Oral administration of chemotherapeutic agents is the mainstay for the treatment of disease. Sustained release formulations have been crucial for the safe and effective dosing of orally administered drugs.[1] Such formulations allow for the prolonged maintenance of therapeutic drug concentrations, reducing the required dosages per day and thereby enhancing patient compliance. Sustained release formulations also provide tighter control over the pharmacokinetics of a drug, thereby minimizing side effects.[1]
Aqueous solubility is likewise a critical attribute for an orally available drug.[2] Robust absorption across the intestinal epithelium relies upon a high concentration of the drug to drive diffusion into enterocytes and, eventually, into the circulatory system. On average, 35–40% of lead compounds have aqueous solubilities of <5 mg/mL, which is defined by the U.S. Pharmacopeia as being slightly soluble or worse.[3] Accordingly, the bioavailability and consequent efficacy of many compounds relies on enhancing their aqueous solubility.[2c]
The formation of a phosphomonoester can improve the oral bioavailability of poorly water-soluble chemotherapeutic agents.[2b,2c,4] Endogenous phosphatases near the surface of enterocytes can catalyze the hydrolysis of the phosphoryl group, releasing the lipophilic drug and allowing for its efficient absorption into the body. Several prodrugs approved by the U.S. Food and Drug Administration rely on this strategy, including estramustine, fosamprenavir, and prednisolone phosphate.[4]
Recently, we reported on the potential utility of a phosphodiester as the pro-moiety for a drug administered intravenously.[5] Specifically, we found that the coupling of 4-hydroxytamoxifen to uridine 3′-phosphate enabled its timed-release in serum by human pancreatic ribonuclease (RNase 1[6]; EC 3.1.27.5). This modification also increased the aqueous solubility of 4-hydroxytamoxifen.
RNase 1 is an ideal endogenous enzyme to elicit pro-moiety release. A major excreted enzyme, RNase 1 has a concentration of 6.4 mg/mL in human pancreatic juice and 0.2 mg/mL in saliva, according to a radioimmunoassay.[7] Moreover, like its renowned homologue bovine pancreatic ribonuclease (RNase A[8]), RNase 1 catalyzes the cleavage of RNA by a transphosphorylation reaction[9] with little specificity for its leaving group.[10] Herein, we report on the utility of several ribonucleoside 3′-phosphates as pro-moieties for a model orally available drug, metronidazole.
Metronidazole is a commonly used antibiotic for a variety of protozoa and anaerobic bacterial infections, including Bacteroides fragilis, Helicobacter pylori, Clostridium difficile, Trichomonas vaginalis, and Entamoeba histolytica.[11] In 1997, Flagyl ER, an extended release formulation of metronidazole, was approved by the FDA as a superior treatment for bacterial vaginosis. Still, metronidazole has several common side effects, such as nausea, diarrhea, and metallic taste. Moreover, metronidazole therapy can occasionally cause more severe side effects, such as pancreatitis, neutropenia, neuropathies, or CNS toxicities.[12] These adverse effects could be attenuated with better control over the pharmacokinetics of metronidazole.[13] Hence, in this proof-of-concept study, we elected to attach metronidazole to ribonucleoside 3′-phosphates to assess the attributes of this promoiety for orally available drugs (Figure 1).
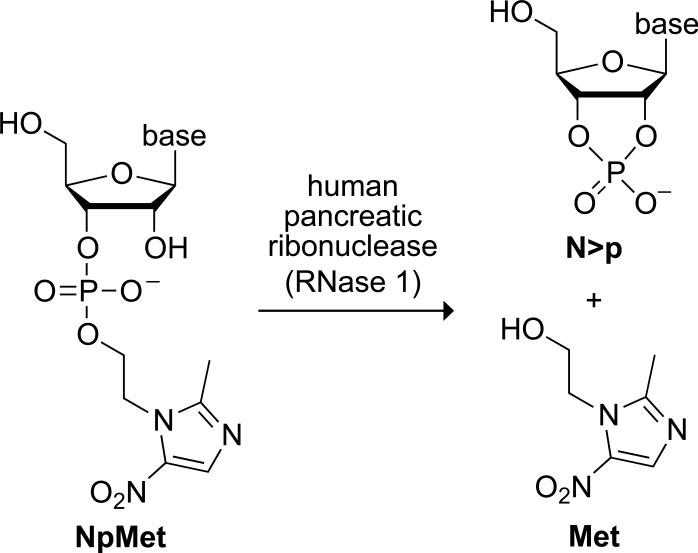
Figure 1.
Scheme showing catalysis of the cleavage of a ribonucleoside 3′-(metronidazole phosphate) (NpMet) by RNase 1 to yield a nucleoside 2′,3′-cyclic phosphate (N>p) and Met.
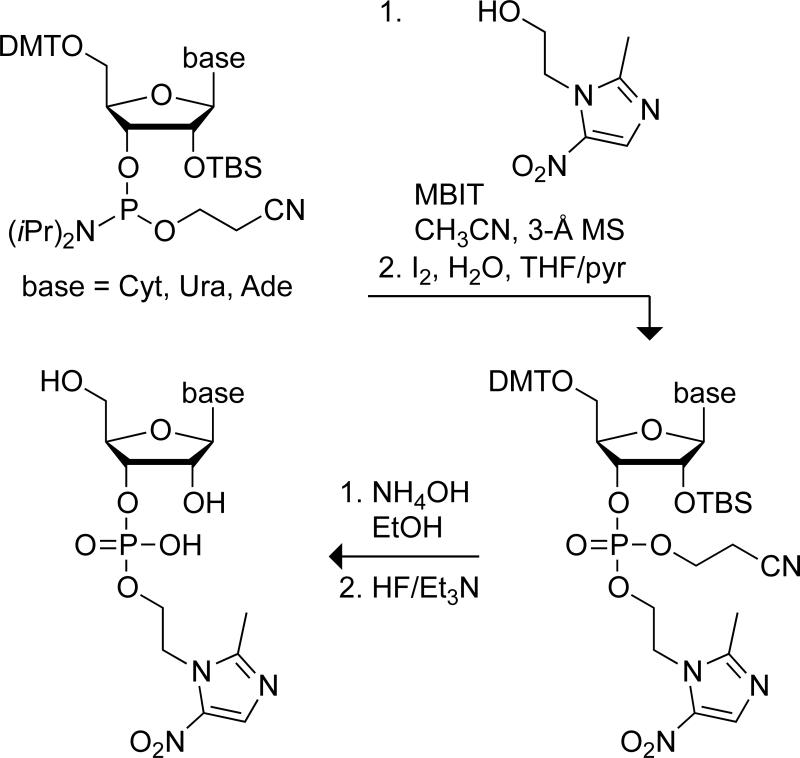
Each ribonucleoside 3′-(metronidazole phosphate) (NpMet) was synthesized in four steps from commercially available metronidazole (Met) and a ribonucleoside phosphoramidite (Scheme 1). Briefly, Met was coupled to the phosphoramidite by using N-methylbenzimidazolium triflate (MBIT) as a catalyst.[14] The coupled product was oxidized with iodine and deprotected stepwise. The final products were purified by chromatography on silica gel. This route was used to synthesize three different NpMets: cytidine 3′-(metronidazole phosphate) (CpMet, 18% non-optimized yield), uridine 3′-(metronidazole phosphate) (UpMet, 80%), and adenosine 3′-(metronidazole phosphate) (ApMet, 64%).
Scheme 1.
Route for the synthesis of NpMets.
We expected the ribonucleoside 3′-phosphate moiety of an NpMet to endow the prodrug with greater hydrophilicity than the parent drug, which could improve its oral bioavailability. To investigate this issue, we calculated the partition (log P) and distribution (log D) coefficients of Met, CpMet, UpMet, and ApMet.[15] The calculated log P and log D values for the NpMets were indeed significantly lower than those of the parent drug (Table 1), indicative of increased hydrophilicity and decreased tendency to aggregate.
Table 1.
Calculated Partition and Distribution Coefficients of Met and NpMets[15]
| cofficient | Met | CpMet | UpMet | ApMet |
|---|---|---|---|---|
| log P | –0.46 | –2.48 | –2.10 | –1.78 |
| log D (pH 7.5) | –0.46 | –4.86 | –4.48 | –4.15 |
| log D (pH 1.1) | –1.49 | –3.73 | –3.20 | –5.57 |
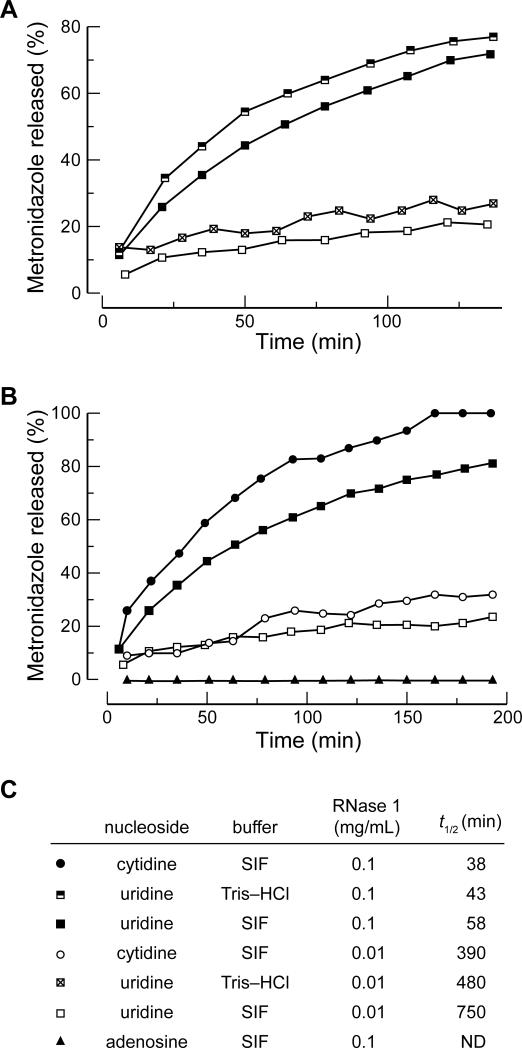
To be the basis for an effective timed-release prodrug strategy, the pro-moiety needs to be released by the activating enzyme over time. Hence, we used 1H NMR spectroscopy to assess the rate at which RNase 1 catalyzed the release of Met from the prodrugs (Figure S1). We assumed that pancreatic juice is diluted in the intestine, which led us to use RNase 1 at concentrations of 0.1 mg/mL and 0.01 mg/mL in these assays. Because inorganic phosphate inhibits RNase A with a Ki value of 2.3 mM,[16] we initially investigated the effect of phosphate in simulated intestinal fluid (SIF) on the rates of UpMet unmasking (Figure 2A). Compared to a buffer with no inorganic phosphate (19.5 mM Tris–HCl, pH 7.4, 2.5% v/v D2O), the rate of Met release in SIF was only marginally slower. RNase 1 cleaves after pyrimidine residues more readily than after purine residues.[6,7] Accordingly, we predicted that RNase 1 would unmask CpMet and UpMet faster than ApMet. For both concentrations of RNase 1, we did indeed observe that the cytidine and uridine prodrugs were unmasked faster than the adenosine prodrug (Figure 2B). Moreover, unlike the uridine 3′-phosphate–4-hydroxytamoxifen conjugate that cleaved spontaneously in aqueous solutions lacking ribonucleases, the NpMet conjugates were stable in SIF, which has pH 7.5, and in simulated gastric fluid (SGF), which has pH 1.1. The absence of appreciable degradation (<5%) in either medium (Figure S2) is attributable to the alkoxyl group of metronidazole being a much worse leaving group than the aryloxyl group of 4-hydroxytamoxifen.
Figure 2.
Progress curves for the release of Met from NpMets under various conditions as determined by 1H NMR spectroscopy. (A) Comparison of UpMet cleavage rates in Tris–HCl buffer, pH 7.4, and simulated intestinal fluid (SIF). (B) Comparison of CpMet, UpMet, and ApMet cleavage rates in SIF. (C) Half-life values of NpMets from panels A and B. Data for UpMet in SIF are reported in both panel A and panel B to facilitate comparisons. ND: Not determined.
Finally, we sought to assess the antimicrobial activity of our NpMet prodrugs on B. fragilis. This penicillin-resistant Gram-negative bacillus is common in anaerobic infections, like those that originate from the gastrointestinal tract. We determined the minimum inhibitory concentration (MIC) of UpMet and ApMet, as well as Met (Figure S3). We found that both UpMet and ApMet had considerably higher MIC values than did Met (Table 2), demonstrating that the prodrugs were relatively inactive. Incubating UpMet with 0.1 mg/mL RNase 1 overnight resulted in an MIC similar to that of Met. Finally, adding UpMet to a culture medium containing RNase 1 gave an intermediate MIC, demonstrating the in situ release of the drug.
Table 2.
MIC Values of NpMets for Bacteroides fragilis
| compound | RNase 1 (mg/mL) | MIC (μg/mL equiv of Met) |
|---|---|---|
| Met | — | 1–0.5 |
| ApMet | — | >128 |
| UpMet | — | 32 |
| Met | 0.1 O/N | 1–0.5 |
| UpMet | 0.1 O/N | 0.5 |
| Met | 0.01 | 1–0.5 |
| UpMet | 0.01 | 2 |
O/N: Preincubation of the compound with RNase 1 overnight.
Conclusions
We have developed a versatile prodrug strategy for orally administered drugs (Figure 1). Our strategy relies on the liberation of a drug from a ribonucleoside 3′-phosphate conjugate by a human ribonuclease. The other product of prodrug cleavage, a nucleoside 2′,3′-cyclic phosphate, is a natural metabolite. We found that the cleavage rate depends on the nucleobase: Cyt > Ura >> Ade, and that the drug was efficacious only in the presence of the ribonuclease. We note that this system is highly versatile. For example, nonnatural nucleobases could provide further rate modulation. Self-immolative linkers could be used to liberate drugs with amino groups. Finally, the 5′-hydroxyl group of the nucleoside could be modified to enhance aqueous solubility, improve pharmacokinetics, or endow other attributes. A variant of RNase 1 is now in a Phase I clinical trial (NCT00818831) as a cancer chemotherapeutic agent.[17] Our work could presage additional clinical utility for ribonucleolytic activity.
Experimental Section
Production and Purification of RNase 1
RNase 1 was produced and purified as reported previously.[17a] The enzyme was concentrated to 6.98 mg/mL in the elution buffer, which was 65% buffer A (50 mM NaOAc and 10 mM EDTA, pH 5.0) and 35% buffer B (50 mM NaOAc, 370 mM NaCl, and 10 mM EDTA, pH 5.0). RNase 1 was diluted subsequently into the buffer appropriate for an assay.
Kinetic Analysis of Prodrug Cleavage
The cleavage of NpMets by RNase 1 was assessed by using 1H NMR spectroscopy. Reaction mixtures contained prodrug (2.1 μmol) and RNase 1 (0.1 or 0.01 mg/mL). NMR experiments were conducted in 19.5 mM Tris–HCl buffer, pH 7.4, containing D2O (2.5% v/v), SIF containing D2O (2% v/v), or SGF (which was 0.10 N HCl, pH 1.1) containing D2O (2% v/v). SIF was USP XXII formulation, without pancreatin, pH 7.5 from the RICCA Chemical Company (Arlington, TX). The spectrometer was shimmed for solvent suppression to a 700-μL sample of the buffer with no NpMet. A 350-μL solution of NpMet in buffer was placed in a quartz NMR tube from Wilmad–LabGlass (Elk Grove Village, IL), which was incubated at 37°C for 5 min. Simultaneously, a solution of RNase 1 in the same buffer was warmed to 37°C for 5 min. At t = 0, 350 μL of the RNase 1 solution was added to the NMR tube and mixed by inversion. The NMR tube was inserted into the spectrometer, and spectra were recorded at known times. Cleavage was assessed by integrating the distinguishable peaks corresponding to the methyl group of the NpMets and Met. Values of t1/2 were calculated from linear regression analysis of plots of 1/[NpMet] versus time.
Preparation of Bacteroides fragilis culture medium
Guidelines for the determination of the MIC of metronidazole on the growth of B. fragilis were from the Clinical and Laboratory Standards Institute.[18] Hemin stock solution was prepared by dissolving 0.10 g of hemin into 2 mL of 1.0 M NaOH, bringing the final volume to 20 mL with distilled water, and sterilizing at 121 °C for 15 min. A solution of vitamin K1 was prepared by adding vitamin K1 (0.20 mL) to 95% ethanol (20 mL). Then, 1 mL of this solution was added to 9 mL of sterile, distilled water, resulting in a 1.0 mg/mL working solution of vitamin K1. Lysed horse blood (50% v/v) was prepared by diluting lysed horse blood from Thermo Fisher Scientific (Waltham, MA) with sterile, distilled water. The solution was clarified by centrifugation at 12,000g for 20 min. The supernatant was decanted through a 40-μm, nylon cell strainer from BD (Franklin Lakes, NJ).
B. fragilis was cultured in supplemented Brucella broth, which consisted of Brucella broth powder (28 g), Hemin stock solution (1.0 mL), and vitamin K1 (1.0 mL of a working solution) in 900 mL of water. After this solution was sterilized at 121°C for 15 min in an autoclave and allowed to cool to <50°C, sterile lysed horse blood (100 mL of a 50% v/v solution) was added to give the culture medium.
Treatment with diethylpyrocarbonate (DEPC[19]) was used to eliminate adventitious ribonucleolytic activity in the culture medium. The 900-mL solution contained DEPC (0.1% v/v) and was stirred at 37°C for 1 h prior to its sterilization in an autoclave. In addition, the 100-mL solution of lysed horse blood contained DEPC (0.1% v/v) and was stirred at 37°C for 1 h prior to being added to the 900-mL solution. DEPC-treatment did not affect the MIC of Met (Figure S4).
Determination of the MIC for NpMet Prodrugs
Concentrations of the NpMets and Met were measured by using 1H NMR spectroscopy. A standard solution of CD3OD containing 7.2 mM dimethylformamide (DMF) was prepared, and the methyl imidazole peak of the NpMets and Met were integrated and compared to the DMF peaks (2.99 and 2.86 ppm) to determine the solution concentration of the NpMets and Met (Figure S5). Serial dilutions were made into 1.7-mL microcentrifuge tubes. The solvent was evaporated with a SpeedVac Concentrator from Thermo Fisher Scientific, and the dried compounds were stored at –20°C until use. On the day of the experiment, the NpMet or Met were dissolved in DEPC-treated B. fragilis culture medium and diluted to create solutions with a known concentration of a n NpMet or Met. Compounds were either incubated in medium only, pre-incubated overnight with RNase 1 (0.1 mg/mL) and then diluted in DEPC-treated medium, or diluted in DEPC-treated medium containing RNase 1 (0.01 mg/mL). Upon dilution of the compounds into the wells of a 96-well plate, the plates were held at room temperature in an anaerobic chamber from Coy Lab Products (Grass Lake, MI) for 4 h prior to inoculation. This chamber was maintained under an atmosphere of 80% v/v N2(g), 10% v/v H2(g), and 10% v/v CO2(g). Any O2(g) was reduced to H2O by Pd on alumina pellets, and the water was removed from the atmosphere using silica gel.
B. fragilis (strain 25285 from ATCC®, Manassas, VA) was grown on Reducible Blood Agar plates from Remel (Lenexa, KS) and then suspended in sterile unsupplemented Brucella broth that had been reduced in the anaerobic chamber for several days. A turbidometer was used to adjust the turbidity to match a 0.5 McFarland standard, which equates to ~1.5 × 108 colony-forming units (CFU)/mL. This suspension of B. fragilis was diluted with additional Brucella broth to a concentration of ~1.0 × 107 CFU/mL, and 10 μL/well of this B. fragilis suspension was used to inoculate each well using a 95-pin inoculator. The final volume of solution in each well was 100 μL, with the indicated final concentration of test compound and ~106 CFU/mL of B. fragilis. The plates were incubated for 4 h at 37°C in the anaerobic chamber. The MIC was defined as the lowest concentration of test compound at which no bacterial growth was observable.
Supplementary Material
Acknowledgements
This work was supported by Grant R01 CA073808 (NIH). M.J.P. was supported by Molecular and Cellular Pharmacology Training Grant T32 GM008688 (NIH) and predoctoral fellowship 09PRE2260125 (American Heart Association). A. K. F. D. was supported by a Hilldale Undergraduate/Faculty Research Fellowship. N.A.M. was supported by postdoctoral fellowship F32 GM096712 (NIH). This study made use of the National Magnetic Resonance Facility at Madison, which is supported by NIH Grants P41 RR02301 (BRTP/NCRR) and P41 GM066326 (NIGMS). Additional equipment was purchased with funds from the University of Wisconsin, the NIH (RR02781, RR08438), the NSF (DMB-8415048, OIA-9977486, BIR-9214394), and the U.S. Department of Agriculture.
References
- 1.a Manzano M, Colilla M, Vallet-Regi M. Expert Opin. Drug Deliv. 2009;6:1383–1400. doi: 10.1517/17425240903304024. [DOI] [PubMed] [Google Scholar]; b Maderuelo C, Zarzuelo A, Lanao JM. J. Control. Release. 2011;154:2–19. doi: 10.1016/j.jconrel.2011.04.002. [DOI] [PubMed] [Google Scholar]; c Rong X, Xie Y, Hao X, Chen T, Wang Y, Liu Y. Curr. Drug Discov. Technol. 2011;8:173–187. doi: 10.2174/157016311796799008. [DOI] [PubMed] [Google Scholar]; d Pan J, Chan SY, Lee WG, Kang L. Biotechnol. J. 2011;6:1477–1487. doi: 10.1002/biot.201100237. [DOI] [PubMed] [Google Scholar]; e Moodley K, Pillay V, Choonara YE, du Toit LC, Ndesendo VM, Kumar P, Cooppan S, Bawa P. Int. J. Mol. Sci. 2012;13:18–43. doi: 10.3390/ijms13010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.a Lipinski CA, Pharmacol J. Toxicol. Methods. 2000;44:235–249. doi: 10.1016/s1056-8719(00)00107-6. [DOI] [PubMed] [Google Scholar]; b Rautio J, Kumpulainen H, Heimbach T, Oliyai R, Oh D, Jarvinen T, Savolainen J. Nat. Rev. Drug Discov. 2008;7:255–270. doi: 10.1038/nrd2468. [DOI] [PubMed] [Google Scholar]; c Jana S, Mandlekar S, Marathe P. Curr. Med. Chem. 2010;17:3874–3908. doi: 10.2174/092986710793205426. [DOI] [PubMed] [Google Scholar]
- 3.Stegemann S, Leveiller F, Franchi D, de Jong H, Linden H. Eur. J. Pharm. Sci. 2007;31:249–261. doi: 10.1016/j.ejps.2007.05.110. [DOI] [PubMed] [Google Scholar]
- 4.a Sousa FJ. CLAO J. 1991;17:282–284. [PubMed] [Google Scholar]; b Bergenheim AT, Henriksson R. Clin. Pharmacokinet. 1998;34:163–172. doi: 10.2165/00003088-199834020-00004. [DOI] [PubMed] [Google Scholar]; c Wire MB, Shelton MJ, Studenberg S. Clin. Pharmacokinet. 2006;45:137–168. doi: 10.2165/00003088-200645020-00002. [DOI] [PubMed] [Google Scholar]
- 5.Ellis GA, McGrath NA, Palte MJ, Raines RT. ACS Med. Chem. Lett. 2012;3:268–272. doi: 10.1021/ml2002554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sorrentino S. FEBS Lett. 2010;584:2194–2200. doi: 10.1016/j.febslet.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 7.Kurihara M, Ogawa M, Ohta T, Kurokawa E, Kitahara T, Kosaki G, Watanabe T, Wada H. Cancer Res. 1982;42:4836–4841. [PubMed] [Google Scholar]
- 8.a Raines RT. Chem. Rev. 1998;98:1045–1065. doi: 10.1021/cr960427h. [DOI] [PubMed] [Google Scholar]; b Cuchillo CM, Nogués MV, Raines RT. Biochemistry. 2011;50:7835–7841. doi: 10.1021/bi201075b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.a Markham R, Smith JD. Biochem. J. 1952;52:552–557. doi: 10.1042/bj0520552. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Cuchillo CM, Parés X, Guasch A, Barman T, Travers F, Nogués MV. FEBS Lett. 1993;333:207–210. doi: 10.1016/0014-5793(93)80654-d. [DOI] [PubMed] [Google Scholar]; c Thompson JE, Venegas FD, Raines RT. Biochemistry. 1994;33:7408–7414. doi: 10.1021/bi00189a047. [DOI] [PubMed] [Google Scholar]
- 10.a Davis AM, Regan AC, Williams A. Biochemistry. 1988;27:9042–9047. doi: 10.1021/bi00425a024. [DOI] [PubMed] [Google Scholar]; b Witmer MR, Falcomer CM, Weiner MP, Kay MS, Begley TP, Ganem B, Scheraga HA. Nucleic Acids Res. 1991;19:1–4. doi: 10.1093/nar/19.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Thompson JE, Raines RT. J. Am. Chem. Soc. 1994;116:5467–5468. doi: 10.1021/ja00091a060. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Stowell JK, Widlanski TS, Kutateladze TG, Raines RT. J. Org. Chem. 1995;60:6930–6936. [Google Scholar]; e Taylorson CJ, Eggelte HJ, Tarragona-Fiol A, Rabin BR, Boyle FT, Hennam JF, Blakey DC, Marsham PR, Heaton DW, Davies DH, Slater AM, Hennequin LFA. US Patent 5985281.
- 11.Lofmark S, Edlund C, Nord CE. Clin. Infect. Dis. 2010;50(Suppl 1):S16–23. doi: 10.1086/647939. [DOI] [PubMed] [Google Scholar]
- 12.Kasten MJ. Mayo Clin. Proc. 1999;74:825–833. doi: 10.4065/74.8.825. [DOI] [PubMed] [Google Scholar]
- 13.Chung MC, Bosquesi PL, dos Santos JL. Curr. Pharm. Des. 2011;17:3515–3526. doi: 10.2174/138161211798194512. [DOI] [PubMed] [Google Scholar]
- 14.Hayakawa Y, Kawai R, Hirata A, Sugimoto JI, Kataoka M, Sakakura A, Hirose M, Noyori R. J. Am. Chem. Soc. 2001;123:8165–8176. doi: 10.1021/ja010078v. [DOI] [PubMed] [Google Scholar]
- 15.ChemAxon. Budapest, Hungary: 2011. Calculator Plugins were used for structure property prediction and calculation, Marvin 5.4.1.1. [Google Scholar]
- 16.Ukita T, Waku K, Irie M, Hoshino O. J. Biochem. 1961;50:405–415. doi: 10.1093/oxfordjournals.jbchem.a127467. [DOI] [PubMed] [Google Scholar]
- 17.a Johnson RJ, McCoy JG, Bingman CA, Phillips GN, Jr., Raines RT. J. Mol. Biol. 2007;368:434–449. doi: 10.1016/j.jmb.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Lomax JE, Eller CH, Raines RT. Methods Enzymol. 2012;502:273–290. doi: 10.1016/B978-0-12-416039-2.00014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria; Approved Standard—Seventh Edition. CLSI document M11-A7. Clinical and Laboratory Standards Institute; Wayne, PA: 2007. [Google Scholar]
- 19.a Wiener SL, Wiener R, Urivetzky M, Meilman E. Biochim. Biophys. Acta. 1972;259:378–385. doi: 10.1016/0005-2787(72)90312-7. [DOI] [PubMed] [Google Scholar]; b Blumberg DD. Methods Enzymol. 1987;152:20–24. doi: 10.1016/0076-6879(87)52005-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.