Abstract
Synaptic function is critical for proper cognition, and synaptopathologies have been implicated in diverse neuropsychiatric disorders. STriatal-Enriched protein tyrosine Phosphatase (STEP) is a brain-enriched tyrosine phosphatase that normally opposes synaptic strengthening by dephosphorylating key neuronal signaling molecules. STEP targets include N-methyl D-aspartate receptors (NMDARs) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs), as well as extracellular signal-regulated kinase (ERK) and the tyrosine kinase Fyn. STEP-mediated dephosphorylation promotes the internalization of NMDARs and AMPARs and the inactivation of ERK and Fyn.
Regulation of STEP is complex, and recent work has implicated STEP dysregulation in the pathophysiology of several neuropsychiatric disorders. Both high levels and low levels of STEP are found in a diverse group of illnesses. This review focuses on the role of STEP in three disorders in which STEP levels are elevated: Alzheimer’s disease, fragile X syndrome, and schizophrenia. The presence of elevated STEP in all three of these disorders raises the intriguing possibility that cognitive deficits resulting from diverse etiologies may share a common molecular pathway.
Keywords: neuropsychiatric disorders, STEP, Alzheimer’s disease, fragile X syndrome, schizophrenia, NMDAR trafficking, synaptic plasticity, translational neuroscience
A Common STEP in the Synaptic Pathology of Diverse Neuropsychiatric Disorders
Synaptic connections provide the physical basis for communication within the brain, and the ability for synapses to strengthen and weaken is critical to maintaining proper cognitive function. Disruptions in synaptic function can thereby lead to impairments in cognition, and synaptopathologies have been implicated in a range of neuropsychiatric disorders, from addiction to mental retardation [1-5]. Dysfunction in proteins that regulate synaptic plasticity likely contributes to the development of neuropsychiatric disorders.
One of these proteins is STriatal-Enriched protein tyrosine Phosphatase (STEP), which is targeted, in part, to synaptic sites. STEP is one of hundreds of proteins present in dendritic spines, and there are many ways that synaptic plasticity can be disrupted. Nevertheless, research on STEP can deepen our understanding of the molecular basis of neuropsychiatric disorders by helping to elucidate mechanisms by which synaptic function is disrupted.
STEP is a protein tyrosine phosphatase enriched in the striatum [6]. After two decades of research, STEP has emerged as an important regulator of signal transduction and synaptic plasticity. Both high and low levels of STEP at synaptic sites would be expected to disrupt synaptic plasticity, and indeed, recent work has demonstrated that dysregulation of STEP is an important pathophysiological feature of several neuropsychiatric disorders. This review focuses on three disorders in which STEP levels are elevated: Alzheimer’s disease, fragile X syndrome, and schizophrenia. The presence of elevated STEP in these illnesses suggests that changes in synaptic levels of STEP provide a common molecular mechanism contributing to cognitive deficits in several neuropsychiatric disorders.
STEP: An Important Regulator of Synaptic Function
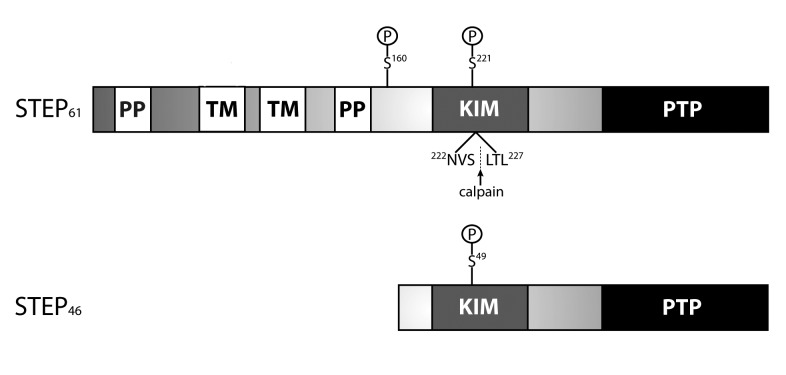
STEP exists as two major alternatively spliced isoforms: STEP61 and STEP46 (Figure 1). STEP61 is membrane-associated and targeted to the postsynaptic density, extrasynaptic sites, and endoplasmic reticulum, while STEP46 is primarily cytoplasmic [7-10]. STEP isoforms are differentially expressed in brain regions: for example, STEP61 is expressed in neurons of the hippocampus, neocortex, and lateral amygdala, while both STEP61 and STEP46 are expressed in the striatum, central nucleus of the amygdala, and optic nerve [11,12].
Figure 1.
STEP isoforms. STEP61 and STEP46 are the major isoforms produced by alternative splicing of a single STEP gene. The kinase-interacting motif (KIM) domain is a substrate-binding domain required for association with all substrates tested to date. STEP46 was the original isoform isolated and is a cytosolic variant. STEP61 contains an additional 172 amino acids in its amino-terminal region that contains two transmembrane (TM) domains, two polyproline-rich (PP) regions, and adjacent PEST sequences (not labeled). The TM regions target STEP61 to the postsynaptic density and endoplasmic reticulum. The KIM domain is required for binding to all substrates, while the PP regions impart substrate specificity. STEP33 is generated by calpain cleavage within the KIM domain between Ser224 and Leu225. Cleavage here destroys the binding domain and prevents STEP33 from interacting with its substrates. Phosphorylation of STEP by PKA occurs within the KIM domain (Ser221 and Ser49 on STEP61 and STEP46, respectively), and at a site adjacent to the PP regions (Ser160 on STEP61). The function of Ser160 on STEP61 remains unclear, and current work is investigating whether phosphorylation at this or other sites signals calpain-mediated cleavage and/or ubiquitination of STEP.
STEP activity and expression is regulated by several known mechanisms, including phosphorylation, proteolysis, dimerization, ubiquitination, and local translation [13-18]. An important theme that emerges from the literature is that different disease states tend to be associated with disruptions of different regulatory mechanisms.
One regulatory mechanism is the phosphorylation of a serine residue (Ser221 in STEP61, Ser49 in STEP46) located in the kinase-interacting motif (KIM) domain [13]. The KIM domain is the site of protein-protein interactions, and phosphorylation at this site sterically prevents the association of STEP with its substrates. Stimulation of dopamine D1 receptors activates cAMP-dependent protein kinase (PKA), which directly phosphorylates STEP to inactivate it and indirectly inactivates STEP by activating DARPP-32, which inhibits the ability of protein phosphatase 1 (PP1) to dephosphorylate and activate STEP [13,19]. In contrast, stimulation of glutamate N-methyl D-aspartate receptors (NMDARs) activates a calcineurin/PP1 pathway that dephosphorylates the regulatory serine residue within the KIM domain and activates STEP [14].
Substrates of STEP include glutamate receptor subunits as well as several protein kinases involved in signal transduction and synaptic plasticity. STEP dephosphorylates the GluN2B subunit (formerly called NR2B) of the NMDAR at Tyr1472, which leads to internalization of the GluN1/GluN2B receptor complex [17,20]. STEP also indirectly regulates Tyr1472 by inactivating Fyn, the tyrosine kinase that phosphorylates GluN2B at Tyr1472 [21]. STEP also appears to regulate α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) internalization by modulating dephosphorylation of the GluA2 subunit [18,22], although the Tyr residue that is dephosphorylated remains to be determined. In addition, STEP dephosphorylates a regulatory Tyr within the activation loop of several kinases and, thereby, inactivates extracellular signal-regulated kinase 1/2 (ERK1/2), Fyn, proline-rich tyrosine kinase 2 (Pyk2), and p38 [14,21,23-25].
The nature of STEP’s substrates suggests that STEP plays a role in the regulation of synaptic plasticity, particularly in the processes of long-term potentiation (LTP) and long-term depression (LTD). Since LTP and LTD are regulated, in part, by the trafficking of NMDARs and AMPARs to and from the postsynaptic membrane [26,27], increased levels of active STEP would be expected to attenuate LTP and promote LTD by reducing the number of NMDARs and AMPARs in the membrane. Indeed, administering STEP onto hippocampal slices prevents the induction of LTP, while hippocampal slices from STEP knockout (KO) mice show enhanced LTP [28,29]. It has also been shown that LTD following stimulation of metabotropic glutamate receptors (mGluRs) requires AMPAR internalization that is mediated in part by STEP [18].
The mitogen-activated protein kinase (MAPK) ERK1/2 also plays a key role in the development of synaptic strengthening, and activation of ERK1/2 is necessary for synaptic plasticity in many brain regions [30-32]. Activation of STEP mediated by GluN2B-containing NMDARs leads to the dephosphorylation and inactivation of ERK1/2 [33], and an inactive, substrate-trapping mutant of STEP disrupts the induction of LTP in the amygdala by binding ERK1/2 and blocking its translocation to the nucleus [34]. Together, these findings suggest a model by which STEP opposes synaptic strengthening through its ability to regulate the expression of glutamate receptors on synaptic membranes and the activity of several protein kinases. Because STEP is a crucial regulator of synaptic function in healthy individuals, it should not be surprising that disruptions in STEP activity are associated with a range of cognitive and neuropsychiatric disorders.
A STEP Too Far: Dysregulation in Neuropsychiatric Disorders
Alzheimer’s Disease
Alzheimer’s disease (AD) is the most common neurodegenerative disorder, with more than 26 million cases worldwide [35]. The risk to develop AD increases with age and occurs most commonly in people age 60 and older. Patients with AD display difficulty in many areas of cognitive function, and deficits are observed in memory, language, and problem-solving [36]. In the United States, AD affects more than 5 million people and is the sixth leading cause of death [37]. The economic impact of AD is enormous, with costs of AD and other dementias accounting for more than 1 percent of the world’s gross domestic product [38]. The human and financial costs are only expected to increase as the population ages in the coming decades.
The classic features of the AD brain are neuritic plaques and neurofibrillary tangles. An earlier hypothesis suggested that the accumulation of these pathological processes leads to neuronal damage. However, cognitive dysfunction did not correlate well with the accumulation of plaques or tangles, and it was found that the best predictor of cognitive deficits was a disruption of synaptic function [39,40]. An important clue pointing to a possible mechanism for AD came from the investigation of inherited forms of the illness. Mutations were found in amyloid precursor protein (APP), and these were shown to lead to the overproduction of β-amyloid (Aβ), suggesting that Aβ may play a role in disease progression [41]. One mechanism by which Aβ disrupts synaptic function is by interfering with the development of LTP [40]. One possible explanation for the disruption in synaptic strengthening is the finding that Aβ decreases the surface expression of NMDARs [20].
STEP plays a role in mediating some of Aβ’s toxic effects (Figure 2a. Aβ increases STEP activity through two distinct mechanisms. First, Aβ binds to the α-7 nicotinic acetylcholine receptor, promoting Ca2+ influx and activation of calcineurin and PP1, which dephosphorylates the regulatory Ser221 in the KIM domain to activate STEP [20]. Second, Aβ inhibits the proteasome degradation pathway [42]; since STEP is normally ubiquitinated and degraded by the proteasome, increased levels of Aβ results in the accumulation of STEP [17].
Figure 2.
The role of STEP in Alzheimer’s disease and Fragile X syndrome. A. Alzheimer’s disease: Two mechanisms have been described that contribute to the increase in STEP activity in Alzheimer’s disease (AD). β-amyloid (Aβ) binds to the α-7 nicotinic acetylcholine receptor [20], leading to calcium influx and activation of calcineurin (PP2B). This leads to activation of protein phosphatase 1 (PP1) and dephosphorylation of STEP at regulatory serine residues (Ser221 in STEP61 and Ser49 in STEP46) within the KIM domain. STEP isoforms can now bind to their substrates. Aβ also inhibits the proteasome [17]. As STEP is normally ubiquitinated and degraded by the proteasome, an Aβ-mediated inhibition of the proteasome results in increased levels of STEP. Activated STEP results in the Tyr dephosphorylation of its substrates. Shown are the dephosphorylation of GluN2B (Tyr1472) and the internalization of the GluN1/GluN2B receptor complex. Activated STEP also results in internalization of AMPARs (GluA1/GluA2) [18,22]. STEP also dephosphorylates and inactivates the tyrosine kinase Fyn (not shown), which phosphorylates GluN2B (Tyr1472) to promote exocytosis of these receptors. Thus, increased levels of active STEP leads to removal of glutamate receptors from synaptic membranes, contributing to the cognitive deficits in AD. B. Fragile X syndrome: STEP mRNA associates with FMRP, which inhibits its translation. Stimulation of mGluR5 receptors increases translation of many synaptic proteins, including STEP and APP. Thus, two mechanisms have been proposed for the increase in STEP levels in FXS. There is a direct increase in STEP translation due to the reduction in FMRP levels. In addition, inhibition of the proteasome by Aβ, as described in (a), would also result in an increase in STEP protein.
As discussed above, elevated STEP levels are associated with decreased phosphorylation and decreased surface expression of GluN2B-containing NMDARs. Indeed, Aβ-induced NMDAR internalization does not occur in cortical cultures that lack STEP [17]. Taken together, these findings suggest a model whereby high levels of Aβ result in increased activity and abundance of STEP, which disrupts synaptic function and contributes to the cognitive deficits present in AD. Consistent with this model, STEP levels are elevated in the cortex of three different AD mouse models [17,29,43], as well as in the prefrontal cortex of human AD patients [17].
To further test this hypothesis, STEP KO mice were crossed with transgenic AD mice, allowing for the study of progeny that still had elevated Aβ levels but were null for STEP. The genetic reduction of STEP reversed the cognitive and biochemical deficits associated with AD, rescuing spatial memory and surface expression of NMDARs [29]. This experiment provides compelling evidence for a role of STEP in the pathophysiology of AD.
Fragile X Syndrome>
Fragile X syndrome (FXS) is the most common inherited form of mental retardation, affecting approximately 1 in 5000 males [44]. It is also the most common known inherited form of autism [45]. Patients with FXS have characteristic behavioral symptoms that include attention deficit, hyperactivity, impulsivity, multiple anxiety symptoms, and seizures [45].
In FXS patients, the Fragile X Mental Retardation 1 (Fmr1) gene is not efficiently transcribed due to a trinucleotide expansion within the 5’-untranslated region. As a result, the production of the encoded Fragile X Mental Retardation Protein (FMRP) is severely decreased [46]. FMRP is an important regulator of signaling through mGluRs and is quickly synthesized upon mGluR stimulation [47]. A normal function of FMRP is to bind to a select population of mRNAs that are transported to synaptic sites and inhibit their translation [48-50]. FMRP thereby puts a brake on mGluR signaling by binding and suppressing translation of mRNAs downstream of mGluR stimulation [50,51]. Since FMRP is functionally absent in FXS, the pathophysiology includes upregulated translation of some synaptic mRNAs [51-53]. These ideas led to the mGluR hypothesis of fragile X syndrome, which posits that many effects of FXS are due to dysregulation of synaptic mRNA translation that is regulated through mGluRs [47]. Consistent with this hypothesis, increased mGluR-dependent LTD is found in FXS [54].
Recent findings indicate a role of STEP in the mechanism of exaggerated LTD in FXS. STEP is a downstream effector of mGluR stimulation, as mGluR agonists cause increased STEP translation [18]. It was recently shown that FMRP normally regulates STEP translation. FMRP binds to and inhibits STEP mRNA, while STEP translation is increased in the brains of Fmr1 KO mice [50,55]. As described previously, STEP plays a role in mGluR-mediated LTD, causing AMPAR endocytosis following mGluR stimulation [18]. Taken together, these findings suggest a model in which the absence of FMRP leads to increased STEP expression, which in turn contributes to a disruption of synaptic strengthening through internalization of glutamate receptors (Figure 2b). It should be noted that the increase in STEP expression described for FXS is different from the mechanism described earlier for the increase in STEP levels in AD. In FXS, increased levels are the result of increased translation of STEP mRNA, while the increase in STEP expression in AD is due to a decrease in its normal degradation.
Interestingly, the mRNA for APP is also regulated by FMRP, and Aβ levels are increased in FXS [56,57]. These findings raise the possibility that a second pathway may contribute to the increased levels of STEP in Fmr1 KO mice due to inhibition of the proteasome by Aβ, although future work will need to test this hypothesis.
The validity of this model was recently tested by producing STEP/Fmr1 double-KO mice. Even though these mice carry the FXS-causing mutation, the absence of STEP rescues some of the observed deficits, as mice with genetically reduced STEP exhibited a decrease in audiogenic seizures, social anxiety, and hyperactivity [55].
Schizophrenia
Schizophrenia (SZ) affects up to 1 percent of the population worldwide [58]. SZ decreases life expectancy by 10 years, mostly related to an increased risk of suicide [59]. SZ usually begins in adolescence, and symptoms typically develop slowly over months or years. People with SZ suffer from several categories of symptoms: positive symptoms, including hallucinations and disordered thinking; negative symptoms, including loss of motivation and social withdrawal; and cognitive deficits, including difficulties with executive function and abstract thinking.
An important model in the field is that SZ results from disruptions of glutamatergic signaling [60]. Specifically, the glutamate hypothesis posits that SZ symptoms result from deficits in glutamate signaling [58,61,62], with recent studies supporting a disruption in NMDAR trafficking [63]. Evidence for this model includes the fact that the drugs phencyclidine (PCP) and ketamine — which are NMDAR antagonists — are psychotomimetic, causing SZ-like symptoms in rodent and primate models as well as in humans [64,65]. However, the molecular mechanisms underlying NMDAR hypofunction in SZ remain unclear.
A recent study by Carty et al. [66] demonstrated elevated STEP levels in SZ brains. STEP is elevated in anterior cingulate cortex and dorsolateral prefrontal cortex of SZ patients, as compared to control tissue. STEP levels are also increased in mice treated with PCP and MK-801, NMDAR antagonists commonly used to model cognitive, behavioral, and biochemical changes associated with SZ. The accumulation of STEP following psychotomimetic treatment involves a disruption of the ubiquitin proteasome system. As noted above, STEP is normally regulated by ubiquitination and proteasomal degradation, and disrupting this regulatory mechanism results in accumulation of STEP in SZ brains.
The elevation of STEP levels is relevant to SZ disease processes. Increased STEP following treatment with psychotomimetics was associated with decreased phosphorylation of GluN2B at the site that STEP regulates, as well as decreased surface expression of GluN2B-containing NMDARs. Increased STEP thus provides a mechanistic link between psychotomimetic drugs and NMDAR hypofunction through decreased NMDAR surface expression.
There is also behavioral evidence that elevated STEP contributes to SZ symptoms. If STEP provides a mechanistic link between psychotomimetics and NMDAR hypofunction, then genetically eliminating STEP should produce mice that are less sensitive to the effects of these drugs. Indeed, STEP KO mice had significantly less cognitive and locomotor deficits after PCP administration compared to wild-type mice [66].
Furthermore, the positive effects of neuroleptics appear to be mediated, at least in part, by the inactivation of STEP. Chronic treatment of mice with both typical and atypical antipsychotic medication leads to increased phosphorylation of STEP61 at the regulatory Ser211 residue within its substrate-binding domain, preventing association of STEP with its substrates. Inactivation of STEP by neuroleptics is associated with the expected downstream effects: increased phosphorylation of GluN2B at the regulatory Tyr1472 residue and a corresponding increase in surface expression of NMDARs [66].
Future Directions and Concluding Remarks
An important question raised by these findings is how elevated STEP could contribute to the cognitive deficits observed in such diverse disorders. One possible explanation is that the specific disease may result from a differential regulation of STEP in different brain regions. For instance, preliminary data on Parkinson’s disease (PD) indicates that the genetic elimination of parkin (an E3 ubiquitin ligase that is mutated in some forms of hereditary PD) is associated with elevated STEP levels in the striatum, but not in the cortex (P. Kurup, M.A. Johnson, P.J. Lombroso, unpublished observations). In contrast, inhibition of the ubiquitin proteasome system in AD is associated with elevated STEP in the cortex. This raises the possibility that different ubiquitin ligases regulate STEP in different brain regions. Consistent with this hypothesis is the finding that differential expression of specific ligases within the ubiquitin proteasome pathway is a feature of SZ [67,68]; disruptions in these mechanisms may also contribute to STEP elevation in specific brain regions.
In general, it is possible that regulatory mechanisms exert differential control of STEP levels according to brain region and/or subcellular localization. According to this model, disrupting one particular mechanism would lead to a distinct pattern of elevated STEP within the brain. Dysfunctional synaptic plasticity within specific neuronal populations could allow STEP to contribute to cognitive deficits of diverse natures. Future work will provide a more complete characterization of STEP expression patterns in neuropsychiatric conditions. For instance, it would be interesting to examine whether STEP levels are differentially elevated in specific neuronal subtypes and neural circuits implicated in SZ pathophysiology.
Another important question is whether STEP could be a useful target for therapeutic interventions. The outlook seems promising for at least three reasons. First, since genetic reductions in STEP are able to rescue deficits in AD and FXS, pharmacologically reducing STEP activity should in principle be a valid therapeutic strategy. Second, therapeutic success has been obtained by inhibiting other protein tyrosine phosphatases (PTPs). For instance, after the discovery that PTP1B knockout mice exhibit increased insulin sensitivity [69], PTP1B inhibitors entered clinical trials for the treatment of type II diabetes. Third, the crystal structure of STEP has been solved, and its distinct molecular features may allow the development of inhibitors that preferentially target STEP as compared to other related PTPs [70].
One key difference between STEP and other PTPs occurs in the so-called WPD loop, which is near the active site of all PTPs. STEP belongs to a class of PTPs with a distinct sequence in the WPD loop, and within this class the WPD loop takes on a unique conformation in STEP [70,71]. Novel chemical strategies can be used to develop inhibitors that exploit these differences to maximize specificity. For instance, the selectivity of PTP1B inhibitors has been enhanced by linking several small molecules together, thereby taking advantage of chemical differences present at multiple sites in the enzyme [69]. Efforts are currently under way to identify and characterize STEP inhibitors. Since STEP levels are down-regulated in a number of disorders, including Huntington’s disease, drug addiction, stroke/ischemia, and inflammatory pain (reviewed in [72]), one potential challenge in employing STEP inhibitors as a therapeutic strategy will be to effectively treat conditions where STEP is elevated without causing deficits associated with other disorders where STEP is down-regulated.
The three neuropsychiatric disorders described in this paper have quite different etiologies and occur at very different times in a person’s life. FXS is present at birth, SZ usually develops in adolescence, and AD is most commonly a disease of the elderly. The cellular and behavioral phenotypes associated with each of these conditions are also quite different. Research that implicates elevated STEP in the pathophysiology of each of these disorders thus raises the intriguing possibility that cognitive deficits resulting from diverse origins may share a common molecular pathway. Researchers and clinicians are hoping that STEP inhibitors will prove to be a new therapeutic approach for these devastating illnesses.
Abbreviations
- STEP
STriatal-Enriched protein tyrosine Phosphatase
- KIM
kinase-interacting motif
- NMDAR
N-methyl D-aspartate receptor
- PP1
protein phosphatase 1
- PKA
cAMP-dependent protein kinase
- AMPAR
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- Pyk2
proline-rich tyrosine kinase 2
- ERK
extracellular signal-regulated kinase
- LTP
long-term potentiation
- LTD
long-term depression
- KO
knockout
- mGluR
metabotropic glutamate receptor
- MAPK
mitogen-activated protein kinase
- AD
Alzheimer’s disease
- APP
amyloid precursor protein
- Aβ
β-amyloid
- FXS
fragile X syndrome
- Fmr1
Fragile X Mental Retardation 1
- FMRP
Fragile X Mental Retardation Protei
- SZ
schizophrenia
- PCP
phencyclidine
- PD
Parkinson’s disease
- PTP
protein tyrosine phosphatase
Author contributions
MAJ wrote and PJL edited the review. Funding sources: MH052711 and MH091037 (PJL).
References
- Huttenlocher PR. Dendritic and synaptic pathology in mental retardation. Pediatr Neurol. 1991;7(2):79–85. doi: 10.1016/0887-8994(91)90001-2. [DOI] [PubMed] [Google Scholar]
- Blanpied TA, Ehlers MD. Microanatomy of dendritic spines: emerging principles of synaptic pathology in psychiatric and neurological disease. Biol Psychiatry. 2004;55(12):1121–1127. doi: 10.1016/j.biopsych.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Fiala JC, Spacek J, Harris KM. Dendritic spine pathology: cause or consequence of neurological disorders? Brain Res Brain Res Rev. 2002;39(1):29–54. doi: 10.1016/s0165-0173(02)00158-3. [DOI] [PubMed] [Google Scholar]
- Oever MCVD, Spijker S, Smit AB. In: Synaptic Plasticity. Kreutz MR, Sala C, editors. Vienna: Springer Vienna; 2012. The Synaptic Plasticity of Drug Addiction; pp. 469–491. [Google Scholar]
- Garner CC, Wetmore DZ. Synaptic pathology of Down syndrome. Adv Exp Med Biol. 2012;970:451–468. doi: 10.1007/978-3-7091-0932-8_20. [DOI] [PubMed] [Google Scholar]
- Lombroso PJ, Murdoch G, Lerner M. Molecular characterization of a protein-tyrosine-phosphatase enriched in striatum. Proc Natl Acad Sci USA. 1991;88(16):7242–7246. doi: 10.1073/pnas.88.16.7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombroso PJ, Naegele JR, Sharma E, Lerner M. A protein tyrosine phosphatase expressed within dopaminoceptive neurons of the basal ganglia and related structures. J Neurosci. 1993;13(7):3064–3074. doi: 10.1523/JNEUROSCI.13-07-03064.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bult A, Zhao F, Dirkx R Jr., Sharma E, Lukacsi E, Solimena M. et al. STEP 61: A member of a family of brain-enriched PTPs is localized to the endoplasmic reticulum. J Neurosci. 1996;16(24):7821–7831. doi: 10.1523/JNEUROSCI.16-24-07821.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama T, Goto S, Nishi T, Sato K, Yamada K, Yoshikawa M. et al. Immunocytochemical localization of the striatal enriched protein tyrosine phosphatase in the rat striatum. Neuroscience. 1995;69(3):869–880. doi: 10.1016/0306-4522(95)00278-q. [DOI] [PubMed] [Google Scholar]
- Goebel-Goody SM, Davies KD, Alvestad Linger RM, Freund RK, Browning MD. Phospho-regulation of synaptic and extrasynaptic N-methyl-d-aspartate receptors in adult hippocampal slices. Neuroscience. 2009;158(4):1446–1459. doi: 10.1016/j.neuroscience.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Boulanger LM, Wahle P, During MJ. Cellular and molecular characterization of a brain-enriched protein tyrosine phosphatase. J Neurosci. 1995;15(2):1532–1544. doi: 10.1523/JNEUROSCI.15-02-01532.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorber B, Berry M, Hendriks W, den Hertog J, Pulido R, Logan A. Stimulated regeneration of the crushed adult rat optic nerve correlates with attenuated expression of the protein tyrosine phosphatases RPTPalpha, STEP, and LAR. Mol Cell Neurosci. 2004;27(4):404–416. doi: 10.1016/j.mcn.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Paul S, Snyder GL, Yokakura H, Picciotto MR, Nairn AC, Lombroso PJ. The Dopamine/D1 receptor mediates the phosphorylation and inactivation of the protein tyrosine phosphatase STEP via a PKA-dependent pathway. J Neurosci. 2000;20(15):5630–5638. doi: 10.1523/JNEUROSCI.20-15-05630.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Nairn AC, Wang P, Lombroso PJ. NMDA-mediated activation of the tyrosine phosphatase STEP regulates the duration of ERK signaling. Nat Neurosci. 2003;6(1):34–42. doi: 10.1038/nn989. [DOI] [PubMed] [Google Scholar]
- Xu J, Kurup P, Zhang Y, Goebel-Goody SM, Wu PH, Hawasli AH. et al. Extrasynaptic NMDA receptors couple preferentially to excitotoxicity via calpain-mediated cleavage of STEP. J Neurosci. 2009;29(29):9330–9343. doi: 10.1523/JNEUROSCI.2212-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb I, Poddar R, Paul S. Oxidative stress-induced oligomerization inhibits the activity of the non-receptor tyrosine phosphatase STEP61. J Neurochem. 2011;116(6):1097–1111. doi: 10.1111/j.1471-4159.2010.07165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurup P, Zhang Y, Xu J, Venkitaramani DV, Haroutunian V, Greengard P. et al. Abeta-mediated NMDA receptor endocytosis in Alzheimer’s disease involves ubiquitination of the tyrosine phosphatase STEP61. J Neurosci. 2010;30(17):5948–5957. doi: 10.1523/JNEUROSCI.0157-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Venkitaramani DV, Gladding CM, Zhang Y, Kurup P, Molnar E. et al. The tyrosine phosphatase STEP mediates AMPA receptor endocytosis after metabotropic glutamate receptor stimulation. J Neurosci. 2008;28(42):10561–10566. doi: 10.1523/JNEUROSCI.2666-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol J-C. et al. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci USA. 2005;102(2):491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY. et al. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8(8):1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Nguyen T-H, Liu J, Lombroso PJ. Striatal enriched phosphatase 61 dephosphorylates Fyn at phosphotyrosine 420. J Biol Chem. 2002;277(27):24274–24279. doi: 10.1074/jbc.M111683200. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kurup P, Xu J, Anderson GM, Greengard P, Nairn AC. et al. Reduced levels of the tyrosine phosphatase STEP block β amyloid-mediated GluA1/GluA2 receptor internalization. J Neurochem. 2011;119(3):664–672. doi: 10.1111/j.1471-4159.2011.07450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Kurup P, Bartos JA, Patriarchi T, Hell JW, Lombroso PJ. Striatal-enriched Protein-tyrosine Phosphatase (STEP) Regulates Pyk2 Kinase Activity. J Biol Chem. 2012;287(25):20942–20956. doi: 10.1074/jbc.M112.368654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz JJ, Tárrega C, Blanco-Aparicio C, Pulido R. Differential interaction of the tyrosine phosphatases PTP-SL, STEP and HePTP with the mitogen-activated protein kinases ERK1/2 and p38alpha is determined by a kinase specificity sequence and influenced by reducing agents. Biochem J. 2003;372(Pt 1):193–201. doi: 10.1042/BJ20021941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poddar R, Deb I, Mukherjee S, Paul S. NR2B-NMDA receptor mediated modulation of the tyrosine phosphatase STEP regulates glutamate induced neuronal cell death. J Neurochem. 2010;115(6):1350–1362. doi: 10.1111/j.1471-4159.2010.07035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Otaño I, Ehlers MD. Homeostatic plasticity and NMDA receptor trafficking. Trends Neurosci. 2005;28(5):229–238. doi: 10.1016/j.tins.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Kerchner G, Nicoll RA. Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci. 2008;9(11):813–825. doi: 10.1038/nrn2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkey KA, Askalan R, Paul S, Kalia LV, Nguyen TH, Pitcher GM. et al. Tyrosine phosphatase STEP is a tonic brake on induction of long-term potentiation. Neuron. 2002;34(1):127–138. doi: 10.1016/s0896-6273(02)00633-5. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kurup P, Xu J, Carty N, Fernandez SM, Nygaard HB. et al. Genetic reduction of striatal-enriched tyrosine phosphatase (STEP) reverses cognitive and cellular deficits in an Alzheimer’s disease mouse model. Proc Natl Acad Sci USA. 2010;107(44):19014–19019. doi: 10.1073/pnas.1013543107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafe GE, Atkins CM, Swank MW, Bauer EP, Sweatt JD, LeDoux JE. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of pavlovian fear conditioning. J Neurosci. 2000;20(21):8177–8187. doi: 10.1523/JNEUROSCI.20-21-08177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14(3):311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Wiegert JS, Bading H. Activity-dependent calcium signaling and ERK-MAP kinases in neurons: a link to structural plasticity of the nucleus and gene transcription regulation. Cell Calcium. 2011;49(5):296–305. doi: 10.1016/j.ceca.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Paul S, Connor J. NR2B-NMDA receptor-mediated increases in intracellular Ca2+ concentration regulate the tyrosine phosphatase, STEP, and ERK MAP kinase signaling. J Neurochem. 2010;114(4):1107–1118. doi: 10.1111/j.1471-4159.2010.06835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Olausson P, Venkitaramani DV, Ruchkina I, Timothy D, Tronson N. et al. The protein tyrosine phosphatase STEP gates long-term potentiation and fear memory in the lateral amygdala. Biol Psychiatry. 2007;61(9):1049–1061. doi: 10.1016/j.biopsych.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement. 2007;3(3):186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148(6):1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimers Association. 2012 Alzheimer’s disease facts and figures. Elsevier Ltd; 2012. [Google Scholar]
- Alzheimer’s Disease International. World Alzheimer Report: The Global Economic Impact of Dementia. 2010
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R. et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30(4):572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298(5594):789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Goate A, Hardy J. Twenty years of Alzheimer’s disease-causing mutations. J Neurochem. 2012;120(Suppl 1):3–8. doi: 10.1111/j.1471-4159.2011.07575.x. [DOI] [PubMed] [Google Scholar]
- Yi JJ, Ehlers MD. Emerging roles for ubiquitin and protein degradation in neuronal function. Pharmacol Rev. 2007;59(1):14–39. doi: 10.1124/pr.59.1.4. [DOI] [PubMed] [Google Scholar]
- Chin J, Palop JJ, Puoliväli J, Massaro C, Bien-Ly N, Gerstein H. et al. Fyn kinase induces synaptic and cognitive impairments in a transgenic mouse model of Alzheimer’s disease. J Neurosci. 2005;25(42):9694–9703. doi: 10.1523/JNEUROSCI.2980-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffee B, Keith K, Albizua I, Malone T, Mowrey J, Sherman SL. et al. Incidence of fragile X syndrome by newborn screening for methylated FMR1 DNA. Am J Hum Genet. 2009;85(4):503–514. doi: 10.1016/j.ajhg.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranfaglia MR. The psychiatric presentation of fragile x: evolution of the diagnosis and treatment of the psychiatric comorbidities of fragile X syndrome. Dev Neurosci. 2011;33(5):337–348. doi: 10.1159/000329421. [DOI] [PubMed] [Google Scholar]
- Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60(2):201–214. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27(7):370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Li Z, Zhang Y, Ku L, Wilkinson KD, Warren ST, Feng Y. The fragile X mental retardation protein inhibits translation via interacting with mRNA. Nucleic Acids Res. 2001;29(11):2276–2283. doi: 10.1093/nar/29.11.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antar LN, Bassell GJ. Sunrise at the synapse: the FMRP mRNP shaping the synaptic interface. Neuron. 2003;37(4):555–558. doi: 10.1016/s0896-6273(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Darnell JC, Van Driesche SJ, Zhang C, Hung KYS, Mele A, Fraser CE. et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell. 2011;146(2):247–261. doi: 10.1016/j.cell.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Antion MD, Hu D, Spencer CM, Paylor R, Klann E. Dynamic translational and proteasomal regulation of fragile X mental retardation protein controls mGluR-dependent long-term depression. Neuron. 2006;51(4):441–454. doi: 10.1016/j.neuron.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Zalfa F, Giorgi M, Primerano B, Moro A, Di Penta A, Reis S. et al. The fragile X syndrome protein FMRP associates with BC1 RNA and regulates the translation of specific mRNAs at synapses. Cell. 2003;112(3):317–327. doi: 10.1016/s0092-8674(03)00079-5. [DOI] [PubMed] [Google Scholar]
- Lu R, Wang H, Liang Z, Ku L, O'Donnell WT, Li W. et al. The fragile X protein controls microtubule-associated protein 1B translation and microtubule stability in brain neuron development. Proc Natl Acad Sci USA. 2004;101(42):15201–15206. doi: 10.1073/pnas.0404995101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci USA. 2002;99(11):7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel-Goody S, Wilson-Wallis ED, Royston S, Tagliatela SM, Naegele JR, Lombroso PJ. Genetic manipulation of STEP reverses behavioral abnormalities in a fragile X syndrome mouse model. Genes Brain Behav. 2012;11(5):586–600. doi: 10.1111/j.1601-183X.2012.00781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westmark CJ, Malter JS. FMRP mediates mGluR5-dependent translation of amyloid precursor protein. PLoS Biology. 2007;5(3):e52. doi: 10.1371/journal.pbio.0050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westmark CJ, Westmark PR, O’Riordan KJ, Ray BC, Hervey CM, Salamat MS. Reversal of fragile X phenotypes by manipulation of AβPP/Aβ levels in Fmr1KO mice. PloS One. 2011;6(10):e26549. doi: 10.1371/journal.pone.0026549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC. Glutamatergic theories of schizophrenia. Isr J Psychiatry Relat Sci. 2010;47(1):4–16. [PubMed] [Google Scholar]
- Rössler W, Salize HJ, van Os J, Riecher-Rössler A. Size of burden of schizophrenia and psychotic disorders B. Eur Neuropsychopharmacol. 2005;15(4):399–409. doi: 10.1016/j.euroneuro.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Goff DC, Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry. 2001;158(9):1367–1377. doi: 10.1176/appi.ajp.158.9.1367. [DOI] [PubMed] [Google Scholar]
- Gao X, Sakai K, Roberts RC, Conley RR, Dean B, Tamminga CA. Ionotropic glutamate receptors and expression of N-methyl- D -aspartate receptor subunits in subregions of human hippocampus: Effects of Schizophrenia. Am J Psychiatry. 2000;157(7):1141–1149. doi: 10.1176/appi.ajp.157.7.1141. [DOI] [PubMed] [Google Scholar]
- Ibrahim HM, Hogg AJ Jr., Healy DJ, Haroutunian V, Davis KL, Meador-Woodruff JH. Ionotropic glutamate receptor binding and subunit mRNA expression in thalamic nuclei in schizophrenia. Am J Psychiatry. 2000;157(11):1811–1823. doi: 10.1176/appi.ajp.157.11.1811. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull. 2009;35(3):509–527. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, D’Souza DC, Petrakis IL, Belger A, Berman RM, Charney DS. et al. NMDA Agonists and antagonists as probes of glutamatergic dysfunction and pharmacotherapies in neuropsychiatric disorders. Harv Rev Psychiatry. 1999;7(3):125–143. [PubMed] [Google Scholar]
- Bubeníková-Valesová V, Horácek J, Vrajová M, Höschl C. Models of schizophrenia in humans and animals based on inhibition of NMDA receptors. Neurosci Biobehav Rev. 2008;32(5):1014–1023. doi: 10.1016/j.neubiorev.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Carty NC, Xu J, Kurup P, Brouillette J, Goebel-Goody SM, Austin DR. et al. The tyrosine phosphatase STEP: implications in schizophrenia and the molecular mechanism underlying antipsychotic medications. Transl Psychiatry. 2012;2:e137. doi: 10.1038/tp.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousman CA, Chana G, Glatt SJ, Chandler SD, May T, Lohr J. et al. Positive symptoms of psychosis correlate with expression of ubiquitin proteasome genes in peripheral blood. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(7):1336–1341. doi: 10.1002/ajmg.b.31106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousman CA, Chana G, Glatt SJ, Chandler SD, Lucero GR, Tatro E. et al. Preliminary evidence of ubiquitin proteasome system dysregulation in schizophrenia and bipolar disorder: convergent pathway analysis findings from two independent samples. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(2):494–502. doi: 10.1002/ajmg.b.31006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr AJ. Protein tyrosine phosphatases as drug targets: strategies and challenges of inhibitor development. Future Med Chem. 2010;2(10):1563–1576. doi: 10.4155/fmc.10.241. [DOI] [PubMed] [Google Scholar]
- Eswaran J, von Kries JP, Marsden B, Longman E, Debreczeni JE, Ugochukwu E. et al. Crystal structures and inhibitor identification for PTPN5, PTPRR and PTPN7: a family of human MAPK-specific protein tyrosine phosphatases. Biochem J. 2006;395(3):483–491. doi: 10.1042/BJ20051931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr AJ, Knapp S. MAPK-specific tyrosine phosphatases: new targets for drug discovery? Trends Pharmacol Sci. 2006;26(10):525–530. doi: 10.1016/j.tips.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Goebel-Goody SM, Baum M, Paspalas CD, Fernandez SM, Carty NC, Kurup P. et al. Therapeutic implications for striatal-enriched protein tyrosine phosphatase (STEP) in neuropsychiatric disorders. Pharmacol Rev. 2012;64(1):65–87. doi: 10.1124/pr.110.003053. [DOI] [PMC free article] [PubMed] [Google Scholar]