Abstract
Cerebral edema plays a central role in the pathophysiology of many diseases of the central nervous system (CNS) including ischemia, trauma, tumors, inflammation, and metabolic disturbances. The formation of cerebral edema results in an increase in tissue water content and brain swelling which, if unchecked, can lead to elevated intracranial pressure (ICP), reduced cerebral blood flow, and ultimately cerebral herniation and death. Despite the clinical significance of cerebral edema, the mechanism of brain water transport and edema formation remain poorly understood. As a result, current therapeutic tools for managing cerebral edema have changed little in the past 90 years. “Malignant ischemic stroke” is characterized by high mortality (~80%) and represents a major clinical problem in cerebrovascular disease. Widespread ischemic injury in these patients causes progressive cerebral edema, increased ICP, and rapid clinical decline. In response to these observations, a series of recent studies have begun to target cerebral edema in the management of large ischemic strokes. During cerebral edema formation, the glial water channel aquaporin-4 (AQP4) has been show to facilitate astrocyte swelling (“cytotoxic swelling”). AQP4 has also been seen to be responsible for the reabsorption of extracellular edema fluid (“vasogenic edema”). In the present review, the role of AQP4 in the development of cerebral edema is discussed with emphasis on its contribution to ischemic edema. We also examine the potential of AQP4 as a therapeutic target in edema associated with stroke.
1 Introduction
Cerebral edema is characterized by the pathological swelling of brain tissue due to progressive increase in brain water content. It is a frequent and feared clinical complication that develops in a broad range of cerebral insults such as ischemia (Ribeiro Mde et al. 2006), trauma (Zador et al. 2007), tumors (Saadoun et al. 2002) and inflammation (Papadopoulos and Verkman 2005). The rigid cranium opposes the progressive swelling of brain tissue, leading to elevated intracranial pressure, decreased cerebral blood flow, and ultimately cerebral herniation and death. Klatzo broadly categorized the mechanisms of brain tissue swelling as cytotoxic edema and vasogenic edema in 1967 (Klatzo 1967). The former process involves progressive cell swelling due to rapid water uptake, whereas in the case of vasogenic edema water leaks into the extracellular space due to defects in the blood–brain barrier. Although these two mechanisms coexist in most brain pathologies, instead of a pure cytotoxic edema or vasogenic edema, there is typically an appreciable dominance of one type over the other in each disease. For example, vasogenic edema seems to dominate in tumors and cerebral abscesses, while cytotoxic edema develops in ischemic stroke and brain trauma.
Cytotoxic edema is a significant clinical problem that can develop in response to a large (“malignant”) middle cerebral artery (MCA) occlusion (Hacke et al. 1996; Bardutzky and Schwab 2007) and has been associated with approximately 80% mortality rate. Cerebral vascular occlusion initiates a sequence of events involving cell swelling, followed by BBB leakage and hemorrhagic conversion of the tissue (Simard et al. 2007). The strategy for the treatment of cerebral edema associated with these large ischemic strokes is limited to the use of intravascular administration of hyperosmolar solutions to remove the excess water from the brain, or removal of a large bone flap to allow the brain to swell outside the rigid cranium (decompressive craniectomy). These methods have remained unchanged for the past 90 years. More recently, the discovery of aquaporin membrane water channels has provided new insights into the molecular mechanisms of edema formation and brain water transport.
The glial membrane water channel aquaporin-4 (AQP4) is largely expressed in astrocytic processes adjacent to cerebral capillaries and pial membranes lining the subarachnoid space (Fig. 1). Such strategic localization at these tissue-water interfaces, and the high water permeability of the channel, makes AQP4 an important route for transporting water to and from the brain. A large body of evidence from transgenic mice deficient in AQP4 has demonstrated the role of this water channel in cytotoxic and vasogenic edema. These findings suggest AQP4 is a potential therapeutic target in the treatment of cerebral edema developing in response to various CNS pathologies including stroke.
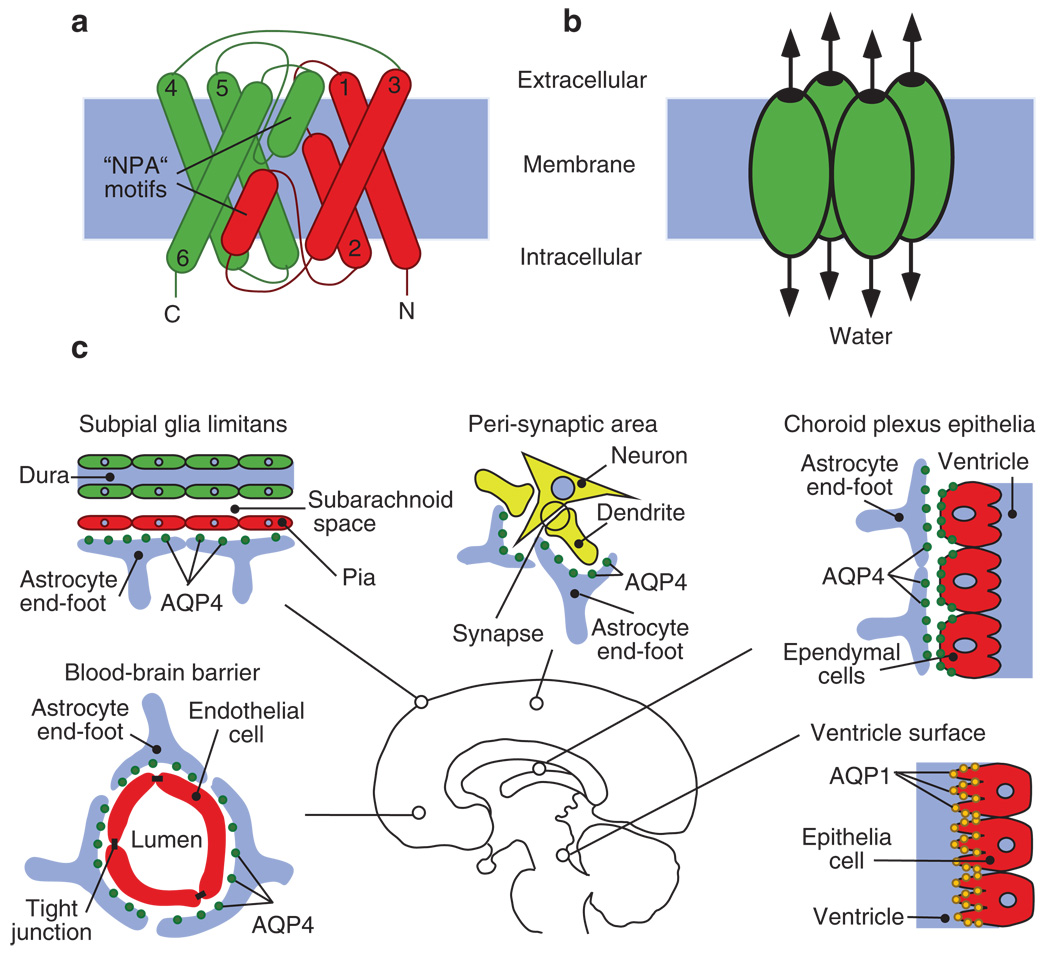
Fig. 1.
(a, b) Diagram depicts the structure of an AQP monomer and its clustering into tetramers. (a) Schematic demonstration of the transmembrane α-helices of AQP4 numbered from 1–6, which surround the highly selective water pore. The highly conserved “NPA” motifs are indicated. (b) AQP organizes into tetramers in the cell membrane with each unit functioning as an independent pore. (c) The distribution of CNS AQPs. AQP4 is polarized at the glial end-feet facing CSF–brain, blood–brain barrier and peri-synaptic areas. Ependymal cells have basolateral expression of AQP4. The apical processes of the choroid plexus cells are rich in AQP1 expression (adapted from Zador and Manley 2008)
2 Aquaporins
Aquaporins are highly permeable water channels widely found in different tissues of the body (Verkman 2005). Structurally, a single AQP channel is formed by a six transmembrane helix protein that forms a water selective pore in the center of the molecule. The channel functions as tetramers in the cell membrane. The structural significance of the AQP4 subtype is that its tetramers organize into large (~100 nm) clusters termed orthogonal array particles (OAPs), visible through freeze fracture electron microscopy of astrocyte processes (Rash et al. 1998)
The first aquaporin (AQP1) was discovered as the membrane water channel responsible for rapid red blood cell swelling in response to osmotic challenge. In addition to facilitating water flux through cell membranes, some family members such as AQP3, AQP7, and AQP9 also allow glycerol transport and may be involved in cell metabolism (Hara-Chikuma and Verkman 2006). Recent data has shown a number of unusual roles for AQPs in cellular functions such as tumor angiogenesis (Saadoun et al. 2005a), glial scar formation (Saadoun et al. 2005b), pain (Oshio et al. 2006) and neuroexcitation (Binder et al. 2006; Padmawar et al. 2005).
2.1 Aquaporins in the Central Nervous System
Aquaporins in the CNS are seen to facilitate water transport between the major compartments of the brain (Fig. 2): (1) the CSF space defined as the cerebral ventricles and subarachnoid space; (2) the brain parenchyma consisting of intracellular and extracellular space; and (3) the intravascular compartment (Zador et al. 2007). Two members of the aquaporin family, AQP1 and AQP4 largely manifest at the interfaces between these compartments where they participate in the maintenance of brain water homeostasis.
Fig. 2.
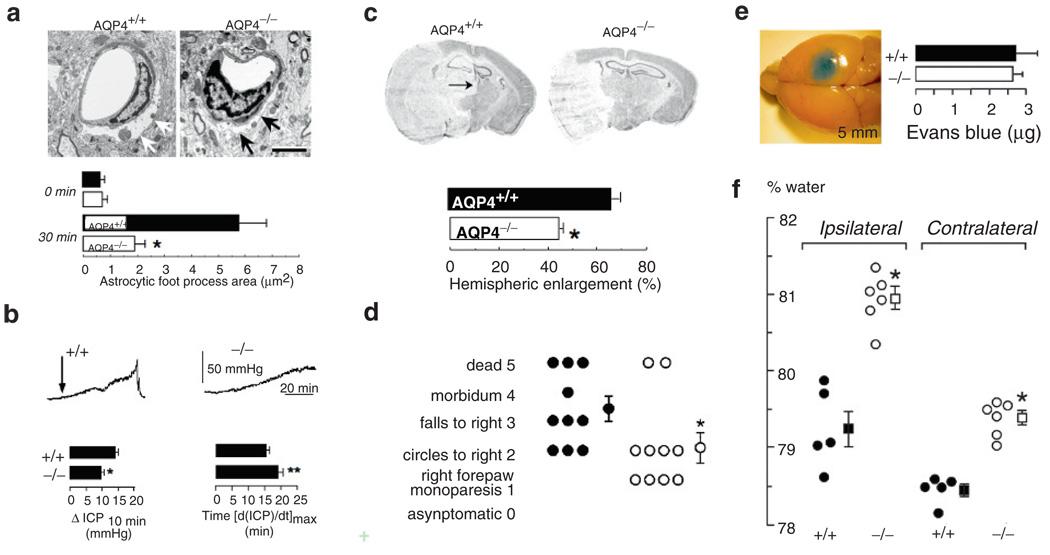
The bimodal role of AQP4 in the pathomechanism of cerebral edema. (a) Electron micrographs demonstrating pronounced perivascular astrocyte foot process swelling (arrows) 30 min after acute water intoxication in wild-type mice, whereas AQP4−/− mice lack cytotoxic cell swelling. (bar=3µm) (b) Wild-type mice show accelerated brain swelling and intracranial pressure increase (ICP) compared to AQP4−/− mice following IP water intoxication. Increased brain water uptake in wild-type mice is demonstrated by higher relative ICP elevation at 10 min (ΔICP10 min) and significantly shorter time required to reach maximal ICP value (time to [dICP/dt]max). (c) Reduced hemispheric enlargement in AQP4−/− mice compared to wild type controls 24 h following permanent MCA occlusion. Note the midline shift in the wild- type brain (arrow). (d) Improved functional outcome 24 h after MCA occlusion in AQP4−/−mice. (e) Cortical freeze injury disrupts the blood–brain barrier as assessed by Evans Blue extravasation and causes vasogenic edema. (f) Brain water content increase in AQP4−/− mice compared to wild-type controls in vasogenic edema
2.1.1 Aquaporin-4 in the Central Nervous System
In the CNS, AQP4 expression is restricted to astrocytes throughout the brain and spinal cord and the ependymal cells that line the cerebral ventricles (Nielsen et al. 1997). There is a characteristic subcellular distribution of AQP4 in astrocytes: it is highly concentrated at cell surfaces of the blood–brain and CSF–brain barriers such as the astrocytic end-feet and glia limitans. At these important water transport sites, AQP4 colocalizes with the inwardly rectifying potassium channel Kir4.1 where it is proposed to act as a water-potassium transport complex (Connors and Kofuji 2002; Nagelhus et al. 2004). The dystroglycan complex (DGC) provides the molecular scaffolding for the polarized colocalization of these two channels. Deletion of one of the DCG complex proteins, alpha-syntrophin, results in the failure of AQP4 to properly colocalize in the plasma membrane without altering overall AQP4 protein expression (Neely et al. 2001). The polarized expression of AQP4 is critical for its function in brain water homeostasis, as the deletion of alpha-syntrophin creates a phenotype similar to that of the AQP4-deficient mice.
2.1.2 Aquaporin-1 in the Central Nervous System
AQP1 is expressed in the apical membrane of the choroid plexus epithelia where it facilitates cerebrospinal fluid (CSF) production. The transcellular water flux through AQP1 contributes approximately 20–30% of CSF volume as demonstrated by AQP1-deficient mice (Oshio et al. 2005). In physiological circumstances, the remaining part of the brain is void of AQP1, in contrast to other organs of the body where it is abundantly expressed in capillary endothelium. However, in pathological states of the CNS such as tumors, there is an upregulation of AQP1 (Papadopoulos et al. 2002) in the endothelia of cerebral capillaries. Based on this finding, it seems that AQP1 is predominantly expressed in the choroid plexus and functions primarily to contribute to CSF production. However in the brain, some AQP1 pathologies are expressed de novo in cerebral endothelia, possibly to aid clearance of edema fluid from the brain.
3 Cerebral Edema in Stroke
Based on their pathomechanism, strokes can be categorized as hemorrhagic or ischemic, and account for 20% and 80% of the cases respectively. Pathways leading to edema formation in hemorrhagic stroke differ from those in ischemic stroke (Fig. 3).
Fig. 3.
Mechanism of edema formation in ischemic and hemorrhagic stroke (see text for details). In ischemic stroke (above) AQP4 facilitates water uptake of perivascular astrocyte end-feet resulting in subsequent compression of the adjacent capillary lumen. As cellular damage evolves, the mechanism shifts into vasogenic edema and later hemorrhagic conversion. During hemorrhagic stroke, factors derived from the clot act on different components of the endothelial tight junction, leading the disruption of the BBB seal. AQP4 facilitates the reabsorption of edema fluid from the extracellular space
3.1 Cerebral Edema in Hemorrhagic stroke
In hemorrhagic stroke, following the initial physical trauma from the hydrostatic effect of intracerebral hemorrhage, perifocal edema formation is initiated by clot derived proteins and vasoactive substances impairing BBB integrity through direct or indirect mechanisms (Xi et al. 2006). The intracerebral injection of blood substrates in animal models show thrombin, plasminogen activator and urokinase contribute to brain edema formation (Matsuoka and Hamada 2002; Lee et al. 1995; Figueroa et al. 1998). The activation of thrombin was shown to cause inflammatory-cell infiltration, and scar formation (Xi et al. 2006) as well as direct disruption of the BBB by inducing endothelial cell retraction (Satpathy et al. 2004). The presence of tissue plasminogen activator and urokinase has been found to enhance this effect, presumably by competing for thrombin inhibitors. The opening of the BBB seal leads to the formation of a proteinacious ultrafiltrate causing vasogenic edema peaking at 10–20 days in humans following ICH (Xi et al. 2006). The complement is introduced through the BBB defect, leading to formation of membrane attack complexes (MAC) and destruction of CNS cells and RBCs (Hua et al. 2000). A subsequent component of edema formation is the lysis of red blood cells followed by liberation of hemoglobin, which inflict cellular damage (hemoglobin toxicity) through caspases (Wang et al. 2002) and oxidative mechanisms (Goldstein et al. 2003). Cytotoxic edema ensues in concert with secondary cellular injury. In later phases of clot evolution and degradation, thrombin, hemoglobin degradation products, inflammatory mediators, interleukins, and metalloproteinases together facilitate both vasogenic and cytotoxic edema (Rincon and Mayer 2004). Vasogenic and cytotoxic edema together last 2–4 weeks. Empirically, intravenous hyperosmolar therapy with mannitol is used in ICH for indications of raised ICP or imaging with findings of significant cerebral edema. Randomized studies have not been conducted and no evidence based conclusions can be made regarding the use of hyperosmolar therapy for acute intracerebral hemorrhage (Bereczki et al. 2000). The role, if any, of AQP expression and function in intracerebral hemorrhage has not been explored.
3.2 Cerebral Edema in Ischemic Stroke
Ischemic stroke initiates a sequence of different edema mechanisms in a stepwise fashion (Simard et al. 2007). The formation of a thrombus occludes the cerebral artery and impairs cellular metabolism resulting in cellular swelling (cytotoxic edema), followed by leakage of the BBB (vasogenic edema) and finally the ischemic tissue undergoes hemorrhagic conversion. The disruption of cerebral blood flow in ischemia leads to the impairment of ATP synthesis, leading to insufficient Na+/K+ ATPase function. The sodium fluxes driven by the transmembrane electrochemical gradient remain unopposed, causing net accumulation of intracellular sodium. The anaerobic glycolysis initiated in response to ischemia cause accumulation of lactate, which acts together with sodium to draw water into the cell creating cytotoxic edema. Further cellular damage caused by ischemia results in BBB disruption through a number of proposed mechanisms such as reverse pinocytosis (Castejon 1984), disputed Ca2+ signaling (Brown and Davis 2002), and actions of other agents such as VEGF (Weis and Cheresh 2005), and MMPs (Asahi 2001). Depending on the depth of ischemia, the BBB may lose its entire physical integrity leading to hemorrhagic conversion: all components of the blood are extravasated into the brain parenchyma leading to catastrophic tissue destruction. The role of AQP4 has been explored in several models of ischemic stroke where it participates in the formation of cerebral edema.
4 AQP4 and Cerebral Edema
Multiple mouse models have been employed to explore the role of AQP4 in the pathogenesis cerebral edema. The main approaches have been either to knockout AQP4 expression completely or to disrupt the polarized subcellular expression of AQP4.
4.1 Mouse Models Lacking AQP4 Expression
Phenotypic analysis of AQP4-deficient mice has provided new insights into the mechanisms of water transport during the development of cerebral edema (Manley et al. 2004). Because AQP4 allows bidirectional water flux through cell membranes, it is unsurprising that it facilitates water transport to and from the CNS. These experiments demonstrate the role of AQP4 in facilitating cellular water uptake as well as clearance of extracellular fluid from the brain.
The loss of AQP4 function has a significant impact on pathological response of the CNS. In disease models, such as acute cerebral ischemia (Manley et al. 2000), water intoxication (Manley et al. 2000; Yang et al. 2008) and traumatic brain injury, water moves into the cell resulting in cytotoxic brain edema. The deletion of AQP4 has shown to impair cell water uptake in all of these models tested to date (Fig. 2a–d), as demonstrated by reduced brain water content, infarct size, lesion volume, and lower ICP values. These favorable measures recorded from AQP4-deficient mice were mirrored by improved survival and better functional outcome compared to wild-type mice.
In another subset of CNS pathologies such as brain tumors, cold brain injury, and persistent ischemia, edema is created via leakage of iso-osmolar fluid through defective blood–brain barrier (BBB) into the brain extracellular space resulting in vasogenic edema. In these models, deletion of AQP4 resulted in worsening of cerebral edema assessed by brain wet to dry weight ratio and intracranial pressure (Papadopoulos et al. 2004). The detrimental effect of AQP4 deletion also translated to lower neurological score. Based on these findings it was concluded that AQP4 facilitates the clearance of vasogenic cerebral edema in pathologies where edema fluid accumulates in the extracellular space.
4.2 Mouse Models Lacking Polarized AQP4 Expression
As an alternative approach to testing AQP4 function, experiments were aimed at disrupting the polarized pattern of AQP4 distribution. The anchoring of AQP4 to the astrocytic foot processes is dependent on the dystrophin-α-syntrophin complex (Amiry-Moghaddam et al. 2003a, 2004a). Dystrophin is the protein mutated in Duchenne Muscular Dystrophy and is part of a large membrane assembly that link the cytoskeleton to the extracellular matrix (Worton 1995). Dystrophin binds to dystrobrevin, which provides a scaffold for syntrophins including α-syntrophin (Peters et al. 1997). The dystrophin complex is localized to many tissues that express AQP4, including perivascular astrocytic foot process, renal collecting duct and skeletal muscle (Neely et al. 2001). This prompted the investigation of expression level of AQP4 in mice deficient in various components of the dytroglycan complex (Neely et al. 2001; Vajda et al. 2002).
Co-immunoprecipitation studies show that AQP4 binds to the dystrophin complex through its interaction with α-syntrophin and Dp71 (Neely et al. 2001). Alpha syntrophin deficient mice lack polarized expression of AQP4 in astrocytic end-feet: Immunogold labeling demonstrates an ~eightfold reduction of AQP4 reactivity at the perivascular astrocytic end-feet of α-syntrophin deficient mice compared to wild-type controls (Neely et al. 2001). Such mislocalization translates into a near equivalent phenotype of AQP4 knock out mice. Following repetitive orthodromic stimulation of hippocampal slides, recovery of extracellular potassium significantly slows in α-syntrophin deficient mice (Amiry-Moghaddam et al. 2003b). The development of cytotoxic edema also significantly retards in α-syntrophin deficient mice following acute hyponatremia (Amiry-Moghaddam et al. 2004b) and transient cerebral ischemia (Amiry-Moghaddam et al. 2003a). The complete deficiency of the distroglycan complex in dmx mice has similar impact, resulting in delayed development of cytotoxic brain edema following acute hyponatremia (Vajda et al. 2002). Although both these mouse strains have similar AQP4 expression as the wild-type controls, the mere mislocalization of AQP4 is sufficient to impair channel function to a significant extent. Recent data has demonstrated marked reduction of AQP4 in glial cells of the retinal (Muller cells) isolated from Dp71- deficient mice (Fort et al. 2008). Electrophysiological studies have shown reduced potassium currents in relation to the reduced AQP4 expression further fortifying the concept of a Kir4.1-AQP4 potassium-water trafficking complex.
Further studies in models of cerebral edema using latter mouse strains could be of merit. The abolishment of AQP4 polarization by modulating the DCG components is a more attractive strategy than manipulating the AQP4 expression directly because it avoids changing AQP4 expression in other tissues.
5 Aquaporin-4 as a Therapeutic Target in Stroke
An important goal in the treatment of stroke is the control and reduction of cerebral edema. While formulating strategies targeting AQP4 in edema therapy the bimodal role of AQP4 in the development of vasogenic and cytotoxic edema has to be borne in mind. The reabsorption of vasogenic edema appearing in hemorrhagic stroke and late ischemic stroke could be facilitated by increased expression of functional AQP4, as the development of cytotoxic edema in early ischemia could be controlled by AQP4 inhibition. Thus, the type of stroke to be treated and the timing of AQP4 modulation will have to be carefully considered in the development of any targeted intervention.
Acknowledgement
Supported by NIH NS050173 and the UCSF Brain and Spinal Injury Center.
References
- Amiry-Moghaddam M, Otsuka T, Hurn PD, et al. An alpha-syntrophin-dependent pool of AQP4 in astroglial end-feet confers bidirectional water flow between blood and brain. Proc Natl Acad Sci U S A. 2003a;100:2106–2111. doi: 10.1073/pnas.0437946100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiry-Moghaddam M, Williamson A, Palomba M, et al. Delayed K+ clearance associated with aquaporin-4 mislocalization: phenotypic defects in brains of alpha-syntrophin-null mice. Proc Natl Acad Sci U S A. 2003b;100:13615–13620. doi: 10.1073/pnas.2336064100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiry-Moghaddam M, Frydenlund DS, Ottersen OP. Anchoring of aquaporin-4 in brain: molecular mechanisms and implications for the physiology and pathophysiology of water transport. Neuroscience. 2004a;129:999–1010. doi: 10.1016/j.neuroscience.2004.08.049. [DOI] [PubMed] [Google Scholar]
- Amiry-Moghaddam M, Xue R, Haug FM, et al. Alpha-syntrophin deletion removes the perivascular but not endothelial pool of aquaporin-4 at the blood-brain barrier and delays the development of brain edema in an experimental model of acute hyponatremia. FASEB J. 2004b;18:542–544. doi: 10.1096/fj.03-0869fje. [DOI] [PubMed] [Google Scholar]
- Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, Fini ME, Lo EH. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001 Oct 1;21(19):7724–7732. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardutzky J, Schwab S. Antiedema therapy in ischemic stroke. Stroke. 2007;38:3084–3094. doi: 10.1161/STROKEAHA.107.490193. [DOI] [PubMed] [Google Scholar]
- Bereczki D, Liu M, Prado GF, Fekete I. Cochrane report: a systematic review of mannitol therapy for acute ischemic stroke and cerebral parenchymal hemorrhage. Stroke. 2000;31:2719–2722. doi: 10.1161/01.str.31.11.2719. [DOI] [PubMed] [Google Scholar]
- Binder DK, Yao X, Zador Z, Sick TJ, Verkman AS, Manley GT. Increased seizure duration and slowed potassium kinetics in mice lacking aquaporin-4 water channels. Glia. 2006;53:631–636. doi: 10.1002/glia.20318. [DOI] [PubMed] [Google Scholar]
- Brown RC, Davis TP. Calcium modulation of adherens and tight junction function: a potential mechanism for blood–brain barrier disruption after stroke. Stroke. 2002;33:1706–1711. doi: 10.1161/01.str.0000016405.06729.83. [DOI] [PubMed] [Google Scholar]
- Castejon OJ. Formation of transendothelial channels in traumatic human brain edema. Pathol Res Pract. 1984;179:7–12. doi: 10.1016/S0344-0338(84)80054-0. [DOI] [PubMed] [Google Scholar]
- Connors NC, Kofuji P. Dystrophin Dp71 is critical for the clustered localization of potassium channels in retinal glial cells. J Neurosci. 2002;22:4321–4327. doi: 10.1523/JNEUROSCI.22-11-04321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa BE, Keep RF, Betz AL, Hoff JT. Plasminogen activators potentiate thrombin-induced brain injury. Stroke. 1998;29:1202–1207. doi: 10.1161/01.str.29.6.1202. discussion 1208. [DOI] [PubMed] [Google Scholar]
- Fort PE, Sene A, Pannicke T, et al. Kir4.1 and AQP4 associate with Dp71- and utrophin-DAPs complexes in specific and defined microdomains of Muller retinal glial cell membrane. Glia. 2008;56:597–610. doi: 10.1002/glia.20633. [DOI] [PubMed] [Google Scholar]
- Goldstein L, Teng ZP, Zeserson E, Patel M, Regan RF. Hemin induces an iron-dependent, oxidative injury to human neuron-like cells. J Neurosci Res. 2003;73:113–121. doi: 10.1002/jnr.10633. [DOI] [PubMed] [Google Scholar]
- Hacke W, Schwab S, Horn M, Spranger M, De Georgia M, von Kummer R. ‘Malignant’ middle cerebral artery territory infarction: clinical course and prognostic signs. Arch Neurol. 1996;53:309–315. doi: 10.1001/archneur.1996.00550040037012. [DOI] [PubMed] [Google Scholar]
- Hara-Chikuma M, Verkman AS. Physiological roles of glycerol-transporting aquaporins: the aquaglyceroporins. Cell Mol Life Sci. 2006;63:1386–1392. doi: 10.1007/s00018-006-6028-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua Y, Xi G, Keep RF, Hoff JT. Complement activation in the brain after experimental intracerebral hemorrhage. J Neurosurg. 2000;92:1016–1022. doi: 10.3171/jns.2000.92.6.1016. [DOI] [PubMed] [Google Scholar]
- Klatzo I. Presidental address. Neuropathological aspects of brain edema. J Neuropathol Exp Neurol. 1967;26:1–14. doi: 10.1097/00005072-196701000-00001. [DOI] [PubMed] [Google Scholar]
- Lee KR, Betz AL, Keep RF, Chenevert TL, Kim S, Hoff JT. Intracerebral infusion of thrombin as a cause of brain edema. J Neurosurg. 1995;83:1045–1050. doi: 10.3171/jns.1995.83.6.1045. [DOI] [PubMed] [Google Scholar]
- Manley GT, Fujimura M, Ma T, et al. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med. 2000;6:159–163. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- Manley GT, Binder DK, Papadopoulos MC, Verkman AS. New insights into water transport and edema in the central nervous system from phenotype analysis of aquaporin-4 null mice. Neuroscience. 2004;129:983–991. doi: 10.1016/j.neuroscience.2004.06.088. [DOI] [PubMed] [Google Scholar]
- Matsuoka H, Hamada R. Role of thrombin in CNS damage associated with intracerebral haemorrhage: opportunity for pharmacological intervention? CNS Drugs. 2002;16:509–516. doi: 10.2165/00023210-200216080-00001. [DOI] [PubMed] [Google Scholar]
- Nagelhus EA, Mathiisen TM, Ottersen OP. Aquaporin-4 in the central nervous system: cellular and subcellular distribution and coexpression with KIR4.1. Neuroscience. 2004;129:905–913. doi: 10.1016/j.neuroscience.2004.08.053. [DOI] [PubMed] [Google Scholar]
- Neely JD, Amiry-Moghaddam M, Ottersen OP, Froehner SC, Agre P, Adams ME. Syntrophin-dependent expression and localization of Aquaporin-4 water channel protein. Proc Natl Acad Sci U S A. 2001;98:14108–14113. doi: 10.1073/pnas.241508198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP. Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci. 1997;17:171–180. doi: 10.1523/JNEUROSCI.17-01-00171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshio K, Watanabe H, Song Y, Verkman AS, Manley GT. Reduced cerebrospinal fluid production and intracranial pressure in mice lacking choroid plexus water channel Aquaporin-1. FASEB J. 2005;19:76–78. doi: 10.1096/fj.04-1711fje. [DOI] [PubMed] [Google Scholar]
- Oshio K, Watanabe H, Yan D, Verkman AS, Manley GT. Impaired pain sensation in mice lacking aquaporin-1 water channels. Biochem Biophys Res Commun. 2006;341:1022–1028. doi: 10.1016/j.bbrc.2006.01.062. [DOI] [PubMed] [Google Scholar]
- Padmawar P, Yao X, Bloch O, Manley GT, Verkman AS. K+ waves in brain cortex visualized using a long-wavelength K+-sensing fluorescent indicator. Nat Methods. 2005;2:825–827. doi: 10.1038/nmeth801. [DOI] [PubMed] [Google Scholar]
- Papadopoulos MC, Verkman AS. Aquaporin-4 gene disruption in mice reduces brain swelling and mortality in pneumococcal meningitis. J Biol Chem. 2005;280:13906–13912. doi: 10.1074/jbc.M413627200. [DOI] [PubMed] [Google Scholar]
- Papadopoulos M, Saadoun S, Krishna S, Bell B, Davies D. The aquaporin-1 water channel protein is abnormally expressed in oedematous human brain tumours. J Anat. 2002;200:531–532. [Google Scholar]
- Papadopoulos MC, Manley GT, Krishna S, Verkman AS. Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB J. 2004;18:1291–1293. doi: 10.1096/fj.04-1723fje. [DOI] [PubMed] [Google Scholar]
- Peters MF, O’Brien KF, Sadoulet-Puccio HM, Kunkel LM, Adams ME, Froehner SC. beta-dystrobrevin, a new member of the dystrophin family. Identification, cloning, and protein associations. J Biol Chem. 1997;272:31561–31569. doi: 10.1074/jbc.272.50.31561. [DOI] [PubMed] [Google Scholar]
- Rash JE, Yasumura T, Hudson CS, Agre P, Nielsen S. Direct immunogold labeling of aquaporin-4 in square arrays of astrocyte and ependymocyte plasma membranes in rat brain and spinal cord. Proc Natl Acad Sci U S A. 1998;95:11981–11986. doi: 10.1073/pnas.95.20.11981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro Mde C, Hirt L, Bogousslavsky J, Regli L, Badaut J. Time course of aquaporin expression after transient focal cerebral ischemia in mice. J Neurosci Res. 2006;83:1231–1240. doi: 10.1002/jnr.20819. [DOI] [PubMed] [Google Scholar]
- Rincon F, Mayer SA. Novel therapies for intracerebral hemorrhage. Curr Opin Crit Care. 2004;10:94–100. doi: 10.1097/00075198-200404000-00003. [DOI] [PubMed] [Google Scholar]
- Saadoun S, Papadopoulos MC, Davies DC, Krishna S, Bell BA. Aquaporin-4 expression is increased in oedematous human brain tumours. J Neurol Neurosurg Psychiatry. 2002;72:262–265. doi: 10.1136/jnnp.72.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadoun S, Papadopoulos MC, Hara-Chikuma M, Verkman AS. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature. 2005a;434:786–792. doi: 10.1038/nature03460. [DOI] [PubMed] [Google Scholar]
- Saadoun S, Papadopoulos MC, Watanabe H, Yan D, Manley GT, Verkman AS. Involvement of aquaporin-4 in astroglial cell migration and glial scar formation. J Cell Sci. 2005b;118:591–598. doi: 10.1242/jcs.02680. [DOI] [PubMed] [Google Scholar]
- Satpathy M, Gallagher P, Lizotte-Waniewski M, Srinivas SP. Thrombin-induced phosphorrylation of the regulatory light chain of myosin II in cultured bovine corneal endothelial cells. Exp Eye Res. 2004;79:477–486. doi: 10.1016/j.exer.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Simard JM, Kent TA, Chen M, Tarasov KV, Gerzanich V. Brain oedema in focal ischaemia: molecular pathophysiology and theoretical implications. Lancet Neurol. 2007;6:258–268. doi: 10.1016/S1474-4422(07)70055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajda Z, Pedersen M, Fuchtbauer EM, et al. Delayed onset of brain edema and mislocalization of aquaporin-4 in dystrophin-null transgenic mice. Proc Natl Acad Sci U S A. 2002;99:13131–13136. doi: 10.1073/pnas.192457099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkman AS. More than just water channels: unexpected cellular roles of aquaporins. J Cell Sci. 2005;118:3225–3232. doi: 10.1242/jcs.02519. [DOI] [PubMed] [Google Scholar]
- Wang X, Mori T, Sumii T, Lo EH. Hemoglobin-induced cytotoxicity in rat cerebral cortical neurons: caspase activation and oxidative stress. Stroke. 2002;33:1882–1888. doi: 10.1161/01.str.0000020121.41527.5d. [DOI] [PubMed] [Google Scholar]
- Weis SM, Cheresh DA. Pathophysiological consequences of VEGF-induced vascular permeability. Nature. 2005;437:497–504. doi: 10.1038/nature03987. [DOI] [PubMed] [Google Scholar]
- Worton R. Muscular dystrophies: diseases of the dystrophin-glycoprotein complex. Science (New York, NY) 1995;270:755–756. doi: 10.1126/science.270.5237.755. [DOI] [PubMed] [Google Scholar]
- Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- Yang B, Zador Z, Verkman AS. Glial cell aquaporin-4 overexpression in transgenic mice accelerates cytotoxic brain swelling. J Biol Chem. 2008;283:15280–15286. doi: 10.1074/jbc.M801425200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zador Z, Bloch O, Yao X, Manley GT. Aquaporins: role in cerebral edema and brain water balance. Prog Brain Res. 2007;161:185–194. doi: 10.1016/S0079-6123(06)61012-1. [DOI] [PubMed] [Google Scholar]
- Zador Zsolt, MD, Yao Xiaoming, MD, Ph.D., Manley Geoffrey T., MD, Ph.D. In: Role of Aquaporins in Non-synaptic Mechanisms of Epilepsy Encyclopedia of Basic Epilepsy Research. Schwartzkroin P, editor. Amsterdam: Elsevier; (In Press) [Google Scholar]