Abstract
It has been estimated that a human cell is confronted with 1 million DNA lesions every day, one fifth of which may originate from the activity of Reactive Oxygen Species (ROS) alone [1,2]. Terminally differentiated neurons are highly active cells with, if any, very restricted regeneration potential [3]. In addition, genome integrity and maintenance during neuronal development is crucial for the organism. Therefore, highly accurate and robust mechanisms for DNA repair are vital for neuronal cells. This requirement is emphasized by the long list of human diseases with neurodegenerative phenotypes, which are either caused by or associated with impaired function of proteins involved in the cellular response to genotoxic stress [4-8]. Ataxia Telangiectasia Mutated (ATM), one of the major kinases of the DNA Damage Response (DDR), is a node that links DDR, neuronal development, and neurodegeneration [2,9-12]. In humans, inactivating mutations of ATM lead to Ataxia-Telangiectasia (A-T) disease [11,13], which is characterized by severe cerebellar neurodegeneration, indicating an important protective function of ATM in the nervous system [14]. Despite the large number of studies on the molecular cause of A-T, the neuroprotective role of ATM is not well established and is contradictory to its general proapoptotic function. This review discusses the putative functions of ATM in neuronal cells and how they might contribute to neuroprotection.
Keywords: ATM, DNA repair, DNA damage response, neurodegeneration, neuronal development, A-T
Introduction
The maintenance of genomic integrity is vital for the survival and proper function of a cell and, consequently, an organism. Cellular DNA is constantly attacked by genotoxic agents, such as UV irradiation, but also endogenous mutagenic metabolic products, most prominently reactive oxygen species (ROS). These radicals are produced during mitochondrial respiration and, unless inactivated, can cause oxidative stress and result in modification of DNA bases [15]. Besides their intriguing and extremely complicated functions, neurons are set apart from other cell types because of their longevity, high energy requirement and transcriptional activity, their complex cellular structure, and limited regeneration potential. Therefore, the neuronal genome is exposed to an increased amount of ROS and DNA damage that accumulates in neurons and may lead to neuronal dysfunction and degeneration.
Indeed, increased genotoxic stress and damage, as well as deregulation of DNA repair mechanisms, have been observed in age-related neurodegenerative diseases [2]. Additionally, inactivation of several key players of the DNA damage response (DDR) is the cause of many human diseases characterized by DNA repair defects and neurodegeneration [4], such as Ataxia Telangiectasia disease (A-T). The frequency of A-T in the United States is about 1 in 100.000 births [16], and it is caused by inactivating mutations of the Ataxia Telangiectasia Mutated (ATM) gene [17,18]. These are mostly nonsense or frameswift mutations that disrupt ATM synthesis, but there are interesting cases of A-T caused by missense or splice site mutations in which the enzymatic activity of ATM is affected [18,19].
ATM is a kinase that forms a central node in the DDR phosphorylation cascade by contributing to the initiation, amplification, and transmission of the DNA damage response (DDR) signal. In particular, ATM is one of the proteins recruited to the sites of DNA lesions, where large protein complexes are formed that initiate the damage signaling. The MRN complex (MRE11/Rad50/NBS1, Meiotic Recombination 11, Radiation repair 50 and Nijmegen breakage syndrome 1 respectively), the BRCA1 and BRCA2 (Breast Cancer 1 and 2, early onset), and MDC1 (Mediator of DNA damage Checkpoint 1) are other factors that accumulate at the lesion sites, and their dysfunction is also associated with radiosensitivity and/or neurodegenerative syndromes [20-34]. The complex formation results in activation of ATM, which in turn phosphorylates additional proteins, such as CHK2 (checkpoint kinase 2), that transmit the DNA damage signal [24,26,28,35]. These initial and intermediate steps consist of positive feedback loops across the phosphorylation cascade, which ensure the rapid transmission and amplification of the signal. Finally, effector proteins are activated that initiate the cellular response, such as p53, cell cycle inhibitors, DNA repair proteins, and caspases [32,35,36].
Since A-T patients lack functional ATM, they are hypersensitive to DNA damage-inducing agents and have a predisposition to cancer [37-39]. However, one of the main hallmarks of A-T is cerebellar neurodegeneration, resulting in impaired coordination and movement control (ataxia) [40-43]. This is already evident in the first years of life and results in wheelchair dependency of most suffering children by the age of 10. The cerebellum of A-T patients is most profoundly affected, but progressive neurodegeneration occurs also in other parts of the central nervous system (CNS) [40,42-44]. This striking neuronal degeneration caused by impaired ATM function has puzzled researchers for decades, and opinions tend to be split between attributing the neuroprotective role of ATM to its DDR-related function and the belief that ATM might have another, so far unclear, role in the CNS [45]. This review aims to address this puzzling question by summarizing the current knowledge on DDR and repair in neuronal cells, with a focus on the role of ATM in neuroprotection.
Topics
Neuronal Defects in DNA Repair-Related Syndromes
The repair of DNA lesions is controlled by a collection of mechanisms that are coordinated depending on the type and the extent of the damage. Malfunction of these mechanisms not only leads to cancer predisposition but also to a series of defects and impairments of neuronal structures [4]. A classical example is A-T disease with severe loss of Purkinje and granule cells of the cerebellum, resulting in uncoordinated movement (ataxia). Inactivating mutations of proteins that work together with ATM in damage recognition and initiation of the DDR also result in neurodegeneration and syndromes similar to A-T. Hypomorphic mutations of MRE11 (Meiotic Recombination 11) lead to A-T-like disease (A-TL) [46], which is similar to mild A-T with later onset and slower progression than classical A-T [47]. Mutations of NBS1 (Nijmegen Breakage Syndrome 1) are the cause of Nijmegen breakage syndrome [48-50], which is characterized by microcephaly rather than cerebellar neurodegeneration.
Interestingly, while NBS1 and MRE11 are part of the same complex (MRN), their mutations lead to different neurological defects. ATM and MRN act together in recognizing DNA lesions and initiating the DDR by assembly at the damaged chromatin and mutual phosphorylation [33,34,51], but they do not actively take part in the repairing process. Mutations of proteins involved in the later steps of repair lead to diseases characterized mainly by cancer predisposition and neurological symptoms affecting other areas of the CNS. For example, Xeroderma Pigmentosum (XP), Cockayne Syndrome (CS), and Trichothiodystrophy (TTD) are caused by mutations in the XP or CS proteins and are associated with microcephaly and wide progressive neurodegeneration [2]. The different phenotypes and neurological findings of these syndromes raise two main questions: Why are different parts of the CNS affected in different ways by the inactivation of a DDR factor, and is there a role for proteins involved in the DDR in neuronal development and neuron maintenance that is not related to DNA repair?
Neuronal Survival or Death — What Makes the Difference?
Part of the answer to the first question probably lies in the fact that the intracellular environment of different types of neurons can vary and the efficacy of damaging agents in the cell can thus vary accordingly. Comparative studies have eloquently shown that different types of neurons, but also the same kind of neurons in distinct areas, can display extremely different sensitivity to oxidative stress and exogenous DNA damaging drugs (reviewed in [52]). Intrinsic cellular factors that may influence this selective response are the endogenous constant levels of oxidative stress, ATP levels, and energy production rates. Furthermore, the occurrence of mitochondrial dysfunction, the expression pattern of DNA repair related and neuroprotective genes, and the extent of using ROS and reactive nitrogen species (RNS) as signaling molecules can affect neuronal sensitivity [52].
Besides the exposure to damaging agents, the extracellular environment also affects neuronal health and the response to DNA damage. In particular, the functionality of glial cells and the control of the inflammatory response are crucial for the maintenance of neuronal homeostasis and protection [52,53]. Notably, neurons relatively sensitive to DNA damage and oxidative stress are those that also degenerate profoundly in aging-related syndromes, such as Parkinson’s (PD) and Alzheimer’s disease (AD). These late onset diseases share some common features with the early onset hereditary diseases like A-T, such as deregulation of DDR and DNA repair, resulting in accumulation of damaged DNA in certain neurons, neuronal dysfunction, and finally degeneration [2,4]. A loss of dopaminergic neurons of the substantia nigra pars compacta was observed in ATM -/- mice, which also displayed several symptoms of PD [54], indicating a connection between early and late onset neurodegenerative diseases, DDR and DNA repair.
It has been speculated that, despite the differential sensitivity of neurons, there is a threshold of accumulated DNA damage beyond which the cell will initiate an apoptotic response [55]. This threshold seems to vary in different neuronal types, leading to the paradox that in neurodegenerative diseases there is substantial loss of certain neuronal cells with the remaining cells having a healthy genome while other neurons may accumulate a higher number of lesions and yet survive [56]. Finally, the extensive duration of cerebellar development and plasticity could also contribute to the sensitivity of the cerebellar neurons to ATM dysfunction.
So far, studies have mostly focused on measuring either the amount of accumulated DNA damage or the DNA repair activity in neurons [2]. It would be of interest to determine the amount of oxidative damage and DNA lesions together with the DNA repair capacity in affected versus resistant neurons to assess the correlation of these two parameters with neuronal survival.
Potential Genomic Rearrangements During Neuronal Development — ATM and DSB
Immunodeficiency is another common pathological finding in individuals with DDR defects. In particular, the majority of A-T patients lack or have reduced levels of certain immunoglobulins and lymphopenia (especially loss of T-lymphocytes) [57,58]. This is generally attributed to the major role of ATM in the recognition of DNA double strand breaks (DSB) and the initiation of their repair. DSB are extremely toxic for the cell, as they can lead to large chromosomal rearrangements, and the cells of the immune system are particularly prone to DSB due to the frequent recombination events such as V(D)J and class switch recombination.
The severe impairment of both the nervous and the immune system in A-T motivated researchers to investigate the occurrence of genomic rearrangements in neuronal development. Indeed, there is evidence supporting recombination events in neuronal cadherin genes, leading to the tempting speculation that these might influence the specificity and plasticity of synapses [59-61]. Importantly, there is a significantly higher accumulation of DSB in the degenerating cerebellum of A-T patients, and these breaks occur in a non-random fashion, supporting the hypothesis of genomic rearrangements during neuronal development [10]. Furthermore, loss of other genes that are crucial for DNA end joining and DSB repair (such as DNA ligase IV) leads to late embryonic lethality in mice and is characterized by massive apoptosis induction in the newly formed post-mitotic neurons [62-64]. These studies exemplify the importance of DNA repair factors and DSB repair in neuronal development.
ATM and Neuronal Degeneration Outside the DDR
The second question raised above was whether ATM and other DDR proteins have other tasks in neuroprotection apart from DNA repair. ATM plays a central role in DDR and as such is very important for the protection of neurons from genomic stress. However, it has become increasingly clear during the last years that ATM might also have neuroprotective functions that are not directly dependent on the accumulation of DNA damage [65-70].
Specifically in A-T, the uncontrolled dephosphorylation of Histone Deacetylase 4 (HDAC4) by PP2A (protein phosphatase 2A, which is normally phosphorylated and thus regulated by ATM) leads to nuclear accumulation of HDAC4. This mislocalization leads to aberrant gene expression and contributes to neurodegeneration [41]. In addition, as a secondary effect of DDR induction, ATM can turn on mitochondrial biogenesis by activating AMPK (AMP Kinase) and thus protect neurons with mitochondrial dysfunction from cell death [71]. Curiously, while in most cells ATM is mainly or exclusively localized in the nucleus, many studies have shown a substantial cytoplasmic amount of this kinase in healthy as well as in damaged neurons [65-70], although an extra-nuclear role for ATM has been questioned [45]. Importantly, cytoplasmic ATM in mouse brain has been shown to associate with synaptic vesicle proteins, namely with VAMP2 and synapsin I, and might play a role in synaptic vesicle cycling [67]. In the same study, Jiali Li et al. demonstrated that cytoplasmic ATM did not respond to DNA damage and concluded that it might play a role in synaptic vesicle docking and fusion and, hence, in the regulation of synaptic function. Da-Qing Yang and colleagues found that upon differentiation of human neuron-like cells, ATM shuttles from the nucleus to the cytoplasm, where it promotes neuronal survival via activation of AKT signaling in an insulin-dependent manner [68,72]. Finally, the group of Yutaka Miura investigated the activation of ATM in deep cerebellar nuclei and found a potential neuroprotective role for cytoplasmic ATM involving ZFHX3 (zinc finger homeobox 3) and PDGFRB (platelet-derived growth factor receptor beta) in response to oxidative stress and excessive excitation [73]. Overall, this brief list of examples illustrates the diverse functions of neuronal ATM that have been proposed so far, as well as the plethora of other proteins that seem to act concomitantly with ATM toward neuroprotection.
Cell Cycle Re-entry, Neurodegeneration, and ATM
An intriguing and so far unclarified phenomenon that precedes cellular death is the attempt of postmitotic neurons to re-enter the cell cycle. This occurs in response to excessive stress, involves expression of cell cycle regulators, such as cyclin D1, CDK4/6 (cyclin dependent kinase 4/6) and members of the E2F family of transcription factors, and can be accompanied by incomplete DNA replication [74,75]. This aberrant cell cycle response culminates in the induction of apoptosis activators. E2F1 is of particular interest because while its primary role is to drive G1 to S phase transition, increased amounts of this protein can induce apoptosis [76,77].
Despite all efforts, the cause and purpose of the abortive cell cycle re-entry, as well as its exact connection to the neurodegeneration that follows, is not clear. It has been speculated that because DNA repair is more efficient in actively dividing cells, neurons with genomic lesions try to mimic the intracellular environment of proliferating cells [2]. However, the G0 to G1 transition of postmitotic neurons is characterized by a confound activation of genes that are normally sequentially expressed in different phases of the cell cycle. This could result in excessive replication stress, as DNA synthesis that takes place is largely incomplete and creates genomic instability finally leading to apoptosis [78]. In addition, the large cytoskeletal structures in neurons would have to be vastly rearranged to allow cell division, thereby posing a further obstacle to completing the cell cycle [79]. Ketamine is an NMDA receptor antagonist that has been associated with neurodegeneration involving aberrant cell cycle re-entry [80]. Soriano et al. showed that the knockdown of cyclin D1 attenuated the proapoptotic response in ketamine-treated neurons, supporting an unfavorable role of cell cycle re-entry for neuronal survival [80].
Interestingly, in postmitotic cortical neurons, only drugs inducing DNA damage were found to reactivate the cell cycle and induce apoptosis in an ATM-dependent manner, while compounds not targeting DNA led to ATM-independent neurodegeneration without cell cycle re-entry attempts. Most importantly, ATM inhibition could rescue neuronal death by genotoxic drugs but not by staurosporine or colchicine [75]. Along this line, caffeine, a PI3-like kinase inhibitor (this family includes the DDR proteins ATM, Ataxia Telangiectasia, and Rad3 related/ATR and DNA protein kinase/DNA-PK) has been shown to exhibit neuroprotective effects mediated by attenuating the ATM-p53-E2F1 pathway. In particular, caffeine inhibited apoptosis and reduced cyclin D1 and E2F1 amounts in cerebellar granule neurons treated with the neurotoxin MMP+ (N-methyl-4-phenylpyridine). However, caffeine had no effect in neurodegeneration induced by serum starvation or potassium withdrawal [81]. These studies suggest that ATM is a central activator of both cell cycle re-entry and apoptosis in the context of DDR following certain types of neuronal stress, but also point out the existence of neurodegenerative pathways that are independent of ATM and cell cycle activation.
Lessons from Animal Models
Toward understanding ATM and the molecular basis of A-T, three ATM knockout mice (ATM-/-) were independently generated and their phenotypes were published in 1996 [82-85]. All these mouse models displayed several features of human A-T, including radiosensitivity, growth defects, infertility, immunodeficiency, and decreased life span, mostly due to the development of thymic lymphomas. Surprisingly, however, the severe neuronal abnormalities of the disease were not observed in mice lacking ATM, placing doubts regarding their use as a model for studying A-T.
Nevertheless, Barlow and colleagues analyzed the oxidative damage levels in ATM-/- mouse tissues and showed that organs that develop pathologic changes in the absence of ATM, including the Purkinje cells, are targets of high oxidative damage [86]. A fourth ATM-/- mouse was generated by Borghesani et al. using gene targeting. These mice had a reduced lymphoma incidence and increased survival rate compared to the previously generated models and were characterized by motor learning deficiency, as well as ectopic and abnormal differentiation of Purkinje cells, although no obvious defects were found in their cerebella [87]. Collectively, these results indicate that, while many aberrations of human A-T can be observed and studied in the mouse ATM-/- models, the neurological findings of the human disease are only marginally observed. Nonetheless, neural cells of these mice display molecular defects that might be important for understanding the role of ATM in A-T. For example, the cerebellar cells of 4-month-old ATM-/- mice were shown to be in a state of continuous stress, which was marked by increased mitochondrial respiration rate and decreased pool of NAD (nicotine adenine dinucleotide) [88], increased p-AMPK, and ROS amounts [89]. In addition, mouse ATM-/- neural progenitor cells (NPC) were genetically unstable and proliferated faster than normal NPC [90]. Hande et al. and Wong et al. pointed out the significance of ATM for telomere maintenance and stability, suggesting that ATM deficiency and telomere dysfunction may act together to reduce cell survival [91,92]. The important function of ATM in telomere protection is evolutionary conserved: In yeast, the ATM homologue Tel1p activates telomere elongation [93], and in the fruitfly, ATM deletion (tefu; telomere fusion) leads to telomere fusion and apoptosis [94,95]. A kinase dead ATM mutant induced neuron and glial cell death in Drosophila brain [96] and radiosensitivity in zebrafish similarly to A-T [97], while knockdown of ATM in zebrafish embryos impaired their development upon ionizing radiation [97,98].
An important contribution to A-T research was the generation of an NBS1 knockout mouse (NBS1-/-), which essentially recapitulated the important motor and neurological defects of human A-T, along with microcephaly, growth retardation, neuronal cell proliferation and survival deficiency, and several other non-CNS related defects of the disease [99,100]. ATM and NBS1 proteins act together during DDR to DSB, where NBS1 contributes to the activation of ATM [34,51]. Furthermore, ATM is required for NBS1 phosphorylation and activation during S phase, when replication stress occurs [47]. The neurological findings of A-T in NBS1-/- mice could therefore imply an important role for the intra-S checkpoint in the development of certain CNS areas and especially for NPC, while the repair of DSB might be crucial for the survival of postmitotic neurons during early life stages. Notably, it seems that ATM likely has a more important function in human than in mouse CNS, as ATM-/- mice do not show the severe neuronal phenotype of A-T. In agreement to that, Da-Qing Yang et al. showed that during differentiation of rat PC12 neuronal cells ATM levels drop, while they remain the same in human SH-SY5Y neuron-like cells [68]. These differences between murine and human neurons indicate that, while mice are an extremely useful model to study A-T, the possibility of diversions in the molecular functions of ATM between the two organisms must be considered. Nevertheless, studies in older ATM-/- mice showed that A-T associated neurological defects occur later in life [101].
Recently, Yamamoto and colleagues [102], as well as Daniel et al. [103], reported their attempts to create a kinase dead (kd) ATM mouse model (ATM kd/kd). The mice could not be generated by either group, and further investigation revealed that they die in utero. This is in stark contrast to the ATM-/- mice, which survive several months after birth [101]. A dominant negative effect of ATM kd on active ATM was excluded, as ATM wt/kd (wild type/kinase dead) heterozygous mice were similar to wild type homozygotes [102,103]. The hypothesis that ATM kd may interfere with other DNA repair pathways was examined with the use of double knockout mouse models [103]. However, these experiments failed to provide a clear explanation for the early embryonic death of ATM kd/kd mice. Nonetheless, a decreased ability to repair DSB and increased genomic instability was detected in ATM kd/kd lymphocytes and ES cells respectively [102]. Both studies concluded that a kinase dead ATM mutant that is expressed in cells has very different consequences for the cell than the mere absence of ATM. They show the importance of an active ATM during embryonic development and possibly provide an explanation for the low frequency of missense kinase dead ATM mutations in human A-T. Finally, these results raise an important issue regarding unsuspected side effects of ATM inhibitors used in cancer therapy.
Conclusions
The importance of ATM in DDR and genome integrity is conserved and well established. Activation of this kinase at genomic lesions is a nodal point of the DDR and involves a cascade of protein-protein interactions, complex formations, and phosphorylation events, which eventually activate the cell cycle checkpoints, initiate the repair of DNA, and decide cellular fate [21,23-26,36]. However, the function of ATM in the development and protection of neuronal cells is not as clear as its role in other cell types. Clearly, the preservation of neuronal function requires efficient DNA repair. Hence, neurodegeneration that occurs in the absence of ATM and is accompanied by accumulation of DSB and oxidative damage arises at least in part due to impaired DDR and repair. On the other hand, an extensive list of publications describes DDR-independent functions of ATM and provides evidence about their significance in maintaining neuronal homeostasis [41,65,67-73,89,104]. Additional ambiguity arises from the two sides of ATM activation: protection from stress and promotion of neuronal survival or initiation of apoptotic pathways and elimination of a damaged cell. Given these two seemingly opposing roles, it is not surprising that there is such diversity in the cellular effects of ATM dysfunction. It has been shown that ATM has a BAX (Bcl2-associated protein X)-dependent proapoptotic role in mouse developing CNS [105]. Furthermore, murine ATM-/- postmitotic neurons initially develop normally only to be degenerated during the first months of life [101,106]. How can the absence of a proapoptotic protein induce neuronal death?
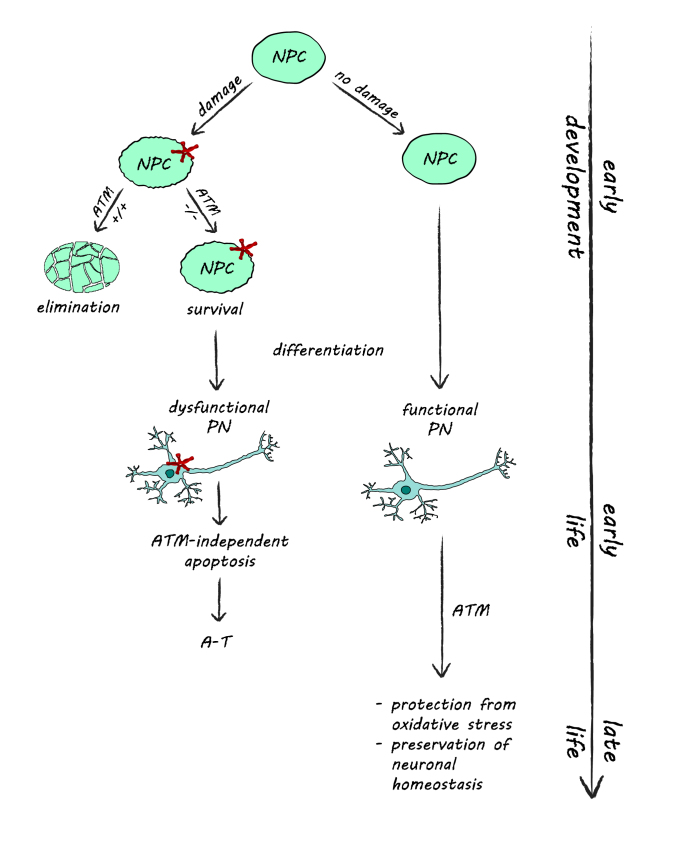
This paradox can be explained by considering a model where ATM initially functions in NPC to eliminate neuronal precursors with extensive DNA damage, thereby preventing them from giving rise to malfunctioning neurons (Figure 1). Thus, non functional ATM would lead to a survival of NPC with accumulated DNA damage in early development, and postmitotic neurons stemming from such cells may have accumulated genomic lesions and hence have an increased risk of malfunctioning. The metabolism of such neurons would soon collapse, and these cells would be eliminated during the first months of their life. Later in development, the predominant role of ATM may be the maintenance of neuronal homeostasis and preventing oxidative stress in postmitotic neurons.
Figure 1.
The role of ATM in neuroprotection. During early development, ATM controls the elimination of dividing NPC with accumulated DNA damage via the induction of apoptosis. In the absence of ATM, damaged NPC survive and give rise to dysfunctional postmitotic neurons, which eventually degenerate, creating the neuronal abnormalities observed in A-T. Later in life, ATM functions to protect the non-dividing postmitotic neurons from oxidative stress and contributes to the maintenance of neuronal homeostasis.
Along the line of investigating the importance of ATM kinase activity, a surprising finding was the phenotype of ATM kd/kd mice. These mutants are early embryonic lethal, thereby revealing a much more devastating effect of having a kinase dead ATM in the cells than its complete absence [102,103]. A suggested explanation for this phenomenon might be that the absence of ATM results in suboptimal DDR and repair of damaged DNA via the activation of alternative mechanisms, thereby preventing lethality. The ATM kd molecules, however, can still sequester common DDR and repair factors and can bind to the damaged DNA [102,103], thus inhibiting other DNA repair pathways and leading to genomic instability, accumulation of DNA damage, and, consequently, cellular death. These studies highlight the interplay between DDR and diverse DNA repair pathways and how tightly they can be balanced to orchestrate the response of cells to genotoxic stress.
In conclusion, there is a plethora of studies supporting the implication of ATM in important nuclear and cytoplasmic processes in neurons, ranging from regulation of chromatin structures [25,41] to synaptic vesicle recycling [67]. Recent data revealing the embryonic lethality of inactivating ATM in mice without disrupting its expression implies an important role for ATM kinase activity during early development [102,103]. Altogether, the gross neuronal abnormalities in human A-T and the multitude of ATM neuronal functions suggest that its role in neuroprotection extends beyond classical DDR. Further investigation of neuronal ATM both in animal models and in human cells should reveal if what we currently see is but the tip of the iceberg.
Acknowledgments
I am very grateful to C.M. Pieper for proofreading this manuscript and for his artistic contribution to Figure 1.
Abbreviations
- A-T
Ataxia Telangiectasia
- ATM
Ataxia Telangiectasia Mutated
- CNS
central nervous system
- DDR
DNA Damage Response
- DSB
Double Strand Breaks
- NBS
Nijmegen Breakage Syndrome
- NPC
Neural Progenitor Cells
- PN
Postmitotic Neuron
- ROS
Reactive Oxygen Species
- PD
Parkinson’s disease
- AD
Alzheimer’s disease
References
- Atamna H, Cheung I, Ames BN. A method for detecting abasic sites in living cells: age-dependent changes in base excision repair. Proc Natl Acad Sci USA. 2000;97(2):686–691. doi: 10.1073/pnas.97.2.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppede F, Migliore L. DNA repair in premature aging disorders and neurodegeneration. Curr Aging Sci. 2010;3(1):3–19. doi: 10.2174/1874609811003010003. [DOI] [PubMed] [Google Scholar]
- Wang S, Okun MS, Suslov O, Zheng T, McFarland NR, Vedam-Mai V. et al. Neurogenic potential of progenitor cells isolated from postmortem human Parkinsonian brains. Brain Res. 2012;1464:61–72. doi: 10.1016/j.brainres.2012.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai A, Biton S, Shiloh Y. The role of the DNA damage response in neuronal development, organization and maintenance. DNA Repair (Amst) 2008;7(7):1010–1027. doi: 10.1016/j.dnarep.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Lavin MF, Kozlov S. DNA damage-induced signalling in ataxia-telangiectasia and related syndromes. Radiother Oncol. 2007;83(3):231–237. doi: 10.1016/j.radonc.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Lehmann AR. DNA repair-deficient diseases, xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy. Biochimie. 2003;85(11):1101–1111. doi: 10.1016/j.biochi.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Varon R, Vissinga C, Platzer M, Cerosaletti KM, Chrzanowska KH, Saar K. et al. Nibrin, a novel DNA double-strand break repair protein, is mutated in Nijmegen breakage syndrome. Cell. 1998;93(3):467–476. doi: 10.1016/s0092-8674(00)81174-5. [DOI] [PubMed] [Google Scholar]
- Vinters HV, Gatti RA, Rakic P. Sequence of cellular events in cerebellar ontogeny relevant to expression of neuronal abnormalities in ataxia-telangiectasia. Kroc Found Ser. 1985;19:233–255. [PubMed] [Google Scholar]
- Canman CE, Lim DS. The role of ATM in DNA damage responses and cancer. Oncogene. 1998;17(25):3301–3308. doi: 10.1038/sj.onc.1202577. [DOI] [PubMed] [Google Scholar]
- Iourov IY, Vorsanova SG, Liehr T, Kolotii AD, Yurov YB. Increased chromosome instability dramatically disrupts neural genome integrity and mediates cerebellar degeneration in the ataxia-telangiectasia brain. Hum Mol Genet. 2009;18(14):2656–2669. doi: 10.1093/hmg/ddp207. [DOI] [PubMed] [Google Scholar]
- Lavin MF, Khanna KK. ATM: the protein encoded by the gene mutated in the radiosensitive syndrome ataxia-telangiectasia. Int J Radiat Biol. 1999;75(10):1201–1214. doi: 10.1080/095530099139359. [DOI] [PubMed] [Google Scholar]
- Tian B, Yang Q, Mao Z. Phosphorylation of ATM by Cdk5 mediates DNA damage signalling and regulates neuronal death. Nat Cell Biol. 2009;11(2):211–218. doi: 10.1038/ncb1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti RA, Berkel I, Boder E, Braedt G, Charmley P, Concannon P. et al. Localization of an ataxia-telangiectasia gene to chromosome 11q22-23. Nature. 1988;336(6199):577–580. doi: 10.1038/336577a0. [DOI] [PubMed] [Google Scholar]
- McKinnon PJ. Ataxia telangiectasia: new neurons and ATM. Trends Mol Med. 2001;7(6):233–234. doi: 10.1016/s1471-4914(01)02035-4. [DOI] [PubMed] [Google Scholar]
- Cooke MS, Evans MD, Dizdaroglu M, Lunec J. Oxidative DNA damage: mechanisms, mutation, and disease. Faseb J. 2003;17(10):1195–1214. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- Swift M, Morrell D, Cromartie E, Chamberlin AR, Skolnick MH, Bishop DT. The incidence and gene frequency of ataxia-telangiectasia in the United States. Am J Hum Genet. 1986;39(5):573–583. [PMC free article] [PubMed] [Google Scholar]
- Savitsky K, Sfez S, Tagle DA, Ziv Y, Sartiel A, Collins FS. et al. The complete sequence of the coding region of the ATM gene reveals similarity to cell cycle regulators in different species. Hum Mol Genet. 1995;4(11):2025–2032. doi: 10.1093/hmg/4.11.2025. [DOI] [PubMed] [Google Scholar]
- Verhagen MM, Last JI, Hogervorst FB, Smeets DF, Roeleveld N, Verheijen F. et al. Presence of ATM protein and residual kinase activity correlates with the phenotype in ataxia-telangiectasia: a genotype-phenotype study. Hum Mutat. 2012;33(3):561–571. doi: 10.1002/humu.22016. [DOI] [PubMed] [Google Scholar]
- Jacquemin V, Rieunier G, Jacob S, Bellanger D, d’Enghien CD, Lauge A. et al. Underexpression and abnormal localization of ATM products in ataxia telangiectasia patients bearing ATM missense mutations. Eur J Hum Genet. 2012;20(3):305–312. doi: 10.1038/ejhg.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Yuan SS, Liu W, Xu Y, Trujillo K, Song B. et al. Radiation-induced assembly of Rad51 and Rad52 recombination complex requires ATM and c-Ab. J Biol Chem. 1999;274(18):12748–12752. doi: 10.1074/jbc.274.18.12748. [DOI] [PubMed] [Google Scholar]
- Chen J. Ataxia telangiectasia-related protein is involved in the phosphorylation of BRCA1 following deoxyribonucleic acid damage. Cancer Res. 2000;60(18):5037–5039. [PubMed] [Google Scholar]
- Chen MJ, Lin YT, Lieberman HB, Chen G, Lee EY. ATM-dependent phosphorylation of human Rad9 is required for ionizing radiation-induced checkpoint activation. J Biol Chem. 2001;276(19):16580–16586. doi: 10.1074/jbc.M008871200. [DOI] [PubMed] [Google Scholar]
- Cortez D, Wang Y, Qin J, Elledge SJ. Requirement of ATM-dependent phosphorylation of brca1 in the DNA damage response to double-strand breaks. Science. 1999;286(5442):1162–1166. doi: 10.1126/science.286.5442.1162. [DOI] [PubMed] [Google Scholar]
- Kastan MB, Lim DS. The many substrates and functions of ATM. Nat Rev Mol Cell Biol. 2000;1(3):179–186. doi: 10.1038/35043058. [DOI] [PubMed] [Google Scholar]
- Kim GD, Choi YH, Dimtchev A, Jeong SJ, Dritschilo A, JungLim M. Sensing of ionizing radiation-induced DNA damage by ATM through interaction with histone deacetylase. J Biol Chem. 1999;274(44):31127–31130. doi: 10.1074/jbc.274.44.31127. [DOI] [PubMed] [Google Scholar]
- Kim ST, Lim DS, Canman CE, Kastan MB. Substrate specificities and identification of putative substrates of ATM kinase family members. J Biol Chem. 1999;274(53):37538–37543. doi: 10.1074/jbc.274.53.37538. [DOI] [PubMed] [Google Scholar]
- Li S, Ting NS, Zheng L, Chen PL, Ziv Y, Shiloh Y. et al. Functional link of BRCA1 and ataxia telangiectasia gene product in DNA damage response. Nature. 2000;406(6792):210–215. doi: 10.1038/35018134. [DOI] [PubMed] [Google Scholar]
- Matsuoka S, Rotman G, Ogawa A, Shiloh Y, Tamai K, Elledge SJ. Ataxia telangiectasia-mutated phosphorylates Chk2 in vivo and in vitro. Proc Natl Acad Sci USA. 2000;97(19):10389–10394. doi: 10.1073/pnas.190030497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273(10):5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- Shafman T, Khanna KK, Kedar P, Spring K, Kozlov S, Yen T. et al. Interaction between ATM protein and c-Abl in response to DNA damage. Nature. 1997;387(6632):520–523. doi: 10.1038/387520a0. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Kodama S, Watanabe M. Recruitment of ATM protein to double strand DNA irradiated with ionizing radiation. J Biol Chem. 1999;274(36):25571–25575. doi: 10.1074/jbc.274.36.25571. [DOI] [PubMed] [Google Scholar]
- Westphal CH, Schmaltz C, Rowan S, Elson A, Fisher DE, Leder P. Genetic interactions between atm and p53 influence cellular proliferation and irradiation-induced cell cycle checkpoints. Cancer Res. 1997;57(9):1664–1667. [PubMed] [Google Scholar]
- Wu X, Ranganathan V, Weisman DS, Heine WF, Ciccone DN, O’Neill TB. et al. ATM phosphorylation of Nijmegen breakage syndrome protein is required in a DNA damage response. Nature. 2000;405(6785):477–482. doi: 10.1038/35013089. [DOI] [PubMed] [Google Scholar]
- Zhao S, Weng YC, Yuan SS, Lin YT, Hsu HC, Lin SC. et al. Functional link between ataxia-telangiectasia and Nijmegen breakage syndrome gene products. Nature. 2000;405(6785):473–477. doi: 10.1038/35013083. [DOI] [PubMed] [Google Scholar]
- Shieh SY, Ahn J, Tamai K, Taya Y, Prives C. The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Dev. 2000;14(3) [PMC free article] [PubMed] [Google Scholar]
- Khanna KK, Keating KE, Kozlov S, Scott S, Gatei M, Hobson K. et al. ATM associates with and phosphorylates p53: mapping the region of interaction. Nat Genet. 1998;20(4):398–400. doi: 10.1038/3882. [DOI] [PubMed] [Google Scholar]
- Chessa L, Petrinelli P, Antonelli A, Fiorilli M, Elli R, Marcucci L. et al. Heterogeneity in ataxia-telangiectasia: classical phenotype associated with intermediate cellular radiosensitivity. Am J Med Genet. 1992;42(5):741–746. doi: 10.1002/ajmg.1320420524. [DOI] [PubMed] [Google Scholar]
- Duhrsen U, Uppenkamp M, Uppenkamp I, Becher R, Engelhard M, Konig E. et al. Chronic T cell leukemia with unusual cellular characteristics in ataxia telangiectasia. Blood. 1986;68(2):577–585. [PubMed] [Google Scholar]
- Ziv Y, Jaspers NG, Etkin S, Danieli T, Trakhtenbrot L, Amiel A. et al. Cellular and molecular characteristics of an immortalized ataxia-telangiectasia (group AB) cell line. Cancer Res. 1989;49(9):2495–2501. [PubMed] [Google Scholar]
- Hoche F, Seidel K, Theis M, Vlaho S, Schubert R, Zielen S. et al. Neurodegeneration in ataxia telangiectasia: what is new? What is evident? Neuropediatrics. 2012;43(3):119–129. doi: 10.1055/s-0032-1313915. [DOI] [PubMed] [Google Scholar]
- Li J, Chen J, Ricupero CL, Hart RP, Schwartz MS, Kusnecov A. et al. Nuclear accumulation of HDAC4 in ATM deficiency promotes neurodegeneration in ataxia telangiectasia. Nat Med. 2012;18(5):783–790. doi: 10.1038/nm.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurov YB, Iourov IY, Vorsanova SG. Neurodegeneration mediated by chromosome instability suggests changes in strategy for therapy development in ataxia-telangiectasia. Med Hypotheses. 2009;73(6):1075–1076. doi: 10.1016/j.mehy.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Lehmann AR, Carr AM. The ataxia-telangiectasia gene: a link between checkpoint controls, neurodegeneration and cancer. Trends Genet. 1995;11(10):375–377. doi: 10.1016/s0168-9525(00)89112-x. [DOI] [PubMed] [Google Scholar]
- Shiloh Y, Rotman G. Ataxia-telangiectasia and the ATM gene: linking neurodegeneration, immunodeficiency, and cancer to cell cycle checkpoints. J Clin Immunol. 1996;16(5):254–260. doi: 10.1007/BF01541389. [DOI] [PubMed] [Google Scholar]
- Biton S, Barzilai A, Shiloh Y. The neurological phenotype of ataxia-telangiectasia: solving a persistent puzzle. DNA Repair (Amst) 2008;7(7):1028–1038. doi: 10.1016/j.dnarep.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Delia D, Piane M, Buscemi G, Savio C, Palmeri S, Lulli P. et al. MRE11 mutations and impaired ATM-dependent responses in an Italian family with ataxia-telangiectasia-like disorder. Hum Mol Genet. 2004;13(18):2155–2163. doi: 10.1093/hmg/ddh221. [DOI] [PubMed] [Google Scholar]
- Taylor AM, Groom A, Byrd PJ. Ataxia-telangiectasia-like disorder (ATLD)-its clinical presentation and molecular basis. DNA Repair (Amst) 2004;3(8-9):1219–1225. doi: 10.1016/j.dnarep.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Kraakman-van der Zwet M, Overkamp WJ, Friedl AA, Klein B, Verhaegh GW, Jaspers NG. et al. Immortalization and characterization of Nijmegen Breakage syndrome fibroblasts. Mutat Res. 1999;434(1):17–27. doi: 10.1016/s0921-8777(99)00009-9. [DOI] [PubMed] [Google Scholar]
- Matsuura S, Tauchi H, Nakamura A, Kondo N, Sakamoto S, Endo S. et al. Positional cloning of the gene for Nijmegen breakage syndrome. Nat Genet. 1998;19(2):179–181. doi: 10.1038/549. [DOI] [PubMed] [Google Scholar]
- Saar K, Chrzanowska KH, Stumm M, Jung M, Nurnberg G, Wienker TF. et al. The gene for the ataxia-telangiectasia variant, Nijmegen breakage syndrome, maps to a 1-cM interval on chromosome 8q21. Am J Hum Genet. 1997;60(3):605–610. [PMC free article] [PubMed] [Google Scholar]
- Uziel T, Lerenthal Y, Moyal L, Andegeko Y, Mittelman L, Shiloh Y. Requirement of the MRN complex for ATM activation by DNA damage. Embo J. 2003;22(20):5612–5621. doi: 10.1093/emboj/cdg541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Michaelis EK. Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci. 2010;2:12. doi: 10.3389/fnagi.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zaidi A, Pal R, Garrett AS, Braceras R, Chen XW. et al. Genomic and biochemical approaches in the discovery of mechanisms for selective neuronal vulnerability to oxidative stress. BMC Neurosci. 2009;10:12. doi: 10.1186/1471-2202-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwin Shackelford R, Manuszak RP, Heard SC, Link CJ, Wang S. Pharmacological manipulation of ataxia-telangiectasia kinase activity as a treatment for Parkinson’s disease. Med Hypotheses. 2005;64(4):736–741. doi: 10.1016/j.mehy.2004.08.029. [DOI] [PubMed] [Google Scholar]
- Mawrin C, Kirches E, Krause G, Schneider-Stock R, Bogerts B, Vorwerk CK. et al. Region-specific analysis of mitochondrial DNA deletions in neurodegenerative disorders in humans. Neurosci Lett. 2004;357(2):111–114. doi: 10.1016/j.neulet.2003.11.073. [DOI] [PubMed] [Google Scholar]
- Barzilai A. DNA damage, neuronal and glial cell death and neurodegeneration. Apoptosis. 2010;15(11):1371–1381. doi: 10.1007/s10495-010-0501-0. [DOI] [PubMed] [Google Scholar]
- Micheli R, Pirovano S, Calandra G, Valotti M, Plebani A, Albertini A. et al. Low thymic output and reduced heterogeneity of alpha/beta, but not gamma/delta, T lymphocytes in infants with ataxia-telangiectasia. Neuropediatrics. 2003;34(3):165–167. doi: 10.1055/s-2003-41280. [DOI] [PubMed] [Google Scholar]
- Giovannetti A, Mazzetta F, Caprini E, Aiuti A, Marziali M, Pierdominici M. et al. Skewed T-cell receptor repertoire, decreased thymic output, and predominance of terminally differentiated T cells in ataxia telangiectasia. Blood. 2002;100(12) doi: 10.1182/blood-2002-03-0976. [DOI] [PubMed] [Google Scholar]
- Yagi T. Diversity of the cadherin-related neuronal receptor/protocadherin family and possible DNA rearrangement in the brain. Genes Cells. 2003;8(1):1–8. doi: 10.1046/j.1365-2443.2003.00614.x. [DOI] [PubMed] [Google Scholar]
- Hamada S, Yagi T. The cadherin-related neuronal receptor family: a novel diversified cadherin family at the synapse. Neurosci Res. 2001;41(3):207–215. doi: 10.1016/s0168-0102(01)00281-4. [DOI] [PubMed] [Google Scholar]
- Hirayama T, Sugino H, Yagi T. Somatic mutations of synaptic cadherin (CNR family) transcripts in the nervous system. Genes Cells. 2001;6(2):151–164. doi: 10.1046/j.1365-2443.2001.00403.x. [DOI] [PubMed] [Google Scholar]
- Barnes DE, Stamp G, Rosewell I, Denzel A, Lindahl T. Targeted disruption of the gene encoding DNA ligase IV leads to lethality in embryonic mice. Curr Biol. 1998;8(25):1395–1398. doi: 10.1016/s0960-9822(98)00021-9. [DOI] [PubMed] [Google Scholar]
- Frank KM, Sekiguchi JM, Seidl KJ, Swat W, Rathbun GA, Cheng HL. et al. Late embryonic lethality and impaired V(D)J recombination in mice lacking DNA ligase IV. Nature. 1998;396(6707):173–177. doi: 10.1038/24172. [DOI] [PubMed] [Google Scholar]
- Frank KM, Sharpless NE, Gao Y, Sekiguchi JM, Ferguson DO, Zhu C. et al. DNA ligase IV deficiency in mice leads to defective neurogenesis and embryonic lethality via the p53 pathway. Mol Cell. 2000;5(6):993–1002. doi: 10.1016/s1097-2765(00)80264-6. [DOI] [PubMed] [Google Scholar]
- Yang DQ, Halaby MJ, Li Y, Hibma JC, Burn P. Cytoplasmic ATM protein kinase: an emerging therapeutic target for diabetes, cancer and neuronal degeneration. Drug Discov Today. 2011;16(7-8):332–338. doi: 10.1016/j.drudis.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Hinz M, Stilmann M, Arslan SC, Khanna KK, Dittmar G, Scheidereit C. A cytoplasmic ATM-TRAF6-cIAP1 module links nuclear DNA damage signaling to ubiquitin-mediated NF-kappaB activation. Mol Cell. 2010;40(1):63–74. doi: 10.1016/j.molcel.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Li J, Han YR, Plummer MR, Herrup K. Cytoplasmic ATM in neurons modulates synaptic function. Curr Biol. 2009;19(24):2091–2096. doi: 10.1016/j.cub.2009.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehrs JK, He J, Halaby MJ, Yang DQ. Constitutive expression and cytoplasmic compartmentalization of ATM protein in differentiated human neuron-like SH-SY5Y cells. J Neurochem. 2007;100(2):337–345. doi: 10.1111/j.1471-4159.2006.04254.x. [DOI] [PubMed] [Google Scholar]
- Barlow C, Ribaut-Barassin C, Zwingman TA, Pope AJ, Brown KD, Owens JW. et al. ATM is a cytoplasmic protein in mouse brain required to prevent lysosomal accumulation. Proc Natl Acad Sci USA. 2000;97(2):871–876. doi: 10.1073/pnas.97.2.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DS, Kirsch DG, Canman CE, Ahn JH, Ziv Y, Newman LS. et al. ATM binds to beta-adaptin in cytoplasmic vesicles. Proc Natl Acad Sci USA. 1998;95(17):10146–10151. doi: 10.1073/pnas.95.17.10146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Wan S, Lyu YL, Liu LF, Qi H. Etoposide induces ATM-dependent mitochondrial biogenesis through AMPK activation. PLoS One. 2008;3(4):e2009. doi: 10.1371/journal.pone.0002009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Xiong H, Yang DQ. Functional switching of ATM: sensor of DNA damage in proliferating cells and mediator of Akt survival signal in post-mitotic human neuron-like cells. Chin J Cancer. 2012;31(3):364–372. doi: 10.5732/cjc.012.10086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TS, Kawaguchi M, Suzuki M, Jung CG, Asai K, Shibamoto Y. et al. The ZFHX3 (ATBF1) transcription factor induces PDGFRB, which activates ATM in the cytoplasm to protect cerebellar neurons from oxidative stress. Dis Model Mech. 2010;3(11-12):752–762. doi: 10.1242/dmm.004689. [DOI] [PubMed] [Google Scholar]
- Kruman II. Why do neurons enter the cell cycle? Cell Cycle. 2004;3(6):769–773. [PubMed] [Google Scholar]
- Kruman II, Wersto RP, Cardozo-Pelaez F, Smilenov L, Chan SL, Chrest FJ. et al. Cell cycle activation linked to neuronal cell death initiated by DNA damage. Neuron. 2004;41(4):549–561. doi: 10.1016/s0896-6273(04)00017-0. [DOI] [PubMed] [Google Scholar]
- Kowalik TF, DeGregori J, Leone G, Jakoi L, Nevins JR. E2F1-specific induction of apoptosis and p53 accumulation, which is blocked by Mdm2. Cell Growth Differ. 1998;9(2):113–118. [PubMed] [Google Scholar]
- Kowalik TF, DeGregori J, Schwarz JK, Nevins JR. E2F1 overexpression in quiescent fibroblasts leads to induction of cellular DNA synthesis and apoptosis. J Virol. 1995;69(4):2491–2500. doi: 10.1128/jvi.69.4.2491-2500.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurov YB, Vorsanova SG, Iourov IY. The DNA replication stress hypothesis of Alzheimer's disease. ScientificWorldJournal. 2011;11:2602–2612. doi: 10.1100/2011/625690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir O, Singh S, Klimaschewski L, Kurnaz IA. From birth till death: neurogenesis, cell cycle, and neurodegeneration. Anat Rec (Hoboken) 2009;292(12):1953–1961. doi: 10.1002/ar.20980. [DOI] [PubMed] [Google Scholar]
- Soriano SG, Liu Q, Li J, Liu JR, Han XH, Kanter JL. et al. Ketamine activates cell cycle signaling and apoptosis in the neonatal rat brain. Anesthesiology. 2010;112(5):1155–1163. doi: 10.1097/ALN.0b013e3181d3e0c2. [DOI] [PubMed] [Google Scholar]
- Alvira D, Yeste-Velasco M, Folch J, Casadesus G, Smith MA, Pallas M. et al. Neuroprotective effects of caffeine against complex I inhibition-induced apoptosis are mediated by inhibition of the Atm/p53/E2F-1 path in cerebellar granule neurons. J Neurosci Res. 2007;85(14):3079–3088. doi: 10.1002/jnr.21427. [DOI] [PubMed] [Google Scholar]
- Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, Collins F. et al. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell. 1996;86(1):159–171. doi: 10.1016/s0092-8674(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Elson A, Wang Y, Daugherty CJ, Morton CC, Zhou F, Campos-Torres J. et al. Pleiotropic defects in ataxia-telangiectasia protein-deficient mice. Proc Natl Acad Sci USA. 1996;93(23):13084–13089. doi: 10.1073/pnas.93.23.13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Ashley T, Brainerd EE, Bronson RT, Meyn MS, Baltimore D. Targeted disruption of ATM leads to growth retardation, chromosomal fragmentation during meiosis, immune defects, and thymic lymphoma. Genes Dev. 1996;10(19):2411–2422. doi: 10.1101/gad.10.19.2411. [DOI] [PubMed] [Google Scholar]
- Xu Y, Baltimore D. Dual roles of ATM in the cellular response to radiation and in cell growth control. Genes Dev. 1996;10(19):2401–2410. doi: 10.1101/gad.10.19.2401. [DOI] [PubMed] [Google Scholar]
- Barlow C, Dennery PA, Shigenaga MK, Smith MA, Morrow JD, Roberts LJ 2nd. et al. Loss of the ataxia-telangiectasia gene product causes oxidative damage in target organs. Proc Natl Acad Sci USA. 1999;96(17):9915–9919. doi: 10.1073/pnas.96.17.9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghesani PR, Alt FW, Bottaro A, Davidson L, Aksoy S, Rathbun GA. et al. Abnormal development of Purkinje cells and lymphocytes in Atm mutant mice. Proc Natl Acad Sci USA. 2000;97(7):3336–3341. doi: 10.1073/pnas.050584897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern N, Hochman A, Zemach N, Weizman N, Hammel I, Shiloh Y. et al. Accumulation of DNA damage and reduced levels of nicotine adenine dinucleotide in the brains of Atm-deficient mice. J Biol Chem. 2002;277(1):602–608. doi: 10.1074/jbc.M106798200. [DOI] [PubMed] [Google Scholar]
- Kuang X, Yan M, Ajmo JM, Scofield VL, Stoica G, Wong PK. et al. Activation of AMP-activated protein kinase in cerebella of Atm-/- mice is attributable to accumulation of reactive oxygen species. Biochem Biophys Res Commun. 2012;418(2):267–272. doi: 10.1016/j.bbrc.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen DM, van Praag H, Ray J, Weaver Z, Winrow CJ, Carter TA. et al. Ataxia telangiectasia mutated is essential during adult neurogenesis. Genes Dev. 2001;15(5):554–566. doi: 10.1101/gad.869001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hande MP, Balajee AS, Tchirkov A, Wynshaw-Boris A, Lansdorp PM. Extra-chromosomal telomeric DNA in cells from Atm(-/-) mice and patients with ataxia-telangiectasia. Hum Mol Genet. 2001;10(5):519–528. doi: 10.1093/hmg/10.5.519. [DOI] [PubMed] [Google Scholar]
- Wong KK, Maser RS, Bachoo RM, Menon J, Carrasco DR, Gu Y. et al. Telomere dysfunction and Atm deficiency compromises organ homeostasis and accelerates ageing. Nature. 2003;421(6923):643–648. doi: 10.1038/nature01385. [DOI] [PubMed] [Google Scholar]
- Ritchie KB, Petes TD. The Mre11p/Rad50p/Xrs2p complex and the Tel1p function in a single pathway for telomere maintenance in yeast. Genetics. 2000;155(1):475–479. doi: 10.1093/genetics/155.1.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva E, Tiong S, Pedersen M, Homola E, Royou A, Fasulo B. et al. ATM is required for telomere maintenance and chromosome stability during Drosophila development. Curr Biol. 2004;14(15):1341–1347. doi: 10.1016/j.cub.2004.06.056. [DOI] [PubMed] [Google Scholar]
- Queiroz-Machado J, Perdigao J, Simoes-Carvalho P, Herrmann S, Sunkel CE. tef: a mutation that causes telomere fusion and severe genome rearrangements in Drosophila melanogaster. Chromosoma. 2001;110(1):10–23. doi: 10.1007/s004120000116. [DOI] [PubMed] [Google Scholar]
- Petersen AJ, Rimkus SA, Wassarman DA. ATM kinase inhibition in glial cells activates the innate immune response and causes neurodegeneration in Drosophila. Proc Natl Acad Sci USA. 2012;109(11):E656–E664. doi: 10.1073/pnas.1110470109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang M, Yong C, Moretti L, Lu B. Zebrafish as a model system to screen radiation modifiers. Curr Genomics. 2007;8(6):360–369. doi: 10.2174/138920207783406497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura S, Kishi S. Molecular cloning and functional characterization of zebrafish ATM. Int J Biochem Cell Biol. 2005;37(5):1105–1116. doi: 10.1016/j.biocel.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Galron R, Gruber R, Lifshitz V, Lu H, Kirshner M, Ziv N. et al. Astrocyte dysfunction associated with cerebellar attrition in a Nijmegen breakage syndrome animal model. J Mol Neurosci. 2011;45(2):202–211. doi: 10.1007/s12031-011-9494-6. [DOI] [PubMed] [Google Scholar]
- Baranes K, Raz-Prag D, Nitzan A, Galron R, Ashery-Padan R, Rotenstreich Y. et al. Conditional inactivation of the NBS1 gene in the mouse central nervous system leads to neurodegeneration and disorganization of the visual system. Exp Neurol. 2009;218(1):24–32. doi: 10.1016/j.expneurol.2009.03.026. [DOI] [PubMed] [Google Scholar]
- Eilam R, Peter Y, Groner Y, Segal M. Late degeneration of nigro-striatal neurons in ATM-/- mice. Neuroscience. 2003;121(1):83–98. doi: 10.1016/s0306-4522(03)00322-1. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Wang Y, Jiang W, Liu X, Dubois RL, Lin CS. et al. Kinase-dead ATM protein causes genomic instability and early embryonic lethality in mice. J Cell Biol. 2012;198(3):305–313. doi: 10.1083/jcb.201204098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JA, Pellegrini M, Lee BS, Guo Z, Filsuf D, Belkina NV. et al. Loss of ATM kinase activity leads to embryonic lethality in mice. J Cell Biol. 2012;198(3):295–304. doi: 10.1083/jcb.201204035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang DQ, Kastan MB. Participation of ATM in insulin signalling through phosphorylation of eIF-4E-binding protein 1. Nat Cell Biol. 2000;2(12):893–898. doi: 10.1038/35046542. [DOI] [PubMed] [Google Scholar]
- Chong MJ, Murray MR, Gosink EC, Russell HR, Srinivasan A, Kapsetaki M. et al. Atm and Bax cooperate in ionizing radiation-induced apoptosis in the central nervous system. Proc Natl Acad Sci USA. 2000;97(2):889–894. doi: 10.1073/pnas.97.2.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuljis RO, Xu Y, Aguila MC, Baltimore D. Degeneration of neurons, synapses, and neuropil and glial activation in a murine Atm knockout model of ataxia-telangiectasia. Proc Natl Acad Sci USA. 1997;94(23):12688–12693. doi: 10.1073/pnas.94.23.12688. [DOI] [PMC free article] [PubMed] [Google Scholar]