Background: The interaction between TGF-β and EGFR signaling in the pathogenesis of pulmonary fibrosis has not been defined.
Results: Amphiregulin (AR), a EGFR ligand, is induced by TGF-β stimulation and regulates TGF-β-induced fibroblast proliferation and pulmonary fibrosis.
Conclusion: AR mediates TGF-β-stimulated pulmonary fibrosis through activation of EGFR signaling pathway.
Significance: AR or AR-activated EGFR signaling is crucial in the pathogenesis of TGF-β-induced pulmonary fibrosis.
Keywords: Animal Models, Epidermal Growth Factor Receptor (EGFR), Fibroblast, Fibrosis, Lung
Abstract
Dysregulated amphiregulin (AR) expression and EGR receptor (EGFR) activation have been described in animal models of pulmonary fibrosis and in patients with idiopathic pulmonary fibrosis. However, the exact role of AR in the pathogenesis of pulmonary fibrosis has not been clearly defined. Here, we show that a potent profibrogenic cytokine TGF-β1 significantly induced the expression of AR in lung fibroblasts in vitro and in murine lungs in vivo. AR stimulated NIH3T3 fibroblast cell proliferation in a dose-dependent manner. Silencing of AR expression by siRNA or chemical inhibition of EGFR signaling, utilizing AG1478 and gefitinib, significantly reduced the ability of TGF-β1 to stimulate fibroblast proliferation and expression of α-smooth muscle actin, collagen, and other extracellular matrix-associated genes. TGF-β1-stimulated activation of Akt, ERK, and Smad signaling was also significantly inhibited by these interventions. Consistent with these in vitro findings, AR expression was impressively increased in the lungs of TGF-β1 transgenic mice, and either siRNA silencing of AR or chemical inhibition of EGFR signaling significantly reduced TGF-β1-stimulated collagen accumulation in the lung. These studies showed a novel regulatory role for AR in the pathogenesis of TGF-β1-induced pulmonary fibrosis. In addition, these studies suggest that AR, or AR-activated EGFR signaling, is a potential therapeutic target for idiopathic pulmonary fibrosis associated with TGF-β1 activation.
Introduction
Fibrotic disorders in lung and other organs affect millions of individuals and result in significant morbidity and mortality (1). In the lung, excessive fibroblast proliferation and extensive extracellular matrix deposition affecting the interstitium are cardinal features of various forms of interstitial lung diseases such as idiopathic pulmonary fibrosis, scleroderma, chronic infection, or radiation-induced pulmonary fibrosis (2, 3). In addition, basement membrane thickening and excessive collagen deposition around the bronchial airways also contribute to the pathogenesis of airway remodeling, a fibrotic process, in airway diseases such as asthma (4). However, therapeutic targets that can be manipulated to control the development of these fibrotic disorders have not been described, and the mechanisms that drive the tissue fibrotic responses in these diseases are poorly understood.
TGF-β family proteins are multifunctional cytokines that have been implicated in diverse biologic processes including cell growth and survival, cell and tissue differentiation, development, inflammation, immunity, hematopoiesis, and tissue remodeling and repair (5). Various studies have shown that TGF-β1 plays a central role in fibrogenesis (6). TGF-β1 contributes to excessive tissue remodeling responses by delayed epithelial wound repair via mechanisms that include inhibition of epithelial proliferation, migration, and increased epithelial apoptosis (7, 8). In addition, TGF-β1 is essential in fibroblast recruitment, myofibroblast differentiation, epithelial mesenchymal transition, and extracellular matrix deposition, which expand the mesenchymal compartment of the lung (9–11). In human fibrotic disorders, bioactive TGF-β1 expression is exaggerated in lungs from patients with idiopathic pulmonary fibrosis (12, 13). Expression of TGF-β receptors are increased in fibroblasts from patients with scleroderma, which leads to enhanced TGF-β1 signaling and elevated production of collagen type I (14). Animal studies have also shown that TGF-β1 is a critical mediator of bleomycin-induced pulmonary fibrosis (15–17).
Interestingly, there is substantial literature to suggest that EGFR signaling is implicated in the pathogenesis of tissue fibrosis, including pulmonary fibrosis. For example, increased expression of EGFR and TGF-α, an EGFR ligand, were found in idiopathic pulmonary fibrosis patients and in the lungs of bleomycin-treated rats, an animal model of pulmonary fibrosis (18, 19). TGF-α knock-out mice are protected from bleomycin-induced fibrosis whereas TGF-α over-expression in the murine lungs spontaneously induces progressive pulmonary fibrosis (20, 21). The use of a selective EGFR tyrosine kinase inhibitor (Gefitinib) prevents the development of bleomycin- or TGF-α-induced pulmonary fibrosis (22, 23). These studies suggest that EGFR signaling plays an important role in the pathogenesis of pulmonary fibrosis. However, it is not clear whether EGFR signaling is involved in the pro-fibrotic pathway of TGF-β, and the interaction between these two growth factors signaling in the pathogenesis of pulmonary fibrosis has not been investigated.
In this study, we found that TGF-β1 stimulated the expression of amphiregulin (AR),3 an EGFR ligand, in NIH3T3 fibroblasts. We further investigated the specific cellular effects and the role of AR in fibroblast proliferation and TGF-β-stimulated responses in vitro and in vivo. In particular, the role of AR in TGF-β1-stimulated signaling and tissue responses was evaluated using AR-specific siRNA silencing and chemical inhibition of EGFR signaling in fibroblasts in vitro, and in the lung of wild type (WT) and TGF-β-overexpressing transgenic (Tg) mice in vivo. These studies showed that AR is induced by TGF-β1 stimulation, and AR plays an essential role in the pathogenesis of TGF-β-induced pulmonary fibrosis through activation of EGFR signaling.
EXPERIMENTAL PROCEDURES
Cell Lines and Recombinant Proteins
NIH3T3, BEAS-2B, and A549 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA). NIH3T3 cells were maintained in ATCC complete medium (ATCC-formulated Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% bovine calf serum, 1% penicillin-streptomycin). BEAS-2B cells were maintained in BEAS-2B growth medium (RPMI 1640 medium with 25 mm HEPES, supplemented with 10% FBS, and 1% penicillin-streptomycin). A549 cells were maintained in DMEM + 2 mm glutamine + 10% FBS. Normal human lung fibroblasts were maintained in Fibroblast Basal Medium (Lonza) + 10% FBS. Primary mouse lung fibroblasts were propagated from C57BL/6 mice in DMEM/F12 + anti/anti and maintained in Medium 199 + 15% FBS (24). All cells were grown and maintained at 37 °C with 5% CO2. Commercially available recombinant (r) AR (Sigma-Aldrich) and rTGF-β1 (R&D Systems) were used to stimulate cells in vitro.
Cell Proliferation Assay
Cells were seeded (4000 cells/well) in 96-well plates. water-soluble tetrazolium salt-1 (WST1) reagent (Roche Applied Science) to detect mitochondrial dehydrogenase enzymes was added directly to cell culture medium 48 h after serum starvation. After 1 h of incubation, the plate was read at 450 nm with equipment reference reading at 630 nm.
In Vitro siRNA Silencing of AR, EGFR, and EGFR Inhibition
To evaluate the role of AR in cellular responses, AR expression was knocked down using siRNA silencing. AR-specific siRNA with >90% silencing efficiency was selected through in vitro screening and used for all experiments (Bioneer Inc., Daejeon, Korea). The following AR-specific and scrambled (Sc) control siRNA sequences were chosen: AR sense, GGA CCU AUC CAA GAU UGC A dTdT; AR antisense, UGC AAU CUU GGA UAG GUC C dTdT; Control sense, CCU ACG CCA CCA AUU UCG U dTdT; Control antisense, ACG AAA UUG GUG GCG UAG G dTdT. Mouse EGFR siRNA was obtained from Santa Cruz Biotechnology. Lipofectamine RNAiMAX Transfection Unit (Invitrogen) was used for transfection of all siRNAs. For chemical inhibition of EGFR signaling, cells were treated with 10 μm AG1478 (EMD Bioscience, San Diego, CA) or gefitinib (Tocris Bioscience, Ellisville, MO). There is no evidence of cytotoxicity by lactate dehydrogenase assay at this concentration in airway epithelial cells (data not shown).
Antibodies for Immunoblot Analysis
Cell lysates were prepared, and Western blot analysis was completed with antibodies that react selectively with p-Akt, total Akt, p-ERK, total ERK, p-Smad, total Smad2/3 (Cell Signaling Technology); α-smooth muscle actin (SMA) (Dako); and β-actin (H-196; Santa Cruz Biotechnology Technology) as described previously (25).
Lung-specific TGF-β1 Tg Mice
TGF-β1 Tg mice previously generated in our laboratory (C57BL/6 background) (25) were utilized in these studies. These mice use the Clara cell 10-kDa protein (CC10) promoter to specifically target bioactive TGF-β1 production in the lung. All animal experiments were approved by the Yale School of Medicine Institutional Animal Care and Use Committee (IACUC) in accordance with federal guidelines.
In Vivo siRNA AR Silencing and Treatment with EGFR Inhibitor
According to the established method in our laboratory (26, 27), 6-week-old TGF-β1 Tg and control littermates were randomized to receive AR-specific or Sc siRNA intranasally once a day for 14 days (3 nmol/mouse per day). For chemical inhibition of EGFR signaling, mice were randomized to receive AG1478 at 1 mg/mouse per day or vehicle (dimethyl sulfoxide) for 7 days. Starting on the second day of treatment, both WT and TGF-β1 Tg mice were given water containing 0.5 mg/ml doxycycline as described previously (25).
Quantification of Lung Collagen
Animals were anesthetized, and median sternotomy was performed, and right heart perfusion was completed with calcium- and magnesium-free PBS. The heart and lungs were then removed. The right lung was frozen in liquid nitrogen and stored at −80 °C until used. Collagen content was determined by quantifying total soluble collagen using the Sircol Collagen Assay kit (Biocolor, Accurate Chemical and Scientific Co., Westbury, NY) according to the manufacturer's instructions.
Histologic Analysis
Lungs were removed en bloc as described above, inflated to 25-cm pressure with PBS containing 0.5% low melting point agarose gel, fixed, embedded in paraffin, sectioned, and stained. Hematoxylin and eosin, and Mallory's trichrome stains, were performed in the Research Histology Laboratory of the Department of Pathology at the Yale University School of Medicine. Bronchoalveolar lavage (BAL) and lung inflammation were assessed as described previously (28).
mRNA Analysis
mRNA was measured using real time RT-PCR as described previously (25, 29). Total cellular RNA from cell lysates or from WT and TGF-β1 Tg mice lungs were obtained using TRIzol reagent (Invitrogen), according to the manufacturer's instructions. The primer sequences for EGFR ligands and extracellular matrix (ECM) genes were obtained from PrimerBank.
ELISA
AR levels in cell culture supernatants and mouse BAL samples were quantified using an ELISA kit (R&D Systems) following the manufacturer's instructions.
Smad Reporter Assay
Canonical TGF-β1 signaling pathways were assessed using dual reporter assays (SABiosciences) according to the manufacturer's instructions. Briefly, a Smad-responsive firefly luciferase construct that constitutively expressed Renilla luciferase was transfected into HEK293 cells. After stimulation with TGF-β, alone or in combination with siRNA, Smad activation was assessed by measuring the dual luciferase activities.
Imunohistochemistry and Immunocytochemistry
Immunohistochemistry was utilized to localize AR and phosphorylated EGFR using antibodies against AR (Thermo Scientific) and EGFR-p (Tyr1068; Cell Signaling) according to the procedures described previously by our laboratories (28). Briefly, rehydrated slides were quenched with 3% hydrogen peroxide, Ag retrieval was performed (Dako) and blocked with blocking buffer (Dako). Slides were incubated with antibodies (1/200 dilution, overnight at 4 °C) and then developed using DakoCytomationEnvision+System (Dako) and counterstained with hematoxylin. Immunocytochemistry was undertaken to determine the expression of α-smooth muscle actin (α-SMA). Cells were fixed with 4% paraformaldehyde, blocked with 5% BSA, and incubated with antibody against α-SMA (1/100 dilution, overnight at 4 °C). Slides were then incubated with Alexa Fluor 555 goat anti-mouse IgG (Invitrogen) then coverslipped with Vectashield with DAPI (Vector Laboratories, Burlingame, CA).
Statistics
Values are expressed as mean ± S.E. As appropriate, groups were compared by ANOVA; follow-up comparisons between groups were conducted using a two-tailed Student t test. A p value of ≤0.05 was considered to be significant.
RESULTS
TGF-β1 Induces AR Expression in Fibroblasts
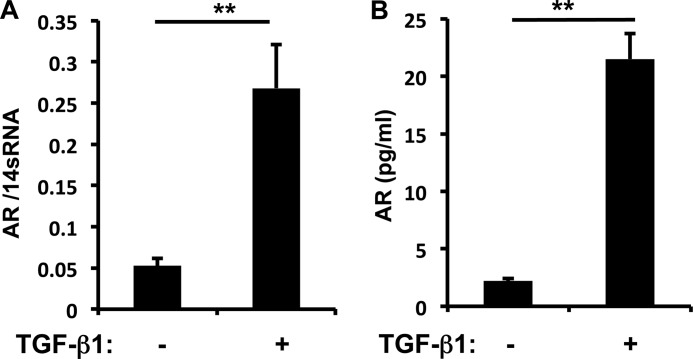
To investigate the regulation of AR expression by TGF-β1, NIH3T3 fibroblasts were incubated with vehicle or recombinant (r) TGF-β1 (10 ng/ml) for 1–16 h. TGF-β1 induction of AR gene expression was noted as early as 6 h, and the levels of mRNA expression and protein production in cell culture supernatants were significantly increased (5-fold) after 16 h of rTGF-β1 stimulation (Fig. 1 and data not shown). Although the bronchial epithelial (BEAS-2B) cell line or A549 lung epithelial cells express high levels of AR at base line, AR expression was not significantly altered by the stimulation of rTGF-β1 (data not shown). These results suggest that fibroblasts are the major cells expressing AR in response to TGF-β1 stimulation.
FIGURE 1.
TGF-β1-stimulated AR expression in NIH3T3 fibroblasts. A, the level of mRNA expression was evaluated by real time PCR after 16 h of TGF-β1 (10 ng/ml) treatment. B, AR protein in cell culture supernatants of NIH3T3 cells was quantitated by ELISA. Values are mean ± S.E. (error bars) with three replicates representative of four separate experiments. **, p ≤ 0.01.
AR Stimulates Fibroblast Proliferation in a Dose-dependent Manner
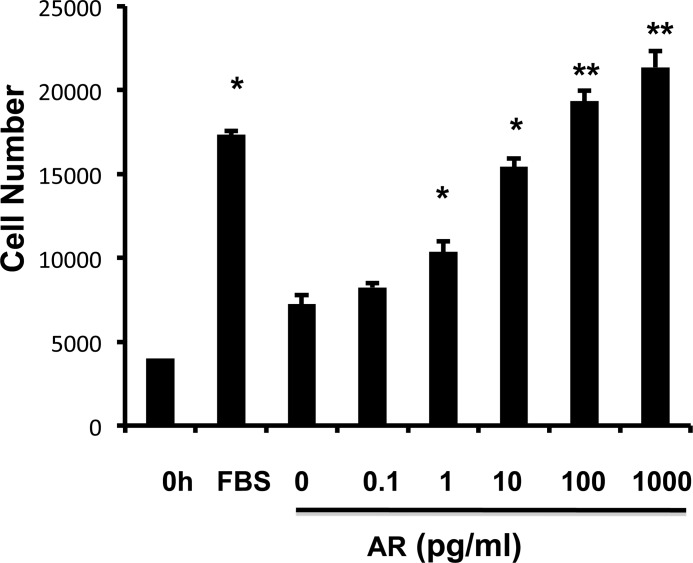
To determine whether AR directly stimulates fibroblast proliferation and myofibroblast transformation, NIH3T3 fibroblasts were treated with rAR for 48 h. rAR treatment significantly enhanced fibroblast proliferation in a dose-dependent manner, as evaluated by the WST1 cell proliferation assay (Fig. 2). No myofibroblast transformation was observed with up to 48-h AR treatment (data not shown).
FIGURE 2.
AR stimulates fibroblast proliferation in a dose-dependent manner. NIH3T3 cells with the indicated doses were stimulated for 48 h, with recombinant AR, and the effect of AR on fibroblast proliferation was measured by WST1 cell proliferation assay. Values are mean ± S.E. (error bars) with four replicates per experiment, representative of two separate experiments. *, p ≤ 0.05; **, p ≤ 0.01 compared with vehicle controls.
AR Mediates TGF-β1-stimulated Fibroblast Proliferation, Myofibroblast Transformation, and ECM-associated Gene Expression
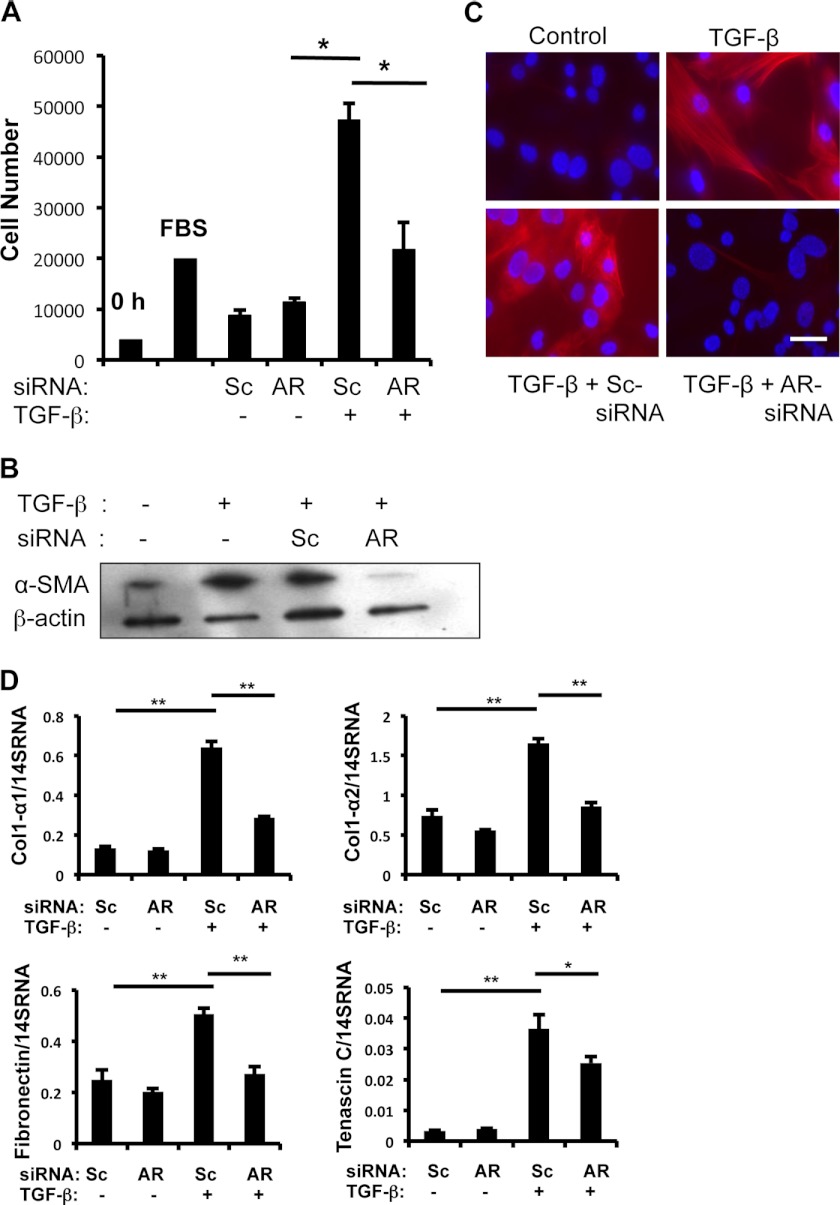
TGF-β1-induced fibroblast proliferation, myofibroblast transformation, and expression of ECM-associated genes are well described (30). Therefore, we investigated whether AR plays a role in TGF-β1-stimulated fibroblast responses. After transfection with Sc or AR-specific siRNA, NIH3T3 cells were treated with rTGF-β1 (10 ng/ml). The AR-specific siRNA treatment significantly reduced AR expression in both protein (>80% inhibition) and mRNA levels (>90%) (supplemental Fig. S1). TGF-β1 stimulated fibroblast proliferation in cells treated with Sc siRNA (Fig. 3A), but TGF-β1-stimulated cell proliferation was abrogated in cells treated with AR-specific siRNA (Fig. 3A). In cells treated with Sc siRNA, TGF-β1 stimulated α-SMA expression significantly (Fig. 3, B and C), whereas TGF-β1-stimulated α-SMA expression was significantly inhibited in NIH3T3 cells treated with AR-specific siRNA (Fig. 3, B and C). These results suggest that AR plays an important role in TGF-β1-induced fibroblast proliferation and myofibroblast transformation. Finally, TGF-β1 stimulated NIH3T3 fibroblasts treated with Sc siRNA expressed ECM-associated genes such as collagen, fibronectin, and tenascin C. TGF-β1-induced expression of these genes was reduced significantly in NIH3T3 cells treated with AR-specific siRNA (Fig. 3D). These results showed that AR plays an essential role in TGF-β1-stimulated fibroblast proliferation, myofibroblast transformation, and ECM gene expression. On the other hand, the role of AR as a downstream mediator TGF-β has been further supported by the observation that rAR effects on fibroblast cell proliferation was not inhibited by the treatment of Smad inhibitor LY2157199 (supplemental Fig. S2A).
FIGURE 3.
AR mediates TGF-β1-stimulated fibroblast proliferation, α-SMA expression, and expression of extracellular matrix-associated genes. A, the role of AR in fibroblast proliferation was evaluated using WST1 assay after silencing of AR by siRNA. NIH3T3 cells were treated with scrambled (Sc) or AR-specific (AR) siRNA, then stimulated with vehicle (PBS) (−) or TGF-β1 (+) (10 ng/ml) for 48 h. B, α-SMA expression was evaluated by Western blot analysis of cell lysates from NIH3T3 cells treated with vehicle (−), TGF-β1(+), Sc- or AR-specific siRNA for 16 h as indicated. β-Actin was used as a loading control. C, immunocytochemical (ICC) evaluation of α-SMA expression in NIH3T3 cells was performed. Red, α-SMA positive cells; blue, DAPI-stained nuclei. D, mRNA levels of α1-procollagen (Col1-α1), α2-procollagen (Col1-α2), fibronectin, and tenascin C expression were evaluated by real time RT-PCR on the NIH3T3 cells treated with vehicle (−), TGF-β (+), Sc, and AR siRNA as indicated. Values in A and D are mean ± S.E. (error bars) with four replicates per experiment. They are representative of two separate experiments. B and C are representative of a minimum of three separate experiments. Values in D are mean ± S.E. evaluations with three replicates and are representative of two separate experiments. Scale bar, 25 μm. *, p ≤ 0.05; **, p ≤ 0.01.
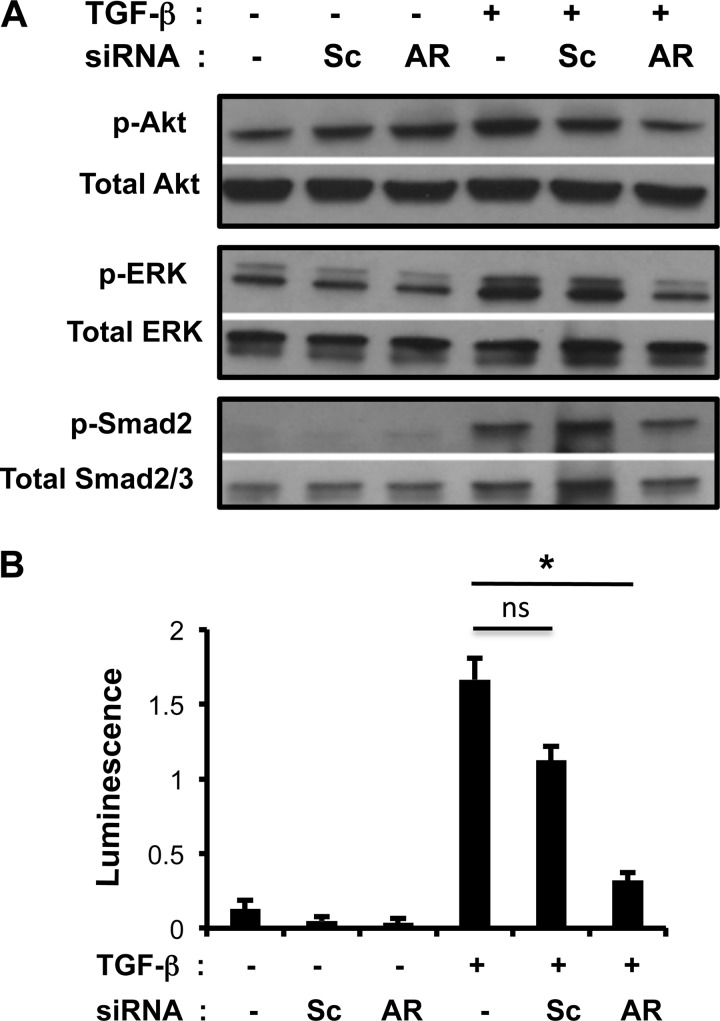
AR Mediates TGF-β1-induced Akt, ERK1/2, and Smad Activation
TGF-β1 binds to a type II receptor, which recruits and phosphorylates a type I receptor to activate Smad-dependent pathways. In addition, TGF-β1 activates Smad-independent pathways such as PI3K-Akt or ERK1/2 MAPK pathways (31–33). Because Akt and ERK1/2 activation are also involved in response to EGFR activation (34, 35), we hypothesized that AR might mediate TGF-β1-stimulated Akt and ERK1/2 activation via EGFR. To investigate the role of AR in TGF-β1-stimulated signaling pathways, NIH3T3 fibroblast cells were transfected with Sc or AR-specific siRNA and stimulated with rTGF-β1 (10 ng/ml) for 16 h. TGF-β1 activated Akt, ERK1/2, Smad, and EGFR phosphorylation, and each of these effects was significantly decreased by siRNA silencing of AR (Fig. 4A and data not shown). TGF-β-induced ERK1/2 phosphorylation was also partly abrogated with AR siRNA knockdown after 30 min, 1 h, and 4 h of TGF-β stimulation, but Akt activation (phosphorylation) was only noted at the 4-h time point, and this activation was also partly abrogated with AR silencing (supplemental Fig. S3). Interestingly, we did not note significant induction of AR at the 30-min and 1-h time points (data not shown). Thus, partial inhibition of ERK and Akt activation by AR silencing might be associated with low level of AR expression at these time points. These results suggest that AR, as a downstream mediator of TGF-β, is not significantly involved in the initiation of TGF-β signaling, but it is an important mediator regulating sustained activation of TGF-β signaling. Utilizing a Smad reporter assay, we showed that TGF-β1-stimulated Smad activation was significantly decreased with siRNA silencing of AR (Fig. 4B). These results suggest that AR mediates TGF-β1-stimulated Akt, ERK1/2, and Smad activation in lung fibroblasts. On the other hand, as a downstream mediator of TGF-β, AR itself did not stimulate Smad activity (supplemental Fig. S2B).
FIGURE 4.
AR modulates TGF-β1-stimulated Akt, ERK, and Smad signaling. A, Western blot of NIH3T3 cell lysates after 16 h treatment with vehicle (−), TGF-β1 (+), scrambled (Sc), or AR-specific (AR) siRNA as indicated. Phosphorylated (p-) and total forms of Akt, ERK, and Smad2 are illustrated. B, HEK293 cells were transfected with Smad reporter constructs, and Smad activation was quantitated by dual luciferase activity assay expressed as a ratio of firefly versus Renilla luminescence. A is representative of two separate experiments. Values in B are mean ± S.E. (error bars) with three replicates and are representative of two separate experiments. *, p ≤ 0.05; ns, not significant.
EGFR Signaling Regulates TGF-β1-stimulated Fibroblast Proliferation, Akt, ERK1/2, and Smad Activation and α-SMA Expression
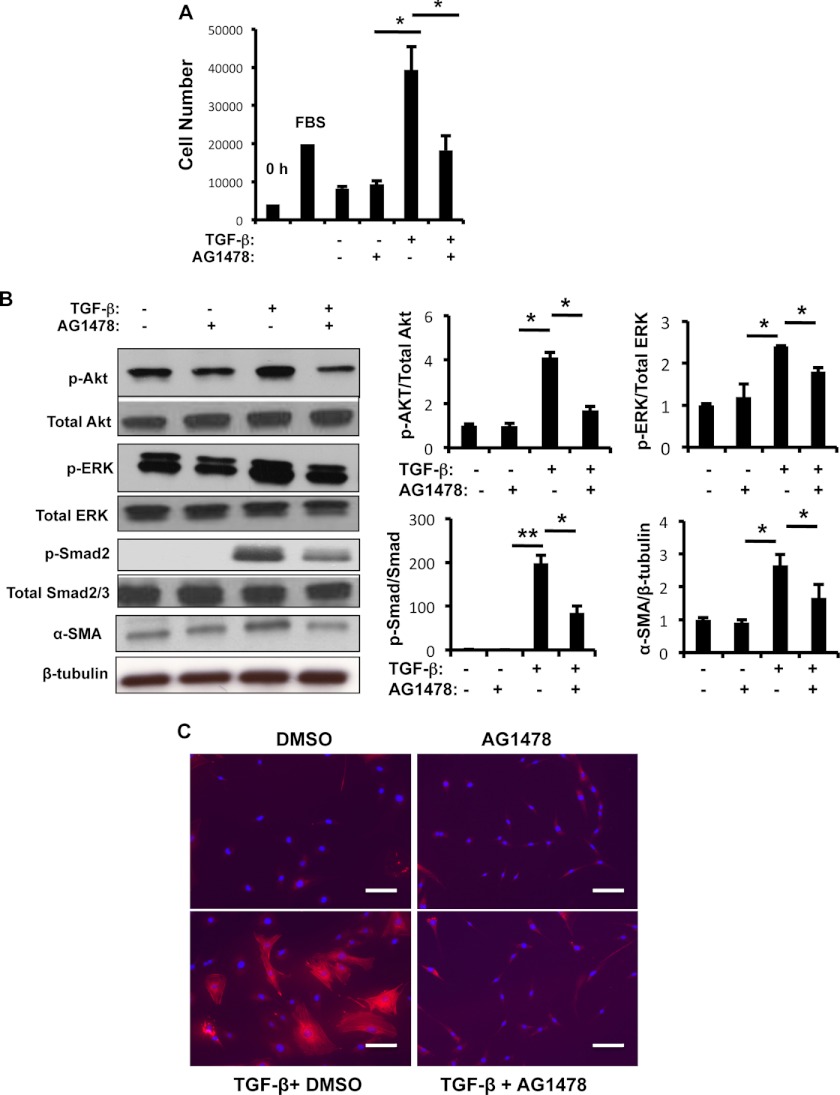
We investigated whether EGFR activation was involved in TGF-β1-stimulated fibroblast responses. TGF-β1 induced fibroblast proliferation and increased Akt, ERK1/2, and Smad activation, and α-SMA expression in NIH3T3 fibroblasts (Fig. 5). In addition, NIH3T3 cells treated with selective EGFR tyrosine kinase inhibitors, AG1478 (10 μm) and gefitinib (10 μm), significantly reduced TGF-β1-stimulated cell proliferation, Akt, ERK1/2, and Smad phosphorylation, and α-SMA expression compared with unstimulated controls (Fig. 5 and supplemental Fig. S4). Immunocytochemistry to evaluate α-SMA showed that TGF-β1-stimulated myofibroblast transformation was abrogated by AG1478 (Fig. 5C). In addition, AG1478 and gefitinib significantly reduced TGF-β1-induced ECM-associated gene expression (supplemental Fig. S5). The specific role of AR and EGFR in lung fibroblasts were further tested by EGFR knocking down using siRNA silencing approach (supplemental Fig. S6). Together, these findings show that EGFR phosphorylation (EGFR-p), activated by AR, was involved in TGF-β1-stimulated fibroblast proliferation, myofibroblast transformation, Akt, ERK1/2, and Smad activation.
FIGURE 5.
EGFR mediates TGF-β1-stimulated fibroblast proliferation, Akt, ERK, and Smad signaling, and α-SMA expression. A, NIH3T3 cells were stimulated with vehicle (−), TGF-β1 (+) alone or in combination with an EGFR inhibitor AG1478 (10 mm) for 48 h, and the effect of AG1478 on TGF-β-stimulated fibroblast cell proliferation was assessed by WST1 assay. B, cell lysates of NIH3T3 cells treated with vehicle (−), TGF-β (+) alone or combined with an EGFR inhibitor AG1478 for 16 h as indicated and activation (phosphorylation, p-) of Akt, ERK1/2 and Smad2/3, and α-SMA expression were evaluated by Western blot analysis. β-Tubulin was used as a loading control. Densitometry analysis was performed on Western blots from three individual experiments. C, immunocytochemical evaluation of α-SMA expression in NIH3T3 cells treated with TGF-β (+) alone and with the addition of an EGFR inhibitor AG1478. Values in A are mean ± S.E. (error bars) evaluations with three replicates and are representative of three separate experiments. B and C are illustrative of a minimum of three separate experiments. *, p ≤ 0.05.
TGF-β1 Stimulates AR Expression and EGFR Phosphorylation in Murine Lung
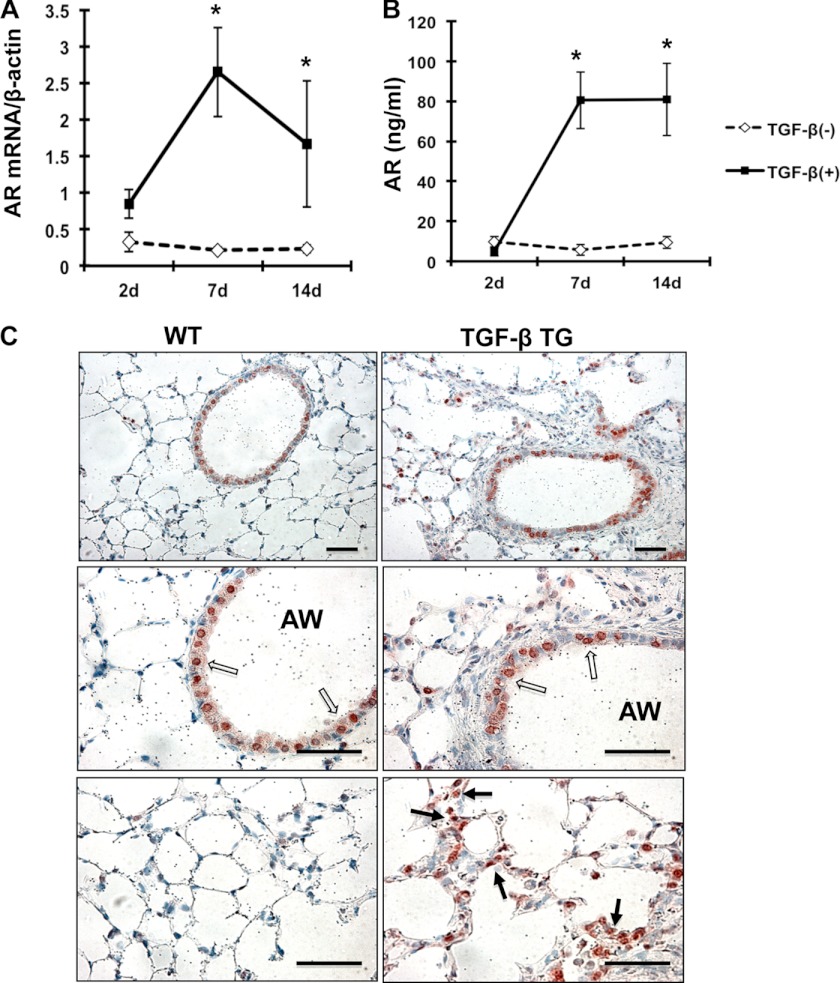
To evaluate the role of TGF-β1 regulation of AR and EGFR signaling in vivo, studies were completed to evaluate the expression of AR and EGFR activation (EGFR-p) in the lungs of TGF-β1 Tg mice, which have active human TGF-β1 that is specifically expressed in the lung (25). Pulmonary fibrosis in these TGF-β1 Tg mice has been extensively characterized in our previous studies (25). To evaluate expression of AR and EGFR-p in this model, mRNA and protein levels of AR were measured in the lungs from TGF-β1 Tg and WT control mice at various time points after transgene activation with doxycycline. These studies showed that TGF-β1 significantly increased AR mRNA expression in the lungs from TGF-β1+ Tg mice compared with TGF-β1− WT control mice (Fig. 6A). The peak induction of AR mRNA expression was seen at 7 days and persisted through 14 days of doxycycline administration (Fig. 6A). In a similar manner, AR protein measured in BAL fluid was increased in the lungs of TGF-β1+ Tg mice compared with control mice (Fig. 6B). Immunohistochemistry showed base-line expression of AR and EGFR-p, primarily in airway epithelial cells in the lungs of WT mice. However, in TGF-β1 Tg mice, AR and EGFR-p induction was most significant in lung parenchyma (Fig. 6C and supplemental Fig. S7). These studies showed that TGF-β1 is a potent stimulator of AR expression and EGFR-p in the murine lung.
FIGURE 6.
TGF-β1 stimulates AR expression in vivo. A, AR transcript was measured in whole lung RNA extracts from WT and TGF-β1 Tg mice using RT-PCR. B, AR protein in BAL fluid from WT and TGF-β1 Tg mice was quantified using ELISA. C, AR expression in the lungs from WT and TGF-β Tg mice was evaluated by immunohistochemistry. Values in A and B are mean ± S.E. (error bars) from a minimum of four mice in each group. C is illustrative of three separate experiments. Arrows indicate AR expressing cells in the airways (AW) (open arrows) and parenchyma (solid arrows). Scale bars, 50 μm. *, p ≤ 0.05.
AR Mediates TGF-β1-stimulated Collagen Accumulation in the Lung
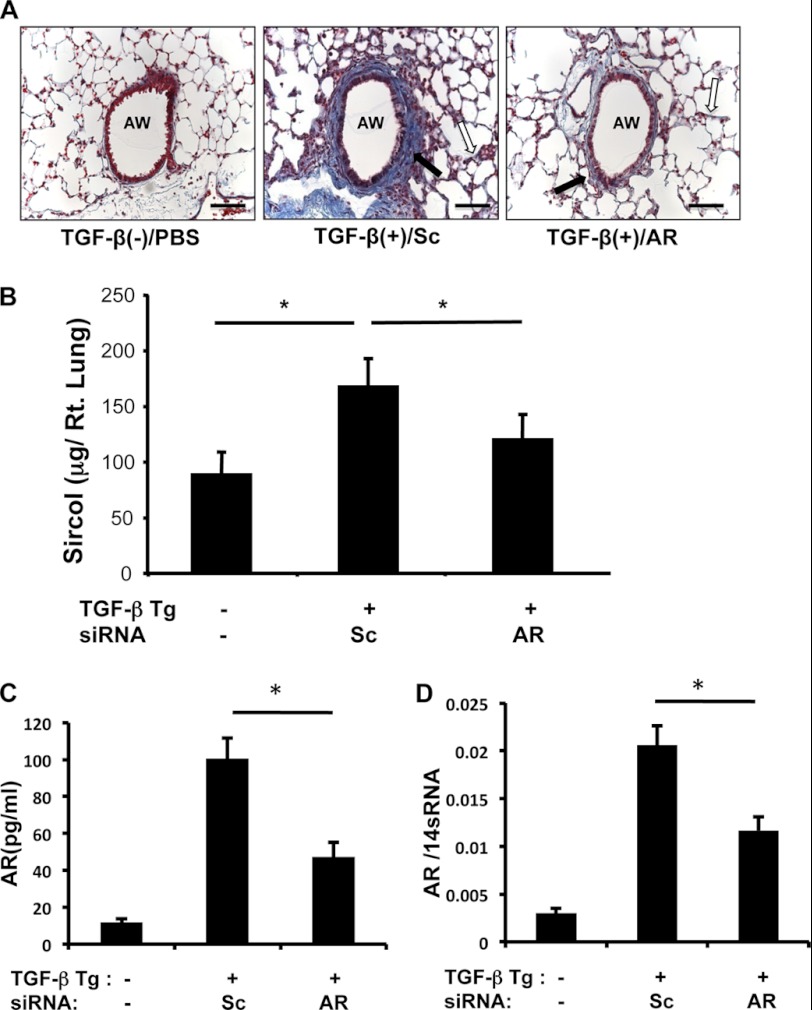
To determine the in vivo role of AR in TGF-β1-induced fibrosis, TGF-β1 Tg and WT mice were treated with Sc or AR-specific siRNA (3 nmol/mouse per day for 14 days), and collagen accumulation in the lungs was measured. Subepithelial (peribronchial) and parenchymal fibrosis was significantly decreased in TGF-β1 Tg mice treated with AR-specific siRNA compared with mice treated with Sc siRNA (Fig. 7A). Consistent with this histologic result, the total amount of collagen in the lungs of TGF-β1 Tg mice was significantly decreased in mice treated with AR-specific siRNA (Fig. 7B) compared with mice treated with Sc siRNA. mRNA and protein analysis showed that both AR transcript and protein levels were significantly decreased with AR siRNA treatment (Fig. 7, C and D). These studies showed that AR plays an important role in TGF-β1-induced collagen accumulation in the murine lung.
FIGURE 7.
AR regulates TGF-β1-induced pulmonary fibrosis. A, Mallory trichrome staining of lungs from WT (TGF-β1 Tg (−)) or TGF-β1 Tg mice treated with scrambled (Sc) or AR-specific siRNA is shown. B, total lung collagen was quantified using Sircol assay. C and D, the expression of AR protein (C) and mRNA (D) was evaluated by ELISA and RT-PCR, respectively. A is representative of a minimum of four mice in each group. Values in B are mean ± S.E. (error bars) with a minimum of four mice in each group. Values in C and D are mean ± S.E. evaluations with three replicates. AW, airway. Scale bars, 50 μm; *, p ≤ 0.05.
EGFR Signaling Plays a Critical Role in TGF-β1-induced Pulmonary Fibrosis
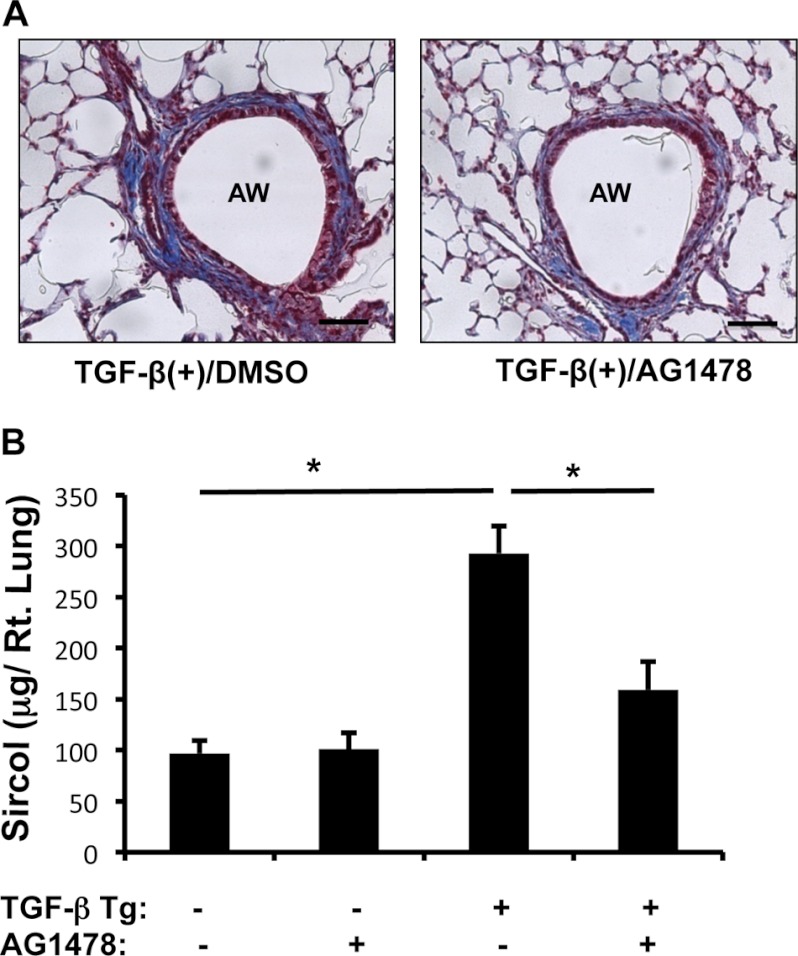
To determine whether EGFR activation is involved in TGF-β1-induced fibrotic responses in vivo, WT and TGF-β1 Tg mice were treated with a selective EGFR tyrosine kinase inhibitor, AG1478 (1 mg/mouse per day intraperitoneally), or vehicle alone (dimethyl sulfoxide). After 7 days of transgene induction, collagen accumulation in the lung was measured by histology and Sircol quantitation. These experiments showed that TGF-β1-induced collagen accumulation in the lung was significantly decreased in mice treated with AG1478 compared with vehicle alone (Fig. 8). These studies showed that EGFR activation plays an essential role in the pathogenesis of TGF-β1-induced collagen accumulation in the lung.
FIGURE 8.
Effect of EGFR inhibition on TGF-β-induced pulmonary fibrosis. A, Mallory trichrome staining of lungs from Tg mice treated with vehicle (DMSO) or AG1478. B, total lung collagen was quantified using Sircol assay. A is representative of a minimum of four mice in each group. Values in B are mean ± S.E. (error bars) with a minimum of four mice in each group. AW, airway. Scale bars, 35 μm. *, p ≤ 0.05.
DISCUSSION
This is the first study to show that AR, an EGFR ligand, is induced by TGF-β1 stimulation and plays a significant role in TGF-β-induced pulmonary fibrosis. In this study, we confirmed that AR stimulated lung fibroblast proliferation (36) and further demonstrated a novel regulatory role for AR that mediates TGF-β1-stimulated fibroblast proliferation, myofibroblast transformation, and ECM accumulation.
As a central mediator of tissue remodeling and fibrosis, canonical TGF-β1 signaling involves receptor activation followed by activation of Smad proteins (Smad2, 3, and 4) that translocate to the nucleus and regulate the expression of a number of TGF-β-specific target genes (37). TGF-β is also known to activate noncanonical signaling pathways, such as PI3K/Akt, and MAPK signaling (33). The interaction between the Smad-dependent and Smad-independent pathways is important to characterize because it may ultimately determine the final outcome of TGF-β-stimulated cellular and tissue responses (38). In support of this interaction, our laboratory and other investigators have shown that activation of PI3K/Akt and ERK1/2 MAPK is critical for the pathogenesis of TGF-β-induced airway remodeling, epithelial mesenchymal transition, myofibroblast transformation, and collagen accumulation in the lung (25, 29, 31). However, the mechanisms or molecules that mediate the interaction between different signaling pathways in the development of specific TGF-β-induced tissue phenotypes, such as pulmonary fibrosis, are still poorly understood. Recent studies have shown that EGFR signaling plays an important role in effector function of TGF-β because EGFR signaling was required for TGF-β1-induced plasminogen activator inhibitor-1 transcription in vascular smooth muscle cells (38) and TGF-β1-induced COX-2 expression in human bronchial epithelial cells (39). These studies suggest that EGFR signaling may be important for TGF-β signaling, and here we showed that intact EGFR signaling was essential for TGF-β1-induced pulmonary fibrosis. We showed that an EGFR ligand, AR, is induced by TGF-β stimulation and modulates both canonical and noncanonical TGF-β1 signaling pathways through activation of EGFR. AR-induced EGFR activation was critical for the optimal effector function of TGF-β1 associated with fibrogenesis because either AR siRNA silencing or chemical inhibition of EGFR signaling significantly reduced TGF-β-stimulated fibroblast proliferation, myofibroblast transformation, and collagen accumulation in the lung. Taken together, these results show that AR is a crucial mediator of TGF-β-induced pulmonary fibrosis, which integrates TGF-β and EGFR signaling pathways. These results are also highlighting the importance of cross-talk between TGF-β and EGFR signaling in the pathogenesis of pulmonary fibrosis.
AR0 is a member of the EGF family (40). AR binds to EGFR, as do other EGFR ligands, such as TGF-α, EGF, HB-EGF, betacellulin, and epiregulin. Ligand-induced EGFR phosphorylation activates normal epithelial, fibroblast, and keratinocyte cell proliferation and differentiation. In the lung, epithelial and mesenchymal cells express AR and differentially responded to AR stimulation in a cell-specific manner in lung branching morphogenesis (41). Although few studies have been undertaken to define the specific role of AR in the pathogenesis of tissue fibrosis, including pulmonary fibrosis, the available results have been conflicting to date. In an animal model of liver fibrosis, AR expression is significantly induced, and the absence of AR markedly reduced α-SMA expression and collagen deposition in the liver, implicating AR contribution in the development or the progression of liver fibrosis (42). In support of this notion, the use of gefitinib, a EGFR blocker, significantly inhibits bleomycin-induced pulmonary fibrosis (22). On the other hand, Fukumoto et al. (43) showed that administration of rAR suppressed bleomycin-induced pulmonary inflammation and fibrosis, suggesting a potential protective role of AR in tissue fibrosis. These potentially contradictory results may simply reflect inherent differences in target organs, cellular compartments, or the nature of injury between different experimental settings. In particular, the complex nature of inflammation and tissue injury and repair responses to bleomycin significantly limit accurate assessment of the specific role of AR in the pathogenesis of tissue fibrosis. In the studies done by Fukumoto's group, AR recombinant protein was delivered mostly during the injury phase (6–10 days after bleomycin stimulation), which might reduce the bleomycin-induced apoptotic cell death response. On the other hand, additional AR treatment would not have significant effects on fibroproliferative repair phase because bleomycin stimulation itself significantly induces endogenous AR. Thus, overall fibrosis could be less in the AR-treated group of mice mainly due to less injury response compared with controls. In the studies done by Ishii's group (22), inhibition of EGFR signaling could have significant effects on the fibroproliferative phase, because EGFR signaling is essential for fibroblast proliferation. Although the inhibition of EGFR signaling may enhance the injury response, overall fibrosis can be protected due to the inhibition of fibroblast proliferation, a critical process in tissue fibrogenesis. Therefore, controlled experiments using transgenic animals may provide better mechanistic insights to assess true biological function of AR. Here, we chose to utilize a Tg mouse model in which biologically active human TGF-β1 is specifically overexpressed in the murine lung in a tightly regulated manner (25). This TGF-β1 Tg model causes a pulmonary phenotype that is consistent with pulmonary fibrosis in humans, characterized by inflammation, airway and parenchymal fibrosis, myocyte and myofibroblast hyperplasia, and alveolar remodeling (25, 29, 37, 44). Utilizing this tightly controlled and lung-specific Tg model, we showed that both AR and intact EGFR signaling are required for the optimal TGF-β1-induced pulmonary fibrosis because both AR siRNA silencing and selective chemical inhibition of EGFR phosphorylation reduced TGF-β1-induced pulmonary fibrosis significantly. Although TGF-β1 Tg mice and fibroblasts stimulated with TGF-β1 did not show significant changes in expression of other EGFR ligands (HB-EGF, TGF-α, and epiregulin) (data not shown), we are not able to completely exclude the possibility these ligands also contribute to EGFR activation in TGF-β1 Tg mice. In particular, tissue activation of TGF-α via TNF-α converting enzyme (45) or increased hyaluronic acid (46) might be implicated in EGFR activation in TGF-β1 Tg mice. The potential contribution of these mechanisms in TGF-β-specific effector functions warrants further investigation in future studies. Interestingly, the clinical use of EGFR inhibitor gefitinib for lung cancer patients can cause interstitial pneumonia or acute lung injury with a global incidence rate of about 1% (47). However, the pathogenetic mechanism of this drug toxicity has not been clearly understood yet. Because apoptotic epithelial cell death could be a contributing factor for pulmonary fibrosis, inappropriate EGFR inhibition, such as high dose and/or chronic administration of EGFR inhibitors, may underlie these adverse effects of interstitial fibrosis. Although we do not have any evidence that EGFR inhibitors induce pulmonary fibrosis with the doses and duration we employed in current in vivo studies, AR inhibition instead of using EGFR inhibitors could be an alternative option to control pulmonary fibrosis to minimize the adverse effects of using EGFR inhibitors.
In summary, these studies showed a novel regulatory role of AR and EGFR activation in fibroblast proliferation and in the pathogenesis of TGF-β1-stimulated pulmonary fibrosis. AR is induced by TGF-β1, and AR expression and production lead to EGFR activation that modulates the effector function TGF-β1. These findings suggest that both AR and EGFR signaling are critically involved in the pathogenesis of TGF-β1-induced pulmonary fibrosis. These studies also suggest that an intervention that targets AR and/or AR-EGFR activation pathway could be a potential therapeutic target in TGF-β-induced pulmonary fibrosis.

This article contains supplemental Figs. S1–S7.
- AR
- amphiregulin
- BAL
- bronchoalveolar lavage
- ECM
- extracellular matrix
- EGFR
- EGF receptor
- r
- recombinant
- Sc
- scrambled
- α-SMA
- α-smooth muscle actin
- Tg
- transgenic
- WST1
- water-soluble tetrazolium salt 1.
REFERENCES
- 1. Ward P. A., Hunninghake G. W. (1998) Lung inflammation and fibrosis. Am. J. Respir. Crit. Care Med. 157, S123–129 [DOI] [PubMed] [Google Scholar]
- 2. Homer R. J., Elias J. A., Lee C. G., Herzog E. (2011) Modern concepts on the role of inflammation in pulmonary fibrosis. Arch. Pathol. Lab. Med. 135, 780–788 [DOI] [PubMed] [Google Scholar]
- 3. Noble P. W., Homer R. J. (2004) Idiopathic pulmonary fibrosis: new insights into pathogenesis. Clin. Chest Med. 25, 749–758, vii [DOI] [PubMed] [Google Scholar]
- 4. Elias J. A., Lee C. G., Zheng T., Ma B., Homer R. J., Zhu Z. (2003) New insights into the pathogenesis of asthma. J. Clin. Invest. 111, 291–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leask A., Abraham D. J. (2004) TGF-β signaling and the fibrotic response. FASEB J. 18, 816–827 [DOI] [PubMed] [Google Scholar]
- 6. Wynn T. A. (2008) Cellular and molecular mechanisms of fibrosis. J. Pathol. 214, 199–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sheppard D. (2006) Transforming growth factor β: a central modulator of pulmonary and airway inflammation and fibrosis. Proc. Am. Thorac. Soc. 3, 413–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Amendt C., Mann A., Schirmacher P., Blessing M. (2002) Resistance of keratinocytes to TGFβ-mediated growth restriction and apoptosis induction accelerates re-epithelialization in skin wounds. J. Cell Sci. 115, 2189–2198 [DOI] [PubMed] [Google Scholar]
- 9. Roy S. G., Nozaki Y., Phan S. H. (2001) Regulation of α-smooth muscle actin gene expression in myofibroblast differentiation from rat lung fibroblasts. Int. J. Biochem. Cell Biol. 33, 723–734 [DOI] [PubMed] [Google Scholar]
- 10. Hu B., Wu Z., Liu T., Ullenbruch M. R., Jin H., Phan S. H. (2007) Gut-enriched Krüppel-like factor interaction with Smad3 inhibits myofibroblast differentiation. Am. J. Respir. Cell Mol. Biol. 36, 78–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee C. G., Homer R. J., Zhu Z., Lanone S., Wang X., Koteliansky V., Shipley J. M., Gotwals P., Noble P., Chen Q., Senior R. M., Elias J. A. (2001) Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor β1. J. Exp. Med. 194, 809–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khalil N., O'Connor R. N., Unruh H. W., Warren P. W., Flanders K. C., Kemp A., Bereznay O. H., Greenberg A. H. (1991) Increased production and immunohistochemical localization of transforming growth factor-β in idiopathic pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 5, 155–162 [DOI] [PubMed] [Google Scholar]
- 13. Khalil N., Parekh T. V., O'Connor R., Antman N., Kepron W., Yehaulaeshet T., Xu Y. D., Gold L. I. (2001) Regulation of the effects of TGF-β1 by activation of latent TGF-β1 and differential expression of TGF-β receptors (TβR-I and TβR-II) in idiopathic pulmonary fibrosis. Thorax 56, 907–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kawakami T., Ihn H., Xu W., Smith E., LeRoy C., Trojanowska M. (1998) Increased expression of TGF-β receptors by scleroderma fibroblasts: evidence for contribution of autocrine TGF-β signaling to scleroderma phenotype. J. Invest. Dermatol. 110, 47–51 [DOI] [PubMed] [Google Scholar]
- 15. Daniels C. E., Wilkes M. C., Edens M., Kottom T. J., Murphy S. J., Limper A. H., Leof E. B. (2004) Imatinib mesylate inhibits the profibrogenic activity of TGF-β and prevents bleomycin-mediated lung fibrosis. J. Clin. Invest. 114, 1308–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nakao A., Fujii M., Matsumura R., Kumano K., Saito Y., Miyazono K., Iwamoto I. (1999) Transient gene transfer and expression of Smad7 prevents bleomycin-induced lung fibrosis in mice. J. Clin. Invest. 104, 5–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yehualaeshet T., O'Connor R., Begleiter A., Murphy-Ullrich J. E., Silverstein R., Khalil N. (2000) A CD36 synthetic peptide inhibits bleomycin-induced pulmonary inflammation and connective tissue synthesis in the rat. Am. J. Respir. Cell Mol. Biol. 23, 204–212 [DOI] [PubMed] [Google Scholar]
- 18. Madtes D. K., Busby H. K., Strandjord T. P., Clark J. G. (1994) Expression of transforming growth factor-α and epidermal growth factor receptor is increased following bleomycin-induced lung injury in rats. Am. J. Respir. Cell Mol. Biol. 11, 540–551 [DOI] [PubMed] [Google Scholar]
- 19. Martinelli M., Pacilli A. M., Rivetti S., Lauriola M., Fasano L., Carbonara P., Mattei G., Valentini I., Scapoli L., Solmi R. (2011) A role for epidermal growth factor receptor in idiopathic pulmonary fibrosis onset. Mol. Biol. Rep. 38, 4613–4617 [DOI] [PubMed] [Google Scholar]
- 20. Madtes D. K., Elston A. L., Hackman R. C., Dunn A. R., Clark J. G. (1999) Transforming growth factor-α deficiency reduces pulmonary fibrosis in transgenic mice. Am. J. Respir. Cell Mol. Biol. 20, 924–934 [DOI] [PubMed] [Google Scholar]
- 21. Korfhagen T. R., Swantz R. J., Wert S. E., McCarty J. M., Kerlakian C. B., Glasser S. W., Whitsett J. A. (1994) Respiratory epithelial cell expression of human transforming growth factor-α induces lung fibrosis in transgenic mice. J. Clin. Invest. 93, 1691–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ishii Y., Fujimoto S., Fukuda T. (2006) Gefitinib prevents bleomycin-induced lung fibrosis in mice. Am. J. Respir. Crit. Care Med. 174, 550–556 [DOI] [PubMed] [Google Scholar]
- 23. Hardie W. D., Davidson C., Ikegami M., Leikauf G. D., Le Cras T. D., Prestridge A., Whitsett J. A., Korfhagen T. R. (2008) EGF receptor tyrosine kinase inhibitors diminish transforming growth factor-α-induced pulmonary fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 294, L1217–1225 [DOI] [PubMed] [Google Scholar]
- 24. Seluanov A., Vaidya A., Gorbunova V. (2010) Establishing primary adult fibroblast cultures from rodents. J. Vis. Exp. 44, 2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee C. G., Cho S. J., Kang M. J., Chapoval S. P., Lee P. J., Noble P. W., Yehualaeshet T., Lu B., Flavell R. A., Milbrandt J., Homer R. J., Elias J. A. (2004) Early growth response gene 1-mediated apoptosis is essential for transforming growth factor β1-induced pulmonary fibrosis. J. Exp. Med. 200, 377–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Siner J. M., Jiang G., Cohen Z. I., Shan P., Zhang X., Lee C. G., Elias J. A., Lee P. J. (2007) VEGF-induced heme oxygenase-1 confers cytoprotection from lethal hyperoxia in vivo. FASEB J. 21, 1422–1432 [DOI] [PubMed] [Google Scholar]
- 27. Bhandari V., Choo-Wing R., Lee C. G., Zhu Z., Nedrelow J. H., Chupp G. L., Zhang X., Matthay M. A., Ware L. B., Homer R. J., Lee P. J., Geick A., de Fougerolles A. R., Elias J. A. (2006) Hyperoxia causes angiopoietin 2-mediated acute lung injury and necrotic cell death. Nat. Med. 12, 1286–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee C. G., Hartl D., Lee G. R., Koller B., Matsuura H., Da Silva C. A., Sohn M. H., Cohn L., Homer R. J., Kozhich A. A., Humbles A., Kearley J., Coyle A., Chupp G., Reed J., Flavell R. A., Elias J. A. (2009) Role of breast regression protein 39 (BRP-39)/chitinase 3-like-1 in Th2 and IL-13-induced tissue responses and apoptosis. J. Exp. Med. 206, 1149–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kang H. R., Lee C. G., Homer R. J., Elias J. A. (2007) Semaphorin 7A plays a critical role in TGF-β1-induced pulmonary fibrosis. J. Exp. Med. 204, 1083–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Evans R. A., Tian Y. C., Steadman R., Phillips A. O. (2003) TGF-β1-mediated fibroblast-myofibroblast terminal differentiation-the role of Smad proteins. Exp. Cell Res. 282, 90–100 [DOI] [PubMed] [Google Scholar]
- 31. Kang H. R., Lee J. Y., Lee C. G. (2008) TGF-b1 as a therapeutic target for pulmonary fibrosis and COPD. Expert Rev. Clin. Pharmacol. 1, 547–558 [DOI] [PubMed] [Google Scholar]
- 32. Mundle S. D., Gao X. Z., Khan S., Gregory S. A., Preisler H. D., Raza A. (1995) Two in situ labeling techniques reveal different patterns of DNA fragmentation during spontaneous apoptosis in vivo and induced apoptosis in vitro. Anticancer Res. 15, 1895–1904 [PubMed] [Google Scholar]
- 33. Derynck R., Zhang Y. E. (2003) Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 425, 577–584 [DOI] [PubMed] [Google Scholar]
- 34. Avraham R., Yarden Y. (2011) Feedback regulation of EGFR signalling: decision making by early and delayed loops. Nat. Rev. Mol. Cell Biol. 12, 104–117 [DOI] [PubMed] [Google Scholar]
- 35. Scartozzi M., Bearzi I., Berardi R., Mandolesi A., Pierantoni C., Cascinu S. (2007) Epidermal growth factor receptor (EGFR) downstream signalling pathway in primary colorectal tumours and related metastatic sites: optimising EGFR-targeted treatment options. Br J. Cancer 97, 92–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang S. W., Oh C. K., Cho S. H., Hu G., Martin R., Demissie-Sanders S., Li K., Moyle M., Yao Z. (2005) Amphiregulin expression in human mast cells and its effect on the primary human lung fibroblasts. J. Allergy Clin. Immunol. 115, 287–294 [DOI] [PubMed] [Google Scholar]
- 37. Kang H. R., Cho S. J., Lee C. G., Homer R. J., Elias J. A. (2007) Transforming growth factor (TGF)-β1 stimulates pulmonary fibrosis and inflammation via a Bax-dependent, Bid-activated pathway that involves matrix metalloproteinase-12. J. Biol. Chem. 282, 7723–7732 [DOI] [PubMed] [Google Scholar]
- 38. Samarakoon R., Higgins P. J. (2008) Integration of non-Smad and Smad signaling in TGF-β1-induced plasminogen activator inhibitor type-1 gene expression in vascular smooth muscle cells. Thromb. Haemost. 100, 976–983 [PMC free article] [PubMed] [Google Scholar]
- 39. Liu M., Yang S. C., Sharma S., Luo J., Cui X., Peebles K. A., Huang M., Sato M., Ramirez R. D., Shay J. W., Minna J. D., Dubinett S. M. (2007) EGFR signaling is required for TGF-β1 mediated COX-2 induction in human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 37, 578–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shoyab M., McDonald V. L., Bradley J. G., Todaro G. J. (1988) Amphiregulin: a bifunctional growth-modulating glycoprotein produced by the phorbol 12-myristate 13-acetate-treated human breast adenocarcinoma cell line MCF-7. Proc. Natl. Acad. Sci. U.S.A. 85, 6528–6532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schuger L., Johnson G. R., Gilbride K., Plowman G. D., Mandel R. (1996) Amphiregulin in lung branching morphogenesis: interaction with heparan sulfate proteoglycan modulates cell proliferation. Development 122, 1759–1767 [DOI] [PubMed] [Google Scholar]
- 42. Perugorria M. J., Latasa M. U., Nicou A., Cartagena-Lirola H., Castillo J., Goñi S., Vespasiani-Gentilucci U., Zagami M. G., Lotersztajn S., Prieto J., Berasain C., Avila M. A. (2008) The epidermal growth factor receptor ligand amphiregulin participates in the development of mouse liver fibrosis. Hepatology 48, 1251–1261 [DOI] [PubMed] [Google Scholar]
- 43. Fukumoto J., Harada C., Kawaguchi T., Suetsugu S., Maeyama T., Inoshima I., Hamada N., Kuwano K., Nakanishi Y. (2010) Amphiregulin attenuates bleomycin-induced pneumopathy in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 298, L131–138 [DOI] [PubMed] [Google Scholar]
- 44. Yamasaki M., Kang H. R., Homer R. J., Chapoval S. P., Cho S. J., Lee B. J., Elias J. A., Lee C. G. (2008) P21 regulates TGF-β1-induced pulmonary responses via a TNF-α-signaling pathway. Am. J. Respir. Cell Mol. Biol. 38, 346–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Borrell-Pagès M., Rojo F., Albanell J., Baselga J., Arribas J. (2003) TACE is required for the activation of the EGFR by TGF-α in tumors. EMBO J. 22, 1114–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Meran S., Luo D. D., Simpson R., Martin J., Wells A., Steadman R., Phillips A. O. (2011) Hyaluronan facilitates transforming growth factor-β1-dependent proliferation via CD44 and epidermal growth factor receptor interaction. J. Biol. Chem. 286, 17618–17630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Forsythe B., Faulkner K. (2004) Overview of the tolerability of gefitinib (IRESSA) monotherapy: clinical experience in non-small-cell lung cancer. Drug Safety 27, 1081–1092 [DOI] [PubMed] [Google Scholar]