| amy3isa3lda | 46.08 ± 5.01 | 47.47 ± 3.10 | 45.09 ± 3.13 |
| gwd1 | 50.39 ± 4.30 | 40.30 ± 0.33b | 36.99 ± 3.40b |
a p ≤ 0.01.
b p ≤ 0.1.
The action of some starch hydrolytic enzymes (specifically the β-amylase BAM3, alone and in combination with ISA3) on the granule surface is strongly enhanced by simultaneous, reversible glucan phosphorylation (13, 14). We compared the effect of blocking hydrolytic starch degradation in isa3, amy3isa3, isa3lda, and amy3isa3lda with the block imposed by a partial or total loss of phosphorylation of the starch granule in pwd (no C3 phosphorylation) and gwd1 (no C6 or C3 phosphorylation) mutants. We measured starch in an independently grown set of plants harvested at the end of the night. As expected, starch was almost depleted in wild-type plants (0.6 ± 0.1 mg/g FW). The mutants contained different amounts of starch: pwd (6.9 ± 0.5 mg/g FW), isa3 (12.2 ± 1.0 mg/g FW), isa3lda (26.9 ± 1.9 mg/g FW), amy3isa3 (40.0 ± 3.6 mg/g FW), amy3isa3lda (58.1 ± 3.4 mg/g FW), and gwd1 (52.4 ± 3.7 mg/g FW). In this case, the amy3isa3 double mutant had slightly higher starch content than isa3lda. The sex phenotype of amy3isa3lda triple mutants was even higher than that of gwd1, which has the most severe phenotype previously reported. Consistent with this, gwd1 plants were able to degrade some of their starch during the extended night experiment (Table 2), although sugar levels derived from starch were very low at the end of the night (Table 1). We also evaluated whether ISA3, LDA, and AMY3 interact physically because complex formation is an important regulatory mechanism and could orchestrate the activities of enzymes that share a common substrate. However, separating proteins from extracts of wild-type plants by gel filtration chromatography (supplemental Fig. S2) did not reveal any evidence for such an interaction.
Starch Structure and Degradation Intermediates upon Loss of Starch-degrading Enzymes
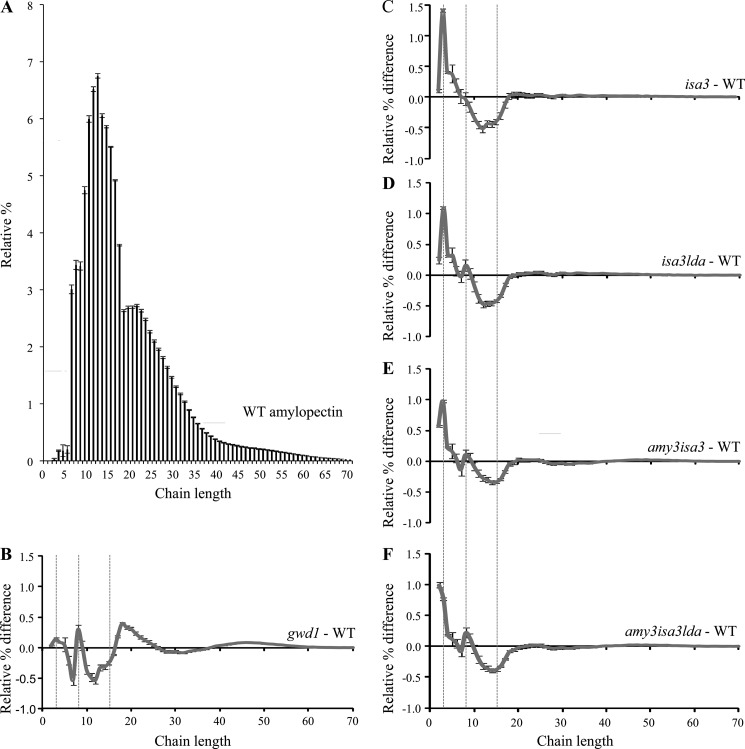
Loss of enzymes in the pathway of starch degradation can be reflected in the structure of the residual starch and/or in the accumulation of starch degradation intermediates (19). Therefore, we first analyzed the fine structure of amylopectin from wild-type, gwd1, isa3, amy3isa3, isa3lda, and amy3isa3lda. Starch was extracted from plants harvested at the end of the day, and the chain length distribution (CLD) was determined (whereby the branched amylopectin is digested in vitro into its constituent linear chains, which are then analyzed by high performance anion exchange chromatography). As reported earlier (19), the CLD profiles from isa3 amylopectin showed a small but highly reproducible enrichment of short chains with a degree of polymerization (d.p.) of 3 compared with the wild type (Fig. 3). This is consistent with the preference of this enzyme for short external chains, as would be generated by β-amylolysis (21, 22). A similar trend was seen in the CLDs of all lines carrying the isa3 mutation. Interestingly, the amy3isa3 and amy3isa3lda CLDs also showed a significant increase in chains of d.p. 2 in comparison with isa3 and isa3lda mutants (Fig. 3, C–F). In contrast, the CLD of gwd1 showed only small differences compared with the wild type and was not enriched in the short chains (11). These data are consistent with the idea that the underlying cause of the sex phenotypes in gwd1 and the amy3isa3lda triple mutant is not identical. Lack of phosphorylation will inhibit the initial β-amylolysis of the granule surface (13). However, the loss of AMY3, ISA3, and LDA would affect later steps in the amylopectin degradation, after some β-amylolysis had occurred.
FIGURE 3.
Changes in amylopectin structure in starch excess mutants. Comparison of chain length distributions of starch from the wild type (WT), isa3, isa3lda, amy3isa3, amy3isa3lda, and gwd1. Starch was extracted from each of four replicate plants harvested at the end of the day. A, the chain length distribution of wild-type amylopectin. B–F, difference plots calculated by subtracting the relative percentages of wild-type amylopectin from those of gwd1 (B), isa3 (C), isa3lda (D), amy3isa3 (E), and amy3isa3lda (F). Chain lengths of d.p. 3, 8, and 15 are indicated by dashed lines. Errors in the difference plots (error bars) are calculated from the S.E. value of the wild type and the individual mutants, with consideration of error propagation (based on the error law of Gauss).
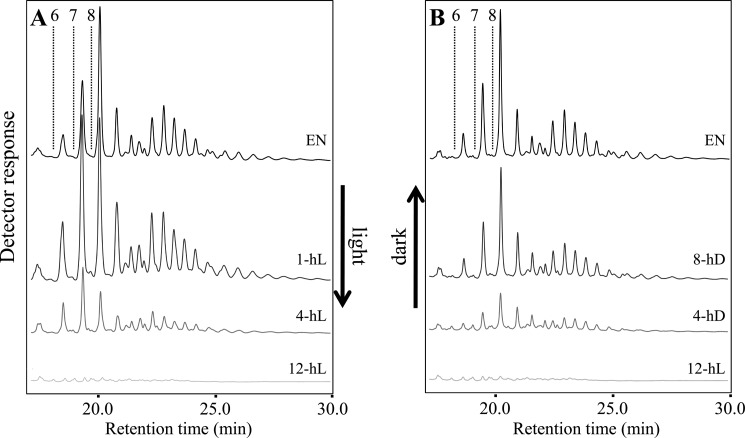
We also investigated the abundance of starch degradation intermediates (i.e. maltose and other small soluble glucans). Maltose was present in all lines at the end of the night (Table 1). Levels were highest in the wild type and the mutants with mild sex phenotypes (isa3 and pwd) and lowest in mutants with stronger sex phenotypes (isa3lda, amy3isa3, amy3isa3lda, and gwd1). Delatte et al. (19) previously showed that small branched glucans accumulate in isa3lda mutants at the end of the night and suggested that they are released from the granule surface by AMY3. We confirmed the presence of these branched glucans in isa3lda at the end of the night. None were detected in the wild type, even when extracts were concentrated 50-fold (supplemental Fig. S3). Branched glucans were also not detected in the single mutants (not shown) or in the amy3lda and amy3isa3 double mutants (not shown). In the amy3isa3lda triple mutant (Fig. 4A), they were also absent. These data strongly support the hypothesis that these glucans are AMY3 products that accumulate in the stroma only in the absence of the two DBEs, ISA3 and LDA.
FIGURE 4.
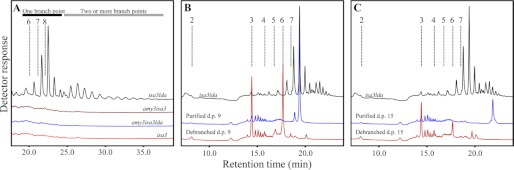
The small branched glucans in the isa3lda double mutant are liberated by AMY3. Plants harvested at the end of the night were immediately frozen in liquid N2. Samples were extracted using perchloric acid. Note the difference in the x axes. A, soluble malto-oligosaccharides in isa3, isa3lda, amy3isa3, and amy3isa3lda mutants, analyzed by HPAEC-PAD. Representative chromatograms are shown. Only isa3lda contains small branched glucans. Elution times of glucans with d.p. 6–8 are indicated by dashed lines. Bars illustrate the bimodal distribution of glucans with one branch point (black bar) and two or more branch points (gray bar; see “Results”). B, soluble malto-oligosaccharides of isa3lda mutants were fractionated by HPAEC. Those eluting between the retention times corresponding to linear chains of d.p. 8 and 9 were collected and debranched enzymatically, yielding linear chains of d.p. 3 and 6. C, chains eluting between the retention times corresponding to linear chains of d.p. 14 and 15 were collected and debranched enzymatically, yielding linear chains of d.p. 3, 6, and 9.
We characterized the nature of these branched intermediates further. The distribution was bimodal, ranging from d.p. 6 to more than d.p. 20 (Fig. 4A). To investigate the number of α-1,6-linkages, we collected one abundant glucan species from each part of the bimodal distribution (Fig. 4A). The first eluted earlier than linear d.p. 9 chains, and the second eluted earlier than linear d.p. 15 chains (branched glucans typically elute slightly earlier than linear glucans with the equivalent number of glucosyl residues). Debranching of the first glucan fraction (Fig. 4B) resulted in degradation products that co-eluted with linear chains of d.p. 3 and 6, suggesting that they were probably singly branched glucans (i.e. a d.p. 6 main chain carrying a d.p. 3 branch close to its non-reducing end. Side branches of d.p. 6 are unlikely because they would be susceptible to β-amylolysis in the stroma). Debranching of the second fraction (Fig. 4C) resulted in products that co-eluted with linear chains of d.p. 3, 6, and 9, suggesting a mixture of species with two or more branch points (e.g. a d.p. 9 main chain with two d.p. 3 branches or a d.p. 6 main chain carrying a d.p. 6 branch, itself carrying a d.p. 3 branch). It is unlikely that the d.p. 15 oligosaccharides were singly branched (e.g. a d.p. 9 main chain with a branch of d.p. 6) because longer side chains would be susceptible to degradation by stromal β-amylases. Based on this experiment, we propose that the first subpopulation of branched glucans in Fig. 4A contains one branch point, whereas the second population contains two (or more). Considering the average relative abundance of each glucan peak and the proposed number of branch points, we calculated an overall average of 8.5 glucosyl units/α-1,6-linkage in the small branched glucan pool. Assuming that the average number of glucose units/α-1,6-linkage in Arabidopsis amylopectin is around 22.5 (27), we estimated that ∼24% of the branch points liberated from the starch granule at night in isa3lda are retained in the small branched glucan pool at the end of the night.
The Involvement of ISA1-ISA2 in Starch Degradation
In one way, the results from our experiments are surprising because they indicate that the remaining DBE activity (the ISA1-ISA2 complex), present in all of the genotypes analyzed thus far, makes little or no contribution to glucan catabolism. Therefore, we performed three experiments in an effort to establish whether this is the case and, if so, why.
First, we followed the dynamics of the small branched glucan pool in isa3lda mutants throughout the diurnal cycle. The amount was the highest at the end of the night, consistent with them being products of starch degradation, but diminished progressively during the day, and almost none were detectable at dusk (Fig. 5A). With the onset of night, the branched glucans accumulated until dawn (Fig. 5B). The relative abundance of the different glucan species did not change as they accumulated, but during the first hour of the day, the distribution shifted slightly toward smaller branched glucans. The disappearance of the branched glucans during the day could imply that ISA1-ISA2 is able to slowly metabolize the α-1,6-linkages.
FIGURE 5.
Diurnal changes of branched glucans in the isa3lda double mutant. Four replicate plants were harvested at each time point indicated during the diurnal cycle and immediately frozen in liquid N2. Malto-oligosaccharides were analyzed by HPAEC-PAD. Representative chromatograms are shown. A, malto-oligosaccharides in isa3lda extracts during the day. EN, end of night. Time in the light is indicated. Elution times of glucans with d.p. 6–8 are indicated by dashed lines. Note the differences in relative abundance of several chain species at the beginning of the day (e.g. glucan species eluting between d.p. 7 and 8). B, malto-oligosaccharides present in isa3lda extracts during the night. ED, end of day. Time in the dark is indicated. Note that there are no differences in relative abundance of the chain species.
Second, we repeated the extended night experiment, comparing isa3lda with the quadruple mutant isa1isa2isa3lda (18). As ISA1-ISA2 modifies amylopectin during starch synthesis to facilitate its crystallization, the quadruple mutant synthesizes primarily phytoglycogen rather than starch (18). In isa3lda, starch was degraded at a linear rate throughout the 60-h night, as before (Table 2). Interestingly, the amount of small branched glucans rose in the first 12 h but then remained constant despite continued starch degradation (supplemental Fig. S4A), suggesting that they are being turned over. In mutants lacking all DBEs, the pattern was different. The amount of phytoglycogen declined in the early part of the extended night but then remained constant, presumably because the remaining enzymes are unable to degrade it further without DBE activity. Small AMY3-derived branched glucans were present, but this pool stayed unchanged (supplemental Fig. S4B) throughout the long night. Although the results from these two genotypes are not directly comparable due to the differences in glucan synthesis and structure, these experiments provide further circumstantial evidence that ISA1-ISA2 may be hydrolyzing the small branched glucans generated in isa3lda mutants.
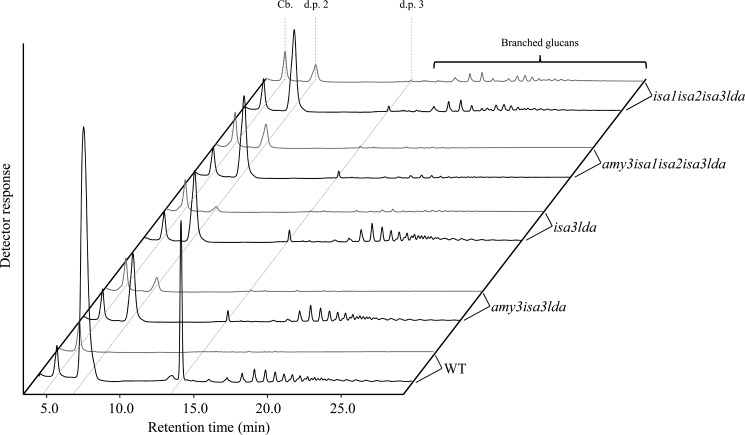
Third, we determined the debranching activity against β-limit dextrin, a substrate produced when amylopectin is enzymatically digested to completion with commercial β-amylase. β-Amylase degrades the external chains of amylopectin close to the branch points, resulting in a branched structure decorated with remaining maltosyl and maltotriosyl stubs (d.p. 2 and 3) of the outer chains. These stubs prevent further degradation of the internal linear chains by the β-amylase. In the assay, β-limit dextrin is incubated with plant extracts in the presence of excess commercial β-amylase. Debranching of the outer branch points releases maltose and maltotriose and exposes formerly protected chains to further degradation by β-amylase, generating more maltose. Maltotriose is thus a direct indication of debranching action, whereas maltose is partly released by debranching but mostly by β-amylase. Delatte et al. (19) showed earlier that isa3lda extracts had a residual 4% maltotriose-releasing activity compared with wild-type extracts. We applied this assay to extracts of the wild type, isa3lda, and isa1isa2isa3lda. Both mutant protein extracts already contain plant-derived small branched glucans produced from AMY3 (see Fig. 6 and Ref. 18). Therefore, to control for potential interference by the chloroplastic α-amylase, we also assayed amy3isa3lda and amy3isa1isa2isa3lda mutant extracts (18). For isa3lda, we found that maltotriose production was decreased to around 3% (Table 3). Similar values were obtained for amy3isa3lda. Plants lacking all DBEs (isa1isa2isa3lda and amy3isa1isa2isa3lda) had even lower maltotriose liberation (around 1% of the wild type activity), suggesting that most of the residual activity is due to the ISA1-ISA2.
FIGURE 6.
Identification of glucans liberated from β-limit dextrin by plant protein extracts. Plants of the wild type (WT), amy3isa3lda, isa3lda, amy3isa1isa2isa3lda, and isa1isa2isa3lda were harvested at the end of the night. Protein extracts were used in the debranching enzyme assay (see Table 3 and “Results”). For each genotype, representative HPAEC-PAD chromatograms of small glucans are shown at time 0 (gray line) and after incubation with substrate (black line). Extracts of the wild type, amy3isa3lda, and isa3lda released branched glucans in addition to maltose (d.p. 2) and maltotriose (d.p. 3), whereas extracts of mutants lacking all debranching enzymes (amy3isa1isa2isa3lda and isa1isa2isa3lda) did not. Note that extracts of isa1isa2isa3lda already contain singly and doubly branched glucans, released in planta by AMY3 (18). Cellobiose (Cb.) served as an internal standard.
TABLE 3.
Determination of debranching enzyme activity
Protein extracts of wild-type, isa3lda, amy3isa3lda, isa1isa2isa3lda, and amy3isa1isa2isa3lda plants were tested for their debranching activity as described under “Experimental Procedures.” Maltose and maltotriose were determined using HPAEC-PAD. Maltose levels reflect the combination of debranching activity and β-amylolytic activity, whereas maltotriose reflects primarily debranching enzyme activity. The values of the two independent experiments (Experiments 1 and 2) are means of two technical replicates from extracts of four plants pooled prior to extraction. The amounts of maltose and maltotriose liberated were set to 100% for wild-type extracts.
| Genotype of plants | Experiment 1 |
Experiment 2 |
||
|---|---|---|---|---|
| Maltose released | Maltotriose released | Maltose released | Maltotriose released | |
| % | % | % | % | |
| Wild type | 100.0 | 100.0 | 100.0 | 100.0 |
| isa3lda | 14.9 | 3.5 | 23.6 | 2.7 |
| amy3isa3lda | 11.4 | 3.0 | 20.9 | 2.2 |
| isa1isa2isa3lda | 15.5 | 1.6 | 47.6 | −0.6 |
| amy3isa1isa2isa3lda | 16.0 | 1.8 | 24.8 | 0.5 |
Although the β-limit dextrin assay confirmed that ISA1-ISA2 has a very low capacity to remove short branches, the enzyme has nevertheless been shown to be very active against β-limit dextrin when incorporated as a substrate in native gels (24). Further HPAEC-PAD analysis of the reaction products reconciled this apparent inconsistency. In addition to maltose and maltotriose, extracts of the wild type generated a population of small branched glucans from the β-limit dextrin (Fig. 6). These were also produced by isa3lda and amy3isa3lda extracts (Fig. 6) but not by isa1isa2isa3lda and amy3isa1isa2isa3lda extracts. This indicates that ISA1-ISA2, rather than attacking the branch points of the short external chains, targets the inner branch points of β-limit dextrin, releasing branched glucans. In contrast, ISA3 and LDA preferentially liberate the short external chains (19, 21, 23). However, despite this activity in vitro on soluble β-limit dextrin as a substrate, it is important to note that ISA1-ISA2 appears not to produce such branched glucans in vivo from the surface of the starch granule because the amy3isa3lda triple mutant has no detectable breakdown and no accumulation of branched intermediates.
DISCUSSION
Refinements to the Model of Starch Degradation
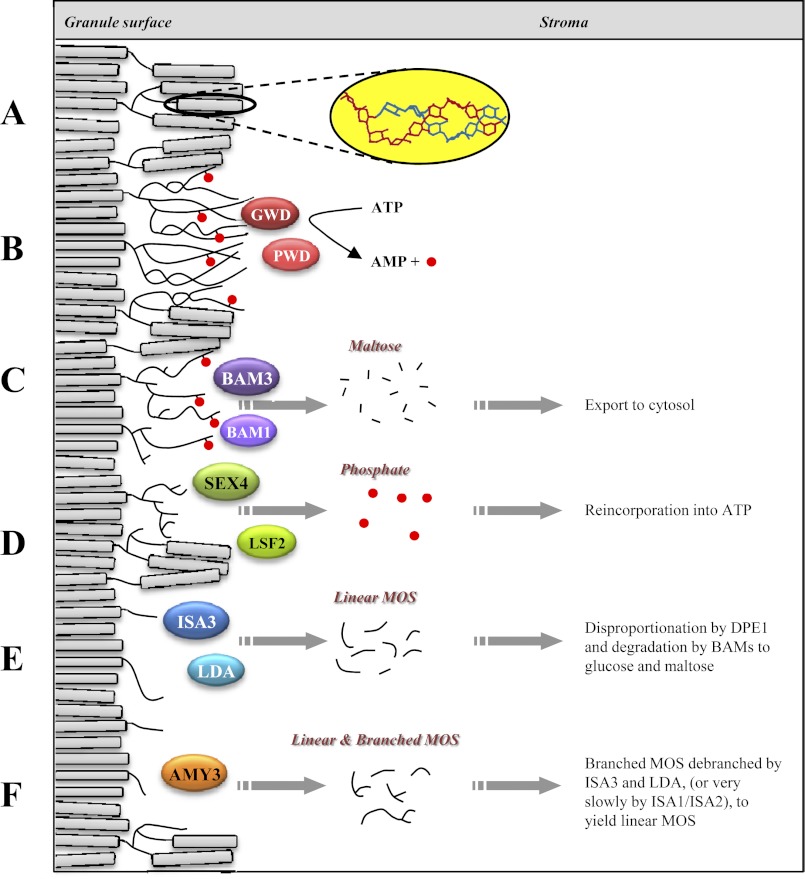
The work presented here shows that three enzymes are critical for transient starch breakdown in Arabidopsis: the two debranching enzymes ISA3 and LDA and the chloroplastic α-amylase AMY3. Our data suggest that each of these three enzymes works independently of the other two, but removal of all three completely blocks starch breakdown, leading to the most severe sex phenotype reported thus far. In Fig. 7, we illustrate how each of these enzymes might fit into the pathway of starch degradation in chloroplasts.
FIGURE 7.
Model for the enzymatic steps in starch degradation in Arabidopsis leaf mesophyll chloroplasts. A, depiction of the starch granule surface with double helices (inset) aligned in crystalline lamellae. B, phosphorylation of glucan chains by GWD1 and PWD, solubilizing the crystalline lamellae. C, β-amylolysis of glucan chains by BAM3 and BAM1 until a phosphate or a branch point is reached. D, SEX4 and LSF2 dephosphorylate glucan chains. E, ISA3 and LDA hydrolyze branch points of stubs left by the BAMs. F, AMY3 liberates small linear and branched glucan chains.
Previous work has illustrated the importance of glucan phosphorylation by GWD1 and PWD in promoting the activities of hydrolytic enzymes, notably β-amylases (13) (Fig. 7, B and C). The gwd1 mutant has a very severe sex phenotype, but we detected some starch degradation when the mutant was kept in an extended night, suggesting that some enzymes can degrade starch to an extent without prior phosphorylation. This is consistent with the analysis of the mex1gwd1 double mutant (28). Maltose accumulates in the stroma of mex1 mutants because they are unable to export the maltose generated during starch degradation. In mex1gwd1, the amount of accumulated maltose is less than half of that observed in the mex1 single mutant but still more than 20-fold in excess of the wild type.
Dephosphorylation of the glucans allows continued degradation by β-amylases until they reach the branch points at the root of each amylopectin cluster (Fig. 7D). We propose that the resulting β-limit structure at the granule surface is a substrate for the three enzymes studied here and that, in the amy3isa3lda triple mutant, degradation stalls at this point. We summarize the role of each of the three enzymes below.
The Function of AMY3
The endoamylase AMY3 can release a range of products from starch (7) (Fig. 7F). It possesses carbohydrate binding modules in its amino terminus that enable it to bind starch (29). Although it cannot hydrolyze α-1,6-linkages, it can theoretically release chains carrying branch points (i.e. small branched glucans) into the chloroplast stroma. Such branched glucans are not seen in a wild type plant, either because they are not produced during a normal day-night cycle or because they are rapidly metabolized. Furthermore, the loss of AMY3 does not have an impact on starch degradation under normal conditions, either because it is not involved or because the activities of other enzymes can compensate for its absence. Our data suggest the latter because loss of AMY3 in the isa3 or isa3lda backgrounds accentuates their sex phenotypes. This shows that, at least in these mutants, AMY3 is required and is consistent with a genotype-dependent requirement for AMY3 reported previously for other lines (14, 18). The accumulation of small branched glucans in the isa3lda double mutant (19), but not in the amy3isa3lda triple mutant (this work), demonstrates the capacity of AMY3 to liberate them from starch. The absence of small branched glucans from the triple mutant data also suggests that no other enzyme generates them at a significant rate (see below).
The Function of LDA
LDA has high activity on soluble β-limit dextrins, removing the external short chain stubs (21–23) (Fig. 7E). Delatte et al. (19) proposed a model in which LDA acts exclusively on stromal branched glucans produced by AMY3. This was because lda mutants have a wild-type phenotype, but when mutated in combination with ISA3, small branched glucans accumulated, and the sex phenotype of the isa3 mutation was enhanced. If the role of LDA was only to metabolize small branched glucans, mutation of AMY3 therefore would be epistatic to that of LDA, because without AMY3, no substrates would be generated for LDA. Hence, the amy3isa3 and amy3isa3lda phenotypes would be identical. However, the loss of LDA in the amy3isa3 background greatly increases the severity of its sex phenotype. This suggests that LDA plays an active role in the amy3isa3 background but argues against its substrate being just small branched glucans in this case. If that were so, the branched glucans would have to be produced by an enzyme other than AMY3 and would accumulate in the triple mutant. Thus, our data suggest that the starch granule surface itself is a substrate for LDA. Indeed, LDA has a multidomain structure that includes CBMs, which may facilitate its action on the starch granule (30). However, direct evidence for granule localization is still required for this enzyme.
The Function of ISA3
Of the three enzymes, ISA3 is the most critical. Like LDA, ISA3 has high activity on soluble β-limit dextrins, removing the external short chain stubs (21–23) (Fig. 7E). Like the other two enzymes, it possesses a carbohydrate binding module (31) and has been localized to the starch granule surface (19). Mutation of ISA3 leads to a sex phenotype, consistent with the idea that neither LDA nor AMY3 can compensate for its loss. In contrast, ISA3 alone appears to be sufficient for branch point removal during starch breakdown because mutations of LDA, of AMY3, or of both LDA and AMY3 together do not cause sex phenotypes. However, loss of ISA3 in the lda, amy3, or amy3lda backgrounds leads to a progressively more severe phenotype. The isa3 mutant also has an increased abundance of short chains in amylopectin. This presumably reflects an inability of the other two enzymes to degrade specific glucan structures generated during the preceding steps of degradation (19). In amy3isa3 and in isa3lda, where the sex phenotypes are more severe than in isa3, the increased abundance of short chains is still measurable. In addition, ISA3 may be able to degrade branched glucans produced by AMY3 in the stroma.
The action of each of the three enzymes would reveal linear chains of new crystalline lamellae below the β-limit surface of the starch granule, which would allow continued degradation. It is intriguing that the severity of the phenotypes is not additive; the impact on starch content, structure, and the accumulation of intermediates is determined by which enzyme is missing and by those enzymes still present. It seems likely that in a wild-type plant, all three enzymes are involved in normal transient starch breakdown but overlap in their functions.
Such overlap or partial redundancy is also seen in other steps in starch metabolism. Arabidopsis plants lacking one of the two major chloroplastic β-amylases (BAM1 and BAM3) have either normal starch levels (bam1) or slightly increased starch contents (bam3) (6), whereas the loss of both leads to a severe sex phenotype approaching that of amy3isa3lda or gwd1 mutants. The phosphoglucan phosphatases SEX4 and LSF2 represent another example. On one hand, sex4 mutants show a sex phenotype, whereas lsf2 mutants do not (15). On the other hand, lsf2 mutants have markedly altered levels of starch-bound phosphate. Double mutants have a further increase in starch content and altered levels of starch-bound phosphate, again pointing toward an overlap in function between the two proteins but not full redundancy.
Another factor that might contribute to the phenotypes we observe is a quantitative difference in the abundance of each enzyme. It is possible that the total remaining enzyme activity in some mutant combinations is insufficient to achieve wild-type rates of starch breakdown rather than the remaining enzyme(s) not being able to act on some of the structures that arise during degradation. This hypothesis could be tested by the overexpression of each of the three enzymes in the amy3isa3lda triple mutant. However, it is likely that plants possess an excess of starch hydrolytic enzyme activity because they are able to perform a higher than normal rate of starch degradation when coping with certain environmental changes (e.g. imposition of photorespiratory conditions or starch degradation after a longer than expected day) (32–34). Thus, degradation does not proceed at its maximal rate and must normally be controlled, either through direct regulation of the enzyme activities or by regulating accessibility to their substrates (e.g. control through glucan phosphorylation mediated by GWD1).
A Role for ISA1-ISA2 in Starch Breakdown?
It is generally accepted that the isoamylase composed of ISA1 and ISA2 subunits is involved in starch synthesis rather than breakdown (16, 18, 35). However, its potential role in starch breakdown was never evaluated. Our data indicate that ISA1-ISA2 can participate in oligosaccharide metabolism but that it has a very minor role in starch breakdown overall. The in vitro debranching assay revealed that ISA1-ISA2 liberates small branched glucans rather than linear chains when acting on soluble β-limit dextrins (Fig. 6), showing that ISA1-ISA2 preferentially hydrolyzes branch points of longer internal chains rather than the short stubs of the external chains. Considering this result, it is theoretically possible that ISA1-ISA2 can release branched glucans from the granule surface during starch breakdown, but our data argue against this. No branched glucans were detected in the amy3isa3lda triple mutant, showing that AMY3, rather than ISA1-ISA2, is their source in the isa3lda double mutant. Furthermore, no net starch degradation was detected in the triple mutant.
The reason for this discrepancy in apparent ISA1-ISA2 activity may lie in the difference in nature between the solubilized β-limit dextrin substrate, accessible to enzymes at virtually any branch point, and the β-limit surface of the intact starch granule, where access to the branch points below the surface may be limited. However, our data do suggest that small branched glucans may be degraded by ISA1-ISA2. Our calculations imply that only one-quarter of the α-1,6-linkages removed from the starch granules in isa3lda is retained in the small branched glucan pool. Furthermore, the small branched glucans decrease in abundance during the day and so may be further metabolized by ISA1-ISA2 (Fig. 5). This provides circumstantial evidence that ISA1-ISA2 may function during both day and night to debranch these glucans. However, the fact that small branched glucans accumulate in isa3lda at all suggests that they are inefficiently degraded and unlikely to be the optimal substrates for ISA1-ISA2 (Fig. 5 and supplemental Fig. S4A). Furthermore, in wild-type leaves, small branched glucans are below the level of detection because they are probably degraded efficiently by ISA3 and LDA, for which these are the preferred substrates.
Implications of Altered Starch Metabolism for Plant Growth
Our data revealed a correlation between the severity of the block in starch degradation and the decrease in sugar contents toward the end of the night (Table 1). Furthermore, plant biomass decreased as the ability to degrade starch was progressively impaired (Figs. 1 and 2). This is presumably because the loss of key enzymes of starch breakdown results in an inability to produce a sufficient amount of energy and metabolic precursors to maintain growth and metabolism during the night (36). This will lead to dramatic metabolic changes, partly explaining the reduced growth (33, 37). However, it was shown previously that the growth inhibition resulting from carbon starvation at night also extends into the subsequent light phase. Growth is not immediately reversed to normal levels when sugar levels are restored (38). All of these factors could contribute to the reduced biomass production in all of the starch excess mutants investigated here.
Can We Generalize the Pathway of Transient Starch Breakdown between Species?
Arabidopsis is the best investigated system for transient starch breakdown, and in general, the enzymes of starch metabolism are highly conserved throughout the plant kingdom. However, relatively few mutants lacking α-amylase and DBEs in other plant species have been characterized. Mutants of LDA in maize and ISA3 in rice both displayed sex phenotypes in leaves (39, 40). Furthermore, repression in rice of an atypical α-amylase (AmyI-1), an N-glycosylated protein that is both secreted in the endosperm of geminated seeds and located in plastids of leaves, leads to increased leaf starch (5). To our knowledge, these are the only three examples in the literature where mutant plants were investigated with respect to leaf starch metabolism. Like our study, they illustrate that the three enzymes are important for normal transient starch breakdown but also that not all phenotypes are equivalent to those observed in Arabidopsis. Thus, the relative contribution of each enzyme might differ between species. This could be due to differences in the relative amount of each enzyme, the properties of the enzymes themselves, the starch structure, or the conditions used for experimentation or a combination of these factors. Validation of the findings with Arabidopsis using another model system (e.g. in a member of the Solanaceae, Leguminoceae, or Poaceae) would be valuable.
Acknowledgments
We thank Barbara Pfister and David Seung for critical reading of the manuscript.
This work was supported by Swiss National Science Foundation Grant 31002AB_131074 (to S. Z.) and by ETH Zurich.

This article contains supplemental Figs. S1–S4.
- BAM
- β-amylase
- DBE
- debranching enzyme
- HPAEC
- high performance anion-exchange chromatography
- PAD
- pulsed amperometric detection
- SEC
- size exclusion chromatography
- FW
- fresh weight
- CLD
- chain length distribution
- d.p.
- degree of polymerization.
REFERENCES
- 1. Gidley M. J., Bulpin P. V. (1987) Crystallization of maltaoses as models of the crystalline forms of starch. Carbohydr. Res. 161, 291–300 [Google Scholar]
- 2. Blennow A., Engelsen S. B. (2010) Helix-breaking news. Fighting crystalline starch energy deposits in the cell. Trends Plant Sci. 15, 236–240 [DOI] [PubMed] [Google Scholar]
- 3. Zeeman S. C., Kossmann J., Smith A. M. (2010) Starch. Its metabolism, evolution, and biotechnological modification in plants. Annu. Rev. Plant Biol. 61, 209–234 [DOI] [PubMed] [Google Scholar]
- 4. Fincher G. B. (1989) Molecular and Cellular Biology Associated with endosperm mobilization in germinating cereal-grains. Annu. Rev. Plant Physiol. Plant Mol. Biol. 40, 305–346 [Google Scholar]
- 5. Asatsuma S., Sawada C., Itoh K., Okito M., Kitajima A., Mitsui T. (2005) Involvement of α-amylase I-1 in starch degradation in rice chloroplasts. Plant Cell Physiol. 46, 858–869 [DOI] [PubMed] [Google Scholar]
- 6. Fulton D. C., Stettler M., Mettler T., Vaughan C. K., Li J., Francisco P., Gil M., Reinhold H., Eicke S., Messerli G., Dorken G., Halliday K., Smith A. M., Smith S. M., Zeeman S. C. (2008) β-AMYLASE4, a noncatalytic protein required for starch breakdown, acts upstream of three active β-amylases in Arabidopsis chloroplasts. Plant Cell 20, 1040–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yu T. S., Zeeman S. C., Thorneycroft D., Fulton D. C., Dunstan H., Lue W. L., Hegemann B., Tung S. Y., Umemoto T., Chapple A., Tsai D. L., Wang S. M., Smith A. M., Chen J., Smith S. M. (2005) α-Amylase is not required for breakdown of transitory starch in Arabidopsis leaves. J. Biol. Chem. 280, 9773–9779 [DOI] [PubMed] [Google Scholar]
- 8. Ritte G., Lloyd J. R., Eckermann N., Rottmann A., Kossmann J., Steup M. (2002) The starch-related R1 protein is an alpha-glucan, water dikinase. Proc. Natl. Acad. Sci. U.S.A. 99, 7166–7171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baunsgaard L., Lütken H., Mikkelsen R., Glaring M. A., Pham T. T., Blennow A. (2005) A novel isoform of glucan, water dikinase phosphorylates prephosphorylated α-glucans and is involved in starch degradation in Arabidopsis. Plant J. 41, 595–605 [DOI] [PubMed] [Google Scholar]
- 10. Kötting O., Pusch K., Tiessen A., Geigenberger P., Steup M., Ritte G. (2005) Identification of a novel enzyme required for starch metabolism in Arabidopsis leaves. The phosphoglucan, water dikinase. Plant Physiol. 137, 242–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yu T. S., Kofler H., Häusler R. E., Hille D., Flügge U. I., Zeeman S. C., Smith A. M., Kossmann J., Lloyd J., Ritte G., Steup M., Lue W. L., Chen J., Weber A. (2001) The Arabidopsis sex1 mutant is defective in the R1 protein, a general regulator of starch degradation in plants, and not in the chloroplast hexose transporter. Plant Cell 13, 1907–1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lorberth R., Ritte G., Willmitzer L., Kossmann J. (1998) Inhibition of a starch-granule-bound protein leads to modified starch and repression of cold sweetening. Nat. Biotechnol. 16, 473–477 [DOI] [PubMed] [Google Scholar]
- 13. Edner C., Li J., Albrecht T., Mahlow S., Hejazi M., Hussain H., Kaplan F., Guy C., Smith S. M., Steup M., Ritte G. (2007) Glucan, water dikinase activity stimulates breakdown of starch granules by plastidial β-amylases. Plant Physiol. 145, 17–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kötting O., Santelia D., Edner C., Eicke S., Marthaler T., Gentry M. S., Comparot-Moss S., Chen J., Smith A. M., Steup M., Ritte G., Zeeman S. C. (2009) STARCH-EXCESS4 is a laforin-like phosphoglucan phosphatase required for starch degradation in Arabidopsis thaliana. Plant Cell 21, 334–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Santelia D., Kötting O., Seung D., Schubert M., Thalmann M., Bischof S., Meekins D. A., Lutz A., Patron N., Gentry M. S., Allain F. H., Zeeman S. C. (2011) The phosphoglucan phosphatase like sex Four2 dephosphorylates starch at the C3-position in Arabidopsis. Plant Cell 23, 4096–4111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Delatte T., Trevisan M., Parker M. L., Zeeman S. C. (2005) Arabidopsis mutants Atisa1 and Atisa2 have identical phenotypes and lack the same multimeric isoamylase, which influences the branch point distribution of amylopectin during starch synthesis. Plant J. 41, 815–830 [DOI] [PubMed] [Google Scholar]
- 17. Wattebled F., Dong Y., Dumez S., Delvallé D., Planchot V., Berbezy P., Vyas D., Colonna P., Chatterjee M., Ball S., D'Hulst C. (2005) Mutants of Arabidopsis lacking a chloroplastic isoamylase accumulate phytoglycogen and an abnormal form of amylopectin. Plant Physiol. 138, 184–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Streb S., Delatte T., Umhang M., Eicke S., Schorderet M., Reinhardt D., Zeeman S. C. (2008) Starch granule biosynthesis in Arabidopsis is abolished by removal of all debranching enzymes but restored by the subsequent removal of an endoamylase. Plant Cell 20, 3448–3466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Delatte T., Umhang M., Trevisan M., Eicke S., Thorneycroft D., Smith S. M., Zeeman S. C. (2006) Evidence for distinct mechanisms of starch granule breakdown in plants. J. Biol. Chem. 281, 12050–12059 [DOI] [PubMed] [Google Scholar]
- 20. Wattebled F., Planchot V., Dong Y., Szydlowski N., Pontoire B., Devin A., Ball S., D'Hulst C. (2008) Further evidence for the mandatory nature of polysaccharide debranching for the aggregation of semicrystalline starch and for overlapping functions of debranching enzymes in Arabidopsis leaves. Plant Physiol. 148, 1309–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Takashima Y., Senoura T., Yoshizaki T., Hamada S., Ito H., Matsui H. (2007) Differential chain-length specificities of two isoamylase-type starch-debranching enzymes from developing seeds of kidney bean. Biosci. Biotechnol. Biochem. 71, 2308–2312 [DOI] [PubMed] [Google Scholar]
- 22. Hussain H., Mant A., Seale R., Zeeman S., Hinchliffe E., Edwards A., Hylton C., Bornemann S., Smith A. M., Martin C., Bustos R. (2003) Three isoforms of isoamylase contribute different catalytic properties for the debranching of potato glucans. Plant Cell 15, 133–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu C., Colleoni C., Myers A. M., James M. G. (2002) Enzymatic properties and regulation of ZPU1, the maize pullulanase-type starch debranching enzyme. Arch. Biochem. Biophys. 406, 21–32 [DOI] [PubMed] [Google Scholar]
- 24. Zeeman S. C., Umemoto T., Lue W. L., Au-Yeung P., Martin C., Smith A. M., Chen J. (1998) A mutant of Arabidopsis lacking a chloroplastic isoamylase accumulates both starch and phytoglycogen. Plant Cell 10, 1699–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smith A. M., Zeeman S. C. (2006) Quantification of starch in plant tissues. Nat. Protoc. 1, 1342–1345 [DOI] [PubMed] [Google Scholar]
- 26. Egli B., Kölling K., Köhler C., Zeeman S. C., Streb S. (2010) Loss of cytosolic phosphoglucomutase compromises gametophyte development in Arabidopsis. Plant Physiol. 154, 1659–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Buléon A., Colonna P., Planchot V., Ball S. (1998) Starch granules. Structure and biosynthesis. Int. J. Biol. Macromol. 23, 85–112 [DOI] [PubMed] [Google Scholar]
- 28. Stettler M., Eicke S., Mettler T., Messerli G., Hörtensteiner S., Zeeman S. C. (2009) Blocking the metabolism of starch breakdown products in Arabidopsis leaves triggers chloroplast degradation. Mol. Plant 2, 1233–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Glaring M. A., Baumann M. J., Abou Hachem M., Nakai H., Nakai N., Santelia D., Sigurskjold B. W., Zeeman S. C., Blennow A., Svensson B. (2011) Starch-binding domains in the CBM45 family–low-affinity domains from glucan, water dikinase and α-amylase involved in plastidial starch metabolism. FEBS J. 278, 1175–1185 [DOI] [PubMed] [Google Scholar]
- 30. Vester-Christensen M. B., Abou Hachem M., Svensson B., Henriksen A. (2010) Crystal structure of an essential enzyme in seed starch degradation. Barley limit dextrinase in complex with cyclodextrins. J. Mol. Biol. 403, 739–750 [DOI] [PubMed] [Google Scholar]
- 31. Machovic M., Janecek S. (2006) The evolution of putative starch-binding domains. FEBS Lett. 580, 6349–6356 [DOI] [PubMed] [Google Scholar]
- 32. Lu Y., Gehan J. P., Sharkey T. D. (2005) Daylength and circadian effects on starch degradation and maltose metabolism. Plant Physiol. 138, 2280–2291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Graf A., Schlereth A., Stitt M., Smith A. M. (2010) Circadian control of carbohydrate availability for growth in Arabidopsis plants at night. Proc. Natl. Acad. Sci. U.S.A. 107, 9458–9463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weise S. E., Schrader S. M., Kleinbeck K. R., Sharkey T. D. (2006) Carbon balance and circadian regulation of hydrolytic and phosphorolytic breakdown of transitory starch. Plant Physiol. 141, 879–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Myers A. M., Morell M. K., James M. G., Ball S. G. (2000) Recent progress toward understanding biosynthesis of the amylopectin crystal. Plant Physiol. 122, 989–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rasse D. P., Tocquin P. (2006) Leaf carbohydrate controls over Arabidopsis growth and response to elevated CO2. An experimentally based model. New Phytol. 172, 500–513 [DOI] [PubMed] [Google Scholar]
- 37. Stitt M., Zeeman S. C. (2012) Starch turnover. Pathways, regulation, and role in growth. Curr. Opin. Plant. Biol. 15, 282–292 [DOI] [PubMed] [Google Scholar]
- 38. Yazdanbakhsh N., Sulpice R., Graf A., Stitt M., Fisahn J. (2011) Circadian control of root elongation and C partitioning in Arabidopsis thaliana. Plant Cell Environ. 34, 877–894 [DOI] [PubMed] [Google Scholar]
- 39. Dinges J. R., Colleoni C., James M. G., Myers A. M. (2003) Mutational analysis of the pullulanase-type debranching enzyme of maize indicates multiple functions in starch metabolism. Plant Cell 15, 666–68012615940 [Google Scholar]
- 40. Yun M. S., Umemoto T., Kawagoe Y. (2011) Rice debranching enzyme isoamylase 3 facilitates starch metabolism and affects plastid morphogenesis. Plant Cell Physiol. 52, 1068–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]