Background: PEPCK is expressed in Leydig cells of testes, but its role has not been studied.
Results: Knockdown and inhibition of PEPCK and Glc-6-Pase inhibited steroidogenesis via activation of AMPK.
Conclusion: PEPCK and Glc-6-Pase are required for steroidogenesis in testicular Leydig cells.
Significance: Male fertility may be adversely affected by conditions and factors that inhibit PEPCK and Glc-6-Pase expression in Leydig cells.
Keywords: AMP-activated Kinase (AMPK), Cyclic AMP (cAMP), Nuclear Receptors, Phosphoenolpyruvate Carboxykinase, Steroidogenesis, DAX-1
Abstract
Cyclic AMP (cAMP) induces steroidogenic enzyme gene expression and stimulates testosterone production in Leydig cells. Phosphoenolpyruvate carboxykinase (PEPCK) is expressed in Leydig cells, but its role has not been defined. In this study, we found that PEPCK and glucose-6-phosphatase (Glc-6-Pase) are increased significantly following cAMP treatment of mouse Leydig cells. Moreover, cAMP treatment increased recruitment of the cAMP-response element-binding transcription factor and decreased recruitment of the corepressor DAX-1 on the pepck promoter. Furthermore, cAMP induced an increase in ATP that correlated with a decrease in phospho-AMP-activated protein kinase (AMPK). In contrast, knockdown or inhibition of PEPCK decreased ATP and increased phospho-AMPK. Treatment with an AMPK activator or overexpression of the constitutively active form of AMPK inhibited cAMP-induced steroidogenic enzyme promoter activities and gene expression. Liver receptor homolog-1 (LRH-1) was involved in cAMP-induced steroidogenic enzyme gene expression but was inhibited by AMPK activation in Leydig cells. Additionally, inhibition or knockdown of PEPCK and Glc-6-Pase decreased cAMP-mediated induction of steroidogenic enzyme gene expression and steroidogenesis. Finally, pubertal mouse (8-week-old) testes and human chorionic gonadotropin-induced prepubertal mouse testes showed increased PEPCK and Glc-6-Pase gene expression. Taken together, these results suggest that induction of PEPCK and Glc-6-Pase by cAMP plays an important role in Leydig cell steroidogenesis.
Introduction
Cytosolic PEPCK2 is strongly expressed in the liver and kidneys and moderately expressed in the testes, prostate, lungs, adrenal glands, stomach, adipose tissue, and small intestine (1). Although its role in gluconeogenesis in the liver and kidney as well as glyceroneogenesis in the adipose tissue is well defined (2), what function it has in the testes has not been defined. The expression of PEPCK is regulated by various transcription factors that bind to response regions in the promoter of the gene (3, 4). The cAMP-responsive element-binding transcription factor (CREB) is a bZIP family transcription factor that modulates the transcription of genes and plays an essential role in the activation of PEPCK by directly binding to the cAMP-response elements (TGACGTCA) of the promoter region (5). CREB regulates transcription coactivator 2 (CRTC2)-regulated glucose homeostasis by inducing PEPCK (3–5). DAX-1 is an unusual member of the nuclear hormone receptor family that lacks a classical DNA-binding domain and a restricted DAX-1 expression pattern whose function is essential for the development of the human adrenal cortex and the onset of puberty (6). Moreover, DAX-1 acts as a negative regulator of nuclear receptors to decrease PEPCK gene expression in the liver (7).
Steroidogenesis is the main function of Leydig cells and is predominantly mediated by luteinizing hormone/human chorionic gonadotropin (LH/hCG) through multiple signaling pathways (8). Steroidogenesis is regulated by steroidogenic enzymes, including the steroidogenic acute regulatory protein (StAR), the cholesterol side chain cleavage enzyme (P450scc), 17α-hydroxylase/C17–20 lyase (P450c17), 3β-hydroxysteroid dehydrogenase (3β-HSD), DAX-1, and Nur77 (7–13). It is well known that cAMP regulates the expression of steroidogenic enzymes in Leydig cells through a variety of transcription factors, including steroidogenic factor-1 (SF-1), DAX-1, CCAAT/enhancer-binding protein-β, liver receptor homolog-1 (LRH-1), Nur77, and the CREB protein (9–14). cAMP signaling regulates StAR gene expression and steroidogenesis via CREB activation (11, 12). DAX-1, a negative regulator of orphan nuclear receptors, decreases StAR gene expression by inhibiting CREB transcriptional activity in Leydig cells (15).
An oxidizable substrate is required for steroidogenesis by testicular interstitial tissue (16, 17) and isolated rat and tumor Leydig cells. Glucose, pyruvate, glutamine, and 3-hydroxybutyrate can satisfy this requirement (17). Despite the need for an energy source, only small amounts of glycogen are stored in Leydig cells (18). Glucose is transported into Leydig cells by glucose transporter 8, which is induced by hCG and insulin-like growth factor-I but repressed by interleukin-1α, tumor necrosis factor-α (TNF-α), and interferon-γ (19–21).
AMP-activated protein kinase (AMPK) is activated under stress or energy-deficient conditions by an increase in the AMP/ATP ratio (22). Moreover, LKB1 is upstream of AMPK, and metformin-mediated activation of AMPK decreases steroidogenesis and MAPK3/MAPK1 phosphorylation levels in bovine granulosa cells (23, 24). Dominant-negative AMPK abolishes the effects of metformin on bovine granulosa cell steroidogenesis (24). Furthermore, the salt-inducible kinase, a serine/threonine kinase that belongs to the AMPK family, decreases the mRNA for the cholesterol side-chain cleavage cytochrome P450 (P450scc) in mouse adrenocortical tumor cells (25). These reports indicate AMPK can regulate steroidogenesis in endocrine tissues, consistent with the requirement for a substrate that can be oxidized for energy.
In this study, we found that cAMP signaling increased PEPCK and Glc-6-Pase gene expression in Leydig cells. Moreover, knockdown or inhibition of these enzymes decreased testosterone production by Leydig cells. These findings suggest that cAMP-mediated reactions catalyzed by PEPCK and Glc-6-Pase are important for Leydig cell steroidogenesis.
EXPERIMENTAL PROCEDURES
Animals and Treatment
C57BL/6J mice (2 and 8 weeks old) were obtained from Korea Research Institute of Bioscience and Biotechnology (Daejeon, South Korea). Animals were sacrificed by CO2 asphyxiation, and testes were extracted for radioimmunoassay (RIA) and immunohistochemical analyses. For the PCR and the real time PCR assay, 14-day-old C57BL/6J mice were injected intraperitoneally with 10 IU of hCG (Sigma) before being sacrificed 6 h later by CO2 asphyxiation. Animal experiments were approved by the Institutional Animal Care and Use Committee. All procedures were performed according to the Guidelines of Chonnam National University.
Reagents and Plasmids
3-Mercaptopicolinic acid (3-MPA) was purchased from TRC (26); 8-bromo-cAMP, was purchased from Sigma; and the derivative of chlorogenic acid, S 3483, was kindly supplied by Hoechst (Frankfurt, Germany) (27). The reporter plasmids, PEPCK-Luc, Glc-6-Pase-Luc, StAR-Luc, 3β-HSD-Luc, P450c17-Luc, and Sft4-Luc (multicopy liver receptor homolog-1/steroidogenic factor-1 binding reporter), were described previously (28, 29). Eight copies of cAMP-responsive element-Luc constructs were prepared by insertion into the pGL2 promoter vector between BglII sites. A series deletion of the pepck promoter luciferase reporters and StAR promoters were constructed previously (30, 31). pRC-RSV-PKA (catalytic subunit) and pRC-RSV-A-CREB were kindly provided by Dr. Richard A. Maurer (Oregon Health and Science University, Portland). DAX-1 (7), constitutively active AMPK (CA-AMPK) (29), and LRH-1 (32) plasmids were described previously. The pcDNA3-FLAG-CREB and pcDNA3-FLAG-CRTC2 plasmids were constructed by subcloning the EcoRI-XhoI cDNA fragments of rat CREB and mouse CRTC2 into pcDNA3-FLAG vector. GST alone (pEBG), GST-DAX-1 (pEBG-DAX-1), and GFP-DAX-1 (pEGFP-DAX-1) were described previously (7).
Cell Culture and Transient Transfection Assay
HeLa and 293T cells were obtained from the American Type Culture Collection (Manassas, VA). MA-10 cells were kindly provided by Dr. Mario Ascoli and maintained as described previously (33, 34). These well studied mouse Leydig tumor cells produce progesterone as the major steroid in response to both LH and cAMP analogs. Briefly, MA-10 cells were cultured with 15% horse serum, RPMI 1640 medium, and antibiotics. Transfections were conducted using the FuGENE HD (Roche Applied Science) transfection reagent with the indicated reporter plasmids together with expression vectors encoding various transcription factors or treated with chemicals. For transfection, cells were incubated for 12 h in customer-ordered glucose-free RPMI 1640 from WelGENE Inc. (Daegu, South Korea) containing 0.5% charcoal-stripped horse serum, 20 mm sodium lactate, 1 mm sodium pyruvate, and 15 mm HEPES without phenol red. Total cDNA used for each transfection was adjusted to 1 μg/well by adding an appropriate amount of empty vector and cytomegalovirus (CMV)-β-galactosidase plasmid as an internal control. Cells were harvested 12–24 h post-transfection for the luciferase and β-galactosidase assays. Luciferase activity was normalized to β-galactosidase activity and expressed as relative luciferase units.
Northern Blotting Analysis
MA-10 cells were maintained for up to 24 h in the indicated media with or without 8-Br-cAMP. Total RNA was isolated from each sample and used for Northern blot analysis, as described previously (7, 9) Probe labeling of each of the cDNAs for pepck, g6pase, fbpase, and gapdh with [α-32P]dCTP was performed using a random primer DNA-labeling system (Amersham Biosciences).
Quantitative RT-PCR
Total RNA was extracted from mouse Leydig cells under various conditions using TRIzol reagent (Invitrogen), according to the manufacturer's protocol. PEPCK, Glc-6-Pase, FBPase, StAR, P450scc, 3β-HSD, and β-actin mRNAs were analyzed by RT-PCR. The primers used for PCR of PEPCK, Glc-6-Pase, FBPase, CRTC2, StAR, P450scc, 3β-HSD, and β-actin are as follows: mouse PEPCK, forward (5′-AACTGTTGGCTGGCTCTC-3′) and reverse (5′-GAACCTGGCGTTGAATGC-3′); mouse Glc-6-Pase, forward (5′-TCCTGGGACAGACACACAAG-3′) and reverse (5′-GAGGACCAAGGAAGCCACAAT-3′); mouse FBPase, forward (5′-ATGGTGGACCATGCACCCTTCGAA-3′) and reverse (5′-TTTCTAATTCCCCGTCGTGGCAAGTCC-3′); mouse CRTC2,forward 5′-AGGTGATGATGGACATCGGCTCCA-3′ and reverse 5′-GTGGTACTGTTGCCCCCACT-3′; mouse StAR, forward 5′-TTGGGCATACTCAACAACCA-3′ and reverse 5′-ATGACACCGCTTTGCTCAG-3′; mouse P450scc, forward (5′-AGGTGTAGCTCAGGACTT-3′) and reverse (5′-AGGAGGCTATAAAGGACACC-3′); mouse 3β-HSD, forward (5′-TTGGTGCAGGAGGAAAGAAC-3′) and reverse (5′-CCGCAAGTATCATGACAGA-3′); and mouse β-actin, forward (5′-GCTCGTCGTCGACAACGGCTC-3′) and reverse (5′-CAAACATGATCTGGGTCATCTTCTC-3′). The sizes of the PEPCK, Glc-6-Pase, FBPase, CRTC2, StAR, P450scc, 3β-HSD and β-actin PCR products were 170, 147, 950,799, 389, 399, 574, and 353 bp, respectively.
Western Blot Analysis
Cell lysates of mouse Leydig cells were prepared, and Western blot analyses were performed using rabbit monoclonal AMPKα (Cell Signaling Technology), rabbit monoclonal phospho-AMPKα (Thr-172) (Cell Signaling Technology), rabbit polyclonal DAX-1 (Cell Signaling Technology), rabbit polyclonal phospho-CREB (Cell Signaling Technology), rabbit polyclonal CREB (Cell Signaling Technology), rabbit polyclonal PEPCK(H-300) (Santa Cruz Biotechnology), and goat polyclonal actin (Santa Cruz Biotechnology) antibodies, as described previously (7, 29).
Knockdown Experiments
PEPCK shRNA antisense 5′-TTCAATTATGTAAAGAAA G-3′ and sense 5′-CTTTCTTTACATAATTGAA-3′ (35) were cloned into BglII/XhoI sites of the pSuper vector. Glc-6-Pase siRNA (sc-145294) and control small interfering RNAs (siRNAs) were purchased (Santa Cruz Biotechnology). MA-10 cells were transfected with PEPCK shRNA or Glc-6-Pase siRNA using FuGENE HD (Roche Applied Science) according to the manufacturer's instructions. The shRNA- or siRNA-treated cells were subjected to reverse transcription-PCR and real time PCR, and the media were used for RIA.
Measurement of ATP Concentration
MA-10 cells were seeded in a 6-well plate. Cells were exposed to cAMP for the times indicated. ATP was measured in total thawed lysed cells via luciferase activity using an ATP bioluminescence assay kit (Roche Applied Bioscience). Results are expressed as the degree of change in control cells (set as unity) not exposed to any chemicals.
Preparation of Recombinant Adenovirus
An adenoviral delivery system was used for ectopic expression of the genes. An adenovirus encoding for AMPKα (29), A-CREB (36), and CRTC2 (36, 37) has been previously described. Briefly, the cDNA encoding LRH-1 and DAX-1 was cloned into the pAdTrack shuttle vector. Recombination of AdTrack-CMV-HA-LRH-1 (38) and AdTrack-CMV-HA-DAX-1 (7) with adenoviral gene carrier vector was performed by transformation into pretransformed AdEasy-BJ21 competent cells.
Confocal Microscopy
Confocal microscopy was performed as described previously (7). Briefly, HeLa cells were grown on uncoated glass coverslips and transfected with pEGFP-DAX-1 and pCDNA3/FLAG-CREB using the Lipofectamine method (Invitrogen). Twenty four hours after transfection, the cells were fixed for 10 min in 3.7% formaldehyde, mounted on glass slides, and observed using a laser-scanning confocal microscope (Olympus Corp., Lake Success, NY) (7).
Immunohistochemistry
Briefly, 5-mm-thick sections of paraffin wax-embedded mouse testis and epididymis were deparaffinized by routine protocols before being exposed to citrate buffer (0.01 m, pH 6.0) and heated in a microwave oven for 3 min. All subsequent steps were performed at room temperature. The sections were treated with 0.3% hydrogen peroxide in methyl alcohol for 20 min to block endogenous peroxidase activity. After three washes in PBS, the sections were blocked with 10% normal goat serum (ABC Elite kit, Vector Laboratories, Burlingame, CA), diluted in PBS for 1 h, and then incubated with rat anti-galectin-3 antibody (1:5,000) for 1 h. After three washes in PBS, the sections were incubated with biotinylated goat anti-rat IgG (1:100; Vector Laboratories) for 45 min. After three washes in PBS, the sections were incubated for 45 min with the avidin-biotin peroxidase complex (Vector Laboratories), prepared according to the manufacturer's instructions. The peroxidase reaction was developed using a peroxidase substrate kit (DAB, SK-4100; Vector Laboratories), according to the manufacturer's instructions. After color development was complete, the sections were counterstained with Harris hematoxylin (Sigma) for 5 s, washed in running tap water for 20 min, dehydrated through a graded ethanol series, cleared with xylene, and mounted with Canada balsam (Sigma). Primary antiserum was omitted from some sections as a control.
Chromatin Immunoprecipitation (ChIP) Assay
The ChIP assay was performed according to the manufacturer's protocol (Upstate, Temecula, CA). Briefly, MA-10 cells were transfected with reporter plasmids, and treatments were performed as indicated. Cells were then fixed in 1% formaldehyde and harvested. Soluble chromatin was immunoprecipitated with rabbit polyclonal phospho-CREB antibody, polyclonal CREB antibody, and polyclonal DAX-1 antibody. After recovering the DNA, quantitative PCR was performed using primers encompassing the mouse pepck promoter (−200/−10). The primers used for PCR are as follows: forward (5′-GATGTAGACTCTGTCCTG-3′) and reverse (5′-GATTGCTCTGCTATGAGT-3′).
In Vivo Interaction Assays
293T cells were grown in the indicated media and were plated in 6-well flat-bottomed microplates at a concentration of 5 × 105 cells/well the day before transfection, as described previously (7). 293T cells were cotransfected with FLAG-CREB, GST-DAX-1 (pEBG-DAX-1), or with GST alone (pEBG). The complex formation (GST purification) and the required amount of FLAG-CREB were used for the in vivo binding assay. Cell lysates were determined by Western blot using the indicated antibodies.
RIA
MA-10 cells were cultured in glucose-free RPMI 1640 medium containing 15% charcoal-stripped horse serum. The inhibitors (3-MPA for PEPCK or S 3483 for Glc-6-Pase) were added 2 h before cAMP treatment. Moreover, MA-10 cells were transfected with shPEPCK or siGlc-6-Pase before cAMP treatment. Additionally, MA-10 cells were incubated with high glucose after knockdown or inhibition of PEPCK and Glc-6-Pase. Testosterone and progesterone concentrations were measured by RIA, as described previously (9, 13).
Statistical Analysis
Data are expressed as mean ± S.D. Statistical analyses were performed using the Student's t test or an analysis of variance followed by Duncan's multiple comparison test. All experiments were performed at least three times. Differences were considered significant when p < 0.05.
RESULTS
Induction of FBPase, PEPCK, and Glc-6-Pase by cAMP in Leydig Cells
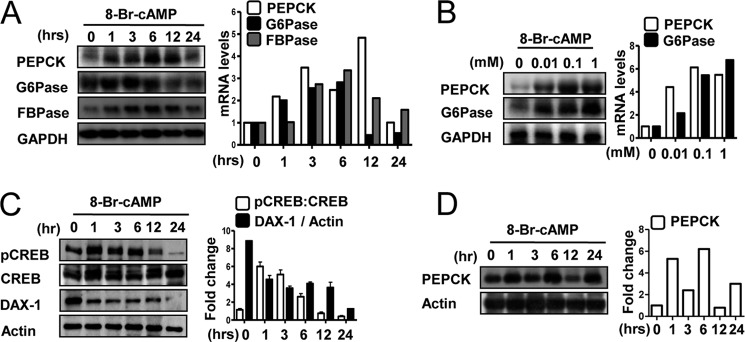
Previous reports have demonstrated that FBPase and PEPCK are expressed in human testis (1). To examine whether cAMP induces expression of these enzymes in testicular Leydig cells, MA-10 cells were treated with cAMP. We observed the maximum induction of pepck, g6pase, and fbpase mRNA by cAMP at ∼6 h at an effective dose of 100 μm (Fig. 1, A and B).
FIGURE 1.
cAMP induces PEPCK, Glc-6-Pase, and FBPase gene expression in Leydig cells. A, MA-10 cells were cultured in 0.5% charcoal-stripped horse serum and glucose-free medium supplemented with 20 mm sodium lactate, 1 mm sodium pyruvate, and 15 mm HEPES and treated with 8-Br-cAMP (100 μm) for the indicated time period. Total RNA was isolated for Northern hybridization, and PEPCK, Glc-6-Pase, and FBPase gene expression was analyzed and normalized to GAPDH gene expression. Results are representative of three independently performed experiments. B, MA-10 cells were treated with 8-Br-cAMP for 6 h in a dose-dependent manner. Total RNA was isolated for Northern hybridization, and PEPCK and Glc-6-Pase gene expressions were analyzed and normalized to GAPDH gene expression. Results are representative of three independently performed experiments. C, MA-10 cells were treated with 8-Br-cAMP (100 μm) for the indicated time period and harvested for Western blot analysis using the indicated antibodies (pCREB, CREB, DAX-1, and actin). Results are representative of three independently performed experiments. D, MA-10 cells were treated with 8-Br-cAMP (100 μm) for the indicated time period and harvested for Western blot analysis using the indicated antibodies (PEPCK and actin). Results are representative of three independently performed experiments.
In the liver, CREB directly regulates both basal and cAMP-mediated expression of the pepck gene (39). CREB also regulates StAR gene expression and steroidogenesis through cAMP signaling in Leydig cells (11, 12). To identify the cAMP-responsive region of the pepck promoter, we utilized serial deletions of pepck promoter constructs. Deletion between −274 and −208 bp resulted in a significant decrease in the induction of pepck promoter activity by cAMP, suggesting that this region is important for the cAMP-mediated effect (supplemental Fig. 1A). A sequence analysis showed that this region contains a potential CREB-binding site (cAMP-response element). Moreover, we performed transient transfection assays using the pepck and g6pase promoters along with expression vectors for protein kinase A (PKA) and dominant-negative CREB (A-CREB) (supplemental Fig. 1B). These results showed that PKA transfection leads to a significant increase in both pepck and g6pase promoters and that cotransfection of A-CREB abolished these effects. Adenovirus-mediated A-CREB overexpression decreased cAMP-induced pepck and g6pase gene expression in a dose-dependent manner (supplemental Fig. 1C). Conversely, adenovirus-mediated CRTC2 (also known as CREB coactivator) overexpression increased pepck and g6pase gene expression in a dose-dependent manner (supplemental Fig. 1D). To further confirm the involvement of CREB with PEPCK gene expression, we examined CREB phosphorylation in mouse Leydig cells by Western blot analysis. Treatment with cAMP rapidly and significantly increased CREB phosphorylation (Fig. 1C). As expected, DAX-1 expression decreased significantly following cAMP treatment (Fig. 1C). Moreover, expression of PEPCK protein in response to cAMP was confirmed by Western blotting. The results clearly show that cAMP increased PEPCK protein level after 1 h with a maximum effect after 6 h (Fig. 1D). Taken together, these results indicate that cAMP rapidly induces pepck expression via CREB in testicular Leydig cells.
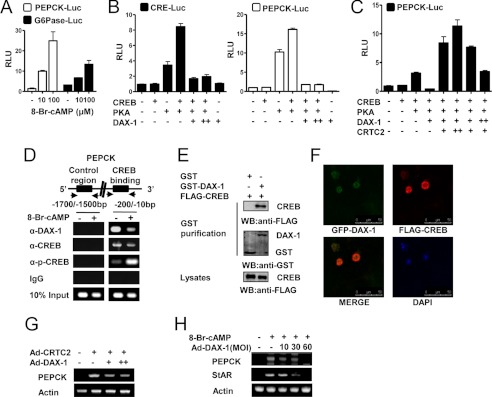
Inhibition of cAMP-induced PEPCK Gene by DAX-1 in Leydig Cells
We demonstrated previously that LH increases steroidogenesis by increasing Nur77 (13). However, LH negatively regulates DAX-1 gene expression, which inhibits StAR gene expression in Leydig cells (13, 15, 40). In transient transfection assays with MA-10 cells, cAMP increased the activities of pepck and g6pase promoters in a dose-dependent manner (Fig. 2A). We next performed transient transfection assays using the cre and pepck promoters and the PKA, CREB, and DAX-1 expression vectors. The increased basal activity of both promoters caused by PKA was significantly decreased by DAX-1 cotransfection (Fig. 2B). Transient transfection assays were performed to determine whether DAX-1 competes with CRTC2 upon CREB transactivation in Leydig cells. As shown in Fig. 2B, CREB transcriptional activity was significantly increased by PKA, and DAX-1 prevented this effect. In transient transfection assays, reduction of CREB transcriptional activity by DAX-1 was significantly relieved by CRTC2 in a dose-dependent manner (Fig. 2C). Next, we examined the recruitment of DAX-1 and CREB by the PEPCK gene promoter under cAMP treatment using the ChIP assay. We detected CREB occupancy on the pepck promoter under basal conditions. However, cAMP significantly increased CREB occupancy on the pepck promoter (Fig. 2D). Conversely, DAX-1 recruitment to the CREB binding region on the pepck promoter was high under basal conditions and significantly lower following cAMP treatment (Fig. 2D). These results indicate an inverse correlation between the recruitment of CREB and DAX-1 on PEPCK under basal and cAMP-stimulated conditions. Moreover, in vivo GST pulldown assays confirmed this interaction in vivo. FLAG-CREB was transfected with the mammalian expression vectors GST (pEBG) or GST-DAX-1 (pEBG DAX-1) into 293T cells. After purifying GST, FLAG-CREB was detected in the coprecipitates only when coexpressed with GST-DAX-1 but not with the negative GST control alone (Fig. 2E).
FIGURE 2.
DAX-1 represses cAMP-induced PEPCK gene expression in Leydig cells. A, MA-10 cells were transfected with PEPCK-Luc (200 ng) and Glc-6-Pase-Luc (200 ng), respectively. Twenty four hours after the transfection, MA-10 cells were cultured in 0.5% charcoal-stripped horse serum and glucose-free medium supplemented with 20 mmol/liter sodium lactate, 1 mmol/liter sodium pyruvate, and 15 mmol/liter HEPES, followed by cAMP treatment at the indicated concentrations for 12 h. Experiments were performed in triplicate, and data are expressed in relative luciferase units (RLU) and as the fold activation relative to the control, representing mean ± S.D. of three individual experiments. B, cells were transfected with CRE-Luc (200 ng), PEPCK-Luc (200 ng), CREB (200 ng), PKA (200 ng), and DAX-1 (200 ng) as indicated. Experiments were performed in triplicate, and data are expressed in relative luciferase units (RLU) and as the fold activation relative to the control. Data represent mean ± S.D. of three individual experiments. C, MA-10 cells were transfected with PEPCK-Luc (200 ng), CREB (200 ng), PKA (200 ng), DAX-1 (200 ng), and CRTC2 (200 ng) as indicated. Experiments were performed in triplicate, and data are expressed in RLU and as the fold activation relative to the control. Data represent mean ± S.D. of three individual experiments. D, MA-10 cells were serum-starved for 12 h followed by cAMP (100 μm) for 12 h. Soluble chromatin was prepared and immunoprecipitated with antibodies against DAX-1, CREB, p-CREB, or IgG only as indicated. Soluble chromatin (10%) was used as input. PCR was performed to determine and quantify CREB binding to the endogenous PEPCK promoter. E, 293T cells were cotransfected with FLAG-CREB expression vectors together with GST DAX-1 (pEBG-DAX-1) or GST alone (pEBG) as a control. The complex formation (top panels, GST purification) and FLAG-CREB (5 μg) used for the in vivo binding assay (bottom panel, lysate) were determined by anti-FLAG antibody immunoblot (WB). The same blot was stripped and reprobed with an anti-GST antibody (middle panel) to confirm the expression levels of the GST fusion protein (GST-DAX-1) and the GST control (GST). F, HeLa cells were transiently transfected with pEGFP-DAX-1 and pCDNA3/FLAG-CREB. The yellow stain in the merged image depicts colocalization of DAX-1 and CREB (×400). Data shown are representative cells from one of three independent experiments. DAPI, 4′,6-diamidino-2-phenylindole dihydrochloride. G, MA-10 cells were infected with the CRTC2 (30 m.o.i.) and DAX-1 (10, 30 m.o.i.) adenoviruses. Total RNA was isolated for semiquantitative RT-PCR analysis. Data represent mean ± S.D. of three individual experiments. H, MA-10 cells were infected with DAX-1 adenovirus at an m.o.i. (MOI) of 10, 30, or 60 for 24 h followed by cAMP (100 μm) treatment for 12 h. Total RNA was isolated for semiquantitative RT-PCR analysis. Data represent mean ± S.D. of three individual experiments.
A confocal microscopic analysis was performed to investigate the subcellular localization of DAX-1 and CREB after transfecting GFP-DAX-1 and FLAG-CREB into HeLa cells. GFP-DAX-1 was scattered in the nucleus and weakly expressed in the cytoplasm, consistent with previous reports (7, 41), whereas FLAG-CREB was distributed only in the nucleus. Moreover, merged images indicated that these two proteins were colocalized in the nucleus (Fig. 2F). Finally, we observed that adenovirus-mediated DAX-1 overexpression decreased Ad-CRTC2 and cAMP-mediated PEPCK gene expression in a dose-dependent manner (Fig. 2, G and H). StAR gene expression was examined as a positive control (Fig. 2H). Overall, these results indicate that DAX-1 repressed PEPCK via inhibition of CREB in Leydig cells.
Regulation of the ATP Level and the Phosphorylation State of AMPK by cAMP and PEPCK
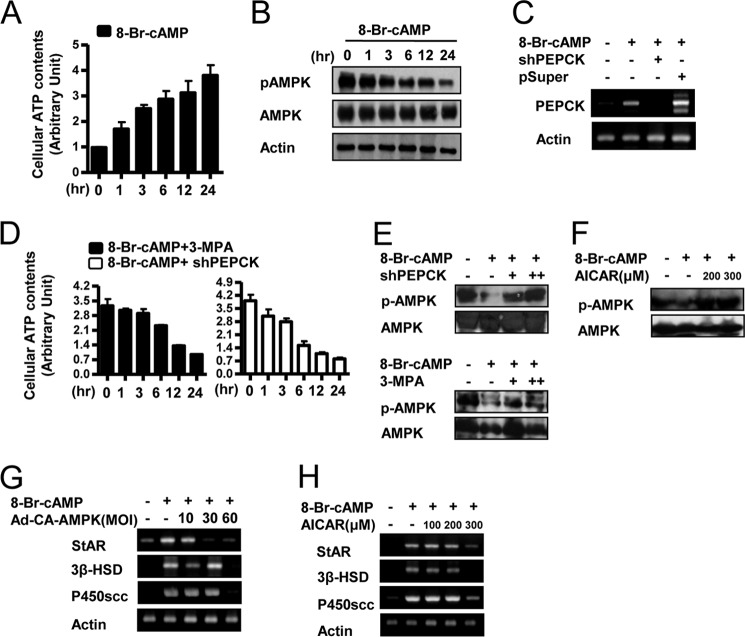
Preliminary experiments indicated that ATP levels are low, and AMPK is heavily phosphorylated and therefore active in resting Leydig cells. Treatment of the cells with cAMP increased ATP levels significantly (∼4-fold) and greatly decreased phospho-AMPK over a 24-h time course (Fig. 3, A and B). These findings show that stimulation of Leydig cells by cAMP reduced phospho-AMPK levels in Leydig cells, most likely by increasing ATP and lowering AMP.
FIGURE 3.
AMPK regulates cAMP-induced steroidogenic gene expression. A, MA-10 cells were treated with cAMP (100 μm) for the indicated time period; cell lysates were extracted, and a cellular ATP assay was performed. Results are representative of three independently performed experiments. B, MA-10 cells were treated with cAMP (100 μm) for the indicated time period; cell lysates were extracted, and Western blot analyses were performed using the indicated antibodies. Results are representative of three independently performed experiments. C, MA-10 cells were transfected with shPEPCK (200 ng) or pSuper vector (200 ng) prior to 8-Br-cAMP (100 μm) treatment. Total RNA was isolated for semiquantitative RT-PCR analysis. Data represent mean ± S.D. of three individual experiments. D, MA-10 cells were incubated with cAMP (100 μm) and then added 3-MPA (10 μm) or shPEPCK (200 ng); cell lysates were extracted, and a cellular ATP assay was performed. Results are representative of three independently performed experiments. E, MA-10 cells were transfected with shPEPCK (200 ng) or pretreated with 3-MPA (10 μm) prior to 8-Br-cAMP (100 μm) treatment. Cells lysates were extracted, and Western blot analyses were performed using p-AMPK and AMPK antibodies. Results are representative of three independently performed experiments. F, MA-10 cells were pretreated with AICAR (200 or 300 μm) prior to cAMP (100 μm) treatment. Cells lysates were extracted, and Western blot analyses were performed using p-AMPK and AMPK. Results are representative of three independently performed experiments. G, MA-10 cells were infected with the CA-AMPK adenovirus at an m.o.i. (MOI) of 10, 30, or 60 for 24 h followed by cAMP (100 μm) treatment for 12 h. Total RNA was isolated for semiquantitative RT-PCR analysis. Data represent mean ± S.D. of three individual experiments. H, MA-10 cells were treated with AICAR (100, 200, or 300 μm) for 12 h followed by a preceding 12 h cAMP (100 μm) treatment. Total RNA was isolated for semiquantitative RT-PCR analysis. Data represent mean ± S.D. of three individual experiments.
To investigate the possible involvement of PEPCK in AMPK phosphorylation, we overexpressed shPEPCK and also treated cells with 3-MPA, a PEPCK enzyme inhibitor. As expected, shPEPCK overexpression decreased cAMP-induced pepck mRNA (Fig. 3C). Treatment of cAMP increased ATP levels, but knockdown or inhibition of PEPCK prevented this increase in ATP levels (Fig. 3D). As a result, AMPK phosphorylation was increased in both conditions (Fig. 3E). These results suggest that PEPCK expression is associated with increased ATP levels and inhibition of AMPK activity.
Regulation of cAMP-induced Steroidogenic Gene Expression by AMPK
To define the role of AMPK in the regulation of steroidogenic enzyme gene expression, the effects of AMPK activation and inhibition on steroidogenic enzyme gene transcription were determined. The AMPK activator, 5-Aminoimidazole-4-carboxamide 1-β-d-ribofuranoside (AICAR) prevented the cAMP-mediated decrease in AMPK phosphorylation (Fig. 3F). cAMP treatment significantly induced steroidogenic enzyme genes (star, p450scc, and 3β-hsd), and these effects were dramatically abolished by adenovirus-mediated CA-AMPK overexpression (Fig. 3G). Moreover, AICAR treatment decreased cAMP-induced star, p450scc, and 3β-hsd gene expression as well (Fig. 3H). Taken together, these results indicate that phospho-AMPK inhibits steroidogenic gene expression and that cAMP regulates steroidogenic enzyme gene expression by decreasing phospho-AMPK levels in Leydig cells.
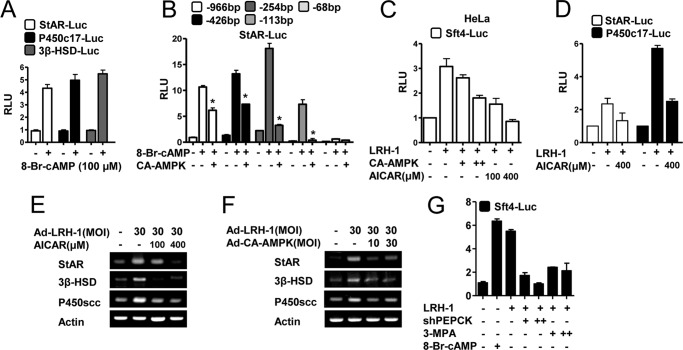
Negative Effect of AMPK on LRH-1-mediated Steroidogenic Gene Promoter Activities and Gene Expression
As expected from the work of others, cAMP treatment significantly increased the activities of steroidogenic enzyme gene promoters for star, p450c17, and 3β-hsd in Leydig cells (Fig. 4A). To elucidate the mechanism of the AMPK-mediated reduction of the positive effect of cAMP on StAR expression, transient transfection assays were performed in MA-10 cells using a serial deletion construct of the StAR gene promoter. Deletion of the StAR promoter sequence from −966 to −113 bp inhibited promoter activity, and further deletion to −68 bp abolished AMPK-mediated repression, suggesting that the region between −113 and −68 bp conferred StAR promoter inhibition by AMPK (Fig. 4B). To investigate whether AMPK inhibits liver receptor homolog-1 (LRH-1), we examined the effects of AMPK on multicopy liver receptor homolog-1/steroidogenic factor-1 binding reporter (Sft4-Luc) activation in HeLa cells. CA-AMPK or AICAR significantly repressed the increase in Sft4-Luc promoter activity by LRH-1 (Fig. 4C) or SF-1 (data not shown) in a dose-dependent manner. LRH-1-mediated StAR-Luc and P450c17-Luc promoter activities were also repressed by AICAR (Fig. 4D). Moreover, adenovirus-mediated overexpression of LRH-1 increased star, p450scc, and 3β-hsd, and these effects were diminished by AICAR treatment or Ad-CA-AMPK overexpression (Fig. 4, E and F). Furthermore, the LRH-1-mediated increase in Sft4-Luc promoter activity was inhibited by shPEPCK overexpression and 3-MPA treatment (Fig. 4G). Taken together, these results suggest that lack of PEPCK leads to activation of AMPK, which inhibits cAMP-induced steroidogenic gene promoter activity and gene expression.
FIGURE 4.
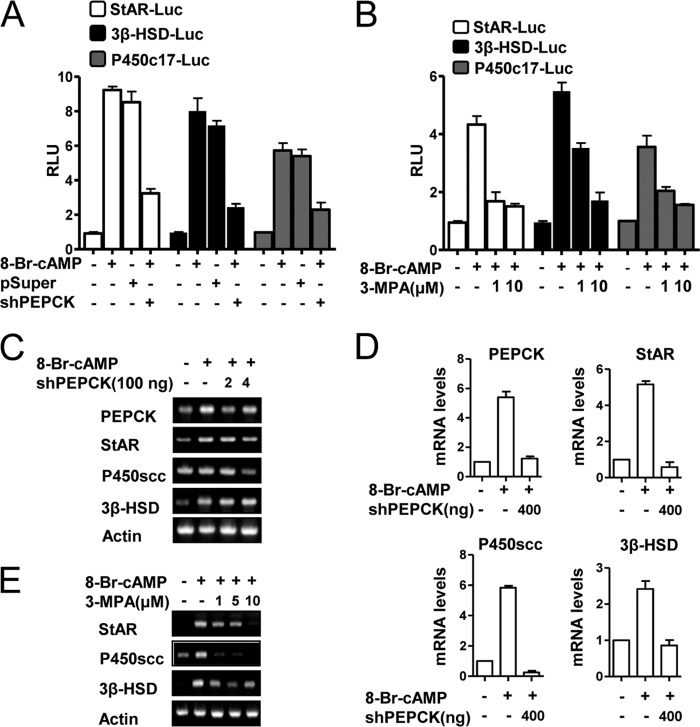
AMPK negatively regulates LRH-1-mediated steroidogenic gene promoter activities and gene expression. A, MA-10 cells were transfected with StAR-Luc (200 ng), P450c17-Luc (200 ng), and 3β-HSD-Luc (200 ng), respectively. Twenty four hours after the transfection, MA-10 cells were cultured in 0.5% charcoal-stripped horse serum and glucose-free medium supplemented with 20 mmol/liter sodium lactate, 1 mmol/liter sodium pyruvate, and 15 mmol/liter HEPES, followed by cAMP treatment at the indicated concentrations for 12 h. Experiments were performed in triplicate, and data are expressed in relative luciferase units (RLU) and as the fold activation relative to the control, representing mean ± S.D. of three individual experiments. B, MA-10 cells were transfected with several deletion constructs of StAR-Luc (200 ng) with or without CA-AMPK and treated with 8-Br-cAMP as indicated for 24 h. Experiments were performed in triplicate, and data are expressed in relative luciferase units (RLU) and as the fold activation relative to the control, representing mean ± S.D. of three individual experiments. *, p < 0.001 compared with untreated control and cAMP treated cells. C, HeLa cells were transfected with Sft4-Luc (200 ng), LRH-1 (200 ng), and CA-AMPK (100 or 200 ng) and were then treated with AICAR (100 or 400 μm). Experiments were performed in triplicate, and data are expressed in RLU and as the fold activation relative to the control. Data represent mean ± S.D. of three individual experiments. D, MA-10 cells were transfected with StAR-Luc (200 ng), P450c17-Luc (200 ng), and LRH-1 (200 ng) as indicated, with or without AICAR (400 μm). Experiments were performed in triplicate, and data are expressed in RLU and as the fold activation relative to the control. Data represent mean ± S.D. of three individual experiments. E and F, MA-10 cells were infected with the LRH-1 adenovirus (30 m.o.i.) for 24 h followed by AICAR (100 and 400 μm) treatment for 12 h or adenovirus CA-AMPK overexpression (10 or 30 m.o.i.). Total RNA was isolated for semiquantitative RT-PCR analysis. Data represent mean ± S.D. of three individual experiments. G, MA-10 cells were transfected with Sft4-Luc (200 ng), LRH-1 (200 ng), and shPEPCK (200 ng) and treated with 3-MPA (10 or 20 μm) or 8-Br-cAMP (100 μm). Experiments were performed in triplicate, and data are expressed in RLU and as the fold activation relative to the control. Data represent mean ± S.D. of three individual experiments.
Role of PEPCK in cAMP-induced Steroidogenic Gene Expression
To confirm that PEPCK plays a critical role in steroidogenesis, we transfected the steroidogenic enzyme gene promoters for star, p450c17, and 3β-hsd into mouse Leydig cells and then treated the cells with 3-MPA or transfected the cells with shPEPCK. Both treatments significantly decreased the activities of the steroidogenic enzyme gene promoters for star, p450c17, and 3β-hsd (Fig. 5, A and B). PEPCK knockdown also decreased cAMP-mediated induction of steroidogenic enzyme gene expression at the protein level (Fig. 5C). Reduced PEPCK protein expression was also confirmed (Fig. 5C). These findings were further confirmed by real time quantitative PCR analysis (Fig. 5D). Similarly, 3-MPA treatment decreased cAMP-induced star, 3β-hsd, and p450scc gene expression (Fig. 5E), thereby suggesting that PEPCK plays a critical role in regulating cAMP-induced steroidogenic gene expression.
FIGURE 5.
PEPCK plays a role in cAMP-induced steroidogenic gene expression. A, MA-10 cells were transfected with StAR-Luc (200 ng), 3β-HSD-Luc (200 ng), P450c17-Luc (200 ng), shPEPCK (200 ng), and pSuper vector (200 ng) for 24 h after transfection; MA-10 cells were serum-starved followed by cAMP (100 μm) treatment. Experiments were performed in triplicate, and data are expressed in relative luciferase units (RLU) and as the fold activation relative to the control. Data represent mean ± S.D. of three individual experiments. B, MA-10 cells were transfected with StAR-Luc (200 ng), 3β-HSD-Luc (200 ng), and P450c17-Luc (200 ng) for 12 h after transfection. MA-10 cells were serum-starved and pretreated with 3-MPA (1 and 10 μm) for 2 h, followed by treatment with cAMP (100 μm). Experiments were performed in triplicate, and data are expressed in relative luciferase units and as the fold activation relative to the control. Data represent mean ± S.D. of three individual experiments. C, MA-10 cells were transfected with shPEPCK (200 or 400 ng) for 24 h after transfection, followed by treatment with cAMP (100 μm) for 12 h. Total RNA was isolated for semiquantitative RT-PCR analysis. Data represent mean ± S.D. of three individual experiments. D, RNA was isolated from C samples for real time PCR analysis. PEPCK, StAR, P450scc, and 3β-HSD were evaluated. Data represent mean ± S.D. of three individual experiments. E, MA-10 cells were pretreated with 3-MPA (1, 5, and 10 μm) for 2 h, followed by treatment with cAMP (100 μm) for 12 h. Total RNA was isolated for semiquantitative RT-PCR analysis. Data represent mean ± S.D. of three individual experiments.
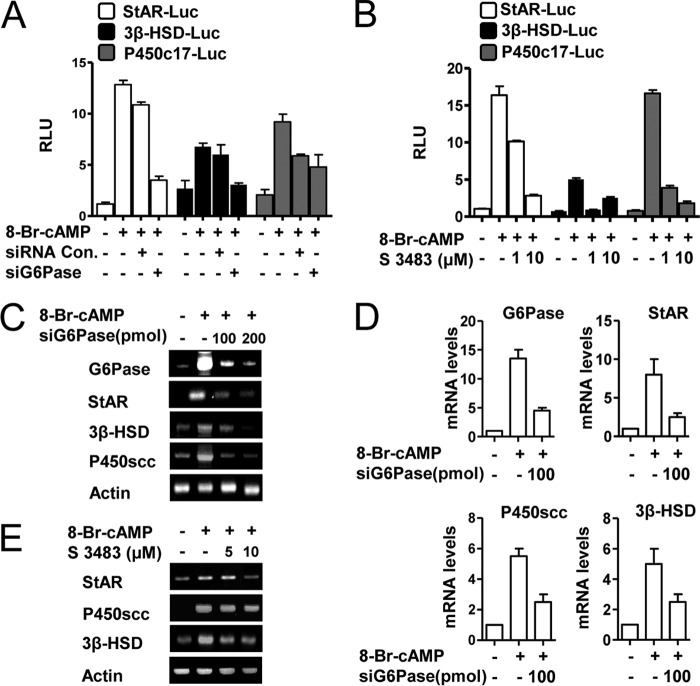
Involvement of Glc-6-Pase in cAMP-induced Steroidogenic Gene Expression
To determine whether Glc-6-Pase also plays a critical role in steroidogenesis, mouse Leydig cells were transfected with gene promoters for star, 3β-hsd, and p450c17 and treated with S 3483 to inhibit Glc-6-Pase activity (27) or transfected with siGlc-6-Pase. Both treatments significantly decreased the activities of the steroidogenic enzyme gene promoters for star, 3β-hsd, and p450c17 (Fig. 6, A and B). Additionally, cAMP-mediated induction of the star, 3β-hsd, and p450scc proteins was reduced by knocking down Glc-6-Pase (Fig. 6C). These findings were confirmed by real time quantitative PCR analysis (Fig. 6D). Treatment with S 3483 also reduced the induction of the star, 3β-hsd, and p450scc proteins by cAMP (Fig. 6E). These findings suggest that Glc-6-Pase plays an important role in cAMP-induced steroidogenic gene expression.
FIGURE 6.
Glc-6-Pase plays a role in cAMP-induced steroidogenic gene expression. A, MA-10 cells were transfected with StAR-Luc (200 ng), 3β-HSD-Luc (200 ng), P450c17-Luc (200 ng), siGlc-6-Pase (100 pmol), and siRNA (100 pmol) for 24 h after transfection. Cells were serum-starved followed by cAMP treatment (100 μm). Experiments were performed in triplicate, and data are expressed in relative luciferase units (RLU) and as the fold activation relative to the control. Data represent mean ± S.D. of three individual experiments. B, MA-10 cells were transfected with StAR-Luc (200 ng), 3β-HSD-Luc (200 ng), and P450c17-Luc (200 ng) for 12 h after transfection. MA-10 cells were serum-starved and pretreated with S 3483 (1 and 10 μm) for 2 h, followed by treatment with cAMP (100 μm). Experiments were performed in triplicate, and data are expressed in relative luciferase units and as the fold activation relative to the control. Data represent mean ± S.D. of three individual experiments. C, MA-10 cells were transfected with siGlc-6-Pase (100 or 200 pmol) for 24 h after transfection, followed by treatment with cAMP (100 μm) for 12 h. Total RNA was isolated for semiquantitative RT-PCR analysis. Data represent mean ± S.D. of three individual experiments. D, total RNA was isolated from C samples for real time PCR analysis. Glc-6-Pase, StAR, P450scc and 3β-HSD were evaluated. Data represent mean ± S.D. of three individual experiments. E, MA-10 cells were pretreated with S 3483 (5 or 10 μm) for 2 h, followed by treatment with cAMP (100 μm) for 12 h. Total RNA was isolated for semiquantitative RT-PCR analysis. Data represent mean ± S.D. of three individual experiments.
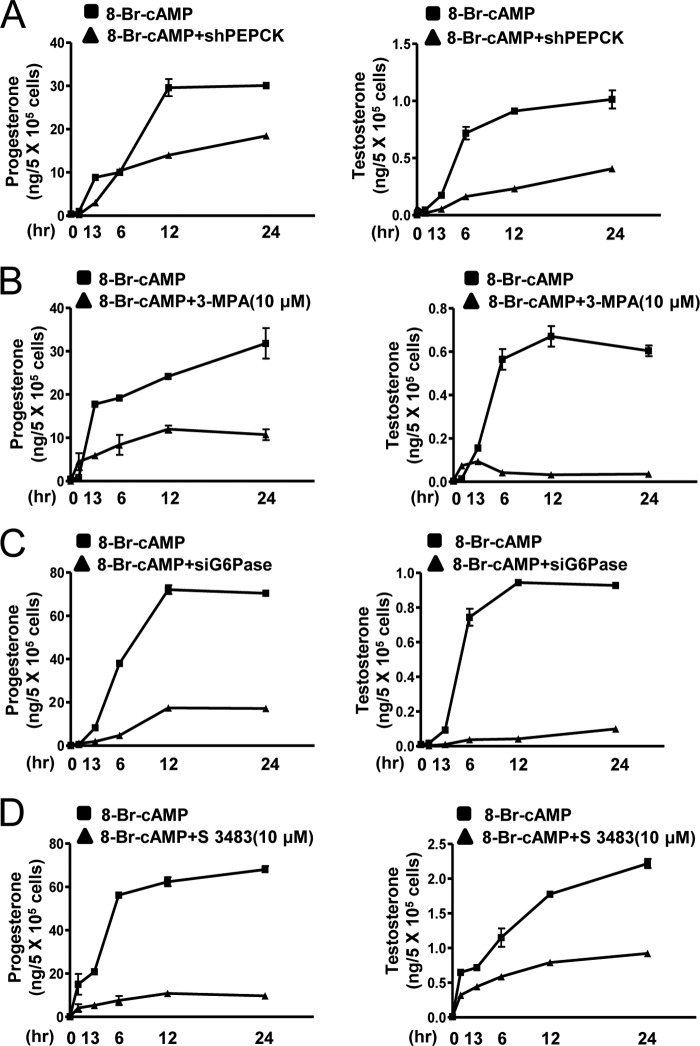
Confirmation that PEPCK and Glc-6-Pase Are Required for the Synthesis of Steroids by Leydig Cells
cAMP treatment significantly increases the synthesis of progesterone and testosterone by Leydig cells (Fig. 7, A–D). PEPCK shRNA and 3-MPA treatment inhibited the increase in the synthesis of progesterone and testosterone by cAMP (Fig. 7, A and B). Glc-6-Pase knockdown and S 3483 treatment also decreased stimulation of progesterone and testosterone by cAMP (Fig. 7, C and D). These results suggest that cAMP-mediated PEPCK and Glc-6-Pase gene expression are required for steroidogenesis in Leydig cells.
FIGURE 7.
PEPCK and Glc-6-Pase play a significant role in cAMP-induced steroidogenesis. A and B, MA-10 cells were transfected with shPEPCK (200 ng) or treated with 3-MPA (10 μm) and cultured in the presence of cAMP (100 μm) for the indicated time period, and media were collected for progesterone (left panel) and testosterone (right panel) measurement by RIA. Each point represents the average concentration of progesterone and testosterone per cell from three independent experiments. Data are representative of two independently conducted experiments. C and D, MA-10 cells were transfected with siGlc-6-Pase (100 pmol) or treated with S 3483 (10 μm) and cultured in the presence of cAMP (100 μm) for the indicated time period, and media were collected for progesterone (left panel) and testosterone (right panel) measurement by RIA. Each point represents the average amount of progesterone and testosterone per cell in three independent experiments. Data are representative of two independently conducted experiments.
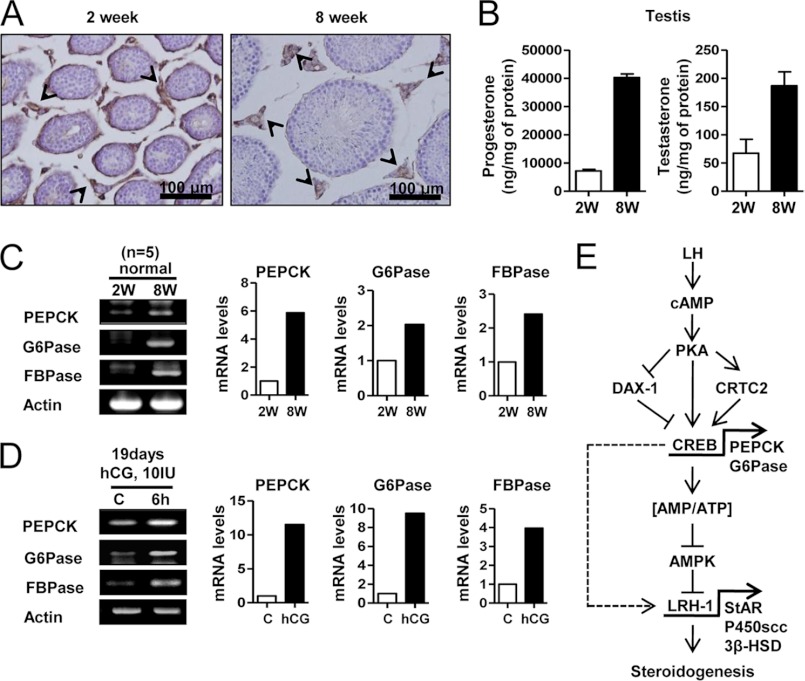
Induction of PEPCK by LH in Mouse Testicular Leydig Cells
The level of PEPCK expression was examined by immunohistochemistry in the pubertal transition in testicular slices from 2- and 8-week-old mice. The LH surge that occurs between prepuberty (2 weeks) and puberty (8 weeks) resulted in an increase in PEPCK (Fig. 8A). The antibody raised against PEPCK only stained Leydig cells (Fig. 8A, arrowheads), indicating the expression of PEPCK is confined to these cells. Moreover, steroidogenic enzyme genes were expressed in postpubertal mice testis (8 weeks) and in hCG-injected mice testis (19 days) (supplemental Fig. 2). Furthermore, postpubertal testis (8 weeks) had higher levels of progesterone and testosterone than prepubertal testis (2 weeks) (Fig. 8B). RT-PCR analysis revealed greater expression of pepck, g6pase, fbpase, and the steroidogenic enzyme genes in the 8-week-old mice (Fig. 8C and supplemental Fig. 2A). A significant increase in pepck, g6pase, and fbpase gene expression further confirmed the hCG-induced increase in prepubertal mouse testis (Fig. 8D). Overall, the LH surge increased the expression of the genes encoding all of these enzymes in testis.
FIGURE 8.
LH increases PEPCK, Glc-6-Pase, and FBPase in mouse testis. A, testes were collected from 2- and 8-week-old C57BL/6J mice for immunohistochemistry assay. The arrowheads show the PEPCK stain in testis. B, testis samples from A was used for RIA. Each point represents the average concentration of progesterone and testosterone from three independent experiments. Data are representative of two independently conducted experiments. C, total RNA was isolated for semiquantitative RT-PCR analysis and real time PCR from 2- and 8-week-old mouse testes. Data represent mean ± S.D. of three individual experiments. D, 19-day-old C57BL/6J mice were injected with hCG (10 IU) for 6 h, and the testes were isolated for semiquantitative RT-PCR analysis and real time PCR. Data represent mean ± S.D. of three individual experiments. E, LH/cAMP/PKA signaling pathway increased expression of PEPCK and Glc-6-Pase via CREB activation, leading to an increased cellular ATP level. Subsequently, AMPK activation decreased in Leydig cells, thereby increasing LRH-1 gene expression, which positively regulates StAR, P450scc, and 3β-HSD steroidogenic enzyme gene expression. Therefore, these effects increase steroidogenesis in mouse testicular Leydig cells.
DISCUSSION
Novel findings in this study include the following evidence. (a) PEPCK and Glc-6-Pase are required for the stimulation of steroidogenic enzyme gene expression and steroidogenesis by Leydig cells. (b) cAMP promotes PEPCK and Glc-6-Pase gene expression in Leydig cells. (c) The positive effects of cAMP on PEPCK gene expression are mediated by the action of PKA on CRTC2, CREB, and DAX-1. (d) PEPCK and Glc-6-Pase are required for maintenance of high cellular ATP levels. (e) cAMP-mediated induction of PEPCK and Glc-6-Pase increases cellular ATP, which reduces the phosphorylation state of AMPK. (f) Phospho-AMPK inhibits stimulation of steroidogenesis by cAMP. (g) LRH-1 is involved in cAMP-mediated stimulation and AMPK-mediated inhibition of steroidogenic enzyme gene expression.
The model given in Fig. 8E is proposed based on these findings. The LH/cAMP/PKA signaling pathway induces PEPCK and Glc-6-Pase through CREB activation. PKA positively regulates CRTC2 and negatively regulates DAX-1. In turn, cAMP decreases AMPK activity via an increase in the ATP/AMP ratio and subsequently increases LRH-1-mediated steroidogenic enzyme gene expression and steroidogenesis in Leydig cells. PKA also directly regulates LRH-1-induced steroidogenesis in Leydig cells (indicated by dashed line, Fig. 8E). The findings summarized in this working model suggest a previously unsuspected role for PEPCK and Glc-6-Pase in steroidogenesis by Leydig cells.
Although the role of rapid StAR phosphorylation in the regulation of steroidogenesis is well established (11, 12), this study showed that chemically inhibiting PEPCK or knockdown of PEPCK expression increased AMPK phosphorylation and decreased steroidogenesis in Leydig cells. Thus, cAMP-mediated steroidogenesis is severely attenuated in the absence of PEPCK and Glc-6-Pase. cAMP inhibited AMPK phosphorylation through an increase in cellular ATP level, but knockdown or inhibition of PEPCK decreased the ATP level in Leydig cells. Therefore, we speculate that cAMP-stimulated steroidogenesis occurs early via direct phosphorylation of StAR, but cAMP-mediated expression of PEPCK and Glc-6-Pase is required for sustained steroidogenesis.
The increase in LH at puberty likely accounts for the greater gene expression of PEPCK, Glc-6-Pase, and FBPase in the testes of 8-week-old mice relative to 2-week-old pups. Moreover, the injection of 19-day-old pups with hCG increased the expression of these enzymes. Furthermore, cAMP increased PEPCK and Glc-6-Pase gene in Leydig cells. These findings are consistent with an important role for PEPCK and Glc-6-Pase in steroidogenesis by Leydig cells.
Although a definitive explanation as to why PEPCK and Glc-6-Pase are required for steroidogenesis by Leydig cells cannot be provided, an important role for these enzymes in maintenance of a high cellular energy state is apparent as evidenced by the effect that a lack of these enzymes has on the ATP concentration and phosphorylation state of AMPK. This is paradoxical because the reaction catalyzed by PEPCK consumes GTP, which would suggest that the lack of this enzyme might increase ATP levels. Instead, the lack of PEPCK results in a drop in ATP levels, thereby eliminating this possibility. The finding that Leydig cells express PEPCK, FBPase, and Glc-6-Pase indicates they have the enzymatic capacity for gluconeogenesis, suggesting that perhaps glucose is required for steroidogenesis. However, the rate of glucose synthesis is low, the synthesis of glucose is expensive in terms of the requirement for ATP, and the addition of a high concentration of glucose (20 mm) to the cell culture media did not eliminate the requirement for PEPCK and Glc-6-Pase (data not shown). The notion that the requirement for PEPCK might reflect a need for the production of glucose 6-phosphate for the pentose phosphate pathway would seem to be eliminated by the requirement for Glc-6-Pase, which should decrease the availability of glucose 6-phosphate. Glyceroneogenesis (43), which is another pathway that requires PEPCK, was also considered. Although it can be imagined that production of glycerol 3-phosphate by this pathway might be important for the esterification of free fatty acids released by the action of cholesteroyl esterase, this would also not explain the requirement for Glc-6-Pase.
Finally, the mechanism we favor is suggested by findings with liver-specific PEPCK KO mice. Studies with these mice suggest PEPCK is important in the integration of mechanisms responsible for regulation of energy metabolism in the liver (44). Indeed, PEPCK deficiency causes inhibition of the citric acid cycle and fatty acid oxidation in the liver (45). This has been suggested to occur because PEPCK is required for the disposal of excess citric acid cycle intermediates (45) by cataplerosis (43). We propose that PEPCK and Glc-6-Pase are important for the disposal of excess citric acid cycle intermediates that may accumulate in Leydig cells as a result of steroidogenesis and the utilization of glutamine as a source of energy. Cleavage of the cholesterol side chain during the synthesis of androgens produces isocaproic acid (46). This six-carbon fatty acid is subsequently oxidized by β-oxidation and the distal steps of the valine catabolic pathway to produce propionyl-CoA, which is converted by an anaplerotic pathway to succinyl-CoA. Because PEPCK converts oxaloacetate to phosphoenolpyruvate, we speculate that the cataplerotic reaction catalyzed by PEPCK is required to prevent expansion of the citric acid cycle. As found for the liver, lack of PEPCK in the Leydig cells may inhibit citric acid cycle activity and the oxidation of fatty acids, including isocaproic acid, resulting in low ATP levels and activation of AMPK. In other words, we propose PEPCK and Glc-6-Pase support a low rate of glucose synthesis in testes to avoid inhibition of the citric acid cycle activity and ATP synthesis. Because Leydig cells can oxidize glutamine to produce ATP (16) and because the entrance of glutamine into the citric acid cycle is anaplerotic, i.e. expands the pool of citric acid cycle intermediates, complete oxidation of glutamine to CO2 and H2O requires cataplerosis. Without a cataplerotic reaction such as that catalyzed by PEPCK, citric acid cycle intermediates would accumulate to inhibitory concentrations (47). Although a full understanding of the mechanism requires further investigation, the findings establish an important role for PEPCK and Glc-6-Pase in maintaining the high cellular energy state necessary for steroidogenesis in Leydig cells.
Acknowledgments
We thank Dr. Seung Bum Park for technical assistance and Dr. Seok-Yong Choi for critical reading of the manuscript.
This work was supported by National Creative Research Initiatives Grant 2011-0018305 from the Korean Ministry of Education, Science, and Technology and the Future-based Technology Development Program (BIO Fields) through the National Research Foundation of Korea funded by the Ministry of Education, Science, and Technology Grant 20100019512.

This article contains supplemental Figs. 1 and 2.
- PEPCK
- phosphoenolpyruvate carboxykinase
- CREB
- cAMP-responsive element-binding protein transcription factor
- CRTC2
- CREB-regulated transcriptional coactivator 2
- Glc-6-Pase
- glucose-6-phosphatase
- FBPase
- fructose-1,6-bisphosphatase
- AMPK
- AMP-activated protein kinase
- DAX-1
- dosage-sensitive sex reversal, adrenal hypoplasia critical region, on chromosome X, gene 1
- 3-MPA
- 3-mercaptopicolinic acid
- LRH-1
- liver receptor homolog-1
- S 3483
- (1–5[2-(4-chlorophenyl)cyclopropyl] methoxy-6–3,4-dihydroxy-5–5[3-(4-hydroxyphenyl)-1-oxo-2-propenyl]oxy-6-cyclohexanecarboxylic acid)
- m.o.i.
- multiplicity of infection
- RIA
- radioimmunoassay
- 3β-HSD
- 3β-hydroxysteroid dehydrogenase
- hCG
- human chorionic gonadotropin
- 8-Br-cAMP
- 8-bromo-cAMP
- CA-AMPK
- constitutively active AMPK
- LH
- luteinizing hormone
- AICAR
- 5-aminoimidazole-4-carboxamide 1-β-d-ribofuranoside.
REFERENCES
- 1. Yánez A. J., Nualart F., Droppelmann C., Bertinat R., Brito M., Concha I. I., Slebe J. C. (2003) Broad expression of fructose-1,6-bisphosphatase and phosphoenolpyruvate carboxykinase provide evidence for gluconeogenesis in human tissues other than liver and kidney. J. Cell. Physiol. 197, 189–197 [DOI] [PubMed] [Google Scholar]
- 2. Hanson R. W., Reshef L. (2003) Glyceroneogenesis revisited. Biochimie 85, 1199–1205 [DOI] [PubMed] [Google Scholar]
- 3. Pilkis S. J., Granner D. K. (1992) Molecular physiology of the regulation of hepatic gluconeogenesis and glycolysis. Annu. Rev. Physiol. 54, 885–909 [DOI] [PubMed] [Google Scholar]
- 4. Hanson R. W., Reshef L. (1997) Regulation of phosphoenolpyruvate carboxykinase (GTP) gene expression. Annu. Rev. Biochem. 66, 581–611 [DOI] [PubMed] [Google Scholar]
- 5. Shaywitz A. J., Greenberg M. E. (1999) CREB. A stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu. Rev. Biochem. 68, 821–861 [DOI] [PubMed] [Google Scholar]
- 6. Lalli E., Alonso J. (2010) Targeting DAX-1 in embryonic stem cells and cancer. Expert Opin. Ther. Targets 14, 169–177 [DOI] [PubMed] [Google Scholar]
- 7. Nedumaran B., Hong S., Xie Y. B., Kim Y. H., Seo W. Y., Lee M. W., Lee C. H., Koo S. H., Choi H. S. (2009) DAX-1 acts as a novel corepressor of orphan nuclear receptor HNF4α and negatively regulates gluconeogenic enzyme gene expression. J. Biol. Chem. 284, 27511–27523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stocco D. M., Wang X., Jo Y., Manna P. R. (2005) Multiple signaling pathways regulating steroidogenesis and steroidogenic acute regulatory protein expression. More complicated than we thought. Mol. Endocrinol. 19, 2647–2659 [DOI] [PubMed] [Google Scholar]
- 9. Ahn S. W., Nedumaran B., Xie Y., Kim D. K., Kim Y. D., Choi H. S. (2008) Bisphenol A bis(2,3-dihydroxypropyl) ether (BADGE·2H2O) induces orphan nuclear receptor Nur77 gene expression and increases steroidogenesis in mouse testicular Leydig cells. Mol. Cells 26, 74–80 [PubMed] [Google Scholar]
- 10. Sewer M. B., Waterman M. R. (2002) cAMP-dependent transcription of steroidogenic genes in the human adrenal cortex requires a dual-specificity phosphatase in addition to protein kinase A. J. Mol. Endocrinol. 29, 163–174 [DOI] [PubMed] [Google Scholar]
- 11. Clark B. J., Ranganathan V., Combs R. (2001) Steroidogenic acute regulatory protein expression is dependent upon post-translational effects of cAMP-dependent protein kinase A. Mol. Cell. Endocrinol. 173, 183–192 [DOI] [PubMed] [Google Scholar]
- 12. Stocco D. M., Clark B. J., Reinhart A. J., Williams S. C., Dyson M., Dassi B., Walsh L. P., Manna P. R., Wang X. J., Zeleznik A. J., Orly J. (2001) Elements involved in the regulation of the StAR gene. Mol. Cell. Endocrinol. 177, 55–59 [DOI] [PubMed] [Google Scholar]
- 13. Song K. H., Park J. I., Lee M. O., Soh J., Lee K., Choi H. S. (2001) LH induces orphan nuclear receptor Nur77 gene expression in testicular Leydig cells. Endocrinology 142, 5116–5123 [DOI] [PubMed] [Google Scholar]
- 14. Freeman D. A., Ontko J. A. (1992) Accumulation and mobilization of triglycerides and cholesteryl esters in Leydig tumor cells. J. Lipid Res. 33, 1139–1146 [PubMed] [Google Scholar]
- 15. Song K. H., Park Y. Y., Park K. C., Hong C. Y., Park J. H., Shong M., Lee K., Choi H. S. (2004) The atypical orphan nuclear receptor DAX-1 interacts with orphan nuclear receptor Nur77 and represses its transactivation. Mol. Endocrinol. 18, 1929–1940 [DOI] [PubMed] [Google Scholar]
- 16. Rommerts F. F., Cooke B. A., Van der Kemp J. W., Van der Molen H. J. (1973) Effect of luteinizing hormone on 3′,5′-cyclic AMP and testosterone production in isolated interstitial tissue of rat testis. FEBS Lett. 33, 114–118 [DOI] [PubMed] [Google Scholar]
- 17. Amrolia P., Sullivan M. H., Garside D., Baldwin S. A., Cooke B. A. (1988) An investigation of glucose uptake in relation to steroidogenesis in rat testis and tumour Leydig cells. Biochem. J. 249, 925–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leiderman B., Mancini R. E. (1969) Glycogen content in the rat testis from postnatal to adult ages. Endocrinology 85, 607–609 [DOI] [PubMed] [Google Scholar]
- 19. Chen Y., Nagpal M. L., Lin T. (2003) Expression and regulation of glucose transporter 8 in rat Leydig cells. J. Endocrinol. 179, 63–72 [DOI] [PubMed] [Google Scholar]
- 20. Doege H., Schürmann A., Bahrenberg G., Brauers A., Joost H. G. (2000) GLUT8, a novel member of the sugar transport facilitator family with glucose transport activity. J. Biol. Chem. 275, 16275–16280 [DOI] [PubMed] [Google Scholar]
- 21. Nehar D., Mauduit C., Boussouar F., Benahmed M. (1998) Interleukin 1α stimulates lactate dehydrogenase A expression and lactate production in cultured porcine Sertoli cells. Biol. Reprod. 59, 1425–1432 [DOI] [PubMed] [Google Scholar]
- 22. Hardie D. G. (2004) The AMP-activated protein kinase pathway–new players upstream and downstream. J. Cell Sci. 117, 5479–5487 [DOI] [PubMed] [Google Scholar]
- 23. Lizcano J. M., Göransson O., Toth R., Deak M., Morrice N. A., Boudeau J., Hawley S. A., Udd L., Mäkelä T. P., Hardie D. G., Alessi D. R. (2004) LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 23, 833–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tosca L., Chabrolle C., Uzbekova S., Dupont J. (2007) Effects of metformin on bovine granulosa cells steroidogenesis. Possible involvement of adenosine 5′-monophosphate-activated protein kinase (AMPK). Biol. Reprod. 76, 368–378 [DOI] [PubMed] [Google Scholar]
- 25. Lin X., Takemori H., Katoh Y., Doi J., Horike N., Makino A., Nonaka Y., Okamoto M. (2001) Salt-inducible kinase is involved in the ACTH/cAMP-dependent protein kinase signaling in Y1 mouse adrenocortical tumor cells. Mol. Endocrinol. 15, 1264–1276 [DOI] [PubMed] [Google Scholar]
- 26. Liang T., Raugi G. J., Blum J. J. (1976) Inhibition of P-enolpyruvate carboxykinase and of glyconeogenesis in Tetrahymena by 3-mercaptopicolinic acid. J. Protozool. 23, 473–477 [DOI] [PubMed] [Google Scholar]
- 27. Arion W. J., Canfield W. K., Ramos F. C., Su M. L., Burger H. J., Hemmerle H., Schubert G., Below P., Herling A. W. (1998) Chlorogenic acid analogue S 3483: a potent competitive inhibitor of the hepatic and renal glucose-6-phosphatase systems. Arch. Biochem. Biophys. 351, 279–285 [DOI] [PubMed] [Google Scholar]
- 28. Hong C. Y., Park J. H., Ahn R. S., Im S. Y., Choi H. S., Soh J., Mellon S. H., Lee K. (2004) Molecular mechanism of suppression of testicular steroidogenesis by proinflammatory cytokine tumor necrosis factor α. Mol. Cell. Biol. 24, 2593–2604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim Y. D., Park K. G., Lee Y. S., Park Y. Y., Kim D. K., Nedumaran B., Jang W. G., Cho W. J., Ha J., Lee I. K., Lee C. H., Choi H. S. (2008) Metformin inhibits hepatic gluconeogenesis through AMP-activated protein kinase-dependent regulation of the orphan nuclear receptor SHP. Diabetes 57, 306–314 [DOI] [PubMed] [Google Scholar]
- 30. Chakravarty K., Leahy P., Becard D., Hakimi P., Foretz M., Ferre P., Foufelle F., Hanson R. W. (2001) Sterol regulatory element-binding protein-1c mimics the negative effect of insulin on phosphoenolpyruvate carboxykinase (GTP) gene transcription. J. Biol. Chem. 276, 34816–34823 [DOI] [PubMed] [Google Scholar]
- 31. Caron K. M., Ikeda Y., Soo S. C., Stocco D. M., Parker K. L., Clark B. J. (1997) Characterization of the promoter region of the mouse gene encoding the steroidogenic acute regulatory protein. Mol. Endocrinol. 11, 138–147 [DOI] [PubMed] [Google Scholar]
- 32. Chanda D., Li T., Song K. H., Kim Y. H., Sim J., Lee C. H., Chiang J. Y., Choi H. S. (2009) Hepatocyte growth factor family negatively regulates hepatic gluconeogenesis via induction of orphan nuclear receptor small heterodimer partner in primary hepatocytes. J. Biol. Chem. 284, 28510–28521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Clem B. F., Hudson E. A., Clark B. J. (2005) Cyclic adenosine 3′,5′-monophosphate (cAMP) enhances cAMP-responsive element-binding (CREB) protein phosphorylation and phospho-CREB interaction with the mouse steroidogenic acute regulatory protein gene promoter. Endocrinology 146, 1348–1356 [DOI] [PubMed] [Google Scholar]
- 34. Ascoli M. (1981) Characterization of several clonal lines of cultured Leydig tumor cells. Gonadotropin receptors and steroidogenic responses. Endocrinology 108, 88–95 [DOI] [PubMed] [Google Scholar]
- 35. Inoue Y., Kurihara R., Tsuchida A., Hasegawa M., Nagashima T., Mori T., Niidome T., Katayama Y., Okitsu O. (2008) Efficient delivery of siRNA using dendritic poly(l-lysine) for loss-of-function analysis. J. Control Release 126, 59–66 [DOI] [PubMed] [Google Scholar]
- 36. Koo S. H., Flechner L., Qi L., Zhang X., Screaton R. A., Jeffries S., Hedrick S., Xu W., Boussouar F., Brindle P., Takemori H., Montminy M. (2005) The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature 437, 1109–1111 [DOI] [PubMed] [Google Scholar]
- 37. Lee J. M., Seo W. Y., Song K. H., Chanda D., Kim Y. D., Kim D. K., Lee M. W., Ryu D., Kim Y. H., Noh J. R., Lee C. H., Chiang J. Y., Koo S. H., Choi H. S. (2010) AMPK-dependent repression of hepatic gluconeogenesis via disruption of CREB·CRTC2 complex by orphan nuclear receptor small heterodimer partner. J. Biol. Chem. 285, 32182–32191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chanda D., Xie Y. B., Choi H. S. (2010) Transcriptional corepressor SHP recruits SIRT1 histone deacetylase to inhibit LRH-1 transactivation. Nucleic Acids Res. 38, 4607–4619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Quinn P. G., Wong T. W., Magnuson M. A., Shabb J. B., Granner D. K. (1988) Identification of basal and cyclic AMP regulatory elements in the promoter of the phosphoenolpyruvate carboxykinase gene. Mol. Cell. Biol. 8, 3467–3475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rommerts F. F., van Roemburg M. J., Lindh L. M., Hegge J. A., van der Molen H. J. (1982) The effects of short term culture and perifusion on LH-dependent steroidogenesis in isolated rat Leydig cells. J. Reprod. Fertil. 65, 289–297 [DOI] [PubMed] [Google Scholar]
- 41. Kim G. S., Lee G. Y., Nedumaran B., Park Y. Y., Kim K. T., Park S. C., Lee Y. C., Kim J. B., Choi H. S. (2008) The orphan nuclear receptor DAX-1 acts as a novel transcriptional corepressor of PPARγ. Biochem. Biophys. Res. Commun. 370, 264–268 [DOI] [PubMed] [Google Scholar]
- 42. Wicks W. D., McKibbin J. B. (1972) Evidence for translational regulation of specific enzyme synthesis by N6,O2′-dibutyryl cyclic AMP in hepatoma cell cultures. Biochem. Biophys. Res. Commun. 48, 205–211 [DOI] [PubMed] [Google Scholar]
- 43. Yang J., Kalhan S. C., Hanson R. W. (2009) What is the metabolic role of phosphoenolpyruvate carboxykinase? J. Biol. Chem. 284, 27025–27029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. She P., Shiota M., Shelton K. D., Chalkley R., Postic C., Magnuson M. A. (2000) Phosphoenolpyruvate carboxykinase is necessary for the integration of hepatic energy metabolism. Mol. Cell. Biol. 20, 6508–6517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Burgess S. C., Hausler N., Merritt M., Jeffrey F. M., Storey C., Milde A., Koshy S., Lindner J., Magnuson M. A., Malloy C. R., Sherry A. D. (2004) Impaired tricarboxylic acid cycle activity in mouse livers lacking cytosolic phosphoenolpyruvate carboxykinase. J. Biol. Chem. 279, 48941–48949 [DOI] [PubMed] [Google Scholar]
- 46. Moyle W. R., Armstrong D. T. (1970) Stimulation of testosterone biosynthesis by luteinizing hormone in transplantable mouse Leydig cell tumors. Steroids 15, 681–693 [DOI] [PubMed] [Google Scholar]
- 47. Owen O. E., Kalhan S. C., Hanson R. W. (2002) The key role of anaplerosis and cataplerosis for citric acid cycle function. J. Biol. Chem. 277, 30409–30412 [DOI] [PubMed] [Google Scholar]