Background: LKB1 is a key signaling kinase that regulates cellular growth.
Results: The reactive aldehyde 4-hydroxynonenal (HNE) forms adducts on LKB1 that directly inhibit kinase activity.
Conclusion: Oxidative modification at Lys-97 by HNE is sufficient for inhibition of LKB1.
Significance: This work describes how a key growth suppressor protein is inactivated during states of oxidant stress.
Keywords: Lipid Peroxidation, LKB, Oxidative Stress, Post-translational Modification, Signal Transduction, HNE, LKB1
Abstract
Oxidative stress is pathogenic in a variety of diseases, but the mechanism by which cellular signaling is affected by oxidative species has yet to be fully characterized. Lipid peroxidation, a secondary process that occurs during instances of free radical production, may play an important role in modulating cellular signaling under conditions of oxidative stress. 4-Hydroxy-trans-2-nonenal (HNE) is an electrophilic aldehyde produced during lipid peroxidation that forms covalent adducts on proteins, altering their activity and function. One such target, LKB1, has been reported to be inhibited by HNE adduction. We tested the hypothesis that HNE inhibits LKB1 activity through adduct formation on a specific reactive residue of the protein. To elucidate the mechanism of the inhibitory effect, HEK293T cells expressing LKB1 were treated with HNE (10 μm for 1 h) and assayed for HNE-LKB1 adduct formation and changes in LKB1 kinase activity. HNE treatment resulted in the formation of HNE-LKB1 adducts and decreased LKB1 kinase activity by 31 ± 9% (S.E.) but had no effect on the association of LKB1 with its adaptor proteins sterile-20-related adaptor and mouse protein 25. Mutation of LKB1 lysine residue 97 reduced HNE adduct formation and attenuated the effect of HNE on LKB1 activity. Taken together, our results suggest that adduction of LKB1 Lys-97 mediates the inhibitory effect of HNE.
Introduction
LKB1 was identified as the loss of function gene product in patients with the familial cancer disorder Peutz-Jeghers syndrome (1) and has since been characterized as a serine/threonine kinase that phosphorylates and activates the AMP-activated protein kinase (AMPK)2 and 12 other AMPK-related kinases (2). AMPK has been shown to regulate mitochondrial biogenesis (3) and autophagy (4) in addition to other cellular processes that promote a net positive energetic balance. Under basal conditions, LKB1 is localized to the nucleus where it has limited catalytic activity. Translocation to the cytosol allows for interaction with the pseudokinase sterile-20-related adaptor (STRAD), thereby enhancing the cytosolic localization of LKB1 by acting as an adaptor between exportins (CRM1 and exportin7) and LKB1 (5). The interaction between LKB1, STRAD, and the scaffolding protein mouse protein 25 (MO25) stabilizes the heterotrimer, forming a constitutively active complex (6). Deacetylation of LKB1 at Lys-48 by the NAD+-dependent class III deacetylase SIRT1 promotes translocation of LKB1 to the cytosol (7), and SIRT3 has been shown to deacetylate and activate LKB1 in the heart (8).
During states of increased oxidative stress, free radical oxygen species interact with polyunsaturated fatty acids to generate lipid peroxyl radicals (9). Decomposition of lipid peroxyl radical species by β-cleavage leads to the generation of α,β-unsaturated aldehydes such as malondialdehyde and 4-hydroxy-trans-2-nonenal (HNE) (10). Accumulation of HNE has been observed in several tissues (11–14), and because of its high reactivity, HNE forms covalent protein adducts on cysteine, histidine, and lysine residues through the Michael reaction and Schiff base formation on lysine and arginine residues (15). Adduction by HNE occurs on proteins involved in cellular signaling (16–19) including LKB1. HNE adducts of LKB1 impair downstream AMPK signaling in MCF-7 cells (20), and in cardiac myocytes, HNE adducts of LKB1 have been reported to promote downstream hypertrophic signaling (21). We recently observed HNE-LKB1 adducts in hearts from mice fed a high fat, high sucrose diet (22).
The role of lysine acetylation and deacetylation has been studied extensively in regard to histone regulation and transcriptional effects, but emerging data suggest that non-histone lysine modifications also play a vital role in cellular energetic homeostasis. Lysine acetylation of non-histone proteins can regulate cellular metabolism (23), and it has been suggested that reversible acetylation may be an evolutionarily conserved mechanism by which organisms respond to changes in nutrient availability (24). In the cardiovascular system, deacetylation of lysine residues on key metabolic proteins can modulate mitochondrial function through changes in apoptotic signaling, respiratory capacity, and ATP synthesis (25). In a previous study, we observed that LKB1 acetylation occurs at several lysine residues that function to regulate LKB1 localization (7). We now test the hypothesis that inhibitory HNE adducts of LKB1 occur at lysine residues capable of being acetylated, thereby regulating enzyme activity. We show that HNE adducts to LKB1 at Lys-96 and Lys-97 and that adduction to Lys-97, but not Lys-96, inhibits LKB1 kinase activity.
EXPERIMENTAL PROCEDURES
Materials
HNE was obtained from Calbiochem, and [γ-32P]ATP was purchased from PerkinElmer Life Sciences. Localization studies were conducted using the NE-PER Nuclear and Cytoplasmic Extraction kit from Thermo Scientific (catalogue number 78833) according to the specifications of the manufacturer. Purity of the cytosolic and nuclear fractions was assessed by protein expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and histone H3, respectively, and fractions with a minimum of 85% purity were used for data analysis.
Cell Culture
Human embryonic kidney 293T (HEK293T) cells were purchased from ATCC (Manassas, VA). The cells were maintained at 37 °C with 5% CO2 and cultured in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) supplemented with 25 mm glucose, 110 mg/liter sodium pyruvate, 10% fetal bovine serum, and 0.1% penicillin-streptomycin.
Antibodies
The antibody recognizing transfected glutathione S-transferase (GST) was obtained from Cell Signaling Technology. The goat polyclonal antibody recognizing HNE and the mouse monoclonal antibody recognizing GAPDH were both from Abcam. FLAG (M2) was from Sigma. The STRAD antibody was from Santa Cruz Biotechnology (S-17). Secondary antibodies coupled to IR 680 or 800 dye were from LI-COR Biosciences (Lincoln, NE).
Synthesis of cDNA Expression Constructs and Site-directed Mutagenesis
The generation of the expression constructs for GST-LKB1, His-STRAD, and FLAG-MO25 and the creation of LKB1 lysine mutations are described in full detail in our previous work (7).
Expression of Plasmids and Protein Purification
HEK293T cells were transiently transfected with 0.5 μg of LKB1, STRAD, and MO25 plasmids in each well of a 6-well plate using the Max-PEI polymer (Polysciences, Inc.). 18 h post-transfection, the cells were used for experimentation.
Western Blot Analysis
Cells were rinsed with cold PBS and lysed with 1× lysis buffer (Cell Signaling Technology) containing 20 mm Tris-HCl (pH 7.5), 150 mm NaCl, 1 mm Na2EDTA, 1 mm EGTA, 1% Triton, 2.5 mm sodium pyrophosphate, 1 mm β-glycerophosphate, 1 mm Na3VO4, 1 μg/ml leupeptin, and 1 mm PMSF. The lysates were cleared by centrifugation at 13,000 rpm for 10 min. To purify GST-tagged protein, the lysates were incubated overnight at 4 °C with glutathione-Sepharose 4B beads (Amersham Biosciences). The purified proteins were washed three times with lysis buffer and resuspended in Laemmli sample loading buffer (Bio-Rad) prior to Western blotting. Protein samples were separated by SDS-PAGE using polyacrylamide gels, transferred to nitrocellulose membranes, and immunoblotted with the indicated antibodies. The probed membranes were visualized using the LI-COR Odyssey IR imager and analyzed by densitometry using Odyssey 2.1 software.
LKB1 Kinase Assay
Immunoprecipitated GST-LKB1 was washed twice with lysis buffer and once with 1× kinase assay buffer (Cell Signaling Technology). The purified proteins were incubated with 1× kinase assay buffer containing the LKBtide synthetic substrate (300 μm; Millipore), ATP (200 μm), and [γ-32P]ATP (5 μCi). After incubation at 30 °C for 20 min, the reaction mixture was spotted on P81 phosphate paper and washed, and radioactivity was measured by liquid scintillation counting.
Statistical Analysis
Statistics were performed with a two-tailed unpaired Student's t test or analysis of variance where appropriate. All data shown represent the results obtained from independent experiments conducted in triplicate (or more as noted in the figure legends), and data are expressed as means ± S.E. Values of p < 0.05 were considered significant.
RESULTS
HNE Forms Inhibitory Covalent Adducts on LKB1 and Inhibits Kinase Activity
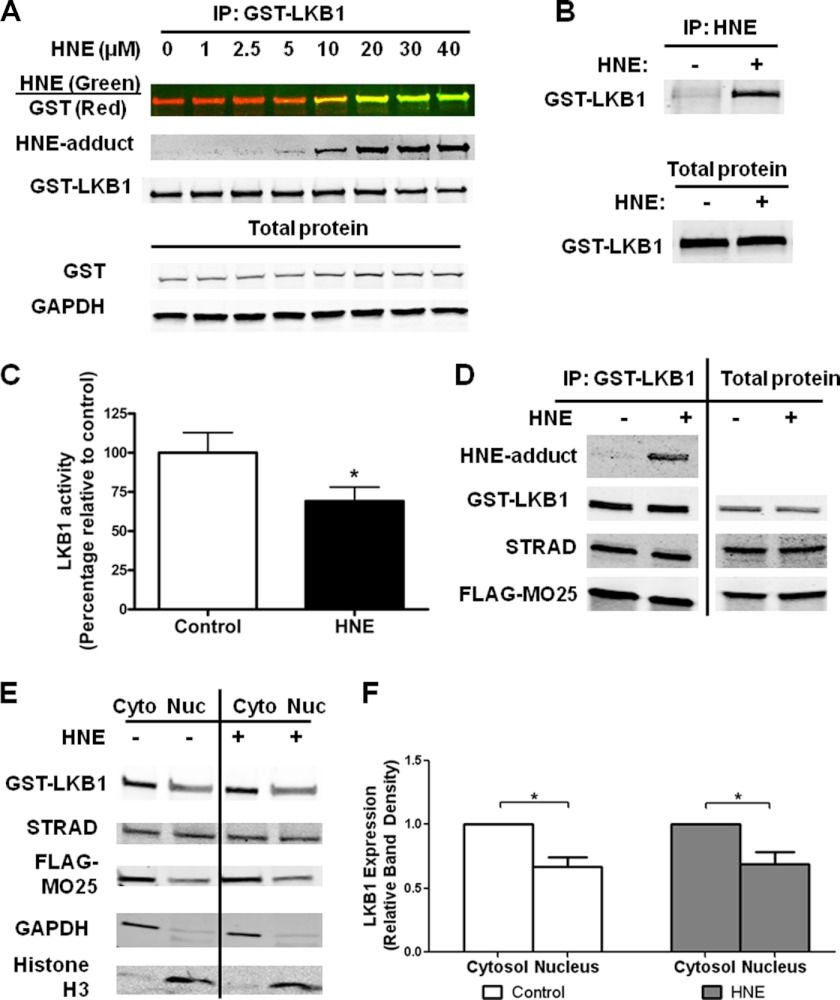
It was reported previously that HNE concentrations of 40 μm form inhibitory adducts on LKB1 in neonatal cardiomyocytes (21). We expressed LKB1 and its binding partners STRAD and MO25 in HEK293T cells and exposed the cells to HNE at concentrations of 1, 2.5, 5, 10, 20, 30, and 40 μm for 1 h. In cells expressing the LKB1 complex, HNE adducts were readily detectable in immunoprecipitated LKB1 with HNE concentrations of 10 μm and higher (Fig. 1A), and accordingly an HNE concentration of 10 μm was used in the remainder of this study. To further confirm the presence of HNE-LKB1 adducts, HNE-bound proteins were immunoprecipitated, and LKB1 was detected among the HNE-modified proteins (Fig. 1B). Coincident with the formation of HNE-LKB1 adducts, exposure to HNE (10 μm for 1 h) caused a 31 ± 9% (S.E.) decrease in LKB1 activity as assessed with a kinase assay using the LKBtide synthetic substrate (Fig. 1C).
FIGURE 1.
HNE forms inhibitory adducts of LKB1 and inhibits kinase activity. HEK293T cells were transiently transfected with GST-LKB1, His-STRAD, and FLAG-MO25, and GST-LKB1 was immunoprecipitated (IP) and immunoblotted with an anti-HNE antibody at the following doses of HNE: 1, 2.5, 5, 10, 20, 30, and 40 μm (n = 3) (A). B, cells treated with 10 μm HNE were immunoprecipitated for HNE-bound proteins and immunoblotted for GST-LKB1 association (n = 3). C, LKB1 activity was assessed using the LKBtide synthetic substrate in the presence of [γ-32P]ATP (n = 9; *, p < 0.01; error bars are mean ± standard error). D, immunoprecipitated GST-LKB1 was immunoblotted for protein-protein interactions of LKB1 with associated proteins STRAD and MO25 (n = 3). E, cytosolic (Cyt) and nuclear (Nuc) fractions of cells expressing the LKB1 complex were isolated and immunoblotted for LKB1 complex proteins (n = 3). F, quantification of LKB1 protein in cytosolic and nuclear cellular fractions, error bars are mean ± standard error.
HNE Adduction Does Not Interfere with LKB1-STRAD-MO25 Complex Formation
Because LKB1 kinase activity is greatly enhanced when LKB1 interacts with STRAD and MO25, we tested whether HNE interferes with LKB1 complex formation and thereby diminishes its activity. Immunoprecipitation of LKB1 and subsequent immunoblotting for STRAD and MO25 demonstrated no effect of HNE on LKB1-STRAD-MO25 complex formation (Fig. 1D). The cellular localization of LKB1 is also known to affect its catalytic activity (6). To determine whether HNE could affect LKB1 localization, protein lysates from cells expressing the LKB1 complex were separated into cytoplasmic and nuclear fractions. HNE treatment had no effect on the localization of LKB1, STRAD, or MO25 (Fig. 1E), and LKB1 was observed to localize primarily in the cytoplasm both with and without HNE (Fig. 1F). Together these data suggest that the inhibitory effect of HNE is due to a direct effect on LKB1 enzymatic function and is not related to changes in complex formation or localization.
LKB1 Lysine Residues 96 and 97 Mediate HNE Adduct Formation
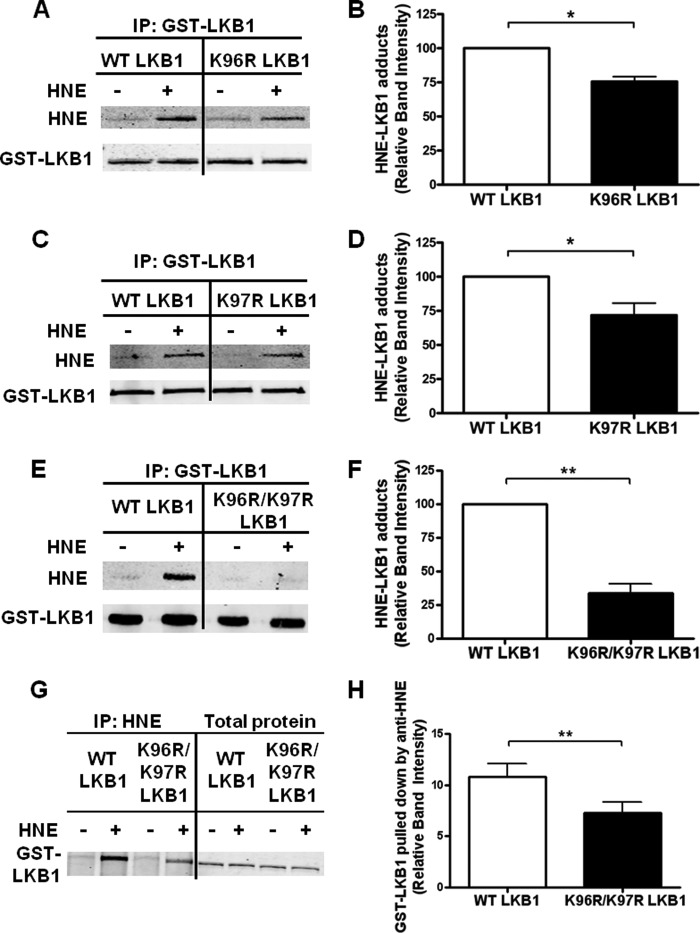
Our previous work demonstrated that several lysine residues of LKB1 can be acetylated both in vitro and in vivo (7). Because lysine residues may possess important regulatory functions, we investigated whether they are targeted for HNE adduction. We used three LKB1 mutants in which lysine residues were mutated to arginine, a mutation that mimics the effects of deacetylation (26) and reduces the chemical affinity of HNE for the mutated residue. The mutants were expressed in HEK293T cells and screened for the ability of HNE to form covalent adducts. Mutation of lysines 96 (K96R) and 97 (K97R) each reduced HNE adduct formation (Fig. 2, A–D). Interestingly, mutation of Lys-96 and Lys-97 together (K96R/K97R) caused a greater decrease in HNE adduct formation than mutation of either Lys-96 or Lys-97 alone (Fig. 2, E and F). Immunoprecipitation of HNE-bound proteins confirmed that there was a decreased amount of HNE-LKB1 adducts in the K96R/K97R mutant (Fig. 2, G and H).
FIGURE 2.
HNE adducts of LKB1 occur at Lys-96 and Lys-97. HEK293T cells were transiently transfected with plasmids encoding wild type or lysine-mutated GST-tagged LKB1, STRAD, and MO25. A–F, Western blots of cells treated with HNE and assayed for HNE adduct formation with the quantification of HNE-LKB1 adducts (n = 3–5; error bars are mean ± standard error). G and H, in cells expressing the K96R/K97R mutant, HNE-modified proteins were immunoprecipitated (IP) with an anti-HNE antibody and immunoblotted for GST-LKB1 association (n = 3; *, p < 0.05; **, p < 0.01. Error bars are mean ± standard error).
LKB1 Lysine Residue 97 Mediates the Inhibitory Effect of HNE on LKB1 Activity
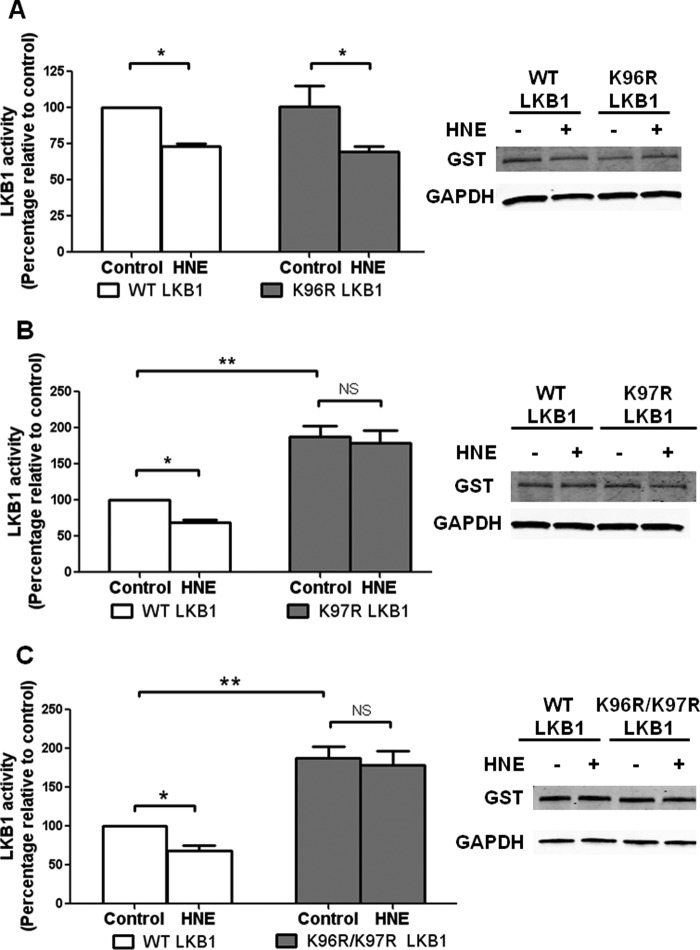
The finding that HNE adduct formation is decreased by mutation of LKB1 lysine 96 or 97 raised the possibility that these mutants would be resistant to the inhibitory effect of HNE. However, the kinase activity of the K96R mutant was inhibited by HNE to the same extent as wild type LKB1 (Fig. 3A). In contrast, kinase activity of the K97R mutant was resistant to inhibition (Fig. 3B) as was the K96R/K97R LKB1 mutant (Fig. 3C). Interestingly, the K97R and K96R/K97R mutants, but not the K96R mutant, exhibited an ∼2-fold increase in basal kinase activity as compared with wild type LKB1.
FIGURE 3.
HNE adduct formation at Lys-97 inhibits LKB1 kinase activity. HEK293T cells expressing WT or mutant LKB1, STRAD, and MO25 were treated with HNE (10 μm for 1 h), and immunoprecipitated LKB1 was assayed for kinase activity. A, K96R (n = 5). B, K97R (n = 6). C, K96R/K97R (n = 6; *, p < 0.05; **, p < 0.01; NS, not significant; one-way analysis of variance, Bonferroni's post-test. Error bars are mean ± standard error).
DISCUSSION
The goal of this study was to determine the mechanism by which HNE inhibits LKB1 kinase activity. Our major findings are that (a) HNE inhibition of LKB1 kinase activity does not involve disruption of LKB1 translocation or complex formation, (b) HNE forms adducts with LKB1 at Lys-96 and Lys-97, and (c) targeted mutation of Lys-97, but not Lys-96, prevents the inhibitory action of HNE.
The ability of HNE to form adducts with LKB1 was demonstrated by immunoprecipitating LKB1 and immunoblotting for HNE adducts and was confirmed by pulling down HNE-modified proteins and probing for associated LKB1. The formation of HNE-LKB1 adducts was first observed in MCF-7 cells (20). It was noted that Cys-210 is an evolutionarily conserved cysteine residue among a number of serine/threonine kinases and is a target for lipid adduction. Although Cys-210 appears capable of mediating lipid adduct formation as demonstrated by loss of the ability of reactive lipids to form adducts against LKB1 in which Cys-210 is mutated to serine, the effects of HNE adduction on LKB1 activity and/or downstream signaling were not determined. More recently, it was shown that HNE can adduct to LKB1 and is associated with inhibition of enzyme activity and increased hypertrophic signaling stemming from changes in the LKB1-AMPK signaling axis (21).
We next confirmed that HNE inhibits LKB1 enzymatic activity. We measured LKB1 kinase activity directly using the synthetic substrate LKBtide, a well validated approach that we (7) and others (21, 27, 28) have used. Phosphorylation at Ser-428 may also be used to measure LKB1 activity as it is correlated with increased activity and is thought to be required for LKB1-dependent functions in some cell types (29). However, phosphorylation at Ser-428 is not necessary for all kinase-dependent functions of the protein (30). Furthermore, a novel alternative LKB1 splice variant that lacks a portion of the C-terminal domain containing Ser-428 but possesses catalytic activity on par with the full-length LKB1 gene product has been identified (31).
To determine the mechanism by which HNE inhibits LKB1 activity, we first examined whether HNE interfered with LKB1 translocation or complex formation. HNE had no effect on the translocation of LKB1 as assessed by the distribution of protein between the nucleus and cytosol. Likewise, HNE inhibited LKB1 activity without disruption of complex formation as measured by the association of LKB1 with its binding partners STRAD and MO25.
We next used site-directed mutants of LKB1 in which lysine is mutated to arginine to test the role of specific lysine residues in mediating the effects of HNE. Because arginine cannot be acetylated, this mutation mimics the effect of deacetylation and also decreases the chemical affinity of the residue for HNE. Mutation of Lys-96 or Lys-97 decreased the formation of HNE adducts to a similar degree, and mutation of Lys-96 and Lys-97 together caused a roughly additive decrease in HNE adduction, indicating that both Lys-96 and Lys-97 are sites of HNE adduction.
In contrast to the similar effects the K96R and K97R mutants have on HNE adduct formation, only the K97R mutant was protected from inhibition by HNE. Enzyme activity was also protected in the K96R/K97R mutant. Thus, although HNE can adduct to both Lys-96 and Lys-97, adduct formation at Lys-97 alone is sufficient to mediate enzyme inhibition. These findings suggest that HNE inhibits LKB1 activity via a direct post-translational modification at Lys-97. Aside from modification by HNE, no other direct oxidative post-translational modifications of LKB1 have been described. The reactive nitrogen species peroxynitrite (ONOO−) has been shown to regulate LKB1 activity indirectly through activity of upstream protein kinase C ζ in the endothelium (32).
Based on these findings, we cannot exclude other sites at which HNE adduction occurs on the LKB1 protein. It is conceivable that amino acid residues aside from Lys-96 and Lys-97 are targets of HNE. Further studies in our laboratory suggest that the GST tag of the protein is not a target, but amino acids in other domains of the protein may be targeted by HNE (data not shown).
It is noteworthy that basal enzyme activity was increased ∼2-fold in both the K97R and K96R/K97R mutants. Lys-97 is a known target of acetylation (7). Modification of this residue by acetylation, methylation, SUMOylation, or ubiquitination could potentially modulate the kinase function of LKB1. More studies will need to be carried out to delineate the precise mechanism by which mutation of Lys-97 increases LKB1 kinase activity.
The structure of HNE contains three functional groups (C C double bond, C
C double bond, C O carbonyl group, and the hydroxyl group) that are responsible for the high reactivity of this electrophilic aldehyde (15). Although carbon 3 is the primary site of nucleophilic attack by thiol or amine groups via the Michael reaction, the carbonyl carbon 1 is a secondary target of primary amines through Schiff base formation. Using synthetic polyamino acid model compounds, HNE was shown to chemically react in the following order: Cys > His > Lys (33). It was noted that this order does not implicate cysteine residues as the preferred targets of HNE. Depending on the overall quaternary protein structure as well as the surrounding amino acid residues, the reactivity of HNE toward amine-containing amino acids can be enhanced.
O carbonyl group, and the hydroxyl group) that are responsible for the high reactivity of this electrophilic aldehyde (15). Although carbon 3 is the primary site of nucleophilic attack by thiol or amine groups via the Michael reaction, the carbonyl carbon 1 is a secondary target of primary amines through Schiff base formation. Using synthetic polyamino acid model compounds, HNE was shown to chemically react in the following order: Cys > His > Lys (33). It was noted that this order does not implicate cysteine residues as the preferred targets of HNE. Depending on the overall quaternary protein structure as well as the surrounding amino acid residues, the reactivity of HNE toward amine-containing amino acids can be enhanced.
A noteworthy aspect of this study is the use of a low concentration of HNE (10 μm). The concentration of HNE in human plasma ranges from 0.3 to 0.7 μm (34) but can reach levels as high as 10 μm under conditions of oxidative stress (35). Studies investigating HNE-mediated changes in LKB1 signaling used an HNE concentration of 40 μm (21), which resulted in significant degradation of the LKB1 protein within 1 h. Pathophysiological responses to HNE treatment in cardiac myocytes with concentrations as high as 400 μm have been reported to include calcium overload and reactive oxygen species generation (36). HNE has been shown to stimulate autophagy (37) and ubiquitin-mediated proteasomal degradation of proteins (38, 39) and to elicit an endoplasmic reticulum stress response in cultured vascular smooth muscle cells (40). By using a relatively low concentration of HNE, our findings are less likely to be confounded by cellular events that may be activated with higher concentrations.
In summary, this study provides compelling evidence that post-translational modification of LKB1 via HNE adduct formation at Lys-97 is responsible for the inhibitory effect of this lipid peroxidation by-product on kinase activity. Inhibition of LKB1 during states of increased oxidative stress and secondary lipid peroxidation could lead to energetic impairment and cellular dysfunction.
Acknowledgments
We thank David Pimentel and Edward Miller for critical evaluations of the data.
This work was supported, in whole or in part, by National Institutes of Health Grants HL-061639 (to W. S. C.) and HL-064750 (to W. S. C.), the NHLBI-sponsored Boston University Cardiovascular Proteomics Center (Contract N01-HV-28178 to W. S. C.), and T32 Cardiovascular Predoctoral Training Grant HL-007969 (to T. D. C.).
- AMPK
- AMP-activated protein kinase
- STRAD
- sterile-20-related adaptor
- MO25
- mouse protein 25
- HNE
- 4-hydroxy-trans-2-nonenal
- SUMO
- small ubiquitin-like modifier.
REFERENCES
- 1. Hemminki A., Markie D., Tomlinson I., Avizienyte E., Roth S., Loukola A., Bignell G., Warren W., Aminoff M., Höglund P., Järvinen H., Kristo P., Pelin K., Ridanpää M., Salovaara R., Toro T., Bodmer W., Olschwang S., Olsen A. S., Stratton M. R., de la Chapelle A., Aaltonen L. A. (1998) A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature 391, 184–187 [DOI] [PubMed] [Google Scholar]
- 2. Alessi D. R., Sakamoto K., Bayascas J. R. (2006) LKB1-dependent signaling pathways. Annu. Rev. Biochem. 75, 137–163 [DOI] [PubMed] [Google Scholar]
- 3. Jäger S., Handschin C., St-Pierre J., Spiegelman B. M. (2007) AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proc. Natl. Acad. Sci. U.S.A. 104, 12017–12022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Egan D. F., Shackelford D. B., Mihaylova M. M., Gelino S., Kohnz R. A., Mair W., Vasquez D. S., Joshi A., Gwinn D. M., Taylor R., Asara J. M., Fitzpatrick J., Dillin A., Viollet B., Kundu M., Hansen M., Shaw R. J. (2011) Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 331, 456–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dorfman J., Macara I. G. (2008) STRADα regulates LKB1 localization by blocking access to importin-α, and by association with Crm1 and exportin-7. Mol. Biol. Cell 19, 1614–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boudeau J., Baas A. F., Deak M., Morrice N. A., Kieloch A., Schutkowski M., Prescott A. R., Clevers H. C., Alessi D. R. (2003) MO25α/β interact with STRADα/β enhancing their ability to bind, activate and localize LKB1 in the cytoplasm. EMBO J. 22, 5102–5114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lan F., Cacicedo J. M., Ruderman N., Ido Y. (2008) SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J. Biol. Chem. 283, 27628–27635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pillai V. B., Sundaresan N. R., Kim G., Gupta M., Rajamohan S. B., Pillai J. B., Samant S., Ravindra P. V., Isbatan A., Gupta M. P. (2010) Exogenous NAD blocks cardiac hypertrophic response via activation of the SIRT3-LKB1-AMP-activated kinase pathway. J. Biol. Chem. 285, 3133–3144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pamplona R. (2008) Membrane phospholipids, lipoxidative damage and molecular integrity: a causal role in aging and longevity. Biochim. Biophys. Acta 1777, 1249–1262 [DOI] [PubMed] [Google Scholar]
- 10. Esterbauer H., Schaur R. J., Zollner H. (1991) Chemistry and biochemistry of 4-hydroxynonenal, malondialdehyde and related aldehydes. Free Radic. Biol. Med. 11, 81–128 [DOI] [PubMed] [Google Scholar]
- 11. Kimura H., Liu S., Yamada S., Uchida K., Matsumoto K., Mukaida M., Yoshida K. (2005) Rapid increase in serum lipid peroxide 4-hydroxynonenal (HNE) through monocyte NADPH oxidase in early endotoxemia. Free Radic. Res. 39, 845–851 [DOI] [PubMed] [Google Scholar]
- 12. Montine K. S., Olson S. J., Amarnath V., Whetsell W. O., Jr., Graham D. G., Montine T. J. (1997) Immunohistochemical detection of 4-hydroxy-2-nonenal adducts in Alzheimer's disease is associated with inheritance of APOE4. Am. J. Pathol. 150, 437–443 [PMC free article] [PubMed] [Google Scholar]
- 13. Ji B., Ito K., Suzuki H., Sugiyama Y., Horie T. (2002) Multidrug resistance-associated protein2 (MRP2) plays an important role in the biliary excretion of glutathione conjugates of 4-hydroxynonenal. Free Radic. Biol. Med. 33, 370–378 [DOI] [PubMed] [Google Scholar]
- 14. Eaton P., Li J. M., Hearse D. J., Shattock M. J. (1999) Formation of 4-hydroxy-2-nonenal-modified proteins in ischemic rat heart. Am. J. Physiol. Heart Circ. Physiol. 276, H935–H943 [DOI] [PubMed] [Google Scholar]
- 15. Schaur R. J. (2003) Basic aspects of the biochemical reactivity of 4-hydroxynonenal. Mol. Aspects Med. 24, 149–159 [DOI] [PubMed] [Google Scholar]
- 16. Ji C., Kozak K. R., Marnett L. J. (2001) IκB kinase, a molecular target for inhibition by 4-hydroxy-2-nonenal. J. Biol. Chem. 276, 18223–18228 [DOI] [PubMed] [Google Scholar]
- 17. Chen J., Henderson G. I., Freeman G. L. (2001) Role of 4-hydroxynonenal in modification of cytochrome c oxidase in ischemia/reperfused rat heart. J. Mol. Cell. Cardiol. 33, 1919–1927 [DOI] [PubMed] [Google Scholar]
- 18. Sampey B. P., Carbone D. L., Doorn J. A., Drechsel D. A., Petersen D. R. (2007) 4-Hydroxy-2-nonenal adduction of extracellular signal-regulated kinase (Erk) and the inhibition of hepatocyte Erk-Est-like protein-1-activating protein-1 signal transduction. Mol. Pharmacol. 71, 871–883 [DOI] [PubMed] [Google Scholar]
- 19. Backos D. S., Fritz K. S., Roede J. R., Petersen D. R., Franklin C. C. (2011) Posttranslational modification and regulation of glutamate-cysteine ligase by the α,β-unsaturated aldehyde 4-hydroxy-2-nonenal. Free Radic. Biol. Med. 50, 14–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wagner T. M., Mullally J. E., Fitzpatrick F. A. (2006) Reactive lipid species from cyclooxygenase-2 inactivate tumor suppressor LKB1/STK11: cyclopentenone prostaglandins and 4-hydroxy-2-nonenal covalently modify and inhibit the AMP-kinase kinase that modulates cellular energy homeostasis and protein translation. J. Biol. Chem. 281, 2598–2604 [DOI] [PubMed] [Google Scholar]
- 21. Dolinsky V. W., Chan A. Y., Robillard Frayne I., Light P. E., Des Rosiers C., Dyck J. R. (2009) Resveratrol prevents the prohypertrophic effects of oxidative stress on LKB1. Circulation 119, 1643–1652 [DOI] [PubMed] [Google Scholar]
- 22. Qin F., Siwik D. A., Luptak I., Hou X., Wang L., Higuchi A., Weisbrod R. M., Ouchi N., Tu V. H., Calamaras T. D., Miller E. J., Verbeuren T. J., Walsh K., Cohen R. A., Colucci W. S. (2012) The polyphenols resveratrol and s17834 prevent the structural and functional sequelae of diet-induced metabolic heart disease in mice. Circulation 125, 1757–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhao S., Xu W., Jiang W., Yu W., Lin Y., Zhang T., Yao J., Zhou L., Zeng Y., Li H., Li Y., Shi J., An W., Hancock S. M., He F., Qin L., Chin J., Yang P., Chen X., Lei Q., Xiong Y., Guan K. L. (2010) Regulation of cellular metabolism by protein lysine acetylation. Science 327, 1000–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang Q., Zhang Y., Yang C., Xiong H., Lin Y., Yao J., Li H., Xie L., Zhao W., Yao Y., Ning Z. B., Zeng R., Xiong Y., Guan K. L., Zhao S., Zhao G. P. (2010) Acetylation of metabolic enzymes coordinates carbon source utilization and metabolic flux. Science 327, 1004–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lu Z., Scott I., Webster B. R., Sack M. N. (2009) The emerging characterization of lysine residue deacetylation on the modulation of mitochondrial function and cardiovascular biology. Circ. Res. 105, 830–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Megee P. C., Morgan B. A., Mittman B. A., Smith M. M. (1990) Genetic analysis of histone H4: essential role of lysines subject to reversible acetylation. Science 247, 841–845 [DOI] [PubMed] [Google Scholar]
- 27. Lizcano J. M., Göransson O., Toth R., Deak M., Morrice N. A., Boudeau J., Hawley S. A., Udd L., Mäkelä T. P., Hardie D. G., Alessi D. R. (2004) LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 23, 833–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hurst D., Taylor E. B., Cline T. D., Greenwood L. J., Compton C. L., Lamb J. D., Winder W. W. (2005) AMP-activated protein kinase kinase activity and phosphorylation of AMP-activated protein kinase in contracting muscle of sedentary and endurance-trained rats. Am. J. Physiol. Endocrinol. Metab. 289, E710–E715 [DOI] [PubMed] [Google Scholar]
- 29. Xie Z., Dong Y., Scholz R., Neumann D., Zou M. H. (2008) Phosphorylation of LKB1 at serine 428 by protein kinase C-ζ is required for metformin-enhanced activation of the AMP-activated protein kinase in endothelial cells. Circulation 117, 952–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fogarty S., Hardie D. G. (2009) C-terminal phosphorylation of LKB1 is not required for regulation of AMP-activated protein kinase, BRSK1, BRSK2, or cell cycle arrest. J. Biol. Chem. 284, 77–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Denison F. C., Hiscock N. J., Carling D., Woods A. (2009) Characterization of an alternative splice variant of LKB1. J. Biol. Chem. 284, 67–76 [DOI] [PubMed] [Google Scholar]
- 32. Xie Z., Dong Y., Zhang M., Cui M. Z., Cohen R. A., Riek U., Neumann D., Schlattner U., Zou M. H. (2006) Activation of protein kinase C ζ by peroxynitrite regulates LKB1-dependent AMP-activated protein kinase in cultured endothelial cells. J. Biol. Chem. 281, 6366–6375 [DOI] [PubMed] [Google Scholar]
- 33. Poli G., Schaur R. J., Siems W. G., Leonarduzzi G. (2008) 4-Hydroxynonenal: a membrane lipid oxidation product of medicinal interest. Med. Res. Rev. 28, 569–631 [DOI] [PubMed] [Google Scholar]
- 34. Strohmaier H., Hinghofer-Szalkay H., Schaur R. J. (1995) Detection of 4-hydroxynonenal (HNE) as a physiological component in human plasma. J. Lipid Mediat. Cell Signal. 11, 51–61 [DOI] [PubMed] [Google Scholar]
- 35. Uchida K. (2003) 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog. Lipid Res. 42, 318–343 [DOI] [PubMed] [Google Scholar]
- 36. Nakamura K., Miura D., Kusano K. F., Fujimoto Y., Sumita-Yoshikawa W., Fuke S., Nishii N., Nagase S., Hata Y., Morita H., Matsubara H., Ohe T., Ito H. (2009) 4-Hydroxy-2-nonenal induces calcium overload via the generation of reactive oxygen species in isolated rat cardiac myocytes. J. Card. Fail. 15, 709–716 [DOI] [PubMed] [Google Scholar]
- 37. Hill B. G., Haberzettl P., Ahmed Y., Srivastava S., Bhatnagar A. (2008) Unsaturated lipid peroxidation-derived aldehydes activate autophagy in vascular smooth-muscle cells. Biochem. J. 410, 525–534 [DOI] [PubMed] [Google Scholar]
- 38. Carbone D. L., Doorn J. A., Petersen D. R. (2004) 4-Hydroxynonenal regulates 26S proteasomal degradation of alcohol dehydrogenase. Free Radic. Biol. Med. 37, 1430–1439 [DOI] [PubMed] [Google Scholar]
- 39. Wang Z., Dou X., Gu D., Shen C., Yao T., Nguyen V., Braunschweig C., Song Z. (2012) 4-Hydroxynonenal differentially regulates adiponectin gene expression and secretion via activating PPARγ and accelerating ubiquitin-proteasome degradation. Mol. Cell. Endocrinol. 349, 222–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vladykovskaya E., Sithu S. D., Haberzettl P., Wickramasinghe N. S., Merchant M. L., Hill B. G., McCracken J., Agarwal A., Dougherty S., Gordon S. A., Schuschke D. A., Barski O. A., O'Toole T., D'Souza S. E., Bhatnagar A., Srivastava S. (2012) The lipid peroxidation product, 4-hydroxy-trans-2-nonenal causes endothelial activation by inducing endoplasmic reticulum stress. J. Biol. Chem. 287, 11398–11409 [DOI] [PMC free article] [PubMed] [Google Scholar]