Abstract
The reactivation of a memory through retrieval can render it subject to disruption or modification through the process of memory reconsolidation. In both humans and rodents, briefly reactivating a fear memory results in effective erasure by subsequent extinction training. Here we show that a similar strategy is equally effective in the disruption of appetitive pavlovian cue–food memories. However, systemic administration of the NMDA receptor partial agonist D-cycloserine under the same behavioural conditions did not potentiate appetitive memory extinction, suggesting that reactivation does not enhance subsequent extinction learning. To confirm that reactivation followed by extinction reflects a behavioural analog of memory reconsolidation, we show that prevention of contextual fear memory reactivation by the LVGCC blocker nimodipine interferes with the amnestic outcome. Therefore, the reconsolidation process can be manipulated behaviourally to disrupt both aversive and appetitive memories.
The retrieval of an established memory is not a passive process. Memory retrieval has long been recognised as an active constructive process1, and is more recently thought of as a means of memory modification2. Under certain circumstances, memory retrieval “reactivates” the memory, destabilising it and engaging a process of post-retrieval plasticity that is known as memory reconsolidation. The long-lasting amnesia that results from disruption of memory reconsolidation has canonically been achieved using cellular or pharmacological interventions in models of pavlovian fear conditioning in both rodents and humans3,4, but also in preclinical models of reward-seeking behaviour5,6. These findings have raised the prospect of reconsolidation-based therapeutic interventions for conditions such as post-traumatic stress disorder7 and drug addiction8.
Recently, a purely behavioural analogue of pharmacologically-induced reconsolidation impairments has been developed, making use of reactivation-induced memory plasticity to diminish pavlovian fear memories through non-reinforced stimulus exposure. In both rodents and humans, this combination of reactivation-induced reconsolidation and behavioural extinction training has resulted in a reduction of fear memory expression that does not recover with normally-effective reminder procedures9-11. The potential translational efficacy of this approach remains unclear, especially given some noted failures to replicate the effects12,13. Moreover, there is also the question of its relevance to appetitive reward-related memories.
The association of a conditioned stimulus (CS) with a rewarding outcome results in long-lasting conditioned reinforcing properties that powerfully influence reward-seeking behaviour14. We have previously shown that the CS–reward memories supporting conditioned reinforcement undergo reconsolidation for both sucrose and cocaine rewards5,6,15, and that disruptive effects in this procedure are reliably predictive of impairments in other translationally-relevant models of reward-seeking behaviour16-18.
The amnestic effect of extinguishing a reactivated memory has been interpreted within the framework of memory reconsolidation9-11. However, it is equally plausible that prior memory reactivation may potentiate subsequent extinction in a similar manner to pharmacological enhancement of extinction. Here we show also that the behavioural approach of combining memory reactivation with extinction training is effective in disrupting appetitive pavlovian memories. Treatment with the NMDA receptor partial agonist D-cycloserine or Fibroblast growth factor 2 enhances fear memory extinction19-21. Importantly, this potentiated memory extinction is not subject to normal recovery, suggesting that extinction enhancement can lead to the same qualitative outcome as reconsolidation impairment. We sought to disambiguate these accounts by selectively disrupting memory reactivation. Contextual fear memory destabilisation upon reactivation depends upon calcium influx at L-type voltage-gated calcium channels (LVGCCs), as shown by the effect of the LVGCC blocker nimodipine to protect against the amnestic effect of protein synthesis inhibition22. Therefore, we show first that memory reactivation and extinction impairs contextual fear memories, and subsequently that this effect depends critically upon LVGCC-mediated memory reactivation.
Results
Appetitive pavlovian memory
First, we tested the effect of combining a brief memory reactivation and, at an interval of 1 hour as demonstrated to be effective in a fear memory paradigm9, a longer memory extinction session upon an appetitive pavlovian light–food memory. This was conducted in a procedure that isolates the acquired pavlovian conditioned reinforcing properties of appetitive conditioned stimuli, tested through the acquisition of discriminated lever pressing for the CS. We selected such a procedure as it is acutely sensitive to disruption of the pavlovian memory, and we have previously used it to demonstrate reconsolidation impairments for both sucrose– and cocaine–associated memories5,6,15. Moreover, disruptive effects in this procedure are reliably predictive of impairments in other translationally-relevant models of reward-seeking behaviour16-18. An overall mixed ANOVA of all the experimental conditions described below revealed significant lever × group (F(5,42)=3.681, p=0.007) and lever × session × group (F(18.3,154.1)=2.130, p=0.007) interactions, as well as a main effect of group (F(5,42)=4.050, p=0.004). This indicates that the groups differed in their acquisition of discriminated responding over the multiple testing sessions. To explore this overall effect, we first analysed the simple session × group interactions for the active and inactive levers independently (mixed ANOVA, p<0.025, using Bonferroni correction for multiple comparisons). For the active lever, there was both a session × group interaction (F(16.5,138.3)=2.316, p=0.004) and a significant effect of group (F(5,42)=4.117, p=0.004). In contrast, there were no group differences upon responding on the inactive lever (session × group: F(19.0,159.9)=1.166, p=0.293; group: F(5,42)=2.619, p=0.038).
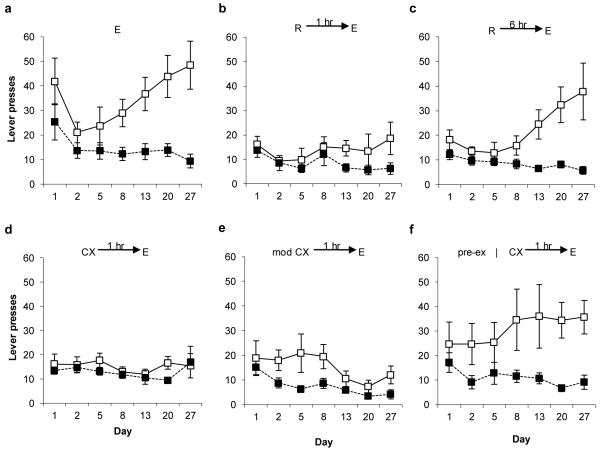
Given the selective effect upon the active lever response rates, post-hoc pairwise comparisons (mixed ANOVA, p<0.01, using Bonferroni correction) were conducted between each experimental group and the control extinction-alone group that received all 70 min of extinction training in a single session. Firstly, the combination of reactivation and extinction (reactivation + extinction) resulted in a marked and persistent reduction in conditioned reinforcement (Figs. 1a-b; session × group: F(2.5,29.7)=2.010, p=0.43; group: F(1,12)=10.045, p=0.008). This disruption was observed through 6 test sessions over 20 days following reactivation + extinction. Moreover, no recovery was seen here in a reinstatement test on day 27. Therefore, there was no evidence of recovery from the memory deficit induced by reactivation + extinction. Similar to fear memories9, there was a critical temporal window following memory reactivation, during which the extinction session must be applied in order produce the amnestic effect. Delaying extinction to 6 hours after reactivation resulted in acquisition of active lever responding that was not different from the control extinction group (Fig. 1c; session × group: F(1.9,24.6)=0.532, p=0.584; group: F(1,13)=3.653, p=0.078).
Fig. 1.
Acquisition of a new response with conditioned reinforcement. Discriminated responding on the active (open symbols) and inactive (filled symbols) levers was compared across experimental conditions. a, Extinction (E) alone, without prior memory reactivation (N=7). b, reactivation (R) followed, 1 hr later, by extinction (N=7). c, reactivation followed, 6 hr later, by extinction (N=8). d, context exposure (CX) followed, 1 hr later, by extinction (N=12). e, exposure to modified (mod) context followed, 1 hr later, by extinction (N=7). f, pretraining context habituation (pre-ex), with posttraining context exposure followed, 1 hr later, by extinction (N=7). Groups b, d & e differed significantly from the control group a. Groups c & f did not differ from group a. Data presented as mean ± SEM.
In a finding notably different from fear memories9, when memory reactivation was replaced by a session in which the rats were simply returned to the experimental context for 10 min without exposure to the light CS (i.e. the nosepoke response had no consequence), its combination with extinction 1 hour later still produced a marked deficit in conditioned reinforcement compared to the extinction controls (Fig. 1d; session × group: F(2.3,39.7)=3.827, p=0.025; group: F(1,17)=17.299, p=0.001). One likely explanation for the capacity of context exposure alone to reactivate the light–food memory in the current study is that the context acts as an occasion-setting stimulus. In this procedure, the context is consistently associated with the light–food memory over 9 days of training, whereas fear conditioning takes place over a single short training session. Alternatively, it is possible that the instrumental setting of the procedure results in nosepoke responding during the context exposure session being sufficient to retrieve and reactivate the CS–food association even the absence of explicit CS exposure. The latter explanation is rendered less likely by the observation that preventing the nosepoke response during context exposure failed to mitigate the deficit in conditioned reinforcement (Fig. 1e; session × group: F(2.3,27.9)=5.026, p=0.011; group: F(1,12)=10.152, p=0.008). Instead, when rats were pre-exposed to the context for 3 hr over two days prior to training, context exposure no longer reactivated the memory sufficiently to induce a subsequent deficit in conditioned reinforcement (Fig. 1f; session × group: F(2.5,29.4)=1.196, p=0.323; group: F(1,12)=0.288, p=0.601). This indicates that context pre-exposure resulted in habituation to the context, slowing or preventing the acquisition of any contextual modulatory impact during training, and hence reduced the capacity of post-training context exposure to reactivate the CS–food memory.
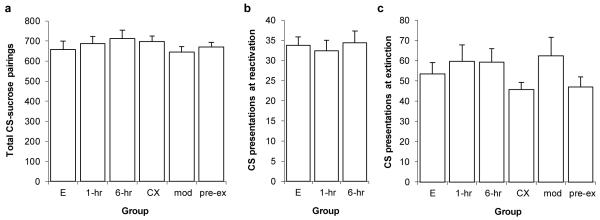
None of the previously-described behavioural effects during the test sessions were easily attributable to prior differences in training history other than the experimental manipulations. The total number of CS–food pairings did not differ between the groups (Fig. 2a; one-way ANOVA; F(5,42)=0.605, p=0.697). Similarly, there was no difference in the number of unreinforced CS presentations during memory reactivation (Fig. 2b; one-way ANOVA; F(2,19)=0.162, p=0.852) or extinction (Fig. 2c; one-way ANOVA; F(5,42)=1.446, p=0.228). Therefore, there is no pattern of differences during training and reactivation/extinction that can account for the observed selective disruption of conditioned reinforcement.
Fig. 2.
No differences between groups prior to acquisition of a new response with conditioned reinforcement. The groups were extinction alone (E; N=7), reactivation + extinction with a 1-hr interval (1-hr; N=7), reactivation + extinction with a 6-hr interval (6-hr, N=8), context exposure followed by extinction (CX; N=12), exposure to modified context prior to extinction (mod; N=7) and pre-exposure to the context before training (pre-ex; N=7). a, Total number of CS–sucrose pairings during training across all groups. b, Number of unreinforced CS presentations during memory reactivation in groups 1-hr and 6-hr compared to the equivalent first 10 min of extinction in group E. c, Number of unreinforced CS presentations during memory extinction across all groups compared to the equivalent final 60 min of extinction in group E. Data presented as mean + SEM.
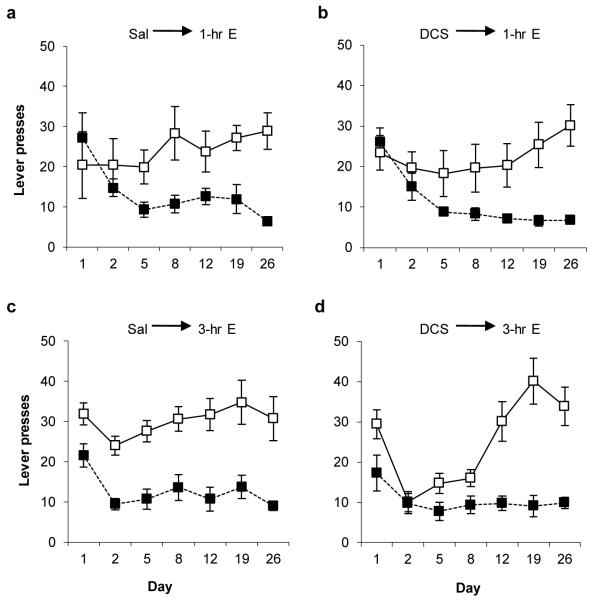
When rats were injected with DCS, instead of being reactivated, prior to the 1-hr memory extinction session, there was no effect upon subsequent acquisition of the new response with conditioned reinforcement. Rats injected with DCS and saline did not differ in lever pressing (Fig. 3a-b; mixed ANOVA; lever × group: F(1,13)=0.004, p=0.949; lever × session × group: F(2.0,25.8)=0.345, p=0.710; group: F(1,13)=0.420, p=0.528), with both groups showing the acquisition of discriminated lever pressing over the course of testing (lever: F(1,13)=19.800, p=0.001; lever × session: F(2.0, 25.8)=8.750, p=0.001). Neither did the groups differ during training or extinction, receiving both similar total numbers of light–food pairings (Saline = 581.5±28.1, DCS = 609.9±29.4; one-way ANOVA; F(1,13)=0.478, p=0.501) and similar numbers of non-reinforced light presentations during extinction (Saline = 63.0±5.8, DCS = 66.6±8.6; one-way ANOVA; F(1,13)=1.297, p=0.275).
Fig. 3.
D-cycloserine potentiates extinction when the extinction session is 3 hr but not 1 hr long. Discriminated responding on the active (open symbols) and inactive (filled symbols) levers was compared across experimental conditions. a, Saline injection prior to 1 hr extinction (N=7). b, DCS injection prior to 1 hr extinction (N=8). c, Saline injection prior to 3 hr extinction (N=7). d, DCS injection prior to 1 hr extinction (N=8). DCS had no effect upon the acquisition of discriminated responding with conditioned reinforcement with the 1-hr extinction session, but did retard acquisition with the 3-hr session. Data presented as mean ± SEM.
The functional effect of DCS to potentiate memory extinction was confirmed in the present appetitive setting when a longer 3-hr extinction session was used (Fig. 3c-d). Rats injected with DCS and saline differed in lever pressing (mixed ANOVA; lever × session × group: F(3.4,40.6)=3.129, p=0.031; lever × group: F(1,12)=1.757, p=0.210; group: F(1,12)=3.176, p=0.100). Analysis of simple effects of group and session by mixed ANOVA revealed a significant effect of DCS upon responding on the active (session × group: F(3.0,36.4)=3.404, p=0.027; group: F(1,12)=4.462, p=0.056), but not the inactive lever (session × group: F(3.1,36.8)=0.477, p=0.704; group: F(1,12)=0.560, p=0.469). Furthermore, the effect of DCS on active lever responding (one-way ANOVA, P<0.007, using Bonferroni correction) was observed on days 2, 5 and 8 only. The groups did not differ during training or extinction, receiving both similar total numbers of light–food pairings (Saline = 662.9±44.5, DCS = 652.0±36.5; one-way ANOVA; F(1,12)=0.038, p=0.848) and similar numbers of non-reinforced light presentations during extinction (Saline = 122.6±11.8, DCS = 129.4±15.2; one-way ANOVA; F(1,12)=0.108, p=0.748). Therefore, DCS potentiated appetitive memory extinction in a quantitative manner. However, the extinguished memory recovered with repeated testing, as observed previously with more extensive extinction training23.
Auditory fear memory
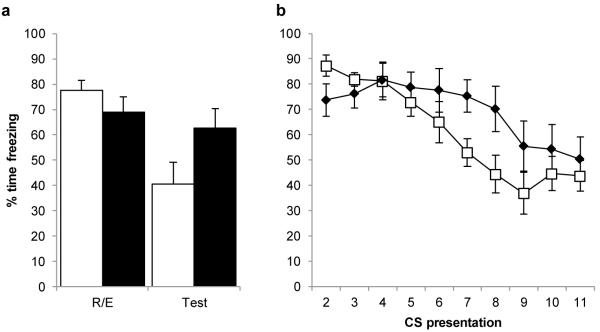
Given that in the appetitive setting, reactivation + extinction results in memory impairment under parametric conditions that render the potentiative effect of DCS ineffective, we tested whether this dissociation between behavioural and pharmacological treatments extends to the aversive domain. We have previously demonstrated that DCS enhances the extinction of an auditory fear memory24. Therefore, we used the same parameters of fear conditioning and extinction to assess the impact of memory reactivation 1 hour prior to extinction. The combination of memory reactivation and extinction did affect subsequent conditioned freezing compared to the control group given extinction training alone (Fig. 4a). However, the direction of the effect was opposite to that which would have been expected for a reactivation + extinction impairment of auditory fear memory. The decrease in freezing from the first CS presentation at reactivation/extinction to the test session was greater in the extinction group than the reactivation + extinction group (mixed ANOVA; session × condition: F(1,14)=7.105, p=0.018). One-way ANOVA revealed that while there were no absolute differences between the conditions at reactivation/extinction (F(1,14)=1.430, p=0.252) or test (F(1,14)=3.693, p=0.075), the extinction alone rats reduced their freezing levels from extinction to test (F(1,7)=14.112, p=0.007), whereas the reactivation + extinction rats did not (F(1,7)=1.068, p=0.336). Moreover, the reduction in conditioned freezing over CS presentations during extinction was retarded in the reactivation + extinction group compared to extinction alone (Fig. 4b). Therefore, memory reactivation prior to limited extinction training impaired both the acquisition and subsequent retention of extinction.
Fig. 4.
Fear conditioning to a discrete clicker. Rats were reactivated and extinguished 1 hr later (filled columns/symbols, N=8) or given extinction alone (open columns/symbols, N=8). a, Freezing to the clicker at reactivation/start of extinction (R/E) and at the post-extinction test. Reactivation and extinction impaired the decrement in memory expression compared to extinction alone. b, Freezing during the extinction session. The final 10 presentations of the CS (i.e. excluding the initial presentation of extinction or the prior reactivation presentation) are presented. The reduction in freezing levels across CS presentations was retarded in the reactivation + extinction condition, compared to the extinction alone condition (mixed ANOVA; presentation × condition: F(4.6,64.3)=3.025, p=0.019; condition: F(1,14)=1.228, p=0.286). Analysis of simple effects (p<0.05) revealed that both conditions reduced their freezing levels across the session, and there was no absolute difference between the conditions at any of the CS presentations. Data presented as mean ± SEM.
Contextual fear memory
In order to disambiguate whether reactivation + extinction results in reconsolidation-mediated memory updating or a potentiation of extinction, we used the LGVCC-blocker nimodipine to prevent the reconsolidation-critical process of memory destabilisation in a contextual fear memory22. Rats were injected with nimodipine or vehicle at differing timepoints. First, we analysed whether the combination of reactivation and extinction produced differing effects from extinction alone (Figs. 5-8; vehicle control groups). Collapsing across the timing of vehicle injection, we compared freezing during the initial post-extinction test and the post-reconditioning test for the two behavioural conditions. While rats that received extinction alone significantly reacquired the contextual fear memory with a less intense footshock, those that received reactivation and extinction showed little evidence of reacquisition (mixed ANOVA; session × condition: F(1,22)=11.815, p=0.002). There were no differences between freezing levels in the two behavioural conditions at reactivation compared to the equivalent time period at the start of extinction (extinction alone = 51.6±6.8, reactivation + extinction = 48.4±5.4; one-way ANOVA; F(1,22)=0.108, p=0.746), indicating that there were no differences in the levels of conditioning between the groups. Moreover, simple effects analysis with separate ANOVAs revealed no group differences at the first test after reactivation and extinction (F(1,22)=0.104, p=0.751). In contrast, rats subjected to reactivation + extinction froze significant less than the extinction alone group at the post-reacquisition test (F(1,22)=11.782, p=0.002). Analysis of the reactivation + extinction condition alone revealed that there was no evidence of reconditioning as the freezing levels did not differ between the two tests (F(1,11)=3.878, p=0.075).
Fig. 5.
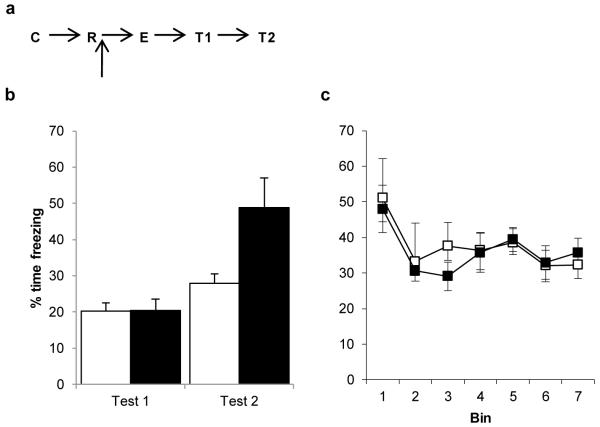
Post-reactivation Nimodipine prevents behavioural memory updating. a, Experimental timeline. Following contextual fear conditioning (C), Nimodipine was injected immediately after memory reactivation (R), and hence 1 hr before extinction (E). b, Subsequent freezing behaviour was compared across the post-reactivation/extinction test (T1/Test 1) and the post-reacquisition test (T2/Test 2). Nimodipine-treated rats (filled columns/symbols) readily reconditioned, whereas vehicle-treated (open columns/symbols) rats showed a persistent impairment in contextual fear. c, Freezing during the extinction session itself did not differ between groups. Contextual freezing was compared across seven 4-min bins corresponding to the final 28 min of extinction after reactivation or the first 2 min of extinction. There were no differences between the groups during extinction (bin × treatment: F(2.0,20.5)=0.321, p=0.733; treatment: F(1,10)=0.374, p=0.554). While there was a trend for both groups to reduce their freezing response to the context over the course of the session, this was not statistically significant (bin: F(2.0,20.5)=5.743, p=0.066). Data presented as mean + SEM. N=6 per group.
Fig. 6.
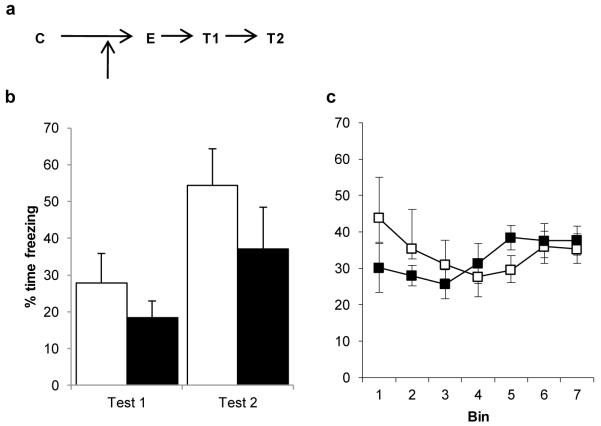
Pre-extinction Nimodipine has no effect upon memory extinction. a, Experimental timeline. Following contextual fear conditioning (C), Nimodipine was injected 1 hr before extinction (E). b, Subsequent freezing behaviour was compared across the post-reactivation/extinction test (T1/Test 1) and the post-reacquisition test (T2/Test 2). Both vehicle- (open columns/symbols) and Nimodipine-treated (filled columns/symbols) rats readily reconditioned. c, Freezing during the extinction session itself did not differ between groups. Contextual freezing was compared across seven 4-min bins corresponding to the final 28 min of extinction after reactivation or the first 2 min of extinction. There were no differences between the groups during extinction (bin × treatment: F(2.2,22.1)=2.038, p=0.150; treatment: F(1,10)=0.020, p=0.889). However, there was also no evidence for a reduction of freezing over the course of the session (bin: F(2.2,22.1)=1.762, p=0.193). Data presented as mean + SEM. N=6 per group.
Fig. 7.
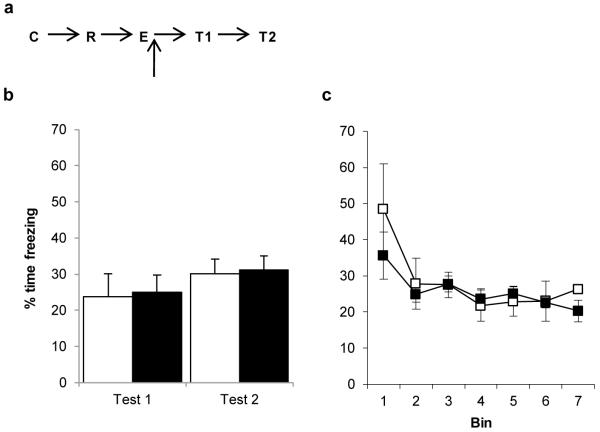
Post-extinction Nimodipine has no effect upon behavioural memory updating. a, Experimental timeline. Following contextual fear conditioning (C) and memory reactivation (R), Nimodipine was injected immediately after extinction (E). b, Subsequent freezing behaviour was compared across the post-reactivation/extinction test (T1/Test 1) and the post-reacquisition test (T2/Test 2). Both vehicle- (open columns/symbols) and Nimodipine-treated (filled columns/symbols) rats readily reconditioned. c, Freezing during the extinction session itself did not differ between groups. Contextual freezing was compared across seven 4-min bins corresponding to the final 28 min of extinction after reactivation or the first 2 min of extinction. There were no differences between the groups during extinction (bin × treatment: F(1.6,15.8)=0.858, p=0.418; treatment: F(1,10)=0.342, p=0.572). Both groups extinguished their freezing response to the context over the course of the session (bin: F(1.6,15.8)=5.743, p=0.018). Data presented as mean + SEM. N=6 per group.
Fig. 8.
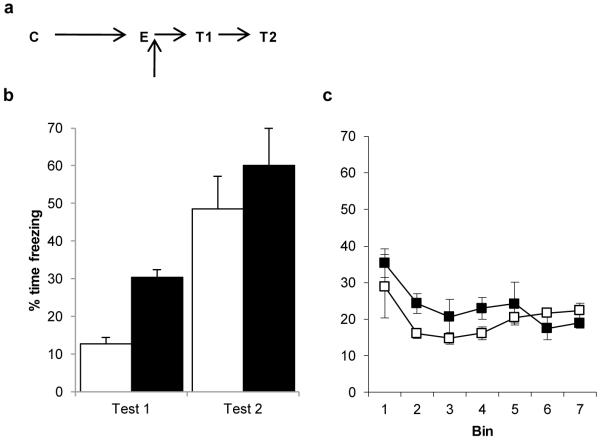
Post-extinction Nimodipine impairs memory extinction. a, Experimental timeline. Following contextual fear conditioning (C), Nimodipine was injected after extinction (E). b, Subsequent freezing behaviour was compared across the post-reactivation/extinction test (T1/Test 1) and the post-reacquisition test (T2/Test 2). Nimodipine-treated rats (filled columns/symbols) were impaired compared to vehicle-treated controls (open columns/symbols) in extinction retention at Test 1. c, Freezing during the extinction session itself did not differ between groups. Contextual freezing was compared across seven 4-min bins corresponding to the final 28 min of extinction after reactivation or the first 2 min of extinction. There were no differences between the groups during extinction (bin × treatment: F(2.4,24.1)=1.421, p=0.262; treatment: F(1,10)=1.608, p=0.234). Both groups showed extinguished their freezing response to the context over the course of the session (bin: F(2.4,24.1)=5.078, p=0.011) Data presented as mean + SEM. N=6 per group.
When nimodipine (16 mg/kg) was injected immediately after memory reactivation, and hence 60 min before the subsequent extinction training, there was a significant disruption of the reactivation + extinction effect upon fear relearning (Fig. 5a; mixed ANOVA; session × treatment: F(1,10)=11.004, p=0.008; treatment: F(1,10)=4.148, p=0.069). The groups did not differ in the levels of contextual fear acquired prior to treatment (vehicle=52.4±11.2, nimodipine=46.9±5.7; one-way ANOVA; F(1,10)=0.229, p=0.643), or during the course of extinction training (Fig. 5b). Moreover, analysis of simple effects with separate ANOVAs revealed that while there was no effect of nimodipine upon freezing at the post-reactivation + extinction test (F(1,10)=0.001, p=0.975), the nimodipine-treated group froze significantly more at the post-reacquisition test (F(1,10)=7.247, p=0.023).
In contrast, injection of nimodipine 60 min before extinction in the extinction-alone condition had no effect upon post-extinction contextual freezing, with both the nimodipine and vehicle groups successfully reacquiring the contextual fear memory to a similar degree (Fig. 6a; mixed ANOVA; session × treatment: F(1,10)=0.382, p=0.550; treatment: F(1,10)=1.517, p=0.246; session: F(1,10)=13.388, p=0.004). Although the rats treated with nimodipine were impaired in the levels of freezing in the first 2 min of extinction (vehicle=64.9±8.7, nimodipine=31.9±8.6; one-way ANOVA; F(1,10)=7.284, p=0.022), they were not impaired in either the acquisition (Fig. 6b) or retention of memory extinction.
When nimodipine was injected immediately after the extinction phase in the reactivation + extinction condition it produced no effect on subsequent contextual freezing and fear memory reacquisition (Fig. 7a; mixed ANOVA; session × treatment: F(1,10)=0.000, p=0.994; treatment: F(1,10)=0.062, p=0.808). A lack of main effect of session (F(1,10)=2.122, p=0.176) revealed that neither group showed an increase in freezing after reacquisition. There were no group differences at memory reactivation (vehicle=44.4±7.6, nimodipine=46.5±7.6; one-way ANOVA; F(1,10)=0.047, p=0.833). Moreover, there was no difference between the groups during extinction training itself (Fig. 7b). Therefore, nimodipine had no impact upon the effect of reactivation + extinction to disrupt the contextual fear memory.
In contrast, nimodipine injection immediately after extinction training impaired the extinction memory (Fig. 8a; mixed ANOVA; session × treatment: F(1,10)=0.275, p=0.611; treatment: F(1,10)=5.067, p=0.048). Given that an impairment in memory extinction would be expected to be most apparent in the post-extinction test, planned one-way ANOVAs analysed the two tests separately. Nimodipine-treated rats froze significantly more than vehicle-treated controls at the post-extinction test (F(1,10)=58.133, p<0.001), but not at the post-reacquisition test (F(1,10)=0.904, p=0.364). There were also no differences between the groups at the start of extinction training, indicating that the differences thereafter were not attributable to pre-existing differences in the level of conditioning (vehicle=38.3±11.2, nimodipine=42.6±4.8; F(1,10)=0.154, p=0.703). Moreover, there was no difference between the groups during extinction training itself (Fig. 8b).
Discussion
The combination of memory reactivation followed 1 hour later by extinction training resulted in a persistent memory impairment in two settings. Firstly, the appetitive pavlovian memory mediating conditioned reinforcement was diminished through several days of testing and reinstatement. Secondly, a contextual fear memory was impaired to the extent that reacquisition was retarded. These effects of reactivation and extinction appear to be mediated by a behavioural updating of the reconsolidation process, rather than a potentiation of extinction.
Memory reconsolidation of sucrose–associated memories in a conditioned reinforcement setting has previously been disrupted by NMDA receptor antagonism using a 10-min reactivation session6. This suggests that the light–food memory in the present study was functionally reactivated by the 10-min reactivation, engaging the reconsolidation process. Adding the extinction training 1 hour later resulted in memory impairment that neither recovered with repeated testing, nor reinstated with pre-test exposure to the food outcome. By contrast, increasing the interval between reactivation and extinction to 6 hours resulted in little evidence of memory impairment. Context re-exposure for 10 min was equally effective in reactivating the appetitive pavlovian. The context re-exposure condition has been used as the control for fear conditioning9, but is clearly inappropriate in the current appetitive setting. The efficacy of context exposure to reactivate memories is mixed. We have previously observed that context exposure does not successfully reactivate a strongly-learned light–cocaine memory, at least as assessed by the amnestic impact of intra-amygdala infusions of Zif268 antisense oligodeoxynucleotides17. However, in human episodic memories, context appears to be the single most important trigger for memories to undergo reconsolidation25. Therefore, the capacity of context exposure to reactivate and destabilise a related discrete memory appears to be variable. Further investigation of this issue will be important, especially given the finding that reconsolidation-based impairments are restricted to the stimulus that is presented at reactivation26, as effective context-mediated reactivation of discrete memories might be particularly useful in targeting sets of stimuli that are important in maintaining maladaptive behaviour.
In contrast to the successful diminishing of appetitive CS–US representations, the combination of reactivation and extinction did not reduce subsequent auditory conditioned freezing. Rather, it seemed to retard or impair extinction relative to an extinction-alone condition. While this observation is in marked contrast to the original demonstration by Monfils et al9, two important points must be noted. First, prior attempts to replicate the findings of Monfils et al9 have yielded contrasting results, with success in mice11, but not in an extensive examination in rats12. Therefore, the conditions under which auditory fear memories can be diminished through reactivation and extinction are poorly understood. Second, the extent of extinction training employed in the present study was intentionally limited in order to replicate the conditions under which DCS potentiates fear memory extinction24. The 10-11 unreinforced CS presentations differs substantially from the 18-19 used by Monfils et al9. Therefore, it is possible that the extent of cued fear extinction training was insufficient to observe the expected effect in the present study. This contrasts with the seemingly more robust extinction training in our contextual fear experiment, which successfully yielded a reactivation + extinction effect.
While it is tempting to characterise the reactivation and extinction impairment as a behavioural updating of the reactivated and reconsolidating memory, the pattern of results is equally consistent with a quantitative and qualitative potentiation of memory extinction, as previously achieved using DCS and FGF-219-21. However, we now have 3 lines of evidence favouring the reconsolidation-based interpretation. First, injecting DCS rather than reactivating the memory prior to appetitive pavlovian extinction training did not affect the subsequent acquisition of a new response with conditioned reinforcement. DCS was able to potentiate appetitive memory extinction, supporting prior observations in other appetitive memory settings27-29, but only when the extinction session was 3 hr long. Moreover, the DCS–induced potentiation of extinction resulted only in a transient deficit in conditioned reinforcement. This spontaneous recovery of the appetitive memory is in marked contrast with the persistent deficit observed following reactivation + extinction. Secondly, for cued fear memories, memory reactivation 1 hour before extinction training and DCS injection in conjunction with the same extinction training produce different effects. This parametric difference between the reactivation + extinction impairment and pharmacological enhancement of extinction is consistent with the interpretation that they are mediated by qualitatively different processes. Finally, we showed that preventing memory reactivation impaired the reactivation + extinction effect in a contextual fear setting.
Using a procedure known to elicit contextual fear memory reconsolidation30, the addition of extinction training 1 hr after memory reactivation seemingly erased the contextual fear memory. This was evidenced by the reduction in freezing levels following reconditioning. Rats that were just extinguished showed high levels of post-reacquisition freezing, likely reflecting a combination of reinstatement of the extinguished memory and the effect of further conditioning. In contrast, rats given reactivation and extinction showed little evidence of reconditioning. This indicates that the original excitatory memory was impaired to the extent that reinstatement was ineffective. Moreover, the failure to recondition suggests that reactivation and extinction may in fact lead to the formation of a memory that the previously-fear conditioned context is now safe9.
LVGCCs are important not only for memory reactivation, but also for the extinction of contextual fear memories22. In order to disambiguate the effects of the LVGCC blocker nimodipine upon memory reactivation and extinction, we administered it after both reactivation and extinction in separate experiments. Nimodipine impaired extinction when given immediately after extinction alone, exactly as previously demonstrated22. In the reactivation + extinction condition, nimodipine was without effect when administered immediately after the extinction phase. When nimodipine was injected 1 hr before extinction it had no effect upon the extinction memory itself, although it did acutely impair memory retrieval. While the acute effect of nimodipine is consistent with some31, but not other32, studies of LVGCCs, the lack of long-term effect on extinction memory suggests that the injection took place too far in advance in order to impact upon extinction learning and consolidation. It has previously been observed that nimodipine injection 20 min before extinction is effective at impairing extinction retention one day later32. However, the alternative antagonist nifedipine has been shown to impair extinction when administered at each of several timepoints up to 4 hours before extinction training31,33. Therefore, a full timecourse analysis of the effects of nimodipine would be required in order to determine the window of vulnerability of contextual fear memory extinction to nimodipine. In contrast to the lack of effect upon extinction alone, nimodipine treatment immediately after memory reactivation, and hence also 60 min prior to the extinction phase, prevented the behavioural memory impairment characteristic of reactivation and extinction. These effects of nimodipine upon post-extinction contextual freezing were not attributable to any differences in extinction learning, as there were no treatment effects upon within-session freezing levels during the extinction sessions themselves. Therefore, the impact of nimodipine was most likely mediated by its effects on extinction consolidation and memory destabilisation.
The divergent impact of nimodipine depending upon the behavioural condition strongly suggests that its effect when given post-reactivation are due to the disruption of memory destabilisation, thereby preventing the later extinction training from behaviourally updating the memory. This means also that the reason nimodipine fails to have an effect when given immediately after the extinction phase of reactivation + extinction is that the contextual fear memory has already been destabilised, and the resultant behavioural updating through non-reinforced context exposure to disrupt the fear memory does not depend upon LVGCC-based mechanisms. This interpretation based upon the dissociable patterns of impairment hold regardless of the true nature of the nimodipine-induced impairment of extinction34. Therefore, the persistent amnestic effect of reactivation and extinction appears to be truly mediated by a behavioural hijacking of the reconsolidation process.
In summary, the present results demonstrate that the memory-impairing impact of reactivation followed by extinction is applicable to appetitive discrete pavlovian memories, as well as aversive contextual fear memories. These effects represent a behavioural method of reconsolidation impairment that utilises the memory-updating function of memory reconsolidation2. Moreover, given that the likely locus of effect of systemically-applied nimodipine is the hippocampus22, these results suggest that reactivation + extinction impacts upon memory mechanisms in the hippocampus as well as in the amygdala11. However, given that the previous demonstrations of persistent cued fear memory disruption with reactivation and extinction9-11 have seemingly failed to be reproduced in a translationally-relevant setting13, it remains to be determined whether the present disruption of pavlovian appetitive food memories will generalise to situations of addictive drug and high-incentive food seeking.
Methods
Subjects
The subjects were 180 experimentally-naive adult male Lister Hooded rats, weighing 200-225g at the start of the experiments. They were housed in groups of 4, in a holding room maintained at 21°C on a normal light cycle (12 hr light: 12 hr dark; lights on at 0700). All rats were given free access to water. Those tested on the appetitive conditioned reinforcement procedure were given restricted access to food (15 g chow per day). Rats that were fear conditioned had free access to food. All procedures were conducted in accordance with the United Kingdom 1986 Animals (Scientific Procedures) Act (PPL 40/3205).
Drug administration
D-cycloserine (DCS) and Nimodipine were both injected intra-peritoneally. DCS was dissolved in saline (15 mg/kg; 1 mg/ml). This dose was effective in potentiating the extinction of fear and drug-associated memories19,24,28,35. Nimodipine (16 mg/kg) was sonicated into 100% Cremophor EL and then diluted to a concentration of 8 mg/ml in a final vehicle of 10% Cremophor and 2.5% DMSO in saline with 1 drop Tween 80 per 3 ml. This dose has previously been demonstrated to impair both the reactivation and extinction of contextual fear memories22,32.
Behavioural Apparatus
The rats were trained and tested in 8 operant chambers (MedAssociates, Vermont) as previously described36. Each chamber had a nosepoke magazine attached to a pellet dispenser, as well as two retractable levers with a stimulus light located above each one. In addition, four of the chambers, used for the fear conditioning experiments, were fitted with clicker modules, shock generators and scramblers, and infra-red video cameras (Viewpoint Life Sciences, France).
Behavioural Procedures
Conditioned reinforcement: In 9 days of appetitive memory acquisition, the rats were trained to nosepoke into the magazine in order to receive a food pellet (45 mg grain-based reward pellet; TestDiet, USA). The delivery of the food pellet was associated with illumination of one of the stimulus lights (right or left, counterbalanced) and extinction of the houselight for 10 s. During this 10-s period, further nosepokes were recorded, but had no programmed consequence. The training sessions lasted 20 min and there was no limit to the number of pellets that could be earned in each session. One group of rats received 2 sessions of context pre-exposure before training began. These sessions were 90 min long on each day, and rats could nosepoke, but there was no programmed consequence. In the day after the final training session, the reactivation and extinction procedures were conducted. Memory reactivation consisted of a 10-min extinction session, in which nosepoke responses were reinforced by the light stimulus, but no pellets were delivered. Extinction was operationally identical, but in a longer session of 60 or 70 min (the latter as the control for total reactivation + extinction time). Groups of rats that were given a context exposure instead of memory reactivation were returned to the operant chambers for 10 min, but nosepokes had no programmed consequence. A final group was subjected to the context exposure, but a transparent Perspex sheet was placed in front of the wall containing the nosepoke magazine and light stimuli in order to prevent the rats from performing the nosepoke response. The rats were returned to their homecages in between reactivation and extinction. For the DCS experiments, there was no memory reactivation or context exposure, and DCS or saline was injected 30 min prior to a 60-min or 180-min extinction session. The testing phase involved the extension of the levers into the chambers for the first time. A response on the active lever on the opposite side to the light stimulus resulted in illumination of the light as a conditioned reinforcer. A response on the inactive lever below the location of the light stimulus, or a nosepoke response, had no programmed consequence. Discriminated responding upon the active lever over and above responding on the inactive lever, over several days of testing is a sensitive measure of the acquired conditioned reinforcing properties of appetitive pavlovian stimuli. The test sessions were 30 min long, and were conducted on 6 occasions over a period of 20 days. For the first 4 sessions, the active lever response was reinforced by the light stimulus under an FR1 schedule. The schedule was increased to FR1-3 for the remaining sessions in order to increase response output and thereby promote the possibility of spontaneous recovery. A final reinstatement test was conducted 7 days after the last test. This session was preceded by a 5-min context exposure, during which the rats were allowed to retrieve and consume 10 pellets that were freely available, in order to test for outcome-induced reinstatement of the diminished memory.
Auditory fear conditioning: Rats were first habituated to the operant chambers for 2 hr on the day before conditioning. For fear conditioning, the rats were again placed in the operant chambers, and after a 30-min habituation period, were subjected to two CS–US pairings with an intertrial interval of 5 min. The CS was an auditory clicker (10 Hz, 80 dB, 60 s) and the US was a mild electric footshock (0.5 mA, 0.5 s). On the day after fear conditioning, the rats were first subjected to a 2-min memory reactivation session, in which the 60-s CS was presented after 60 s. One hour later, the rats received a 20-min extinction session, involving 10 CS presentations with an interstimulus interval of 60 s. The control group received a single 22-min extinction session with 11 CS presentations. No footshocks were delivered in any of the reactivation or extinction sessions. On the next day, the final fear memory test was undertaken, consisting of a single presentation of the 60-s CS after 60 s in a 2-min session. Freezing behaviour was automatically scored throughout all the sessions using Videotrack software (Viewpoint Life Sciences, France).
Contextual fear conditioning: For fear conditioning, the rats were placed into the operant chambers for 3 min, and after 2 min a single footshock (0.5 mA, 2 s) was delivered. On the next day, the contextual fear memory was reactivated by returning the rats to the chamber for 2 min. One hour later, the rats received a 28-min extinction session in the same context. Extinction-alone controls received a single 30-min extinction session. The next day, all rats were tested for contextual fear memory and were also reconditioned using a less intense footshock (0.35 mA, 2 s) to test for savings. This took place in a single 3-min conditioning session, with the initial 2 min exposure serving as the test period, at the end of which the footshock was delivered. Finally, there was a 2-min post-reconditioning test on day 4. The rats were injected with Nimodipine (16 mg/kg) or vehicle either immediately after the extinction phase, or immediately after reactivation/60 min before extinction.
Statistical analysis
The appetitive data were analysed using mixed factorial ANOVA with factors lever (active vs. inactive), session (days of testing) and group (behavioural condition) as appropriate. The data were check for normality, using the Shapiro-Wilk test. The Greenhouse-Geisser correction was employed in all analyses as the assumption of sphericity was always violated. Analysis of the training, reactivation and extinction data consisted of individual one-way ANOVAs on the total number of light–food pairings and the numbers of non-reinforced light presentations across groups. For the freezing data, the % time freezing was analysed using mixed factorial ANOVA with factors session, behavioural condition and treatment (nimodipine vs. vehicle) as appropriate. As the anticipated effects of nimodipine were expected to differ between the two test sessions, planned comparisons for the effect of treatment at each test were carried out. A significance level of p<0.05 was used for all analyses.
Supplementary Material
Acknowledgements
The work was supported by a grant from the Medical Research Council (grant no. G0700991).
Footnotes
Competing Financial Interests
We have no competing financial interests to disclose.
References
- 1.Schacter DL, Norman KA, Koutstaal W. The cognitive neuroscience of constructive memory. Annu Rev Psychol. 1998;49:289–318. doi: 10.1146/annurev.psych.49.1.289. [DOI] [PubMed] [Google Scholar]
- 2.Lee JLC. Reconsolidation: maintaining memory relevance. Trends Neurosci. 2009;32:413–420. doi: 10.1016/j.tins.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- 4.Kindt M, Soeter M, Vervliet B. Beyond extinction: erasing human fear responses and preventing the return of fear. Nat Neurosci. 2009;12:256–258. doi: 10.1038/nn.2271. [DOI] [PubMed] [Google Scholar]
- 5.Lee JLC, Di Ciano P, Thomas KL, Everitt BJ. Disrupting reconsolidation of drug memories reduces cocaine seeking behavior. Neuron. 2005;47:795–801. doi: 10.1016/j.neuron.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Lee JLC, Everitt BJ. Appetitive memory reconsolidation depends upon NMDA receptor-mediated neurotransmission. Neurobiol Learn Mem. 2008;90:147–154. doi: 10.1016/j.nlm.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Brunet A, et al. Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. J Psychiatr Res. 2008;42:503–506. doi: 10.1016/j.jpsychires.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Milton AL, Everitt BJ. The psychological and neurochemical mechanisms of drug memory reconsolidation: implications for the treatment of addiction. Eur J Neurosci. 2010;31:2308–2319. doi: 10.1111/j.1460-9568.2010.07249.x. [DOI] [PubMed] [Google Scholar]
- 9.Monfils MH, Cowansage KK, Klann E, LeDoux JE. Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science. 2009;324:951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schiller D, et al. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2010;463:49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clem RL, Huganir RL. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science. 2010;330:1108–1112. doi: 10.1126/science.1195298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan WY, Leung HT, Westbrook RF, McNally GP. Effects of recent exposure to a conditioned stimulus on extinction of Pavlovian fear conditioning. Learn Mem. 2010;17:512–521. doi: 10.1101/lm.1912510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soeter M, Kindt M. Disrupting Reconsolidation: Pharmacological and Behavioral Manipulations. Learn Mem. 2011;18:357–366. doi: 10.1101/lm.2148511. [DOI] [PubMed] [Google Scholar]
- 14.Everitt BJ, Cardinal RN, Hall J, Parkinson JA, Robbins TW. In: The Amygdala: A Functional Analysis. Aggleton JP, editor. OUP; 2000. pp. 353–390. [Google Scholar]
- 15.Milton AL, Lee JLC, Everitt BJ. Reconsolidation of appetitive memories for both natural and drug reinforcement is dependent on b-adrenergic receptors. Learn Mem. 2008;15:88–92. doi: 10.1101/lm.825008. [DOI] [PubMed] [Google Scholar]
- 16.Milton AL, Lee JLC, Butler VJ, Gardner R, Everitt BJ. Intra-amygdala and systemic antagonism of NMDA receptors prevents the reconsolidation of drug-associated memory and impairs subsequently both novel and previously acquired drug-seeking behaviors. J Neurosci. 2008;28:8230–8237. doi: 10.1523/JNEUROSCI.1723-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JLC, Milton AL, Everitt BJ. Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J Neurosci. 2006;26:5881–5887. doi: 10.1523/JNEUROSCI.0323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JLC, Everitt BJ. Reactivation-dependent amnesia for appetitive memories is determined by the contingency of stimulus presentation. Learn Mem. 2008;15:390–393. doi: 10.1101/lm.976108. [DOI] [PubMed] [Google Scholar]
- 19.Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear- potentiated startle in rats. J Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ledgerwood L, Richardson R, Cranney J. D-cycloserine and the facilitation of extinction of conditioned fear: consequences for reinstatement. Behav Neurosci. 2004;118:505–513. doi: 10.1037/0735-7044.118.3.505. [DOI] [PubMed] [Google Scholar]
- 21.Graham BM, Richardson R. Fibroblast growth factor-2 enhances extinction and reduces renewal of conditioned fear. Neuropsychopharmacology. 2010;35:1348–1355. doi: 10.1038/npp.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki A, Mukawa T, Tsukagoshi A, Frankland PW, Kida S. Activation of LVGCCs and CB1 receptors required for destabilization of reactivated contextual fear memories. Learn Mem. 2008;15:426–433. doi: 10.1101/lm.888808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Ciano P, Everitt BJ. Conditioned reinforcing properties of stimuli paired with self-administered cocaine, heroin or sucrose: implications for the persistence of addictive behaviour. Neuropharmacology. 2004;47:202–213. doi: 10.1016/j.neuropharm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Lee JLC, Milton AL, Everitt BJ. Reconsolidation and extinction of conditioned fear: inhibition and potentiation. J Neurosci. 2006;26:10051–10056. doi: 10.1523/JNEUROSCI.2466-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hupbach A, Hardt O, Gomez R, Nadel L. The dynamics of memory: context-dependent updating. Learn Mem. 2008;15:574–579. doi: 10.1101/lm.1022308. [DOI] [PubMed] [Google Scholar]
- 26.Doyere V, Debiec J, Monfils MH, Schafe GE, Ledoux JE. Synapse-specific reconsolidation of distinct fear memories in the lateral amygdala. Nat Neurosci. 2007;10:414–416. doi: 10.1038/nn1871. [DOI] [PubMed] [Google Scholar]
- 27.Botreau F, Paolone G, Stewart J. d-Cycloserine facilitates extinction of a cocaine-induced conditioned place preference. Behav Brain Res. 2006;172:173–178. doi: 10.1016/j.bbr.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 28.Nic Dhonnchadha BA, et al. D-cycloserine deters reacquisition of cocaine self-administration by augmenting extinction learning. Neuropsychopharmacology. 2010;35:357–367. doi: 10.1038/npp.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thanos PK, Bermeo C, Wang GJ, Volkow ND. D-cycloserine facilitates extinction of cocaine self-administration in rats. Synapse. 2011;65:938–944. doi: 10.1002/syn.20922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JLC, Everitt BJ, Thomas KL. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science. 2004;304:839–843. doi: 10.1126/science.1095760. [DOI] [PubMed] [Google Scholar]
- 31.Waltereit R, Mannhardt S, Nescholta S, Maser-Gluth C, Bartsch D. Selective and protracted effect of nifedipine on fear memory extinction correlates with induced stress response. Learn Mem. 2008;15:348–356. doi: 10.1101/lm.808608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki A, et al. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cain CK, Blouin AM, Barad M. L-type voltage-gated calcium channels are required for extinction, but not for acquisition or expression, of conditional fear in mice. J Neurosci. 2002;22:9113–9121. doi: 10.1523/JNEUROSCI.22-20-09113.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schafe GE. Rethinking the role of L-type voltage-gated calcium channels in fear memory extinction. Learn Mem. 2008;15:324–325. doi: 10.1101/lm.996908. [DOI] [PubMed] [Google Scholar]
- 35.Ledgerwood L, Richardson R, Cranney J. Effects of D-cycloserine on extinction of conditioned freezing. Behav Neurosci. 2003;117:341–349. doi: 10.1037/0735-7044.117.2.341. [DOI] [PubMed] [Google Scholar]
- 36.Lee JLC. Memory reconsolidation mediates the updating of hippocampal memory content. Front Behav Neurosci. 2010;4:168. doi: 10.3389/fnbeh.2010.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.