Background: The PI3K-AKT pathway is an important signaling pathway that may be affected by viral infections.
Results: HCV transiently activates AKT during the early stage of viral infection. The suppression of the PI3K-AKT pathway inhibits HCV entry.
Conclusion: HCV transiently activates PI3K-AKT to enhance its entry into the host cell.
Significance: The studies provide important information for understanding HCV-host interactions.
Keywords: Akt, Hepatitis C Virus, PI 3-Kinase (PI3K), Receptors, Virus Entry, AKT Activation, HCV E2 Protein, HCV Co-receptors, HCV Entry
Abstract
The PI3K-AKT signaling pathway plays an important role in cell growth and metabolism. Here we report that hepatitis C virus (HCV) transiently activates the PI3K-AKT pathway. This activation was observed as early as 15 min postinfection, peaked by 30 min, and became undetectable at 24 h postinfection. The activation of AKT could also be mediated by UV-inactivated HCV, HCV pseudoparticle, and the ectodomain of the HCV E2 envelope protein. Because antibodies directed against CD81 and claudin-1, but not antibodies directed against scavenger receptor class B type I or occludin, could also activate AKT, the interaction between HCV E2 and its two co-receptors CD81 and claudin-1 probably triggered the activation of AKT. This activation of AKT by HCV was important for HCV infectivity, because the silencing of AKT by siRNA or the treatment of cells with its inhibitors or with the inhibitor of its upstream regulator PI3K significantly inhibited HCV infection, whereas the expression of constitutively active AKT enhanced HCV infection. The PI3K-AKT pathway is probably involved in HCV entry, because the inhibition of this pathway could inhibit the entry of HCV pseudoparticle but not the VSV pseudoparticle into cells. Furthermore, the treatment of cells with the AKT inhibitor AKT-V prior to HCV infection inhibited HCV infection, whereas the treatment after HCV infection had no obvious effect. Taken together, our studies indicated that HCV transiently activates the PI3K-AKT pathway to facilitate its entry. These results provide important information for understanding HCV replication and pathogenesis and raised the possibility of targeting this cellular pathway to treat HCV patients.
Introduction
Hepatitis C virus (HCV)2 is an important blood-borne pathogen that can cause severe liver diseases, including cirrhosis and hepatocellular carcinoma (1). This virus is an enveloped positive-stranded RNA virus belonging to the Flaviviridae family. Its 9.6-kb genome encodes a polyprotein, which is slightly more than 3000 amino acids in length, as well as a small protein named F protein that uses an alternative reading frame. The HCV polyprotein is cleaved by cellular and viral proteases into 10 different proteins named core, E1, E2, p7, NS2, NS3, NS4A, NS4B, NS5A, and NS5B (2–5). The HCV virion is composed of the viral RNA genome, the core protein, a lipid envelope, and the two major viral envelope proteins, E1 and E2. The virion is also associated with the very low density lipoprotein, which plays an important role in viral attachment to the cell surface (6–8).
HCV entry into its host cell is a tightly regulated process that involves a number of cell surface molecules in sequential steps (8–14). It has been shown that the initial attachment of HCV to the cell is mediated by its associated apolipoprotein E (apoE), which binds to heparan sulfate on the cell surface (8). It has been also shown that the scavenger receptor class B type I (SR-BI) is a critical co-receptor for HCV entry (10). SR-BI is a receptor for lipoproteins and can bind to the HCV E2 protein. It mediates HCV entry in a manifold manner, including its interaction with the lipoproteins associated with HCV and its modification of the lipid composition of the plasma membrane (15–22). The tetraspanin protein CD81 is another co-receptor for HCV, which interacts with the HCV glycoprotein E2 at a postattachment step. A recent study determined that the maximal half-time for CD81-mediated HCV entry was 17 min, suggesting a role of CD81 in HCV entry in the immediate early step after binding (23). The tight junction protein claudin-1 is mainly expressed in the liver, and its first extracellular loop is responsible for the interaction with CD81. Based on the observation that the maximal half-time for the anti-claudin-1 antibody to inhibit HCV entry was 73 min, it was suggested that claudin-1, which could form a complex with CD81 (24), played a role in a later step of HCV entry after the CD81 and E2 interaction. Occludin is another tight junction protein that is required for HCV entry at a postattachment step. Both CD81 and occludin restrict the host range of HCV, because they cannot be replaced by their murine homologues to mediate viral entry (25, 26).
The class I phosphatidylinositol 3-kinase (PI3K) is activated by G protein-coupled receptors and tyrosine kinase receptors. Upon its activation, it converts phosphatidylinositol 4,5-biphosphate to phosphatidylinositol 3,4,5-triphosphate (27), which binds to and recruits AKT to the membrane for its phosphorylation by PDK1 at threonine 308 and mTORC2 (mTOR complex 2) at serine 473 (28). This phosphorylation by PDK1 and mTORC2 activates AKT, which then regulates the activities of its many downstream effectors to affect cell survival, proliferation, migration, differentiation, and apoptosis (29). There are three different isoforms of AKT, which share a high degree of sequence homology. Whereas AKT1 and AKT2 are ubiquitous, AKT3 is only detected in the brain. AKT1 is anti-apoptotic and involved in cell survival, and AKT2 is a key effector of the insulin signaling pathway and regulates cellular metabolism (30).
Many viruses regulate the PI3K-AKT pathway for their replication (31). Picornaviruses and paramyxoviruses have been shown to activate the PI3K-AKT pathway to promote viral replication. The influenza A virus also activates the PI3K-AKT pathway to enhance viral replication at a postentry step, and its NS1 protein can bind to and activate PI3K to suppress apoptosis (32). The avian leukosis virus (33) and Ebola virus (34) utilize the PI3K-AKT pathway to facilitate their entry. The replication complex of Sindbis virus stimulates PI3K-AKT to promote viral protein translation (35). Arenavirus activates PI3K-AKT to enhance viral RNA synthesis (36). Human cytomegalovirus (37), coxsackievirus B3 (38), and varicella-zoster virus (39) require PI3K-AKT for efficient infection as well. Although PI3K-AKT pathway promotes viral infection in most cases, it suppresses hepatitis B virus replication (40).
The possible relationship between HCV and the PI3K-AKT pathway has also been studied. It has been shown that the HCV NS5A protein binds to PI3K and activates the PI3K-AKT signaling pathway (41, 42). In addition, the HCV core protein as well as HCV infection can induce the phosphorylation of serine 473 of AKT with no apparent effect on the phosphorylation of threonine 308 and hence impair the insulin signaling pathway (43, 44). However, little is known regarding how the effect of HCV on the PI3K-AKT signaling pathway might affect HCV replication. In this report, we describe our studies on the relationship between HCV and the PI3K-AKT pathway. Surprisingly, we found that HCV rapidly and transiently activated AKT in the early stage of infection to enhance its entry. We further found that this activation was mediated by the interaction between the HCV E2 envelope protein and its co-receptors, CD81 and claudin-1.
EXPERIMENTAL PROCEDURES
Cell Lines, Virus Stock, Antibodies, and Reagents
Huh7.5 and 293T cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% non-essential amino acids (Invitrogen). The HCV genotype 2a JFH1 adaptive mutant, which produces a high level of infectious virus particles, has been recently described (45).
AKT, pAKT-473, and pAKT-308 antibodies were purchased from Cell Signaling. The rabbit anti-core antibody was produced in rabbits using the HCV core protein expressed in Escherichia coli (46). Soluble E2 (sE2), the neutralizing CBH-5 human mAb against HCV E2, and the isotype-matched control human mAb R04 have been described previously (47). The rabbit anti-actin antibody, the mouse anti-SR-BI antibody, and a control mouse IgG were purchased from Abcam. The mouse anti-CD81 antibody was purchased from BD Biosciences; the mouse anti-claudin-1 antibody was from Invitrogen; and the mouse anti-occludin antibody was obtained from Calbiochem. The monoclonal anti-HA antibody was prepared at the Caltech Hybridoma Core. Horseradish peroxidase (HRP)-conjugated goat anti-rabbit and goat anti-mouse secondary antibodies were purchased from Abcam. The Alexa 488-conjugated goat anti-rabbit antibody was obtained from Thermo Scientific. The mounting medium FluoroShield with DAPI was purchased from Sigma. The PI3K inhibitor wortmannin was purchased from Sigma. AKT inhibitor II and V were obtained from EMD Millipore and Calbiochem, respectively.
Analysis of the Effects of sE2 and Antibodies on AKT Activation
To determine the effect of sE2 on AKT, Huh7.5 cells were incubated with different amounts of sE2 for 2 h and then lysed for the immunoblot analysis of AKT. For the anti-E2 antibody neutralization assay, the HCV virus stock or sE2 was incubated with the CBH-5 anti-E2 antibody or the isotype control R04 on ice for 1 h prior to the incubation with Huh7.5 cells. Cells were lysed 2 h postincubation for the immunoblot analysis of AKT. For the analysis of the effects of anti-co-receptor antibodies on AKT, Huh7.5 cells were incubated with the control mouse IgG or the antibody directed against CD81, SR-BI, claudin-1, or occludin (5 μg/ml) for 2 h. Cell lysates were then collected for the AKT immunoblot analysis.
AKT Kinase Assay
The AKT kinase assay was conducted using the assay kit from Cell Signaling. Briefly, 200 μg of Huh7.5 cell lysates with or without HCV infection for 2 h were incubated with Sepharose beads conjugated with the anti-pAKT-473 antibody overnight at 4 °C. After washing, beads were resuspended and incubated with 1 μl of 10 mm ATP and 1 μg of the recombinant GSK-3 substrate at 30 °C for 30 min. The AKT kinase activity was analyzed by immunoblot analysis of the level of phosphorylated GSK-3.
UV Inactivation of HCV
HCV was irradiated with the UV light for 5 min and then used to infect Huh7.5 cells. Cells were lysed at 2 and 24 h postinfection for immunoblot analysis.
Pseudotyped Virus Particle Infection Assay
Pseudoparticles of HCV (HCVpp) and vesicular stomatitis virus (VSVpp) that carried the firefly luciferase reporter gene were generated in 293T cells (14). Huh7.5 cells seeded in 6-well plates and pretreated with DMSO, AKT-V, or wortmannin for 3 h were inoculated with the same amount of HCVpp, VSVpp, or EnV− pseudoparticles. After washing with phosphate-buffered saline (PBS) to remove the pseudoparticles, cells were lysed 72 h postinfection for the luciferase assay using the luciferase assay kit (Promega). The pseudoviral infectivity was determined after the subtraction of the mean EnV− signal from the mean HCVpp or VSVpp signal.
siRNA Silencing of AKT
Pooled siRNAs against AKT1, AKT2, and AKT3 (Sigma) were transfected with Lipofectamine 2000 (Invitrogen) into Huh7.5 cells following the manufacturer's instructions. Briefly, 4 × 104 cells seeded in a 35-mm dish were transfected with 10 μl of AKT siRNAs (20 μm each) for 5 h and then transfected 24 h later with AKT siRNAs one more time for another 5 h. After the second transfection, cells were inoculated with HCV (MOI = 1) for 24 h and then lysed for immunoblotting or fixed for immunofluorescence staining.
Expression of myristoylated AKTs (myr-AKTs)
4 × 104 Huh7.5 cells were seeded in a 35-mm dish and then transfected the next day with 2 μg of myr-AKT1, myr-AKT2 (generous gifts from Dr. Deborah Johnson), or the control pCDNA3.1 expression vector using Lipofectamine 2000. Five hours later, the transfection medium was replaced with the fresh DMEM containing 10% FBS. Twenty-four hours later, cells were inoculated with HCV (MOI = 1) for 24 h and lysed for immunoblotting or fixed for immunofluorescence staining.
Immunoblotting
Cells were rinsed with PBS and lysed with the radioimmune precipitation assay buffer (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 1% Triton X-100, 1 mm PMSF). After the sonication, cell lysates were subjected to centrifugation to remove cell debris. An aliquot of the supernatant containing 50 μg of proteins was used for gel electrophoresis. After the semi-wet transfer, the membrane was blocked with 5% milk for 30 min and incubated with the primary antibody for 4 h. After three washes with PBS containing 1% Tween 20 (PBST), the membrane was incubated with the HRP-conjugated secondary antibody for 2 h. After further washes with PBST, chemiluminescent substrates (Pierce) were applied on the membrane, and the image was captured using the LAS-4000 imaging system (FujiFilm).
Immunofluorescence Staining and Confocal Microscopy
Huh7.5 cells were seeded on a coverslip in a 6-well plate (5 × 105 cells/well). The next day, cells were rinsed with PBS and fixed with 4% paraformaldehyde in PBS for 15 min. After washing with PBS, cells were incubated in the blocking solution (PBS, 5% bovine serum albumin (BSA)) for 30 min and incubated with the control mouse IgG or the antibody directed against CD81, SR-BI, claudin-1, or occludin (1:200 dilution) for 2 h. Cells were washed with PBS three times and then incubated with the goat anti-mouse Alexa-488 secondary antibody (1:200 dilution) for 2 h. After washing with PBS, the coverslip was mounted onto a glass slide with the mounting medium FluroShield for confocal microscopy.
Quantification of HCV Infectivity
2 × 104 Huh7.5 cells were seeded per chamber in the 8-well chamber slide and inoculated with serially diluted HCV the next day. Forty-eight hours after infection, cells were rinsed with PBS and fixed with 4% paraformaldehyde for 15 min. After the PBS wash, cells were incubated with the blocking solution (PBS, 5% BSA, 0.3% Triton X-100) for 30 min followed by incubation with the rabbit anti-core antibody for 2 h. Cells were washed with PBT (PBS, 0.3% Triton X-100) three times and incubated with the goat anti-rabbit Alexa-488 for 2 h. After three washes with PBT, cells on the slide were mounted with FluroShield with DAPI. The HCV core-positive cells were counted under the microscope for titration.
Cell Viability Assay
Huh7.5 cells were treated with DMSO or the inhibitors to PI3K and AKT for 24 h. Cells were then trypsinized and mixed 1:9 with 0.4% trypan blue for the trypan blue exclusion assay. The cell viability was quantified using a hemocytometer.
RESULTS
Transient Activation of AKT by HCV
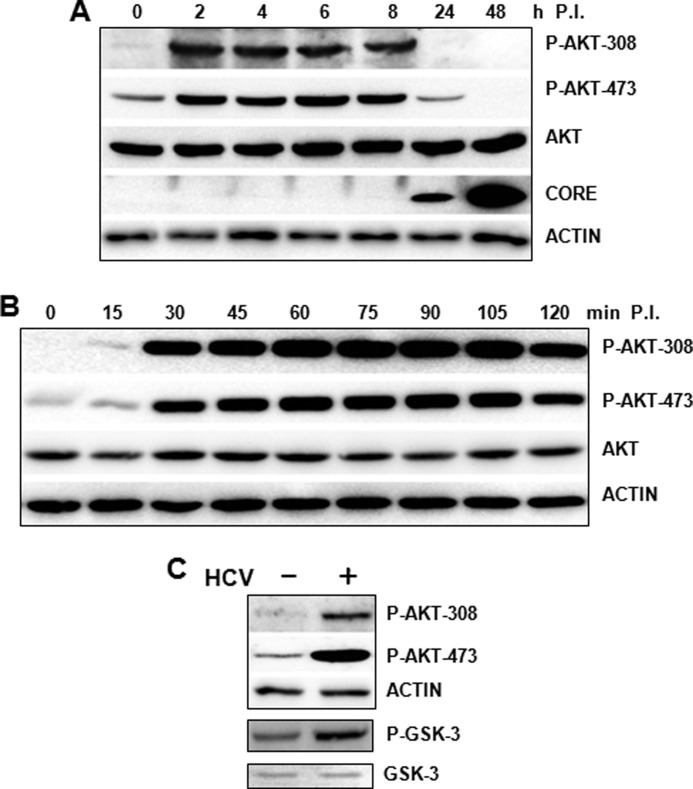
To determine whether HCV could affect the PI3K-AKT pathway, we infected Huh7.5 cells with a cell culture-adapted HCV JFH1 variant, which produced a significantly higher level of infectious virions than its parental JFH1 virus (45), and analyzed AKT at different time points after infection over a 2-day period. As shown in Fig. 1A, HCV did not affect the overall protein level of AKT in infected cells. However, it induced the phosphorylation of AKT at threonine 308 and serine 473 between 2 and 8 h postinfection. By 24 h postinfection, when the HCV core protein became detectable in infected cells, the induction of AKT phosphorylation was no longer visible, and instead, a suppression of AKT phosphorylation at threonine 308 and serine 473 was observed. This suppression of ATK phosphorylation by HCV was even more pronounced at 48 h postinfection. Because the induction of AKT phosphorylation peaked within 2 h of infection, we decided to further analyze the AKT status at earlier time points after HCV infection. As shown in Fig. 1B, the induction of AKT phosphorylation at threonine 308 and serine 473 was visible as early as 15 min postinfection, peaked at 30 min, and was slightly reduced at 2 h postinfection. To ensure that this induction of AKT phosphorylation by HCV indeed increased the AKT kinase activity, we immunoprecipitated the phosphorylated AKT from Huh7.5 cells with and without HCV infection using the anti-pAKT-473 antibody and analyzed the kinase activity of AKT in vitro using its substrate protein, GSK-3. As shown in Fig. 1C, HCV-infected cells indeed exhibited a higher AKT kinase activity, confirming the activation of AKT by HCV.
FIGURE 1.
Transient activation of AKT by HCV. A, Huh7.5 cells were infected with HCV at an MOI of 1 for 0, 2, 4, 6, 8, 24, and 48 h, as indicated, followed by the analysis of AKT phosphorylation at threonine 308 (P-AKT-308) and serine 473 (P-AKT-473) by immunoblot. The total AKT level and HCV core protein were also analyzed. B, Huh7.5 cells were infected with HCV as in A with the exception that the cells were lysed at the time points indicated. The actin was also analyzed to serve as the loading control in both A and B. C, Huh7.5 cells with or without HCV infection (MOI = 1) for 2 h were lysed and immunoprecipitated with the pAKT-473 antibody. The immunoprecipitated AKT was then analyzed for its kinase ability using its substrate, GSK-3. The phosphorylated GSK-3 was analyzed by immunoblot using the anti-phospho-GSK-3 antibody. The total GSK-3 was also analyzed to serve as the control.
Induction of AKT Activation by HCV E2 and Its Co-receptors CD81 and Claudin-1
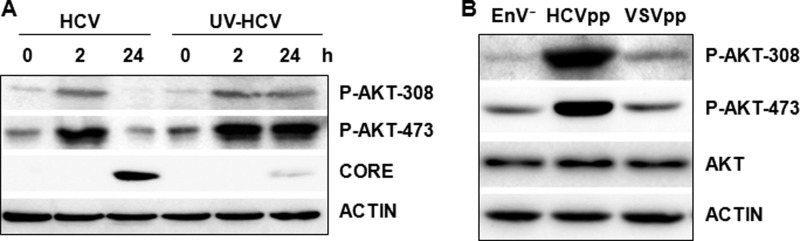
The observation that the AKT activation was detected as early as 15 min and peaked by 30 min after HCV infection indicated the involvement of an early HCV infection event, possibly the interaction between HCV and its co-receptors, in the activation of AKT. To test this possibility, we infected Huh7.5 cells with UV-irradiated HCV. The UV irradiation does not affect the interaction between HCV and its co-receptors, but it damages the viral genomic RNA and disrupts the HCV life cycle after the step of viral entry. As shown in Fig. 2A, the UV treatment caused a drastic reduction of the HCV core protein level in cells 24 h postinfection, which was expected. However, this UV treatment did not abolish the phosphorylation of AKT at threonine 308 and serine 473. Interestingly, although the AKT phosphorylation was reduced to slightly below the basal level in cells infected by the non-UV-treated virus at 24 h postinfection, the AKT phosphorylation remained at a high level in cells infected by the UV-irradiated virus at that time point. These results indicated that an early event during HCV entry triggered the activation of AKT, and a subsequent step after HCV entry was required to inactivate AKT. To further confirm that the AKT activation requires only the initial interaction between HCV and the host cell, we produced lentiviral pseudoparticles HCVpp and VSVpp and determined their effects on AKT activation. HCVpp and VSVpp were identical with the exception that the envelope of HCVpp contained the HCV E1 and E2 proteins, whereas that of VSVpp contained the VSV G protein. As shown in Fig. 2B, the infection of Huh7.5 cells by HCVpp, but not VSVpp, could induce the phosphorylation of AKT at threonine 308 and serine 473. These results lent further support to the possibility that the activation of AKT might be mediated by the interaction between HCV and its co-receptors.
FIGURE 2.
Effect of UV irradiation and HCVpp on AKT activation by HCV. A, HCV particles with and without UV irradiation for 5 min were used to infect Huh7.5 cells. Cells were lysed at 0, 2, and 24 h postinfection for the analysis of phospho-AKT and the HCV core protein. B, Huh7.5 cells were inoculated with the control EnV− particle, HCVpp, or VSVpp for 3 h, and cell lysates were collected for immunoblot analysis. The actin was also analyzed to serve as the loading control in A and B.
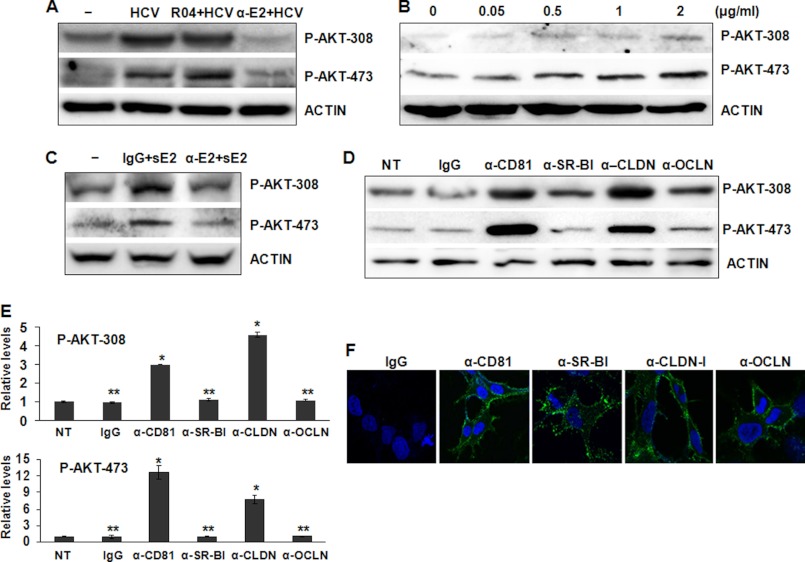
To further investigate whether the activation of AKT by HCV was indeed due to the interaction between HCV and its co-receptors, we preincubated HCV with the R04 isotype control or the CBH-5 anti-E2 antibody, previously shown to neutralize the infectivity of HCV (48), prior to the infection of Huh7.5 cells. Cells were then lysed for the immunoblot analysis 2 h postinfection. As shown in Fig. 3A, the HCV-induced AKT activation was not affected by the control antibody, but it was abolished by the anti-E2 antibody. This result indicated an important role of HCV E2 in the activation of AKT. To test whether HCV E2 by itself would be sufficient to activate AKT, we incubated Huh7.5 cells with sE2 (i.e. the ectodomain of HCV E2) for 2 h. Indeed, sE2 by itself was able to induce the phosphorylation of AKT at threonine 308 and serine 473 in a dose-dependent manner (Fig. 3B), and this induction could be blocked by the anti-E2 antibody but not by the control antibody (Fig. 3C).
FIGURE 3.
Effects of soluble E2 and antibodies directed against HCV E2 and co-receptors on the activation of AKT. A, HCV was preincubated with the control antibody R04 or the anti-E2 antibody CBH-5 (10 μg/ml) for 1 h on ice prior to infection of Huh7.5 cells. Mock-infected cells and HCV not preincubated with any antibodies were used as the controls. Cell lysates were collected at 2 h postinfection and analyzed by immunoblotting for the phospho-AKT levels. The actin was also analyzed to serve as the loading control. B, Huh7.5 cells were incubated with different concentrations of sE2 for 2 h. Cell lysates were collected for the immunoblot analysis of phospho-AKT. C, sE2 (2 μg/ml) was preincubated with the control antibody R04 or the anti-E2 antibody CBH-5 (1.25 μg/ml) on ice for 1 h and then used to treat Huh7.5 cells, which were lysed 2 h later for the immunoblot analysis of AKT. D, Huh7.5 cells were treated with the control mouse IgG or the mouse antibodies directed against CD81, SR-BI, claudin-1, and occludin (5 μg/ml) for 2 h. Cells were then lysed for the immunoblot analysis of phospho-AKT. The actin served as a loading control. NT, cells not treated with antibodies. E, quantitative analysis of phospho-AKT levels shown in D. The phospho-AKT levels in non-treated cells were arbitrarily defined as 1. *, p < 0.001; **, p > 0.05. F, Huh7.5 cells were fixed and stained with the control IgG or the antibodies directed against CD81, SR-BI, claudin-1, and occludin. Nuclei were stained with DAPI. Images were captured using the Zeiss LSM 510 confocal microscope. Error bars, S.D.
HCV entry is mediated by its co-receptors SR-BI, CD81, claudin-1, and occludin. To further test the roles of these co-receptors in the activation of AKT by HCV, we treated naive Huh7.5 cells with monoclonal antibodies directed against CD81, SR-BI, claudin-1, and occludin. We then conducted the immunoblot analysis to determine the effects of these antibodies on AKT. As shown in Fig. 3, D and E, although the control IgG, the anti-SR-BI antibody, and the anti-occludin antibody had no apparent effect on the activation of AKT, both anti-CD81 and anti-claudin-1 antibodies activated AKT. Because all of the anti-co-receptor antibodies could bind to their respective target proteins on the cell surface, as revealed by the immunofluorescence staining assay (Fig. 3F), these results strongly indicated that the activation of AKT by HCV was mediated by the interaction between its E2 protein and its cellular co-receptors CD81 and claudin-1.
Positive Effect of AKT on HCV Infection
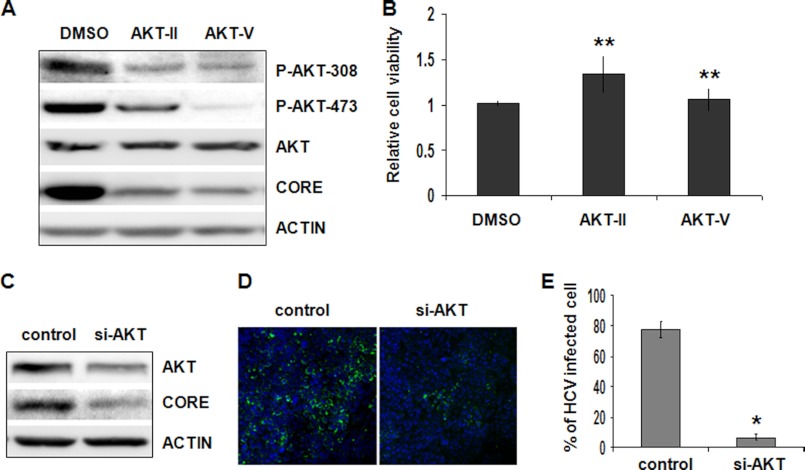
The observation that HCV could activate AKT prompted us to investigate the possible effect of AKT on HCV infection. We first treated Huh7.5 cells with AKT inhibitors ATK-II and AKT-V for 3 h and then infected cells with HCV to assess the possible effect of AKT on HCV infectivity. As shown in Fig. 4A, the AKT phosphorylation at threonine 308 and serine 473 was significantly inhibited by either of the two AKT inhibitors. When the HCV core protein was analyzed, a significant reduction of the core protein level by these two AKT inhibitors was observed. This reduction of the HCV core protein level was not due to the cytotoxicity of AKT inhibitors, because there was no apparent effect of these two drugs on cell viability under the experimental conditions (Fig. 4B). These results suggested a positive role of AKT in HCV infection. To rule out the possibility of nonspecific effects of AKT inhibitors, we also used a pool of siRNAs that targeted all three AKT isoforms to inhibit the expression of AKT for 24 h prior to the infection of cells with HCV. Cells were lysed a day after infection for the analysis of AKT and HCV core protein levels. As shown in Fig. 4C, the AKT siRNAs inhibited the expression of AKT and in the meantime significantly reduced the HCV core protein level, again supporting a positive role of AKT in HCV infection. To further confirm these results, we also performed the immunofluorescence staining analysis. As shown in Fig. 4, D and E, the inhibition of AKT expression with the siRNAs reduced the number of HCV-positive cells by ∼70%, confirming a positive role of AKT in HCV infection.
FIGURE 4.
Analysis of the role of AKT on HCV infectivity. A, Huh7.5 cells were pretreated with the control DMSO or the AKT inhibitor AKT-II (5 μm) or AKT-V (20 μm) for 3 h followed by infection with HCV (MOI = 1) for 24 h. Cell lysates were collected and analyzed by immunoblot. B, Huh7.5 cells were treated with DMSO, AKT-II (5 μm), or AKT-V (20 μm) for 24 h, and live cells were quantified by the trypan blue exclusion assay. The results represent the average of three independent experiments. The statistical difference of cell viability was analyzed by Student's t test. There was no difference among different samples. **, p > 0.05. C, Huh7.5 cells were transfected with the control siRNA or the pooled siRNAs targeting AKT1, AKT2, and AKT3 for 1 day. The siRNA transfection was repeated one more time on the second day, and, after replacing medium, cells were infected with HCV (MOI = 1) and lysed 24 h later for immunoblot analysis for AKT, core protein, and actin. D, Huh7.5 cells transfected with the control siRNA or pooled siRNAs against AKT1, AKT2, and AKT3 were infected with HCV (MOI = 1) as described in C. Cells were fixed 24 h postinfection and immunostained with the anti-HCV core antibody. E, quantification of HCV core-positive cells in D. The results represent the average of three independent experiments. *, p < 0.001. Error bars, S.D.
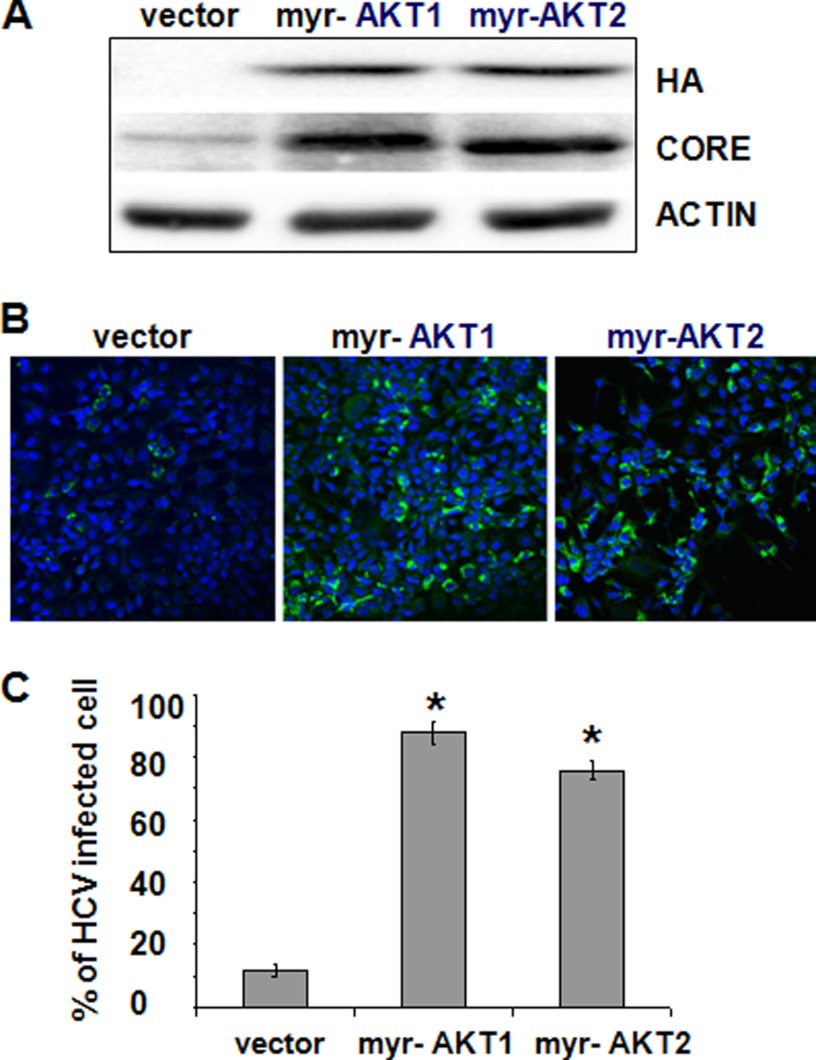
To further test the role of AKT in HCV infection and to rule out the possibility that the effect of AKT siRNAs on HCV was due to nonspecific off-target effects, we also expressed myr-AKT1 and myr-AKT2 in Huh7.5 cells and then infected the cells with HCV the second day after DNA transfection. Both myr-AKT1 and myr-AKT2 are constitutively active AKTs (49). We used a lower MOI for infection (MOI = 0.1) so that the enhancing effect of myr-AKTs could be easily analyzed. As shown in Fig. 5A, the expression of either myr-AKT1 or myr-AKT2 markedly elevated the expression level of the HCV core protein. Similarly, when the immunofluorescence staining assay was conducted, either myr-AKT1 or myr-AKT2 increased the number of HCV-positive cells ∼7-fold (Fig. 5, B and C). These results were again in support of the results shown in Fig. 3 and confirmed that AKT played a positive role in HCV infection.
FIGURE 5.
Effect of constitutively active AKT on HCV infection. A, Huh7.5 cells were transfected with the control vector pCDNA3.1 and the expression vector for the HA-tagged myr-AKT1 or myr-AKT2 and infected with HCV (MOI = 0.1) 24 h later. Cell lysates were collected 24 h after infection for immunoblot analysis of HA-tagged myr-AKTs, the core protein, and actin. B, Huh7.5 cells transfected with pCDNA3.1 or the expression plasmid for HA-tagged myr-AKT1 or myr-AKT2 were infected with HCV (MOI = 0.1) as described in A. Cells were fixed and immunostained with the anti-HCV core antibody. C, quantification of HCV core-positive cells in B. The results represent the average of three independent experiments. The difference of the results between vector-transfected cells and myr-AKT1-transfected cells and between vector-transfected cells and myr-AKT2-transfected cells was statistically significant. *, p < 0.001. Error bars, S.D.
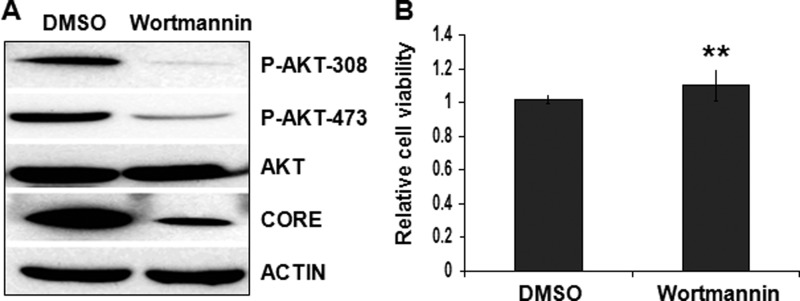
PI3K is the upstream regulator of AKT. Thus, if AKT plays a positive role in HCV infection, then the inhibition of the PI3K activity should also inhibit HCV infection. To test this possibility, we pretreated Huh7.5 cells with wortmannin, a well characterized inhibitor of PI3K, followed by HCV infection. As shown in Fig. 6A, wortmannin markedly inhibited the AKT phosphorylation and reduced the HCV core protein level. Similarly, this effect of wortmannin on HCV was not due to the cytotoxicity of the drug, because wortmannin had no apparent effect on cell viability under our experimental conditions (Fig. 6B). These results further confirmed that the PI3K-AKT pathway played a positive role in HCV infection.
FIGURE 6.
Analysis of the PI3K-AKT pathway in HCV infection. A, Huh7.5 cells were pretreated with the control DMSO or the PI3K inhibitor wortmannin (2.5 μm) for 3 h followed by infection with HCV (MOI = 1) for 24 h. Cell lysates were collected and analyzed by immunoblot. B, Huh7.5 cells were treated with DMSO or wortmannin (2.5 μm) for 24 h, and live cells were quantified by the trypan blue exclusion assay. The results represent the average of three independent experiments. **, p > 0.05. Error bars, S.D.
Enhancement of HCV Entry by the Activated PI3K-AKT Pathway
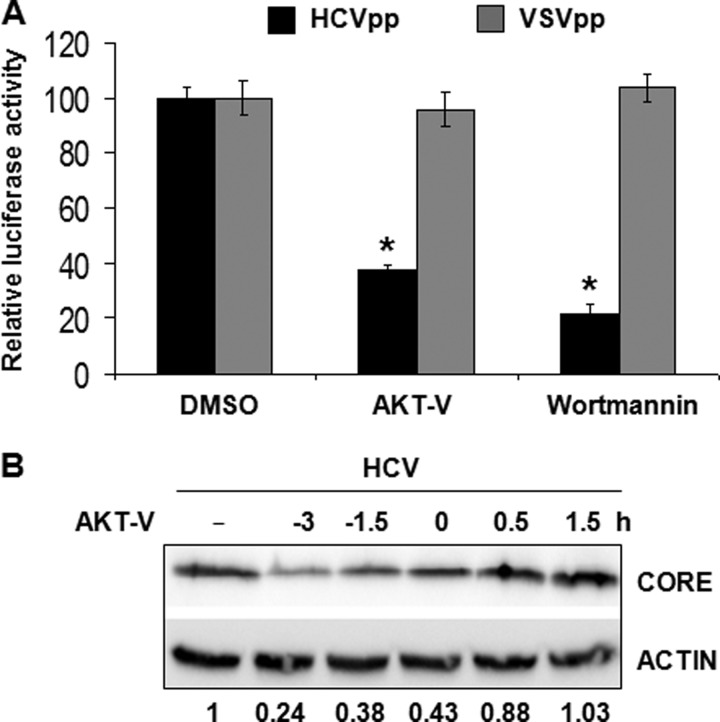
To further investigate how the activated PI3K-AKT pathway supported HCV infection, we used HCVpp and VSVpp, which carried the firefly luciferase reporter gene, to assess the possible effect of AKT on HCV entry. Huh7.5 cells were pretreated with AKT-V or wortmannin, a PI3K inhibitor, for 3 h and then infected with HCVpp or VSVpp for 72 h. Cells were then lysed for the luciferase assay. As shown in Fig. 7A, the pretreatment of Huh7.5 cells with either the AKT inhibitor AKT-V or the PI3K inhibitor wortmannin reduced the infectivity of HCVpp by ∼60 and 80%, respectively, without affecting the infectivity of VSVpp. Because the only difference between HCVpp and VSVpp was their envelope proteins, these results strongly suggested that the activation of the PI3K-AKT pathway was required to enhance HCV entry.
FIGURE 7.
Analysis of the effect of the PI3K-AKT pathway on HCV entry. A, Huh7.5 cells were treated with AKT-V (20 μm) or wortmannin (2.5 μm) for 3 h and then infected with HCVpp or VSVpp. Cell lysates were collected for the luciferase assay to determine the infectivity of HCVpp and VSVpp. The luciferase activity of cells treated with DMSO was arbitrarily defined as 100%. The results represent the average of three independent experiments. The differences among the VSVpp-infected cells are statistically insignificant. In contrast, for HCVpp-infected cells, the difference between cells treated with DMSO and AKT-V and between cells treated with DMSO and wortmannin is statistically significant. *, p < 0.001. B, Huh7.5 cells were treated with AKT-V (20 μm) at the time points indicated before, during, or after HCV infection (MOI = 1). Cell lysates were collected 24 h postinfection for the immunoblot analysis. The level of the core protein was quantified with ImageJ and normalized against the actin-loading control, and the relative core protein levels are shown below the gel. The core protein level of cells not treated (NT) with AKT-V was arbitrarily defined as 1. Error bars, S.D.
To further test the possible role of PI3K-AKT in HCV entry, we treated Huh7.5 cells with AKT-V before, during, and after HCV infection and assessed the effect of the AKT inhibition on HCV replication. As shown in Fig. 7B, the effect of AKT-V on HCV infectivity, as determined by the HCV core protein level, was dependent on when AKT-V was used to treat cells. The treatment of cells with AKT-V 3 h prior to HCV infection reduced the HCV core protein level to ∼24%. The core protein level was increased to about 38% if AKT-V was added 1.5 h prior to HCV infection and to about 43% when it was added together with HCV to the cells. The inhibitory effect of the drug on HCV was marginal if it was added 0.5 h after HCV infection and not observed if it was added 1.5 h postinfection. These time-dependent inhibitory effects of AKT-V on HCV again supported the notion that the PI3K-AKT pathway was required for efficient HCV entry.
DISCUSSION
The PI3K-AKT signaling pathway plays an important role in the regulation of cell survival, proliferation, migration, differentiation, apoptosis, and metabolism (29). Many viruses have explored this signaling pathway to enhance their replication (31–39). In the present study, we demonstrated that HCV could activate AKT as early as 15 min after infection and prominently by 30 min of infection. This activation was transient and no longer visible at 24 h after infection (Fig. 1). The observation that the UV-inactivated HCV and HCVpp, but not VSVpp, could also activate AKT suggested a possible role of the interaction between HCV and its co-receptors in the activation of AKT (Fig. 2). This possibility was bolstered by the observations that the HCV sE2 protein could also activate AKT and that this effect could be blocked by the anti-E2 antibody CBH-5 (Fig. 3, B and C). Because either the anti-CD81 or the anti-claudin-1 antibody could replace HCV or sE2 to activate AKT (Fig. 3D), it is highly likely that the interaction between HCV E2 and these two HCV co-receptors led to the activation of AKT. Recently, it was demonstrated that claudin-1 could activate the PI3K-AKT pathway to inhibit the expression of E-cadherin in colon cancer cells (50). Thus, although HCV E2 does not bind directly to claudin-1, it may activate claudin-1 indirectly through the binding to CD81, which forms a complex with claudin-1 after engaging HCV (24, 51), to result in the activation of the PI3K-AKT pathway.
Interestingly, although the UV irradiation, which inactivated HCV, did not abolish the ability of HCV to activate AKT, it abolished its ability to inactivate AKT 24 h after infection (Fig. 2A). The sustained activation of AKT by UV-irradiated HCV indicated that HCV could inactivate AKT via a postentry mechanism. Because the inactivation of AKT coincided with the detection of HCV core protein (Fig. 1), it is conceivable that this inactivation of AKT might be mediated by HCV gene products. Previously, it was demonstrated that the ectopically expressed HCV NS5A could bind to the p85 subunit of PI3K to activate the PI3K-AKT pathway (41, 42). It is unlikely that the activation of PI3K-AKT was due to the activity of NS5A, because the activation occurred in the very early stage of HCV infection and could also be mediated by sE2 or HCVpp, which did not express NS5A. It remains to be seen, however, whether in the context of HCV infection, this binding to PI3K by NS5A may actually inhibit the PI3K activity instead. It has also been shown that the core protein could impair the AKT phosphorylation at threonine 308 (43, 44). Thus, it is also possible that the inactivation of AKT by HCV may involve the HCV core protein. This may also explain why AKT was constitutively activated in HCV subgenomic RNA replicon cells (42) but not in our HCV infection studies. Alternatively, it has been demonstrated that the clustering of CD81 on the membrane or its lack of expression could result in the deactivation of AKT in hematopoietic stem cells (52). Thus, it is also possible that the clustering of CD81 and claudin-1 after HCV binding or their degradation in the endocytic pathway might also lead to the inactivation of AKT. In any case, the observation that AKT was inactivated 24 h after HCV infection indicates that this kinase might be dispensable for HCV replication in the later stages of the HCV life cycle.
The activation of AKT by HCV apparently enhanced the permissiveness of the cell for HCV infection, because the silencing of AKT with siRNAs inhibited HCV infection, whereas the expression of constitutively active AKT1 and AKT2 enhanced HCV infection (Figs. 4 and 5). Because the inhibition of the PI3K-AKT pathway with either the PI3K inhibitor wortmannin or the AKT inhibitor AKT-V reduced the infection of HCVpp but not VSVpp (Fig. 7A), it is highly likely that HCV activated the PI3K-AKT pathway to enhance its entry into cells. This possibility was further supported by the observation that only the treatment of cells with AKT-V prior to or at the time of HCV infection could significantly reduce the HCV infectivity (Fig. 7B). The transient activation of the PI3K-AKT pathway by HCV was also consistent with a role of this pathway in the early stage of HCV infection. It has been demonstrated that the activation of the PI3K-AKT pathway is important for the entry of the African swine fever virus and pneumococci into cells via endocytosis. In both cases, this activation of the PI3K-AKT pathway was associated with the reorganization of actin filaments (53, 54). It will be interesting to determine whether the activation of PI3K-AKT by HCV also enhances the actin reorganization and endocytosis of the virus. It should be noted that HCV appears to have evolved different mechanisms to enhance its entry, because it has also been reported that HCV infection activates protein kinase A (PKA) in a cAMP-dependent manner to retain claudin-1 on the plasma membrane, which then promotes the entry of HCV (55).
In conclusion, our results demonstrated that HCV could transiently activate the PI3K-AKT pathway to enhance its entry into the host cells. The continuous assault by HCV on this important pathway that regulates cellular growth and metabolism probably plays an important role in HCV pathogenesis. Our results also raised the possibility of targeting this pathway to treat HCV patients.
Acknowledgments
We thank Dr. Deborah Johnson (University of Southern California, Los Angeles) for providing the myr-AKT expression plasmids and Dr. Charles Rice for the Huh7.5 cell line.
This work was supported, in whole or in part, by National Institutes of Health Grants DK094652 and AI083025.
- HCV
- hepatitis C virus
- VSV
- vesicular stomatitis virus
- SR-BI
- scavenger receptor class B type I
- sE2
- soluble E2
- HCVpp
- pseudoparticles of HCV
- VSVpp
- pseudoparticles of VSV
- MOI
- multiplicity of infection
- myr-AKT
- myristoylated AKT.
REFERENCES
- 1. Shepard C. W., Finelli L., Alter M. J. (2005) Global epidemiology of hepatitis C virus infection. Lancet Infect. Dis. 5, 558–567 [DOI] [PubMed] [Google Scholar]
- 2. Bartenschlager R., Frese M., Pietschmann T. (2004) Novel insights into hepatitis C virus replication and persistence. Adv. Virus Res. 63, 71–180 [DOI] [PubMed] [Google Scholar]
- 3. Bartenschlager R., Sparacio S. (2007) Hepatitis C virus molecular clones and their replication capacity in vivo and in cell culture. Virus Res. 127, 195–207 [DOI] [PubMed] [Google Scholar]
- 4. Brass V., Moradpour D., Blum H. E. (2006) Molecular virology of hepatitis C virus (HCV). 2006 update. Int. J. Med. Sci. 3, 29–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Penin F., Brass V., Appel N., Ramboarina S., Montserret R., Ficheux D., Blum H. E., Bartenschlager R., Moradpour D. (2004) Structure and function of the membrane anchor domain of hepatitis C virus nonstructural protein 5A. J. Biol. Chem. 279, 40835–40843 [DOI] [PubMed] [Google Scholar]
- 6. Gastaminza P., Cheng G., Wieland S., Zhong J., Liao W., Chisari F. V. (2008) Cellular determinants of hepatitis C virus assembly, maturation, degradation, and secretion. J. Virol. 82, 2120–2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ye J. (2007) Reliance of host cholesterol metabolic pathways for the life cycle of hepatitis C virus. PLoS Pathog. 3, e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jiang J., Cun W., Wu X., Shi Q., Tang H., Luo G. (2012) Hepatitis C virus attachment mediated by apolipoprotein E binding to cell surface heparan sulfate. J. Virol. 86, 7256–7267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pileri P., Uematsu Y., Campagnoli S., Galli G., Falugi F., Petracca R., Weiner A. J., Houghton M., Rosa D., Grandi G., Abrignani S. (1998) Binding of hepatitis C virus to CD81. Science 282, 938–941 [DOI] [PubMed] [Google Scholar]
- 10. Scarselli E., Ansuini H., Cerino R., Roccasecca R. M., Acali S., Filocamo G., Traboni C., Nicosia A., Cortese R., Vitelli A. (2002) The human scavenger receptor class B type I is a novel candidate receptor for the hepatitis C virus. EMBO J. 21, 5017–5025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Evans M. J., von Hahn T., Tscherne D. M., Syder A. J., Panis M., Wölk B., Hatziioannou T., McKeating J. A., Bieniasz P. D., Rice C. M. (2007) Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature 446, 801–805 [DOI] [PubMed] [Google Scholar]
- 12. Liu S., Yang W., Shen L., Turner J. R., Coyne C. B., Wang T. (2009) Tight junction proteins claudin-1 and occludin control hepatitis C virus entry and are downregulated during infection to prevent superinfection. J. Virol. 83, 2011–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ploss A., Evans M. J., Gaysinskaya V. A., Panis M., You H., de Jong Y. P., Rice C. M. (2009) Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature 457, 882–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Benedicto I., Molina-Jiménez F., Bartosch B., Cosset F. L., Lavillette D., Prieto J., Moreno-Otero R., Valenzuela-Fernández A., Aldabe R., López-Cabrera M., Majano P. L. (2009) The tight junction-associated protein occludin is required for a postbinding step in hepatitis C virus entry and infection. J. Virol. 83, 8012–8020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rigotti A., Edelman E. R., Seifert P., Iqbal S. N., DeMattos R. B., Temel R. E., Krieger M., Williams D. L. (1996) Regulation by adrenocorticotropic hormone of the in vivo expression of scavenger receptor class B type I (SR-BI), a high density lipoprotein receptor, in steroidogenic cells of the murine adrenal gland. J. Biol. Chem. 271, 33545–33549 [DOI] [PubMed] [Google Scholar]
- 16. Reaven E., Nomoto A., Leers-Sucheta S., Temel R., Williams D. L., Azhar S. (1998) Expression and microvillar localization of scavenger receptor, class B, type I (a high density lipoprotein receptor) in luteinized and hormone-desensitized rat ovarian models. Endocrinology 139, 2847–2856 [DOI] [PubMed] [Google Scholar]
- 17. Reaven E., Zhan L., Nomoto A., Leers-Sucheta S., Azhar S. (2000) Expression and microvillar localization of scavenger receptor class B, type I (SR-BI) and selective cholesteryl ester uptake in Leydig cells from rat testis. J. Lipid Res. 41, 343–356 [PubMed] [Google Scholar]
- 18. Reaven E., Leers-Sucheta S., Nomoto A., Azhar S. (2001) Expression of scavenger receptor class B type 1 (SR-BI) promotes microvillar channel formation and selective cholesteryl ester transport in a heterologous reconstituted system. Proc. Natl. Acad. Sci. U.S.A. 98, 1613–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kellner-Weibel G., de La Llera-Moya M., Connelly M. A., Stoudt G., Christian A. E., Haynes M. P., Williams D. L., Rothblat G. H. (2000) Expression of scavenger receptor BI in COS-7 cells alters cholesterol content and distribution. Biochemistry 39, 221–229 [DOI] [PubMed] [Google Scholar]
- 20. Williams D. L., Wong J. S., Hamilton R. L. (2002) SR-BI is required for microvillar channel formation and the localization of HDL particles to the surface of adrenocortical cells in vivo. J. Lipid Res. 43, 544–549 [PubMed] [Google Scholar]
- 21. Huang Z. H., Gu D., Lange Y., Mazzone T. (2003) Expression of scavenger receptor BI facilitates sterol movement between the plasma membrane and the endoplasmic reticulum in macrophages. Biochemistry 42, 3949–3955 [DOI] [PubMed] [Google Scholar]
- 22. Peng Y., Akmentin W., Connelly M. A., Lund-Katz S., Phillips M. C., Williams D. L. (2004) Scavenger receptor BI (SR-BI) clustered on microvillar extensions suggests that this plasma membrane domain is a way station for cholesterol trafficking between cells and high-density lipoprotein. Mol. Biol. Cell 15, 384–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bertaux C., Dragic T. (2006) Different domains of CD81 mediate distinct stages of hepatitis C virus pseudoparticle entry. J. Virol. 80, 4940–4948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harris H. J., Davis C., Mullins J. G., Hu K., Goodall M., Farquhar M. J., Mee C. J., McCaffrey K., Young S., Drummer H., Balfe P., McKeating J. A. (2010) Claudin association with CD81 defines hepatitis C virus entry. J. Biol. Chem. 285, 21092–21102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Flint M., von Hahn T., Zhang J., Farquhar M., Jones C. T., Balfe P., Rice C. M., McKeating J. A. (2006) Diverse CD81 proteins support hepatitis C virus infection. J. Virol. 80, 11331–11342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kohaar I., Ploss A., Korol E., Mu K., Schoggins J. W., O'Brien T. R., Rice C. M., Prokunina-Olsson L. (2010) Splicing diversity of the human OCLN gene and its biological significance for hepatitis C virus entry. J. Virol. 84, 6987–6994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Toker A., Cantley L. C. (1997) Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature 387, 673–676 [DOI] [PubMed] [Google Scholar]
- 28. Wullschleger S., Loewith R., Hall M. N. (2006) TOR signaling in growth and metabolism. Cell 124, 471–484 [DOI] [PubMed] [Google Scholar]
- 29. Gingras A. C., Kennedy S. G., O'Leary M. A., Sonenberg N., Hay N. (1998) 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway. Genes Dev. 12, 502–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Juntilla M. M., Patil V. D., Calamito M., Joshi R. P., Birnbaum M. J., Koretzky G. A. (2010) AKT1 and AKT2 maintain hematopoietic stem cell function by regulating reactive oxygen species. Blood 115, 4030–4038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cooray S. (2007) Apoptosis, Cell Signaling, and Human Diseases: Molecular Mechanisms, pp. 57–80 Humana Press Inc., Totowa, NJ [Google Scholar]
- 32. Shin Y. K., Liu Q., Tikoo S. K., Babiuk L. A., Zhou Y. (2007) Effect of the phosphatidylinositol 3-kinase/Akt pathway on influenza A virus propagation. J. Gen. Virol. 88, 942–950 [DOI] [PubMed] [Google Scholar]
- 33. Feng S. Z., Cao W. S., Liao M. (2011) The PI3K/Akt pathway is involved in early infection of some exogenous avian leukosis viruses. J. Gen. Virol. 92, 1688–1697 [DOI] [PubMed] [Google Scholar]
- 34. Saeed M. F., Kolokoltsov A. A., Freiberg A. N., Holbrook M. R., Davey R. A. (2008) Phosphoinositide-3 kinase-Akt pathway controls cellular entry of Ebola virus. PLoS Pathog. 4, e1000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Patel R. K., Hardy R. W. (2012) Role for the phosphatidylinositol 3-kinase-Akt-TOR pathway during sindbis virus replication in arthropods. J. Virol. 86, 3595–3604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Urata S., Ngo N., de la Torre J. C. (2012) The PI3K/Akt pathway contributes to arenavirus budding. J. Virol. 86, 4578–4585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yu Y., Alwine J. C. (2002) Human cytomegalovirus major immediate-early proteins and simian virus 40 large T antigen can inhibit apoptosis through activation of the phosphatidylinositide 3′-OH kinase pathway and the cellular kinase Akt. J. Virol. 76, 3731–3738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Esfandiarei M., Luo H., Yanagawa B., Suarez A., Dabiri D., Zhang J., McManus B. M. (2004) Protein kinase B/Akt regulates coxsackievirus B3 replication through a mechanism which is not caspase dependent. J. Virol. 78, 4289–4298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rahaus M., Desloges N., Wolff M. H. (2007) Varicella-zoster virus requires a functional PI3K/Akt/GSK-3α/β signaling cascade for efficient replication. Cell. Signal. 19, 312–320 [DOI] [PubMed] [Google Scholar]
- 40. Guo H., Zhou T., Jiang D., Cuconati A., Xiao G. H., Block T. M., Guo J. T. (2007) Regulation of hepatitis B virus replication by the phosphatidylinositol 3-kinase-Akt signal transduction pathway. J. Virol. 81, 10072–10080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Street A., Macdonald A., McCormick C., Harris M. (2005) Hepatitis C virus NS5A-mediated activation of phosphoinositide 3-kinase results in stabilization of cellular β-catenin and stimulation of β-catenin-responsive transcription. J. Virol. 79, 5006–5016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Street A., Macdonald A., Crowder K., Harris M. (2004) The hepatitis C virus NS5A protein activates a phosphoinositide 3-kinase-dependent survival signaling cascade. J. Biol. Chem. 279, 12232–12241 [DOI] [PubMed] [Google Scholar]
- 43. Banerjee S., Saito K., Ait-Goughoulte M., Meyer K., Ray R. B., Ray R. (2008) Hepatitis C virus core protein upregulates serine phosphorylation of insulin receptor substrate-1 and impairs the downstream Akt/protein kinase B signaling pathway for insulin resistance. J. Virol. 82, 2606–2612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bose S. K., Shrivastava S., Meyer K., Ray R. B., Ray R. (2012) Hepatitis C virus activates the mTOR/S6K1 signaling pathway in inhibiting IRS-1 function for insulin resistance. J. Virol. 86, 6315–6322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jiang J., Luo G. (2012) Cell culture-adaptive mutations promote viral protein-protein interactions and morphogenesis of infectious hepatitis C virus. J. Virol. 86, 8987–8997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lo S. Y., Masiarz F., Hwang S. B., Lai M. M., Ou J. H. (1995) Differential subcellular localization of hepatitis C virus core gene products. Virology 213, 455–461 [DOI] [PubMed] [Google Scholar]
- 47. Keck Z. Y., Xia J., Cai Z., Li T. K., Owsianka A. M., Patel A. H., Luo G., Foung S. K. (2007) Immunogenic and functional organization of hepatitis C virus (HCV) glycoprotein E2 on infectious HCV virions. J. Virol. 81, 1043–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Keck Z. Y., Saha A., Xia J., Wang Y., Lau P., Krey T., Rey F. A., Foung S. K. (2011) Mapping a region of hepatitis C virus E2 that is responsible for escape from neutralizing antibodies and a core CD81-binding region that does not tolerate neutralization escape mutations. J. Virol. 85, 10451–10463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang C., Comai L., Johnson D. L. (2005) PTEN represses RNA polymerase I transcription by disrupting the SL1 complex. Mol. Cell Biol. 25, 6899–6911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Singh A. B., Sharma A., Smith J. J., Krishnan M., Chen X., Eschrich S., Washington M. K., Yeatman T. J., Beauchamp R. D., Dhawan P. (2011) Claudin-1 up-regulates the repressor ZEB-1 to inhibit E-cadherin expression in colon cancer cells. Gastroenterology 141, 2140–2153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zeisel M. B., Fofana I., Fafi-Kremer S., Baumert T. F. (2011) Hepatitis C virus entry into hepatocytes. Molecular mechanisms and targets for antiviral therapies. J. Hepatol. 54, 566–576 [DOI] [PubMed] [Google Scholar]
- 52. Lin K. K., Rossi L., Boles N. C., Hall B. E., George T. C., Goodell M. A. (2011) CD81 is essential for the re-entry of hematopoietic stem cells to quiescence following stress-induced proliferation via deactivation of the Akt pathway. PLoS Biol. 9, e1001148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sánchez E. G., Quintas A., Pérez-Núñez D., Nogal M., Barroso S., Carrascosa A. Ĺ., Revilla Y. (2012) African Swine Fever virus uses macropinocytosis to enter host cells. PLoS Pathog. 8, e1002754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Agarwal V., Hammerschmidt S. (2009) Cdc42 and the phosphatidylinositol 3-kinase-Akt pathway are essential for PspC-mediated internalization of pneumococci by respiratory epithelial cells. J. Biol. Chem. 284, 19427–19436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Farquhar M. J., Harris H. J., Diskar M., Jones S., Mee C. J., Nielsen S. U., Brimacombe C. L., Molina S., Toms G. L., Maurel P., Howl J., Herberg F. W., van Ijzendoorn S. C., Balfe P., McKeating J. A. (2008) Protein kinase A-dependent step(s) in hepatitis C virus entry and infectivity. J. Virol. 82, 8797–8811 [DOI] [PMC free article] [PubMed] [Google Scholar]