Abstract
Scope
Understanding the molecular mechanisms through which natural products and dietary supplements exhibit anticancer properties is crucial and can lead to drug discovery and chemoprevention. The current study sheds new light on the mode of action of Resveratarol (RES), a plant-derived polyphenolic compound, against EL-4 lymphoma growth.
Methods and results
Immuno-compromised NOD/SCID mice injected with EL-4 tumor cells and treated with RES (100 mg/kg body weight) showed delayed development and progression of tumor growth and increased mean survival time. RES caused apoptosis in EL4 cells through activation of aryl hydrocarbon receptor (AhR) and upregulation of Fas and FasL expression in vitro. Blocking of RES-induced apoptosis in EL4 cells by FasL mAb, cleavage of caspases and PARP, and release of cytochorme c, demonstrated the participation of both extrinsic and intrinsic pathways of apoptosis. RES also induced upregulation of SIRT1 and downregulation of NF-kB in EL4 cells. SiRNA-mediated down regulation of SIRT1 in EL4 cells increased the activation of NF-kB but decreased RES-mediated apoptosis, indicating the critical role of SIRT1 in apoptosis via blocking activation of NF-kB.
Conclusion
These data suggest that RES-induced SIRT1 upregulation promotes tumor cell apoptosis through negative regulation of NF-kB, leading to suppression of tumor growth.
Keywords: Cancer, Lymphoma, Resveratrol, AhR, Fas, FasL, Apoptosis, Extrinsic pathway, Intrinsic pathway
1. Introduction
RES (trans-3,5,4’-trihydorxystilbene) is a naturally occurring polyphenolic phytoalexin compound found in a large number of plant products including red grapes, mulberries, peanuts, and Japanese knotweed [1]. RES possesses structural similarities to estradiol and diethylstilbestrol [2], and is a member of plant antibiotic compounds produced as a part of a plant's defense system against fungal infection [3]. Ko-jo-kon, an oriental medicine used to treat diseases of the blood vessels, heart [3], and liver [3] contains RES as an essential component. Although, RES received attention initially as a compound in red wine responsible for the "French Paradox" [4], in recent years, it has gained more attention for its anticancer [5,6], antioxidant [7], anti-inflammatory [8,9], and anti-aging properties [10,11].
RES has been the focus of many studies for its anticancer and chemopreventive properties against various types of cancer [6,12–14]. Armour et al proposed that some of the beneficial effects of RES may be due to its suppressing property of autopagy [15]. RES has been shown to suppress proliferation of various types of cancer cells, including breast, lung, colon, pancreas, liver, stomach, prostrate, leukemia, and medulloblastoma [5,16]. This may result from induction of apoptosis by RES in cancer cells [5,6], although the precise mechanisms and pathways involved remain unclear.
RES is well known for its ability to induce the expression of SIRT, a NAD+-dependent histone deacetylase that plays a role in chromatin silencing, longevity and genomic stability [17]. The question as to whether SIRT1 acts as a tumor promoter or tumor suppressor is much debated [18]. SIRT1 is overexpressed in certain types of cancer [19], which led to the hypothesis that SIRT1 may serve as a tumor promoter. In contrast, SIRT1 expression was also shown to be reduced in many other types of cancers [20]. Thus, it is likely that increased expression of SIRT1 may be a consequence, rather than a cause, of tumorigenesis [18]. The fact that SIRT1 serves as a tumor suppressor was suggested by the findings that overexpression of SIRT1 in APCmin/+ mice reduces development of colon cancer [21]. Thus, clearly, additional studies are necessary to understand the mode of action of RES and its impact on SIRT1 expression and consequent effect on tumor growth. In the current study, we investigated the effect of RES on a T cell lymphoma line, EL4. We demonstrate that the anticancer property of RES against EL4, can be attributed, at least in part, to its ability to induce apoptosis in EL4 cells through reciprocal regulation in the expression of SIRT1 (upregulation) and NF-kB (downregulation).
2. Materials and Methods
2.1 Mice and Cell line
We purchased NOD/SCID/γcnull mice from Jackson Laboratory (Bar Harbor, Maine). The animals were housed in University of South Carolina Animal facility and care and maintenance of the animals were in accordance with guide for the care and use of laboratory animals and according to the declaration of Helsinki and as adopted by Institutional and NIH guidelines.
Mouse T lymphoma cell line (EL4 cells) was maintained in complete RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum, 10 mM L-Glutamine, 10 mM HEPES, and 100 µg/ml penicillin/streptomycin at 37°C and 5% CO2.
2.2 Reagents and Antibodies
Culture medium and its reagents (RPMI 1640, L-Glutamine, HEPES, Gentamicin, DMEM, PBS, and FBS) were purchased from Invitrogen Life Technologies (Carlsbad, CA) and ConA from Sigma-Aldrich (St. Louis, MO). The following mAbs: anti-mouse IgG-PE, FcBlock, CD3-PE (chain) purified anti-FasL (k-10), anti-FasL-PE (Kay-10), and anti-Fas-PE (Jo2) were purchased from BD Pharmingen (Carlsbad, CA). The following primary Abs: caspase-2, caspase-3, caspase-8, caspase-9 (Cell Signaling, Danvers, MA), cytochrome-c (Cell Signaling, Danvers, MA), PARP (Cell Signaling, Danvers, MA), Bax (Cell Signaling, Danvers, MA), Bid (R & D System, Minneapolis, MN), and β-actin (Sigma-Aldrich, St. Louis, MO)) for Western blots were used. All antibodies used for Western blots were diluted 1:1000 fold except β-actin, which was used at 1:5000 dilution. HRP-conjugated secondary Ab (Cell Signaling, Danvers, MA) was used at 1:1000 dilution. Inhibitors against Caspase 3 (Z-DEVD), Caspase 8 (Z-IETD-FMK), Caspase 9 (Z-LEHD-FMK) were purchased from R&D Systems (Minneapolis, MN). RNeasy Mini kit and iScript cDNA synthesis kit were purchased from Qiagen (Valencia, CA). Epicentre’s PCR premix F and Platinum Taq Polymerase kits were purchased from Invitrogen Life Technologies (Carlsbad, CA). TUNEL kits were purchased from Roche (Indianapolis, IN). RES was purchased from Sigma-Aldrich (St. Louis, MO). RES suspended in DMSO was used in the in vitro studies and suspended in water was used for in vivo studies, as described [22].
2.3 EAE-induced tumorigenesis in NOD/SCID mice and RES treatment
NOD/SCID mice on the background of BALB/c were subcutaneously injected with freshly cultured EL4 cells (1×106/mice) suspended in 100 µl 1X PBS. Five days post injection, vehicle or various doses of RES suspended in water (10, 50, and 100 mg/kg body weight) were administered orally every day. The mice were scored for tumor growth every alternate day and tumor size was documented by direct measurement in two perpendicular directions using a Max-Cal caliper (Cole Parmer Instrument Co.). The experiments were terminated when the tumors reached 18–20 mm in diameter, or severe ulceration and bleeding had developed. The measurements were recorded as tumor area (mm2) from groups of 6 mice each. The experiments were repeated with various groups three times.
2.4 RES induced apoptosis in EL4 cells
To determine RES-induced apoptosis in EL4 cells in vitro, freshly cultured EL4 cells were treated with vehicle (DMSO) or different concentrations (1–100 µM) of RES for 6, 12, and 24 hrs. Apoptosis in EL4 cells post-RES treatment was determined by performing TUNEL assays (FITC-dUTP nick-end labeling) using In situ Cell-Death Detection kit (Roche, Indianapolis, IN) as described previously [23].
2.5 Reverse Transcriptase PCR (RT-PCR) to determine the expression of AhR, Fas, and FasL in EL4 cells
First strand cDNA synthesis was performed using total RNA (2 µg) isolated from EL4 cells treated with vehicle (DMSO) or RES using iScript Kit and following the protocol of the manufacturer (Bio-Rad). To detect the expression AhR, Fas, and FasL, sets of primers specific to mouse AhR, Fas, and FasL as described earlier [22] were used and PCR was performed as described earlier [22]. The PCR products, generated from mouse AhR, Fas, and FasL primer pairs, were normalized against PCR products generated from mouse 18S forward (5'-GCCCGAGCCGCCTGGATAC-3') and reverse (5'-CCGGCGGGTCATGGGAATAAC-3') primers after electrophoresis on 1.5% agarose gel and visualization with UV light. The band intensity of PCR products was determined using BioRad image analysis system (BioRad, Hercules, CA).
2.6 Role of FasL in RES-induced EL4 cell apoptosis
EL4 cells were cultured in the absence or presence of antibody against mouse FasL (5 µg/ml) 1 hr prior to RES treatment. Apoptosis in EL4 cells was determined by performing TUNEL assays as described earlier [22]. At least three independent experiments were performed and the data shown represent one representative experiment. Data from 3 to 4 independent experiments were also depicted as mean fluorescence unit ± SEM.
2.7 Analysis of caspase 3/7, caspase 8, and caspase 9 activity in EL4 cells post RES treatments
To determine the role of caspases 3/7, 8, and 9 in RES-induced apoptosis in EL4 cells, activity of these caspases were determined using the Apo-ONE Homogeneous Caspase-3/7, caspase-8, and caspase-9 Assays according to manufacturer’s instructions (Promega Corporation, Madison, WI) and as described earlier [22].
2.8 Role of various caspases in RES-induced apoptosis in EL4 cells
To investigate the role and participation of caspases in RES-induced apoptosis in EL4 cells, we performed in vitro assays as described earlier [22] and inhibitors specific to mouse caspase-3 (Z-DEVD), caspase-8 (Z-IETD-FMK), and caspase-9 (Z-LEHD-FMK) at a concentration of 20 µM. EL4 cells were incubated with various caspase inhibitors for at least 1 hr prior to RES treatment. The cells were harvested 24 hrs post-vehicle or RES treatment and TUNEL assays were performed to determine apoptosis as described earlier. At least three independent experiments were performed and the data shown represent mean of three independent experiments.
2.9 Immunoblot analysis
Immunoblotting was performed as described previously [22]. The following antibodies: caspase-3, caspase-8, caspase-9, PARP, cytochrome-c, Bax, caspase-2, and β-actin were used at dilution of 1:2000 except β-actin, which was used at dilution of 1:5000. HRP-conjugated secondary Ab was used 1:4000 dilution (Cell Signaling). The proteins fractionated in SDS-PAGE were transferred onto PVDF membranes using a dryblot apparatus (BioRad, Hercules, CA) and was first, incubated in blocking buffer for 1 hr at RT, followed by incubation in primary antibody at 4°C overnight. After washing several times with washing buffer, the membrane was then incubated for 1 hr in HRP-conjugated secondary antibody (Cell Signaling Technology, Danvers, MA). The membranes after washing several times were incubated in developing solution (ECL Western Blotting Detection Reagents, GE Life Sciences, England) and signal was detected using ChemiDoc System (BioRad). Densitometric analyses of the Western blots were performed using ChemiDoc software (BioRad).
2.10 Mitochondrial membrane potential (Δψm) analysis
To examine the role of intrinsic pathway (mitochondrial pathway) in RES-mediated apoptosis in EL4 cell, we determined mitochondrial membrane potential (Δψm) of EL4 cells post-vehicle (DMSO) or RES treatment using 3,3’-dihexyloxacarboeczyme (DiOC6) dye as described earlier [22]. Propidium iodide (PI) was used to differentiate the dead cells. At least three independent experiments were performed.
2.11 Expression SIRT1 in EL4 cells and effect of RES
To determine expression of SIRT1 in EL4 cells, we performed RT-PCR using mouse SIRT1-specific forward and reverse primers as described earlier [24]. In brief, total RNA from EL4 cells untreated or treated with vehicle or RES were prepared and cDNAs were synthesized as described earlier [22]. PCR for SIRT1 expression in EL4 cells was performed as described earlier [24]. The PCR products, generated from mouse SIRT1 primer pairs, were normalized against PCR products generated from mouse 18S (215 bp) as described earlier [22] after electrophoresis on 1.5% agarose gel and visualization with UV light. The band intensity of PCR products was determined using Bio Rad image analysis system (Bio Rad Hercules, CA). The fold increase mRNA in each sample was evaluated by using the ChemiDoc software (Bio-Rad, Hercules, CA).
2.12 Western blot analysis for expression of SIRT1 and p-IκBα-Ser32 in EL4 cells
We performed immunoblotting to determine the expression of SIRT1 and phosphorylated IκBα following the protocol as described earlier [24]. The proteins from various treated groups of EL4 cells were fractionated in 12% SDS-PAGE and transferred onto PVDF membranes using a DryBlot apparatus (BioRad, Hercules, CA). The membrane after washing 3 times (10–15 min) with washing buffer (PBS + 0.2% Tween 20) were incubated in developing solution (ECL Western Blotting Detection Reagents, Amersham Biosciences England) and signal was detected using Chemi Doc System (Bio-Rad, Hercules CA). The protein on the membrane was quantified using Densitometry analyses of the western blots and Chemi Doc Software (Bio-Rad, Hercules CA).
2.13 Transfection of EL4 cells with mouse SIRT1 siRNA
Freshly cultured EL4 cells (5 × 106) were transfected with 1.7 µmol/L mouse SIRT1-specific siRNAs (Dharmacon RNA Technologies, Lafayette, CO) using nucleofection of EL4 cells with EL4 Nucleofector Transfection Reagent kit and Nucleofactor II electroporation system and following the protocols of the company (Amaxa, Inc., Gaithersburg, MD). As a control, 5 × 106 EL4 cells were transfected with mouse-specific control SiGLO RISK-free siRNA (3 µg; designated control siRNA) unconjugated and pmaxGFP plasmid (2 µg). Transfected T cells were cultured for 48 hours in complete medium at 37°C and 5% CO2. We observed >85% cell viability after transfection and observed >65% cells expressing green fluorescent protein when examined by flow cytometry (Cytomics FC 500, Beckman Coulter). EL4 cells, untransfected or transfected with control siRNA or mouse-specific SIRT1 siRNAs, were treated with vehicle or RES (25 µM/ml). EL4 cells, 24 hours after treatment, were harvested and apoptosis was determined by performing TUNEL assay and using flow cytometry (Cytomics FC 500, Beckman Coulter).
2.14 Electrophoretic Mobility Shift Assay (EMSA)
To determine the expression of NF-kB post RES treatment, EMSA assays using mouse-specific NF-kB probes and nuclear proteins harvested from EL4 cells post vehicle or RES treatment were performed following the protocol as described previously [25].
Preparation of nuclear proteins/extracts from EL4 cells
Nuclear proteins/extracts from EL4 cells treated with vehicle or RES or SIRT1siRNA-transfected and not treated or treated with RES for EMSA were prepared as described previously [25]. The supernatant containing nuclear proteins/extracts was collected and the protein concentration was measured using the BCA protein determination kit from Pierce, snap frozen in liquid nitrogen, and stored at −80 °C.
Generation of NF-κB oligo probes, radio-labeling and EMSA assays
We used double stranded mouse NF-kB-specific wild-type and mutant oligo probes in this study. The double stranded wild- types and mutant NF-κB oligonucleotide probes were 5-end-labeled as described previously [25]. Radiolabeled (50,000 cpm) NF-kB probes were then incubated together with nuclear protein (3–5 µg) in a reaction mix containing 1 µl binding buffer (10 mM Tris, 1 mM EDTA, 1 mM DTT, 100 mM KCl, 10% (v/v) glycerol) and 1 µg poly (dI-dC) (Amersham Biosciences) as a nonspecific inhibitor in a final volume of 25 µl for 30 min at 25 °C. The samples were resolved on a 6% polyacrylamide gel in Tris borate EDTA that had been pre run for 30 min. The gels were dried and exposed to X-ray film.
2.15 Statistical analysis
Results presented here represent at least three independent experiments and are presented as the mean ± SEM. Statistical analyses were performed using Student's t-test or two-factor ANOVA as appropriate, with a P-value of ≤0.05 considered to be statistically significant. For tumor size, significant difference between control and experimental groups was determined using the Mann-Whitney U test (*p<0.01).
3 Results
3.1 RES suppresses EL4 tumor growth in NOD/SCID mice
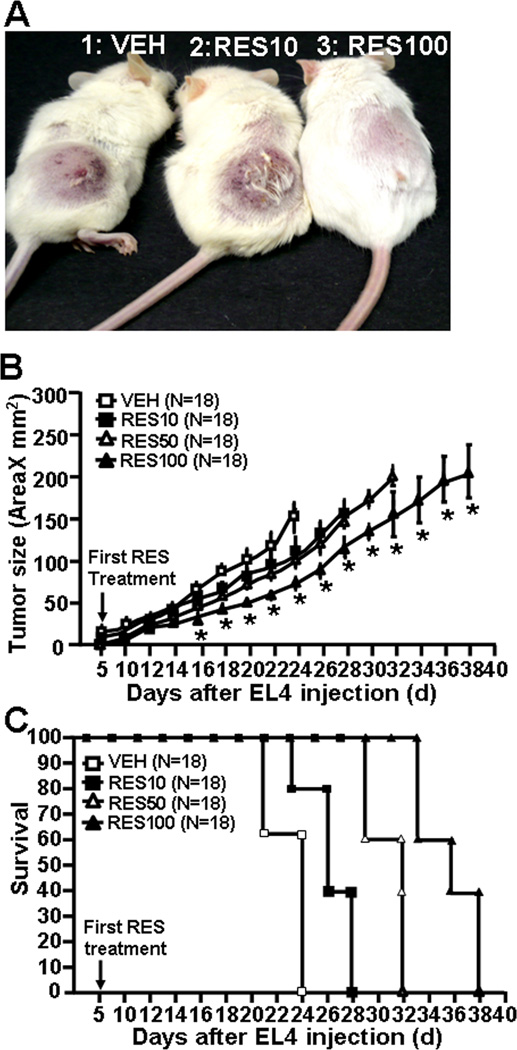
To assess the efficacy of RES against EL4-mediated tumorigenesis in vivo, independent of the immune components, an immuno-compromised murine (NOD/SCID) model was used. NOD/SCID mice were challenged s.c. into the right flank with a lethal dose of EL4 cells (1 × 106) and generation and development of tumor growth was monitored for 40 days. All the NOD/SCID mice that received vehicle treatment post EL4 challenge developed tumor (Fig. 1A and B) whereas the mice that received various doses of RES treatments, responded in accordance with the dose of RES (Fig. 1A and B). The mice that received lower dose (10 mg/kg bw) of RES showed minimal effect on tumor development and survival of the mice (Fig. 1A and B). Mice that received 50 mg/kg bw RES showed moderate effects of RES on tumor burden (Fig. 1A and B). However, mice that received highest dose (100 mg/kg bw) of RES treatment, showed significant effects as there was a delay in tumor growth and significantly lower tumor burden (Fig. 1A and B). Also, RES showed a dose-dependent increase in survival time of mice when compared to those treated with vehicle, which died by day 24 (Fig 1C). These data together suggested that RES inhibits tumor growth as well as prolongs the survival of EL4 bearing mice.
Figure 1.
RES treatment suppresses EL4 tumor growth in NOD/SCID mice. A, EL4 cells (1×106) were subcutaneously injected on to dorsal surface of NOD/SCID mice. These mice received daily dose of 10, 50 and 100 mg/kg of RES orally from day 5 till the termination of the experiment. B, Measurement of tumor size in NOD/SCID mice. C, survival time of NOD/SCID mice post EL4 injection. Data represent mean ± SEM of 18 animals and asterisks (*) represent significant differences (p < 0.05) between RES-treated groups when compared to vehicle controls.
3.2 RES induces apoptosis in EL4 cells
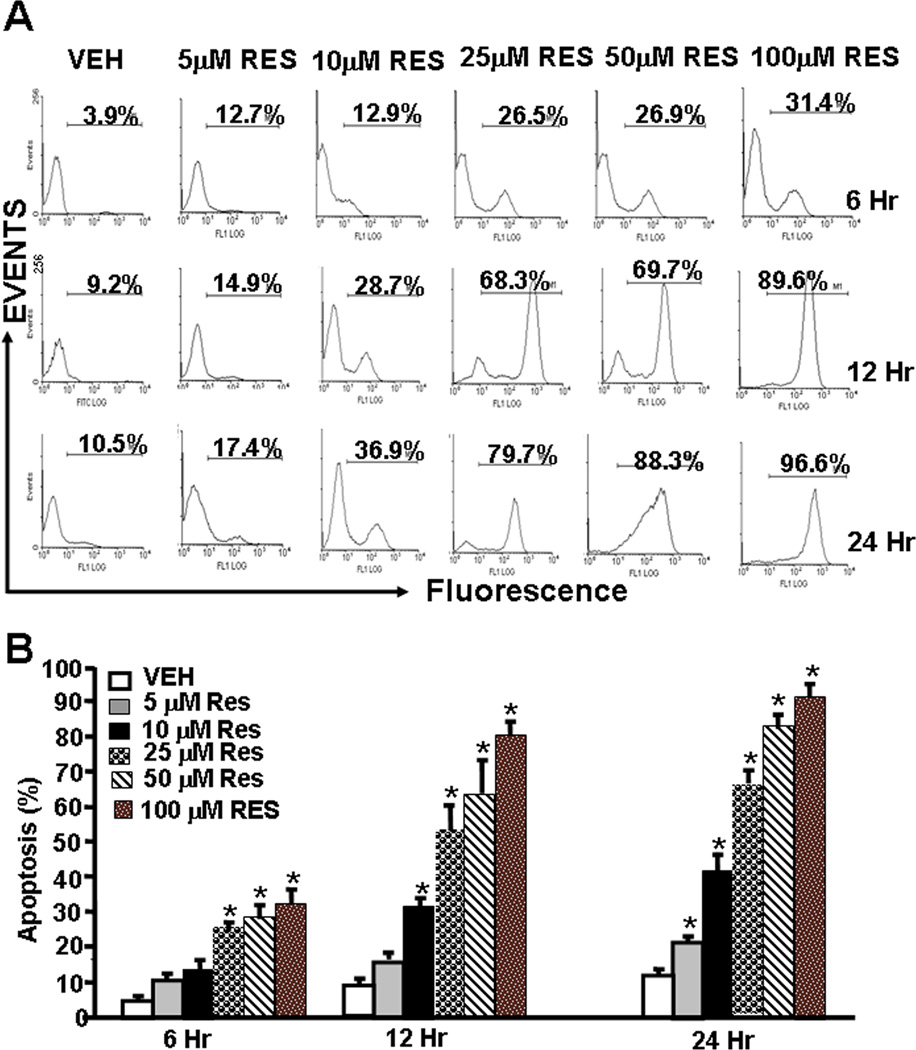
To understand the mechanisms through which RES inhibited EL4 tumor growth, we performed a series of in vitro assays. To this end, EL4 cells were treated with various doses of RES (5–100 µM). The data obtained from TUNEL assays demonstrated that EL4 cells cultured in the presence of RES underwent significant levels of apoptosis in a dose-dependent fashion (Fig. 2A and B). Apoptosis was demonstrable as early as 6 hr and peaked at 24 hr. At 24 hrs, particularly at higher concentrations, RES induced 80–90% apoptosis in EL4 cells.
Figure 2.
RES triggers apoptosis in EL4 cells. EL4 cells were treated with RES or vehicle for 6 –24 hrs (Panels A–B). Panel A demonstrates representative experiment for apoptosis and panel B shows mean ± SEM of 4 independent experiments. Asterisks (*) indicate statistically significant (p < 0.05) difference between RES-treated groups when compared to vehicle controls.
3.3 RES upregulates AhR, Fas, and FasL expression in EL4 cells
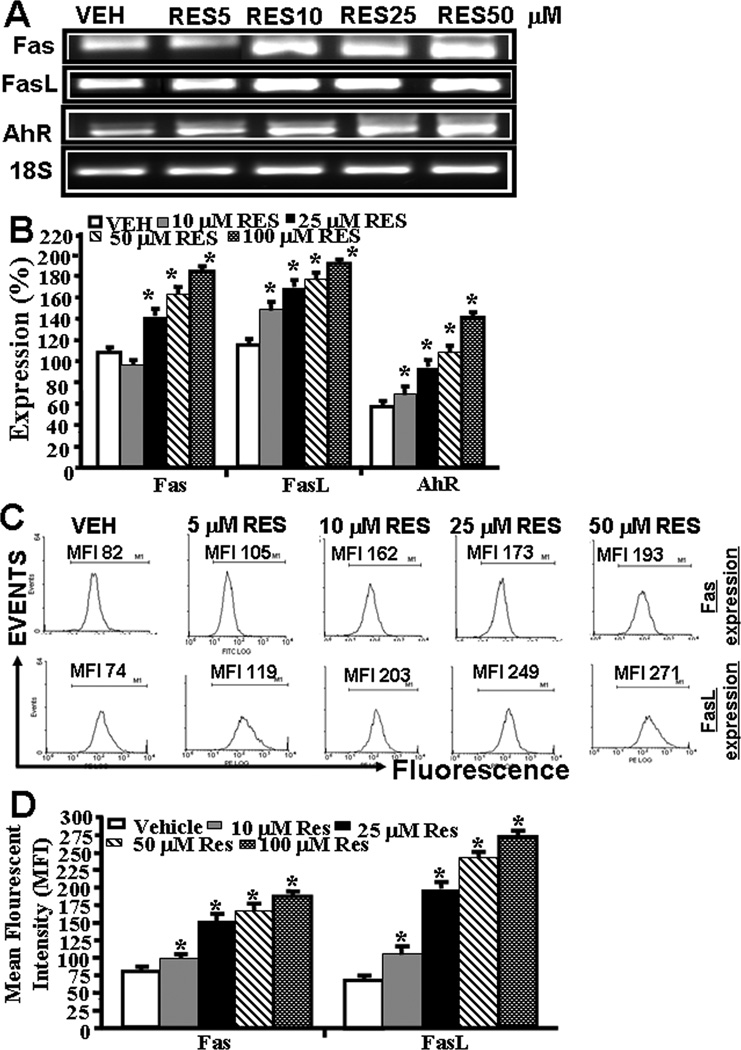
Next, we determined the expression of AhR, Fas, and FasL in EL4 cells in vitro post vehicle or RES treatment. To this end, we first performed RT-PCR to determine the expression of AhR, Fas and FasL in EL4 cells. We also stained EL4 cells with mouse-specific anti-Fas and FasL antibodies respectively to determine the expression Fas and FasL in EL4 cells. Data obtained from RT-PCRs showed constitutive expression of AhR, Fas, and FasL in EL4 cells. However, post RES-treatment, there was a significant increase in expression of all the three genes in EL4 cells (Fig. 3A and B) when compared to vehicle-treated EL4 cells (Fig. 3A and B). Importantly, the increase in their expression in the presence of RES was dose-dependent (Fig. 3A and B). We observed similar 18S (a house keeping gene) expression between RES- and vehicle-treated EL4 cells (Fig. 3A). Upon examination of Fas and FasL expression in EL4 cells post RES/vehicle treatments, by flow cytometry, data corroborated with RT-PCR data for increased expression of Fas and FasL (Fig 3C and D).
Figure 3.
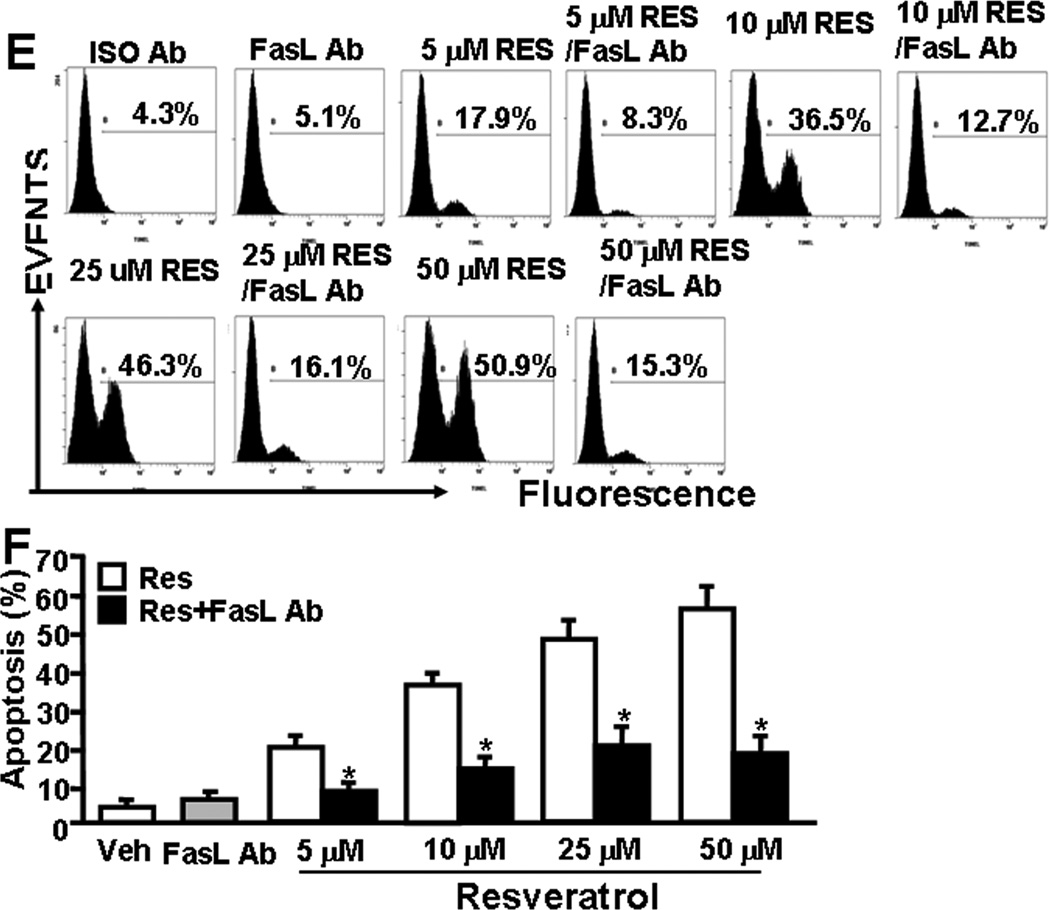
RES-induced upregulation of AhR, Fas, and FasL in EL4 cells and role of FasL in induction of death-receptor pathway of apoptosis in EL4. Expression of AhR, Fas, and FasL in EL4 cells was determined by performing RT-PCR and staining the cells with anti-mouse Fas-PE and anti-mouse FasL-PE antibodies and analyzed by Flow cytometry. A, Expression of AhR, Fas, and FasL in EL4 cells 24 hrs post RES or vehicle treatment (RT-PCR). 18S, a house keeping, was used as a positive control. B, represents mean ± SEM of three independent experiments and asterisks (*) represent significant (p < 0.05) difference between RES-treated groups when compared to vehicle controls. C, Mean fluorescent intensity of Fas and FasL expression post RES or vehicle treatment. Panel D represents mean ± SEM of three independent experiments and asterisks (*) represent significant (p < 0.05) difference between RES-treated groups when compared to vehicle controls. Panels E and F: Apoptosis in EL4 cells in the absence or presence of mouse-specific anti-FasL Ab was determined by TUNEL assays. The data presented in panel E are representative of 3 independent experiments. Panel F represents mean of 3 independent experiments and asterisks (*) represent significant (p < 0.05) reduction in RES-induced apoptosis of T cells cultured in the presence of FasL Ab when compared to the controls.
3.4 FasL plays a role in initiating death-receptor pathway during RES-induced apoptosis in EL4 cells
To test the role of FasL in RES-induced apoptosis, EL4 cells were cultured in the absence or presence of anti-mouse FasL mAb (5 µg/ml) and various doses of RES (5–50 µM). There was significant reduction in RES-induced apoptosis in EL4 cells when Ab against FasL was added to the culture (Fig. 3E and F). Addition of isotype control Ab failed to exhibit any significant effect on RES-induced apoptosis (Fig. 3E and F). These data suggested a role for FasL in initiating RES-mediated death-receptor pathway leading to apoptosis in EL4 cells.
3.5 RES triggers both extrinsic (death-receptor) and intrinsic (mitochondrial) pathways of apoptosis in EL4 cells
We next investigated the role of various apoptotic pathways in RES-induced apoptosis in EL4 cells. To this end, we determined the role of various caspases (Caspase-3/7, caspase-8, and caspase–9) in RES-induced apoptosis in EL4 cells. To this end, we performed standard enzymatic assays in EL4 cells post vehicle or RES treatment measuring caspases as described earlier [22]. Increased enzymatic activities were observed for all three caspases examined in RES-treated EL4 cells when compared to vehicle-treated EL4 cells (data not shown). These data were similar to our previous studies in which activated T cells were shown to undergo apoptosis following resveratrol treatment that was mediated by caspases [22]. The role of various caspases was further confirmed by blocking caspase activity using various caspase inhibitors (caspase-3; Z-DEVD, caspase-8; Z-IETD-FMK, and caspase-9; Z-LEHD-FMK). These data demonstrated significant blocking of RES-induced apoptosis in EL4 cells the presence of caspase-3 inhibitor and partial blocking in the presence of caspase-8 or caspase-9 inhibitors (data not shown), thereby suggesting that RES-induced apoptosis in EL4 cells was caspase-dependent involving both intrinsic and extrinsic pathways. To further corroborate the role of caspases in RES-induced apoptosis in EL4 cells, Western blot analysis for various caspases, PARP, BAX, Bid, and CYT-c was performed. The data demonstrated that RES treatment caused cleavage of caspases-8, -3, -9, -2, and PARP in EL4 cells and these data were similar to that reported by us earlier [22]. Increased expression of BAX, Bid, and cytochrome-c was also observed in RES-treated EL4 cells when compared to vehicle controls (data not shown). The increase in Bid expression in RES-treated EL4 cells demonstrated its role in initiating cross-talk between death-receptor and mitochondrial pathways. Moreover, release of cytochrome c from nucleus to cytoplasm in RES-treated EL4 cells, corroborated the role of mitochondrial pathway in RES-induced apoptosis in EL4 cells.
To further corroborate the role of mitochondrial pathway in RES-induced apoptosis in EL4 cells, we examined loss of mitochondrial membrane potential (Δψm) using DIOC6 dye post-RES or vehicle treatment. Reduction in Δψm in RES-treated EL4 cells compared to vehicle-treated EL4 cells was noted (data not shown), similar to our previous data on the effect of RES on T cells[22]. However, even at a high dose of 50 µM RES, we noted moderate levels of reduction in Δψm (data not presented) thereby suggesting that mitochondrial pathway plays only a partial role in RES-mediated apoptosis in EL4 cells.
3.6 RES reciprocally regulates SIRT1 and NF-kB expression in EL4 cells
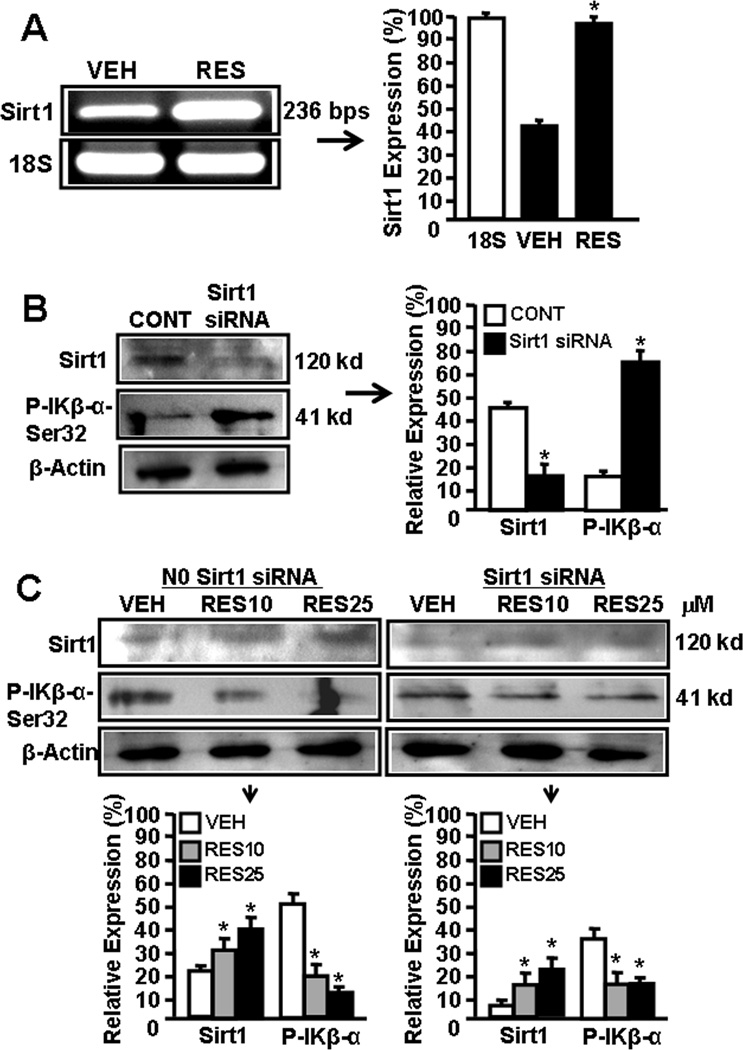
There are reports demonstrating RES-mediated reciprocally regulates SIRT1 and NF-kB expression [26–29]. To determine the effect of RES on SIRT1 and NF-kB activation in EL4 cells, we performed a series of in vitro assays and determined the expression of SIRT1, phosphorylated IkK, and NF-kB in EL4 cells. As shown in Fig 4A, EL4 cells express moderate-level of SIRT1 but upon RES treatment, SIRT1 expression was significantly upregulated (Fig. 4A).
Figure 4.
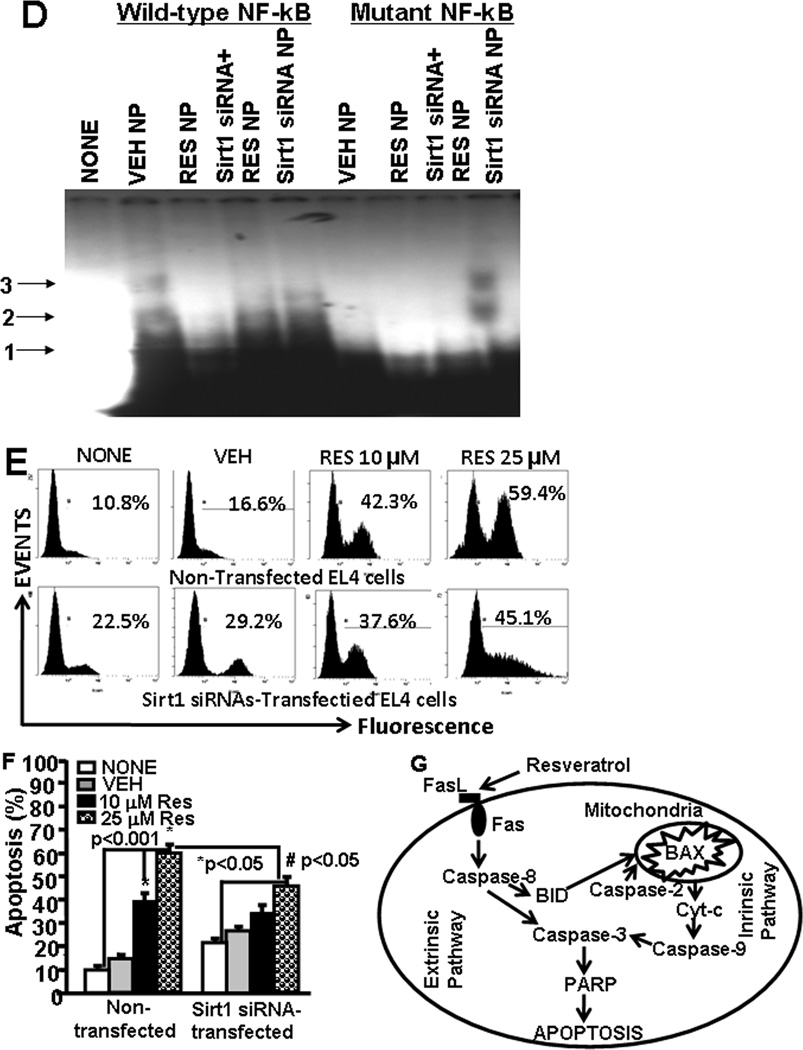
SIRT1 expression in EL4 cells regulates NF-kB activation and apoptosis. Expression of SIRT1 in EL4 cells was determined by performing RT-PCR. A, Expression of SIRT1 in EL4 cells 6 hrs post vehicle or RES treatment (RT-PCR). 18S, a house keeping gene, was used as a positive control. In panel B, the bars represents mean ± SEM of three independent experiments and asterisks (*) represent significant (p < 0.05) difference between RES-treated EL4 cells when compared to vehicle-treated EL4 cells. B, cytosolic proteins from EL4 cell untransfected or transfected with mouse SIRT1-specific siRNAs were fractionated in polyacrylamide gel and expression of SIRT1 and p-IKβα-Ser32 was analyzed by Western blotting using antibody against mouse-specific SIRT1 and p-IKβα-Ser32. Expressions of SIRT1 and and p-IKβα-Ser32 is presented as percentage of β-Actin expression on Y-axis and expression of β -Actin was considered to be 100% for each experiment. Vertical bars represent mean +/− SEM of three independent experiments and asterisks (*) represent statistically significant (p < 0.002) reduction in expression of SIRT1 (untransfected control; CONT vs SIRT1 siRNA transfected EL4 cells) and an increase in expression of p-IKβ-α-Ser32 (untransfected control; CONT vs SIRT1 siRNA transfected EL4 cells) in EL4 cells. C, cytosolic proteins from EL4 cell untransfected or transfected with mouse SIRT1-specific siRNAs and treated with vehicle or RES were fractionated in polyacrylamide gel and expression of SIRT1 and p- IKβα-Ser32 in EL4 cells was analyzed by Western blotting using antibody against mouse-specific SIRT1 and p-IKβα-Ser32. Left panel in C represents expressions of SIRT1 and p-IKβα-Ser32 in untransfected EL4 cells and treated with vehicle or RES whereas right panel represents expressions of SIRT1 and p-IKβα-Ser32 in EL4 cells transfected with SIRT1 siRNA and treated with vehicle or RES. The vertical bars represent percentage of β-actin expression which was considered to be 100%. Also, vertical bars represent mean +/− SEM of three independent experiments and asterisks (*) represent statistically significant (p < 0.002) increase in expression of SIRT1 (VEH vs RES10 or RES25 µM) and decrease in p-IKβα-Ser32 (VEH vs RES10 or RES25 µM). D, EMSA analysis of NF-kB motif. Double stranded probes containing wild-type NF-kB or mutant NF-kB motifs were generated and nuclear extracts (4–5 µg) generated from vehicle- or RES-treated untransfected or SIRT1 siRNA-transfected EL4 cells not treated or treated with RES was used in each reaction. Radiolabeled (P32) wild-type or mutant NF-kB1 probes were used either without incubation with nuclear extracts or after incubation with nuclear extracts. Arrows 1 represents free probes and arrows 2 and 3 represent NF-kB+ nuclear protein (NP) complexes. E, EL4 cells non-transfected or transfected with SIRT1 siRNA were cultured in the presence of vehicle or RES (10 or 25 µM) for 24 hrs and apoptosis was determined using TUNEL assay. F, Vertical bars in the left panel of E represent mean +/− SEM of three independent experiments and asterisks (*) represent statistically significant (p< 0.001) increase in apoptosis in non-transfected EL4 cells or SIRT1-siRNA transfected EL4 cells post RES treatment compared to vehicle-treated cells and (#) represents statistically significant (p< 0.05) decrease in apoptosis in SIRT1-siRNA transfected EL4 cells post RES treatment compared to untransfected EL4 cells. G, Schematic diagram demonstrating pathways involved in RES-mediated apoptosis in EL4 cells.
Furthermore, we used mouse SIRT1-specific siRNAs to silence the expression of SIRT1 in EL4 cells and examined the expression of SIRT1 in the absence or presence of RES. As shown in Fig 4B, there was significant blocking of SIRT1 expression in EL4 cells two days post transfection with SIRT1 siRNAs. However, RES treatment of SIRT1 siRNA-transfected EL4 cells led to upregulation of SIRT1 expression in transfected EL4 cells although these levels were less than that seen in wild-type EL-4 cells (Fig 4C). We also examined the posphorylation of IκBα which leads to release NF-kB that migrates to nucleus to regulate various genes. We observed lower level of phosphorylation of IκBα (Ser 32) upon RES treatment in these cells when compared to vehicle treatment (Fig 4C) demonstrating that RES-mediated induction in expression of SIRT1 blocks phosphorylation of IκBα (Ser 32) which in turn influences expression and release of NF-kB.
We also determined the activation of NF-kB in the nucleus. To this end, we performed EMSA assays using mouse NF-kB-specific probe and nuclear protein prepared from untreated or SIRT1 siRNA transfected EL4 cells treated with vehicle or RES. As shown in Fig 4D, we observed shift in NF-kB probe when nuclear protein form vehicle-treated EL4 cells was used. However, there was lesser shift in NF-kB probes when nuclear proteins from Resveatrol-treated EL4 cells were used (Fig 4D). Moreover, when nuclear protein from SIRT1 siRNA-transfected EL4 cells was used, we observed relatively more shift in NF-kB probes but RES treatment of transfected EL4 cells significantly affected the shift of NF-kB probes (Fig. 4D). The nuclear extracts from various treated groups of EL4 cells did not show a shift in mutant NF-kB probes demonstrating NF-kB-specific binding of nuclear extracts of EL4 cells (Fig. 4D). The data obtained from these studies demonstrated that RES treatment of EL4 cells upregulated SIRT1 expression but downregulated NF-kB expression.
3.7 SIRT1 expression affects RES-mediated apoptosis of EL4 cells
We next assessed the effects of SIRT1 on RES-mediated apoptosis in EL4 cells. To this end, we performed in vitro assays using EL4 cells untransfected or tranfected with SIRT1 siRNA and treated with vehicle or RES. We observed that untransfected EL4 cells were more susceptible to RES-induced apoptosis (Fig. 4E and F), when compared to EL4 cells transfected with SIRT1 siRNAs, particularly when the background apoptosis seen with vehicle was subtracted from RES-induced apoptosis (Fig. 4E and F). These data demonstrated that SIRT1 expression influences RES-mediated apoptosis in EL4 cells.
4 Discussion
Development of anti-cancer drugs with high efficacy is a major challenge. The resistance of many cancers to various treatments throws obstacles and constitutes major problems in cancer therapy. Usually, the abnormalities in apoptotic pathways of cancer cells make them resistant to various chemopreventive drugs. Successful induction of apoptosis in cancer cells, therefore, is a key for the development of anti-cancer drugs and therapies. Chemoprevention is considered as a promising strategy to control cancer development [30,31]. There are many natural products and/or dietary supplements that have been shown to act as chemopreventive agents and inhibit experimental carcinogenesis [32]. In recent years, RES, a natural plant-derived phytoalexin compound, which possesses anti-cancer properties, has been the focus for its use as a chemopreventive agent against various types of cancer [5,6]. RES has been shown to suppress the growth of transformed cells through induction of apoptosis [33]. There are studies demonstrating RES-mediated apoptosis in HL60 leukaemia cells as well as in T47D breast carcinoma cells [6]. RES-mediated apoptosis has also been shown in human T-cell acute lymphoblastic leukemia MOLT-4 cells [34].
In the current study, we investigated the chemopreventive and anti-cancer properties of RES against EL4, a T lymphoma cell line, and evaluated its efficacy against EL4-induced tumorigenesis in immunodeficient NOD/SCID mouse model. We used this model to directly test the effect of RES on tumor cells because it is well known that RES also exhibits immunomodulatory properties, including the ability to enhance perforin expression and NK cell cytotoxicity through NKG2D-dependent pathways [36]. We noted that RES treatment significantly suppressed EL4-tumor growth and increased the mean survival time. These results correlated with the ability of RES to induce apoptosis in EL4 tumor cells. Analysis of apoptotic pathways revealed that RES triggered both death-receptor (Fas/FasL-mediated) and mitochondrial pathways in EL4 cells. Although both death-receptor and mitochondrial pathways played a role in RES-mediated apoptosis in EL4 cells, the fact that only a small proportion of apoptotic cells showed loss of mitochondrial membrane potential suggested that death-receptor pathway may play more critical role in RES-mediated apoptosis in EL4 cells. The mitochondrial pathway may be activated through cross-talk via Bid as we observed presence of cleaved Bid (22 kd) in EL4 cells in the presence of RES. We also observed the presence of cleaved caspase-2, which can be activated by cytotoxic stress, and caspase-2 is required for the permeabilization of mitochondria [35]. RES has been shown to trigger CD95 signaling-dependent apoptosis in human tumor cells [6]. RES may also induce FasL-mediated apoptosis through Cdc42 activation of ASK1/JNK-dependent signaling pathway in human leukemia HL-60 cells [37]. Dorrie et al have shown that RES induces apoptosis by depolarizing mitochondrial membrane and activating caspase-9 in acute lymphoblastic leukemia cells [5].
We and others have shown that RES also acts as a AhR mixed agonist/antagonist [22,38]. In this study, we observed RES-mediated the activation of AhR in EL4 cells. Previously, we have shown that activated AhR induced Fas and FasL expression in T cells leading to apoptosis [22,23]. The earlier study from our laboratory has also shown that AhR upon activation upregulates the expression of Fas by interacting with dioxin response element (DRE) present as a regulatory element in murine Fas promoter, and FasL by interacting with NF-kB regulatory elements present in murine FasL promoter [25]. Thus, RES not only induces AhR but also acts as an AhR ligand which may trigger the induction of Fas and FasL leading to apoptosis. There are reports demonstrating that RES can act as a ligand for aryl hydrocarbon receptor (AhR) thereby regulating the expression of various genes [39]. In summary, we propose a model demonstrating participation of both extrinsic and intrinsic pathways in RES-mediated apoptosis in EL4 cells (Fig. 4G).
RES is one of the most potent natural compounds that activate SIRT1 [40,41]. Earlier studies have demonstrated that SIRT1 acts as a longevity factor and its overexpression increases the life span of many organisms tested [42]. Many of the SIRT1 substrates are transcription factors and key regulators described to take part in cancer development, such as the nuclear factor-κB (NF-κB), the DNA repair factor Ku70, the tumor suppressor gene p53, and the forkhead transcription factors (FoxOs) [43,44]. The relationship between SIRT1 activity and tumorigenesis is still open to debate [43]. Boily and others have reported that SIRT1 does not behave like a classical tumor-suppressor gene but the antitumor activity of RES is mediated, at least in part, by SIRT1 [45]. There are other reports demonstrating high expression of SIRT1 in several types of tumors [46] and may be responsible for the development of chemotherapy resistance [47]. SIRT1 is also an important mediator in calorie restriction-associated tumor prevention [48]. Although some studies have suggested that SIRT1 may function as a tumor promoter because of its increased expression in some types of cancers, other studies have demonstrated that SIRT1 levels are reduced in some other types of cancers thereby acting as a tumor suppressor [18]. Moreover, SIRT1 deficiency promotes tumorigenesis, and overexpression of SIRT1 attenuates cancer formation in mice heterozygous for tumor suppressor p53 [49,50] or APC [45]. Thus, clearly, additional studies are necessary to address the precise role of SIRT1 in tumor growth and metastasis. In the current study we noted that upon RES treatment, SIRT1 expression was significantly increased in EL4 tumor cells. It was also striking that there was a significant decrease in p-IκBα expression and NF-κB nuclear translocation in EL4 cells. These data are consistent with the observation that IκBα levels were elevated in mouse embryonic fibroblasts derived from SIRT1−/− mice under basal conditions as well as in response to TNFα, thereby suggesting that SIRT1 suppresses the expression of NF-κB-dependent genes [51]. Moreover, SIRT1 regulates the transcriptional activity of NF-κB by physically interacting with the RelA/p65 subunit of NF-κB and inhibiting transcription by deacetylating RelA/p65 [52]. Together, these studies suggest that RES by enhancing SIRT1 expression may downregulate NF-κB activation and thereby making tumor cells susceptible to RES-induced apoptosis, specifically because NF-κB exhibits anti-apoptotic properties.
In the current study, we used a dose of 10, 50 and 100 mg/kg of RES administered by oral gavage. Previous in vivo studies to treat cancer suggested that low doses such as 40 mg/kg of RES are not effective while higher doses such as 80 mg/kg were partially effective [53]. In another study, RES was shown to inhibit tumor growth in Balb/c mice at high doses such as 500, 1000 and 1500 mg/kg in a dose dependent manner when administered for 10 days [54]. Also, in a rat model, 100 mg/kg of RES was very effective in delaying tumorigenesis [55]. In this study, we observed that lower dose (10 mg/kg) was not effective against EL4 tumor growth while a higher dose (100 mg/kg) was very effective. It should be noted that the dose that we have used is feasible to achieve in humans because the human equivalent dose of 100 mg/kg in mouse is 486 mg, considering an average human weight of 60 kg. Currently, there are several nutraceutical companies selling purified resveratrol in 500-mg quantities in capsule form. Thus, our doses reflect the potential pharmacological/dietary supplement dose available in market.
In summary, our study provides important information regarding the reciprocal regulation of SIRT1 and NF-κB in tumor apoptosis in vitro and tumor growth in vivo. Our study demonstrates that exposure of a T cell lymphoma line to RES induces SIRT1 and decreases NF-κB activation thereby making the cells more susceptible to apoptosis and inhibition of tumor growth in vivo. Thus, our studies suggest that RES may serve as a chemopreventive agent and make the tumor cells more susceptible to apoptosis when used either directly or in combination with other anti-cancer agents.
Acknowledgements
N.P.S. designed and performed experiments, analyzed results, prepared the figures, and wrote the manuscript; U.P.S. helped in design of experiments and discussion, VGH, performed apoptotic assays and analyzed data, HG, performed in vitro experiments and analyzed data, LH, participated in discussion to examine data, M.N. designed the experiments, edited the manuscript, and provided the resources, and P.S.N. assisted in project conception, design of experiments, provided resources, and edited the manuscript.
All authors read and approved the final manuscript.
This work was funded in part by National Institutes of Health Grants P01-AT03961, R01-ES09098, R01-DA016545).
Abbreviations used in this paper
- Resveratrol (RES)
trans-3,5,4'-trihydroxystilbene
- SIRT1
silent mating type information regulation 2 homolog, 1
- NF-kB
Nuclear Factor Kappa B
- AhR
aryl hydrocarbon receptor
Footnotes
The authors have declared no conflicts of interests
References
- 1.Jang JH, Surh YJ. Protective effects of resveratrol on hydrogen peroxide-induced apoptosis in rat pheochromocytoma (PC12) cells. Mutat Res. 2001;496:181–190. doi: 10.1016/s1383-5718(01)00233-9. [DOI] [PubMed] [Google Scholar]
- 2.Gehm BD, McAndrews JM, Chien PY, Jameson JL. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc Natl Acad Sci U S A. 1997;94:14138–14143. doi: 10.1073/pnas.94.25.14138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soleas GJ, Diamandis EP, Goldberg DM. Wine as a biological fluid: history, production, and role in disease prevention. J Clin Lab Anal. 1997;11:287–313. doi: 10.1002/(SICI)1098-2825(1997)11:5<287::AID-JCLA6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kopp P. Resveratrol, a phytoestrogen found in red wine. A possible explanation for the conundrum of the 'French paradox'? Eur J Endocrinol. 1998;138:619–620. doi: 10.1530/eje.0.1380619. [DOI] [PubMed] [Google Scholar]
- 5.Dorrie J, Gerauer H, Wachter Y, Zunino SJ. Resveratrol induces extensive apoptosis by depolarizing mitochondrial membranes and activating caspase-9 in acute lymphoblastic leukemia cells. Cancer Res. 2001;61:4731–4739. [PubMed] [Google Scholar]
- 6.Clement MV, Hirpara JL, Chawdhury SH, Pervaiz S. Chemopreventive agent resveratrol, a natural product derived from grapes, triggers CD95 signaling-dependent apoptosis in human tumor cells. Blood. 1998;92:996–1002. [PubMed] [Google Scholar]
- 7.Ovesna Z, Kozics K, Bader Y, Saiko P, Handler N, Erker T, Szekeres T. Antioxidant activity of resveratrol, piceatannol and 3,3',4,4',5,5'-hexahydroxy-trans-stilbene in three leukemia cell lines. Oncol Rep. 2006;16:617–624. [PubMed] [Google Scholar]
- 8.Cignarella A, Minici C, Bolego C, Pinna C, Sanvito P, Gaion RM, Puglisi L. Potential pro-inflammatory action of resveratrol in vascular smooth muscle cells from normal and diabetic rats. Nutr Metab Cardiovasc Dis. 2006;16:322–329. doi: 10.1016/j.numecd.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Palsamy P, Subramanian S. Ameliorative potential of resveratrol on proinflammatory cytokines, hyperglycemia mediated oxidative stress, and pancreatic beta-cell dysfunction in streptozotocin-nicotinamide-induced diabetic rats. J Cell Physiol. 224:423–432. doi: 10.1002/jcp.22138. [DOI] [PubMed] [Google Scholar]
- 10.Mousa SA, Gallati C, Simone T, Dier E, Yalcin M, Dyskin E, Thangirala S, Hanko C, Rebbaa A. Dual targeting of the antagonistic pathways mediated by Sirt1 and TXNIP as a putative approach to enhance the efficacy of anti-aging interventions. Aging (Albany NY) 2009;1:412–424. doi: 10.18632/aging.100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orallo F. Trans-resveratrol: a magical elixir of eternal youth? Curr Med Chem. 2008;15:1887–1898. doi: 10.2174/092986708785132951. [DOI] [PubMed] [Google Scholar]
- 12.Harikumar KB, Kunnumakkara AB, Sethi G, Diagaradjane P, Anand P, Pandey MK, Gelovani J, Krishnan S, Guha S, Aggarwal BB. Resveratrol, a multitargeted agent, can enhance antitumor activity of gemcitabine in vitro and in orthotopic mouse model of human pancreatic cancer. Int J Cancer. 127:257–268. doi: 10.1002/ijc.25041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 14.Mao QQ, Bai Y, Lin YW, Zheng XY, Qin J, Yang K, Xie LP. Resveratrol confers resistance against taxol via induction of cell cycle arrest in human cancer cell lines. Mol Nutr Food Res. 54:1574–1584. doi: 10.1002/mnfr.200900392. [DOI] [PubMed] [Google Scholar]
- 15.Armour SM, Baur JA, Hsieh SN, Land-Bracha A, Thomas SM, Sinclair DA. Inhibition of mammalian S6 kinase by resveratrol suppresses autophagy. Aging (Albany NY) 2009;1:515–528. doi: 10.18632/aging.100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71:1397–1421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 17.North BJ, Verdin E. Sirtuins: Sir2-related NAD-dependent protein deacetylases. Genome Biol. 2004;5:224. doi: 10.1186/gb-2004-5-5-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng CX. SIRT1, is it a tumor promoter or tumor suppressor? Int J Biol Sci. 2009;5:147–152. doi: 10.7150/ijbs.5.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cha EJ, Noh SJ, Kwon KS, Kim CY, Park BH, Park HS, Lee H, Chung MJ, Kang MJ, Lee DG, Moon WS, Jang KY. Expression of DBC1 and SIRT1 is associated with poor prognosis of gastric carcinoma. Clin Cancer Res. 2009;15:4453–4459. doi: 10.1158/1078-0432.CCR-08-3329. [DOI] [PubMed] [Google Scholar]
- 20.Wang RH, Sengupta K, Li C, Kim HS, Cao L, Xiao C, Kim S, Xu X, Zheng Y, Chilton B, Jia R, Zheng ZM, Appella E, Wang XW, Ried T, Deng CX. Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice. Cancer Cell. 2008;14:312–323. doi: 10.1016/j.ccr.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Firestein R, Blander G, Michan S, Oberdoerffer P, Ogino S, Campbell J, Bhimavarapu A, Luikenhuis S, de Cabo R, Fuchs C, Hahn WC, Guarente LP, Sinclair DA. The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth. PLoS One. 2008;3:e2020. doi: 10.1371/journal.pone.0002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh NP, Hegde VL, Hofseth LJ, Nagarkatti M, Nagarkatti P. Resveratrol (trans-3,5,4'-trihydroxystilbene) ameliorates experimental allergic encephalomyelitis, primarily via induction of apoptosis in T cells involving activation of aryl hydrocarbon receptor and estrogen receptor. Mol Pharmacol. 2007;72:1508–1521. doi: 10.1124/mol.107.038984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Camacho IA, Singh N, Hegde VL, Nagarkatti M, Nagarkatti PS. Treatment of mice with 2,3,7,8-tetrachlorodibenzo-p-dioxin leads to aryl hydrocarbon receptor-dependent nuclear translocation of NF-kappaB and expression of fas ligand in thymic stromal cells and consequent apoptosis in T cells. J Immunol. 2005;175:90–103. doi: 10.4049/jimmunol.175.1.90. [DOI] [PubMed] [Google Scholar]
- 24.Singh UP, Singh NP, Singh B, Hofseth LJ, Price RL, Nagarkatti M, Nagarkatti PS. Resveratrol (trans-3,5,4'-trihydroxystilbene) induces silent mating type information regulation-1 and down-regulates nuclear transcription factor-kappaB activation to abrogate dextran sulfate sodium-induced colitis. J Pharmacol Exp Ther. 332:829–839. doi: 10.1124/jpet.109.160838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh NP, Nagarkatti M, Nagarkatti PS. Role of dioxin response element and nuclear factor-kappaB motifs in 2,3,7,8-tetrachlorodibenzo-p-dioxin-mediated regulation of Fas and Fas ligand expression. Mol Pharmacol. 2007;71:145–157. doi: 10.1124/mol.106.028365. [DOI] [PubMed] [Google Scholar]
- 26.Gracia-Sancho J, Villarreal G, Jr, Zhang Y, Garcia-Cardena G. Activation of SIRT1 by resveratrol induces KLF2 expression conferring an endothelial vasoprotective phenotype. Cardiovasc Res. 85:514–519. doi: 10.1093/cvr/cvp337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 28.Markus MA, Morris BJ. Resveratrol in prevention and treatment of common clinical conditions of aging. Clin Interv Aging. 2008;3:331–339. [PMC free article] [PubMed] [Google Scholar]
- 29.Yang J, Kong X, Martins-Santos ME, Aleman G, Chaco E, Liu GE, Wu SY, Samols D, Hakimi P, Chiang CM, Hanson RW. Activation of SIRT1 by resveratrol represses transcription of the gene for the cytosolic form of phosphoenolpyruvate carboxykinase (GTP) by deacetylating hepatic nuclear factor 4alpha. J Biol Chem. 2009;284:27042–27053. doi: 10.1074/jbc.M109.047340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez-Nieto S, Zhivotovsky B. Role of alterations in the apoptotic machinery in sensitivity of cancer cells to treatment. Curr Pharm Des. 2006;12:4411–4425. doi: 10.2174/138161206779010495. [DOI] [PubMed] [Google Scholar]
- 31.Testa U, Riccioni R. Deregulation of apoptosis in acute myeloid leukemia. Haematologica. 2007;92:81–94. doi: 10.3324/haematol.10279. [DOI] [PubMed] [Google Scholar]
- 32.Kelloff GJ, Crowell JA, Steele VE, Lubet RA, Malone WA, Boone CW, Kopelovich L, Hawk ET, Lieberman R, Lawrence JA, Ali I, Viner JL, Sigman CC. Progress in cancer chemoprevention: development of diet-derived chemopreventive agents. J Nutr. 2000;130:467S–471S. doi: 10.1093/jn/130.2.467S. [DOI] [PubMed] [Google Scholar]
- 33.Ferry-Dumazet H, Garnier O, Mamani-Matsuda M, Vercauteren J, Belloc F, Billiard C, Dupouy M, Thiolat D, Kolb JP, Marit G, Reiffers J, Mossalayi MD. Resveratrol inhibits the growth and induces the apoptosis of both normal and leukemic hematopoietic cells. Carcinogenesis. 2002;23:1327–1333. doi: 10.1093/carcin/23.8.1327. [DOI] [PubMed] [Google Scholar]
- 34.Cecchinato V, Chiaramonte R, Nizzardo M, Cristofaro B, Basile A, Sherbet GV, Comi P. Resveratrol-induced apoptosis in human T-cell acute lymphoblastic leukaemia MOLT-4 cells. Biochem Pharmacol. 2007;74:1568–1574. doi: 10.1016/j.bcp.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Lassus P, Opitz-Araya X, Lazebnik Y. Requirement for caspase-2 in stress-induced apoptosis before mitochondrial permeabilization. Science. 2002;297:1352–1354. doi: 10.1126/science.1074721. [DOI] [PubMed] [Google Scholar]
- 36.Lu CC, Chen JK. Resveratrol enhances perforin expression and NK cell cytotoxicity through NKG2D-dependent pathways. J Cell Physiol. 223:343–351. doi: 10.1002/jcp.22043. [DOI] [PubMed] [Google Scholar]
- 37.Su JL, Lin MT, Hong CC, Chang CC, Shiah SG, Wu CW, Chen ST, Chau YP, Kuo ML. Resveratrol induces FasL-related apoptosis through Cdc42 activation of ASK1/JNK-dependent signaling pathway in human leukemia HL-60 cells. Carcinogenesis. 2005;26:1–10. doi: 10.1093/carcin/bgh220. [DOI] [PubMed] [Google Scholar]
- 38.de Medina P, Casper R, Savouret JF, Poirot M. Synthesis and biological properties of new stilbene derivatives of resveratrol as new selective aryl hydrocarbon modulators. J Med Chem. 2005;48:287–291. doi: 10.1021/jm0498194. [DOI] [PubMed] [Google Scholar]
- 39.Casper RF, Quesne M, Rogers IM, Shirota T, Jolivet A, Milgrom E, Savouret JF. Resveratrol has antagonist activity on the aryl hydrocarbon receptor: implications for prevention of dioxin toxicity. Mol Pharmacol. 1999;56:784–790. [PubMed] [Google Scholar]
- 40.Albani D, Polito L, Batelli S, De Mauro S, Fracasso C, Martelli G, Colombo L, Manzoni C, Salmona M, Caccia S, Negro A, Forloni G. The SIRT1 activator resveratrol protects SK-N-BE cells from oxidative stress and against toxicity caused by alpha-synuclein or amyloid-beta (1–42) peptide. J Neurochem. 2009;110:1445–1456. doi: 10.1111/j.1471-4159.2009.06228.x. [DOI] [PubMed] [Google Scholar]
- 41.Sulaiman M, Matta MJ, Sunderesan NR, Gupta MP, Periasamy M, Gupta M. Resveratrol, an activator of SIRT1, upregulates sarcoplasmic calcium ATPase and improves cardiac function in diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol. 298:H833–H843. doi: 10.1152/ajpheart.00418.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- 43.Saunders LR, Verdin E. Sirtuins: critical regulators at the crossroads between cancer and aging. Oncogene. 2007;26:5489–5504. doi: 10.1038/sj.onc.1210616. [DOI] [PubMed] [Google Scholar]
- 44.Blander G, Guarente L. The Sir2 family of protein deacetylases. Annu Rev Biochem. 2004;73:417–435. doi: 10.1146/annurev.biochem.73.011303.073651. [DOI] [PubMed] [Google Scholar]
- 45.Boily G, He XH, Pearce B, Jardine K, McBurney MW. SirT1-null mice develop tumors at normal rates but are poorly protected by resveratrol. Oncogene. 2009;28:2882–2893. doi: 10.1038/onc.2009.147. [DOI] [PubMed] [Google Scholar]
- 46.Huffman DM, Grizzle WE, Bamman MM, Kim JS, Eltoum IA, Elgavish A, Nagy TR. SIRT1 is significantly elevated in mouse and human prostate cancer. Cancer Res. 2007;67:6612–6618. doi: 10.1158/0008-5472.CAN-07-0085. [DOI] [PubMed] [Google Scholar]
- 47.Chu F, Chou PM, Zheng X, Mirkin BL, Rebbaa A. Control of multidrug resistance gene mdr1 and cancer resistance to chemotherapy by the longevity gene sirt1. Cancer Res. 2005;65:10183–10187. doi: 10.1158/0008-5472.CAN-05-2002. [DOI] [PubMed] [Google Scholar]
- 48.Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- 49.Peck B, Chen CY, Ho KK, Di Fruscia P, Myatt SS, Coombes RC, Fuchter MJ, Hsiao CD, Lam EW. SIRT inhibitors induce cell death and p53 acetylation through targeting both SIRT1 and SIRT2. Mol Cancer Ther. 9:844–855. doi: 10.1158/1535-7163.MCT-09-0971. [DOI] [PubMed] [Google Scholar]
- 50.Yi J, Luo J. SIRT1 and p53, effect on cancer, senescence and beyond. Biochim Biophys Acta. 1804:1684–1689. doi: 10.1016/j.bbapap.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kwon HS, Brent MM, Getachew R, Jayakumar P, Chen LF, Schnolzer M, McBurney MW, Marmorstein R, Greene WC, Ott M. Human immunodeficiency virus type 1 Tat protein inhibits the SIRT1 deacetylase and induces T cell hyperactivation. Cell Host Microbe. 2008;3:158–167. doi: 10.1016/j.chom.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. Embo J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao X, Xu YX, Divine G, Janakiraman N, Chapman RA, Gautam SC. Disparate in vitro and in vivo antileukemic effects of resveratrol, a natural polyphenolic compound found in grapes. J Nutr. 2002;132:2076–2081. doi: 10.1093/jn/132.7.2076. [DOI] [PubMed] [Google Scholar]
- 54.Liu HS, Pan CE, Yang W, Liu XM. Antitumor and immunomodulatory activity of resveratrol on experimentally implanted tumor of H22 in Balb/c mice. World J Gastroenterol. 2003;9:1474–1476. doi: 10.3748/wjg.v9.i7.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bhat KP, Lantvit D, Christov K, Mehta RG, Moon RC, Pezzuto JM. Estrogenic and antiestrogenic properties of resveratrol in mammary tumor models. Cancer Res. 2001;61:7456–7463. [PubMed] [Google Scholar]