Abstract
Background
Recently described neuronal intermediate filament inclusion disease (NIFID) shows considerable clinical heterogeneity.
Objective
To assess the spectrum of the clinical and neuropathological features in 10 NIFID cases.
Methods
Retrospective chart and comprehensive neuropathological review of these NIFID cases was conducted.
Results
The mean age at onset was 40.8 (range 23 to 56) years, mean disease duration was 4.5 (range 2.7 to 13) years, and mean age at death was 45.3 (range 28 to 61) years. The most common presenting symptoms were behavioral and personality changes in 7 of 10 cases and, less often, memory loss, cognitive impairment, language deficits, and motor weakness. Extrapyramidal features were present in 8 of 10 patients. Language impairment, perseveration, executive dysfunction, hyperreflexia, and primitive reflexes were frequent signs, whereas a minority had buccofacial apraxia, supranuclear ophthalmoplegia, upper motor neuron disease (MND), and limb dystonia. Frontotemporal and caudate atrophy were common. Histologic changes were extensive in many cortical areas, deep gray matter, cerebellum, and spinal cord. The hallmark lesions of NIFID were unique neuronal IF inclusions detected most robustly by antibodies to neurofilament triplet proteins and α-internexin.
Conclusion
NIFID is a neuropathologically distinct, clinically heterogeneous variant of frontotemporal dementia (FTD) that may include parkinsonism or MND. Neuronal IF inclusions are the neuropathological signatures of NIFID that distinguish it from all other FTD variants including FTD with MND and FTD tauopathies.
Neuronal intermediate filament inclusion disease (NIFID) is a distinctive neurologic disease with a clinically heterogeneous phenotype, including atypical dementia, pyramidal and extrapyramidal signs presenting at a young age.1–8 The clinical variability of this disease represents a diagnostic challenge. Grossly, there is focal atrophy of the frontal lobes, and to a lesser degree, the temporal and parietal lobes, and the caudate is frequently affected. Microscopically, the disease is characterized by hallmark lesions, neuronal IF inclusions, which contain neither tau nor α-synuclein but are variably ubiquitinated. The histopathology is variable, and the clinical features relate to the density and anatomic distribution of the neuronal IFs and other pathologies. In this study, we provide a detailed clinical and neuropathological description of the disease caused by the abnormal aggregation of neuronal IF proteins in 10 cases of NIFID.
Neuronal IFs are cytoskeletal proteins which include the neurofilament (NF) triplet of light (NF-L), medium (NF-M), and heavy (NF-H) subunits of approximately 68 kd (light), 145 kd (medium), and 200 kd (heavy).9,10 All of the NFs are phosphorylated, and the majority of the phosphorylation sites are located in the tail domain of NF-H.11 Immunohistochemistry using phosphorylation-dependent and -independent antibodies to NF epitopes has facilitated the molecular dissection of these proteins and has revealed that NFs within axons are heavily phosphorylated while NFs in the perikaryon and proximal segments of axons and dendrites are normally hypophosphorylated. 12 Abnormal accumulations of phosphorylated NF proteins in the cell body have been reported in several neurodegenerative diseases including Alzheimer disease (AD), Parkinson disease (PD), dementia with Lewy bodies (DLB), and motor neuron disease (MND), but the functions of phosphorylated NF proteins within the cytoplasm are unclear.12–15,33 Phosphorylation may have a toxic effect by impeding axonal transport, while the constitutive phosphorylation of NFs may have a protective effect by inhibiting proteolysis.16 Abnormal accumulations of NFs have also been described in cases of MND and the peripheral neuropathy Charcot–Marie–Tooth (CMT) disease which are associated with mutations in the NF-H and NF-L genes, respectively.17 Experimental transgenic mouse models which over express NF proteins such as the NFH/LacZ mouse also display some features of the pathology of MND.18
The neuronal IF protein α-internexin and the three NF triplet proteins have been classified as type IV IFs.19 α-Internexin is expressed by most, if not all, neurons during development and it precedes the expression of the NF triplet proteins.20 In comparison to the NF proteins, α-internexin is expressed at relatively low levels in the adult brain and there is selective neuronal expression with greater immuno-reactivity being seen in thin-caliber axons of the cerebellum and in the neuron cell bodies and processes of cortical layer II neurons. The NF triplet proteins and α-internexin may co-assemble.21 Over expression of rat α-internexin in a transgenic mouse model has been shown to cause abnormal neurofilamentous accumulations and motor coordination deficits.22 We have previously shown that α-internexin is a major component of the pathologic hallmark of NIFID,2 and the diagnostic specificity of neuronal α-internexin inclusions for NIFID was underlined by our observations that α-internexin immunoreactivity was only a minor component of the pathologic neuronal inclusions in other neurodegenerative disorders.3
However, given the apparent heterogeneity of NIFID as it is emerging in descriptions in recent publications, this report evaluates the phenotypic variations in the clinical symptoms and pathology caused by the abnormal aggregation of neuronal IF proteins in 10 cases of NIFID and reinforces the view that NIFID is a distinct variant of frontotemporal dementia (FTD).
Methods
Clinical, neuropsychological, and neuroimaging reports from 10 cases of NIFID were obtained from Canada, Norway, Spain, and Japan (1 case from each), and from France, the United Kingdom, and the United States (2 cases from each). All displayed the histologic hallmarks of NIFID, previously described.1–8 Age at onset was defined as the age at which an individual first demonstrated symptoms affecting personality, behavior, memory, language, or motor abilities sufficient to cause functional impairment and was estimated from clinical records. Following local hospital ethics committees’ guidelines and individual informed consent, blood specimens were used for genetic analyses. After death, the consent of the next of kin was obtained for brain autopsy.
Brain tissue was preserved in buffered 10% formalin or 4% paraformaldehyde; when available, tissue was frozen at −70°C for biochemistry. Tissue blocks were taken from the frontal, temporal, parietal, and occipital lobes, hippocampus, amygdala, basal ganglia, thalamus, midbrain, pons, medulla oblongata, cerebellum, and spinal cord. Sections were stained with hematoxylin and eosin, cresyl fast violet, Luxol fast blue, and periodic acid–Schiff and impregnated with silver according to the Bodian and modified Bielschowsky techniques. Following antigen retrieval methods, immunostaining was performed using the antibodies to all class IV neuronal IF proteins: phosphorylation-dependent neurofilament heavy subunit (RMO 2412 and SMI 312; Sternberger Monoclonals Inc., Lutherville, MD), non-phosphorylation-dependent (RMd 0912), phosphorylation-independent (RMO 189) NF-M23, phosphorylation-independent NF-L (NFL10 and NR4; Sigma- Aldrich, St. Louis, MO), and α-internexin (Zymed Laboratories Inc., San Francisco, CA). Ubiquitin antibodies were purchased from Chemicon International Inc. (Temecula, CA). The distribution and severity of lesions were assessed semiquantitatively using well-established methods for grading pathologic lesions in multiple fields. Ultrastructural studies were performed on formalin-fixed, paraffin wax-embedded, and frozen tissue following standard procedures.24,25
Results
Clinical features
The mean age at onset was 40.8 (range 23 to 56) years and the mean duration of disease was 4.5 (range 2.7 to 13) years. The mean age at death was 45.3 (range 28 to 61) years. The clinical symptoms are summarized in table 1 and table E-1 (available on the Neurology Web site at www.neurology.org). Four cases were women and six men. There was neither family history of psychiatric or neurologic disorder, nor exposure to environmental toxins. Presenting symptoms included personality change, apathy, blunted affect, and disinhibition in 7 of 10 patients. There was also memory loss, cognitive impairment, and language deficits present in 5 of 10 patients. Motor weakness was evident at presentation in 3 of 10 patients. Extrapyramidal features were present in 8 of 10 patients, with 5 of 10 patients showing a buccofacial apraxia and 5 patients with a supranuclear ophthalmoplegia. Personality and behavioral changes were eventually seen in 7 of the 10 patients for whom longitudinal neuro-psychiatric data were recorded. Memory deficits were reported in 9 of 10 patients, and language deficits in 7 of 9 patients. Hyperorality or weight gain was reported in 2 of 9 patients and weight loss in 1 of 9 patients. Hyperreflexia was present in 9 of 9 patients. Primitive reflexes were present in 6 of 7 patients. Nine of 10 patients became mute with advanced disease.
Table 1.
Clinical features
| Variable | Patient 11 | Patient 237 | Patient 35 | Patient 41 | Patient 55 | Patient 61 | Patient 76 | Patient 87 | Patient 91 | Patient 104 | Summary |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Demographic features | |||||||||||
| Gender | F | F | M | F | M | M | M | M | F | M | 4 F/6 M |
| Age at onset, y | 23 | 25 | 32 | 38 | 39 | 47 | 48 | 48 | 52 | 56 | 40.8; SD 11.3 |
| Duration, y | 5 | 4 | 3 | 3 | 3.5 | 3 | 4 | 13 | 2.7 | 4 | 4.5; SD 3.0 |
| Family history | N | N* | N | N | N | N | N | N | N | N | 0/10 |
| Symptoms and signs | |||||||||||
| Presenting symptom(s) | Apathy, dysphasia | Disinhibition, dysphasia, apathy |
Left arm weakness |
Memory, behavior |
Apathy | Disinhibition | Loss of fine motor dexterity, rigidity of left hand |
Disinhibition, motor weakness |
Memory, apathy |
Memory | |
| Language deficit | Y | Y | N | Y | Y | — | N | Y | Y | Y | 7/9 |
| Memory loss | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | 9/10 |
| Executive dysfunction | — | Y | — | Y | Y | Y | Y | Y | Y | Y | 8/8 |
| Mutism | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | 9/10 |
| Behavioral change | Y | Y | N | Y | Y | Y | N | N | Y | Y | 7/10 |
| Frontal lobe signs | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | 9/10 |
| Loss of insight | Y | — | Y | Y | Y | Y | — | Y | Y | — | 7/7 |
| Pyramidal signs | Y | Y | Y | Y | Y | Y | Y | Y | Y | — | 9/9 |
| Extrapyramidal signs | N | N | Y | Y | Y | Y | Y | Y | Y | Y | 8/10 |
| Oculomotor abnormalities | N | Y | Y | Y | Y | N | — | Y | N | — | 5/8 |
| CT/MRI atrophy | F, T, S | Atrophy | F | F, T | F | F, T | N | F, T, S | F, T, S | F, T | 9/10 |
| Clinical diagnoses | Atypical dementia | FTD, MND | CBD, PLS | Pick disease | FTD | FTD | PLS | Pick disease | FTD | FTD, parkinsonism | — |
A weak family history of late-onset dementia was recorded (see table E-1 on the Neurology Web site).
Y = present; N = absent; (—) = not available; F = frontal lobe; T = temporal lobe; S = striatum; FTD = frontotemporal dementia; MND = motor neuron disease; CBD = corticobasal degeneration; PLS = primary lateral sclerosis.
Structural brain imaging showed atrophy of the frontal lobe in 9 of 10 patients, and of the temporal lobe in 6 of 10 patients. In three cases, the caudate was atrophied. Three patients had had functional neuroimaging, with two exhibiting frontal hypometabolism and one showing parietal hypometabolism. No expansion in the Huntington gene was reported, and blood tests revealed no HIV infection. EEG recordings were unremarkable. Clinical vignettes of Patients 3 and 4 are described here (See also Vignettes V-1 to V-3 and V-5 to V-10 on the Neurology Web site at www.neurology.org). A summary of the neuropathology in these NIFID cases follows here.
Patient 3
This right-handed man had developed progressive motor difficulties in his left arm at age 32. Examination showed motor weakness with brisk reflexes in the left arm together with a marked spastic hypertonia with a plastic component. A mild parkinsonism with expression-less facies and left akinesia were also noticed. Neuropsychological evaluation at age 33 revealed initiation difficulties and depression. Memory was not impaired. Oculomotor testing showed slowness of saccades and distractibility suggestive of a pre-frontal impairment. MRI showed moderate asymmetric frontal atrophy. The patient gradually developed diffuse and hypertonic motor weakness, pseudobulbar palsy with dysarthria, swallowing difficulties, uncontrollable crying and laughing, supranuclear palsy, grasping reflex, and global cognitive impairment. There was no clinical or EMG evidence of anterior horn cell disease. Muscular biopsy and CSF examination (with 14-3-3 protein) were normal, and enzymatic assays did not find any metabolic abnormality suggestive of a gangliosidosis. Two clinical diagnoses were considered: atypical primary lateral sclerosis and corticobasal degeneration. The patient died at age 35.
Patient 4
This 37-year-old woman developed slurred speech and a decrease in her usual level of enthusiasm and social involvement. On examination in the first year of her illness, she was noted to have a scanning dysarthria and to be withdrawn. Subsequent mental status examinations found poor verbal fluency but intact literacy and arithmetic skills, impaired verbal and visual memory, orolingual apraxia but no limb apraxia, and disinhibition. Examinations also found abnormal saccadic eye movements, primitive reflexes, brisk tendon reflexes, mild parkinsonism, gegenhalten, and a stooped gait with poor arm swing. She gradually lost all speech such that she only was able to grunt or say yes or no. EEG and EMG were normal. Imaging revealed atrophy of the frontal and temporal lobes. The patient was diagnosed with Pick disease.
Neuropathology of 10 NIFID brains
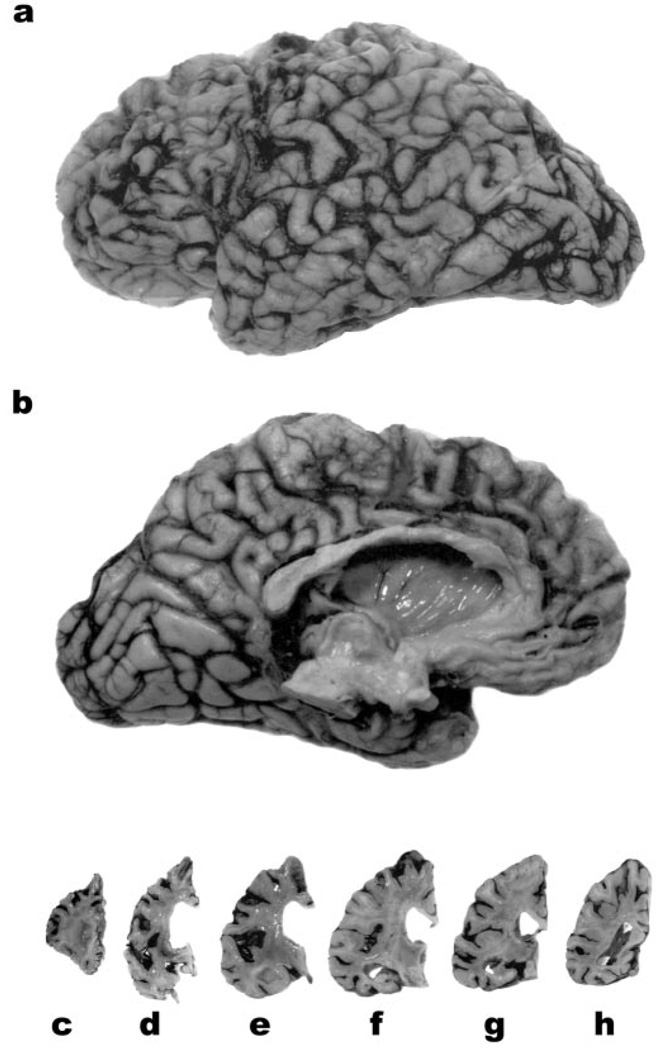
The mean brain weight was 983 g (range = 710 to 1,310 g). At the time of death, atrophy was much more pronounced than that visualized by imaging techniques during the course of the disease. Macroscopic examination showed frontal atrophy in all 10 cases and temporal atrophy in 9 of 10 cases with mild parietal atrophy in 4. The caudate nucleus was markedly atrophied in nine cases. Whereas the severity and pattern of atrophy varied from case to case, the frontal and temporal poles and caudate nucleus often were severely affected. In all cases, there was variable atrophy of subcortical nuclei including the basal ganglia, amygdala, and thalamus. The brainstem, cerebellum, and spinal cord appeared unremarkable. Coronal slices further confirmed frontotemporal atrophy: The gyri were narrowed and the intervening sulci widened with enlargement of the lateral ventricles (figure 1). In the more severely affected cases the cortical ribbon was thinned. In some cases there was striking atrophy of the amygdala and hippocampus. The histologic changes included the stereotypic lesions of FTDs: neuronal loss, status spongiosus, and astrocytosis in temporal and frontal isocortex. Swollen achromatic neurons were also seen. However, the most striking feature was the presence of inclusions containing neuronal IF proteins, which are composed of, the NF triplet proteins and α-internexin (figure 2 through figure 4; also see figures E-1 and E-2 on the Neurology Web site). The severity of each of these histologic abnormalities varied from case to case. Results are summarized in table 2.
Figure 1.
The lateral (a) and medial (b) aspects of the left hemibrain of 27-year-old woman with neuronal intermediate filament inclusion disease. There is pronounced atrophy of the frontal and anterior temporal lobes. Coronal slices of the left hemisphere (c through h) reveal enlargement of the lateral ventricle, and marked atrophy of the striatum (d). The Sylvian fissure (e) is widened and the amygdala (e) and hippocampus (f, g) are atrophied. The parietal (a, b, h) and occipital (a, b) lobes are relatively well preserved.
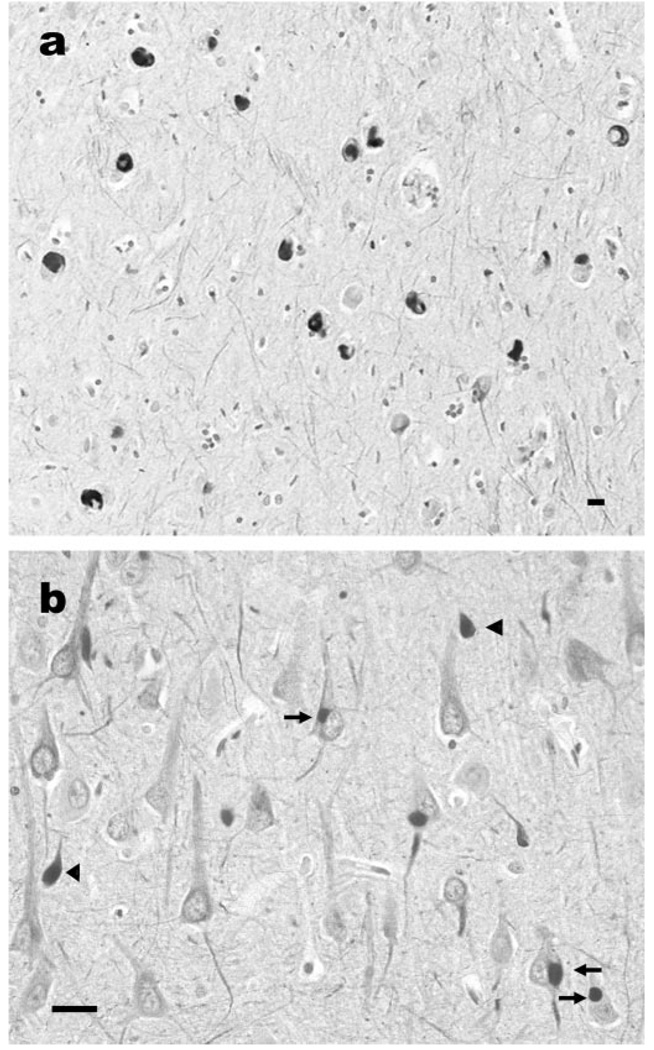
Figure 2.
Neuronal intermediate filament inclusions in the temporal lobe of a case of neuronal intermediate filament inclusion disease. There are pleomorphic neuronal inclusions in the subiculum (a) and in the perikarya of pyramidal neurons (b, arrows) and in swollen axons and spheroids (b, arrowheads) of hippocampal subfield CA1. α-Internexin immunohistochemistry. Scale bar = 10 µm.
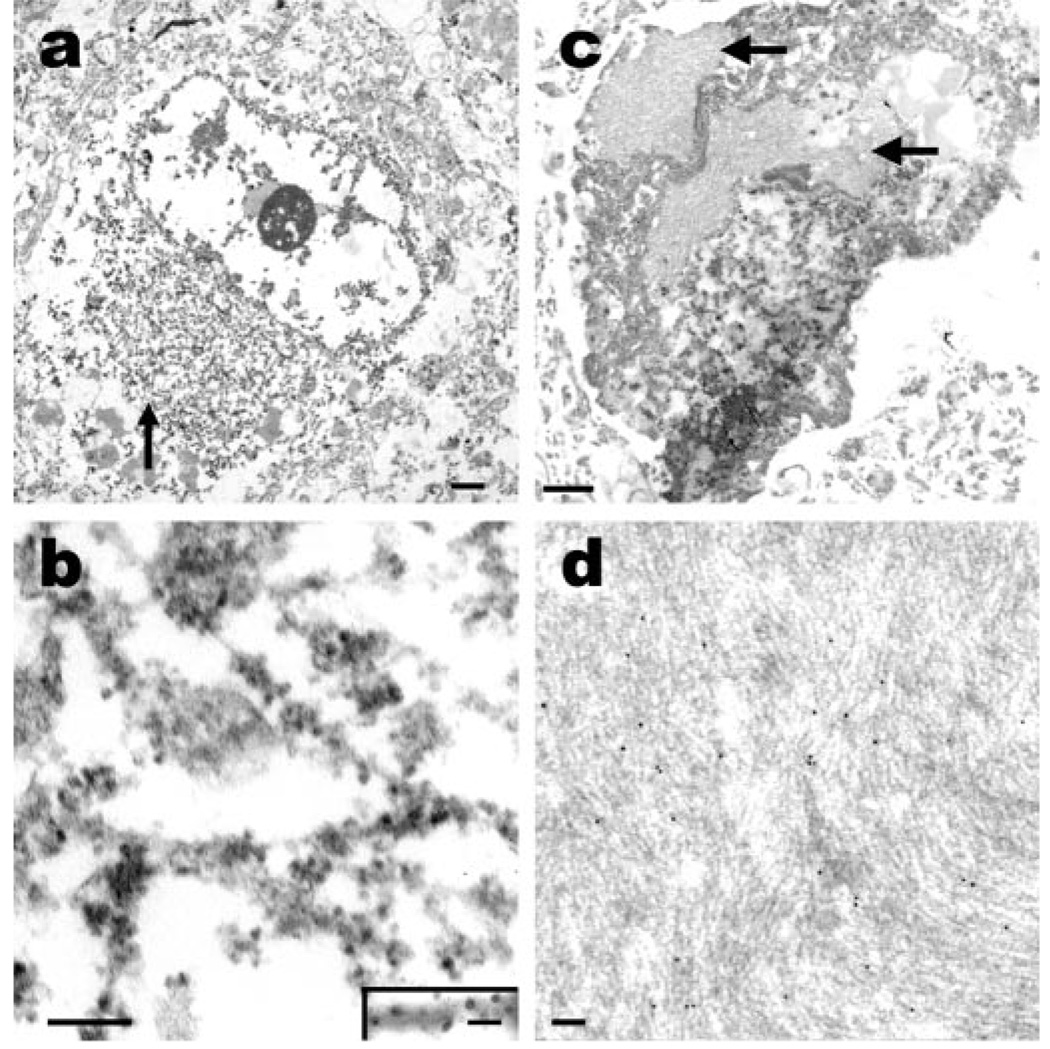
Figure 4.
Fine structure of inclusions in NIFID. (a) An electron micrograph of a neuronal cytoplasmic inclusion (arrow) in the frontal lobe. The inclusion is not membrane bound and contains granular filamentous material with a diameter of 10 to 25 nm (b). Immunoelectron microscopy (b, inset) reveals that the filaments of the inclusion contain epitopes of heavy-subunit neurofilament (RMO 24, immunoperoxidase-labeling). Hyaline bodies in NIFID contain dense fibrillar arrays. (c) A pyknotic neuron containing hyaline inclusions of closely packed arrays of filaments (arrows). (d) Immunoelectron microscopy of hyaline bodies reveals that the filaments contain epitopes of neurofilament proteins (SMI 312 labeled with 10-nm gold particles). (a, c) Scale bar = 1 µm; (b, d) scale bar = 100 nm and (b, inset) scale bar = 20 nm.
Table 2.
Neuropathology
| Variable | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | Case 10 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brain weight, g | 860 | 710 | NA | 904 | 950 | 1,200 | 1,310 | 850 | 813 | 1,250 | ||||||||||
| Atrophy | F, T, P, C | F, T, P, C | F, T, C | F, T, C | F, T, C | F, T, C | F | F, T, C | F, T, P, C, A | F, T, P, C, A | ||||||||||
| NL and I | NL | I | NL | I | NL | I | NL | I | NL | I | NL | I | NL | I | NL | I | NL | I | NL | I |
| Anterior frontal lobe, BA 9 | +++ | ++ | +++ | +++ | ++ | ++ | +++ | ++ | +++ | ++ | +++ | + | + | ++ | +++ | ++ | +++ | + | ++ | ++ |
| Posterior frontal lobe, BA 4 | ++ | ++ | +++ | +++ | + | ++ | +++ | ++ | +++ | ++ | +++ | ++ | +++ | ++ | +++ | ++ | + | +++ | ++ | +++ |
| Superior temporal lobe, BA 22 | ++ | ++ | ++ | ++ | + | + | + | ++ | ++ | + | ++ | ++ | ++ | ++ | ++ | ++ | + | +++ | + | ++ |
| Parahippocampal gyrus, BA 28 | ++ | ++ | 0 | + | + | ++ | ++ | + | ++ | ++ | + | ++ | ++ | +++ | ++ | ++ | +++ | + | +++ | ++ |
| Hippocampus CA1–4 | ++ | + | 0 | + | + | + | + | ++ | ++ | + | ++ | + | + | ++ | ++ | ++ | ++ | ++ | + | ++ |
| Dentate gyrus | ++ | + | 0 | + | + | + | + | + | + | + | ++ | ++ | + | + | ++ | ++ | + | + | + | + |
| Amygdala | + | + | NA | NA | NA | NA | + | + | ++ | + | NA | NA | + | + | + | + | +++ | + | +++ | ++ |
| Parietal lobe, BA 7 | ++ | ++ | + | + | 0 | + | ++ | ++ | + | + | NA | NA | 0 | + | + | + | + | + | + | + |
| Occipital lobe, BA 17 | + | + | + | + | 0 | 0 | 0 | + | 0 | 0 | NA | NA | 0 | + | + | + | 0 | + | + | + |
| Caudate nucleus | +++ | ++ | +++ | ++ | ++ | ++ | ++ | + | ++ | + | +++ | + | +++ | + | +++ | + | +++ | + | ++ | + |
| Putamen | +++ | + | + | ++ | + | + | ++ | + | + | + | +++ | + | ++ | + | +++ | + | +++ | ++ | + | + |
| Globus pallidus | + | + | +++ | + | + | + | + | + | + | + | NA | NA | ++ | + | +++ | + | + | + | + | + |
| Thalamus | + | + | + | ++ | + | ++ | + | ++ | ++ | ++ | NA | NA | + | ++ | + | + | + | ++ | + | + |
| Subthalamus | NA | NA | + | + | NA | NA | + | + | NA | NA | NA | NA | + | ++ | +++ | + | + | ++ | + | + |
| Red nucleus | + | + | + | + | + | + | + | + | + | + | NA | NA | + | + | + | + | + | + | + | 0 |
| Substantia nigra | + | 0 | ++ | + | + | + | + | + | + | + | NA | NA | + | + | +++ | + | + | + | + | 0 |
| Locus ceruleus | + | + | + | 0 | 0 | 0 | + | + | + | 0 | NA | NA | 0 | + | 0 | 0 | + | + | 0 | 0 |
| Pontine base | + | + | + | + | + | + | + | + | + | + | NA | NA | 0 | + | 0 | 0 | + | ++ | + | + |
| Inferior olivary nucleus | + | + | + | + | + | + | + | + | + | + | NA | NA | + | + | 0 | 0 | + | + | + | + |
| Hypoglossal nucleus | 0 | 0 | 0 | 0 | 0 | 0 | + | + | 0 | 0 | NA | NA | 0 | 0 | 0 | 0 | + | + | 0 | 0 |
| Cerebellar Purkinje cells | + | 0 | + | 0 | 0 | 0 | + | 0 | + | 0 | NA | NA | 0 | 0 | 0 | 0 | + | 0 | 0 | 0 |
| Dentate nucleus | + | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | NA | NA | 0 | 0 | 0 | 0 | 0 | 0 | + | 0 |
| Spinal cord | NA | NA | 0 | + | 0 | 0 | NA | NA | 0 | 0 | NA | NA | + | + | NA | NA | NA | NA | + | + |
Atrophy: A = amygdala, C = caudate–putamen, F = frontal, T = temporal, P = parietal lobes; NL = neuronal loss and gliosis: +++ = severe, ++ = moderate, + = mild, 0 = none; I = neuronal cytoplasmic inclusion: +++ = abundant, ++ = moderate, + = few, 0 = none; BA = Brodmann area.
Microscopy revealed extensive neuronal loss and reactive astrocytosis in the frontal, medial temporal, and parietal lobes and varying degrees of loss in subcortical nuclei including the caudate nucleus, putamen, globus pallidus, amygdala, substantia nigra, and locus ceruleus. A variable degree of neuronal loss and axonal swellings was present in the cerebellum. Occasional swollen achromatic neurons were observed in affected areas. In areas of neuronal loss, faintly eosinophilic, intraneuronal, cytoplasmic inclusions were present (see figure 3, a through c). In both hematoxylin and eosin- and cresyl violet-stained sections, hyaline aggregates were observed. In some hyaline bodies, but not all, a central core was stained producing a target-like structure (see figure 3, c and d). The inclusions were variably argyrophilic with modified Bielschowsky and Bodian silver impregnation (see figure 3 e through h).
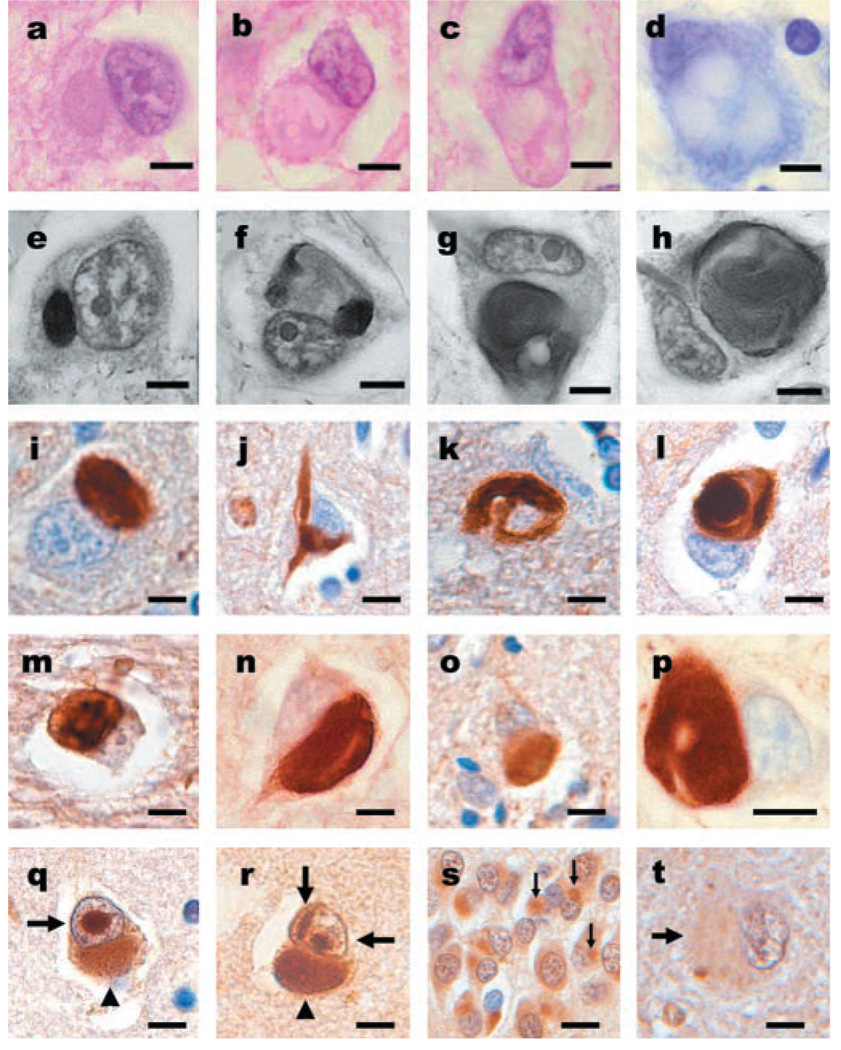
Figure 3.
Neuronal inclusions in neuronal intermediate filament inclusion disease (NIFID) are pleomorphic and contain all class IV IF proteins. (a) An eosinophilic, cytoplasmic inclusion in the frontal lobe. (b, c) Neurons with globular or elongated inclusions with hyaline compartments of variable eosinophilia. (a through c) Hematoxylin and eosin. (d) Only the central core of a hyaline inclusion is visible with cresyl violet stain. (e through h) Inclusions are variably argyrophilic and pleomorphic: some are spheroidal (a), others have stained and unstained compartments (f), and others have serpiginous and complex forms (g, h). Modified Bielschowsky silver impregnation. (i) Pick body-like inclusions are the most common morphological type. Inclusions contain all neuronal IF proteins. (j) A flame-shaped inclusion. (k) A filamentous serpiginous inclusion. (l) A globose inclusion. (i through l) α-Internexin immunohistochemistry. Epitopes of phosphorylation-dependent NF-H (RMO 24) (m), non-phosphorylation-dependent NF-H (RMd 09) (n), phosphorylation-independent NF (NF-M; RMO 189) (o), phosphorylation-independent NF (NF-L; NR4) (p), are present in the inclusions. (m through p) Neurofilament immunohistochemistry. Neuronal inclusions in NIFID are variably ubiquitinated. (q, r) Neuronal cytoplasmic (arrowhead) and intranuclear inclusions (arrows) are present in the same neurons. The intranuclear inclusions appear as round or elongated structures either as a single inclusion (q) or as multiple forms (r). (s) Inclusions in the neurons of the dentate gyrus are ubiquitinated (arrows). (t) In a 61-year-old case of NIFID, a hyaline neuronal fibrillary inclusion is weakly stained. (q through t) Ubiquitin immunohistochemistry. (a through h) Scale bars = 5 µm; (i through t) scale bars = 10 µm.
Neuronal IF pathology was both extensive and varied (see figure 2 and figure 3, i through p; also see figures E-1 and E-2 on the Neurology Web site). Immunohistochemistry demonstrated the presence within the inclusions of all class IV IF proteins (NF-H, NF-M, NF-L, and α-internexin; figure 3, i through p). The morphology of the inclusions was extremely variable throughout the neuraxis. Spherical inclusions were the most abundant morphological type and could be seen in superficial and deep cortical layers (figure 3i). Other morphological forms of neuronal cytoplasmic inclusions were seen in cortical (see figure 3, j through l) and subcortical nuclei (see figure E-1 on the Neurology Web site) and spinal cord (see figure E-2 on the Neurology Web site). Axonal swellings, similar to those found in ALS and normal aging, but which are not specific to any neurodegenerative disease, were also seen in affected areas and in underlying white matter, corticospinal tracts, and other white matter tracts (see figure E-2 on the Neurology Web site).
In three of five cases, there was evidence of corticospinal tract degeneration in the spinal cord (see figure E-2 on the Neurology Web site). However, motor neurons in the anterior horns were relatively spared and the number of pyknotic neurons (see figure E-2 on the Neurology Web site) was much lower than that typically seen in lower MND. Intraneuronal inclusions in the spinal cord were most commonly seen in the central gray, and these contained aggregates of neuronal IF proteins (see figure E-2 on the Neurology Web site). Axonal swellings and spheroids commonly seen in MND were present in the cortico-spinal tracts of the spinal cord, and an occasional Bunina body was seen in a motor neuron in the anterior horn (see figure E-2 on the Neurology Web site), although no skein-like or Lewy-body-like inclusions were seen in the spinal cords of cases of NIFID.
The cytoplasmic inclusions were variably ubiquitinated as demonstrated by immunohistochemistry. Ubiquitinated and neuronal IF-positive inclusions were most abundant in the youngest cases (Patients 1 and 2). Generally, more compact spherical inclusions were ubiquitinated (figure 3, q and r). Ubiquitinated inclusions were found in the neurons of the dentate gyrus in all cases. Rare single (see figure 3q) and multiple (see figure 3 r) round and elongated intranuclear inclusions were observed in neurons containing cytoplasmic inclusions, and these were present in 4 of 10 cases and present in cases of frontotemporal lobar degeneration (FTLD) with MND-type inclusions. The intranuclear inclusions in NIFID were not stained with IF antibodies.
Ultrastructural study of the neuronal cytoplasmic inclusions in NIFID revealed aggregates of granular filamentous material with no apparent limiting membrane (see figure 4a). The granular material resembled the morphology of ribosomes, and the filaments had an apparent diameter of 10 to 25 nm (see figure 4b). Immunoelectron microscopy demonstrated that the filaments of the inclusions contain epitopes of neuronal IF proteins (see figure 4b inset). The hyaline bodies observed in hematoxylin and cresyl violet-stained sections contained more compact arrays of neuronal IFs (see figure 4c), and immunoelectron microscopy revealed that these filaments also contain epitopes of neuronal IF proteins (see figure 4d).
Discussion
The NIFID patients described here developed a spectrum of clinical symptoms with features of FTD, FTD with upper MND, upper MND, and corticobasal degeneration, not unlike the clinical manifestations of other FTDs including FTD tauopathies.26–29 The most common macroscopic finding was frontotemporal and caudate atrophy with loss of neurons, status spongiosus, and gliosis. The hallmark lesions are neuronal IF inclusions. The severity and anatomic distribution of the lesions relate to the clinical features.
The 10 patients described here have phenotypic similarities and differences. In all cases, the disease was insidious, of early onset, and steadily progressive. Frontal lobe signs, decline in social and personal conduct, and language and memory difficulty were frequent and prominent. Blunting of emotions and depression occurred in six of nine patients. Most patients had no or little insight into the nature of their disease. There were frequent behavioral changes, including a decline in personal hygiene and hyperorality in some cases. Limb motor weakness, buccofacial apraxia, and supranuclear ophthalmoplegia were early and prominent features in a minority of cases. Two cases presented with an asymmetric limb weakness, and limb dystonia was present in one case, with upper motor neuron and pyramidal signs similar to the phenotype of corticobasal degeneration (CBD).28 Two patients developed psychotic signs and anxiety. Primitive reflexes were a common finding indicating frontal lobe dysfunction. Brisk tendon reflexes were frequent. Difficulty with swallowing was a common observation. Parkinsonism was a common finding in this series and in four previously reported cases.8 In most cases, brain imaging revealed focal changes in the frontal and, to a lesser degree, the parietal and temporal lobes. The basal ganglia were often shrunken in those cases where imaging was available. EEG was normal. All of the patients were mute and bedridden and required constant nursing care in the terminal stage of the disease.
The relatively short duration of disease in NIFID is shorter than most cases of FTD.25 The relatively early onset of the disease is consistent with that seen in other sporadic and familial cases of FTD.26,30 Brain imaging showing atrophy of the frontal lobe and basal ganglia in NIFID was comparable to that seen in cases of FTLD, Pick disease, CBD, and FTLD with MND-type inclusions (FTLD-MND). However, the temporal lobe, particularly the hippocampus, was generally less atrophied in NIFID than in Pick disease and other FTDs. Thus, the clinical features of NIFID were most like those found in FTDs and unlike these of other neurodegenerative dementing diseases including AD, DLB, and prion disease.
Although there is clinical overlap between FTDs, NIFID can be easily distinguished neuropathologically from all other FTD subtypes including FTD tauopathies such as Pick disease, CBD, progressive supranuclear palsy, and FTD with parkinsonism linked to chromosome 17 caused by tau gene mutations that are characterized by widespread and prominent neuronal and glial tau inclusions. However, tau is not seen in NIFID inclusions and α-internexin immunohistochemistry readily distinguishes between the inclusions of NIFID and those in all tauopathies.
Superficially, the histopathology of NIFID resembles that seen in cases of MND with dementia and FTLD with MND-type inclusions. However, the ubiquitinated inclusions in NIFID are much more variable in shape, density and distribution. More importantly, NIFID can be distinguished from both MND with dementia and FTLD with MND-type inclusions by α-internexin, which is a signature of the inclusions in NIFID.2,3
Immunohistochemistry and electron microscopy link the neuropathology of NIFID most closely to MND since phosphorylated NF proteins are found in chromatolytic neurons and as fibrillary inclusions in affected neurons of both disorders.12,30–32 In both MND and MND with dementia, chromatolytic neurons, cytoplasmic fibrillary inclusions, axonal swellings, and large swellings called spheroids are found most frequently in the spinal cord and they contain predominantly phosphorylated NF proteins.33 Globules and swellings are found in normal aged spinal cords, but they are far more numerous in MND.12 α-Internexin is a major component of the inclusions in NIFID, but is largely absent from the neuronal inclusions of MND,3 whereas accumulations of NFs may contribute to NIFID and MND.34
Lewy body-like hyaline inclusions have been reported in motor and extramotor cortices of sporadic cases of MND,31,35 and these are also present in NIFID. Neuronal IF immunohistochemistry reveals that these structures contain all class IV IF proteins and are called, more appropriately, neuronal IF inclusions. Bunina bodies are considered to possess disease specificity for MND,35 and rare examples (see figure E-2 on the Neurology Web site) were present in one case of NIFID with primary lateral sclerosis, suggesting an overlap between MND and NIFID. However, the basophilic cytoplasmic inclusions seen in juvenile and sporadic ALS31 were not observed in any of the NIFID cases reported here.
NF accumulations have been reported in several diseases including MND, PD, DLB, progressive supranuclear palsy, Charcot–Marie–Tooth (CMT) disease, diabetic neuropathy, and giant axonal neuropathy. 17 NIFID is the first neurodegenerative disease in which another neuronal IF, α-internexin, is the major component of the pathologic inclusion.2 Although mutations in NF genes have been associated with CMT disease, PD, and MND,17 none of our cases had a family history of neurologic or psychiatric disease. On the other hand, the early age at onset might signify that NIFID is a recessive genetic disorder; this idea remains to be examined in more detail.
The mechanisms leading to neuronal IF aggregation in the cytoplasm and proximal axons of NIFID are unknown. This and our previous studies show that α-internexin is a major component of the pathologic inclusions in NIFID.2,3 Although NFs aggregate in neurodegenerative diseases, the role of α-internexin in this process is currently unknown. It is possible that a failure of axonal transport may contribute to the abnormal cytoplasmic accumulation and transgenic mice over expressing α-internexin cause abnormal NF accumulations, indicating that this is a primary cause of accumulation. 22 Toxic insults, including elevated levels of the neurotransmitter glutamate may also contribute to decreased axonal transport and NF accumulation.17,36 The role of α-internexin in the pathogenesis of NIFID and other neurodegenerative disorders characterized by pathologic aggregates of IF remains to be elucidated. The identification of other cases of NIFID will extend understanding of the phenotype and its prevalence.
Acknowledgment
The authors thank the staff of the Center for Neurodegenerative Disease Research of the University of Pennsylvania and the Department of Neuropathology, Newcastle General Hospital, Newcastle-upon-Tyne, UK, for technical support and the families of patients for their participation.
Supported by grants from the National Institute on Aging, NIH (AG-09215, AG-10124, and AG-17586 to V.M.-Y.L. and J.Q.T. and AG-10130 and ES12068 to M.G.), and from the Wellcome Trust (GR066166AIA) to N.J.C., J.R.T., and S.M. V.M.-Y.L. is the John H. Ware 3rd Professor of Alzheimer’s Research, and J.Q.T. is the William Maul Measey-Truman G. Schnabel, Jr., MD, Professor of Geriatric Medicine and Gerontology.
Footnotes
Additional material related to this article can be found on the Neurology Web site. Go to www.neurology.org and scroll down the Table of Contents for the October 26 issue to find the link for this article.
References
- 1.Cairns NJ, Perry RH, Jaros E, et al. Patients with a novel neurofilamantopathy: dementia with neurofilament inclusions. Neurosci Lett. 2003;341:177–180. doi: 10.1016/s0304-3940(03)00100-9. [DOI] [PubMed] [Google Scholar]
- 2.Cairns NJ, Zhukareva V, Uryu K, et al. α-Internexin is present in the pathological inclusions of neuronal intermediate filament inclusion disease. Am J Pathol. 2004;164:2153–2161. doi: 10.1016/s0002-9440(10)63773-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cairns NJ, Uryu K, Bigio E, et al. α-Internexin aggregates are abundant in neuronal intermediate filament inclusion disease (NIFID) but rare in other neruodegenerative diseases. Acta Neuropathol. 2004;108:213–233. doi: 10.1007/s00401-004-0882-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bigio EH, Lipton AM, White CL, III, et al. Frontotemporal dementia and motor neurone degeneration with neurofilament inclusion bodies: additional evidence for overlap between FTD and ALS. Neuropathol Appl Neurobiol. 2003;29:239–253. doi: 10.1046/j.1365-2990.2003.00466.x. [DOI] [PubMed] [Google Scholar]
- 5.Duyckaerts C, Mokhtari K, Fontaine B, et al. Maladie de Pick généralisée: une démence mal nommée caractérisée par des inclusions neurofilamentaires. Rev Neurol (Paris) 2003;159:219–219. [Google Scholar]
- 6.Gearing M, Castellano AA, Hunter SB, et al. Unusual neuropathologic findings in a case of primary lateral sclerosis. J Neuropathol Exp Neurol. 2003;62:555–555. [Google Scholar]
- 7.Yokoo H, Oyama T, Hirato J, et al. A case of Pick’s disease with unusual neuronal inclusions. Acta Neuropathol. 1994;88:267–272. doi: 10.1007/BF00293404. [DOI] [PubMed] [Google Scholar]
- 8.Josephs KA, Holton JL, Rossor MN, et al. Neurofilament inclusion body disease: a new proteinopathy? Brain. 2003;126:2291–2303. doi: 10.1093/brain/awg231. [DOI] [PubMed] [Google Scholar]
- 9.Lee K, Cleveland DW. Neuronal intermediate filaments. Ann Rev Neurosci. 1996;19:187–217. doi: 10.1146/annurev.ne.19.030196.001155. [DOI] [PubMed] [Google Scholar]
- 10.Carden MJ, Trojanowski JQ, Schlaepfer WW, et al. Two-stage expression of neurofilament polypeptides during rat neurogenesis with early establishment of adult phosphorylation patterns. J Neurosci. 1987;7:3499–3504. doi: 10.1523/JNEUROSCI.07-11-03489.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee VM-Y, Carden MJ, Schlaepfer WW, et al. Monoclonal antibodies distinguish several differentially phosphorylated states of the two largest rat neurofilament subunits (NF-H and NF-M) and demonstrate their existence in the normal nervous system of adult rats. J Neurosci. 1987;7:3478–3488. doi: 10.1523/JNEUROSCI.07-11-03474.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt ML, Carden MJ, Lee VM-Y, et al. Phosphate dependent and independent neurofilament epitopes in the axonal swellings of patients with motor neuron disease and controls. Lab Invest. 1987;56:282–294. [PubMed] [Google Scholar]
- 13.Schmidt ML, Lee VM-Y, Trojanowski JQ. Relative abundance of tau and neurofilament epitopes in hippocampal neurofibrillary tangles. Am J Pathol. 1990;136:1069–1075. [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt ML, Lee VM-Y, Trojanowski JQ. Comparative epitope analysis of neuronal cytoskeletal proteins in Alzheimer’s disease senile plaque neurites and neuropil threads. Lab Invest. 1991;64:352–357. [PubMed] [Google Scholar]
- 15.Schmidt ML, Murray J, Lee VM-Y, et al. Epitope map of neurofilament protein domains in cortical and peripheral nervous system Lewy bodies. Am J Pathol. 1991;139:53–65. [PMC free article] [PubMed] [Google Scholar]
- 16.Goldstein E, Sternberger NM, Sternberger LA. Phosphorylation protects neurofilaments against proteolysis. J Neuroimmunol. 1987;14:149–160. doi: 10.1016/0165-5728(87)90049-x. [DOI] [PubMed] [Google Scholar]
- 17.Al-Chalabi A, Miller CCJ. Neurofilaments and neurological disease. BioEssays. 2003;25:346–365. doi: 10.1002/bies.10251. [DOI] [PubMed] [Google Scholar]
- 18.Galvin JE, Nakamura M, McIntosh TK, et al. Neurofilament-rich intra-neuronal inclusions exacerbate neurodegenerative sequalae of brain trauma in NFH/LacZ transgenic mice. Exp Neurol. 2000;165:77–89. doi: 10.1006/exnr.2000.7461. [DOI] [PubMed] [Google Scholar]
- 19.Ching GY, Liem RKH. Structure of the gene for the neuronal intermediate filament protein α-internexin and functional analysis of its promoter. J Biol Chem. 1991;29:19459–19468. [PubMed] [Google Scholar]
- 20.Fliegner KH, Kaplan MP, Wood TL, et al. Expression of the gene for the neuronal intermediate filament protein α-internexin coincides with the onset of neuronal differentiation in the developing rat nervous system. J Comp Neurol. 1994;342:161–173. doi: 10.1002/cne.903420202. [DOI] [PubMed] [Google Scholar]
- 21.Ching GY, Liem RKH. Roles of head and tail domains in α-internexin’s self-assembly and coassembly with the neurofilament triplet proteins. J Cell Science. 1998;111:321–333. doi: 10.1242/jcs.111.3.321. [DOI] [PubMed] [Google Scholar]
- 22.Ching GY, Chien C-L, Flores R, et al. Overexpression of α-internexin causes abnormal neurofilamentous accumulations and motor coordination deficits in transgenic mice. J Neurosci. 1999;19:2974–2986. doi: 10.1523/JNEUROSCI.19-08-02974.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee VM-Y, Otvos L, Jr, Schmidt ML, et al. Alzheimer disease tangles share immunological similarities with multiphosphorylation repeats in the two large neurofilament proteins. Proc Natl Acad Sci USA. 1988;85:7384–7388. doi: 10.1073/pnas.85.19.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duda JE, Giasson BI, Mabon ME, et al. Novel antibodies to synuclein show abundant striatal pathology in Lewy body diseases. Ann Neurol. 2002;52:205–210. doi: 10.1002/ana.10279. [DOI] [PubMed] [Google Scholar]
- 25.Thorpe JR, Morley SJ, Rulten SL. Utilizing the peptidyl-prolyl cis-trans isomerase pin1 as a probe of its phosphorylated target proteins. Examples of binding to nuclear proteins in a human kidney cell line and to tau in Alzheimer’s diseased brain. J Histochem Cytochem. 2001;49:97–108. doi: 10.1177/002215540104900110. [DOI] [PubMed] [Google Scholar]
- 26.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 27.Neary D, Snowden JS, Mann DMA, et al. Frontal lobe dementia and motor neuron disease. J Neurol Neurosurg Psychiat. 1990;53:23–32. doi: 10.1136/jnnp.53.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boeve BF, Lang AE, Lang AE. Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Neurology. 2003;54(suppl):S15–S19. doi: 10.1002/ana.10570. [DOI] [PubMed] [Google Scholar]
- 29.Brooks BR. El Escorial World Federation of Neurology Criteria for the Diagnosis of Amyotrophic Lateral Sclerosis. J Neurol Sci. 1994;124(suppl):96–107. doi: 10.1016/0022-510x(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 30.Kertesz A, Kawarai T, Rogaeva E, et al. Familial frontotemporal dementia with ubiquitin-positive, tau-negative inclusions. Neurology. 2000;54:818–827. doi: 10.1212/wnl.54.4.818. [DOI] [PubMed] [Google Scholar]
- 31.Leigh PN, Dodson A, Swash M, et al. Cytoskeletal abnormalities in motor neuron disease: an immunohistochemical study. Brain. 1989;112:521–535. doi: 10.1093/brain/112.2.521. [DOI] [PubMed] [Google Scholar]
- 32.Okamoto K, Hirai S, Yamazaki T, et al. New ubiquitin-positive intraneuronal inclusions in the extra-motor cortices in patients with amyotrophic lateral sclerosis. Neurosci Lett. 1991;129:233–236. doi: 10.1016/0304-3940(91)90469-a. [DOI] [PubMed] [Google Scholar]
- 33.Manetto V, Sternberger NH, Perry G, et al. Phosphorylation of neuro-filaments is altered in amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 1988;47:642–653. doi: 10.1097/00005072-198811000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Strong MJ, Strong WL, Jaffe H, et al. Phosphorylation state of the native high-molecular-weight neurofilament subunit protein from cervical spinal cord in sporadic amyotrophic lateral sclerosis. J Neurochem. 2001;76:1315–1325. doi: 10.1046/j.1471-4159.2001.00094.x. [DOI] [PubMed] [Google Scholar]
- 35.Ikemoto A, Hirano A, Akiguchi I. Neuropathology of amyotrophic lateral sclerosis with extra-motor system degeneration: characteristics and differences in the molecular pathology between ALS with dementia and Gumanian ALS. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:97–104. doi: 10.1080/14660820050515395. [DOI] [PubMed] [Google Scholar]
- 36.Durham HD. Aggregation of intermediate filaments by 2,5-hexanedione: comparison of effects on neurofilaments, GFAP filaments and vimentin filaments in dissociated cultures of mouse spinal cord dorsal root ganglia. J Neuropathol Exp Neurol. 1988;47:432–442. doi: 10.1097/00005072-198807000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Mackenzie IRA, Feldman H. Neurofilament inclusion body disease with early onset frontotemporal dementia and primary lateral sclerosis. Clin Neuropathol. 2004;23:183–193. [PubMed] [Google Scholar]