Background: The mechanistic relationship of Parkin and mitofusins in mitochondria quality control is not known.
Results: CCCP-induced mitophagy and mitochondrial spheroid formation differentially require Parkin and mitofusins.
Conclusion: Parkin and mitofusins reciprocally regulate mitophagy and mitochondrial spheroid formation.
Significance: This study revealed a default response of mitochondria to oxidative stress and a molecular mechanism by which Parkin primes the mitochondria for mitophagy.
Keywords: Autophagy, Cell Biology, Mitochondria, Oxidative Stress, Parkin
Abstract
Mitochondrial homeostasis via mitochondrial dynamics and quality control is crucial to normal cellular functions. Mitophagy (mitochondria removed by autophagy) stimulated by a mitochondrial uncoupler, carbonyl cyanide m-chlorophenylhydrazone (CCCP), requires Parkin, but it is not clear why Parkin is crucial to this process. We found that in the absence of Parkin, carbonyl cyanide m-chlorophenylhydrazone induced the formation of mitochondrial spheroids. Mitochondrial spheroid formation is also induced in vivo in the liver by acetaminophen overdose, a condition causing severe oxidative mitochondrial damages and liver injury. Mitochondrial spheroids could undergo a maturation process by interactions with acidic compartments. The formation of this new structure required reactive oxygen species and mitofusins. Parkin suppressed these mitochondrial dynamics by promoting mitofusin degradation. Consistently, genetic deletion of mitofusins without concomitant expression of Parkin was sufficient to prevent mitochondrial spheroid formation and resumed mitophagy. Mitochondrial spheroid formation and mitophagy could represent different strategies of mitochondrial homeostatic response to oxidative stress and are reciprocally regulated by mitofusins and Parkin.
Introduction
Maintaining mitochondria homeostasis is vital to cellular function (1–6). Cells have developed mechanisms, known as the mitochondrial quality control, to harness dysfunctional mitochondria caused by pathological conditions. Fusion between mitochondria can serve as one such mechanism (1, 3, 7). Damaged mitochondria may be revitalized, or their detrimental effects may be diluted after they fuse with the healthy mitochondria to regain functional components. Another quality control mechanism engages the autophagy machinery, and the damaged mitochondria are engulfed by autophagosomes for lysosomal degradation (4, 6, 8, 9). This process, known as mitophagy, may be more appropriate for mitochondria that cannot be repaired through fusion or when doing so may harm other mitochondria.
A well studied mitophagy model utilizes carbonyl cyanide m-chlorophenylhydrazone (CCCP)3, which is a reversible mitochondria uncoupler. CCCP rapidly causes mitochondrial depolarization and fragmentation, which in turn can broadly affect mitochondrial functions and trigger multiple responses (3, 10). Mitochondrial damage likely occurs as mitophagy is rapidly triggered (11–17), which is largely dependent on the expression of Parkin, an E3 ligase whose mutations have been linked to the etiology of certain forms of Parkinson's disease. However, the fate of the damaged mitochondria in the absence of Parkin under CCCP treatment is not known.
Here we report that in the absence of Parkin, CCCP triggers a unique form of mitochondrial dynamic process. This allows the formation of ball-like mitochondria, termed mitochondrial spheroid, that wraps the cytoplasm and other organelles. These structures can acquire lysosomal markers, become acidic, and undergo limited degradation of the mitochondrial proteins. This structural change of mitochondria is dependent on mitofusin as well as reactive oxygen species but not the autophagy machinery. Parkin acts as a switch to inhibit mitochondrial spheroid formation via the degradation of mitofusins and to promote mitophagy at the same time. Moreover, mitochondrial spheroids could also be found in hepatocytes following acetaminophen overdose in vivo, a condition causing severe oxidative mitochondrial damage and liver injury. These observations indicate that mitochondrial spheroid formation and mitophagy may represent alternative strategies of mitochondrial response to oxidative stress, which are reciprocally regulated by mitofusin and Parkin.
EXPERIMENTAL PROCEDURES
Chemicals and Antibodies
Chemicals were obtained from Sigma-Aldrich or Invitrogen. Primary antibodies were against Tom20 (Santa Cruz Biotechnology), voltage-dependent anion channel (VDAC, Calbiochem), cytochrome c (BD Biosciences), COX-IV (MitoScience), Mfn1 (Santa Cruz Biotechnology), Mfn2 (Sigma), cyclophilin D (MitoScience), Parkin (Santa Cruz Biotechnology), Lamp1 and Lamp2 (Developmental Studies Hybridoma Bank, Iowa City, IA), LC3B (18), β-Actin (Sigma), and GAPDH (Cell Signaling). Secondary antibodies were HRP-conjugated goat anti-mouse or HRP-conjugated goat anti-rabbit antibodies (JacksonImmunoResearch) for immunoblot assay. Secondary antibodies for immunofluorescence were conjugated with Alexa Fluor 488 (Invitrogen) or Cy3 (Jackson ImmunoResearch). Secondary antibodies for immuno-EM were conjugated with 5 nm or 10 nm of gold particles (GE Healthcare).
Cell Lines and Cell Culture
Atg5−/− MEFs were generously provided by Dr. N. Mizushima (Tokyo Medical and Dental University, Japan). Atg7−/− and Atg3−/− MEFs were a generous gifts from Dr. M. Komatsu (Tokyo Metropolitan Institute of Medical Science, Japan). Mfn1−/− and Mfn2−/− were kindly provided by Dr. D. C. Chan (California Institute of Technology, CA). Mfn1 and Mfn2 double knockout MEFs and the matched wild-type MEFs were purchased from the ATCC. Lamp1 and Lamp2 double knockout MEFs were reported previously (19). HEK-293 and HeLa cells were used as in our previous work (14). All cells were maintained in DMEM with 10% fetal bovine serum (Invitrogen) supplemented with l-glutamine and penicillin/streptomycin.
Cells were treated with CCCP (30 μm) for 6 h unless otherwise indicated in the figure legend with or without chloroquine (20 μm), E64D (10 μm), pepstatin (10 μm), N-acetyl cysteine (5 mm), H2O2 (250 μm), or bafilomycin A1 (50 nm). In some experiments, cells were preloaded with MitoTracker Green (MTG, 50 nm), or LysoTracker Red (LTR) (50 nm) for 15 min, or with adenoviral GFP-LC3 overnight before treatment.
Animal Experiments
All procedures were approved by the Institutional Animal Care and Use Committee. Wild-type C57BL/6 mice were given saline (33 ml/kg) or acetaminophen (500 mg/kg) intraperitoneally and sacrificed 6 h later for analysis.
Immunoblot and Immunofluorescence Microscopy
These procedures were performed as described previously (18). Twenty micrograms of protein was separated by SDS-PAGE and transferred to PVDF membranes. The membranes were further developed with Super Signal West Pico chemiluminescent substrate (Pierce). For immunostaining, all cellular images were obtained using an Olympus inverted confocal microscope (Olympus Fluoview 1000) or an epifluorescence microscope (Nikon, TE 200).
Conventional Electron Microscopy
Cells or liver tissues were fixed in 2.5% glutaraldehyde in 0.1 m phosphate buffer (pH 7.4) followed by 1% OsO4. Sections were examined with a JEM 1011CX electron microscope (JEOL, Peabody, MA). Images were acquired digitally. The total number of mitochondrial structures, including the normal mitochondria and the mitochondrial spheroids, per section was determined, and the percentage of mitochondrial spheroids was determined. Autophagosomes were quantified in the same way.
Immuno-electron Microscopy
Cells were fixed in 2% paraformaldehyde/0.01% glutaraldehyde in PBS. Cells were pelleted in 3% gelatin in PBS and solidified on ice. Small blocks (∼0.5 mm) of the cell pellet were prepared and infiltrated with 2.3 m sucrose at 4 °C overnight. Blocks were mounted on aluminum stubs, frozen, and sectioned. The sections (60 nm) were picked up in drops of 2.3 m sucrose and collected on Formvar-coated mesh grids. After blocking in 1% BSA in PBS, the sections were incubated with a primary rabbit anti-Tom20 antibody or a rat anti-Lamp2 antibody and subsequently incubated with a secondary antibody conjugated with 5-nm or 10-nm gold particles. The sections were fixed in 1% glutaraldehyde and stained with ice-cold 0.4% uranyl acetate/1% methyl cellulose (pH 4) and dried. The samples were viewed in a Tecnai transmission electron microscope (FEI, Hillsboro, OR).
Statistics
Animal studies had at least three mice per experimental group. Cellular studies were repeated at least three times. Fluorescence microscopic quantifications were conducted in 35–60 cells per experiment. EM quantification was obtained from 11–34 cell profiles in each experiment. The Western blot densitometry assay was based on four different experiments. Experimental data were expressed as mean ± S.E. Statistical analysis was conducted with Student's t test, z test, or one-way analysis of variance where appropriate. The significance level was set at p < 0.05.
RESULTS
CCCP Induces Autophagy-independent Formation of Mitochondrial Spheroids in the Absence of Parkin
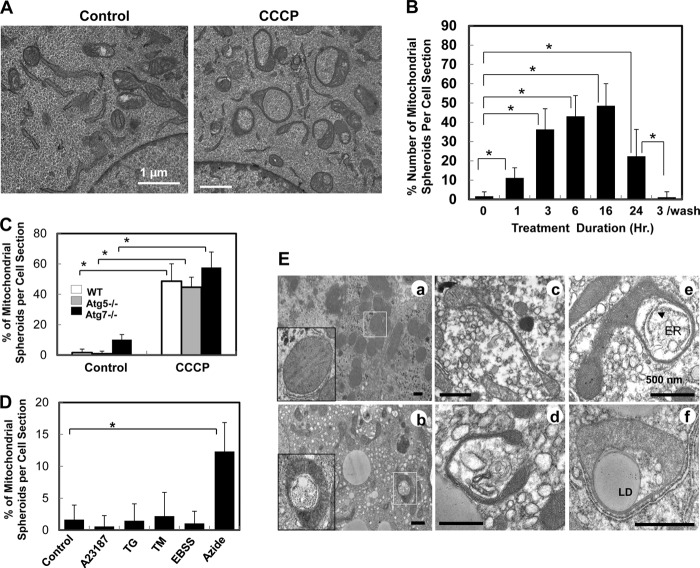
CCCP-triggered mitophagy was largely dependent on the expression of Parkin (12–15, 17, 20, 21). Thus, although autophagy was robustly activated following CCCP in MEFs, the level of mitophagy was minimal because the level of Parkin was below detection (14, 21). Although autophagy was activated, EM examination revealed few autophagosomes that contained mitochondria (see below). Unexpectedly, we found that many mitochondria became ring-shaped, which was rarely detected in non-treated cells (Fig. 1A). The proportion of the ring-shaped mitochondria increased in a time-dependent fashion, reaching a peak of nearly 50% at 16 h after CCCP treatment, and dropped by 24 h (Fig. 1B). This process also seemed to be reversible, and the level of ring-shaped mitochondria reduced to that in untreated cells after CCCP was washed away (Fig. 1B).
FIGURE 1.
Formation of mitochondrial spheroids in oxidative stress. A, wild-type MEFs were treated with vehicle control or CCCP for 6 h followed by EM. B, the percentage of mitochondrial spheroids in all mitochondrial structures was determined in each cell section at different times after CCCP treatment. The washout experiment was conducted with a 3-hour treatment, followed by replacement with new medium and continuous culture for another 21 h (a total of 24 h of culture). C, the percentage of mitochondrial spheroids was determined in MEFs of different genotypes treated with CCCP for 6 h. D, wild-type MEFs were treated with A23187 (2.5 μm), thapsigargin (TG) (0.5 μm), or tunicamycin (TM) (2.5 μm) for 6 h; subjected to Earle's Balanced Salt Soultion (EBSS) for 2 h; or treated with sodium azide (10 μm) for 6 h followed by EM. Mitochondrial spheroid formation was quantified as in A. E, C57BL/6 mice were treated with saline (a) or APAP (b–f) for 6 h. EM examination of livers showed multiple examples of mitochondrial spheroids with a narrowing segment and engulfing cytosolic components including the endoplasmic reticulum (ER) (e), lipid droplets (LD, f) and other membranes (d) in APAP treatment. *, p < 0.05.
In a separate study we conducted detailed structural studies using serial sectioning and electron tomography (22). The ring-shaped mitochondria resulted from a three-dimensional transformation into a spherical structure enclosing cytoplasmic materials in a newly formed lumen, which was surrounded by the mitochondrial membranes. Thus, this mitochondrial structure was operationally named as a mitochondrial spheroid.
Unlike autophagosome formation, mitochondrial spheroid formation did not require the participation of the autophagy conjugation system and could occur in MEFs deficient in Atg7, Atg5, or Atg3 (Fig. 1C and supplemental Fig. S1A). Common autophagy inducers, such as starvation and endoplasmic reticulum stress, did not induce mitochondrial spheroid formation (Fig. 1D and supplemental Fig. S1B). However, another mitochondrial toxin, sodium azide, a complex IV inhibitor, could do so (Fig. 1D and supplemental Fig. S1B), suggesting that a direct mitochondria insult was needed.
To determine whether mitochondrial spheroids could form in vivo, we examined a classical acetaminophen overdose model in mouse. Acetaminophen overdose in humans is the most common cause of drug-induced liver injury, which involves severe oxidative mitochondrial damage (23, 24). Indeed, mitochondrial spheroids were readily detected in the livers of mice that received an overdosed amount of acetaminophen (Fig. 1E). They were structurally similar to those observed in the cultured cells and wrapped multiple types of intracellular materials. These results indicate that the formation of mitochondrial spheroids represents a mitochondrial stress response to adverse conditions.
Mitochondrial Spheroid Formation Is a Response to Oxidative Stress in Vitro and in Vivo
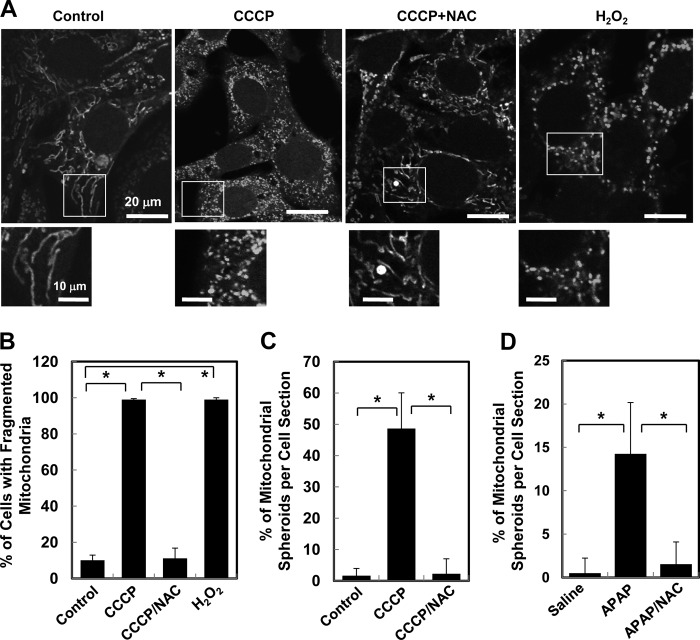
In addition to mitochondrial depolarization, treatment of cells with CCCP could lead to increased ROS generation (14, 25) and mitochondrial fragmentation (14, 21, 26). Depolarization and oxidative stress have been shown to inhibit mitochondrial fusion and/or to promote mitochondrial fragmentation (3, 27–29). Indeed, CCCP treatment led to mitochondrial fragmentation, which was not dependent on the autophagy machinery (Fig. 2A and supplemental Fig. S2). In addition, CCCP-induced fragmentation was suppressed by N-acetyl cysteine (NAC), a potent antioxidant (Fig. 2, A and B) even in the presence of CCCP. Thus ROS may prevent refusion of mitochondria or promote fragmentation.
FIGURE 2.
ROS contribute to mitochondria fragmentation and mitochondrial spheroid formation. A, wild-type MEFs preloaded with MitoTracker Red were treated with CCCP or H2O2 for 6 h, followed by confocal microscopy. The insets were enlarged in the lower panels for each treatment. B, the percentage of cells with fragmented mitochondria was quantified. C, wild-type MEFs treated with CCCP in the presence or absence of NAC for 6 h were examined by EM. The percentage of mitochondrial spheroids in each section was quantified. D, C57BL/6 mice were treated with saline, APAP, or APAP plus NAC for 6 h. Livers were processed for EM. The percentage of mitochondrial spheroids in each section was quantified. *, p < 0.05.
Notably, ROS was also required for the formation of mitochondrial spheroids because NAC completely blocked the structural change (Fig. 2C). Consistently, acetaminophen-induced mitochondrial spheroids in the liver were also completely suppressed by the coadministration of NAC (Fig. 2D), confirming the critical role of ROS in this process. These observations suggest a potential connection among ROS, mitochondrial fragmentation, and mitochondrial spheroid formation.
Interaction of the Mitochondrial Spheroid with Acidic Compartments
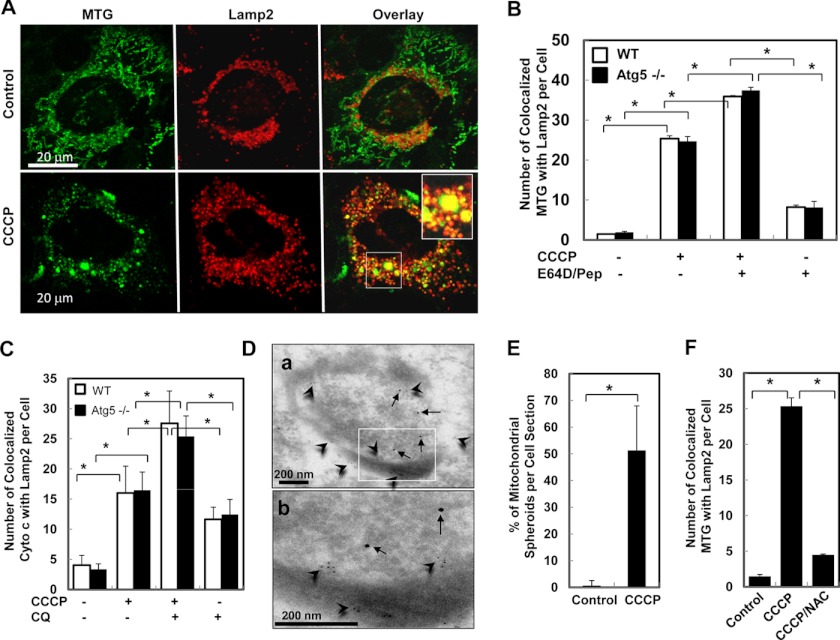
Maturation of the autophagosomes and heterophagosomes is accompanied with acidification and fusion with the lysosome. To track the fate of the mitochondrial spheroids, we examined whether they may interact with the acidic compartment as well. We found that in CCCP-treated MEFs, a high level of mitochondria, as defined by MTG staining (Fig. 3, A and B, and supplemental Fig. S3, A, B, and E) or by anti-cytochrome c staining (Fig. 3C and supplemental Fig. S3, C and D), were colocalized with the lysosomal markers, Lamp2 (Fig. 2, A–C, and Fig. S3, A–D), or Lamp1 (supplemental Fig. S3E). Such colocalization was significantly stimulated by CCCP treatment but was unlikely due to canonical mitophagy because of the lack of Parkin expression in these cells and because of the independence of this colocalization on Atg5 or Atg7 (Fig. 3, B and C, and supplemental Fig. S3). Dual-labeling immuno-EM with anti-Tom20 and anti-Lamp2 confirmed the presence of both markers on the same mitochondrial spheroid (Fig. 3D), indicating that the markers were not merely adjacent to each other. The colocalization of the mitochondrial spheroids with these markers thus likely occurred subsequently to their formation, which could represent fusion with the lysosome. However, Lamp1 and Lamp2 were not required for the formation of the mitochondrial spheroid (Fig. 3E and supplemental Fig. S4).
FIGURE 3.
CCCP-induced colocalization of mitochondria with lysosomes is dependent on ROS but not autophagy. A–C, wild-type (A–C) or Atg5−/− (B and C) MEFs preloaded with MTG (A and B) or without the dye (C) were treated as indicated and immunostained with anti-Lamp2 alone (A and B) or together with anti-cytochrome c (C) followed by confocal microscopy. Colocalization of the mitochondria with Lamp2 was quantified (B and C). CQ, chloroquine. D, wild-type MEFs were treated with CCCP and processed for immuno-EM with anti-Tom20 and anti-Lamp2. Arrows, Lamp2 (10-nm gold particles); arrowheads, Tom20 (5-nm gold particles). The inset is enlarged in b. E, Lamp1/2−/− MEFs were treated with vehicle control or CCCP followed by EM, and the percentage of mitochondrial spheroid formation was quantified. F, wild-type MEFs preloaded with MTG were treated as indicated for 6 h, immunostained for Lamp2, and subjected to confocal microscopy. The colocalization signals were quantified. *, p < 0.05.
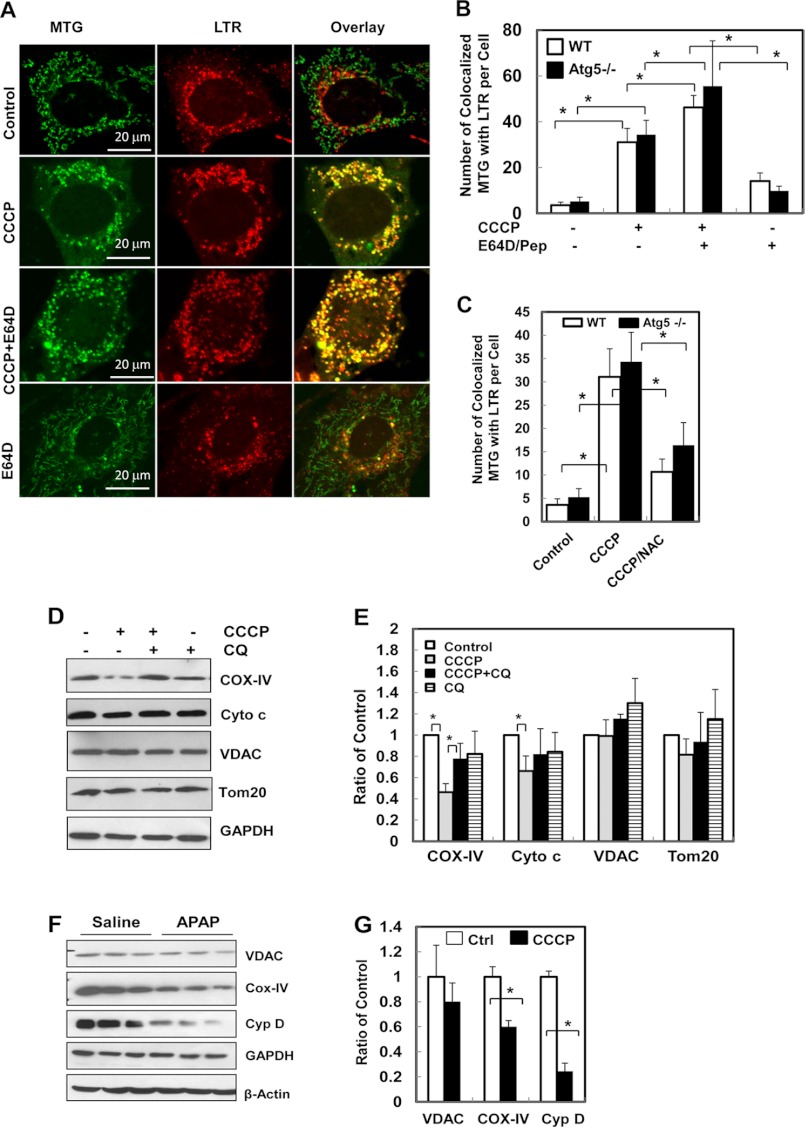
Further supporting the hypothesis that mitochondrial spheroids interact with the acidic compartment, mitochondrial spheroids became acidic, as indicated by the significantly increased colocalization with LTR following CCCP treatment (Fig. 4, A and B, and supplemental Fig. S3F). This process was also independent on Parkin and was not affected by autophagy because it could be detected in Parkin-negative and Atg5-negative MEFs.
FIGURE 4.
CCCP induces mitochondrial interactions with acidic compartments and degradation of mitochondrial components. A–C, wild-type (A–C) and Atg5 (B–C) MEFs were first loaded with MTG (50 nm) and LTR (50 nm) for 15 min followed by the indicated treatment for 6 h. The cells were then subjected to confocal microscopy. The number of mitochondria (MTG signals) colocalized with LTR in each cell was quantified (B and C). D and E, wild-type MEFs were treated as indicated for 24 h. Total cell lysates were subjected to immunoblot analysis for the indicated proteins (D). Densitometry analysis was conducted with data from four experiments and standardized to the untreated controls. CQ, chloroquine. F and G, C57BL/6 mice were treated with vehicle control or APAP (500 mg/kg) for 6 h. Total liver lysates were subjected to immunoblot analysis for the indicated mitochondrial proteins (F). Densitometry analysis was conducted with data from three mice per condition, which were standardized to the untreated controls (G). *, p < 0.05.
Notably, ROS was also required for the autophagy-independent mitochondrial colocalization with Lamp2 (Fig. 2F) or with LTR (Fig. 4C and supplemental Fig. S5). This observation supported the notion that the mitochondrial structures that were colocalized with the acidic compartment were regulated by ROS, as indicated above (Fig. 2).
Interestingly, the colocalized signals of MTG or cytochrome c with Lamp2 or LTR were further increased in the presence of lysosomal inhibitors, E64D, pepstatin A, or chloroquine (Fig. 3, B and C; Fig. 4, A and B; and supplemental Fig. S3, A, C, D, and F), suggesting that mitochondrial components could be degraded by acidic proteases. This was not due to mitophagy as Parkin was not present, and the enhancement was also observed in the absence of autophagy.
The immunoblotting analysis indicated that some mitochondrial components could be degraded in CCCP-treated MEFs to various degrees. Although the outer membrane proteins, such as VDAC and Tom 20, were not usually degraded, consistent with the notion that Parkin would be required for this process (20, 21, 26), proteins located in the inner membranes and the intermembrane space, such as cytochrome c oxidase subunit IV (COX-IV) and cytochrome c, could experience a detectable level of degradation (Fig. 4, D and E). Furthermore, mitochondrial inner membrane and matrix proteins were also found to undergo limited degradation in vivo in acetaminophen-treated livers (Fig. 4, F and G).
Parkin and Mfn1/2 Control the Switch between Mitochondrial Spheroid Formation and Mitophagy
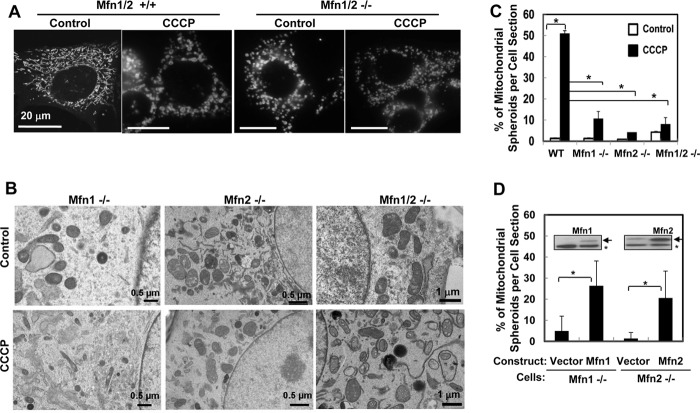
It seemed that CCCP-induced ROS generation, mitochondrial fragmentation, and mitochondrial spheroid formation had intricate mechanistic relationships (Fig. 2). Mitofusin (Mfn) 1 and 2 are integral proteins of the mitochondrial outer membrane (MOM) and are both essential to the mitochondrion-mitochondrion fusion, although their functions may not be redundant (30–32). MEFs deficient in Mfn1 and/or Mfn2 presented spontaneous mitochondrial fragmentation (Fig. 5A) but did not form mitochondrial spheroids either spontaneously or following CCCP treatment (Fig. 5, B and C). This indicated that mitochondrial fragmentation alone was not sufficient to trigger mitochondrial spheroid formation and that mitofusins were required for this process. Reconstitution of Mfn1 in the Mnf1−/− cells or Mfn2 in the Mfn2−/− cells restored this capacity (Fig. 5D), proving the importance of the mitofusins in mitochondrial spheroid formation.
FIGURE 5.
CCCP-induced mitochondrial spheroid formation requires mitofusins. A, wild-type and Mfn1/2−/− MEFs were preincubated with MitoTracker Red were treated CCCP. Mitochondrial fragmentation was examined by confocal microscopy. B–D, MEFs of different genotypes, with or without the reconstitution of Mfn1 or Mfn2 (D, arrows in the inset; asterisk, nonspecific band), were treated by CCCP for 6 h. Cells were subjected to EM examination, and the percentage of mitochondrial spheroids was quantified.
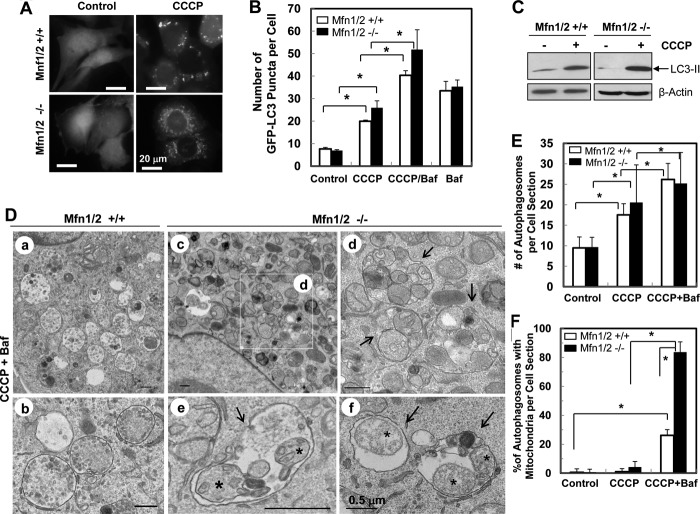
On the other hand, mitofusins were not required for the canonic autophagy induced by CCCP (Fig. 6, A–C) and the total numbers of autophagosomes were comparable in MEFs with or without mitofusins (Fig. 6, D and E). However, mitophagy was not detected in CCCP-treated wild-type MEFs at any significant level by EM, even in the presence of bafilomycin A1 (Baf, Fig. 6, D and F), which inhibited autophagic degradation. This was expected, considering the lack of Parkin expression in MEFs (14, 21). Unexpectedly, the majority of autophagosomes in CCCP-treated Mfn1/2-deficient MEFs were filled with mitochondria (Fig. 6, D and F), most of which were in degrading conditions but without obviously disrupted outer membranes. The latter was typical of Parkin-mediated degradation (21). This observation indicated that deletion of Mfn1/2 led to increased mitophagy and decreased mitochondrial spheroid formation following CCCP treatment. Thus, these two processes were mechanistically linked and reciprocally regulated by mitofusins.
FIGURE 6.
Loss of mitofusins promotes CCCP-induced mitophagy. A–C, MEFs of different genotypes were infected with ad-GFP-LC3 and treated as indicated. The level of GFP-LC3 puncta (A and B) and LC3-II in cell lysates (C) were determined. D–F, treated MEFs were subjected to EM examination (D). a and b were from wild-type MEFs, whereas c–f were from Mfn1/2−/− MEFs. An inset in c is enlarged in d. The arrows indicate autophagosomes containing different subcellular components. Asterisks indicated possible mitochondrial remnants (e and f). The number of autophagosomes (E) and the percentage of autophagosomes containing mitochondrial structures (F) were quantified. *, p < 0.05.
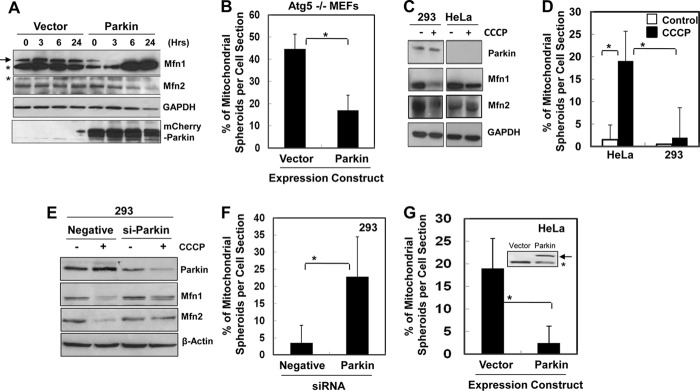
Mfn1 and Mfn2 are among the MOM proteins that can be ubiquitinated by Parkin and degraded by the proteasome in response to CCCP (20, 21, 26). We reasoned that Parkin could regulate mitochondrial spheroid formation through its effect on mitofusins. To examine this hypothesis, we first looked at MEFs where both mitofusins remained at constant levels following CCCP treatment unless Parkin was introduced exogenously (Fig. 7A). Indeed, although the canonical mitophagy was increased by the enhanced Parkin expression as reported by others and us (12–14, 20), the autophagy-independent formation of mitochondrial spheroids was inhibited instead (Fig. 7B).
FIGURE 7.
Parkin inhibits mitochondrial spheroid formation by degrading mitofusins. A and B, Atg 5−/− MEFs transfected with mCherry-Parkin or the vector were treated with CCCP and analyzed by immunoblotting (A) or EM for quantification of the mitochondrial spheroids (B). C–D, HEK-293 and HeLa cells were treated as indicated and analyzed by immunoblotting (C) or EM (D). E and F, siRNA-transfected HEK-293 cells were treated as indicated and analyzed by immunoblotting (E) or EM (F). G, EM analysis of CCCP-treated HeLa cells expressing a vector or mCherry-Parkin (arrow in the inset; *, nonspecific band). *, p < 0.05.
Next, we compared the response of HEK-293 cells, in which the endogenous Parkin was readily detected, and in HeLa cells, in which endogenous Parkin was below the detectable level (14, 20, 26) (Fig. 7C). Consistently, following CCCP treatment, Mfn1 and Mfn2 were degraded preferentially in HEK-293 cells, and mitochondrial spheroids were detected in HeLa cells but not in HEK-293 cells (Fig. 7D and supplemental Fig. S6). siRNA-mediated knockdown of Parkin in HEK-293 cells resulted in blockage of mitofusin degradation (Fig. 7E) and appearance of mitochondrial spheroids (Fig. 7F) following CCCP treatment. Conversely, as in the MEFs, introduction of Parkin into HeLa cells followed by CCCP treatment led to diminished generation of mitochondrial spheroids (Fig. 7G), whereas, as reported in previous studies including ours, this genetic manipulation increased Mfn1 and Mfn2 degradation (20, 26) and increased mitophagy (12, 14, 20).
Taken together, these findings suggested that Mfn1 and Mfn2 are required for mitochondrial spheroid formation, which could be the default response of the mitochondria to CCCP in the absence of Parkin. Expression of Parkin can disable the mitochondrial spheroid pathway as the result of mitofusin degradation, which also directs the damaged mitochondria to the canonical mitophagy pathway. Consistently, deletion of Mfn1/2 alone without a concomitant expression of Parkin in the MEFs was sufficient to significantly increase mitophagy with a simultaneous decrease in mitochondrial spheroid formation.
DISCUSSION
Formation of the Mitochondrial Spheroid
Mitochondrial morphology can vary in different species, tissues, respiratory functional status, and pathological conditions, which affects the shape, size, and cristae arrangement (1, 3, 7). The formation of a spherical mitochondrial structure would likely reflect a response to the environment with functional consequences.
Formation of the mitochondria spheroid is not a process of autophagy or mitophagy but a response to oxidative stress. CCCP-induced spherical mitochondria disappeared when CCCP was removed, suggesting that this phenomenon can be reversible (Fig. 1B). This is consistent with CCCP being a reversible uncoupler and with other reversible CCCP-induced phenotypes, such as mitochondrial fragmentation and autophagy induction (data not shown). ROS are required for these unique mitochondrial dynamics, as antioxidants suppress it. In the presence of ROS, fragmented mitochondria would be prevented from refusion with each other even in the presence of mitofusins. This, together with the absence of Parkin, could create a condition where mitofusins are engaged in a self-fusion process for the mitochondrial spheroid to form.
Mitofusins are known to function in intermitochondrial tethering and fusion. Our studies would suggest that mitofusins are equally important in the self-fusion of the mitochondrial membrane during the formation of mitochondrial spheroids. Because both Mfn1 and Mfn2 are required, it is possible that the Mfn1-Mfn2 hetero-oligomers (31) are needed for this process. On the other hand, the inner membrane protein, OpA1, although required for intermitochondrial fusion (3, 33), may not be important for CCCP-induced mitochondrial spheroid formation, as it is rapidly degraded upon mitochondrial depolarization. However, there would likely be other molecular events to affect the membrane curvature and the dynamics for such a process to initiate and to complete.
It has been reported recently that mitochondria could respond to oxidative stress by the formation of the so-called mitochondria-derived vesicles (34). At the time of mitochondria-derived vesicle generation, the mitochondrial network remains intact, and membrane potentials can be well kept. However, CCCP-induced mitochondrial spheroids are accompanied by mitochondrial depolarization and fragmentation. In addition, mitochondria-derived vesicle formation is not affected by the fission-fusion mechanism, whereas mitochondrial spheroid formation requires mitofusins. By conventional EM we did not observe small vesicles (70–100 nm) budding off from, or surrounding, normal or spherical mitochondria upon CCCP treatment. It seems that mitochondria-derived vesicles and mitochondrial spheroid formation are mediated by different mechanisms.
Interaction of Mitochondrial Spheroids with the Acidic Environment
Once formed, mitochondrial spheroids can interact with the acidic compartment and acquire the lysosome markers, Lamp1 and Lamp2, as shown by immunofluorescence staining and by immuno-EM analysis. Although other possibilities may not be ruled out, the acquisition could be due to the fusion with the lysosomes, which can also result in acidification. The latter would not likely be caused by the dissipation of the ΔpH gradient per se because the immediate action of CCCP on mitochondrial pH only leads to its decrease to the near neutral level but not to the acidic level (35, 36).
In the fusion scenario, the outer membrane at the periphery of the mitochondrial spheroid (the outside outer membranes) (22) would be fused with the lysosomal membrane, and the lysosome enzymes can enter into the intermembrane space. This may lead to the degradation of the proteins within the boundary defined by the outer membranes (Fig. 4, D–G). However, the degradation occurring in the intermembrane space and inner membranes seemed to be limited. Additionally, it is not clear whether the luminal contents could be degraded by the lysosome enzymes. The exact mechanism of the fusion and the significance of the degradation have thus yet to be fully determined in future studies. Nevertheless, the interaction with the acidic compartment suggests that certain elements of the maturation pathway of the endosomes and phagosomes could be also conserved in the progression of mitochondrial spheroids.
Coupled Regulation of Mitophagy and Mitochondrial Spheroid Formation by Parkin and Mitofusins
In the absence of Parkin, CCCP triggers autophagy, but mitophagy does not occur in any significant way. Parkin is required for mitophagy to occur (12–14, 20). However, it is not clear how Parkin promotes mitophagy. Its activity is mostly related to mitochondrial priming, a process facilitating the recognition of the mitochondria by the autophagosome, but not related to autophagy activation (14). The major effect of Parkin on mitochondria is the ubiquitination of the MOM proteins, which causes their degradation by the proteasome and the disruption of the outer membrane (20, 21, 26). How this broad activation of MOM degradation facilitates mitophagy is not clear.
Not all MOM proteins seem to be equally susceptible to Parkin-mediated degradation. In SH-SY5Y cells that express an endogenous level of Parkin, only Mfn1 and Mfn2, but not other MOM proteins, such as VDAC and Fis1, were degraded (26). In Parkin-overexpressing HeLa cells and MEFs, additional MOM proteins were degraded along with Mfn1/Mfn2 (20, 21). These observations suggest that mitofusins could be the preferential targets of Parkin at a more physiological level of expression. Consequently, Parkin may promote autophagosomal recognition of the mitochondria via the degradation of mitofusins and thus the inhibition of mitochondrial spheroid formation. Indeed, deletion of the mitofusins alone is sufficient to induce a significant increase in CCCP-induced mitophagy in the absence of Parkin (Fig. 6). There could be important contributions to mitophagy by the degradation of additional MOM proteins (20), but it seems that degradation of mitofusins could be the most critical.
We thus speculate that the critical role of Parkin in CCCP-induced mitophagy is to degrade mitofusins so that the mitochondria will not become mitochondrial spheroids, which may prevent autophagosome recognition. In the absence of Parkin, mitochondrial spheroid formation seems to be a default pathway following CCCP treatment because of the presence of mitofusins. Indeed, genetic deletion of mitofusins seems to be sufficient to allow CCCP-induced mitophagy to occur, even in the absence of Parkin (Fig. 6, D–F). In this manner, the levels of Parkin and mitofusins control whether mitophagy or mitochondrial spheroid formation will occur following CCCP treatment.
How this role of Parkin and mitofusins is involved in a condition where mitochondrial spheroids are stimulated by non-CCCP signals has yet to be defined. In the case of APAP-induced liver injury, Parkin is normally present in the liver. However, mitochondrial spheroids were found in restricted areas adjacent to necrotic regions where hypoxia and oxidative stress are anticipated. It is possible that the level of Parkin in these susceptible regions could be different from that in normal areas. This hypothesis could be examined in future studies.
Potential Pathophysiological Significance of Mitochondrial Spheroids
The significance of the mitochondrial spheroids has yet to be fully determined. This study indicates that it can occur in vitro and in vivo under oxidative mitochondrial stress. Earlier studies have also found that similar structures could be found in livers of rats subjected to long-term alcohol consumption (37). Interestingly, they could be also found in apparently normal tissues to various degrees, with an increase in older animals (38–40). This could reflect a local stress and/or an aging process (40), which is known to be accompanied by increased oxidative stress.
Mutations of Parkin and its activating molecule PINK1 are found in certain familial forms of Parkinson's disease, and some of them are associated with the failure to engage mitophagy (15, 17). Mitochondrial dysfunction has been well implicated in the pathogenesis of Parkinson's disease (41), but how PINK1/Parkin and mitophagy could contribute to this process is still unknown. Being regulated by the same molecules, mitochondrial spheroids could potentially play a role in the pathogenesis of Parkinson's disease.
Mitochondrial spheroids may not necessarily be detrimental to cell homeostasis. It is equally possible that this process could serve as an alternative mechanism to limit the influence of mitochondrial damage. It is known that deletion of mitofusins could render cells more susceptible to insults (3). Consistently, CCCP induced more significant cell death in Mfn1/2-deficient cells than in the wild-type cells (data not shown). It has yet to be determined whether the increased susceptibility is related to the incapacity of forming mitochondrial spheroids.
Taken together, the coordinated regulation of mitochondrial spheroid formation by ROS, mitofusins, and Parkin links it closely to other mitochondrial dynamics and mitophagy, which can be important for the control of mitochondrial damage and oxidative stress under pathological conditions.
Acknowledgments
We thank N. Mizushima (Tokyo Medical and Dental University, Japan) for Atg5−/− MEFs, M. Komatsu (Tokyo Metropolitan Institute of Medical Science, Japan) for Atg7−/− and Atg3−/− MEFs, and D. C. Chan (California Institute of Technology, CA) for Mfn1−/− and Mfn2−/− MEFs. We also thank Ms Barbara Fegley (University of Kansas Medical Center Electron Microscopy Research Laboratory) for excellent assistance with the EM studies; Teri Johnson and Ting Xie (Stowers Institute for Medical Research, MO); and Paul Saftig (University of Kiel, Germany) for support with reagents and facility use.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 AA020518-01, R21AA017421, P20 RR021940, and P20 RR016475 (to W. X. D.) and R01CA111456 and R01CA 83817 (to X. M. Y.).

This article contains supplemental Figs. S1–S6.
- CCCP
- carbonyl cyanide m-chlorophenylhydrazone
- LTR
- LysoTrackerRed
- MEF
- murine embryonic fibroblast
- APAP
- acetamenophen
- MTG
- MitoTracker Green
- NAC
- N-acetyl cysteine
- MOM
- mitochondrial outer membrane
- APAP
- acetaminophen
- ROS
- reactive oxygen species
- VDAC
- voltage-dependent anion channel.
REFERENCES
- 1. Westermann B. (2010) Mitochondrial fusion and fission in cell life and death. Nat. Rev. Mol. Cell Biol. 11, 872–884 [DOI] [PubMed] [Google Scholar]
- 2. Wallace D. C., Fan W., Procaccio V. (2010) Mitochondrial energetics and therapeutics. Annu. Rev. Pathol 5, 297–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chan D. C. (2006) Mitochondria. Dynamic organelles in disease, aging, and development. Cell 125, 1241–1252 [DOI] [PubMed] [Google Scholar]
- 4. Lemasters J. J. (2005) Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res. 8, 3–5 [DOI] [PubMed] [Google Scholar]
- 5. Green D. R., Galluzzi L., Kroemer G. (2011) Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science 333, 1109–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Youle R. J., Narendra D. P. (2011) Mechanisms of mitophagy. Nat. Rev. Mol. Cell Biol. 12, 9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scheffler I. E. (1999) in Mitochondria (Scheffler I. E. ed) pp.15–47, Wiley-Liss, New York [Google Scholar]
- 8. Tolkovsky A. M. (2009) Mitophagy. Biochim. Biophys. Acta 1793, 1508–1515 [DOI] [PubMed] [Google Scholar]
- 9. Kanki T., Klionsky D. J. (2010) The molecular mechanism of mitochondria autophagy in yeast. Mol. Microbiol. 75, 795–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ricci J. E., Waterhouse N., Green D. R. (2003) Mitochondrial functions during cell death, a complex (I-V) dilemma. Cell Death Differ. 10, 488–492 [DOI] [PubMed] [Google Scholar]
- 11. Sandoval H., Thiagarajan P., Dasgupta S. K., Schumacher A., Prchal J. T., Chen M., Wang J. (2008) Essential role for Nix in autophagic maturation of erythroid cells. Nature 454, 232–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Narendra D., Tanaka A., Suen D. F., Youle R. J. (2008) Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 183, 795–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Geisler S., Holmström K. M., Skujat D., Fiesel F. C., Rothfuss O. C., Kahle P. J., Springer W. (2010) PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat. Cell Biol. 12, 119–131 [DOI] [PubMed] [Google Scholar]
- 14. Ding W. X., Ni H. M., Li M., Liao Y., Chen X., Stolz D. B., Dorn G. W., 2nd, Yin X. M. (2010) Nix is critical to two distinct phases of mitophagy, reactive oxygen species-mediated autophagy induction and Parkin-ubiquitin-p62-mediated mitochondrial priming. J. Biol. Chem. 285, 27879–27890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matsuda N., Sato S., Shiba K., Okatsu K., Saisho K., Gautier C. A., Sou Y. S., Saiki S., Kawajiri S., Sato F., Kimura M., Komatsu M., Hattori N., Tanaka K. (2010) PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J. Cell Biol. 189, 211–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vives-Bauza C., Zhou C., Huang Y., Cui M., de Vries R. L., Kim J., May J., Tocilescu M. A., Liu W., Ko H. S., Magrané J., Moore D. J., Dawson V. L., Grailhe R., Dawson T. M., Li C., Tieu K., Przedborski S. (2010) PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc. Natl. Acad. Sci. U.S.A. 107, 378–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee J. Y., Nagano Y., Taylor J. P., Lim K. L., Yao T. P. (2010) Disease-causing mutations in parkin impair mitochondrial ubiquitination, aggregation, and HDAC6-dependent mitophagy. J. Cell Biol. 189, 671–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ding W. X., Ni H. M., Gao W., Yoshimori T., Stolz D. B., Ron D., Yin X. M. (2007) Linking of autophagy to ubiquitin-proteasome system is important for the regulation of endoplasmic reticulum stress and cell viability. Am. J. Pathol. 171, 513–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eskelinen E. L., Schmidt C. K., Neu S., Willenborg M., Fuertes G., Salvador N., Tanaka Y., Lüllmann-Rauch R., Hartmann D., Heeren J., von Figura K., Knecht E., Saftig P. (2004) Disturbed cholesterol traffic but normal proteolytic function in LAMP-1/LAMP-2 double-deficient fibroblasts. Mol. Biol. Cell 15, 3132–3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chan N. C., Salazar A. M., Pham A. H., Sweredoski M. J., Kolawa N. J., Graham R. L., Hess S., Chan D. C. (2011) Broad activation of the ubiquitin-proteasome system by Parkin is critical for mitophagy. Hum. Mol. Genet. 20, 1726–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yoshii S. R., Kishi C., Ishihara N., Mizushima N. (2011) Parkin mediates proteasome-dependent protein degradation and rupture of the outer mitochondrial membrane. J. Biol. Chem. 286, 19630–19640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ding W. X., Li M., Biazik J. M., Morgan D. G., Guo F., Ni H. M., Goheen M., Eskelinen E. L., Yin X. M. (2012) Electron microscopic analysis of a spherical mitochondrial structure. J. Biol. Chem. 287, 42373–42378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tujios S., Fontana R. J. (2011) Mechanisms of drug-induced liver injury. From bedside to bench. Nat. Rev. Gastroenterol. Hepatol. 8, 202–211 [DOI] [PubMed] [Google Scholar]
- 24. Jaeschke H., McGill M. R., Williams C. D., Ramachandran A. (2011) Current issues with acetaminophen hepatotoxicity. A clinically relevant model to test the efficacy of natural products. Life Sci. 88, 737–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Izeradjene K., Douglas L., Tillman D. M., Delaney A. B., Houghton J. A. (2005) Reactive oxygen species regulate caspase activation in tumor necrosis factor-related apoptosis-inducing ligand-resistant human colon carcinoma cell lines. Cancer Res. 65, 7436–7445 [DOI] [PubMed] [Google Scholar]
- 26. Tanaka A., Cleland M. M., Xu S., Narendra D. P., Suen D. F., Karbowski M., Youle R. J. (2010) Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J. Cell Biol. 191, 1367–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pletjushkina O. Y., Lyamzaev K. G., Popova E. N., Nepryakhina O. K., Ivanova O. Y., Domnina L. V., Chernyak B. V., Skulachev V. P. (2006) Effect of oxidative stress on dynamics of mitochondrial reticulum. Biochim. Biophys. Acta 1757, 518–524 [DOI] [PubMed] [Google Scholar]
- 28. Liot G., Bossy B., Lubitz S., Kushnareva Y., Sejbuk N., Bossy-Wetzel E. (2009) Complex II inhibition by 3-NP causes mitochondrial fragmentation and neuronal cell death via an NMDA- and ROS-dependent pathway. Cell Death Differ. 16, 899–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tian C., Murrin L. C., Zheng J. C. (2009) Mitochondrial fragmentation is involved in methamphetamine-induced cell death in rat hippocampal neural progenitor cells. PLoS ONE 4, e5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen H., Detmer S. A., Ewald A. J., Griffin E. E., Fraser S. E., Chan D. C. (2003) Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J. Cell Biol. 160, 189–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Detmer S. A., Chan D. C. (2007) Complementation between mouse Mfn1 and Mfn2 protects mitochondrial fusion defects caused by CMT2A disease mutations. J. Cell Biol. 176, 405–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Koshiba T., Detmer S. A., Kaiser J. T., Chen H., McCaffery J. M., Chan D. C. (2004) Structural basis of mitochondrial tethering by mitofusin complexes. Science 305, 858–862 [DOI] [PubMed] [Google Scholar]
- 33. Ishihara N., Fujita Y., Oka T., Mihara K. (2006) Regulation of mitochondrial morphology through proteolytic cleavage of OPA1. EMBO J. 25, 2966–2977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Soubannier V., McLelland G. L., Zunino R., Braschi E., Rippstein P., Fon E. A., McBride H. M. (2012) A vesicular transport pathway shuttles cargo from mitochondria to lysosomes. Curr. Biol. 22, 135–141 [DOI] [PubMed] [Google Scholar]
- 35. Llopis J., McCaffery J. M., Miyawaki A., Farquhar M. G., Tsien R. Y. (1998) Measurement of cytosolic, mitochondrial, and Golgi pH in single living cells with green fluorescent proteins. Proc. Natl. Acad. Sci. U.S.A. 95, 6803–6808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Matsuyama S., Llopis J., Deveraux Q. L., Tsien R. Y., Reed J. C. (2000) Changes in intramitochondrial and cytosolic pH. Early events that modulate caspase activation during apoptosis. Nat. Cell Biol. 2, 318–325 [DOI] [PubMed] [Google Scholar]
- 37. Kiessling K. H., Tobe U. (1964) Degeneration of liver mitochondria in rats after prolonged alcohol consumption. Exp. Cell Res. 33, 350–354 [DOI] [PubMed] [Google Scholar]
- 38. Christensen A. K., Chapman G. B. (1959) Cup-shaped mitochondria in interstitial cells of the albino rat testis. Exp. Cell Res. 18, 576–579 [DOI] [PubMed] [Google Scholar]
- 39. Stephens R. J., Bils R. F. (1965) An atypical mitochondrial form in normal rat liver. J. Cell Biol. 24, 500–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lauber J. K. (1982) Retinal pigment epithelium. Ring mitochondria and lesions induced by continuous light. Curr. Eye Res. 2, 855–862 [DOI] [PubMed] [Google Scholar]
- 41. Winklhofer K. F., Haass C. (2010) Mitochondrial dysfunction in Parkinson's disease. Biochim. Biophys. Acta 1802, 29–44 [DOI] [PubMed] [Google Scholar]