Background: Reactivation of ERK1/2 frequently underlies acquired resistance to RAF inhibitors.
Results: NRAS mutations are acquired during resistance to RAF inhibitors and promote CRAF and SHOC2-modulated ERK1/2 pathway re-activation.
Conclusion: NRAS mutations in mutant BRAF cells alter RAF isoform and SHOC2 usage in the presence of RAF inhibitor.
Significance: These studies delineate mechanisms mediating RAF inhibitor resistance in mutant BRAF cells.
Keywords: Cell Signaling, MAP Kinases (MAPKs), Melanoma, Raf, Ras, BRAF, NRAS, PLX4032, PLX4720, SHOC2
Abstract
ERK1/2 signaling is frequently dysregulated in tumors through BRAF mutation. Targeting mutant BRAF with vemurafenib frequently elicits therapeutic responses; however, durable effects are often limited by ERK1/2 pathway reactivation via poorly defined mechanisms. We generated mutant BRAFV600E melanoma cells that exhibit resistance to PLX4720, the tool compound for vemurafenib, that co-expressed mutant (Q61K) NRAS. In these BRAFV600E/NRASQ61K co-expressing cells, re-activation of the ERK1/2 pathway during PLX4720 treatment was dependent on NRAS. Expression of mutant NRAS in parental BRAFV600 cells was sufficient to by-pass PLX4720 effects on ERK1/2 signaling, entry into S phase and susceptibility to apoptosis in a manner dependent on the RAF binding site in NRAS. ERK1/2 activation in BRAFV600E/NRASQ61K cells required CRAF only in the presence of PLX4720, indicating a switch in RAF isoform requirement. Both ERK1/2 activation and resistance to apoptosis of BRAFV600E/NRASQ61K cells in the presence of PLX4720 was modulated by SHOC-2/Sur-8 expression, a RAS-RAF scaffold protein. These data show that NRAS mutations confer resistance to RAF inhibitors in mutant BRAF cells and alter RAF isoform and scaffold molecule requirements to re-activate the ERK1/2 pathway.
Introduction
RAS activation of the extracellular signal-regulated kinase (ERK)3/mitogen-activated protein (MAP) kinase cascade plays a critical role in the proliferation and survival of normal and malignant cells (1–3). In this pathway, GTP-loading of RAS isoforms (HRAS, NRAS, KRAS) leads to the membrane recruitment of RAF serine-threonine kinases (ARAF, BRAF, CRAF). RAS binds RAF and facilitates RAF activation and initiation of a kinase cascade that signals to the dual specificity kinases, MAPK/ERK kinases 1 and 2 (MEK1/2), and subsequently to ERKs 1 and 2. The activity at each level of the cascade is fine tuned by additional layers of control. Substitute MAP3Ks, such as Cot1/TPL2, can activate MEKs in certain conditions (4). Splice variants have been identified for pathway components including BRAF (5), MEK (6), and ERK1 (7), which display altered activity and/or specificity. Negative feedback pathways including ERK1/2-dependent up-regulation of sprouty proteins and dual-specificity phosphatases serve to dampen pathway output at various levels (8–11). Additionally, scaffold molecules interact with pathway components and co-ordinate their activation at distinct subcellular locales. Among these scaffolds is Soc-2 (suppressor of clear) homolog, SHOC2/Sur8. This leucine-rich repeat protein was originally identified in Caenorhabditis elegans-based screens for regulators of growth factor receptor-RAS signaling (12, 13). In mammalian cells, SHOC2 promotes RAS-RAF association and positively enhances signaling through the ERK1/2 pathway (14).
Signaling downstream of mutant BRAF represents one context in which it is critical to understand the complex interactions in the ERK1/2 pathway. Approximately, 8% of human tumors harbor a BRAF mutation, the majority of which are phosphomimetic V600E substitutions within the activation loop of BRAF (15). High frequencies of BRAF mutations have been identified in melanomas, thyroid carcinomas, and colorectal tumors (15). BRAFV600E signals as a monomer and in a RAS-independent manner to constitutively activate MEK (16). Small molecule inhibitors, such as vemurafenib (PLX4032) and its tool analog, PLX4720, have been developed. Despite being pan RAF inhibitors in vitro, vemurafenib selectively inhibits BRAFV600E signaling in cells (17–19). The majority of late stage, mutant BRAF melanoma patients treated with vemurafenib display short-term tumor shrinkage (18, 20, 21). Furthermore, vemurafenib gives improved overall survival and progression-free survival compared with standard chemotherapy (22). Unfortunately, the long-term effects of vemurafenib are frequently hampered by resistance mechanisms (23–25).
While multiple modes of resistance to vemurafenib are likely to occur, one over-arching mechanism involves re-activation of the ERK1/2 pathway (25). Mutations in NRAS and BRAF are usually mutually exclusive; however, attention has focused on mutations in NRAS in the resistance to vemurafenib. This is largely based on the paradoxical effects of RAF inhibitors in cells with mutant or activated RAS (19, 26–28). Mutations in NRAS were recently identified in two tumor re-growths in one mutant BRAF melanoma patient (23) and four out of nineteen patients (29) following progression on vemurafenib. In the former study, NRASQ61R and NRASQ61K mutations were associated with ERK1/2 pathway re-activation (23). Despite this knowledge, the mechanism whereby mutant NRAS prevents vemurafenib from inhibiting MEK-ERK1/2 signaling in cells expressing mutant BRAF remains unknown. Here, we isolated mutant BRAF melanoma cell lines with secondary resistance to PLX4720 (the tool compound for vemurafenib). A subset of the resistant cells co-expressed NRASQ61K and BRAFV600E. In parental cells, expression of NRASQ61K was sufficient to provide resistance to PLX4720 in a manner dependent on the RAF binding domain. ERK1/2 reactivation in resistant cells was dependent on NRAS and regulated by both BRAF and CRAF. Furthermore, ERK1/2 reactivation in the presence of PLX4720 was reduced following depletion of the RAS-RAF scaffold molecule, SHOC2. SHOC2 was required, at least partially, for the survival of BRAFV600E/NRASQ61K cells in the presence of PLX4720. These data provide novel insight into a mechanism of ERK1/2 pathway re-activation in RAF inhibitor-resistant cells.
EXPERIMENTAL PROCEDURES
Inhibitors
PLX4720 was kindly provided by Dr. Gideon Bollag and Plexxikon Inc. (Berkeley, CA). AZD6244 was purchased from Selleck Chemicals LLC (Houston, TX).
Cloning and Stable Cell Line Generation
Wild-type and mutant NRAS were cloned from cDNA using the following primers: forward CACCATGACTGAGTACAAACTGGTGGTG and reverse TTACATCACCACACATGGCAATCCC. NRASQ61K, T35S and NRASQ61K, E37G were constructed following the QuikChange protocol (Stratagene, La Jolla, CA). NRASQ61K was the template for the following primers: T35S forward GTAGATGAATATGATCCCTCCATAGAGGATTCTTACAGA and T35S reverse TCTGTAAGAATCCTCTATGGAGGGATCATATTCATCTAC and E37G forward GAATATGATCCCACCATAGGGGATTCTTACAGAAACAA and E37 reverse TTGTTTTCTGTAAGAATCCCCATGGTGGGATCATATTC. All DNA constructs were sequence verified. Lentiviral particles and stable cell lines were made as previously described (30). Transgene expression was induced with 0.1 μg/ml doxycycline.
Cell Culture
Melanoma cells were cultured, as previously described (31). WM793, WM115, WM1366, Sbcl2, and 1205Lu were cultured in MCDB 153 containing 20% Leibovitz L-15 medium, 2% FBS, 5 μg/ml insulin and penicillin/streptomycin. A375 cells were cultured in DMEM with 10% FBS.
Generation of Resistant Cell Lines
WM793 cells were cultured in the aforementioned MCDB 153 medium in the presence of 5 μm PLX4720 for 4 weeks. Medium containing PLX4720 was replenished every 2 days. Resistant clonal lines were generated by seeding cells at low density and allowing isolated colonies to form. These colonies were picked, and cells expanded in the continued presence of PLX4720.
siRNA Transfections
WM793 cells were transfected with siRNAs using Lipofectamine RNAiMAX (Invitrogen, Carlsbad, MA). Non-targeting control, B-RAF #1, and C-RAF sequences were as previously described (26). NRAS (CACCAUAGAGGAUUCUUAC) and SHOC2 (#1: GACCUUAGCUAGAAAUUGC; #2: GAAAUUGGUACACUGGAGA; #3: GAGGUAGUAUAGUUAGAUA) siRNAs were purchased from Dharmacon (Lafayette, CO). Cells were transfected for 72 h with a final siRNA concentration of 25 nm before subsequent treatment or analysis.
Western Blotting
Western blotting was performed, as previously described (26). Immunoreactivity was detected using peroxidase-conjugated secondary antibodies and chemiluminescence substrate (Pierce). Chemiluminescence was detected using a Versadoc Imaging system (Bio-Rad). The following antibodies were purchased from Cell Signaling Technology, (Beverley, MA): phospho-ERK1/2 (Thr-202/Tyr-204, #4377), MEK1 (#9124), phospho-MEK1 (#9121), phospho-Akt (Thr-308, #2965) phospho-Akt (Ser-473, #6942) total Akt (#2965), and total FAK (BD Biosciences, San Jose, CA). B-RAF (sc-5284), C-RAF/RAF-1 (sc-133), ERK1/2 (sc-094), ERK2 (sc-1647), and NRAS (sc-31) antibodies were purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). Shoc2/Sur8 (ab32982) antibody was purchased from Abcam (Cambridge, MA). Phospho-FAK-S910 was purchased from BIOSOURCE Int. (Camarillo, CA).
Three-dimensional (3-D) Collagen Gels and Apoptosis Assays
Collagen gels were cast by mixing the following on ice: Eagle's Minimum Essential Medium (Lonza, Inc. Walkersville, MD), 2 mm l-glutamine, 2% FBS, 0.15% sodium bicarbonate, and 0.8 mg/ml bovine type-I collagen (Organogenesis Inc., Canton, MA). Cells were seeded in 2 ml collagen gels and incubated at 37 °C for 30 min. After polymerization, the collagen gel lattice was overlaid with 2 ml of medium at 37 °C for 48 h. 3-D collagen gels were dissolved in 1 mg/ml collagenase (Sigma-Aldrich) solution to release cells. Cells were resuspended in 100 μl of binding buffer (10 mm HEPES, 0.14 m sodium chloride, 2.5 mm calcium chloride), stained with 5 μl of Annexin V-APC (BD Biosciences) for 15 min and finally an additional 400 μl of binding buffer was added. Apoptosis was analyzed by flow cytometry on the FACSCalibur (BD Biosciences). Data were analyzed using Flowjo software (Three Star, Inc. Ashland, OR).
EdU (5-Ethynyl-2′-deoxyuridine) Incorporation Assays
Parental WM793 cells and WM793-Res NRAS cells were cultured overnight in the absence of PLX4720. Cells were treated with DMSO or PLX4720 for 48 h before the addition of 10 μm EdU for another 16 h. Cells were then processed using the Click-iTTM EdU Alexa Fluor 647 Flow Cytometry Assay kit (Invitrogen, Carlsbad, CA) for flow cytometry analysis.
Soft Agar Assays
Cells (3 × 103 cells/ml) were grown in 0.3% soft agar, as previously described (30). Cells were grown for 14 days, replacing the medium every 3 days. Five random fields per chamber were acquired using NIS-Elements software from Nikon.
Statistical Analysis
Statistical analysis of the data was performed using an unpaired Student's t test assuming unequal variance.
RESULTS
Acquired Resistance of Mutant BRAFV600E Melanoma Cells to PLX4720 Is Associated with Mutational Activation of NRAS
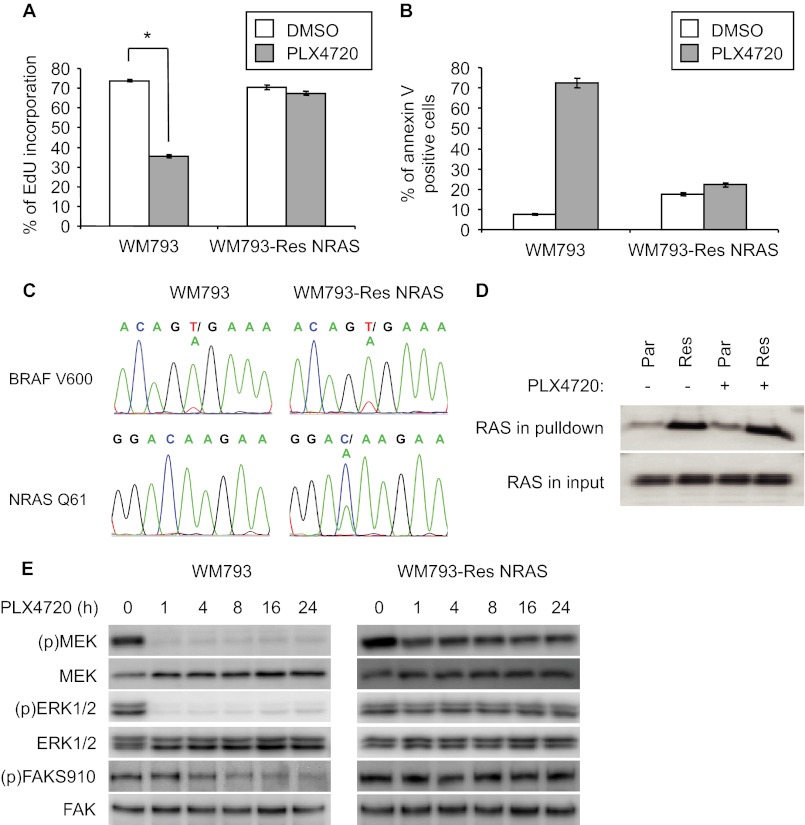
To examine acquisition of resistance to RAF inhibitors, we treated mutant BRAFV600E harboring WM793 melanoma cells with PLX4720 for 4 weeks at which time resistant cells grew out. PLX4720 is the tool compound for vemurafenib and elicits comparable actions to its clinical grade counterpart (19). A sub-population of the resistant cells displayed a distinctive compact morphology. These resistant cells (termed WM793-Res NRAS, vide infra) displayed comparable incorporation of EdU, a thymidine analog, in the presence and absence of PLX4720 (Fig. 1A). Also, when embedded in 3-D type I collagen, no significant increase in annexin V staining was observed when treated with PLX4720 (Fig. 1B). As expected, parental WM793 cells treated with PLX4720 displayed a significant reduction of EdU incorporation and increased annexin V staining (Fig. 1, A and B). DNA sequencing of RAS-RAF pathway genes revealed a C to A mutation in NRAS, which leads to a glutamine to lysine substitution at position 61 (NRASQ61K), in the resistant cells (Fig. 1C). No NRASQ61K mutation was detected in parental cells. The mutational status of BRAF was unchanged in WM793-Res NRAS cells (Fig. 1C). Consistent with the known effect of the Q61K mutation of reducing the rate of intrinsic GTP hydrolysis in RAS, WM793-Res NRAS cells displayed increased RAS activity compared with WM793 cells in both the presence and absence of PLX4720 (Fig. 1D). Furthermore, whereas PLX4720 treatment of parental WM793 cells resulted in inhibition of MEK1/2 and ERK1/2 phosphorylation throughout a 24 h time course, the phosphorylation of MEK and ERK1/2 remained elevated in WM793-Res NRAS cells (Fig. 1E). Comparable findings were observed upon analysis of serine 910 phosphorylation of focal adhesion kinase (FAK), a direct ERK1/2 phosphorylation site (32). Together, these data indicate that mutation of NRAS is associated with MEK-ERK1/2 reactivation and acquired resistance to PLX4720.
FIGURE 1.
Mutation in NRAS associated with acquired resistance to RAF inhibitor in mutant BRAF cells. A, WM793 and WM793-Res NRAS cells were cultured overnight in the absence of PLX4720 and then treated with DMSO and 1 μm PLX4720 for 48 h. Finally, EdU incorporation was performed for 16 h in the presence/absence of PLX4720. Assays were performed in triplicate. Error bars, S.D. *, p < 0.05. B, WM793 and WM793-Res cells were cultured overnight in the absence of PLX4720, embedded in 3-D collagen and then treated with either DMSO or 1 μm PLX4720 for 48 h. Apoptosis was quantitated using annexin V staining. Assays were performed in triplicate. Error bars, S.D. C, sequencing of WM793-Res pooled cells for BRAF and NRAS. D, WM793 parental and WM793-Res NRAS cells were incubated in the absence of PLX4720 overnight and then in the absence/presence of 5 μm PLX4720 for an additional 24 h. RAS pull-down assays using GST-RAF-RBD (RAS binding domain) were performed. Shown are Western blot data for NRAS from the input and pulldown samples. E, WM793 and WM793-Res NRAS cells were seeded overnight in the absence of PLX4720 and then treated with 1 μm PLX4720 for times ranging from 0 to 24 h. Samples were analyzed by Western blotting for phospho-MEK, total MEK, phospho-ERK1/2, total ERK1/2, phospho-S910 FAK, and total FAK.
NRAS Is Required for Resistance to PLX4720
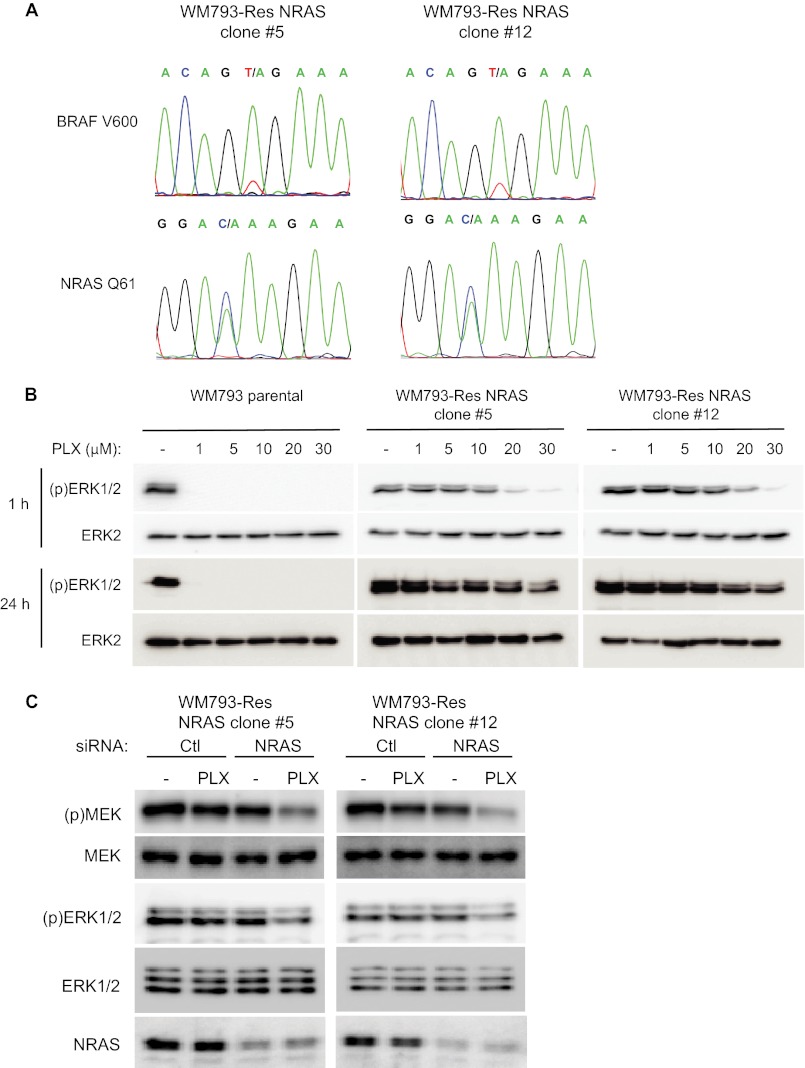
To further analyze the role of NRAS, we isolated clonal cell lines that were heterozygous for the mutant NRAS allele (Fig. 2A). Out of 13 clones isolated, 8 (61.5%) harbored NRAS mutations and 5 (38.5%) were wild-type for NRAS. Two representative clones (5 and 12) were utilized for subsequent experiments. WM793-Res NRAS clonal cells maintained high levels of phospho-ERK1/2 over a range of PLX4720 concentrations at both 1 and 24 h treatment time points (Fig. 2B). We did observe inhibition of phospho-ERK1/2 in WM793-Res NRAS cells at high doses (30 μm) of PLX4720 after 1 h but the activation rebounded by 24 h.
FIGURE 2.
Activation of MEK-ERK1/2 in resistant clones is dependent on NRAS in the presence of RAF inhibitor. A, sequencing of WM793-Res NRAS clones #5 and #12 for BRAF exon 15 and NRAS exon 2. B, Parental WM793, WM793Res NRAS clone #5, and WM793Res NRAS clone #12 were seeded overnight in the absence of PLX4720 and then treated with 0, 1, 5, 10, 20, 30 μm PLX4720 for 1 h or 24 h, as indicated. Cell lysates were analyzed by Western blotting for phospho-ERK1/2 and total ERK2. C, WM793-Res NRAS cells (clones #5 and #12) were transfected with control (Ctl) or NRAS siRNA. Post-transfection, cells were treated with either DMSO (−) or 5 μm PLX4720 for 72 h. Inhibitor was replenished after 48 h. Lysates were analyzed by Western blotting for phospho-MEK, total MEK, phospho-ERK1/2, total ERK1/2, and NRAS.
Next, we performed RNA interference experiments to determine the requirement for NRAS in the ability of resistant cells to by-pass the inhibitory effects of PLX4720. For these experiments, we utilized prolonged exposure to PLX4720 at the concentration (5 μm) at which the drug resistance was generated. siRNA-mediated knockdown of NRAS in WM793-Res NRAS#5 and #12 cells had only a minor effect on the basal levels of MEK and ERK1/2 phosphorylation in the absence of PLX4720 (Fig. 2C). By contrast, PLX4720 treatment of NRAS knockdown WM793-Res NRAS cells effectively inhibited MEK and ERK1/2 phosphorylation (Fig. 2C). These findings show that NRAS is required for continued ERK1/2 activation in BRAFV600E/NRASQ61K co-expressing cells in the presence of PLX4720.
Mutant NRAS Is Sufficient Prevent PLX4720 Inhibition of the ERK1/2 Pathway in Mutant BRAF Cells
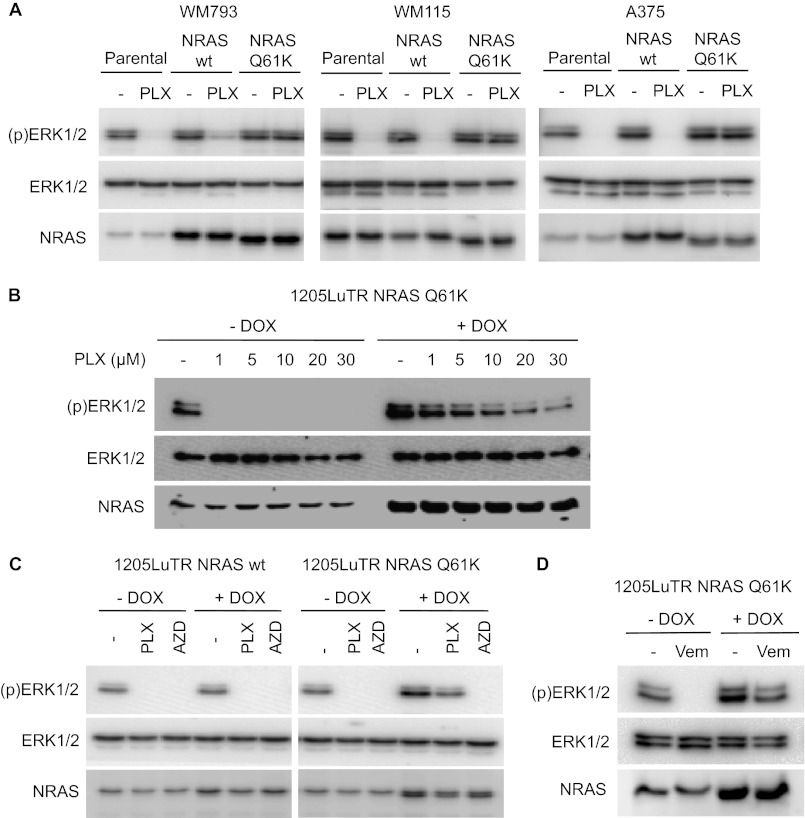
We next tested whether NRASQ61K was sufficient to confer resistance to PLX4720 inactivation of ERK1/2. WM793, A375 (both BRAFV600E), and WM115 (BRAFV600D) cells were engineered to co-express either wild-type or Q61K NRAS. The concentration of 1 μm PLX4720 was utilized for these experiments since this dose was effective at inhibiting ERK1/2 signaling in parental WM793 but not resistant cells (Fig. 2B). Parental and wild-type NRAS-expressing WM793, WM115 and A375 cells showed a marked reduction in ERK1/2 phosphorylation with PLX4720, whereas ectopic NRASQ61K-expressing versions of all three lines did not show down-regulation of ERK1/2 phosphorylation (Fig. 3A). The effects of NRAS occur regardless of the retention of one wild-type BRAF allele, since A375 cells are homozygous for BRAFV600E (supplemental Fig. S1A). Because various KRAS mutants confer differing responses to chemotherapies (33) and multiple Q61 substitutions occur in melanoma, we also tested whether expression of other Q61 NRAS mutants could also promote resistance to PLX4720. Expression of Q61H, Q61R, and Q61L NRAS mutations in WM793 cells all resulted in resistance to PLX4720-induced ERK1/2 inactivation (supplemental Fig. S1B). Comparable results were seen in A375 cells when NRASQ61H and NRASQ61R mutations were expressed (supplemental Fig. S1C); NRASQ61L was not analyzed in this line.
FIGURE 3.
NRASQ61K expression is sufficient for resistance to PLX4720/vemurafenib inhibition of ERK1/2. A, WM793, WM115, and A375 parental cells and lines constitutively expressing NRAS wild-type (wt) and NRASQ61K were treated with DMSO or 1 μm PLX4720 for 1 h. Western blots were performed for phospho and total ERK1/2. B, 1205LuTR-Lu NRASQ61K cells were cultured with 100 ng/ml doxycycline (+DOX) for 16 h to induce ectopic NRAS expression and then treated with DMSO or a range of concentrations of PLX4720 for 1 h. Western blot analysis was performed on the lysates with the indicated antibodies. C, inducible 1205LuTR-Lu NRAS wild-type and NRASQ61K cells were treated −/+ DOX for 16 h and then with DMSO, 5 μm PLX4720 or 3.3 μm AZD for 16 h. Lysates were subjected to Western blot analysis. D, inducible 1205LuTR-Lu NRASQ61K cells were treated −/+ DOX for 16 h to induce NRAS expression. Cells were then treated with DMSO or 1 μm vemurafenib for 1 h and subjected to Western blot analysis.
To rule out the possibility of adaption to constitutive expression of mutant NRAS, we generated isogenic, doxycycline-inducible lines for short-term expression of wild-type or Q61K NRAS in BRAFV600E 1205Lu cells. PLX4720 inhibition of ERK1/2 was prevented by inducible expression of NRASQ61K over a range of PLX4720 concentrations (Fig. 3B) and after prolonged drug treatment (Fig. 3C). Similarly, induction of NRASQ61K but not wild-type NRAS prevented ERK1/2 inactivation by vemurafenib, the clinical grade inhibitor (Fig. 3D). By contrast the MEK inhibitor, AZD6244, inhibited ERK1/2 phosphorylation in both NRASQ61K- and NRASWT-induced cells (Fig. 3C). In summary, expression of mutant NRAS in BRAFV600E/D cells counteracts PLX4720 inhibition of the MEK-ERK1/2 pathway.
Mutant NRAS Prevents PLX4720 Inhibition of S Phase Entry and Induction of Apoptosis in Mutant BRAF Cells
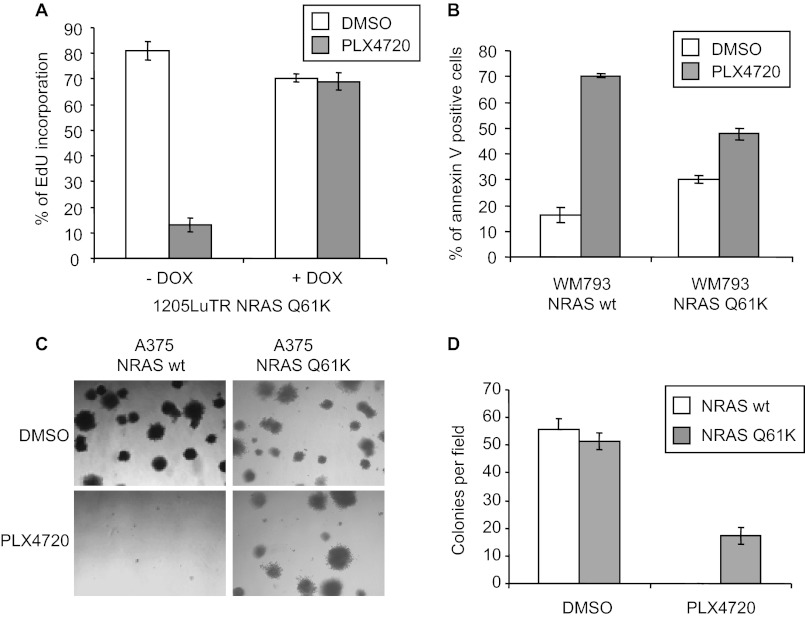
We extended the analysis of ectopic expression of mutant NRAS in BRAFV600E melanoma cells to cell cycle progression and cell survival. First, we analyzed effects on S phase entry in inducible 1205LuTR NRASQ61K cells. In the absence of RAS induction, treatment of 1205LuTR NRASQ61K cells with PLX4720 significantly inhibited the incorporation of EdU compared with vehicle treatment (Fig. 4A). Induction of NRASQ61K expression prevented PLX4720 inhibition of S-phase entry (Fig. 4A). To determine effects on cell survival, we utilized WM793 cells, which are susceptible to PLX4720-induced apoptosis in 3-D type I collagen (34). Compared with expression of wild-type NRAS, WM793 cells ectopically expressing NRASQ61K were more resistant to PLX4720-induced apoptosis (Fig. 4B). To determine if mutant NRAS expression alters PLX4720 inhibition of malignant properties of melanoma cells, soft agar assays were performed using A375 cells, which readily form colonies in these assays (30). In the absence of PLX4720, both wild-type and mutant NRAS expressing A375 cells formed similar numbers of colonies (Fig. 4C and quantitated in 4D). In the presence of PLX4720, only A375 cells expressing mutant NRAS formed colonies, albeit at lower numbers than without PLX4720 treatment (Fig. 4, C and D). Collectively, these data suggest that expression of mutant NRAS promotes malignant properties of mutant BRAFV600E melanoma cells treated with PLX4720.
FIGURE 4.
Expression of NRASQ61K promotes proliferation and inhibits apoptosis in the presence of PLX4720. A, 1205Lu-TR NRASQ61K cells were cultured + DOX (100 ng/ml) for 16 h. Cells were then treated with 1 μm PLX4720 or DMSO for 48 h. EdU was added and analysis was performed after 16 h. Assays were performed in triplicate. Error bars, standard deviation. B, WM793 and WM115 cells expressing NRAS wild-type and NRASQ61K cells were embedded into 3-D collagen and treated with either DMSO or 1 μm PLX4720 for 48 h. Assays were performed in triplicate and apoptosis was quantitated using annexin V staining. Error bars, S.D. C, A375 cells expressing wild-type NRAS or NRASQ61K were embedded into soft agar and treated with either DMSO or 1 μm PLX4720. Representative pictures were taken after 2 weeks. D, five independent fields were used to determine the average number of colonies per field. Error bars, S.D.
NRAS-RAF Association Correlates with Resistance to PLX4720
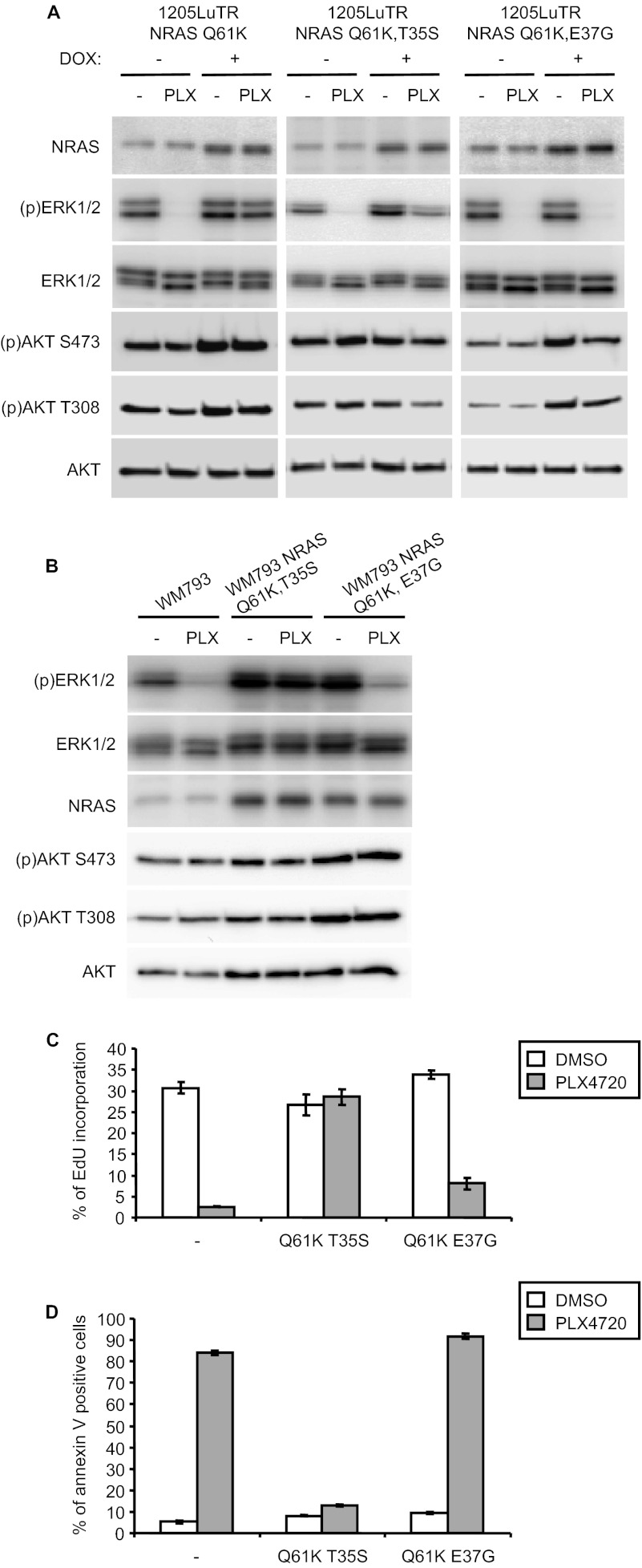
We next determined whether maintenance of the NRAS-RAF interaction was associated with resistance to PLX4720 by initially utilizing NRAS effector domain mutants. We generated doxycycline inducible NRASQ61K,T35S and NRASQ61K, E37G expression systems in the mutant BRAF cell line, 1205Lu. RASQ61K,T35S interacts with RAF but not PI-3 kinase or Ral-GDS; whereas RASQ61K,E37G interacts with PI-3 kinase and Ral-GDS but not RAF (14, 35). In the absence of exogenous NRAS expression, ERK1/2 phosphorylation was inhibited by PLX4720 in 1205LuTR NRASQ61K,T35S and 1205LuTR NRASQ61K,E37G cell lines (Fig. 5A). Following transgene induction, NRASQ61K,T35S led to a partial block of PLX4720-induced ERK1/2 inactivation, whereas NRASQ61K,E37G did not prevent PLX4720 inhibition of phospho-ERK1/2 (Fig. 5A). NRASQ61K,E37G induction promoted Akt activity demonstrating biological activity of this effector domain mutant in this system (Fig. 5A) Similarly, constitutive expression of NRASQ61K,T35S but not NRASQ61K,E37G in WM793 prevented PLX4720 inhibition of phospho-ERK1/2 (Fig. 5B). In this system, NRASQ61K,T35S completely prevented the inhibitory effects of PLX4720. These data suggest that NRASQ61K-mediated resistance to PLX4720 is associated with the RAF binding site.
FIGURE 5.
NRASQ61K-mediated resistance is associated with RAF binding. A, 1205LuTR-Lu NRASQ61K, NRASQ61K,T35S, and NRASQ61K,E37G cells were cultured +DOX for 16 h to induce NRAS expression. Cells were then treated with 1 μm PLX4720 or DMSO (−) for 1 h and cell lysates subjected to Western blot analysis for the indicated proteins. B, Same as A, except that WM793 cells constitutively expressing NRASQ61K effector domain mutants were utilized. C, 1205Lu cells (parental, expressing NRASQ61K T35S, or expressing NRASQ61K E37G) were analyzed for EdU incorporation as in Fig. 1A. D, WM793 cells (parental, expressing NRASQ61K T35S, or expressing NRASQ61K E37G) were analyzed for annexin V staining in 3-D collagen as in Fig. 1B.
To test if association with RAF is required for mutant N-RAS to promote S-phase entry in the presence of PLX4720, EdU incorporation assays were performed on 1205LuTR cells expressing NRASQ61K effector domain mutations. In the presence of PLX4720, NRASQ61K,T35S promoted S-phase progression, whereas NRASQ61K,E37G was unable to promote S-phase progression in the presence of PLX4720 (Fig. 5C). We also examined resistance to apoptosis in the presence of PLX4720 using WM793 cells embedded in 3-D type-I collagen. WM793 NRASQ61K,E37G-expressing cells were susceptible to PLX4720-induced increases in annexin V staining, whereas N-RASQ61K,T35S-expressing cells displayed only a slight increase in annexin V in the presence of PLX4720 (Fig. 5D).
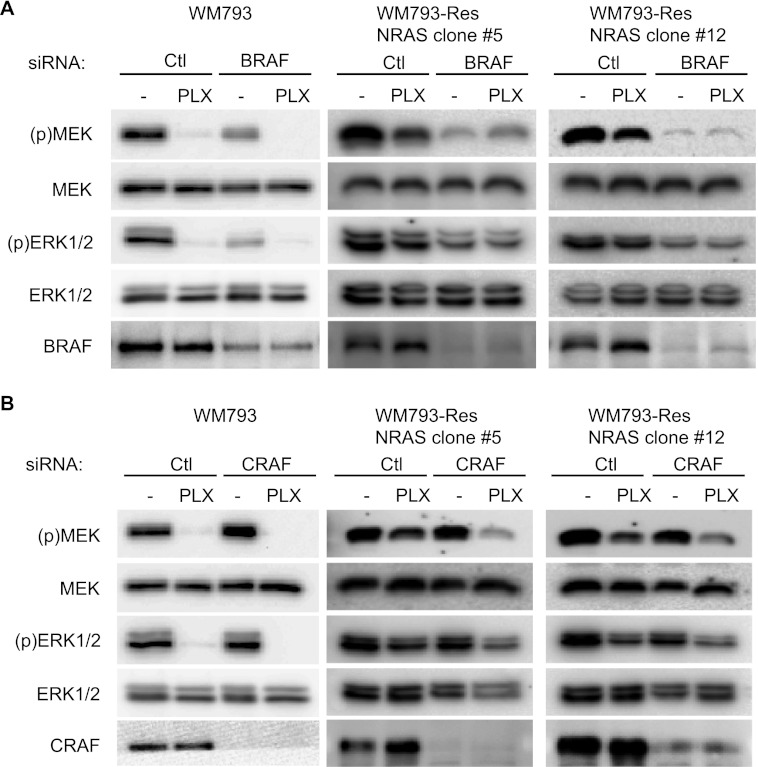
Consistent with prior studies, activation of the MEK-ERK1/2 pathway in parental WM793 cells requires BRAF but not CRAF (Fig. 6, A and B) (36). We next determined the involvement of RAF isoforms in MEK-ERK1/2 signaling in WM793-Res NRAS clonal cells. Compared with the control siRNA knockdowns, BRAF depletion decreased MEK and ERK1/2 phosphorylation in the absence and presence of PLX4720 in both clone 5 and clone 12 (Fig. 6A). By contrast, depletion of CRAF did not affect basal levels of phospho-MEK and phospho-ERK1/2 in the absence of PLX4720 but did inhibit MEK and ERK1/2 phosphorylation following PLX4720 treatment compared with control knockdowns (Fig. 6B). Together, these data indicate that WM793-Res NRAS cells primarily signal to MEK-ERK1/2 through BRAF in the absence of PLX4720 but utilize both CRAF and BRAF in the presence of PLX4720.
FIGURE 6.
ERK1/2 activity in WM793-Res NRAS cells is regulated by both BRAF and CRAF. A, parental WM793 and WM793-Res NRAS cells (clones #5 and #12) were transfected with control (Ctl) or BRAF siRNA. Post-transfection, cells were treated with either DMSO (−) or 5 μm PLX4720 for 72 h with drug replenished for the last 24 h. Lysates were analyzed by Western blotting for phospho-MEK, total MEK, phospho-ERK1/2, total ERK1/2, and BRAF. B, as for A, except that cells were transfected with CRAF siRNAs and analyzed for CRAF expression.
Depletion of SHOC2 Enables PLX4720-induced Inhibition of ERK1/2 and Apoptosis in PLX4720-resistant Cells
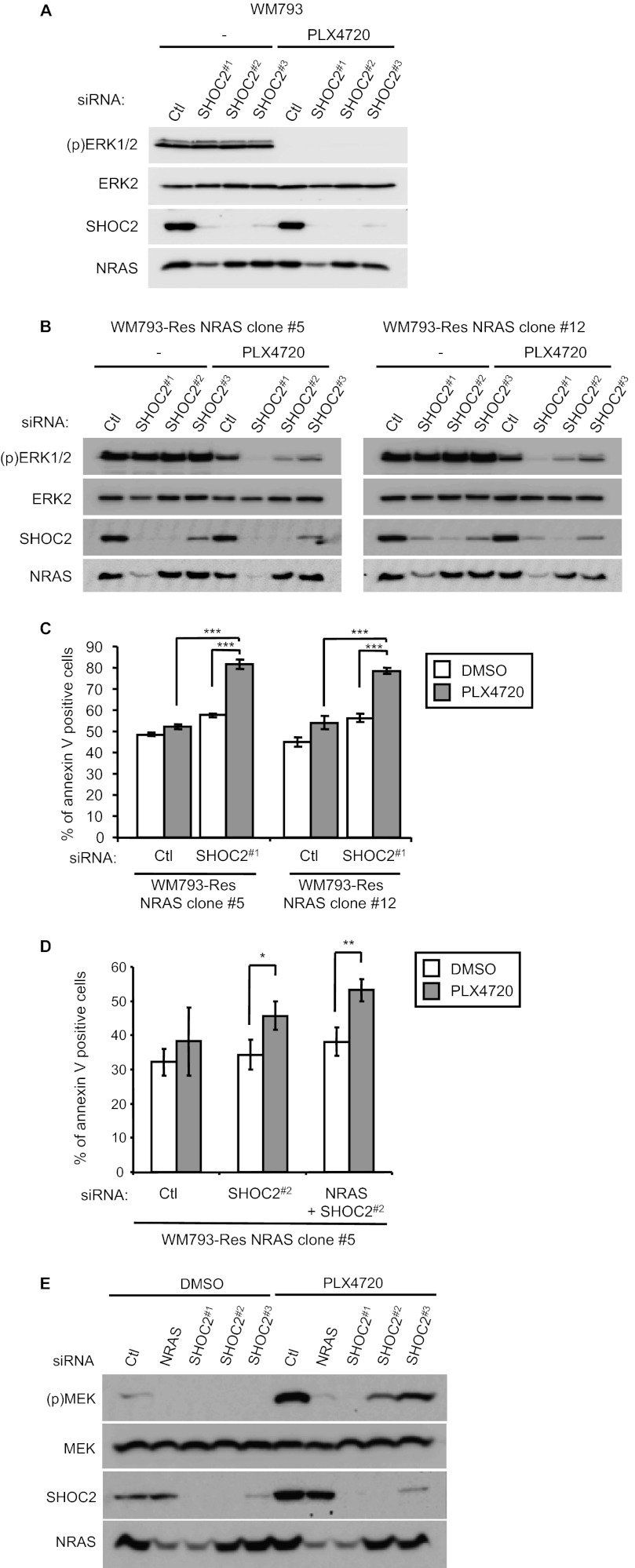
Scaffold proteins influence RAS-RAF signaling but how these molecules impact on the effect of RAF inhibitors remains poorly understood. We examined the role of the scaffold molecule, SHOC2/Sur8, which has been shown to bind to multiple RAS isoforms (13, 14, 37) and positively enhance growth factor and mutant RAS-induced signaling through the ERK1/2 pathway by promoting RAS-RAF association (14). SHOC2 depletion with multiple siRNAs did not alter levels of phosphorylated ERK1/2 in parental WM793 cells (Fig. 7A). In WM793-Res NRAS clones, basal ERK1/2 phosphorylation was not dramatically altered by knockdown of SHOC2 in the absence of PLX4720 but SHOC2 depletion did enhance the inhibition of ERK1/2 phosphorylation in PLX4720-treated WM793-Res NRAS clones (Fig. 7B). Effects were observed with multiple independent SHOC2 siRNAs (Fig. 7B) indicating that mutant NRAS signaling to MEK-ERK1/2 in PLX4720-resistant cells is partially dependent on SHOC2.
FIGURE 7.
The role of NRAS and SHOC2 in ERK1/2 activation and resistance to apoptosis in WM793-Res NRAS cells. A, parental WM793 cells were transfected with control (Ctl) or SHOC2 (sequences #1, #2, and #3) siRNAs. Cells were treated with either DMSO (−) or 5 μm PLX4720 for 1 h. Lysates were analyzed by Western blotting for phospho-ERK1/2, total ERK2, SHOC2 and NRAS. B, as for A, except that WM793-Res NRAS cells (clones #5 and #12) were analyzed. C, WM793-Res NRAS cells (clone #5 and #12) were transfected with siRNA for control and SHOC2 (sequence #1). Post-transfection, cells were treated with DMSO and 1 μm PLX4720 for 48 h and annexin V analysis was performed. Assays were performed in triplicate. Error bars, S.D. ***, p < 0.001. D, same as C, except that WM793-Res NRAS clone #5 cells were transfected SHOC2 siRNA#2 and NRAS siRNA. Assays were performed in triplicate and apoptosis was quantitated using annexin V staining. Error bars, S.D. *, p < 0.05, **, p < 0.01. E, mutant NRAS melanoma cells, WM1366, were transfected with Ctl, NRAS, or SHOC2 (sequences #1, #2, #3) siRNA. Post-transfection, cells were treated with either DMSO (−) or 1 μm PLX4720 for 72 h (inhibitor was replenished for the last 24 h). Lysates were analyzed by Western blotting, as indicated.
We next determined the requirement of SHOC2 on resistance to apoptosis induced by PLX4720. Control or SHOC2 knockdown WM793-Res NRAS cells were embedded in 3-D type I collagen in the presence of DMSO and PLX4720 for 48 h before annexin V analysis. Compared with control, SHOC2 knockdown did not lead to increased apoptosis in either clone 5 or clone 12 cells (Fig. 7C). However, the combination of SHOC2 knockdown and PLX4720 treatment resulted in a statistical increase in apoptosis (Fig. 7C). Since the levels of NRAS were altered with SHOC2 siRNA #1 (Fig. 7, A and B), we performed similar experiments with a second SHOC2 siRNA (#2) which did not affect NRAS levels. SHOC2 knockdown with siRNA #2 caused a small but consistent enhancement of apoptosis in WM793-Res NRAS cells in the presence of PLX4720 (Fig. 7D). This effect was further enhanced with NRAS co-knockdown. Together, these data indicate that NRAS is required for MEK-ERK1/2 signaling and resistance to RAF inhibitor-induced apoptosis in co-mutant BRAFV600E/NRASQ61K cells and that NRAS effects are likely mediated in part by SHOC2.
Because paradoxical signaling is induced by RAF inhibitors in cells with elevated RAS activity (19, 26–28, 38), we determined the involvement of SHOC2 in the paradoxical activation of MEK-ERK1/2 in mutant NRAS cells. As we have previously shown (26), treatment with PLX4720 enhanced phospho-MEK levels in mutant NRAS-harboring melanoma lines, WM1366 (Fig. 7E) and Sbcl2 (supplemental Fig. S2, A and B). Knockdown of NRAS or SHOC2 in these lines reduced both basal levels and PLX4720-induced phospho-MEK and phospho-ERK1/2 levels. Similar effects were observed by depleting SHOC2 with multiple independent siRNAs. Thus, MEK-ERK1/2 signaling in PLX4720-treated WM793-Res NRAS cells and parental mutant NRAS cells is regulated by SHOC2.
DISCUSSION
The RAF-MEK-ERK1/2 pathway is aberrantly activated in many human tumor types. In particular, the high frequency of activating BRAF mutations in melanomas, thyroid carcinomas, and colorectal tumors has focused attention on this serine-threonine kinase as a therapeutic target (15). Strong clinical efficacy has been shown with vemurafenib (PLX4032) in mutant BRAF melanoma patients leading to its FDA-approval (20–22). However, many tumors eventually progress, a process that is frequently associated with ERK1/2 re-activation (reviewed in Ref. 25). It is important to elucidate the intricate wiring of the ERK1/2 signaling pathway to understand the response to targeted therapies and, thus, the clinical use of RAF inhibitors.
From a screen for acquisition of resistance to PLX4720 (the tool compound for vemurafenib), we isolated cell lines co-expressing BRAFV600E and NRASQ61K. The latter alteration activates NRAS by reducing the rate of intrinsic GTP hydrolysis. This cell-based finding is consistent with initial findings from melanoma patients with acquired resistance to vemurafenib. Nazarian et al. described one patient (patient 55) with an isolated, left groin metastasis that initially shrank with vemurafenib treatment but subsequently re-grew (23). A Q61K NRAS mutation was detected in the re-growing tumor. The same patient developed additional nodal metastases, one of which was associated with a Q61R NRAS mutation. More recently, NRAS mutations were identified in 4 out of 19 samples from patients progressing on vemurafenib (29). The exact frequency of acquired NRAS mutations is currently being analyzed in larger patient cohorts at multiple centers. Nonetheless, these data underscore the patient relevance of mutations in NRAS associated with resistance to PLX4032.
How NRASQ61K mediates resistance to RAF inhibitors remains unclear. PLX4720 inhibits cell cycle progression and enhances cell apoptosis in mutant BRAF melanoma cells (17, 34). Co-expression of NRASQ61K negates the inhibitory effects of PLX4720 on entry into S phase and PLX4720-initiated apoptosis in mutant BRAF melanoma lines. These effects are associated with the inability of PLX4720 (and vemurafenib) to inhibit MEK and ERK1/2 activation. Recent studies on KRAS signaling indicate that distinct activating mutations and even amino acid substitutions may mediate differential signaling and response to chemotherapeutics (33). Multiple NRAS Q61 substitutions have been identified in melanoma. Our studies herein show that Q61H, Q61R, and Q61L substitutions in NRAS were all sufficient and equivalent in their ability to promote ERK1/2 reactivation. Thus, multiple mutations in NRAS at codon 61 are able to change the response of mutant BRAF harboring cells to RAF inhibitors.
We further investigated the mechanism underlying mutant NRAS-mediated resistance. Effector domain studies show a requirement for the RAF binding site in mutant NRAS in the bypass of PLX4720 inhibitory effects. Our knockdown data suggest that PLX4720 causes a switching of the requirement for RAF isoforms in resistant cells harboring NRASQ61K. In the absence of PLX4720, signaling to MEK occurs via BRAF and is independent of CRAF. This BRAF dependence is similar to that observed in the parental cells (39, 40). By contrast, in NRASQ61K resistant cells treated with PLX4720, activation of MEK-ERK1/2 require both BRAF and CRAF. The altered requirement of RAF isoforms upon expression of mutant NRAS is consistent with published data showing that mutant, active HRAS induces heterodimerization of BRAF with CRAF (41). This condition is also similar to the mechanism underlying paradoxical activation of the ERK1/2 in which RAF inhibitors hyperactivate the pathway in cells with elevated RAS activity via drug inactivated BRAF binding to and trans-activating CRAF (26, 28, 38). Knockdown of CRAF alone was not sufficient to inhibit entry into S phase in the WM793-Res NRAS cells since the requirement is partial (data not shown). This is likely due to flexible switching between all RAF isoforms during resistance to RAF inhibitors (42).
Signaling through the ERK1/2 pathway is fine-tuned by scaffold proteins; however, the extent that scaffolding regulates the response to targeted therapies is poorly described. We investigated the RAS-RAF binding protein, SHOC2. Although reports on SHOC2 have yielded differing conclusions regarding the RAS isoforms to which SHOC2 binds (13, 14, 37), they agree that SHOC2 positively enhances growth factor and mutant RAS-induced signaling through the MEK-ERK1/2 pathway by promoting RAS-RAF association. We show that SHOC2 is not required for activation of ERK1/2 when signaling through mutant BRAF is dominant, i.e. parental mutant BRAF melanoma cells or resistant mutant NRAS-expressing cells in the absence of PLX4720. By contrast, our initial data suggest that SHOC2 modulates ERK1/2 activation when a CRAF-dependent mechanism is dominant such as in resistant BRAFV600E/NRASQ61K treated with PLX4720 or parental mutant NRAS melanoma cells. This is consistent with a previous report showing a requirement for SHOC2 in MEK-ERK1/2 activation of a mutant NRAS cell line and the G463V BRAF/mutant KRAS harboring MDA-MB-231 cells (37). G463V BRAF has intermediate kinase activity and is likely to act via binding to CRAF (16). Overall, these findings suggest that acquired mutation of NRAS re-wires RAS-RAF signaling in response to RAF inhibitor.
In summary, we describe melanoma cells with resistance to a clinically relevant RAF inhibitor that is associated with a mutation in NRAS. We provide evidence that these mutant NRAS resistant cells alter their signaling connections in response to RAF inhibitor resulting in a shift to dependence on CRAF and a role for the RAS-RAF scaffold molecule, SHOC2.
Acknowledgments
We thank Dr. Gideon Bollag and Plexxikon Inc. for providing PLX4720, and Dr. Meenhard Herlyn (Wistar Institute, Philadelphia, PA) for supplying the WM melanoma cell lines. The Kimmel Cancer Center is funded by National Cancer Institute Support Grant 1P30CA56036.
This work was supported, in whole or in part, by National Institutes of Health Grants GM067893 and CA125103, Pennsylvania Commonwealth Universal Research Enhancement Grant AF0301, and Department of Defense Grant CA101019. This work was also supported by fellowships from Outrun the Sun (to Y. S.) and the Joanna M. Nicolay Melanoma Research Foundation (to E. A.), respectively.

This article contains supplemental Figs. S1 and S2.
- ERK
- extracellular signal-regulated kinase
- MAPK
- mitogen-activated kinase
- MEK1/2
- MAPK/ERK kinases 1 and 2
- EdU
- 5-ethynyl-2′-deoxyuridine
- FAK
- focal adhesion kinase.
REFERENCES
- 1. Sturgill T. W., Ray L. B., Erikson E., Maller J. L. (1988) Insulin-stimulated MAP-2 kinase phosphorylates and activates ribosomal protein S6 kinase II. Nature 334, 715–718 [DOI] [PubMed] [Google Scholar]
- 2. Boulton T. G., Nye S. H., Robbins D. J., Ip N. Y., Radziejewska E., Morgenbesser S. D., DePinho R. A., Panayotatos N., Cobb M. H., Yancopoulos G. D. (1991) ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell 65, 663–675 [DOI] [PubMed] [Google Scholar]
- 3. Robinson M. J., Cobb M. H. (1997) Mitogen-activated protein kinase pathways. Curr. Opin. Cell Biol. 9, 180–186 [DOI] [PubMed] [Google Scholar]
- 4. Salmeron A., Ahmad T. B., Carlile G. W., Pappin D., Narsimhan R. P., Ley S. C. (1996) Activation of MEK-1 and SEK-1 by Tpl-2 proto-oncoprotein, a novel MAP kinase kinase kinase. EMBO J. 15, 817–826 [PMC free article] [PubMed] [Google Scholar]
- 5. Papin C., Denouel-Galy A., Laugier D., Calothy G., Eychène A. (1998) Modulation of kinase activity and oncogenic properties by alternative splicing reveals a novel regulatory mechanism for B-Raf. J. Biol. Chem. 273, 24939–24947 [DOI] [PubMed] [Google Scholar]
- 6. Shaul Y. D., Gibor G., Plotnikov A., Seger R. (2009) Specific phosphorylation and activation of ERK1c by MEK1b: a unique route in the ERK cascade. Genes Dev. 23, 1779–1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aebersold D. M., Shaul Y. D., Yung Y., Yarom N., Yao Z., Hanoch T., Seger R. (2004) Extracellular signal-regulated kinase 1c (ERK1c), a novel 42-kilodalton ERK, demonstrates unique modes of regulation, localization, and function. Mol. Cell Biol. 24, 10000–10015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sun H., Charles C. H., Lau L. F., Tonks N. K. (1993) MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell 75, 487–493 [DOI] [PubMed] [Google Scholar]
- 9. Muda M., Boschert U., Dickinson R., Martinou J. C., Martinou I., Camps M., Schlegel W., Arkinstall S. (1996) MKP-3, a novel cytosolic protein-tyrosine phosphatase that exemplifies a new class of mitogen-activated protein kinase phosphatase. J. Biol. Chem. 271, 4319–4326 [DOI] [PubMed] [Google Scholar]
- 10. Hanafusa H., Torii S., Yasunaga T., Nishida E. (2002) Sprouty1 and Sprouty2 provide a control mechanism for the Ras/MAPK signalling pathway. Nat. Cell Biol. 4, 850–858 [DOI] [PubMed] [Google Scholar]
- 11. Sasaki A., Taketomi T., Kato R., Saeki K., Nonami A., Sasaki M., Kuriyama M., Saito N., Shibuya M., Yoshimura A. (2003) Mammalian Sprouty4 suppresses Ras-independent ERK activation by binding to Raf1. Nat. Cell Biol. 5, 427–432 [DOI] [PubMed] [Google Scholar]
- 12. Selfors L. M., Schutzman J. L., Borland C. Z., Stern M. J. (1998) soc-2 encodes a leucine-rich repeat protein implicated in fibroblast growth factor receptor signaling. Proc. Natl. Acad. Sci. U.S.A. 95, 6903–6908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sieburth D. S., Sun Q., Han M. (1998) SUR-8, a Conserved Ras-Binding Protein with Leucine-Rich Repeats, Positively Regulates Ras-Mediated Signaling in C. elegans. Cell 94, 119–130 [DOI] [PubMed] [Google Scholar]
- 14. Li W., Han M., Guan K.-L. (2000) The leucine-rich repeat protein SUR-8 enhances MAP kinase activation and forms a complex with Ras and Raf. Genes Dev. 14, 895–900 [PMC free article] [PubMed] [Google Scholar]
- 15. Wellbrock C., Karasarides M., Marais R. (2004) The Raf proteins take centre stage. Nat. Rev. Mol. Cell Biol. 5, 875–885 [DOI] [PubMed] [Google Scholar]
- 16. Wan P. T., Garnett M. J., Roe S. M., Lee S., Niculescu-Duvaz D., Good V. M., Jones C. M., Marshall C. J., Springer C. J., Barford D., Marais R. (2004) Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell 116, 855–867 [DOI] [PubMed] [Google Scholar]
- 17. Tsai J., Lee J. T., Wang W., Zhang J., Cho H., Mamo S., Bremer R., Gillette S., Kong J., Haass N. K., Sproesser K., Li L., Smalley K. S., Fong D., Zhu Y. L., Marimuthu A., Nguyen H., Lam B., Liu J., Cheung I., Rice J., Suzuki Y., Luu C., Settachatgul C., Shellooe R., Cantwell J., Kim S. H., Schlessinger J., Zhang K. Y., West B. L., Powell B., Habets G., Zhang C., Ibrahim P. N., Hirth P., Artis D. R., Herlyn M., Bollag G. (2008) Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc. Natl. Acad. Sci. U.S.A. 105, 3041–3046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bollag G., Hirth P., Tsai J., Zhang J., Ibrahim P. N., Cho H., Spevak W., Zhang C., Zhang Y., Habets G., Burton E. A., Wong B., Tsang G., West B. L., Powell B., Shellooe R., Marimuthu A., Nguyen H., Zhang K. Y. J., Artis D. R., Schlessinger J., Su F., Higgins B., Iyer R., D'Andrea K., Koehler A., Stumm M., Lin P. S., Lee R. J., Grippo J., Puzanov I., Kim K. B., Ribas A., McArthur G. A., Sosman J. A., Chapman P. B., Flaherty K. T., Xu X., Nathanson K. L., Nolop K. (2010) Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature 467, 596–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Poulikakos P. I., Zhang C., Bollag G., Shokat K. M., Rosen N. (2010) RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature 464, 427–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Flaherty K. T., Puzanov I., Kim K. B., Ribas A., McArthur G. A., Sosman J. A., O'Dwyer P. J., Lee R. J., Grippo J. F., Nolop K., Chapman P. B. (2010) Inhibition of mutated, activated BRAF in metastatic melanoma. New Eng. J. Med. 363, 809–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sosman J. A., Kim K. B., Schuchter L., Gonzalez R., Pavlick A. C., Weber J. S., McArthur G. A., Hutson T. E., Moschos S. J., Flaherty K. T., Hersey P., Kefford R., Lawrence D., Puzanov I., Lewis K. D., Amaravadi R. K., Chmielowski B., Lawrence H. J., Shyr Y., Ye F., Li J., Nolop K. B., Lee R. J., Joe A. K., Ribas A. (2012) Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. New Eng. J. Med. 366, 707–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chapman P. B., Hauschild A., Robert C., Haanen J. B., Ascierto P., Larkin J., Dummer R., Garbe C., Testori A., Maio M., Hogg D., Lorigan P., Lebbe C., Jouary T., Schadendorf D., Ribas A., O'Day S. J., Sosman J. A., Kirkwood J. M., Eggermont A. M. M., Dreno B., Nolop K., Li J., Nelson B., Hou J., Lee R. J., Flaherty K. T., McArthur G. A., and BRIM-3 Study Group (2011) Improved survival with Vemurafenib in melanoma with BRAF V600E mutation. New Eng. J. Med. 364, 2507–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nazarian R., Shi H., Wang Q., Kong X., Koya R. C., Lee H., Chen Z., Lee M.-K., Attar N., Sazegar H., Chodon T., Nelson S. F., McArthur G., Sosman J. A., Ribas A., Lo R. S. (2010) Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature 468, 973–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johannessen C. M., Boehm J. S., Kim S. Y., Thomas S. R., Wardwell L., Johnson L. A., Emery C. M., Stransky N., Cogdill A. P., Barretina J., Caponigro G., Hieronymus H., Murray R. R., Salehi-Ashtiani K., Hill D. E., Vidal M., Zhao J. J., Yang X., Alkan O., Kim S., Harris J. L., Wilson C. J., Myer V. E., Finan P. M., Root D. E., Roberts T. M., Golub T., Flaherty K. T., Dummer R., Weber B. L., Sellers W. R., Schlegel R., Wargo J. A., Hahn W. C., Garraway L. A. (2010) COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature 468, 968–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aplin A. E., Kaplan F. M., Shao Y. (2011) Mechanisms of resistance to RAF inhibitors in melanoma. J. Invest. Dermatol. 131, 1817–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaplan F. M., Shao Y., Mayberry M. M., Aplin A. E. (2011) Hyperactivation of MEK-ERK1/2 signaling and resistance to apoptosis induced by the oncogenic B-RAF inhibitor, PLX4720, in mutant N-RAS melanoma cells. Oncogene 30, 366–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Halaban R., Zhang W., Bacchiocchi A., Cheng E., Parisi F., Ariyan S., Krauthammer M., McCusker J. P., Kluger Y., Sznol M. (2010) PLX4032, a selective BRAF V600E kinase inhibitor, activates the ERK pathway and enhances cell migration and proliferation of BRAF WT melanoma cells. Pigment Cell Melanoma Res. 23, 190–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heidorn S. J., Milagre C., Whittaker S., Nourry A., Niculescu-Duvas I., Dhomen N., Hussain J., Reis-Filho J. S., Springer C. J., Pritchard C., Marais R. (2010) Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell 140, 209–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Poulikakos P. I., Persaud Y., Janakiraman M., Kong X., Ng C., Moriceau G., Shi H., Atefi M., Titz B., Gabay M. T., Salton M., Dahlman K. B., Tadi M., Wargo J. A., Flaherty K. T., Kelley M. C., Misteli T., Chapman P. B., Sosman J. A., Graeber T. G., Ribas A., Lo R. S., Rosen N., Solit D. B. (2011) RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E). Nature 480, 387–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Abel E. V., Aplin A. E. (2010) FOXD3 is a mutant B-RAF-regulated inhibitor of G1/S progression in melanoma cells. Cancer Res. 70, 2891–2900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hu R., Aplin A. E. (2010) alphaB-crystallin is mutant B-RAF regulated and contributes to cyclin D1 turnover in melanocytic cells. Pigment Cell Melanoma Res. 23, 201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hunger-Glaser I., Fan R. S., Perez-Salazar E., Rozengurt E. (2004) PDGF and FGF induce focal adhesion kinase (FAK) phosphorylation at Ser-910: dissociation from Tyr-397 phosphorylation and requirement for ERK activation. J. Cell Physiol. 200, 213–222 [DOI] [PubMed] [Google Scholar]
- 33. Stinchcombe T. E., Der C. J. (2011) Are all KRAS mutations created equal? Lancet Oncology 12, 717–718 [DOI] [PubMed] [Google Scholar]
- 34. Shao Y., Aplin A. (2010) Akt3-mediated resistance to apoptosis in B-RAF targeted melanoma cells. Cancer Res. 70, 6670–6681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. White M. A., Nicolette C., Minden A., Polverino A., Van Aelst L., Karin M., Wigler M. H. (1995) Multiple ras functions can contribute to mammalian cell transformation. Cell 80, 533–541 [DOI] [PubMed] [Google Scholar]
- 36. Hingorani S. R., Jacobetz M. A., Robertson G. P., Herlyn M., Tuveson D. A. (2003) Suppression of BRAF(V599E) in human melanoma abrogates transformation. Cancer Res. 63, 5198–5202 [PubMed] [Google Scholar]
- 37. Rodriguez-Viciana P., Oses-Prieto J., Burlingame A., Fried M., McCormick F. (2006) A phosphatase holoenzyme comprised of Shoc2/Sur8 and the catalytic subunit of PP1 functions as an M-Ras effector to modulate Raf activity. Mol. Cell 22, 217–230 [DOI] [PubMed] [Google Scholar]
- 38. Hatzivassiliou G., Song K., Yen I., Brandhuber B. J., Anderson D. J., Alvarado R., Ludlam M. J. C., Stokoe D., Gloor S. L., Vigers G., Morales T., Aliagas I., Liu B., Sideris S., Hoeflich K. P., Jaiswal B. S., Seshagiri S., Koeppen H., Belvin M., Friedman L. S., Malek S. (2010) RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature 464, 431–435 [DOI] [PubMed] [Google Scholar]
- 39. Karasarides M., Chiloeches A., Hayward R., Niculescu-Duvaz D., Scanlon I., Friedlos F., Ogilvie L., Hedley D., Martin J., Marshall C. J., Springer C. J., Marais R. (2004) B-RAF is a therapeutic target in melanoma. Oncogene 23, 6292–6298 [DOI] [PubMed] [Google Scholar]
- 40. Boisvert-Adamo K., Aplin A. E. (2006) B-RAF and PI-3 kinase signaling protect melanoma cells from anoikis. Oncogene 25, 4848–4856 [DOI] [PubMed] [Google Scholar]
- 41. Weber C. K., Slupsky J. R., Kalmes H. A., Rapp U. R. (2001) Active ras induces heterodimerization of cRaf and BRaf. Cancer Res. 61, 3595–3598 [PubMed] [Google Scholar]
- 42. Villanueva J., Vultur A., Lee J. T., Somasundaram R., Fukunaga-Kalabis M., Cipolla A. K., Wubbenhorst B., Xu X., Gimotty P. A., Kee D., Santiago-Walker A. E., Letrero R., D'Andrea K., Pushparajan A., Hayden J. E., Brown K. D., Laquerre S., McArthur G. A., Sosman J. A., Nathanson K. L., Herlyn M. (2010) Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell 18, 683–695 [DOI] [PMC free article] [PubMed] [Google Scholar]