Case report
We report on a 45-year-old Irishman who presented with a 9-month history of progressive weakness and wasting of his hands followed by dysarthria, falls, weight loss, and slowness. His family reported personality change characterized by apathy, rigid adherence to routines, and, later, temper outbursts. He developed a perseverative mannerism of pointing and shaking his right finger when speaking (see Video).
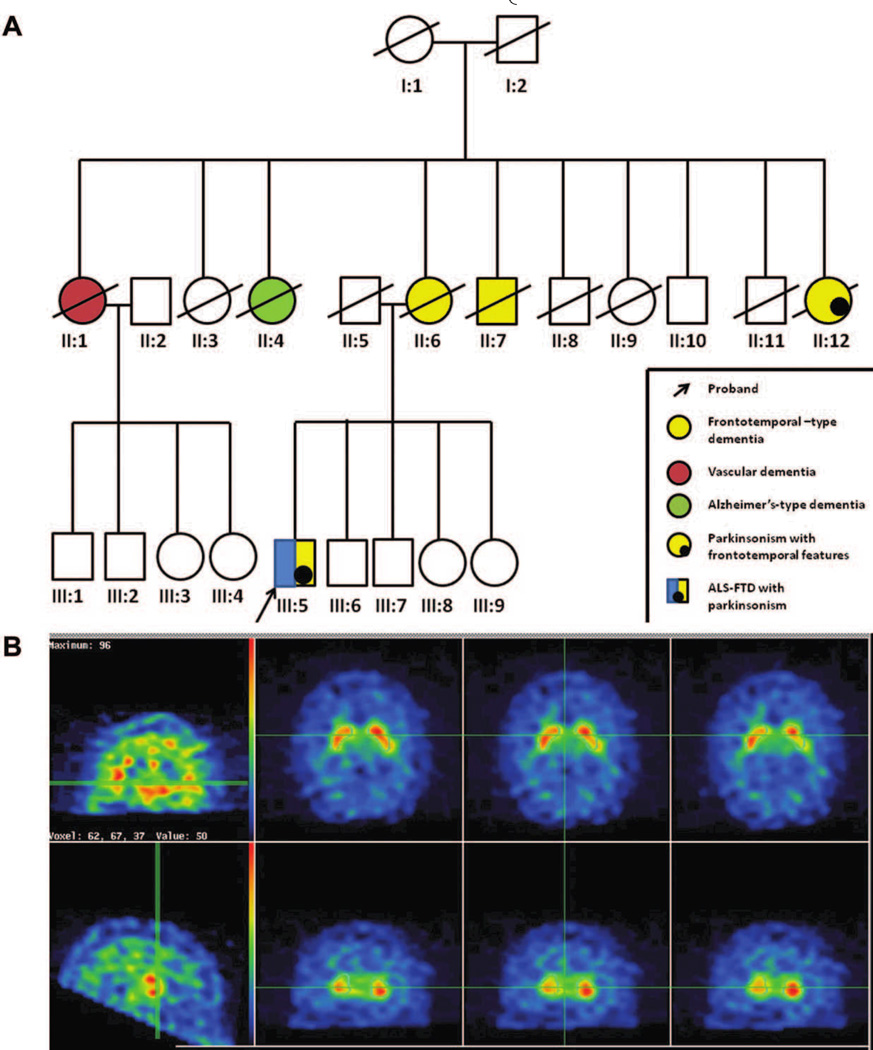
The patient’s mother (case II:6) developed dementia in her seventies characterized by compulsive behavior, hoarding and aggression, progressing to mutism, and death within 3 years (see Fig. 1A). His maternal aunt (case II:12) developed similar features and parkinsonism in her late sixties and died within 4 years; a maternal uncle (case II:7) developed behavior and personality change before dying in a care institution in his fifties. Two other maternal aunts had dementia: one labeled as vascular dementia (Case II:1), and the other as senile dementia (case II:4). None of the affected relatives had an autopsy.
FIG. 1.
(A) Pedigree demonstrating clustering and overlap of neurodegenerative syndromes. Affected individuals are represented by filled symbols (key indicates color coding of syndromes); deceased individuals are marked by a slash. (B) DAT scan demonstrating reduced uptake in the right striatum.
Physical exam revealed bradyphrenia, dysarthria, dysfluent speech, wasting in the hands, and widespread fasciculations. Left-sided bradykinesia was out of proportion to weakness (see Video). There was mild left-sided rigidity in addition to spasticity, no tremor, brisk reflexes, downgoing plantars, and a normal sensory examination. Posture was stooped with reduced arm swing, and the patient turned “en bloc” and had an abnormal “pull” test (see Video). Neuropsychological testing revealed executive dysfunction as well as poor confrontational naming, working memory, and semantic encoding.
Electromyography (EMG) showed widespread partial denervation with normal sensory studies. Brain MRI revealed mild, symmetrical frontotemporal atrophy. Dopamine transporter (DAT) scan showed reduced radiotracer uptake in the right striatum (see Fig. 1B). We found a pathogenic hexanucleotide (GGGGCC) expansion, of at least 40 repeat units, in the noncoding region of the C9ORF72 gene.
A hexanucleotide repeat expansion in the C9ORF72 gene on chromosome 9p21 has been identified as the most common genetic abnormality in both familial amyotrophic lateral sclerosis (ALS) and familial frontotemporal dementia (FTD).1,2 Pathologically, these patients have unique characteristics, including neuronal cytoplasmic inclusions in cerebellar granular neurons and hippocampal pyramidal layer that are not present in patients without the C9ORF72 expansion.3,4 Though the mechanism of disease is unknown, it is postulated that the gene expansion gives rise to toxic RNA that impairs gene transcription.1,2 Additional genetic or environmental factors may influence the predilection of one phenotype over another. The coexistence of ALS, frontotemporal dysfunction, and parkinsonism, although very rare, has been described.5 Western Pacific ALS/parkinsonism/dementia complex may manifest with any, or all of the three, syndromes.6 Likewise, mutations in the microtubule-associated protein tau gene on chromosome 17 can give rise to a spectrum of phenotypes, including parkinsonism, FTD, and, rarely, amyotrophy.7 Our report is the first to describe parkinsonism in detail with video footage and DAT imaging in the setting of the C9ORF72 mutation and further highlights the phenotypic heterogeneity attributable to a single-gene mutation as well as the genetic heterogeneity in ALS/parkinsonism/dementia.
Supplementary Material
Video 1. The patient’s stooped posture with rounded shoulders is evident; he walks with reduced arm swing and turns en bloc. The facies is hypomimic. The pull test is abnormal. Bradykinesia is evident with slower shoulder shrug and left-sided alternating movements; the decrement in response underlines that extrapyramidal features are present in addition to spasticity. The patient has a repeated mannerism of pointing and shaking his finger. Signe d’applause is positive. Speech is dysarthric with perseveration.
Acknowledgments
The authors wish to thank the patient and his family for their willingness to participate in this research study.
Funding agencies
This work was supported by The Motor Neuron Disease Association (grant no.: 8047), The Irish Institute of Clinical Neuroscience, and the Mater College of Postgraduate Research, Dublin. This work was funded in part by the Intramural Research Program of the NIH, National Institute on Aging (Z01-AG000949-02) and NINDS. Bryan J. Traynor was funded by the ALS Association, The Packard Center for ALS Research, FIGC, and The Myasthenia Gravis Foundation. A patent is pending for C9ORF72, with Bryan J. Traynor, Nigel M. Williams, and Huw R. Morris listed as inventors.
Footnotes
Relevant conflicts of interest/financial disclosures: Timothy Lynch has received educational grants from Bayer-Schering, Biogen, Boerhinger-Ingelheim, Lundbeck, Orion and Merck Serona; and for payment for advisory boards and lectures from Abbott, Biogen, Lundbeck, Novartis and UCB.
References
- 1.Renton AE, Majounie E, Waite A, et al. A Hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dejesus-Hernandez M, Mackenzie IR, Boeve BF, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray ME, DeJesus-Hernandez M, Rutherford NJ, et al. Clinical and neuropathalogical heterogeneity of C9FTD/ALS associated with hexanucleotide repeat expansion in C9ORF72. Acta Neuropathol. 2011;122:673–690. doi: 10.1007/s00401-011-0907-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Sarraj S, King A, Troakes C, et al. p62 positive, TDP-43 negative, neuronal cytoplasmic, and intranuclear inclusions in the cerebellum and hippocampus define the pathology of C9ORF72-linked FTLD and MND/ALS. Acta Neuropathol. 2011;122:691–702. doi: 10.1007/s00401-011-0911-2. [DOI] [PubMed] [Google Scholar]
- 5.Hudson AJ. Amyotrophic lateral sclerosis and its association with dementia, parkinsonism, and other neurological disorders: a review. Brain. 1981;104:217–247. doi: 10.1093/brain/104.2.217. [DOI] [PubMed] [Google Scholar]
- 6.Hirano A, Kurland LT, Krooth RS, Lessell S. Parkinsonism-dementia complex, an endemic disease on the island of Guam. I. Clinical features. Brain. 1961;84:642–661. doi: 10.1093/brain/84.4.642. [DOI] [PubMed] [Google Scholar]
- 7.Lynch T, Sano M, Marder KS, et al. Clinical characteristics of a family with chromosome 17-linked disinhibition-dementia-parkinsonism-amyotrophy complex. Neurology. 1994;44:1878–1884. doi: 10.1212/wnl.44.10.1878. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1. The patient’s stooped posture with rounded shoulders is evident; he walks with reduced arm swing and turns en bloc. The facies is hypomimic. The pull test is abnormal. Bradykinesia is evident with slower shoulder shrug and left-sided alternating movements; the decrement in response underlines that extrapyramidal features are present in addition to spasticity. The patient has a repeated mannerism of pointing and shaking his finger. Signe d’applause is positive. Speech is dysarthric with perseveration.