Background: Circulating vitamin D relationship with insulin resistance is not resolved.
Results: 1,25(OH)2D3 (active form of vitamin D) up-regulates GLUT4 translocation and glucose utilization and is inhibited by chemical inhibition or silencing of cystathionine-γ-lyase in high glucose-treated adipocytes.
Conclusion: 1,25(OH)2D3-induced GLUT4 translocation and glucose utilization are mediated by cystathionine-γ-lyase activation and H2S formation.
Significance: 1,25(OH)2D3 up-regulates the insulin signaling for maintenance of glucose homeostasis in diabetes.
Keywords: Adipocyte, Diabetes, GLUT4, Hydrogen Sulfide, Vitamin D
Abstract
A scientific explanation for the beneficial role of vitamin D supplementation in the lowering of glycemia in diabetes remains to be determined. This study examined the biochemical mechanism by which vitamin D supplementation regulates glucose metabolism in diabetes. 3T3L1 adipocytes were treated with high glucose (HG, 25 mm) in the presence or absence of 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) (25, 50 nm), the active form of vitamin D. 1,25(OH)2D3 treatment caused significant up-regulation of GLUT4 total protein expression and its translocation to cell surface, and an increase in glucose uptake as well as glucose utilization in HG-treated cells. 1,25(OH)2D3 also caused cystathionine-γ-lyase (CSE) activation and H2S formation in HG-treated adipocytes. The effect of 1,25(OH)2D3 on GLUT4 translocation, glucose utilization, and H2S formation was prevented by propargylglycine, an inhibitor of CSE that catalyzes H2S formation. Studies using antisense CSE also demonstrated the inhibition of GLUT4 translocation as well as glucose uptake and utilization in 1,25(OH)2D3-supplemented CSE-siRNA-transfected adipocytes compared with controls. 1,25(OH)2D3 treatment along with insulin enhanced GLUT4 translocation and glucose utilization compared with either insulin or 1,25(OH)2D3 alone in HG-treated adipocytes. 1,25(OH)2D3 supplementation also inhibited monocyte chemoattractant protein-1 and stimulated adiponectin secretion in HG-treated adipocytes, and this positive effect was prevented in propargylglycine-treated or CSE-knockdown adipocytes. This is the first report to demonstrate that 1,25(OH)2D3 up-regulates GLUT4 translocation and glucose utilization and decreases inflammatory markers, which is mediated by CSE activation and H2S formation in adipocytes. This study provides evidence for a novel molecular mechanism by which 1,25(OH)2D3 can up-regulate the GLUT4 translocation essential for maintenance of glucose metabolism.
Introduction
Vitamin D is a steroid hormone that regulates calcium and phosphate levels in the bloodstream and promotes healthy bone growth (1). A review of the literature suggests that higher concentrations of circulating vitamin D are associated with a substantial decrease in cardiovascular disease, type 2 diabetes, and metabolic syndrome (2). Supplementation with vitamin D decreases blood glucose and HbA1c in both type 2 and type 1 diabetic patients (3, 4). These studies in humans support a strong case for the association between vitamin D status and hyperglycemia in diabetes. The relationship among vitamin D, insulin resistance, and obesity is not completely resolved. In addition, a scientific explanation for the beneficial role of vitamin D supplementation in the lowering of glycemia in diabetes remains to be determined.
Hydrogen sulfide (H2S)2 is an important signaling molecule formed mainly by the enzyme cystathionine-γ-lyase (CSE) in the cardiovascular system (5–7). Recent reviews discuss the potential benefits of H2S in biological systems and in the protection of mitochondrial function and cardiovascular pathophysiology (5–7). Glucose transporter 4 (GLUT4) is a key player in glucose metabolism and maintenance of glucose homeostasis in the body (8, 9). Studies using GLUT4 heterozygous knock-out mice showed that a decrease in GLUT4 expression led to increased levels of serum glucose and insulin, reduced muscle glucose uptake, hypertension, and diabetic complications similar to those seen in the diabetic population (8–10). GLUT4 activation has been a therapeutic target for pharmacological intervention strategies to control diabetic hyperglycemia (8).
Adipose tissue is a major site of glucose metabolism and plays a critical role in the maintenance of glucose homeostasis. This study reports for the first time that 1,25(OH)2D3 up-regulates GLUT4 translocation to the cell surface and glucose uptake and utilization in adipocytes treated with high glucose (HG). 1,25(OH)2D3 also up-regulates CSE activation and H2S formation in HG-treated adipocytes. The effect of 1,25(OH)2D3 on GLUT4 up-regulation and glucose utilization, as well as levels of inflammatory markers, was significantly inhibited by propargylglycine (PAG), an inhibitor of CSE, or in CSE-knockdown adipocytes. These results demonstrate that the effect of 1,25(OH)2D3 on GLUT4 translocation and glucose utilization and levels of inflammatory markers, is mediated by CSE activation and H2S formation in adipocytes. To our knowledge, this is the first report to demonstrate a direct effect of 1,25(OH)2D3 on up-regulation of GLUT4 translocation and glucose utilization at a cellular level. This study provides a promising lead to investigate vitamin D supplementation in boosting blood levels of H2S and as an adjuvant therapy in reducing hyperglycemia and its associated complications.
EXPERIMENTAL PROCEDURES
3T3L1 Adipocyte Cell Culture and Differentiation
The murine 3T3L1 fibroblast cell line was obtained from American Type Culture Collection (ATCC). These cells were cultured and differentiated into adipocytes following the method described earlier (11). The cells were incubated with serum-free low glucose DMEM during the experiments.
Treatment with Control Glucose (CG), HG, and 1,25(OH)2D3
Cells were treated with CG (7 mm) or HG (25 mm) with or without 1,25(OH)2D3, the active form of vitamin D. Cells were pretreated with different concentrations (25 or 50 nm) of 1,25(OH)2D3 for 2 h followed by CG or HG exposure for the next 20 h. Mannitol was used as an osmolarity control. In the mannitol group, cells were exposed to 18 mm mannitol because the medium contains 7 mm glucose. The viability of treated cells was determined using the Alamar Blue reduction bioassay (Alamar Biosciences, Sacramento, CA). In this study control cells are incubated in medium with a glucose concentration of 7 mm. In the human body glucose is continuously degraded and re-formed to maintain a 5 mm blood glucose level. However, in cell culture studies, we observed that incubating cells in medium with a 5 mm glucose concentration for 24 h caused the concentration of glucose in the medium to drop to levels lower than 2 mm. In cell culture studies, glucose is metabolized but not replaced. Our experience has shown us that a glucose level of 7 mm does not lead to glucose deficiency after 24 h of treatment, and thus we incubated the cells in medium with a glucose concentration of 7 mm. In the HG study, cells were exposed to a medium having a HG concentration of 25 mm. Many previous studies have reported that glucose concentrations as high as 50 mm have been found in the blood of patients with uncontrolled diabetes (12). It is true that blood glucose levels in patients are not likely to stay as high as 25 mm for 24 h. However, tissue damage in diabetic patients occurs over many years of countless hyperglycemic episodes. Thus, the glucose concentration of 25 mm used in this cell culture study does not seem unreasonable. Doses of 1,25(OH)2D3 used in this study are in the physiological range (13).
Determination of CSE Activity
Intracellular CSE activity was determined following the method of Xu et al. (14) using an enzyme-coupled assay with lactate dehydrogenase. Using pyridoxal phosphate as a coenzyme, CSE first catalyzes the α,γ-elimination of cystathionine to give cysteine, which is then converted into pyruvate, ammonia, and H2S by the action of the same enzyme (CSE). Exogenous addition of lactate dehydrogenase then catalyzes the conversion of pyruvate into lactate with concomitant formation of NAD+ from NADH. The oxidation rate of NADH was monitored at 340 nm for 15 min at 37 °C as an index of CSE activity. After treatment, cells were homogenized in 50 mm potassium phosphate buffer (pH 6.9) containing 1 mm EDTA and 1:100 (v/v) protease inhibitor mixture (Calbiochem) followed by centrifugation at 15,000 × g for 30 min at 4 °C. The resulting supernatant was used for CSE activity. The reaction mixture (100 μl) contained 100 mm potassium phosphate buffer (pH 7.4), 4.0 mm l-cystathionine, 0.125 mm pyridoxal 5′-phosphate, 0.32 mm NADH, 1.5 units lactate dehydrogenase, and 10 μl of cell homogenate. The decrease in optical density at an absorbance of 340 nm was kinetically monitored with a microplate reader (Spectramax-5; Molecular Devices) at 37 °C for 30 min. Blank reactions were performed in the same way except that l-cystathionine was omitted. Maximum velocities were calculated from the linear portion of the graphs, and the results were expressed as nmol min−1 mg protein−1.
Glucose Transporter Cell Membrane Translocation Assay
GLUT4 and GLUT1 translocation from cytoplasm to the cell surface were determined using flow cytometry as described earlier (15). After treatment cells were washed in FACS buffer (PBS without Mg2+ and Ca2+, with the addition of 10% fetal bovine serum and 0.1% sodium azide), centrifuged, suspended in FACS buffer, and incubated for 2 h at 4 °C with either anti-GLUT4 (Santa Cruz Biotechnology, sc-53566) or anti-GLUT1 (Santa Cruz Biotechnology, sc-7903) primary antibody at a 1:50 dilution. The cells were then washed in washing buffer for FACS (PBS containing 1% BSA and 0.1% sodium azide) and incubated with a FITC-conjugated appropriate secondary antibody (Abcam) at a 1:40 dilution on ice for 30 min in the dark. After the incubation, 1 ml of washing buffer for FACS was added to each sample. The samples were then vortexed and centrifuged, the supernatant was removed, and 0.5 ml of FACS buffer was added. In each experiment, a minimum of 15,000 cells was analyzed (per treatment condition) by FACSCalibur flow cytometer (BD Biosciences) equipped with multicolor analysis capability. Gates were set to exclude nonviable cells, cell debris, and cells of abnormal size and shape. Results were expressed as mean fluorescence intensity/15,000 cells.
Glucose Utilization, Glucose Uptake, H2S Concentration, and Cytokine Secretion Assays
Glucose assays were done at 0 h and at other specified times. The glucose utilization level was determined by subtracting glucose values at specified times (leftover glucose) from the 0 h glucose level. All assays were done in duplicate at each time point. An Advantage Accu-check glucometer (Roche Applied Science) was used for the glucose assay. The glucose utilization values were expressed in nmol/ml per min unit. The glucose uptake assay was performed using 6-NBDG (Invitrogen), a fluorescent analog of 2-deoxyglucose, following the method of Jung et al. (16). Briefly, after treatment, cells were incubated with serum-free low glucose medium containing 6-NBDG (20 μm) for 30 min. After the incubation, cells were washed with PBS and then lysed with 70 μl of PBS containing 1% Triton X-100 and kept at dark for 10 min. Then 30 μl of dimethyl sulfoxide was added in each sample and homogenized by pipetting up and down, and the plate was read immediately using a microplate reader at an excitation/emission wave length of 466/540 nm. Results were expressed as relative fluorescence units. H2S concentrations in the cell culture supernatant were measured following the formation of methylene blue (17). Briefly, 100 μl of medium was mixed with 250 μl of 1% (w/v) zinc acetate and 425 μl of distilled water, followed by addition of 133 μl of N-dimethyl-p-phenylenediamine sulfate (20 mm in 7.2 m HCl) and 133 μl of FeCl3 in (30 mm in 1.2 m HCl) to the test tube. The mixture was incubated at room temperature for 10 min. The protein in the plasma was removed by adding 250 μl of 10% trichloroacetic acid to the reaction mixture which was pelleted by centrifugation. The absorbance of the resulting solution was measured at 670 nm in a 96-well plate with a microplate reader. Concentration of H2S formation was calculated using a calibration curve of standard Na2S. The monocyte chemoattractant protein-1 (MCP-1) and adiponectin levels were measured in the supernatants of treated cells by the sandwich ELISA method using a commercially available kit from R&D Systems and ALPCO Diagnostics (Salem, NH), respectively. All appropriate controls and standards as specified by each manufacturer's kit were used. In the cytokine assay, control samples were analyzed each time to check the variation from plate to plate on different days of analyses.
Immunoblotting
For the immunoblotting, after treatment, cells were lysed in radioimmunoprecipitation assay buffer (50 mm Tris, pH 8, 150 mm NaCl, 1% Nonidet P-40, 0.5% deoxycholic acid, 0.1% SDS) supplemented with protease and phosphatase inhibitors (1 mm PMSF, 5 μg/ml leupeptin, 2 μg/ml aprotinin, 1 mm EDTA, 10 mm NaF, and 1 mm NaVO4). Lysates were cleared by centrifugation, and total protein concentrations were determined using a BCA assay kit (Pierce/Thermo Scientific). All samples contained approximately the same amount of protein (∼20–30 μg) and were used for immunoblotting with either anti-GLUT4 (1:1000) (Abcam), anti-GLUT1 (1:1000) (Abcam), or anti-CSE (1:1000) (Sigma) primary antibody and appropriate HRP-conjugated secondary antibody (1:5000 dilution). The intensity of each immunoblotting band was measured using the histogram tool of Adobe Photoshop CS5.
All chemicals were purchased from Sigma unless otherwise mentioned. Data were analyzed statistically using one-way ANOVA with Sigma Stat software (Jandel Scientific, San Rafael, CA). When data passed a normality test, all groups were compared using the Student-Newman-Keuls method. p < 0.05 was considered significant.
RESULTS
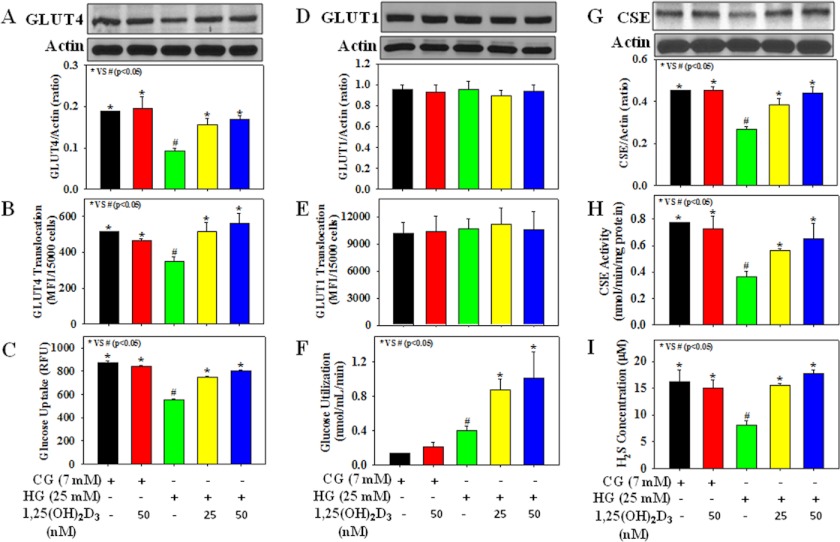
Fig. 1 illustrates the effect of 1,25(OH)2D3 supplementation on GLUT4 translocation, glucose utilization, CSE activation, and H2S formation in HG-treated 3T3L1 adipocytes. It shows that HG exposure caused a reduction in GLUT4 total protein expression, as well as its cell surface translocation, and in glucose uptake. In addition, HG exposure also caused a significant (p < 0.05) decrease in CSE protein expression as well as its activity and a concomitant reduction in H2S formation in adipocytes. Treatment with 1,25(OH)2D3 (25 or 50 nm) up-regulated the GLUT4 total protein expression and its translocation to cell membrane, glucose uptake, and glucose utilization and prevented reduction in CSE protein expression, CSE activity, and H2S concentration in HG-treated adipocytes. In addition to GLUT4, GLUT1 also plays an important role in basal glucose transport in 3T3L1 adipocytes. However, a significant difference in GLUT1 total protein expression as well as its translocation to cell surface was not observed among different experimental groups. HG concentration (25 mm) showed an inhibition of uptake of tracer glucose (a fluorescent analog of 2-deoxyglucose), although the actual glucose uptake and utilization were increased at this HG concentration. Thus, cells treated with HG (25 mm) were showing lower uptake of tracer glucose but higher glucose utilization compared with those seen in cells treated with control glucose (7 mm). Mannitol used as an osmolarity control did not affect CSE activation, H2S formation, GLUT4 translocation, and glucose utilization in adipocytes (data not shown). Different treatments did not cause any change in cell viability (data not shown).
FIGURE 1.
Effect of 1,25(OH)2D3 supplementation on the glucose transporters (GLUT4 and GLUT1) total protein expression and their translocation to cell surface, glucose uptake and glucose utilization, CSE protein expression and its activity, and H2S formation in 3T3L1 adipocytes exposed to CG or HG. A, GLUT4 total protein expression. B, GLUT4 translocation. C, glucose uptake. D, GLUT1 total protein expression. E, GLUT1 translocation. F, glucose utilization. G, CSE protein expression. H, CSE activity. I, H2S formation. Cells were pretreated with 1,25(OH)2D3 for 2 h followed by CG (7 mm) or HG (25 mm) exposure for the next 20 h. Values are mean ± S.E. (error bars; n = 3).
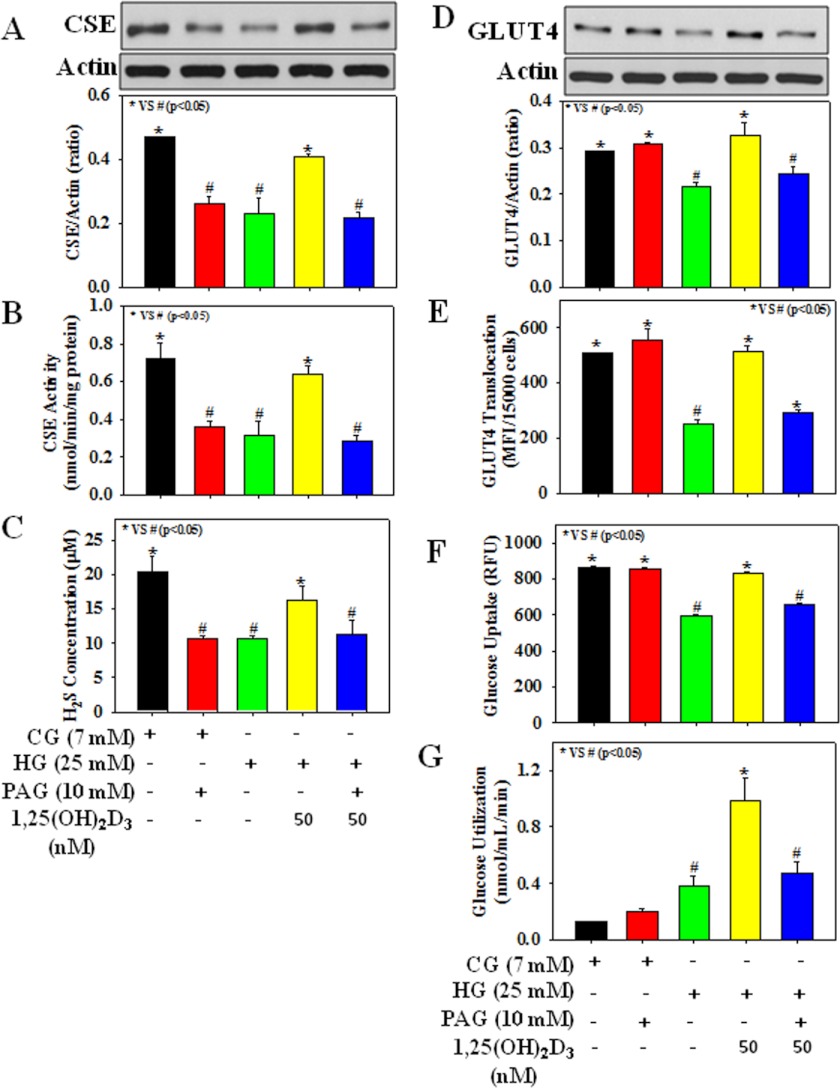
Fig. 2 shows the effect of PAG, an inhibitor of CSE that catalyzes H2S formation, on CSE activation, H2S formation, GLUT4 translocation, and glucose utilization in HG-treated adipocytes. The role of H2S was demonstrated when the presence of PAG lowered CSE protein expression and its activity, H2S concentration, GLUT4 total protein expression and its translocation, glucose utilization, and glucose uptake in 1,25(OH)2D3-supplemented cells exposed to HG (Fig. 2). Fig. 3 demonstrates that PAG supplementation after 1,25(OH)2D3 treatment also caused a reduction in both CSE and GLUT4 total protein expression in adipocytes exposed to HG (25 mm). Control cells treated with PAG alone showed a decrease in CSE protein expression and its activity, and H2S formation, but had no effect on GLUT4 total protein expression or its cell surface translocation, glucose uptake, or glucose utilization.
FIGURE 2.
Effect of PAG on CSE protein expression and its activity, H2S formation, GLUT4 total protein expression and its translocation, and glucose uptake and glucose utilization in 3T3L1 adipocytes exposed to 1,25(OH)2D3 and CG or HG. A, CSE protein expression. B, CSE activity. C, H2S formation. D, GLUT4 total protein expression. E, GLUT4 translocation. F, glucose uptake. G, glucose utilization. Cells were treated with PAG (10 mm) 5 min prior to 1,25(OH)2D3 (50 nm) supplementation followed by CG (7 mm) or HG (25 mm) exposure for the next 20 h. Values are mean ± S.E. (error bars; n = 3).
FIGURE 3.
Effect of PAG supplementation after 1,25(OH)2D3 treatment on the protein expression of both CSE and GLUT4 in 3T3L1 adipocytes exposed to CG or HG. A, CSE protein expression. B, GLUT4 total protein expression. Cells were treated with PAG (10 mm) 5 min after 1,25(OH)2D3 (50 nm) supplementation followed by CG (7 mm) or HG (25 mm) exposure for the next 20 h. Values are mean ± S.E. (error bars; n = 3).
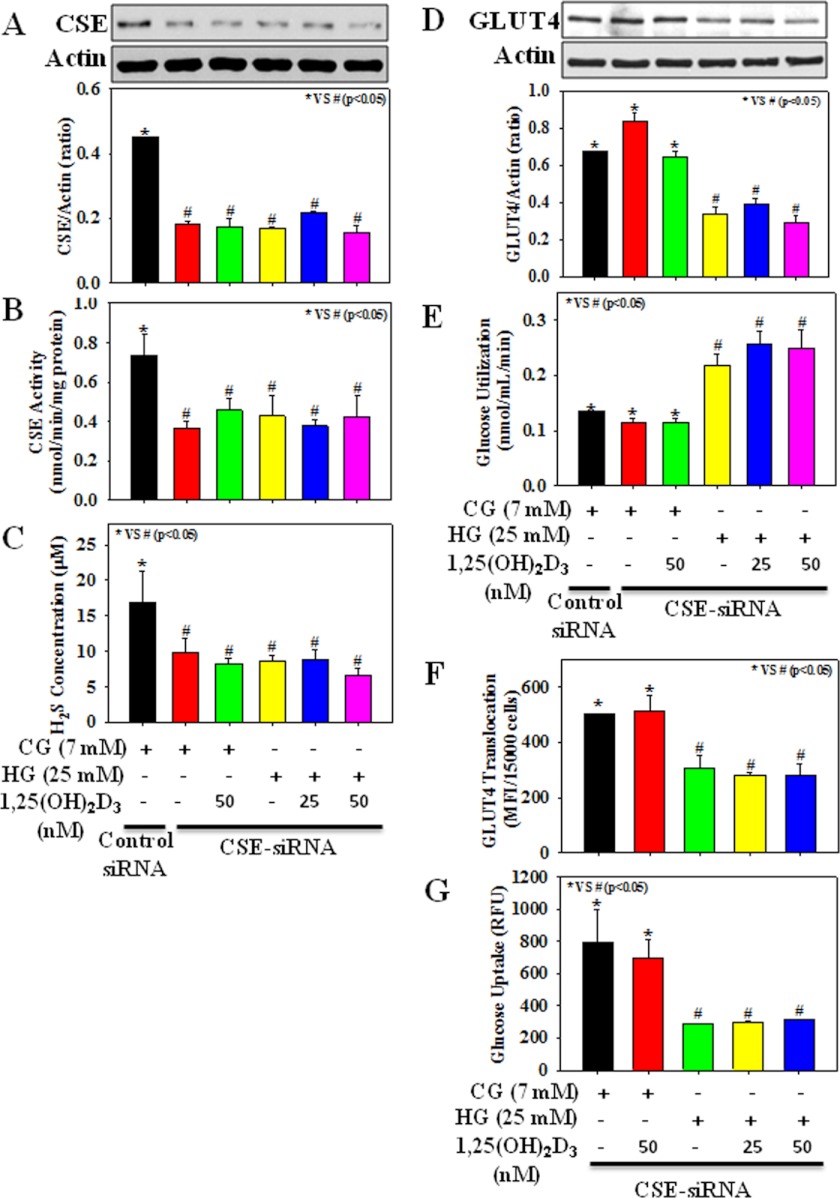
Fig. 4 shows the signal silencing studies with antisense CSE. We observed that in control cells, transient transfection with CSE-siRNA decreased CSE protein expression and its activity, as well as H2S formation, compared with cells transfected with nontargeted control siRNA; however, neither GLUT4 total protein expression nor glucose utilization was significantly different compared with those of control siRNA-transfected cells. Treatment with HG caused a reduction in GLUT4 total protein expression as well as its cell surface translocation, glucose uptake, and glucose utilization in CSE-siRNA-transfected adipocytes compared with those of CSE-siRNA transfected control cells. Interestingly, the effect of HG on GLUT4 translocation, glucose uptake, and glucose utilization could not be reversed by 1,25(OH)2D3 supplementation.
FIGURE 4.
Effect of 1,25(OH)2D3 supplementation on CSE protein expression and its activity, H2S formation, GLUT4 total protein expression and its translocation to cell surface, and glucose uptake and glucose utilization in 3T3L1 adipocytes exposed to CG or HG after transfection with CSE siRNA (100 nm). A, CSE protein expression. B, CSE activity. C, H2S formation. D, GLUT4 total protein expression. E, glucose utilization. F, GLUT4 translocation. G, glucose uptake. Cells transfected with CSE-siRNA were treated with 1,25(OH)2D3 for 2 h followed by CG (7 mm) or HG (25 mm) exposure for the next 20 h. Control siRNA is a fluorescein-conjugated scrambled nonspecific RNA duplex with no sequence homology with any of the genes. Values are mean ± S.E. (error bars; n = 3).
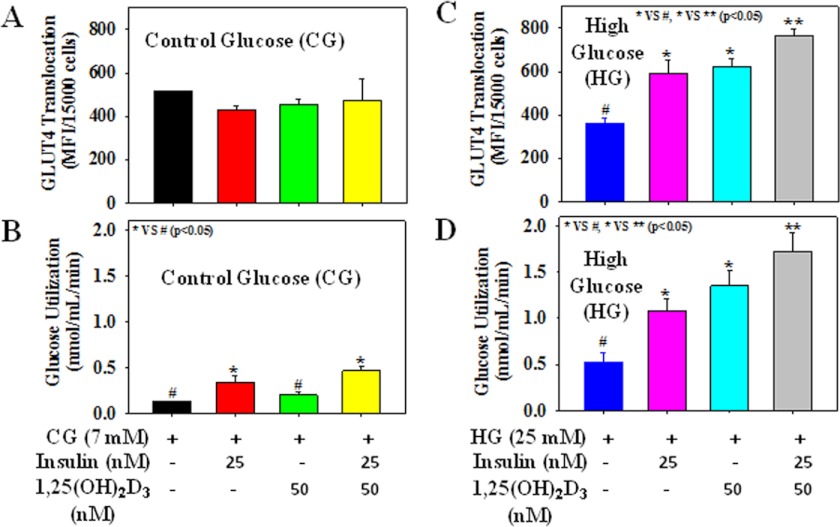
Fig. 5 shows the effect of insulin (25 nm) alone or in combination with 1,25(OH)2D3 supplementation (50 nm) on GLUT4 translocation and glucose utilization in cells treated with either CG or HG. Treatment with insulin alone increased the GLUT4 translocation and glucose utilization in HG-treated cells. Interestingly, 1,25(OH)2D3 supplementation along with insulin significantly boosted the GLUT4 translocation and glucose utilization compared with the either insulin or 1,25(OH)2D3 alone supplementation in HG-treated cells. Insulin alone or in combination with 1,25(OH)2D3 supplementation also increased the glucose utilization levels compared with control cells or 1,25(OH)2D3 alone-treated cells exposed to CG. However, different treatments did not cause any significant difference among the GLUT4 cell surface translocation in CG-treated cells. The outcome of this study suggests that vitamin D can increase the insulin action in diabetes.
FIGURE 5.
Effect of insulin and 1,25(OH)2D3 supplementation either alone or in combination on the GLUT4 translocation and glucose utilization in 3T3L1 adipocytes exposed to CG or HG. A and C, GLUT4 translocation. B and D, glucose utilization. Cells were pretreated with insulin (25 nm) or 1,25(OH)2D3 (50 nm) either alone or in combination for 2 h followed by CG (7 mm) or HG (25 mm) exposure for the next 20 h. Values are mean ± S.E. (error bars; n = 4).
HG exposure caused an increase in MCP-1 secretion and decrease in adiponectin levels in adipocytes, and that was prevented by 1,25(OH)2D3 (Fig. 6). 1,25(OH)2D3 did not affect the HG-induced increase in MCP-1 secretion and decrease in adiponectin levels in either PAG-treated or CSE-siRNA-transfected adipocytes.
FIGURE 6.
Effect of 1,25(OH)2D3 supplementation on MCP-1 and adiponectin secretion in 3T3L1 adipocytes exposed to CG or HG either alone or in the presence of PAG or in CSE-siRNA transfection. A, MCP-1. B, adiponectin. C and D, effect of PAG on MCP-1 (C) and adiponectin levels (D). E and F, adiponectin (E) and MCP-1 (F) levels in CSE-siRNA-transfected cells treated with 1,25(OH)2D3 for 2 h followed by CG (7 mm) or HG (25 mm) exposure for the next 20 h. Values are mean ± S.E. (error bars; n = 3).
DISCUSSION
Epidemiological studies show a negative association between circulating blood vitamin D concentration and insulin resistance in diabetic patients. However, the role of adiposity in relationship between vitamin D and insulin resistance has not been resolved (18). Nor do we know the molecular mechanisms by which vitamin D can directly regulate the glucose homeostasis. Adipose tissue plays a critical role in glucose metabolism and the maintenance of glucose homeostasis in our body. This study reports for the first time that 1,25(OH)2D3 supplementation increased CSE activity and H2S formation, which in turn caused GLUT4 translocation and glucose utilization in adipocytes exposed to HG. Using an adipocyte cell model, this finding provides a novel link among vitamin D, CSE activation, H2S formation, GLUT4 translocation, and glucose utilization. This suggests that circulating vitamin D levels can directly and positively regulate GLUT4, a key player in glucose metabolism.
Blood levels of H2S have been shown to be lower in various diseases associated with a higher incidence of vascular inflammation, such as those seen in diabetic animals and patients, and hypertensive animals and patients (19–21). H2S is produced in vivo from l-cysteine by the action of two main enzymes, CSE and cystathionine β-synthase (5–7). Exogenous H2S supplementation reduces blood pressure, decreases atherosclerotic plaque size compared with those of controls in apolipoprotein E knock-out mice, and prevents progression of diabetic nephropathy in spontaneously hypertensive rats and endothelial dysfunction in diabetic rats (22, 23). Several studies demonstrate that H2S is an antioxidant that regenerates GSH and lowers oxidative stress (5–7, 11). Our recent study reported that supplementation with H2S increases glucose utilization and decreases secretion of the proinflammatory biomarkers IL-8 and MCP-1 in adipocyte and monocyte cell models (11, 19). These studies demonstrate that malfunctioning H2S homeostasis can contribute to the pathogenesis of vascular inflammation and impaired glucose metabolism.
GLUT4 plays an important role in the regulation of whole body glucose homeostasis (8). Conditional depletion of GLUT4 in either adipose tissue or skeletal muscle causes insulin resistance (8–10). Both insulin and exercise acutely stimulate GLUT4 translocation to the cell surfaces of muscle and adipose cells independent of transcription or translation (8). The insulin-responsive GLUT4 translocation is mediated by activation of the insulin receptor substrate (IRS)/PI3K/PIP3 (phosphatidylinositol-3,4,5-trisphosphate)/AKT (serine/threonine protein kinase) signaling cascade (8). In our earlier study (11), we observed that exogenous supplementation with Na2S (a source of H2S) up-regulated insulin stimulated glucose utilization via the IRS/PI3K/PIP3/AKT/GLUT4 signaling pathway. Impaired responsiveness of GLUT4 to the insulin signaling pathway in obesity and diabetes is also mediated through the actions of fatty acids, cytokines, and the endoplasmic reticulum stress response via down-regulation of tyrosine phosphorylation of IRS by insulin (8). A decrease in GLUT4 expression in GLUT4 heterozygous knock-out mice led to increased levels of serum glucose and insulin, reduced muscle glucose uptake, hypertension, and diabetic complications in the heart and liver similar to those seen in the diabetic population (9).
In the basal state, GLUT1 is present both at the cell surface and in intracellular sites, but GLUT4 remains sequestered almost entirely within the cell (24). Liao et al. (25) reported that siRNA-mediated knockdown of GLUT4 reduced glucose uptake by 50–60%. However, introduction of siGLUT1 in GLUT4-deficient 3T3L1 adipocytes abolished the insulin-stimulated glucose uptake, suggesting that GLUT1 plays a role as an important glucose transporter in these cells. Overexpression of the constitutively active form of AKT stimulates GLUT4, but not GLUT1, translocation in 3T3L1 adipocytes (26). This study observed a higher GLUT1 total protein expression and its surface expression compared with those of GLUT4. However, a significant difference in GLUT1 total protein expressions as well as its surface expression was not observed among different experimental groups. It has been reported that, although both GLUT1 and GLUT4 facilitate glucose uptake following docking and fusion at the plasma membrane, the GLUT4 system is more sensitive and rapid compared with that of GLUT1 in 3T3L1 adipocytes (27).
1,25(OH)2D3 is the biologically active form and a stable indicator of vitamin D status in the body (1). Vitamin D may up-regulate insulin receptors and activate peroxisome proliferator-activated receptor γ expression (28). Vitamin D may increase the synthesis of calbindin, which can alter calcium-dependent insulin secretion by pancreatic β-cells (22). Vitamin D can also affect IL-12 secretion via inhibition of NF-κB in macrophages and dendritic cells, as well as inhibition of proinflammatory cytokine secretion by modulation of monocyte/macrophage and preadipocyte differentiation (1, 28–31).
This study reports that 1,25(OH)2D3 up-regulates GLUT4 translocation and glucose uptake and utilization in HG-treated adipocytes (Fig. 7). 1,25(OH)2D3 also up-regulated CSE activation and H2S formation in HG-treated adipocytes. The effect of 1,25(OH)2D3 on GLUT4 translocation, H2S formation, and glucose uptake and utilization was significantly inhibited in the presence of PAG (an inhibitor of CSE) or in CSE knockdown adipocytes. In addition, treatment with insulin alone also increased the GLUT4 translocation and glucose utilization in HG-treated cells. Interestingly, 1,25(OH)2D3 along with insulin significantly increased the GLUT4 translocation and glucose utilization compared with supplementation with either insulin or 1,25(OH)2D3 alone in HG-treated cells. This suggests that vitamin D can increase the insulin sensitivity in diabetes. This study has not examined the lower dose of 1,25(OH)2D3 needed to promote the insulin action on the GLUT4 translocation and glucose utilization in adipocytes. MCP-1 plays an important role in vascular inflammation, and adiponectin is a positive regulator of insulin sensitivity. 1,25(OH)2D3 supplementation inhibited MCP-1 and stimulated adiponectin secretion in adipocytes treated with HG. The effect of 1,25(OH)2D3 on inhibition of MCP-1 and stimulation of adiponectin secretion was also inhibited in PAG-treated or CSE-knockdown adipocytes compared with those of controls. These two different approaches demonstrate that 1,25(OH)2D3-induced up-regulation of GLUT4 translocation and glucose utilization is mediated by CSE activation and H2S formation in adipocytes.
FIGURE 7.
Schematic diagram of the proposed mechanism of 1,25(OH)2D3 effects on GLUT4 translocation and glucose utilization mediated by CSE up-regulation and H2S formation in 3T3L1 adipocytes.
The discovery of this novel link among vitamin D, GLUT4, and glucose utilization will allow for better understanding of and care for the excess diabetes and cardiovascular disease associated with the vitamin D-deficient population (1, 2). Future clinical trials are needed to investigate whether vitamin D supplementation increases blood CSE activity and H2S concentrations and is also associated with lower glycemia and vascular inflammation in the type 2 diabetic patient population.
Acknowledgment
We thank Georgia Morgan for excellent editing.
This work was supported by National Institutes of Health Grant R01 DK072433 through the NIDDK and the Office of Dietary Supplements. This work was also supported by the Malcolm Feist Chair in Diabetes and the Malcolm Feist Cardiovascular research fellowship.
- H2S
- hydrogen sulfide
- CG
- control glucose
- CSE
- cystathionine-γ-lyase
- GLUT4
- glucose transporter 4
- IRS
- insulin receptor substrate
- MCP-1
- monocyte chemoattractant protein-1
- 6-NBDG
- 6-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxy-d-glucose
- 1,25(OH)2D3
- 1,25-dihydroxyvitamin D3
- PAG
- propargylglycine.
REFERENCES
- 1. Rosen C. J., Adams J. S., Bikle D. D., Black D. M., Demay M. B., Manson J. E., Murad M. H., Kovacs C. S. (2012) The nonskeletal effects of vitamin D: an endocrine society scientific statement. Endocr. Rev. 33, 456–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grant W. B., Peiris A. N. (2010) Possible role of serum 25-hydroxyvitamin D in black-white health disparities in the United States. J. Am. Med. Dir. Assoc. 11, 617–628 [DOI] [PubMed] [Google Scholar]
- 3. Schwalfenberg G. (2008) Vitamin D and diabetes: improvement of glycemic control with vitamin D3 repletion. Can. Fam. Physician. 54, 864–866 [PMC free article] [PubMed] [Google Scholar]
- 4. Aljabri K. S., Bokhari S. A., Khan M. J. (2010) Glycemic changes after vitamin D supplementation in patients with type 1 diabetes mellitus and vitamin D deficiency. Ann. Saudi Med. 30, 454–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kimura H., Shibuya N., Kimura Y. (2012) Hydrogen sulfide is a signaling molecule and a cytoprotectant. Antioxid. Redox Signal. 17, 45–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kabil O., Banerjee R. (2010) Redox biochemistry of hydrogen sulfide. J. Biol. Chem. 285, 21903–21907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu Y. H., Lu M., Hu L. F., Wong P. T., Webb G. D., Bian J. S. (2012) Hydrogen sulfide in the mammalian cardiovascular system. Antioxid. Redox Signal. 17, 141–185 [DOI] [PubMed] [Google Scholar]
- 8. Huang S., Czech M. P. (2007) The GLUT4 glucose transporter. Cell Metab. 5, 237–252 [DOI] [PubMed] [Google Scholar]
- 9. Stenbit A. E., Tsao T. S., Li J., Burcelin R., Geenen D. L., Factor S. M., Houseknecht K., Katz E. B., Charron M. J. (1997) GLUT4 heterozygous knockout mice develop muscle insulin resistance and diabetes. Nat. Med. 3, 1096–1101 [DOI] [PubMed] [Google Scholar]
- 10. Li J., Houseknecht K. L., Stenbit A. E., Katz E. B., Charron M. J. (2000) Reduced glucose uptake precedes insulin signaling defects in adipocytes from heterozygous GLUT4 knockout mice. FASEB J. 14, 1117–1125 [DOI] [PubMed] [Google Scholar]
- 11. Manna P., Jain S. K. (2011) Hydrogen sulfide and l-cysteine increase phosphatidylinositol 3,4,5-trisphosphate (PIP3) and glucose utilization by inhibiting phosphatase and tensin homolog (PTEN) protein and activating phosphoinositide 3-kinase (PI3K)/serine/threonine protein kinase (AKT)/protein kinase Cζ/λ (PKCζ/λ) in 3T3L1 adipocytes. J. Biol. Chem. 286, 39848–39859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Candiloros H., Muller S., Zeghari N., Donner M., Drouin P., Ziegler O. (1995) Decreased erythrocyte membrane fluidity in poorly controlled IDDM: influence of ketone bodies. Diabetes Care 18, 549–551 [DOI] [PubMed] [Google Scholar]
- 13. Yaturu S., Davis J. (2011) Prevalence of decreased vitamin D levels is high among veterans with diabetes and/or CKD. ISRN Endocrinol. 2011, 109458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu Z., Prathapasinghe G., Wu N., Hwang S. Y., Siow Y. L., O K. (2009) Ischemia-reperfusion reduces cystathionine-β-synthase-mediated hydrogen sulfide generation in the kidney. Am. J. Physiol. Renal Physiol. 297, F27-F35 [DOI] [PubMed] [Google Scholar]
- 15. Koshy S., Alizadeh P., Timchenko L. T., Beeton C. (2010) Quantitative measurement of GLUT4 translocation to the plasma membrane by flow cytometry. J. Vis. Exp. 45, e2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jung D. W., Ha H. H., Zheng X., Chang Y. T., Williams D. R. (2011) Novel use of fluorescent glucose analogues to identify a new class of triazine-based insulin mimetics possessing useful secondary effects. Mol. Biosyst. 7, 346–358 [DOI] [PubMed] [Google Scholar]
- 17. Zhu Y. Z., Wang Z. J., Ho P., Loke Y. Y., Zhu Y. C., Huang S. H., Tan C. S., Whiteman M., Lu J., Moore P. K. (2007) Hydrogen sulfide and its possible roles in myocardial ischemia in experimental rats. J. Appl. Physiol. 102, 261–268 [DOI] [PubMed] [Google Scholar]
- 18. Lamendola C. A., Ariel D., Feldman D., Reaven G. M. (2012) Relations between obesity, insulin resistance, and 25-hydroxyvitamin D. Am. J. Clin. Nutr. 95, 1055–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jain S. K., Bull R., Rains J. L., Bass P. F., Levine S. N., Reddy S., McVie R., Bocchini J. A. (2010) Low levels of hydrogen sulfide in the blood of diabetic patients and streptozotocin-treated rats cause vascular inflammation. Antioxid. Redox Signal. 12, 1333–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yan H., Du J., Tang C. (2004) The possible role of hydrogen sulfide on the pathogenesis of spontaneous hypertension in rats. Biochem. Biophys. Res. Commun. 313, 22–27 [DOI] [PubMed] [Google Scholar]
- 21. Whiteman M., Gooding K. M., Whatmore J. L., Ball C. I., Mawson D., Skinner K., Tooke J. E., Shore A. C. (2010) Adiposity is a major determinant of plasma levels of the novel vasodilator hydrogen sulfide. Diabetologia 53, 1722–1726 [DOI] [PubMed] [Google Scholar]
- 22. Wang Y., Zhao X., Jin H., Wei H., Li W., Bu D., Tang X., Ren Y., Tang C., Du J. (2009) Role of hydrogen sulfide in the development of atherosclerotic lesions in apolipoprotein E knockout mice. Arterioscler. Thromb. Vasc. Biol. 29, 173–179 [DOI] [PubMed] [Google Scholar]
- 23. Suzuki K., Olah G., Modis K., Coletta C., Kulp G., Gerö D., Szoleczky P., Chang T., Zhou Z., Wu L., Wang R., Papapetropoulos A., Szabo C. (2011) Hydrogen sulfide replacement therapy protects the vascular endothelium in hyperglycemia by preserving mitochondrial function. Proc. Natl. Acad. Sci. U.S.A. 108, 13829–13834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang J., Holman G. D. (1993) Comparison of GLUT4 and GLUT1 subcellular trafficking in basal and insulin-stimulated 3T3-L1 cells. J. Biol. Chem. 268, 4600–4603 [PubMed] [Google Scholar]
- 25. Liao W., Nguyen M. T., Imamura T., Singer O., Verma I. M., Olefsky J. M. (2006) Lentiviral short hairpin ribonucleic acid-mediated knockdown of GLUT4 in 3T3-L1 adipocytes. Endocrinology 147, 2245–2252 [DOI] [PubMed] [Google Scholar]
- 26. Foran P. G., Fletcher L. M., Oatey P. B., Mohammed N., Dolly J. O., Tavaré J. M. (1999) Protein kinase B stimulates the translocation of GLUT4 but not GLUT1 or transferrin receptors in 3T3-L1 adipocytes by a pathway involving SNAP-23, synaptobrevin-2, and/or cellubrevin. J. Biol. Chem. 274, 28087–28095 [DOI] [PubMed] [Google Scholar]
- 27. Rudich A., Konrad D., Török D., Ben-Romano R., Huang C., Niu W., Garg R. R., Wijesekara N., Germinario R. J., Bilan P. J., Klip A. (2003) Indinavir uncovers different contributions of GLUT4 and GLUT1 towards glucose uptake in muscle and fat cells and tissues. Diabetologia 46, 649–658 [DOI] [PubMed] [Google Scholar]
- 28. Pittas A. G., Dawson-Hughes B. (2010) Vitamin D and diabetes. J. Steroid Biochem. Mol. Biol. 121, 425–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maestro B., Campión J., Dávila N., Calle C. (2000) Stimulation by 1,25-dihydroxyvitamin D3 of insulin receptor expression and insulin responsiveness for glucose transport in U-937 human promonocytic cells. Endocr. J. 47, 383–391 [DOI] [PubMed] [Google Scholar]
- 30. D'Ambrosio D., Cippitelli M., Cocciolo M. G., Mazzeo D., Di Lucia P., Lang R., Sinigaglia F., Panina-Bordignon P. (1998) Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3: involvement of NF-κB down-regulation in transcriptional repression of the p40 gene. J. Clin. Invest. 101, 252–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Willheim M., Thien R., Schrattbauer K., Bajna E., Holub M., Gruber R., Baier K., Pietschmann P., Reinisch W., Scheiner O., Peterlik M. (1999) Regulatory effects of 1α,25-dihydroxyvitamin D3 on the cytokine production of human peripheral blood lymphocytes. J. Clin. Endocrinol. Metab. 84, 3739–3744 [DOI] [PubMed] [Google Scholar]