Coordinated expression of TSG-6 and PTX3 in myelomonocytic cells and MDDC support the hypothesis that these two proteins may have a role in extracellular matrix remodeling at sites of inflammation.
Keywords: extracellular matrix, inflammation, acute-phase reactants, dendritic cells, neutrophils
Abstract
The prototypic long PTX3 is a multifunctional protein involved in innate resistance to pathogens and in controlling inflammation. TSG-6 is a hyaluronan-binding protein that is involved in ECM remodeling and has anti-inflammatory and chondroprotective functions. PTX3 and TSG-6 are coregulated by growth differentiation factor-9 in granulosa cells, where they are produced during the periovulatory period and play essential roles in the incorporation of hyaluronan into the ECM during cumulus expansion. The present study was designed to assess whether PTX3 and TSG-6 are coregulated in leukocytes, in particular, in phagocytes and DC. Monocytes, macrophages, and myeloid DC were found to produce high levels of TSG-6 and PTX3 in response to proinflammatory mediators (LPS or cytokines). Unstimulated neutrophil polymorphonuclear granulocytes expressed high levels of TSG-6 mRNA, but not PTX3 transcript, and stored both proteins in granules. In contrast, endothelial cells expressed substantial amounts of PTX3 mRNA and low levels of TSG-6 transcript under the conditions tested. Anti-inflammatory cytokines, such as IL-4, dampened LPS-induced TSG-6 and PTX3 expression. Divergent effects were observed with IL-10, which synergizes with TLR-mediated PTX3 induction but inhibits LPS-induced TSG-6 transcription. Immunohistochemical analysis confirms the colocalization of the two proteins in inflammatory infiltrates and in endothelial cells of inflamed tissues. Thus, here we show that myelomonocytic cells and MoDC are a major source of TSG-6 and that PTX3 and TSG-6 are coregulated under most of the conditions tested. The coordinated expression of PTX3 and TSG-6 may play a role in ECM remodeling at sites of inflammation.
Introduction
PTXs are a superfamily of evolutionarily conserved proteins that are characterized by the presence of a structural motif termed the PTX domain. Short PTXs, C-reactive protein, and serum amyloid P component are acute-phase proteins in man and mouse, respectively, produced mainly by the liver in response to inflammatory stimuli, such as IL-6 [1]. PTX3 was the first long PTX described as an IL-1β-inducible gene in endothelial cells. A variety of cell types produces PTX3 in response to proinflammatory stimuli; these include endothelial cells, smooth muscle cells, adipocytes, fibroblasts, mononuclear phagocytes, DC, and granulosa cells [1]. Recently, we found that PTX3 is stored in neutrophil-specific granules and undergoes release in response to microbial recognition and inflammatory signals [2]. PTX3 binds selected ligands, such as C1q, and specific microorganisms and microbial moieties, thereby initiating an innate immune response, possibly through complement activation [3–5]. Studies in vivo with PTX3-deficient mice have revealed that PTX3 plays a number of nonredundant roles in innate resistance to selected pathogens [6] and during inflammation [4, 7]. In humans, increased levels of PTX3 are found in different infectious diseases [8, 9], in autoimmune disorders [10, 11], and in inflammatory conditions, reflecting, in particular, the involvement of the vascular bed [12, 13].
TSG-6, also known as TNFAIP6, is a 35-kDa-secreted protein, comprised almost entirely of a Link module and a CUB_C domain, where the former binds to a wide range of ECM components including HA, chondroitin-4-sulfate, and aggrecan (reviewed in refs. [14, 15]), and the latter has been shown recently to bind fibronectin [16]. TSG-6 is up-regulated rapidly in response to proinflammatory cytokines (e.g., IL-1 and TNF-α) and various growth factors, and it is produced by a wide range of cell types, including chondrocytes, synoviocytes, and smooth muscle cells. High levels of TSG-6 are found in the synovial fluids of patients with RA and osteoarthritis and also in sera of individuals with bacterial sepsis and other inflammatory diseases [17, 18]. PTX3 and TSG-6 are expressed by granulosa cells and cumulus cells following the luteinizing hormone surge and are associated with the ECM surrounding the oocyte during the periovulatory period. Moreover, PTX3 binds to TSG-6 and to IαI and is believed to form a multimolecular complex that might support HA cross-linking [19, 20]. These three proteins have crucial roles in the structural organization of cumulus oophorus ECM, which is essential for in vivo fertilization. Indeed, the deficiency of any one of these three molecules causes defective cumulus oophorus expansion and female subfertility or sterility as a result of defective in vivo fertilization [19, 21–23]. PTX3 and TSG-6 transcripts were also found to be induced in decidual stromal cells by the trophoblast in in vitro systems that mimic the alteration of the local immune environment induced by the trophoblast in the process of embryo implantation [24, 25]; however, their role in this context has not been elucidated yet.
TSG-6 has been implicated in the process of HA cross-linking, which might potentially occur through a number of different mechanisms, first, by acting as cofactor and catalyst in the covalent transfer of the HC of IαI onto HA (thereby forming HC•HA complexes, which are more aggregated than free HA [26, 27]), and second, more speculatively, by forming multimolecular complexes with PTX3 that act as foci for the attachment of multiple HA chains via the Link module of TSG-6 [19, 28]. It has been shown recently that HC • HA complexes are likely to associate directly with PTX3, leading to an alternative method of cross-linking [20]. Besides its role in HA cross-linking, TSG-6 has anti-inflammatory and chondroprotective roles in arthritis [29–31], which might relate to its abilities to potentiate the anti-plasmin and anti-tissue kallicrein activities of IαI [31–35] and to inhibit neutrophil migration [36–38].
The aim of this study was to analyze the expression and coregulation of TSG-6 and PTX3 in different cell types and in particular, in myelomonocytic cells, which are the major producers of PTX3 [39]. Here, we show that myelomonocytic cells and MoDC are a major source of TSG-6 and that PTX3 and TSG-6 are coregulated under most of the conditions tested. The coordinated expression of PTX3 and TSG-6 may play a role in ECM remodeling at sites of inflammation.
MATERIALS AND METHODS
Reagents
For cell culture, we used the following reagents: RPMI-1640 medium (Biochrom, Berlin, Germany), L-glutamine (Biochrom), and aseptically collected FCS (Hyclone Laboratories, Logan, UT, USA). hIL-13 was a gift from Dr. Adrian Minty (Sanofi Elf Bio Recherches, Labege, France), and rhGM-CSF was a gift from Novartis (Milan, Italy). rhM-CSF was from Peprotech (London, UK). LPS from Escherichia coli strain 055:B5 was obtained from Sigma-Aldrich (St. Louis, MO, USA). rhIL-1β was from Dompè (L'Aquila, Italy). rhIFN-γ and IL-4 were purchased from Peprotech. rhIL-10 was kindly provided by Prof. Giorgio Trinchieri (Schering Plough, Kenilworth, NJ, USA). Actinomycin D (Sigma-Aldrich) was used at 1 μg/ml. All reagents contained <0.125 endotoxin unit/ml as checked by the Limulus amoebocyte lysate assay (BioWhittaker, Inc., Walkersville, MD, USA).
Cell culture
Monocytes were isolated from fresh buffy coats of healthy donors (Centro Trasfusionale Ospedale Niguarda, Milan, Italy) using Ficoll (Biochrom) and Percoll (Amersham, Uppsala, Sweden). Nonadherent cells were discarded, and the purified monocytes were incubated with different stimuli as indicated. To obtain MoDC, purified monocytes were cultured for 6 days at 106 cells/ml with 50 ng/ml GM-CSF and 20 ng/ml IL-13 [40]. To obtain macrophages, monocytes were cultured at 2 × 106 cell/ml in Petriperm dishes (Petriperm Hydrophobic, Heraeus Instruments GmbH, Munich, Germany) for 5 days with 40 ng/ml M-CSF [39]. Circulating human lymphocytes and PMN were obtained from peripheral blood of healthy donors. Cells were separated by Percoll gradient after centrifugation as described [2]. Purity, determined by FACS analysis on forward-scatter/side-scatter parameters, was routinely >98%. T lymphocytes were stimulated for 48 h with 100 U/ml PHA. NK cells were obtained through Ficoll gradient, followed by monocyte depletion and discontinuous Percoll gradient. Dr. Angela Santoni (University “La Sapienza,” Rome, Italy) provided primary cells prepared in this way, as well as NK 925 and YT6 NK cell lines. Total and large B cells were prepared from tonsils as described in ref. [41]. HUVEC were obtained as described in ref. [42] and cultured with LPS (10 ng/ml) or IL-1β (20 ng/ml). HDMEC were obtained from Dr. Moshe Arditi (Cedars-Sinai Medical Center and UCLA School of Medicine, Los Angeles, CA, USA) [43]. The human lung fibroblast cell line W138 was obtained from American Type Culture Collection (Manassas, VA, USA). Dr. Mario Luppi (University of Modena and Reggio Emilia, Italy) provided A549, a human lung carcinoma cell line, and BEAS-2B, a nontumorogenic bronchial epithelial cell line. Cells were cultured in RPMI-1640 medium containing 10% (v/v) FCS.
To study PTX3 and TSG-6 expression, cells were plated at 106 cell/ml, 3 ml/well and incubated for the times indicated with the following reagents: LPS (100, 10, or 1 ng/ml), IFN-γ (5000 or 500 U/ml), IL-13, IL-10, or IL-4 (20 ng/ml for all). LPS was added to relevant cultures 30 min after exposure to other stimuli. Three to four donors were tested for each condition.
Northern blot analysis
Total RNA was extracted using TRIzol, according to the manufacturer's instructions (Invitrogen, Life Technologies, Carlsbad, CA, USA), blotted, and hybridized as described [39]. The probes hPTX3, a full-length hPTX3 cDNA, and hTSG-6, a cDNA corresponding to the Link module of hTSG-6 (Link_TSG6) [44], were labeled with (α-32P) dCTP (3000 Ci/mmol) (Amersham, Buckinghamshire, UK) using the Megaprime DNA labeling system (Amersham, Buckinghamshire, UK).
Detection of TSG-6 and PTX3 by ELISA and Western blot
To determine the presence of TSG-6 or PTX3 in supernatants, cells were cultured in RPMI-1640 medium containing 3% (v/v) FCS (106/ml DC and 5×106/ml PMN). To detect TSG-6 by ELISA, 96-well Maxisorp plates (Nunc, Denmark) were coated overnight at room temperature with 125 ng/well rat anti-hTSG-6 mAb (A38) [45] in 20 mM Na2CO3, pH 9.6. Plates were washed three times with PBS/0.05% (v/v) Tween-20 after this and all subsequent steps. Nonspecific sites were blocked with 0.25% (w/v) BSA in PBS/0.05% (v/v) Tween-20 (blocking buffer) for 1 h at 37°C. Wells were then incubated for 2 h at room temperature with dilutions of rhTSG-6 [46] in blocking buffer (50 μl/well) or with DC or PMN supernatants (100 μl/well), followed by 10 ng/well biotinylated goat anti-hTSG-6 antibody (R&D Systems, Minneapolis, MN, USA) in blocking buffer for 90 min at room temperature. Bound antibody was detected by incubation for 30 min with ExtrAvidin alkaline phosphatase (Sigma-Aldrich), diluted 1:10,000 in blocking buffer and then with Sigma Fast p-nitrophenyl phosphate. Absorbance values at 405 nm were read after 45 min.
For Western blot analysis, DC culture supernatants (1 ml) were mixed with 10 μl Strataclean resin (Stratagene, La Jolla, CA, USA) for 30 s at room temperature. The resin was collected by centrifugation, and proteins were fractionated on 10% (w/v) Tris/Tricine/SDS-polyacrylamide gels following reduction with 5% (v/v) β-ME in SDS protein sample buffer (5 min at 100°C) and then transferred onto polyvinylidene difluoride membrane (Amersham). Aliquots, 50 ng-purified rhTSG-6, were run as controls. TSG-6 was detected on Western blots using biotinylated R-C21 antibody [1:2000 dilution; raised against residues 135–150 of murine TSG-6 and kindly provided by Prof. Katalin Mikecz (Chicago, IL, USA], followed by HRP-conjugated strepavidin (GE Healthcare, Waukesha, WI, USA) and ECL Advance detection reagents (GE Healthcare).
ELISA for PTX3 was performed as described [2].
Detection of TSG-6 and PTX3 by confocal microscopy
Neutrophils were freshly isolated or cultured in the presence of 100 ng/ml LPS for 30 min or 4 h and seeded on precoated (poly-L-lysine) glass coverslips or cytospun, fixed with 4% (v/v) PFA and permeabilized for 5 min with 0.3% (v/v) Triton (Sigma-Aldrich) and 0.1% (v/v) SDS in PBS, pH 7.4, before incubation for 1 h at 4°C with 10% (v/v) normal goat serum (Sigma-Aldrich) and glycine 0.1% (v/v) in PBS. Specimens were incubated with 1 μg/ml affinity-purified polyclonal RAH-1 [47] or purified preimmune rabbit serum as control, followed by incubation with Alexa Fluor 488-conjugated anti-rabbit IgG (1:2000, Molecular Probes, Eugene, OR, USA). Cells were postfixed with 1% (v/v) PFA for 5 min at room temperature, followed by incubation with biotin-conjugated, affinity-purified rabbit IgG against hPTX3 (0.5 μg/ml) [2], mouse anti-lactoferrin mAb (1 mg/ml; HyCult Biotechnology, Uden, The Netherlands), or IgG isotype control mAb for 1 h at 4°C. After each step, cells were washed with 0.2% (v/v) BSA and 0.05% (v/v) Tween-20 in PBS (pH 7.4). Sections were mounted with FluorSave™ reagent (Calbiochem, San Diego, CA, USA) and analyzed with an Olympus Fluoview FV1000 laser-scanning confocal microscope. Images (1024×1024 pixels) were acquired with a 100× 1.4 NA Plan-Apochromat oil immersion objective (Olympus, Hamburg, Germany).
Immunohistochemistry
The expression patterns of PTX3 and TSG-6 were studied by immunohistochemistry in colon tissues from two patients with inflammatory bowel disease. Samples were collected and frozen in liquid nitrogen. Sections (9 μm) were cut and mounted on poly-L-lysine-coated slides. After fixation with acetone/chloroform for 3 min, sections were incubated for 2 h with the following antibodies: affinity-purified rabbit IgG against hPTX3 (250 ng/ml) [2] and polyclonal RAH-1 (7 μg/ml) [47]. Binding to target protein was determined using the nonbiotin peroxidase detection system with 3,3′ diaminobenzidine freebase as chromogen. The primary antibody was omitted from negative controls. TSG-6-producing cells were identified using cytological and morphological parameters and by performing double-immunohistochemical reactions on consecutive slides with polyclonal rabbit anti-hTSG-6 along with monoclonal mouse anti-hCD15 (Dako, Denmark, to detect PMN), CD68 (Dako, to detect macrophages), or CD31 (Dako, to detect endothelial cells). TSG-6 was visualized in red (with Alexa Fluor 594-conjugated goat anti-rabbit as secondary antibody, Molecular Probes), and the anti-PMN, -macrophages, and -endothelial cells were developed in green (using Alexa Fluor 488-conjugated goat anti-mouse as secondary antibody, Invitrogen-Molecular Probes). Nuclei were stained with 4′,6-diamidino-2-phenylindole (Invitrogen-Molecular Probes). Slides were mounted with FluorSave (Calbiochem) and analyzed by immunofluorescence microscopy.
RESULTS
Expression of TSG-6 and PTX3 mRNA in human cells
To study TSG-6 and PTX3 mRNA expression in human cells, we performed Northern blot analysis of total RNA using radiolabeled hTSG-6 and hPTX3 cDNAs as probes. When comparing TSG-6 and PTX3 expression under identical conditions, blots were first hybridized with the TSG-6 probe and then, after stripping, with the PTX3 probe.
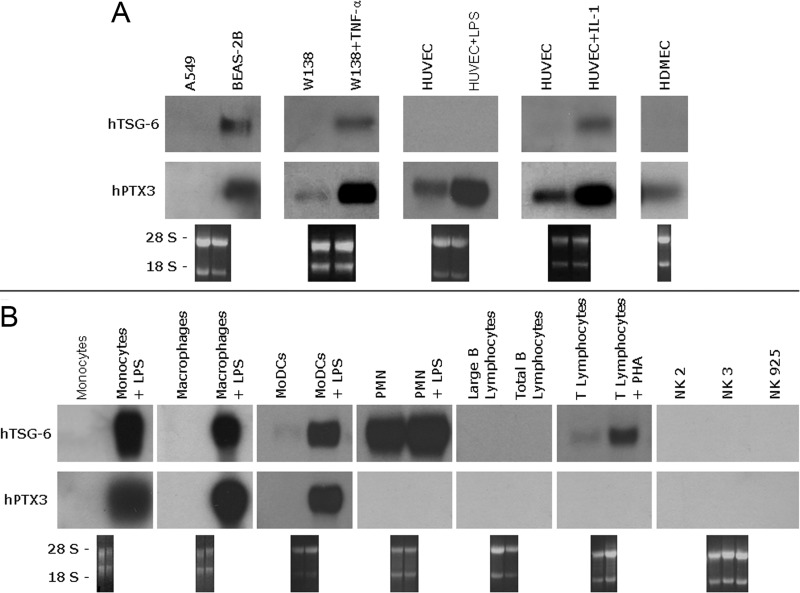
Analysis of material from unstimulated cell lines (Fig. 1, A and B) generally revealed little or no expression of TSG-6 or PTX3 mRNA. TSG-6 was detectable only in the bronchial epithelial cell line BEAS-2B (Fig. 1A), and PTX3 was evident in endothelial cells (HUVEC and HDMEC) and fibroblasts as well as BEAS-2B cells. TNF-α-treated fibroblasts and IL-1β-treated HUVECs showed up-regulation of PTX3 and to a lesser extent, TSG-6 mRNA, and LPS induced the production of PTX3 but not TSG-6 in HUVECs.
Figure 1. TSG-6 and PTX3 expression in different human cell lines and leukocyte subpopulations.
(A) Total RNA was extracted using TRIzol from bronchial epithelial cells (A549), transformed bronchial epithelial cells (BEAS-2B), fibroblasts (W138), and endothelial cells (HUVEC, HDMEC), cultured in the presence of LPS, TNF-α, or IL-1β where specified and analyzed by Northern blotting. Total RNA (10 μg) was run in each lane, and the lower panels show the ethidium bromide-stained membranes to confirm mRNA transfer. Blots were first hybridized with a human Link_TSG6 cDNA probe (hTSG-6) and then, after stripping, with hPTX3. (B) Total RNA was extracted from freshly isolated human monocytes, cultured macrophages, MoDC, PMN, B and T lymphocytes, and NK cells. Where indicated, cells were incubated with LPS (10 ng/ml) for 4 h or in the case of lymphocytes, with PHA (100 U/ml) for 48 h. Northern blot analysis with hTSG-6 and hPTX3 probes was performed as described for A.
To compare TSG-6 and PTX3 expression in leukocyte populations, we isolated fresh human monocytes, T lymphocytes, NK cells, and PMN from the peripheral blood of healthy donors. Macrophages and MoDC were differentiated from monocytes as described above, and B cells were prepared from tonsils. As shown in Figure 1B, under resting conditions, there was no evidence of TSG-6 transcript in monocytes, macrophages, circulating B cells, large B cells from tonsils, or NK cells (freshly isolated or cultured cell lines). In contrast, freshly isolated PMN exhibited a strong signal for TSG-6 mRNA, which almost disappeared after 20 h in culture (see Fig. 2). T lymphocytes and to a lesser extent, immature MoDC also expressed low levels of TSG-6 mRNA (Fig. 1B). None of the cell types tested expressed PTX3 mRNA in resting conditions (Fig. 1B).
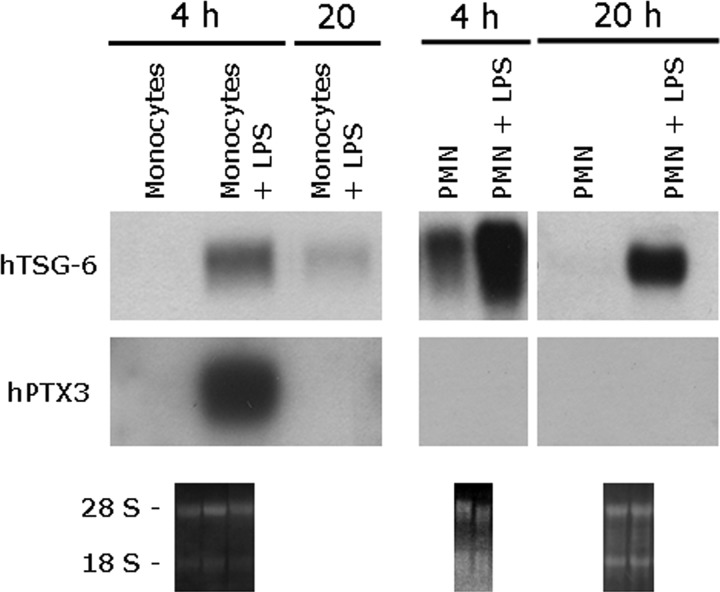
Figure 2. Kinetics of PTX3 and TSG-6 expression in human monocytes and PMN.
Monocytes and PMN were isolated from buffy coats and incubated with LPS (100 ng/ml) for 4 h or 20 h. RNA was extracted, and the expression of TSG-6 was analyzed by Northern blot analysis. Membranes were subsequently stripped and hybridized with the PTX3 probe. The lower panels show ethidium bromide-stained membranes.
The stimulation of lymphocytes with PHA induced TSG-6 expression but had no effect on PTX3 (Fig. 1B). In contrast, TSG-6 and PTX3 transcripts were induced in monocytes, macrophages, and MoDC following the engagement of TLR4 by LPS (see Figs. 1B and 2). In MoDC, which are the major producers of PTX3 upon TLR engagement [39], PTX3 and TSG-6 were induced by LPS in a dose-dependent manner with expression being detectable from a dose of 1 ng/ml (see below). Analysis of the kinetics of TSG-6 and PTX3 expression in monocytes after LPS stimulation (Fig. 2) revealed that the induction of both mRNAs was rapid and transient, peaking at 4 h and being strongly reduced after 20 h of stimulation. LPS also caused up-regulation of TSG-6 expression in PMN after 4 h of incubation, which persisted after 20 h (Fig. 2); PTX3 message was not detected in PMN in response to any of the stimuli tested, as expected [2], and was not studied further.
Regulation of TSG-6 and PTX3 expression by pro- and anti-inflammatory stimuli
We next analyzed whether LPS-induced TSG-6 and PTX3 mRNA levels in leukocytes were comodulated by a series of pro- and anti-inflammatory cytokines.
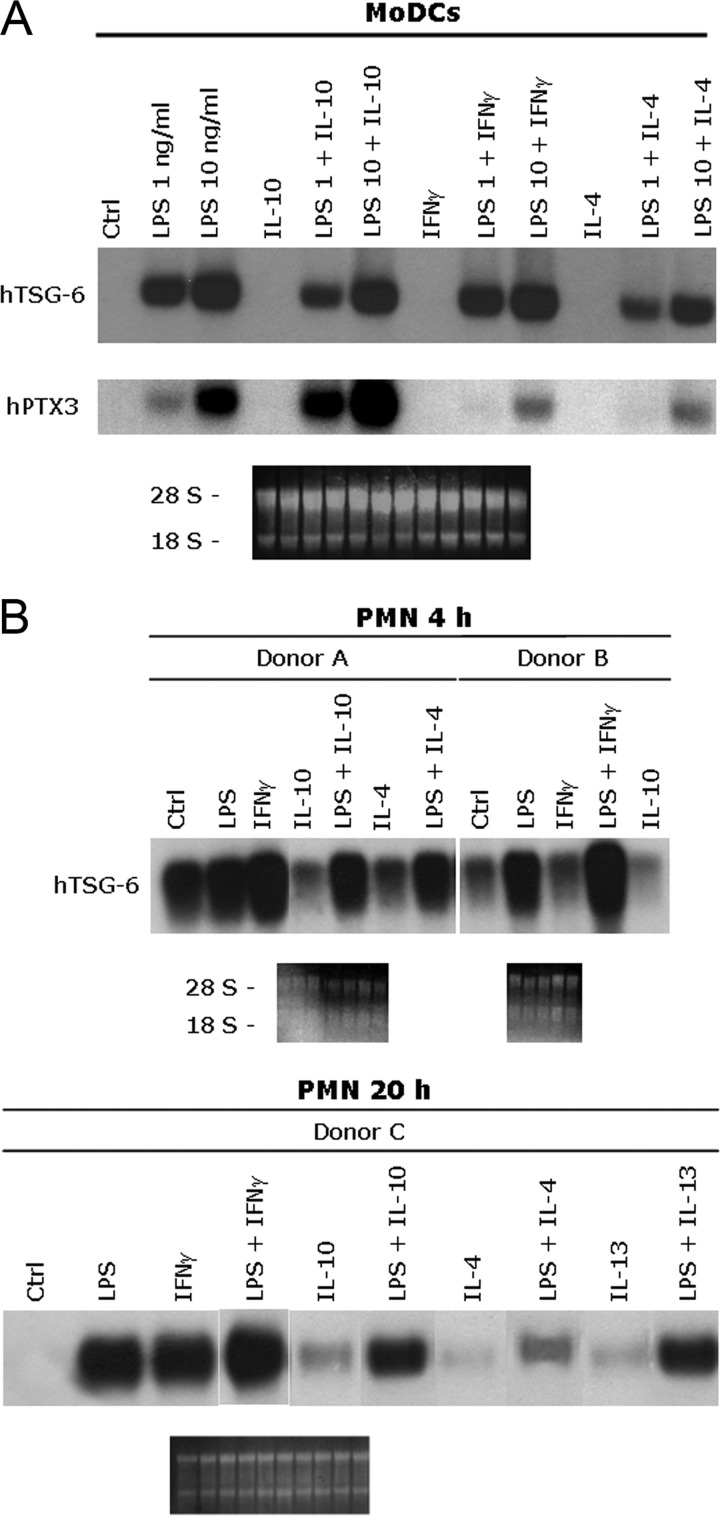
Treatment with IL-4 alone did not modify TSG-6 or PTX3 expression in monocytes (not shown), MoDC, or PMN (Fig. 3). However, IL-4 was seen to inhibit LPS-induced expression of TSG-6 and PTX3 in MoDC (Fig. 3A) and less efficiently, in monocytes (not shown). In MoDC, this inhibitory effect of IL-4 was particularly strong in the case of PTX3, which was barely detectable when the high or low doses of LPS (10 and 1 ng/ml, respectively) were used. TSG-6 was strongly induced in MoDC even with the lower dose of LPS, and the inhibitory effect of IL-4 was substantially less than that seen for PTX3 (Fig. 3A). In PMN incubated for 4 h with LPS and IL-4, the TSG-6 mRNA level was only reduced slightly compared with that in cells treated with LPS alone (Fig. 3B). However, after 20 h incubation, IL-4-mediated inhibition of this LPS activity was pronounced. PMN were also treated with IL-13, which inhibited LPS-induced TSG-6 message expression, although to a lesser extent than IL-4 (Fig. 3B). These, along with previous results for PTX3 [40], suggest that TSG-6 and PTX3 are coregulated by the anti-inflammatory cytokines IL-4 and IL-13 in leukocytes.
Figure 3. Comparison of TSG-6 and PTX3 expression in human leukocytes stimulated with pro- and anti-inflammatory stimuli.
(A) MoDC were differentiated from monocytes and incubated for 4 h with the stimuli indicated; two different concentrations of LPS were used (1 and 10 ng/ml), where this was added to the cultures after 30 min preincubation with IL-10, IL-4 (20 ng/ml), or IFN-γ (5000 U/ml). After total mRNA extraction, TSG-6 and PTX3 expression was determined by Northern blot analysis. The lower panels show the ethidium bromide-stained membranes. The figure shows PTX3 and TSG-6 expression in MoDC in one donor out of the three tested, where these all gave similar results. (B) hPMN were incubated for 4 h or 20 h with LPS (100 ng/ml), alone or in combination with IFN-γ (5000 U/ml), IL-10, IL-4, or IL-13 (20 ng/ml), where the latter was added to cultures 30 min prior to LPS. Ctrl, Control.
It has been demonstrated that in apparent contrast to its general anti-inflammatory role, IL-10 alone slightly induces PTX3 mRNA expression in monocytes and DC; furthermore, it synergizes with LPS in up-regulating PTX3 transcript and protein levels [40, 48]. IL-10 was not able to induce TSG-6 expression in monocytes (not shown) or MoDC (Fig. 3A) or PMN (Fig. 3B) and actually exerted a slight negative effect on LPS-induced TSG-6 transcription in monocytes (not shown), MoDC, and PMN (Fig. 3, A and B).
IFN-γ is a negative regulator of TLR-mediated induction of PTX3 transcription and protein release [49]. Therefore, it is not surprising that IFN-γ alone has no effect on PTX3 mRNA production and that it down-regulates LPS-induced transcription in MoDC (Fig. 3A). Interestingly, LPS-induced TSG-6 expression was variably modulated by IFN-γ, depending on the cell type analyzed; we observed some induction of TSG-6 message by IFN-γ alone in monocytes (not shown) and to a greater extent in PMN after 20 h of incubation and some enhancement of LPS-induced transcription in PMN (Fig. 3B). In macrophages (not shown) and MoDC (Fig. 3A), IFN-γ had no effect on TSG-6 transcription, alone or in combination with LPS.
Release of TSG-6 by MoDC and PMN
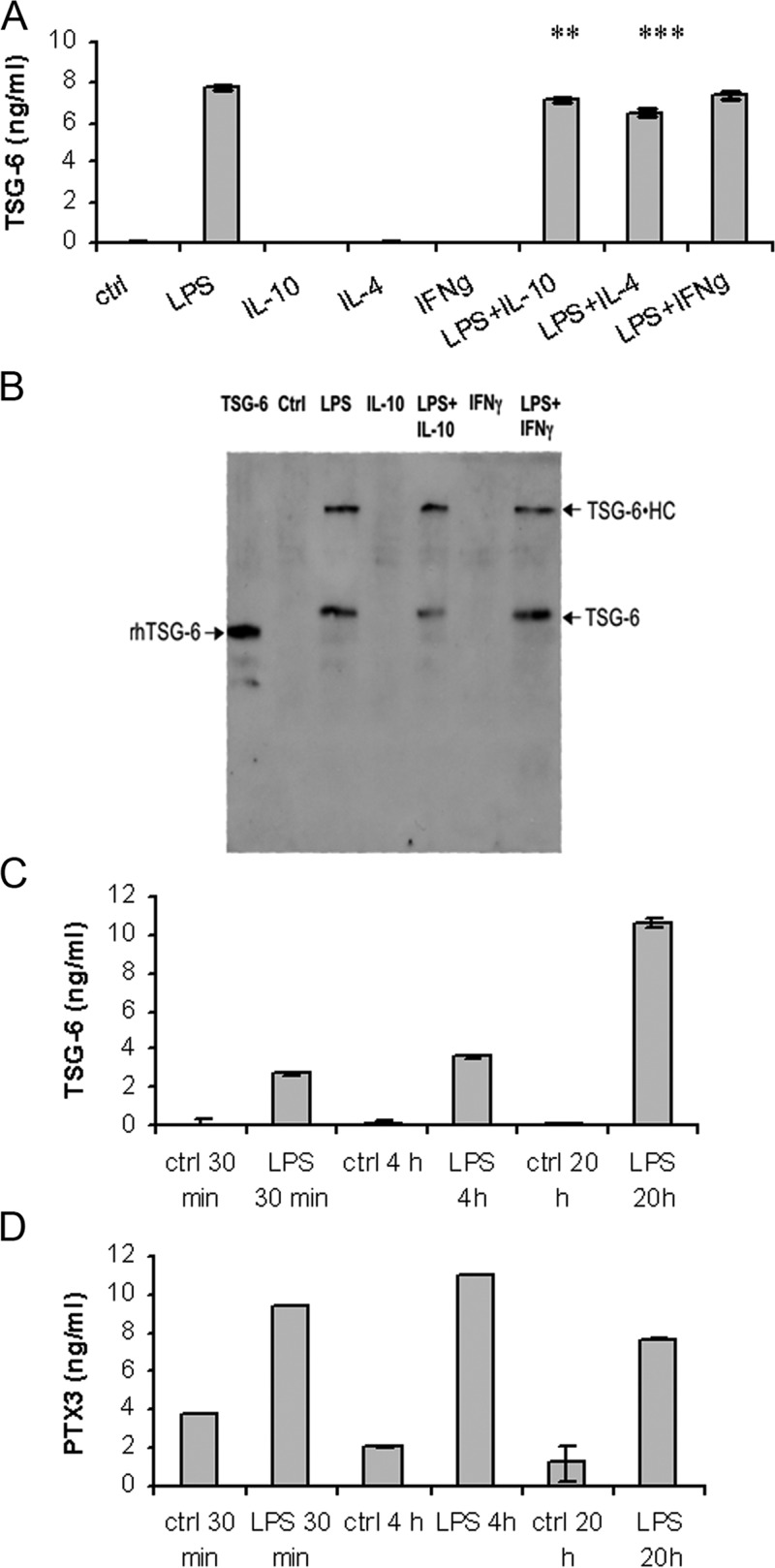
MoDC have been studied extensively in relation to the production of PTX3 upon TLR engagement and the regulation of this effect by pro- and anti-inflammatory cytokines [39, 40]. As illustrated in Figure 4A, which shows one out of three experiments performed, we measured TSG-6 levels in MoDC supernatants, cultured for 24 h with LPS as an inflammatory stimulus in the absence or presence of IL-4, IL-10, or IFN-γ as modulators. The secretion of TSG-6 into the supernatants of LPS-treated MoDC was confirmed by Western blot analysis (Fig. 4B); in addition to free TSG-6 protein (∼35 kDa), a species of ∼120 kDa, which is likely to correspond with a covalent complex of IαI HC with TSG-6 (i.e., TSG-6•HC), was also detected as a result of the presence of bovine serum in the culture medium [27, 50, 51]. In agreement with the mRNA data (Fig. 3A), LPS-induced TSG-6 release was not modified by treatment with IFN-γ. IL-4 and IL-10 had small but significant inhibitory effects on LPS-induced TSG-6 expression. IFN-γ, IL-4, or IL-10 alone did not induce protein secretion.
Figure 4. Secretion of TSG-6 by DC and neutrophils.
(A) TSG-6 was detected in DC culture supernatants by sandwich ELISA, using A38 as capture antibody and biotinylated anti-TSG-6 (R&D Systems) for detection. Values from one donor out of three tested are shown. Values are plotted as ng/ml (n=4) ± se. **, P < 0.01; ***, P < 0.001 (Student's t-test). (B) Proteins from 1 ml aliquots of DC culture supernatants (or rhTSG-6 as control) were fractionated on SDS-PAGE, and TSG-6 was detected on Western blots using biotinylated R-C21 antibody. Samples shown are 50 ng rhTSG-6 and supernatants from untreated DC (control) and cells treated with LPS (10 ng/ml), IL-10 (20 ng/ml), LPS + IL-10, IFN-γ (5000 U/ml), or LPS + IFN-γ. Free TSG-6 (∼35 kDa) and TSG-6 • HC complexes (∼120 kDa) are detectable in the LPS-treated samples. (C) TSG-6 and (D) PTX3 were detected in PMN culture supernatants (5×106 cells/ml) by sandwich ELISA at 30 min, 4 h, and 20 h after LPS stimulation (100 ng/ml). TSG-6 and PTX3 values are plotted as ng/ml (n=4) ± se. Data for one donor out of five tested are shown.
As shown in Figure 4, C and D, which illustrates the results for one out of five donors tested, TSG-6 as well as PTX3 are released by PMN upon engagement of TLR4 by LPS. The kinetics of TSG-6 release is in agreement with transcription data and with experiments performed in the presence of actinomycin D (not shown), which suggest degranulation followed by de novo transcription. As reported previously [2], the time scale of the PTX3 increase in the supernatant indicates rapid release and absence of transcription.
Localization of TSG-6 in neutrophils
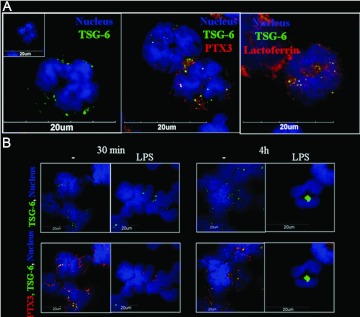
PTX3 is stored in neutrophil-specific granules [2]. Here, confocal microscopy was used to identify the localization of TSG-6 within freshly isolated and cultured neutrophils. The images shown in Figure 5A point to the presence of TSG-6 in PMN granules of resting PMN, where it colocalizes partially with PTX3 in lactoferrin-positive PMN granules, i.e., specific granules. Thirty minutes after LPS treatment, TSG-6 and PTX3 staining in granules was reduced, suggesting release by degranulation (Fig. 5B). After 4 h, immunoreactivity for TSG-6 in granules had disappeared but was now present in the cytoplasm, localized in vesicular structures (Fig. 5B). In contrast, no immunoreactivity for PTX3 was observed at this time-point after LPS treatment. These results are in agreement with the mRNA studies described above, which indicated LPS-induced TSG-6 transcription; on the other hand, PTX3 mRNA is expressed only at the promyelocytic stage, where the protein is stored in granules and released upon TLR engagement [2].
Figure 5. Localization of TSG-6 and PTX3 within neutrophil granules.
Neutrophils were freshly isolated (A) or cultured in the presence of 100 ng/ml LPS for 30 min or 4 h (B). Cells were fixed and stained for hTSG-6 (green), PTX3 (red), or lactoferrin (red; see Materials and Methods). Nuclei were stained with Hoechst 33258. A representative cell is shown in each panel. Specific granules were identified as lactoferrin positive.
Immunohistochemistry
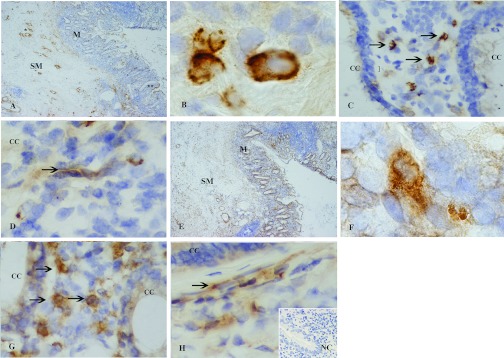
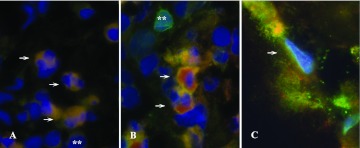
To assess PTX3 and TSG-6 localization in inflamed tissues in vivo, their expression was analyzed by immunohistochemistry in biopsies of colon mucosa from patients with inflammatory bowel disease. As shown in Figure 6, a huge inflammatory infiltrate, involving the mucosa and the superficial layers of the submucosa, is associated with detachment and distortion of the crypts. PTX3 expression (Fig. 6A) is observed in the inflammatory cells of the lamina propria and also beneath the surface mucosa and in the perivascular spaces, with an interstitial and extracellular pattern of distribution. TSG-6 staining (Fig. 6E) is diffusely localized in the inflammatory infiltrate in the mucosa and submucosa. PTX3 and TSG-6 were observed in the cytoplasm of macrophages (Fig. 6, B and F), granulocytes (Fig. 6, C and G), and in some endothelial cells of the lamina propria (Fig. 6, D and H). For both proteins, no immunostaining was observed in the absence of primary antibodies. Double immunofluorescence analysis indicated that there was TSG-6 protein expression in CD15+ neutrophils (Fig. 7A), CD68+ macrophages (Fig. 7B), and CD31+ endothelial cells (Fig. 7C).
Figure 6. PTX3 and TSG-6 are expressed at inflammatory sites in vivo.
PTX3 (A–D) and TSG-6 (E–H) were determined by immunohistochemistry using 3′-diaminobenzidine tetrahydrochloride detection with hematoxylin counterstaining in colon mucosa from a patient with inflammatory bowel disease. (A) PTX3 expression in the inflammatory cells of the lamina propria, beneath the surface mucosa (**) and in perivascular spaces (*). (E) TSG-6 staining in the inflammatory infiltrate in the mucosa (M) and in the submucosa (SM). (B–D and F–H) Cellular localization of PTX3 and TSG-6 in the cytoplasm of macrophages (B and F), granulocytes (C and G, arrows), and in some endothelial cells of the lamina propria (D and H, arrows). No immunochemical signal was observed when the primary antibody was omitted. CC, Colonic crypts; NC, negative control. The original magnifications used are: ×4 (A and E), ×100 (B and F), and ×40 (C, D, G, and H).
Figure 7. Cellular localization of PTX3 and TSG-6 by immunofluorescence.
Double immunofluorescence analysis of TSG-6 expression by neutrophils (A), macrophages (B), and endothelial cells (C, arrows). TSG-6 is denoted in red and CD15, CD68, and CD31 in green. **, Rare granulocytes (A) and macrophages (B) not producing TSG-6. The original magnification used is ×100.
These results suggest that PTX3 and TSG-6 are expressed in inflammatory conditions in vivo by leukocytes and endothelial cells.
DISCUSSION
PTX3 is a multifunctional soluble pattern recognition receptor involved in inflammation and innate immunity [1]. Similarly, TSG-6, which has anti-inflammatory and chondroprotective effects, is produced in the context of inflammatory processes and diseases (reviewed in refs. [14, 15]). Both of these proteins are up-regulated specifically during cumulus oophorus expansion in response to hormonal ovulatory stimuli and oocyte soluble factors, such as the TGF-β family member growth differentiation factor-9 [21]. PTX3 and TSG-6 colocalize in the cumulus matrix and play a crucial role in cumulus expansion, where deficiency of either protein causes instability of the cumulus ECM as a result of defective hyaluronan incorporation and female infertility [19, 22]. The results reported here provide new insights into the expression of the TSG-6 and PTX3 genes in various cell types, including different leukocyte populations, in response to pro- and anti-inflammatory mediators and demonstrate the expression of both proteins in leukocytes and endothelial cells in inflammatory conditions.
In this study, we generally observed little or no basal expression of TSG-6 or PTX3 mRNA in any of the cell types analyzed, with the exception of TSG-6 in neutrophils. However, both mRNAs were induced rapidly by proinflammatory stimuli, consistent with their involvement in inflammation and innate immune responses.
Our attention has been focused particularly on leukocytes, as although these cells are known to be a major source of PTX3, TSG-6 has not been studied extensively in this context [14]. TSG-6 has emerged, in expression-profiling analyses, among the inflammation-inducible genes in leukocytes [52, 53], but to our knowledge, after the original description by Lee and colleagues [54], who observed TSG-6 mRNA in PBMCs after treatment with TNF, Con A, or PHA, this is the first systematic analysis of TSG-6 expression and regulation in leukocyte subpopulations.
Mononuclear phagocytes responded to LPS engagement of TLR4 by rapidly producing TSG-6 and PTX3 in a dose-dependent manner. Thus, similarly to PTX3, TSG-6 behaves as an immediate early gene in leukocytes. In particular, we observed high levels of TSG-6 mRNA in monocytes, macrophages, and MoDC, which was rapidly detectable even upon stimulation with low levels of LPS, and this result was confirmed at the protein level, in vitro and in inflamed tissues. Freshly isolated PMN were the only cell type analyzed that had high basal levels of TSG-6 mRNA expression; this was elevated following treatment with LPS but was lost after 20 h in culture without stimulation. In contrast, PMN incubated with LPS and/or IFN-γ maintained high levels of TSG-6 mRNA after 20 h of culture.
PTX3 mRNA is not detectable in mature PMN under any of the conditions analyzed, whereas it is produced by pro-myelocytes [2]; the protein is stored in neutrophil-specific granules and undergoes release in response to microbial recognition and inflammatory stimuli [2], resulting in pathogen recognition and clearance. Confocal analysis suggested that TSG-6 is also present in specific granules, where it partially colocalizes with PTX3, and both proteins are released upon LPS stimulation. Finally, immunohistochemistry demonstrated the coexpression of TSG-6 and PTX3 in leukocytes at inflammatory sites in vivo.
TSG-6 is an anti-inflammatory mediator, which inhibits neutrophil recruitment in different models of inflammation [36–38], an activity that is mediated by the Link module and might be a result of modulation of neutrophil adhesion to the endothelium. Interestingly, PTX3 has also been shown to have regulatory effects on inflammatory reactions [7, 55]. The rapid availability of PTX3 at the onset of inflammation could be important for the development of an appropriate innate immune response to invading pathogens, whereas the local expression of TSG-6 could serve to control neutrophil migration, thus preventing host tissue damage [35]. Furthermore, the combined actions of PTX3 and TSG-6 might support wound healing through the deposition of HA-rich ECM; this would be in line with the role suggested for neutrophils in tissue remodeling based on the transcriptional profiles seen in innate responses [56]. The secretion of TSG-6 protein by neutrophils is also of interest in relation to its recently reported role as an inhibitor of RANKL-mediated osteoclastic bone resorption [57]. RANKL is expressed by normal and inflammatory neutrophils, and its receptor (RANK) and also osteoprotegrin (a soluble inhibitor of RANKL) are expressed only by neutrophils exposed to inflammatory stimuli, e.g., in synovial fluids from patients with RA [58]. Therefore, TSG-6 can be added to the list of molecules expressed by neutrophils that modulate bone remodeling, where these cells may have an important role in the regulation of bone turnover at inflammatory sites [59].
In phagocytes, we observed that preincubation of cells with anti-inflammatory cytokines (IL-4 and IL-13) resulted in marked reductions in LPS-induced expression of TSG-6 and PTX3 mRNA. In contrast, pretreatment with IL-10 resulted in differential regulation of the two genes, where IL-10 acted synergistically with LPS in stimulating PTX3 expression [40, 48] but inhibited the induction of TSG-6. PTX3 acts at an early stage in the immune response (i.e., before production of antibodies), being induced as a result of the recognition of pathogens and promoting complement activation and phagocytosis (as a result of opsonization). Thus, IL-10 contributes to the activation of the humoral arms of the innate and adaptive immune responses via PTX3 and antibodies, respectively [40, 60]. Moreover, IL-10 is involved in chronic inflammation and in the resolution of inflammatory activity [60] as well as playing a role in tissue remodeling by modulating the expression of genes involved in the synthesis and breakdown of ECM in connective tissue [60]. Given that PTX3 and TSG-6 are involved in the assembly of HA-rich matrices, the divergent effects of IL-10 in modulating their expression suggest that these two proteins might have distinct roles in tissue remodeling during chronic inflammation.
The in vitro and ex vivo results reported here suggest that mononuclear phagocytes, DC, and neutrophils may be important sources of TSG-6 at sites of inflammation, where this protein is understood to participate in the regulation of leukocyte recruitment and in tissue repair and remodeling.
ACKNOWLEDGMENTS
This work was supported by Associazione Italiana per la Ricerca sul Cancro (AIRC), Ministero Istruzione Università e Ricerca (MIUR), European Commission (MUGEN, EMBIC), the Arthritis Research Campaign (grant 16539), and the Medical Research Council.
Footnotes
- DC
- dendritic cell(s)
- ECM
- extracellular matrix
- h
- human
- HA
- hyaluronan
- HC
- heavy chain(s)
- HDMEC
- human dermal microvascular endothelial cells
- IαI
- inter-α-inhibitor
- MoDC
- monocyte-derived DC
- PFA
- paraformaldehyde
- PMN
- polymorphonuclear neutrophils
- PTX3
- pentraxin 3
- r
- recombinant
- RA
- rheumatoid arthritis
- RAH-1
- rabbit anti-hTSG-6
- RANKL
- receptor activator of NF-κB ligand
- TSG-6
- TNF-α-stimulated gene-6
REFERENCES
- 1. Garlanda C., Bottazzi B., Bastone A., Mantovani A. (2005) Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu. Rev. Immunol. 23, 337–366 [DOI] [PubMed] [Google Scholar]
- 2. Jaillon S., Peri G., Delneste Y., Fremaux I., Doni A., Moalli F., Garlanda C., Romani L., Gascan H., Bellocchio S., Bozza S., Cassatella M. A., Jeannin P., Mantovani A. (2007) The humoral pattern recognition receptor PTX3 is stored in neutrophil granules and localizes in extracellular traps. J. Exp. Med. 204, 793–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nauta A. J., Bottazzi B., Mantovani A., Salvatori G., Kishore U., Schwaeble W. J., Gingras A. R., Tzima S., Vivanco F., Egido J., Tijsma O., Hack E. C., Daha M. R., Roos A. (2003) Biochemical and functional characterization of the interaction between pentraxin 3 and C1q. Eur. J. Immunol. 33, 465–473 [DOI] [PubMed] [Google Scholar]
- 4. Jeannin P., Bottazzi B., Sironi M., Doni A., Rusnati M., Presta M., Maina V., Magistrelli G., Haeuw J. F., Hoeffel G., Thieblemont N., Corvaia N., Garlanda C., Delneste Y., Mantovani A. (2005) Complexity and complementarity of outer membrane protein A recognition by cellular and humoral innate immunity receptors. Immunity 22, 551–560 [DOI] [PubMed] [Google Scholar]
- 5. Cotena A., Maina V., Sironi M., Bottazzi B., Jeannin P., Vecchi A., Corvaia N., Daha M. R., Mantovani A., Garlanda C. (2007) Complement dependent amplification of the innate response to a cognate microbial ligand by the long pentraxin PTX3. J. Immunol. 179, 6311–6317 [DOI] [PubMed] [Google Scholar]
- 6. Garlanda C., Hirsch E., Bozza S., Salustri A., De Acetis M., Nota R., Maccagno A., Riva F., Bottazzi B., Peri G., Doni A., Vago L., Botto M., De Santis R., Carminati P., Siracusa G., Altruda F., Vecchi A., Romani L., Mantovani A. (2002) Non-redundant role of the long pentraxin PTX3 in anti-fungal innate immune response. Nature 420, 182–186 [DOI] [PubMed] [Google Scholar]
- 7. Salio M., Chimenti S., De Angelis N., Molla F., Maina V., Nebuloni M., Pasqualini F., Latini R., Garlanda C., Mantovani A. (2008) Cardioprotective function of the long pentraxin PTX3 in acute myocardial infarction. Circulation 117, 1055–1064 [DOI] [PubMed] [Google Scholar]
- 8. Mairuhu A. T., Peri G., Setiati T. E., Hack C. E., Koraka P., Soemantri A., Osterhaus A. D., Brandjes D. P., van der Meer J. W., Mantovani A., van Gorp E. C. (2005) Elevated plasma levels of the long pentraxin, pentraxin 3, in severe dengue virus infections. J. Med. Virol. 76, 547–552 [DOI] [PubMed] [Google Scholar]
- 9. Muller B., Peri G., Doni A., Torri V., Landmann R., Bottazzi B., Mantovani A. (2001) Circulating levels of the long pentraxin PTX3 correlate with severity of infection in critically ill patients. Crit. Care Med. 29, 1404–1407 [DOI] [PubMed] [Google Scholar]
- 10. Luchetti M. M., Piccinini G., Mantovani A., Peri G., Matteucci C., Pomponio G., Fratini M., Fraticelli P., Sambo P., Di Loreto C., Doni A., Introna M., Gabrielli A. (2000) Expression and production of the long pentraxin PTX3 in rheumatoid arthritis (RA). Clin. Exp. Immunol. 119, 196–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fazzini F., Peri G., Doni A., Dell'Antonio G., Dal Cin E., Bozzolo E., D'Auria F., Praderio L., Ciboddo G., Sabbadini M. G., Manfredi A. A., Mantovani A., Querini P. R. (2001) PTX3 in small-vessel vasculitides: an independent indicator of disease activity produced at sites of inflammation. Arthritis Rheum. 44, 2841–2850 [DOI] [PubMed] [Google Scholar]
- 12. Latini R., Maggioni A. P., Peri G., Gonzini L., Lucci D., Mocarelli P., Vago L., Pasqualini F., Signorini S., Soldateschi D., Tarli L., Schweiger C., Fresco C., Cecere R., Tognoni G., Mantovani A., Lipid Assessment Trial Italian Network (LATIN) Investigators (2004) Prognostic significance of the long pentraxin PTX3 in acute myocardial infarction. Circulation 110, 2349–2354 [DOI] [PubMed] [Google Scholar]
- 13. Cetin I., Cozzi V., Pasqualini F., Nebuloni M., Garlanda C., Vago L., Pardi G., Mantovani A. (2006) Elevated maternal levels of the long pentraxin 3 (PTX3) in preeclampsia and intrauterine growth restriction. Am. J. Obstet. Gynecol. 194, 1347–1353 [DOI] [PubMed] [Google Scholar]
- 14. Milner C. M., Day A. J. (2003) TSG-6: a multifunctional protein associated with inflammation. J. Cell Sci. 116, 1863–1873 [DOI] [PubMed] [Google Scholar]
- 15. Milner C. M., Higman V. A., Day A. J. (2006) TSG-6: a pluripotent inflammatory mediator? Biochem. Soc. Trans. 34, 446–450 [DOI] [PubMed] [Google Scholar]
- 16. Kuznetsova S. A., Mahoney D. J., Martin-Manso G., Ali T., Nentwich H. A., Sipes J. M., Zeng B., Vogel T., Day A. J., Roberts D. D. (2008) TSG-6 binds via its CUB_C domain to the cell-binding domain of fibronectin and increases fibronectin matrix assembly. Matrix Biol. 27, 201–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wisniewski H. G., Maier R., Lotz M., Lee S., Klampfer L., Lee T. H., Vilcek J. (1993) TSG-6: a TNF-, IL-1-, and LPS-inducible secreted glycoprotein associated with arthritis. J. Immunol. 151, 6593–6601 [PubMed] [Google Scholar]
- 18. Wisniewski H. G., Vilcek J. (1997) TSG-6: an IL-1/TNF-inducible protein with anti-inflammatory activity. Cytokine Growth Factor Rev. 8, 143–156 [DOI] [PubMed] [Google Scholar]
- 19. Salustri A., Garlanda C., Hirsch E., De Acetis M., Maccagno A., Bottazzi B., Doni A., Bastone A., Mantovani G., Beck Peccoz P., Salvatori G., Mahoney D. J., Day A. J., Siracusa G., Romani L., Mantovani A. (2004) PTX3 plays a key role in the organization of the cumulus oophorus extracellular matrix and in in vivo fertilization. Development 131, 1577–1586 [DOI] [PubMed] [Google Scholar]
- 20. Scarchilli L., Camaioni A., Bottazzi B., Negri V., Doni A., Deban L., Bastone A., Salvatori G., Mantovani A., Siracusa G., Salustri A. (2007) PTX3 interacts with inter-{α}-trypsin inhibitor: implications for hyaluronan organization and cumulus oophorus expansion. J. Biol. Chem. 282, 30161–30170 [DOI] [PubMed] [Google Scholar]
- 21. Varani S., Elvin J. A., Yan C., DeMayo J., DeMayo F. J., Horton H. F., Byrne M. C., Matzuk M. M. (2002) Knockout of pentraxin 3, a downstream target of growth differentiation factor-9, causes female subfertility. Mol. Endocrinol. 16, 1154–1167 [DOI] [PubMed] [Google Scholar]
- 22. Fulop C., Szanto S., Mukhopadhyay D., Bardos T., Kamath R. V., Rugg M. S., Day A. J., Salustri A., Hascall V. C., Glant T. T., Mikecz K. (2003) Impaired cumulus mucification and female sterility in tumor necrosis factor-induced protein-6 deficient mice. Development 130, 2253–2261 [DOI] [PubMed] [Google Scholar]
- 23. Zhuo L., Yoneda M., Zhao M., Yingsung W., Yoshida N., Kitagawa Y., Kawamura K., Suzuki T., Kimata K. (2001) Defect in SHAP-hyaluronan complex causes severe female infertility. A study by inactivation of the bikunin gene in mice. J. Biol. Chem. 276, 7693–7696 [DOI] [PubMed] [Google Scholar]
- 24. Hess A. P., Hamilton A. E., Talbi S., Dosiou C., Nyegaard M., Nayak N., Genbecev-Krtolica O., Mavrogianis P., Ferrer K., Kruessel J., Fazleabas A. T., Fisher S. J., Giudice L. C. (2007) Decidual stromal cell response to paracrine signals from the trophoblast: amplification of immune and angiogenic modulators. Biol. Reprod. 76, 102–117 [DOI] [PubMed] [Google Scholar]
- 25. Popovici R. M., Betzler N. K., Krause M. S., Luo M., Jauckus J., Germeyer A., Bloethner S., Schlotterer A., Kumar R., Strowitzki T., von Wolff M. (2006) Gene expression profiling of human endometrial-trophoblast interaction in a coculture model. Endocrinology 147, 5662–5675 [DOI] [PubMed] [Google Scholar]
- 26. Yingsung W., Zhuo L., Morgelin M., Yoneda M., Kida D., Watanabe H., Ishiguro N., Iwata H., Kimata K. (2003) Molecular heterogeneity of the SHAP-hyaluronan complex. Isolation and characterization of the complex in synovial fluid from patients with rheumatoid arthritis. J. Biol. Chem. 278, 32710–32718 [DOI] [PubMed] [Google Scholar]
- 27. Rugg M. S., Willis A. C., Mukhopadhyay D., Hascall V. C., Fries E., Fulop C., Milner C. M., Day A. J. (2005) Characterization of complexes formed between TSG-6 and inter-α-inhibitor that act as intermediates in the covalent transfer of heavy chains onto hyaluronan. J. Biol. Chem. 280, 25674–25686 [DOI] [PubMed] [Google Scholar]
- 28. Day A. J., de la Motte C. A. (2005) Hyaluronan cross-linking: a protective mechanism in inflammation? Trends Immunol. 26, 637–643 [DOI] [PubMed] [Google Scholar]
- 29. Mindrescu C., Thorbecke G. J., Klein M. J., Vilcek J., Wisniewski H. G. (2000) Amelioration of collagen-induced arthritis in DBA/1J mice by recombinant TSG-6, a tumor necrosis factor/interleukin-1-inducible protein. Arthritis Rheum. 43, 2668–2677 [DOI] [PubMed] [Google Scholar]
- 30. Bardos T., Kamath R. V., Mikecz K., Glant T. T. (2001) Anti-inflammatory and chondroprotective effect of TSG-6 (tumor necrosis factor-α-stimulated gene-6) in murine models of experimental arthritis. Am. J. Pathol. 159, 1711–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Glant T. T., Kamath R. V., Bardos T., Gal I., Szanto S., Murad Y. M., Sandy J. D., Mort J. S., Roughley P. J., Mikecz K. (2002) Cartilage-specific constitutive expression of TSG-6 protein (product of tumor necrosis factor α-stimulated gene 6) provides a chondroprotective, but not antiinflammatory, effect in antigen-induced arthritis. Arthritis Rheum. 46, 2207–2218 [DOI] [PubMed] [Google Scholar]
- 32. Wisniewski H. G., Hua J. C., Poppers D. M., Naime D., Vilcek J., Cronstein B. N. (1996) TNF/IL-1-inducible protein TSG-6 potentiates plasmin inhibition by inter-α-inhibitor and exerts a strong anti-inflammatory effect in vivo. J. Immunol. 156, 1609–1615 [PubMed] [Google Scholar]
- 33. Forteza R., Casalino-Matsuda S. M., Monzon M. E., Fries E., Rugg M. S., Milner C. M., Day A. J. (2007) TSG-6 potentiates the antitissue kallikrein activity of inter-α-inhibitor through bikunin release. Am. J. Respir. Cell Mol. Biol. 36, 20–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mindrescu C., Dias A. A., Olszewski R. J., Klein M. J., Reis L. F., Wisniewski H. G. (2002) Reduced susceptibility to collagen-induced arthritis in DBA/1J mice expressing the TSG-6 transgene. Arthritis Rheum. 46, 2453–2464 [DOI] [PubMed] [Google Scholar]
- 35. Szanto S., Bardos T., Gal I., Glant T. T., Mikecz K. (2004) Enhanced neutrophil extravasation and rapid progression of proteoglycan-induced arthritis in TSG-6-knockout mice. Arthritis Rheum. 50, 3012–3022 [DOI] [PubMed] [Google Scholar]
- 36. Getting S. J., Mahoney D. J., Cao T., Rugg M. S., Fries E., Milner C. M., Perretti M., Day A. J. (2002) The link module from human TSG-6 inhibits neutrophil migration in a hyaluronan- and inter-α -inhibitor-independent manner. J. Biol. Chem. 277, 51068–51076 [DOI] [PubMed] [Google Scholar]
- 37. Wisniewski H. G., Naime D., Hua J. C., Vilcek J., Cronstein B. N. (1996) TSG-6, a glycoprotein associated with arthritis, and its ligand hyaluronan exert opposite effects in a murine model of inflammation. Pflugers Arch. 431, R225–R226 [DOI] [PubMed] [Google Scholar]
- 38. Cao T. V., La M., Getting S. J., Day A. J., Perretti M. (2004) Inhibitory effects of TSG-6 Link module on leukocyte-endothelial cell interactions in vitro and in vivo. Microcirculation 11, 615–624 [DOI] [PubMed] [Google Scholar]
- 39. Doni A., Peri G., Chieppa M., Allavena P., Pasqualini F., Vago L., Romani L., Garlanda C., Mantovani A. (2003) Production of the soluble pattern recognition receptor PTX3 by myeloid, but not plasmacytoid, dendritic cells. Eur. J. Immunol. 33, 2886–2893 [DOI] [PubMed] [Google Scholar]
- 40. Doni A., Michela M., Bottazzi B., Peri G., Valentino S., Polentarutti N., Garlanda C., Mantovani A. (2006) Regulation of PTX3, a key component of humoral innate immunity in human dendritic cells: stimulation by IL-10 and inhibition by IFN-γ. J. Leukoc. Biol. 79, 797–802 [DOI] [PubMed] [Google Scholar]
- 41. Bourke E., Bosisio D., Golay J., Polentarutti N., Mantovani A. (2003) The Toll-like receptor repertoire of human B lymphocytes: inducible and selective expression of TLR9 and TLR10 in normal and transformed cells. Blood 102, 956–963 [DOI] [PubMed] [Google Scholar]
- 42. Breviario F., d'Aniello E. M., Golay J., Peri G., Bottazzi B., Bairoch A., Saccone S., Marzella R., Predazzi V., Rocchi M., et al. (1992) Interleukin-1-inducible genes in endothelial cells. Cloning of a new gene related to C-reactive protein and serum amyloid P component. J. Biol. Chem. 267, 22190–22197 [PubMed] [Google Scholar]
- 43. Faure E., Equils O., Sieling P. A., Thomas L., Zhang F. X., Kirschning C. J., Polentarutti N., Muzio M., Arditi M. (2000) Bacterial lipopolysaccharide activates NF-κB through Toll-like receptor 4 (TLR-4) in cultured human dermal endothelial cells. Differential expression of TLR-4 and TLR-2 in endothelial cells. J. Biol. Chem. 275, 11058–11063 [DOI] [PubMed] [Google Scholar]
- 44. Day A. J., Aplin R. T., Willis A. C. (1996) Overexpression, purification, and refolding of link module from human TSG-6 in Escherichia coli: effect of temperature, media, and mutagenesis on lysine misincorporation at arginine AGA codons. Protein Expr. Purif. 8, 1–16 [DOI] [PubMed] [Google Scholar]
- 45. Lesley J., English N. M., Gal I., Mikecz K., Day A. J., Hyman R. (2002) Hyaluronan binding properties of a CD44 chimera containing the link module of TSG-6. J. Biol. Chem. 277, 26600–26608 [DOI] [PubMed] [Google Scholar]
- 46. Nentwich H. A., Mustafa Z., Rugg M. S., Marsden B. D., Cordell M. R., Mahoney D. J., Jenkins S. C., Dowling B., Fries E., Milner C. M., Loughlin J., Day A. J. (2002) A novel allelic variant of the human TSG-6 gene encoding an amino acid difference in the CUB module. Chromosomal localization, frequency analysis, modeling, and expression. J. Biol. Chem. 277, 15354–15362 [DOI] [PubMed] [Google Scholar]
- 47. Fujimoto T., Savani R. C., Watari M., Day A. J., Strauss J. F., III (2002) Induction of the hyaluronic acid-binding protein, tumor necrosis factor-stimulated gene-6, in cervical smooth muscle cells by tumor necrosis factor-α and prostaglandin E(2). Am. J. Pathol. 160, 1495–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Perrier P., Martinez F. O., Locati M., Bianchi G., Nebuloni M., Vago G., Bazzoni F., Sozzani S., Allavena P., Mantovani A. (2004) Distinct transcriptional programs activated by interleukin-10 with or without lipopolysaccharide in dendritic cells: induction of the B cell-activating chemokine, CXC chemokine ligand 13. J. Immunol. 172, 7031–7042 [DOI] [PubMed] [Google Scholar]
- 49. Polentarutti N., Picardi G., Basile A., Cenzuales S., Rivolta A., Matteucci C., Peri G., Mantovani A., Introna M. (1998) Interferon-γ inhibits expression of the long pentraxin PTX3 in human monocytes. Eur. J. Immunol. 28, 496–501 [DOI] [PubMed] [Google Scholar]
- 50. Wisniewski H. G., Burgess W. H., Oppenheim J. D., Vilcek J. (1994) TSG-6, an arthritis-associated hyaluronan binding protein, forms a stable complex with the serum protein inter-α-inhibitor. Biochemistry 33, 7423–7429 [DOI] [PubMed] [Google Scholar]
- 51. Sanggaard K. W., Karring H., Valnickova Z., Thogersen I. B., Enghild J. J. (2005) The TSG-6 and I α I interaction promotes a transesterification cleaving the protein-glycosaminoglycan-protein (PGP) cross-link. J. Biol. Chem. 280, 11936–11942 [DOI] [PubMed] [Google Scholar]
- 52. Malcolm K. C., Arndt P. G., Manos E. J., Jones D. A., Worthen G. S. (2003) Microarray analysis of lipopolysaccharide-treated human neutrophils. Am. J. Physiol. Lung Cell. Mol. Physiol. 284, L663–L670 [DOI] [PubMed] [Google Scholar]
- 53. Chaussabel D., Semnani R. T., McDowell M. A., Sacks D., Sher A., Nutman T. B. (2003) Unique gene expression profiles of human macrophages and dendritic cells to phylogenetically distinct parasites. Blood 102, 672–681 [DOI] [PubMed] [Google Scholar]
- 54. Lee T. H., Wisniewski H. G., Vilcek J. (1992) A novel secretory tumor necrosis factor-inducible protein (TSG-6) is a member of the family of hyaluronate binding proteins, closely related to the adhesion receptor CD44. J. Cell Biol. 116, 545–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Soares A. C., Souza D. G., Pinho V., Vieira A. T., Nicoli J. R., Cunha F. Q., Mantovani A., Reis L. F., Dias A. A., Teixeira M. M. (2006) Dual function of the long pentraxin PTX3 in resistance against pulmonary infection with Klebsiella pneumoniae in transgenic mice. Microbes Infect. 8, 1321–1329 [DOI] [PubMed] [Google Scholar]
- 56. Theilgaard-Monch K., Knudsen S., Follin P., Borregaard N. (2004) The transcriptional activation program of human neutrophils in skin lesions supports their important role in wound healing. J. Immunol. 172, 7684–7693 [DOI] [PubMed] [Google Scholar]
- 57. Mahoney D. J., Mikecz K., Ali T., Mabilleau G., Benayahu D., Plaas A., Milner C. M., Day A. J., Sabokbar A. (2008) TSG-6 regulates bone remodeling through inhibition of osteoblastogenesis and osteoclast activation. J. Biol. Chem. 283, 25952–25962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Poubelle P. E., Chakravarti A., Fernandes M. J., Doiron K., Marceau A. A. (2007) Differential expression of RANK, RANK-L, and osteoprotegerin by synovial fluid neutrophils from patients with rheumatoid arthritis and by healthy human blood neutrophils. Arthritis Res. Ther. 9, R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Haynes D. R. (2007) Inflammatory cells and bone loss in rheumatoid arthritis. Arthritis Res. Ther. 9, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Moore K. W., de Waal Malefyt R., Coffman R. L., O'Garra A. (2001) Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19, 683–765 [DOI] [PubMed] [Google Scholar]