Summary
The efficacy of many vaccines against intracellular bacteria depends on the generation of cell-mediated immunity, but studies to determine the duration of immunity are usually confounded by re-exposure. The causative agent of tularemia, Francisella tularensis, is rare in most areas and, therefore, tularemia vaccination is an interesting model for studies of the longevity of vaccine-induced cell-mediated immunity. Here lymphocyte proliferation and cytokine production in response to F. tularensis were assayed in two groups of 16 individuals, vaccinated 1-3 or 27-34 years previously. As compared to naïve individuals, vaccinees of both groups showed higher proliferative responses and, out of 17 cytokines assayed, higher levels of MIP-1β, IFN-γ, IL-10, and IL-5 in response to recall stimulation. The responses were very similar in the two groups of vaccinees. A statistical model was developed to predict the immune status of the individuals and by use of two parameters, proliferative responses and levels of IFN-γ, 91.1% of the individuals were correctly classified. Using flow cytometry analysis, we demonstrated that during recall stimulation, expression of IFN-γ by CD4+CCR7+, CD4+CD62L+, CD8+CCR7+, and CD8+CD62L+ cells significantly increased in samples from vaccinated donors. In conclusion, cell-mediated immunity was found to persist three decades after tularemia vaccination without evidence of decline.
Keywords: Francisella tularensis, vaccination, persistence, cell-mediated immunity
Introduction
Francisella tularensis, the causative agent of tularemia, is a highly virulent Gram-negative coccobacillus. Irrespective of disease manifestation, tularemia results in effective protective immunity, and only a few cases of reinfection are known from the literature [1]. Also, vaccination with live attenuated strains of F. tularensis affords effective protection [2]. For example, when vaccination with the F. tularensis live vaccine strain (LVS) was introduced at the US Army Medical Research Institute of Infectious Diseases, Fort Detrick, Maryland, the incidence of tularemia among the laboratory staff decreased by 95% [1]. F. tularensis-specific immunity is T cell-dependent [3-5] and the capacity of the responding T cells to produce IFN-γ is crucial for protection [6-7]. The F. tularensis-specific cell-mediated immune response is extremely long-lived. After an outbreak of tularemia in Sweden, T cell responses to various F. tularensis antigens were demonstrated in almost all of 52 former tularemia patients 25 years later [5].
These findings also imply that F. tularensis vaccination may lead to long-lived cell-mediated immunity. The latter is an important issue to consider when establishing recommendations regarding revaccination of laboratory staff and other individuals at risk. A previous study demonstrated a proliferative T cell response to F. tularensis up to nine years after LVS vaccination [9]. Data on the immune response for longer periods are not available. There are examples of historical guidelines regarding revaccination. In the Soviet Union during the 1950s, e.g., there was an official recommendation of a five-year interval between revaccinations, since cases of tularemia [10].
In the present study, recall responses to F. tularensis were assayed in individuals vaccinated with LVS at least 27 years ago and these responses were compared quantitatively and qualitatively to responses of individuals vaccinated at most three years before testing.
Results and Discussion
Lymphocyte proliferation assay (LPA)
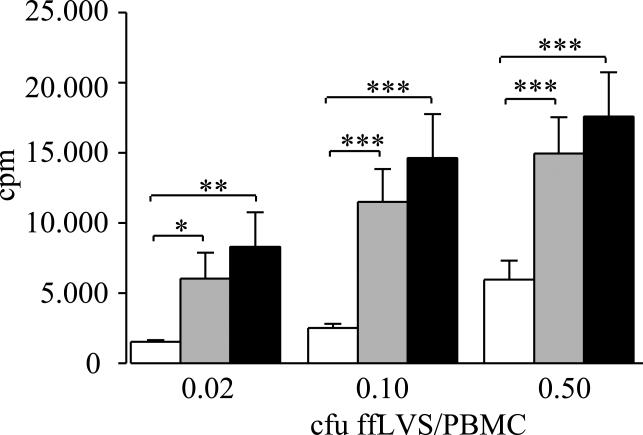
The proliferative response of T cells to formalin-fixed F. tularensis LVS (ffLVS) was assayed in PBMC cultures from 16 individuals vaccinated with LVS within the last three years (svc), 16 individuals vaccinated 27 to 34 years before testing (lvc), and 13 non-vaccinated individuals. Although the magnitude of responses varied widely within the groups, each group of vaccinees showed a higher proliferative response (P <0.05) than did the group of non-vaccinated subjects. There were no significant differences between the two groups of vaccinees (P >0.10) at any of three antigen concentrations tested (figure 1).
Figure 1.
Proliferative responses of PBMC from naive and from LVS vaccinated donors to recall antigen. Lymphocyte proliferation was measured by incorporation of [3H]-thymidine upon stimulation with indicated concentrations of formalin-fixed LVS/PBMC for five days. PBMC were from donors vaccinated 27-34 years ago (black bars) or less than three years ago (grey bars) or from naïve individuals (white bars). Mean values ± SEM of samples from 13-16 individuals per group are shown. P-values were determined using Wilcoxon's test (* P<0.05, ** P<0.01; *** P<0.005). The median background levels ± SEM for the groups were 1,557 ± 161 cpm (naïve), 580 ± 70 cpm (svc) and 1,527 ± 600 cpm (lvc).
Cytokine production in response to F. tularensis antigen
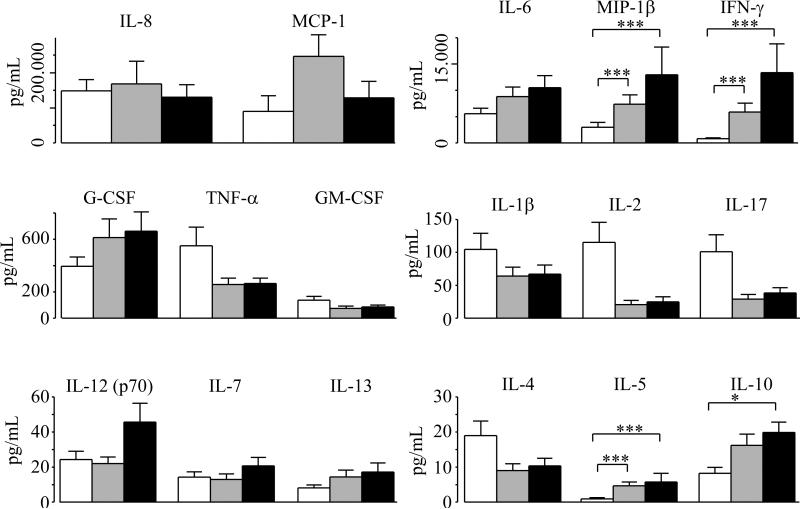
Supernatants of PBMC cultures used for LPA were assayed for 17 cytokines. Most cytokines were present at higher levels after five days than after three days of recall stimulation and the cytokine results for the former time point was therefore compared between the three groups. At an antigen concentration of 0.1 cfu ffLVS/PBMC, levels of MIP-1β, IFN-γ, IL-5 and IL-10 were higher in cultures from one or both groups of vaccinees than in cultures of the nonvaccinated group (P<0.05, figure 2). In addition, at 0.02 cfu ffLVS/PBMC, MCP-1 also showed significantly higher levels in the two groups of vaccinees than in non-vaccinated subjects (P<0.005, data not shown). When cytokine levels were compared between lvc and svc for all 17 cytokines and the three antigen concentrations, we observed only a single statistically significant difference. After stimulation with 0.02 cfu ffLVS/PBMC the levels of IL-12 were significantly higher in samples from svc (P< 0.05, not shown).
Figure 2.
Levels of secreted cytokines after five days of recall stimulation with 0.1 cfu ffLVS/PBMC in cell samples from naïve and from LVS vaccinated donors (black bars: donors vaccinated 27-34 years ago; grey bars: donors vaccinated less than three years ago). Cytokines were measured using a 17-plex Bio-Plex kit. Mean values ± SEM of samples from 13-16 individuals per group are presented. P-values were determined using Wilcoxon's test (* P<0.05, ** P<0.01; *** P<0.005).
Three out of 17 cytokines showed more rapid kinetics, i.e. they had higher levels after three days of recall stimulation than after five days. These cytokines were IL-17, TNF-α and IL-2; all three were secreted at significantly higher levels by PBMC from vaccinees than by cells from non-vaccinated individuals (P < 0.05) and there were no significant differences between cells from svc and from lvc. The mean levels (pg/ml) ± SEM for vaccinees on day three were: IL-17 46.4 ± 8.2, TNF-α 882.6 ± 156.0, IL-2 80.8 ± 14.3 and for non-vaccinated individuals: IL-17 11.7 ± 5.2, TNF-α 271.8 ± 121.6, IL-2 22.9 ± 10.2. Our result for IL-17 is in line with a recent study of the T cell memory in LVS vaccinees vaccinated six to nine months prior to testing [11]. These authors demonstrated a Th17 cell response and increased levels of IL-17 in the culture medium after three days of recall stimulation. Interestingly, the differentiation of Th17 cells appears to be negatively regulated by IFN-γ [12-13]. Indeed, our results showed a reciprocal relationship between IL-17 and IFN-γ; high levels of IL-17 and low levels of IFN-γ on day 3, and vice versa on day 5 (not shown). In addition, after five days of stimulation the IL-17 levels in samples from vaccinees were lower than the ones in samples from non-vaccinated donors (figure 2). Thus IFN-γ may have negatively regulated the Th17 cell differentiation, leading to lower levels of IL-17 than in cultures from non-vaccinated subjects.
Although known to be critical to host resistance to the agent, the role of TNF-α in the secondary response to the agent is less carefully studied. In the acute phase of human tularemia, F. tularensis antigen-induced TNF-α has been demonstrated at high level during the first few weeks after onset of disease, however, with a decline to low levels within four months [14]. Our observation that IL-2 levels were higher after three days than after five days of recall stimulation is in concordance with previous studies that showed a peak in IL-2 production after 12-18 h of stimulation [15] and maximum expression of IL-2 receptor after 48-72 h of stimulation [16].
Predicting the vaccination status of an individual
A pair-wise analysis showed that at 0.1 cfu ffLVS/PBMC, levels of the cytokines IFN-γ, MIP-1β, IL-5 and IL-10 in cultures from any given individual were highly correlated (range of Spearman correlation coefficient 0.59-0.76 with P< 0.001), while correlations between the cytokine levels and the proliferation data were considerably lower (range 0.04-0.34 with P>0.80). We then asked whether these results could be used to distinguish between the different donor groups. Multivariate data analyses based on lymphocyte proliferation and the cytokines that showed significant differences suggested a good separation of naïve from vaccinated donors, but no separation of lvc and svc (data not shown).
We wanted to develop a model based on a minimum number of parameters to identify vaccinees, as a practical means to validate the development of specific immunity after vaccination. A classifier predicting the immune status of an individual was built using five parameters: the significantly increased cytokines, IFN-γ, MIP-1β, IL-5, IL-10, together with the LPA data. The individuals were divided into two groups: vaccinees and naïve donors. The final model included LPA results and data on secreted IFN-γ. The probability p that an individual showed a significant vaccine take was expressed as follows:
The relevance of the model was verified in that 91.1% of the individuals were correctly classified. It should be noted that several more complex models including three out of the five aforementioned variables (IFN-γ, MIP-1β, IL-5, IL-10, and LPA) also discriminated between vaccinees and naïve donors with similar degrees of predicting power as the suggested model.
Intracellular IFN-γ–expression during recall responses to F. tularensis antigens
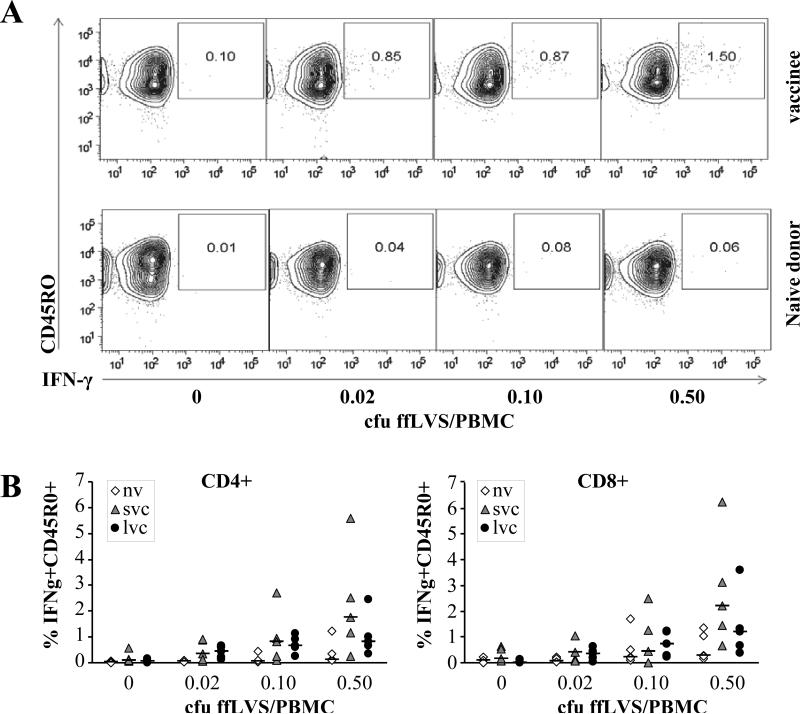
To describe the phenotypes of the F. tularensis-specific T cells in detail, we performed polychromatic flow cytometry analysis on PBMC samples from five individuals in each donor group. Given the significance of IFN-γ in host resistance to F. tularensis, we determined the frequency of IFN-γ-producing CD4 and CD8 memory T cells after stimulation with various antigen concentrations. PBMC from vaccinated donors demonstrated an antigen-dependent increase in the frequency of IFN-γ+ CD4 and CD8 memory T cells, while cells from naïve individuals did not. For CD4 T cells, regardless of antigen concentration, this increase in the IFN-γ+ cell population was significantly higher in vaccinees than in naïve individuals (P < 0.05, figure 3), while levels of IFN-γ expression by CD8 T cells were not significantly increased (P > 0.42). Regardless of T cell population, we did not observe any significant differences between the two groups of vaccinees (P > 0.84).
Figure 3.
Frequency of IFNγ expressing memory T cells in recall stimulated PBMC. A) Representative elevation curve plots for samples from one vaccinee (upper panels) and one naïve individual (lower panels). B) Scatter plots summarizing results from five individuals in each donor group (nv: PBMC from naïve individuals, svc: PBMC from were from donors vaccinated less than three years ago, lvc: PBMC from 27-34 years ago). Horizontal bars mark the mean of each sample group. Intracellular cytokine staining and flow cytometry analysis of T cell subsets was performed after 48 h of stimulation with indicated concentrations of cfu ffLVS/PBMC. Lymphocytes were gated from live cells according to forward and side scatter and subsequently CD4 and CD8 T cells were gated as CD3+CD4+ or CD3+CD8+, respectively.
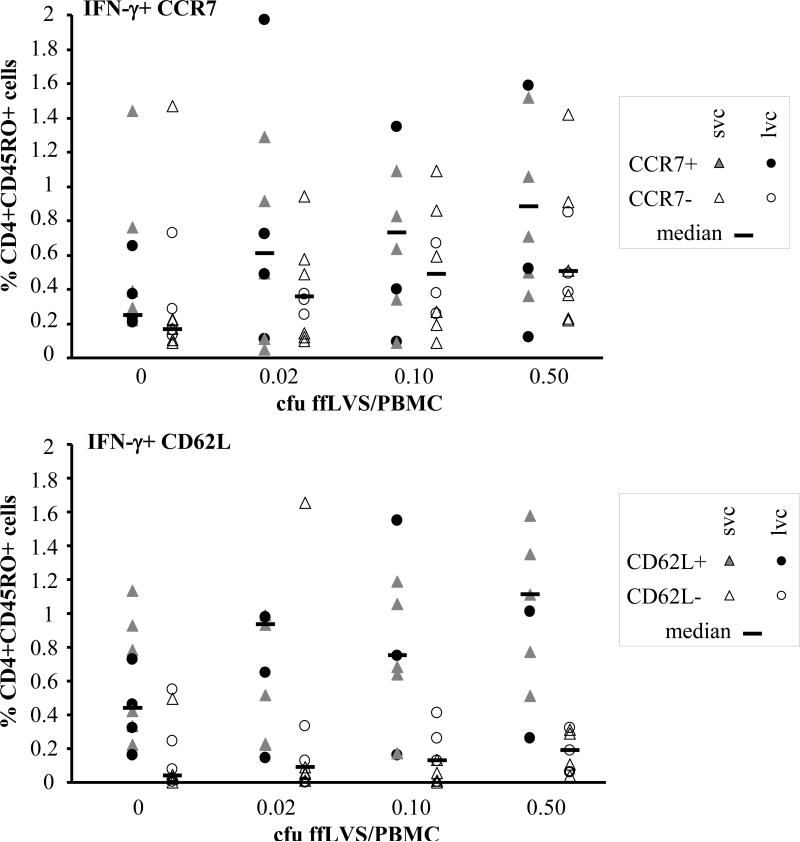
Chemokine receptor 7 (CCR7) is a molecule controlling homing to secondary lymphoid organs and, together with CD62L, it distinguishes central memory (CCR7+CD62L+) T cells from effector memory (CCR7-CD62L-) T cells [17]. We used detection of CCR7 or CD62L in combination with CD45RO and IFN-γ to further characterize the F. tularensis-specific T cells. Analysis of PBMC samples from ten vaccinated donors (six svc, four lvc) showed that the frequency of IFN-γ producing CD45RO+ CCR7+ or CD45RO+ CD62L+ cells increased in an antigen-dependent manner in both, the CD4 (see figure 4) and the CD8 population (data not shown). In almost all cases, the corresponding effector memory T cell subsets increased as well, with the exception of CD8 CD45RO+ CCR7- IFN-γ+. In the case of IFN-γ producing CD4 T cells, the increase in CD62L+ cells was higher than in CD62L- cells. In comparison, the absolute increase in the CCR7+ population was more prominent than in the CD62L+ population, however, they were highly correlated (P<0.0001). We did not find any significant differences between the two groups of vaccines (figure 4). These findings agree with the capability of these cells to proliferate vigorously to antigen upon secondary stimulation as shown in the lymphocyte proliferation assay. Proliferation from T cell receptor triggering in vitro has been shown earlier to be stronger in CCR7+ central memory cells than either naïve (CD45RA, CCR7+) or effector memory T cells (CD45RO, CCR7-, CD62Llow) [17].
Figure 4.
Characterization of CD4+CD45RO+ T cells by flow cytometric detection of CCR7 or CD62L and IFN-γ. Scatter plots summarizing results from ten vaccinated individuals (six svc: triangles; four lvc: circles). Open symbols indicate the frequency of IFN-γ producing CD4+CD45RO+ T cells that were CCR7- or CD62L-negative, filled symbols mark the percentage of IFN-γ producing CD4+CD45RO+ T cells that were CCR7- or CD62L-positive cells, horizontal bars show the median of each T cell subset.
Intracellular cytokine staining and flow cytometry analysis of T cell subsets was performed after 48 h of stimulation with indicated concentrations of ffLVS/PBMC. CD4+CD45RO+ cells were identified and thereafter gated for expression or lack of expression of CCR7 or CD62L. The percentage of cells expressing intracellular IFN-γ in each of the four subsets was then determined.
Effector memory cells are believed to participate in host defense during a specific infection whereas central memory cells constitute a population primed for subsequent encounters with the pathogen. Altogether, our results demonstrate a long-term survival for both these T cell populations after tularemia vaccination.
There is compelling evidence that immunological memory can be long-lived, but studies on its longevity in humans are usually complicated by periodic re-exposure of individuals to most pathogens. It is important to understand, however, how long the memory is sustained, not only from a theoretical perspective, but also with regard to immunity to pathogens of low prevalence. Knowledge about the longevity of cell-mediated immunity in humans to pathogens is incomplete despite a wealth of recent findings on the molecular mechanisms of T cell function and maintenance [18]. A study on Native Americans demonstrated preserved T cell immunity 60 years after vaccination with BCG [18]. Our results also resemble previous observations on human T cell memory to certain viral infections. After smallpox vaccination, 90 % of the individuals maintained substantial cellular immunity even 50 years later [19] and measles-specific CD4 and CD8 T cells were detected 34 years after vaccination [20].
In view of our present findings, cell-mediated immunity to F. tularensis after vaccination with LVS may also be life-long. It should be remarked, however, that no specific correlate to protective immunity against intracellular bacteria exists. At present it can only be argued that cell-mediated immunity is important for protection to agents such as F. tularensis. A caveat in interpreting the present results is the possibility of natural re-exposure of vaccinated individuals to F. tularensis. However, during the last 40 years, tularemia has been reported very rarely in the Umeå region of Sweden, where our donors lived and only one individual had worked in a laboratory handling F. tularensis. It seems unlikely that exposure to cross-reacting microbial antigens can be a reason for the long-lived immunity, as also evidenced by the minimal reactivity among the control individuals. Altogether, our findings indicate that long-term immunity to F. tularensis persists after tularemia vaccination independently of re-exposure to the bacterium, which is similar to the situation after natural infection [5]. This is also in agreement with recent findings on T cell memory demonstrating that a minor fraction of antigen-specific T cells, about 5% of the peak frequency, survives to become long-lived central memory cells [21]. These cells are sustained through contact with IL-7 and IL-15 but independently of encounters with specific antigen or MHC [21].
There was a highly significant difference in the average age of the lvc versus the svc. It is well established that there are characteristic changes of the immune system during aging, including a progressive reduction of the CD4+ and CD8+ T cells and up-regulation of the inflammatory responses [22]. However, no differences in proliferative capacity were found between young, middle-aged, and centenarians [23]. In accordance, the lvc and svc investigated here showed similar magnitudes of T-cell responses to F. tularensis antigen.
A model was developed based on multivariate analysis to determine a minimal number of parameters that could predict vaccine take, since this would be a practical way of identifying immunoconversion after vaccination. A model with high discriminatory power was developed based on the results of LPA and secretion of IFN-γ. The model correctly classified 91% of the individuals.
Revaccination with live attenuated bacterial vaccines is a controversial issue. Since there is a lack of evidence of efficacy after BCG vaccination in adults, the World Health Organization does not recommend revaccination against tuberculosis [24]. In view of our findings, even theoretically the need for revaccination appears questionable since an intact T cell memory may prevent bacterial replication and thereby effective boosting of immunity. The only exception would be individuals at risk of exposure to F. tularensis that show no specific cell-mediated immune responses after vaccination.
Conclusions
Similar to previous findings after natural infection [5], a wide individual variation in the magnitude of proliferative and cytokine responses was found in LVS vaccinees; however, the overall responses were much higher in vaccinees than in naïve individuals. A remarkable longevity of the cell-mediated immune responses to F. tularensis in LVS-vaccinated individuals was observed. The proliferative response and cytokine patterns were essentially the same in lvc (27-34 years) as in svc (<3 years). This longevity of T cell memory to F. tularensis after vaccination is comparable to what has been found 25 years after recovery from tularemia [5].
Materials and Methods
Individuals included in the study
Blood donors had either been vaccinated with F. tularensis LVS or had no anamnestic data on LVS vaccination, tularemia, or occupational exposure to F. tularensis (naïve). All vaccinees had been given the same variant of LVS, designated NDBR 101, lot no. 11 (National Drug Company, Philadelphia, PA), by scarification, either >27 (lvc) or <3 years ago (svc). In the former group, only one individual had worked in a clinical microbiological laboratory, thus for the remaining individuals, occupational exposure to F. tularensis was highly unlikely. Ethical approval, 05-166M, was obtained from the Regional Ethical Review Board in Umeå, Sweden, and an informed consent was obtained from all individuals included in the study. The mean age and sex distribution of each group was the following: lvc - 60.4 years (13 females, 3 males), svc - 40.0 years (12 females, 4 males), naïve - 36.6 years (5 females, 8 males).
PBMC collection
Venous blood from donors was collected using CPT-tubes (Becton Dickinson, NJ, USA) and PBMC were prepared according to the manufacturer's recommendations.
Lymphocyte proliferation assay (LPA)
For LPA and multiplex cytokine analysis, PBMC were seeded at 2×105 in 100μL RPMI 1640 (GIBCO/Invitrogen) with 10% heat-inactivated human serum and 40μg/mL gentamicin in 96-well plates. For flow cytometry analysis, 4 ×105 cells per well was used. Cells were stimulated with antigen at final concentrations of 0.02, 0.1, or 0.5 cfu ffLVS/PBMC or without antigen and incubated for two (flow cytometry) or three and five days (LPA, multiplex cytokine analysis) in a humidified atmosphere with 5% CO2 at 37°C. LPA was performed as described previously [5].
Multiplex cytokine analysis
Cell culture supernatants, 80μL, were collected and stored frozen at -80° C until analyzed. Supernatants were diluted 1:10 and analyzed using a commercial 17-plex kit and a Bio-Plex 200 system (BioRad Laboratories Inc, Hercules, CA, USA) according to manufacturer's instructions. Samples were analyzed in duplicates.
Flow cytometry analysis of surface markers and intracellular IFN-γ
After 44 h, Brefeldin A was added to the cultures. Four hours later, plates were centrifuged 3 min at 500 × g and supernatants removed. Cells were prepared for labeling with cell surface marker monoclonal antibodies (mAb) or intracellular cytokine mAb as recommended by Becton-Dickinson. The following mAb conjugates were used: CD3-AlexaFlour700 (clone UCHT1, Becton-Dickinson), CD4-PE Texas red (clone S3.5, Caltag/Invitrogen) or –APCCy7 (clone RPA-T4), CD8-PerCPCy5.5 (clone SK1), CD45R0-PECy7 (clone UCHL-1), IFNγ-FITC (clone 25723.11, Becton-Dickinson), CCR7-PE (clone 3D12), CD62LAPCAlexa750 or -PeCy5 (clones DREG-56). A minimum of 50,000 cells were acquired for each analysis using a LSRII flow cytometer (Becton-Dickinson). Results were analyzed using FACS Diva (Becton-Dickinson) or FlowJo (Tree Star) software.
Data analysis and statistical methods
Wilcoxon's test was used to calculate significant differences in cytokine levels and in lymphocyte proliferation between different donor groups of individuals. Spearman's rank correlation was used to estimate association. In the multivariate analyses hierarchical clustering using log-transformed and non-standardized data, Manhattan distance and Ward's method were used together with principal component analysis. Logistic regression, using log-transformed data, was used for modeling. Model selection was applied using Akaike's information criterion and the complexity of the model as selection criteria. Only models with less than four variables were considered. The prediction ability of the final model was estimated using “leave one out”- cross validation.
Acknowledgements
This work has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, Contract No. HHSN266200500041C. The authors declare no conflict of interest.
Abbreviations
- LVS
live vaccine strain
- ffLVS
formalin-fixed LVS
- PBMC
peripheral blood mononuclear cells
- Svc
short-term vaccinee
- Lvc
long-term vaccinee
References
- 1.Burke DS. Immunization against tularemia: analysis of the effectiveness of live Francisella tularensis vaccine in prevention of laboratory-acquired tularemia. J Infect Dis. 1977;135:55–60. doi: 10.1093/infdis/135.1.55. [DOI] [PubMed] [Google Scholar]
- 2.Conlan JW, Oyston PC. Vaccines against Francisella tularensis. Ann N Y Acad Sci. 2007;1105:325–350. doi: 10.1196/annals.1409.012. [DOI] [PubMed] [Google Scholar]
- 3.Kostiala AA, McGregor DD, Logie PS. Tularaemia in the rat. I. The cellular basis on host resistance to infection. Immunology. 1975;28:855–869. [PMC free article] [PubMed] [Google Scholar]
- 4.Anthony LS, Kongshavn PA. Experimental murine tularemia caused by Francisella tularensis, live vaccine strain: a model of acquired cellular resistance. Microb Pathog. 1987;2:3–14. doi: 10.1016/0882-4010(87)90110-0. [DOI] [PubMed] [Google Scholar]
- 5.Ericsson M, Sandstrom G, Sjöstedt A, Tärnvik A. Persistence of cell-mediated immunity and decline of humoral immunity to the intracellular bacterium Francisella tularensis 25 years after natural infection. J Infect Dis. 1994;170:110–114. doi: 10.1093/infdis/170.1.110. [DOI] [PubMed] [Google Scholar]
- 6.Anthony LS, Ghadirian E, Nestel FP, Kongshavn PA. The requirement for gamma interferon in resistance of mice to experimental tularemia. Microb Pathog. 1989;7:421–428. doi: 10.1016/0882-4010(89)90022-3. [DOI] [PubMed] [Google Scholar]
- 7.Leiby DA, Fortier AH, Crawford RM, Schreiber RD, Nacy CA. In vivo modulation of the murine immune response to Francisella tularensis LVS by administration of anticytokine antibodies. Infect Immun. 1992;60:84–89. doi: 10.1128/iai.60.1.84-89.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tärnvik A. Nature of protective immunity to Francisella tularensis. Rev Infect Dis. 1989;11:440–451. [PubMed] [Google Scholar]
- 9.Tärnvik A, Löfgren ML, Löfgren S, Sandström G, Wolf-Watz H. Long-lasting cell-mediated immunity induced by a live Francisella tularensis vaccine. J Clin Microbiol. 1985;22:527–530. doi: 10.1128/jcm.22.4.527-530.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piotrovskaia SA. [Certain indices of immunity in children vaccinated against tularemia.]. Zh Mikrobiol Epidemiol Immunobiol. 1958;29:129. [PubMed] [Google Scholar]
- 11.Paranavitana C, Zelazowska E, DaSilva L, Pittman PR, Nikolich M. Th17 cytokines in recall responses against Francisella tularensis in humans. J Interferon Cytokine Res. 2010;30:471–476. doi: 10.1089/jir.2009.0108. [DOI] [PubMed] [Google Scholar]
- 12.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 13.Nakae S, Iwakura Y, Suto H, Galli SJ. Phenotypic differences between Th1 and Th17 cells and negative regulation of Th1 cell differentiation by IL-17. J Leukoc Biol. 2007;81:1258–1268. doi: 10.1189/jlb.1006610. [DOI] [PubMed] [Google Scholar]
- 14.Surcel HM, Syrjala H, Karttunen R, Tapaninaho S, Herva E. Development of Francisella tularensis antigen responses measured as T-lymphocyte proliferation and cytokine production (tumor necrosis factor alpha, gamma interferon, and interleukin-2 and -4) during human tularemia. Infect Immun. 1991;59:1948–1953. doi: 10.1128/iai.59.6.1948-1953.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gullberg M, Ivars F, Coutinho A, Larsson EL. Regulation of T cell growth factor production: arrest of TCGF production after 18 hours in normal lectin-stimulated mouse spleen cell cultures. J Immunol. 1981;127:407–411. [PubMed] [Google Scholar]
- 16.Cantrell DA, Smith KA. Transient expression of interleukin 2 receptors. Consequences for T cell growth. J Exp Med. 1983;158:1895–1911. doi: 10.1084/jem.158.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 18.Steward-Tharp SM, Song YJ, Siegel RM, O'Shea JJ. New insights into T cell biology and T cell-directed therapy for autoimmunity, inflammation, and immunosuppression. Ann N Y Acad Sci. 2010;1183:123–148. doi: 10.1111/j.1749-6632.2009.05124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 20.Naniche D, Garenne M, Rae C, Manchester M, Buchta R, Brodine SK, Oldstone MB. Decrease in measles virus-specific CD4 T cell memory in vaccinated subjects. J Infect Dis. 2004;190:1387–1395. doi: 10.1086/424571. [DOI] [PubMed] [Google Scholar]
- 21.Boyman O, Letourneau S, Krieg C, Sprent J. Homeostatic proliferation and survival of naive and memory T cells. Eur J Immunol. 2009;39:2088–2094. doi: 10.1002/eji.200939444. [DOI] [PubMed] [Google Scholar]
- 22.Sansoni P, Vescovini R, Fagnoni F, Biasini C, Zanni F, Zanlari L, Telera A, Lucchini G, Passeri G, Monti D, Franceschi C, Passeri M. The immune system in extreme longevity. Exp Gerontol. 2008;43:61–65. doi: 10.1016/j.exger.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 23.Sansoni P, Fagnoni F, Vescovini R, Mazzola M, Brianti V, Bologna G, Nigro E, Lavagetto G, Cossarizza A, Monti D, Franceschi C, Passeri M. T lymphocyte proliferative capability to defined stimuli and costimulatory CD28 pathway is not impaired in healthy centenarians. Mech Ageing Dev. 1997;96:127–136. doi: 10.1016/s0047-6374(97)01887-3. [DOI] [PubMed] [Google Scholar]
- 24.Global tuberculosis programme and global programme on vaccines. Statement on BCG revaccination for the prevention of tuberculosis. Wkly Epidemiol Rec. 1995;70:229–231. [PubMed] [Google Scholar]