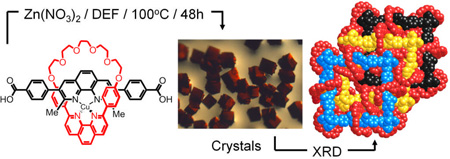
Abstract
MOFs on the move: A copper coordinated [2]pseudorotaxanate which reacts with zinc nitrate to form an extended structure, consisting of three-fold interpenetrated networks, retains most of its solution-state chemistry including its ability to undergo electronic switching of some of the copper(I) ions under redox control.
Keywords: extended structures, mechanostereochemistry, reticular chemistry, robust dynamics, rotaxanes
The incorporation of mechanically interlocked molecules (MIMs), such as rotaxanes and catenanes, into porous metal-organic frameworks (MOFs) has recently been recognized[1] as a strategy for introducing dynamics into otherwise rigid, robust frameworks. In this manner, the interlocked nature of these switchable components allows dynamics to be expressed independently of the MOF backbone and, therefore, without compromising the fidelity of the whole system. Accordingly, π-electron-rich crown ethers, capable of binding π-electron-deficient substrates,[2] and degenerate donor-acceptor [2]catenanes[3] have already been introduced into MOFs. In addition, [2]rotaxanes, wherein the dumbbell components function as struts in the frameworks, have also been incorporated[4] into MOFs. While this strategy has been applied[4a–f] to the preparation of a number of 2D and 3D frameworks from [2]pseudorotaxanate subunits, it remains a challenge to locate switchable rotaxanes and catenanes inside extended frameworks. Metal-containing MIMs—particularly those based on the well-known[5] copper (I)-templated systems—offer the opportunity to locate switches within MOFs. Herein, we report the successful incorporation of copper(I)-complexed [2]pseudorotaxanate struts into a MOF (MOF-1040). Each of the struts is composed of a rigid rod-like fragment containing a 1,10-phenanthroline unit which is encircled by a coordinating 30-membered ring. We present (i) the synthesis and solid-state structure of the strut in the form of a [2]pseudorotaxanate, (ii) the preparation of MOF-1040 and its single crystal structure, along with (iii) data supporting the oxidation of copper(I) in the struts to copper(II), as well as (iv) the demetalation of the strut, creating rotaxanes with free coordination sites within the solid-state structure of MOF-1040.
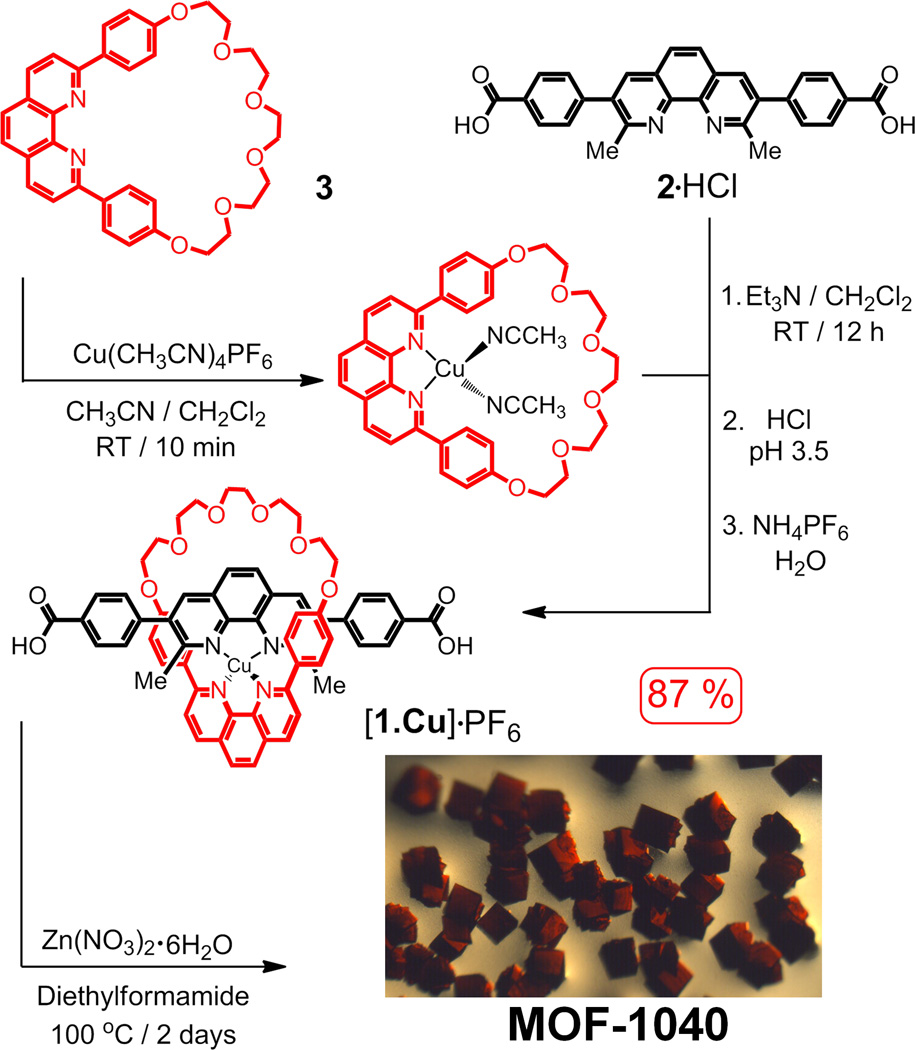
The synthesis of the copper-coordinated pseudorotaxanate [1·Cu]·PF6 and the subsequent preparation of MOF-1040 employing this strut are summarized in Scheme 1. The [2]pseudorotaxanate [1·Cu]·PF6 was prepared—using the effective Cu(I)-templated “gathering-and-threading” approach[5a, 5b] of a copper(I) catenate— by (i) mixing of 2·HCl[6] with Et3N, thus generating the dicarboxylate of 2, followed by (ii) the addition of the copper complex[7] of 3, which was prepared by (iii) the reaction of the coordinating ring with Cu(CH3CN)4PF6 in stoichiometric amounts, followed by (iv) reprotonation of the dicarboxylate and counterion exchange with saturated aqueous NH4PF6 solution, resulted in the production of [1·Cu]·PF6 in 87% yield.
Scheme 1.
Synthesis of the [2]pseudorotaxanate [1•Cu]·PF6 and MOF-1040. The synthesis of the [2]pseudorotaxanate was achieved using metal-templated protocols—the copper-complexed ring 3 was added to a solution of 2 in CH2Cl2 to form the [2]pseudorotaxanate [1•Cu]·PF6. Red cubic crystals, suitable for X-ray crystallographic analysis, were obtained by mixing [1•Cu]·PF6 and Zn(NO3)2.6H2O in N,N-diethylformamide in a sealed tube at 100 °C for 2 days.
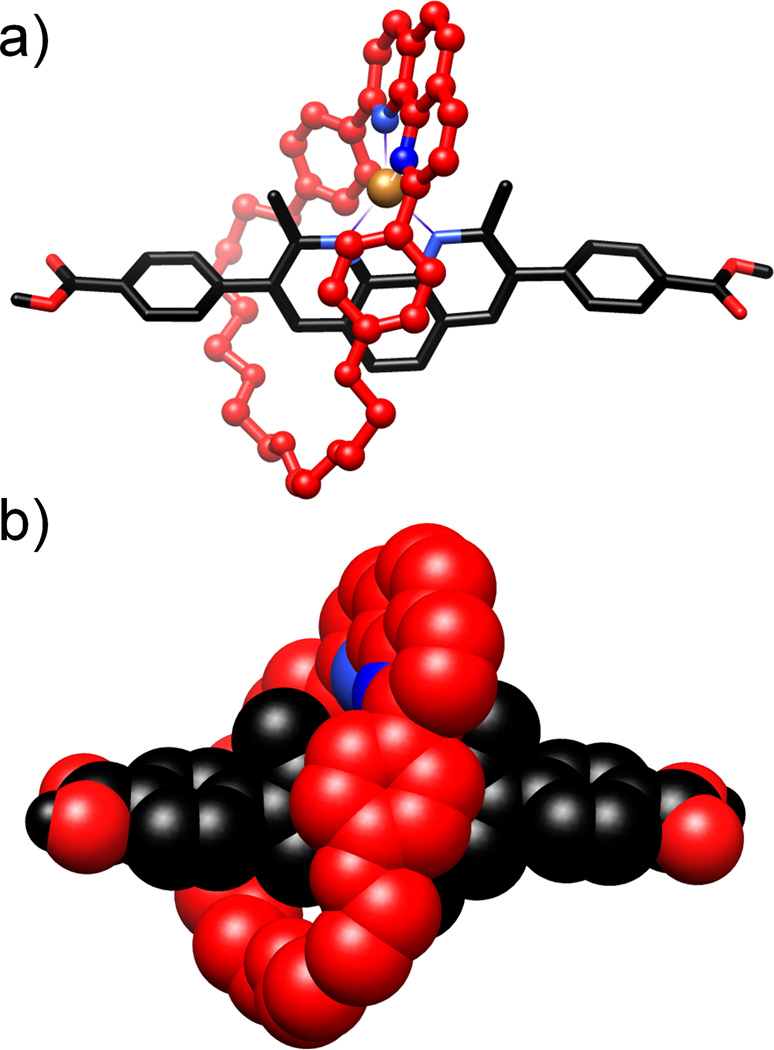
Dark red single crystals of the copper-coordinated [2]pseudorotaxanate were grown by diffusing Et2O into a solution of [1·Cu]·PF6 in CH2Cl2. The solid-state superstructure[8] (Figure 1), which occupies the triclinic space group P1̅, comprises three independent [2]pseudorotaxanates, in addition to disordered PF6− anions and CH2Cl2 solvent molecules. The Cu-N bond distances are typical [2.014 to 2.080 Å] of such complexes, and, while the N-Cu-N angles are in some cases distorted significantly from ideal tetrahedral geometry [81 to 139°], this occurrence is in agreement with previously reported examples.[9] It would appear that these distortions occur to enable more efficient packing, as well as stabilizing intramolecular interactions, e.g., one of the phenoxy moieties of the ring component participates in [π⋯π] stacking interactions with the phenanthroline ring system of the rod component. The three independent [2]pseudorotaxanates found in the unit cell arise primarily from the changes in the arrangement of the two phenanthroline ring systems and the intramolecular interactions they seek to satisfy.
Figure 1.
Solid-state (super)structure of the copper-complexed [2]pseudorotaxanate [1•Cu]·PF6 in (a) ball-and-stick and (b) space-filling representations. The copper(I)-complex is distorted significantly from ideal tetrahedral geometry on account of the [π⋯π] stacking interactions between one of the phenoxy moieties of the ring and the phenanthroline ring system in the thread component. The hydrogen atoms and counterions have been omitted for clarity.
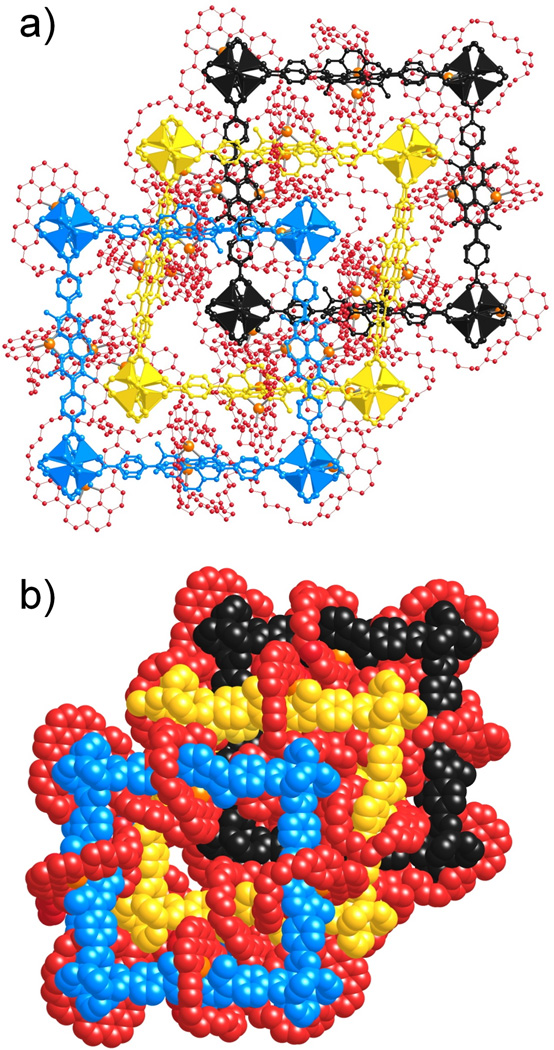
Mixing [1·Cu]·PF6 and Zn(NO3)2·6H2O in diethylformamide (DEF) at 100 °C in a sealed tube for 48 h resulted (see Experimental Section) in the formation of red cubic crystals of MOF-1040. The single crystal X-ray diffraction study was performed using synchrotron radiation in the beamline 24-ID-C at NECAT, in the Advanced Photon Source (APS) at Argonne National Laboratory. The crystal structure was solved in the monoclinic space group P21/c,[10] revealing a structure (Figure 2) consisting of three fold interpenetrated networks. The inorganic Zn4O secondary building units (SBUs) are linked by the [1·Cu] complex struts, producing a framework with pcu topology.[11] The inorganic SBUs, the Cu complexes and the rest of the aromatic rings in the organic strut were unambiguously determined from the single-crystal structural analysis of MOF-1040. In common with other crown-ether containing MOFs,[2] the polyether part of the coordinating ring were found to be highly disordered in the structure (see Supporting Information, SI, for details). In addition, the interstitial spaces of MOF-1040 are not only occupied by disordered solvent molecules, but also by disordered PF6− ions. The polyether loops of the coordinating rings in the structure were modeled using material studio.[10] Nonetheless, the crystal structure of MOF-1040 confirms that the integrity of the copper(I)-complexed [2]pseudorotaxanate is maintained after the formation of the MOF.
Figure 2.
(a) Ball-and-stick representation of the crystal structure of MOF-1040 which has three-fold interpenetrated networks shown in blue, yellow, and black, respectively, with coordinating rings represented by red balls and wires, the copper atoms are represented in gold. (b) Space-filling representations of the three-fold interpenetrated networks of MOF-1040 shown in blue, yellow and black, respectively, with coordinating rings represented in red. The polyether parts of coordinating ring in the structure were modeled by using material studio.[10]
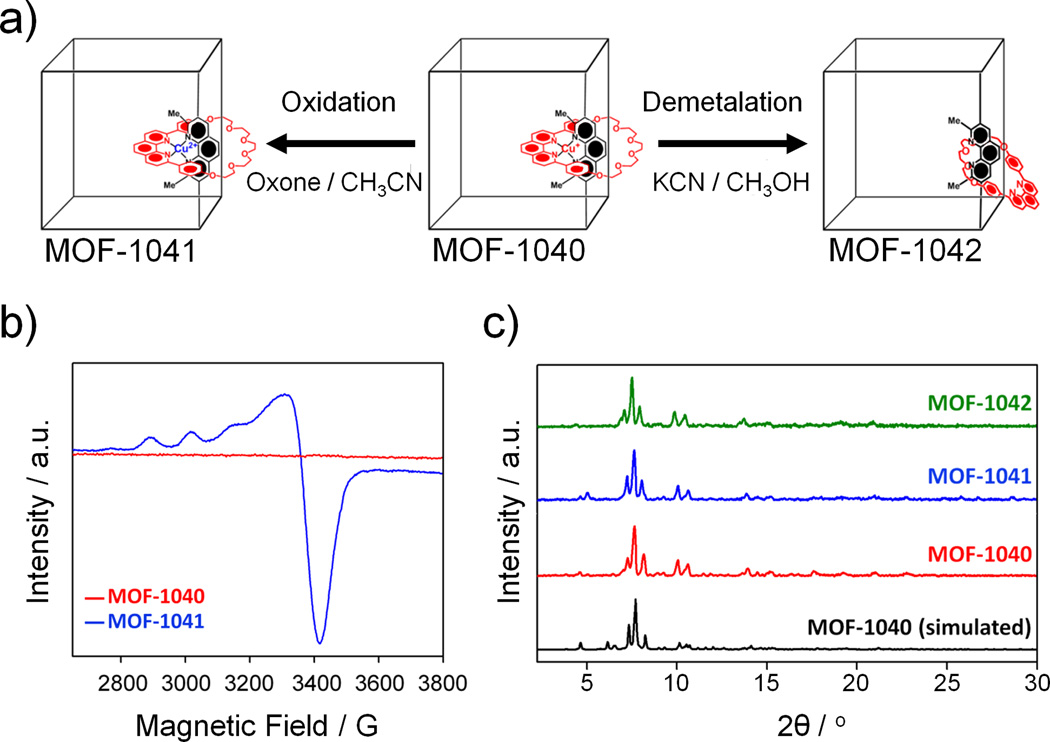
The stable Cu(I)N4+ complex of MOF-1040 with four-coordinate d10 metal (pseudotetrahedral) is oxidized (Figure 3a) by the addition of oxone solution in CH3CN (0.05 M) at room temperature, forming the paramagnetic copper (II) complex—Cu(II)N42+ (d9 configuration) in the oxidized MOF-1040, is referred to as MOF-1041. The oxidation of Cu(I) to Cu(II) has been monitored (Figure 3b) by EPR spectroscopy. While it is important to note that the EPR experiment does not allow us to quantify the degree of change in the redox state of copper ions, those Cu(I) ions which happen to be oxidized to Cu(II) can be regarded as local electronic switches which will undoubtedly be accompanied by significant[12] alterations in the coordination geometries surrounding these copper ions located inside MOF-1040/1041. The resulting spectrum displays characteristic features of a d9 Cu(II)N4+ complex with g-factors of g‖ = 2.290, g⊥ = 2.064 and A‖ = 11.1 mT, values expected for four-coordinate Cu(II) in a distorted tetrahedral arrangement. These values are in perfect agreement with those obtained[13] in the solution studies of related complexes consisting of the same chelating units—namely 2,9-diphenyl-1,10-phenanthroline. Additionally, we have performed inductively coupled plasma mass spectrometry (ICP-MS) in order to quantify the amount of copper within the framework. MOF-1041 revealed a decrease in the amount of copper with the calculated molar ratios [Zn]/[Cu] of 1.33 and 1.50, before and after oxidation, respectively. These numbers support the fact that the complex of the copper(II) ion in a four-coordinate environment is a thermodynamically unstable species and can be dissociated easily.[13b, 14] Furthermore, the powder X-ray diffraction (PXRD) pattern (Figure 3c) of MOF-1041 proves conclusively that the structure of the framework is maintained after oxidation has occurred.
Figure 3.
(a) Graphical representation of the oxidation of Cu(I) to Cu(II)—using oxone solution (0.05 M) in CH3CN—resulted in the formation of MOF-1041 with the Cu(II)N42+ rotaxane complexes and the demetalation of MOF-1040 using KCN (5×10−3 M in anhydrous CH3OH) affording MOF-1042 in which 60% of rotaxanes are demetalated. (b) Solid-state EPR spectra of MOF-1040 (red line) and its oxidized form (MOF-1041) (blue line) recorded at room temperature at 9.78 GHz. (c) PXRD patterns of as-synthesized MOF-1040 (red), after oxidation reaction (MOF-1041) (blue) and after demetalation (MOF-1042) (green) compared with calculated pattern (black) derived from the crystal structure, reveal that MOF-1040 maintained its structural topology throughout the oxidation and the demetalation.
In addition to the switching experiments, we have carried out demetalation (Figure 3) by following well-established literature procedures already developed for the molecular strut,[14] wherein Cu(I)-complexed rotaxanes and catenanes can be demetalated by reaction with CN− ion under mild reaction conditions, leading to rotaxanes and catenanes with free coordination sites. In order to demetalate the rotaxane in MOF-1040, a reaction between MOF-1040 crystals and KCN (5×10−3 M) in CH3OH has been performed (see SI, for details). After the reaction, a sample analyzed by ICP-MS revealed a 60% decrease in the amount of Cu(I), compared with the original sample—indicating that not all of the Cu(I)-complexing sites within the framework are accessible.[15] The partially demetalated MOF-1040 (termed MOF-1042) maintains (Figure 3c) its structural topology throughout the reaction as evidenced by PXRD measurements.
We have demonstrated that metal-templated [2]pseudorotaxanates bearing carboxyl groups can be incorporated into metal-organic frameworks. The structural characterization of MOF-1040, based on X-ray structural analysis, shows that these struts form three-fold interpenetrated pcu networks. While both the oxidation and demetalation experiments have been demonstrated to occur without disrupting the crystallinity of the frameworks, the fact that at least some of the copper ions can be oxidized to their dicationic states indicates the presence of electronic switches which are presumably accompanied by geometrical changes involving shrinking and flattening in the coordination sphere of the copper ions in question. These observations allow us to contemplate incorporating molecular switches into the metal-organic frameworks, thus uniting the concepts of mechanostereochemistry,[16] chemical topology[17] and reticular chemistry[18] to create robust, yet dynamic systems.[1d] They mark a significant step towards transferring the solution-state chemistry of mechanically interlocked molecules into the solid-state in the form of a porous extended structure.
Experimental Section
[1·Cu]·PF6: Cu(CH3CN)4PF6 (37 mg, 0.1 mmol) was dissolved in anhydrous CH3CN (5 mL) under a N2 atmosphere and added, using a cannula, to a solution of 3 (58 mg, 0.1 mmol) in CH2Cl2 (2.5 mL), affording a dark orange solution, which was stirred at room temperature for 10 min. This solution, when added to a solution of 2 (50 mg, 0.1 mmol) in CH2Cl2 (5 mL) containing Et3N (0.3 mmol, 45 µL), formed a dark red solution, which was stirred for 12 h. It was then concentrated under vacuum, before being dissolved in the minimum amount of CH3CN (3 mL) and added to H2O (200 mL) at pH 3.5. Counterion exchange with a saturated aqueous NH4PF6 solution afforded analytically pure [1·Cu]·PF6 as a red solid (106 mg, 87 %). FT-IR (KBr, 3500-400 cm−1): 2920(w), 2870(w), 1715(s), 1605(s), 1481(m), 1390(br), 1250(s), 1173(m), 1111(m), 941(w), 840(s), 648(w). 1H NMR (500 MHz, CD3CN, 298 K): δ = 2.13 (s, 6H, Me), 3.38–3.69 (m, 20H), 6.05 (d, J = 8.5 Hz, 4H), 7.55 (d, J = 8.5 Hz, 4H), 7.60 (d, J = 8.0 Hz, 4H), 8.17 (d, J = 8 Hz, 4H), 8.20–8.23 (m, 6H), 8.51 (s, 2H), 8.78 (d, J = 8.5 Hz, 2H). 13C NMR (125 MHz, CD3CN, 298 K): δ = 24.1, 66.5, 67.9, 69.9, 70.0, 70.3, 112.6, 123.9, 126.0, 126.5, 127.3, 127.7, 129.1, 129.2, 129.5, 133.1, 136.9, 137.0, 137.2, 141.0, 143.2, 143.3, 154.0, 157.4, 158.7, 166.1. ESI-HRMS m/z calcd for C62H54CuN4O10: 1077.3136 ([M – PF6]+); found: 1077.3171 ([M – PF6]+). Single Crystals of the copper(I)-complexed pseudorotaxanate were grown by diffusing Et2O in to a solution of [1·Cu]·PF6 in CH2Cl2 at room temperature.
MOF-1040: Strut [1·Cu]·PF6 (6.50 mg, 5.22 × 10−6 mol) and Zn(NO3)2·6H2O (10.00 mg, 3.36 × 10−5 mol) were dissolved in DEF (1.0 mL) in a sealed tube which was placed in an isothermal oven at 100 °C for 48 h, after which time it was removed from the oven and allowed to cool to room temperature. Red cubic crystals were collected and rinsed with fresh DEF (3 × 2 mL). Elemental analysis: calc: C 56.15%, N 3.97%, H 5.71%, [Zn]/[Cu] = 1.33,; found: C 56.36%, N 3.88%, H 5.70 %, [Zn]/[Cu] = 1.37. FT-IR (KBr, 3500-400 cm−1): 2916(w), 2870(w), 1615(s), 1550(s), 1388(br), 1250(s), 1179(m), 1110(m), 946(w), 843(s), 671(w).
Footnotes
This work was supported by both the U.S. Department of Defense (DTRA: HDTRA1-08-10023) at UCLA, the U.S. Department of Defence under award number W911NF-07-R-001-04 at Northwestern University and the Microelectronics Advanced Research Corporation (MARCO) and its Center on Functional Engineered Nano Architectonics (FENA). The research reported herein is also supported under the auspices of an international collaboration supported in the US by the National Science Foundation under grant CHE-0924620 and in the UK by the Engineering and Physical Sciences Research Council under grant EP/H003517/1. J.F.S. and O.M.Y. were supported by the WCU Program (NRF R-31-2008-000-10055-0) funded by the Ministry of Education, Science and Technology, Korea. We gratefully acknowledge Drs. Siddhartha Das, Robert E. Taylor, Armando Durazo (Department of Chemistry and Biochemistry at University of California, Los Angeles) and Dr Duilio Cascio (UCLA–Department of Energy (DOE) Institute of Genomics and Proteomics) for experimental assistance and invaluable discussions. The authors thank M. Capel, K. Rajashankar, F. Murphy, J. Schuermann, and I. Kourinov at NE-CAT beamline 24-ID-C at APS, which is supported by National Institutes of Health Grant RR-15301 (NCRR). G.B. would like to thank the EFRC for a NERC Fellowship and the International Center for Diffraction Data for the award of a 2011 Ludo Frevel Crystallography Scholarship.
Contributor Information
Ali Coskun, Department of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, IL 60208 (USA); NanoCentury KAIST Institute and Graduate School of EEWS (WCU), Korea Advanced Institute of Science and Technology (KAIST), 373-1 Guseong Dong, Yuseong Gu, Daejeon 305-701 (Republic of Korea).
Mohamad Hmadeh, Department of Chemistry and Biochemistry, University of California, Los Angeles, 607 Charles E. Young Drive East, Los Angeles, CA 90095 (USA).
Gokhan Barin, Department of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, IL 60208 (USA).
Felipe Gándara, Department of Chemistry and Biochemistry, University of California, Los Angeles, 607 Charles E. Young Drive East, Los Angeles, CA 90095 (USA).
Qiaowei Li, Department of Chemistry and Biochemistry, University of California, Los Angeles, 607 Charles E. Young Drive East, Los Angeles, CA 90095 (USA).
Eunwoo Choi, Department of Chemistry and Biochemistry, University of California, Los Angeles, 607 Charles E. Young Drive East, Los Angeles, CA 90095 (USA).
Nathan L. Strutt, Department of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, IL 60208 (USA)
David B. Cordes, School of Chemistry and Centre for Bimolecular Sciences, University of St. Andrews, North Haugh, St. Andrews, KY16 9ST (UK)
Alexandra M. Z. Slawin, School of Chemistry and Centre for Bimolecular Sciences, University of St. Andrews, North Haugh, St. Andrews, KY16 9ST (UK), Fax: (+44) 01334-467280, amzs@st-andrews.ac.uk
J. Fraser Stoddart, Department of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, IL 60208 (USA); NanoCentury KAIST Institute and Graduate School of EEWS (WCU), Korea Advanced Institute of Science and Technology (KAIST), 373-1 Guseong Dong, Yuseong Gu, Daejeon 305-701 (Republic of Korea).
Jean-Pierre Sauvage, Department of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, IL 60208 (USA); Institut de Science et d'Ingénierie Supramoléculaires, Université de Strasbourg, 8 Allée Gaspard Monge, 67000 Strasbourg (France).
Omar M. Yaghi, Department of Chemistry and Biochemistry, University of California, Los Angeles, 607 Charles E. Young Drive East, Los Angeles, CA 90095 (USA); NanoCentury KAIST Institute and Graduate School of EEWS (WCU), Korea Advanced Institute of Science and Technology (KAIST), 373-1 Guseong Dong, Yuseong Gu, Daejeon 305-701 (Republic of Korea).
References
- 1.a) Kim K. Chem. Soc. Rev. 2002;31:96–107. doi: 10.1039/a900939f. [DOI] [PubMed] [Google Scholar]; b) Loeb SJ. Chem. Commun. 2005:1511–1518. doi: 10.1039/b416609d. [DOI] [PubMed] [Google Scholar]; c) Loeb SJ. Chem. Soc. Rev. 2007;36:226–235. doi: 10.1039/b605172n. [DOI] [PubMed] [Google Scholar]; d) Deng HX, Olson MA, Stoddart JF, Yaghi OM. Nature Chem. 2010;2:439–443. doi: 10.1038/nchem.654. [DOI] [PubMed] [Google Scholar]
- 2.Li QW, Zhang WY, Miljanić OŠ, Sue CH, Zhao YL, Liu LH, Knobler CB, Stoddart JF, Yaghi OM. Science. 2009;325:855–859. doi: 10.1126/science.1175441. [DOI] [PubMed] [Google Scholar]
- 3.a) Zhao YL, Liu LH, Zhang WY, Sue CH, Li QW, Miljanić OŠ, Yaghi OM, Stoddart JF. Chem. Eur. J. 2009;15:13356–13380. doi: 10.1002/chem.200902350. [DOI] [PubMed] [Google Scholar]; b) Li Q, Sue C-H, Basu S, Shveyd AK, Zhang W, Barin G, Fang L, Sarjeant A, Stoddart JF, Yaghi OM. Angew. Chem. 2010;122:6903–6907. doi: 10.1002/anie.201003221. Angew. Chem. Int. Ed.2010, 49, 6751–6755; [DOI] [PubMed] [Google Scholar]; c) Li QW, Zhang WY, Miljanić OŠ, Knobler CB, Stoddart JF, Yaghi OM. Chem. Commun. 2010;46:380–382. doi: 10.1039/b919923c. [DOI] [PubMed] [Google Scholar]
- 4.a) Whang D, Kim K. J. Am. Chem. Soc. 1997;119:451–452. [Google Scholar]; b) Lee E, Heo J, Kim K. Angew. Chem. 2000;112:2811–2813. Angew. Chem. Int. Ed.2000, 39, 2699–2701; [Google Scholar]; c) Lee E, Kim J, Heo J, Whang D, Kim K. Angew. Chem. 2001;113:413–416. Angew. Chem. Int. Ed.2001, 40, 399–402; [Google Scholar]; d) Davidson GJE, Loeb SJ. Angew. Chem. 2003;115:78–81. Angew. Chem. Int. Ed.2003, 42, 74–77; [Google Scholar]; e) Hoffart DJ, Loeb SJ. Angew. Chem. 2005;117:923–926. doi: 10.1002/anie.200461707. Angew. Chem. Int. Ed.2005, 44, 901–904; [DOI] [PubMed] [Google Scholar]; f) Vukotic VN, Loeb SJ. Chem. Eur. J. 2010;16:13630–13637. doi: 10.1002/chem.201002542. [DOI] [PubMed] [Google Scholar]; g) Gong H-Y, Rambo BM, Cho W, Lynch VM, Oh M, Sessler JL. Chem. Commun. 2011;47:5973–5975. doi: 10.1039/c1cc10272a. [DOI] [PubMed] [Google Scholar]
- 5.a) Dietrich-Buchecker CO, Sauvage J-P, Kintzinger JP. Tetrahedron Lett. 1983;24:5095–5098. [Google Scholar]; b) Chambron JC, Dietrich-Buchecker CO, Nierengarten JF, Sauvage J-P. J. Chem. Soc., Chem. Commun. 1993:801–804. [Google Scholar]; c) Coronado E, Forment-Aliaga A, Gaviña P, Romero FM. Inorg. Chem. 2003;42:6959–6961. doi: 10.1021/ic034797h. [DOI] [PubMed] [Google Scholar]; d) Baranoff ED, Voignier J, Yasuda T, Heitz V, Sauvage J-P, Kato T. Angew. Chem. 2007;119:4764–4767. doi: 10.1002/anie.200700308. Angew. Chem. Int. Ed.2007, 46, 4680–4683; [DOI] [PubMed] [Google Scholar]; e) Champin B, Mobian P, Sauvage J-P. Chem. Soc. Rev. 2007;36:358–366. doi: 10.1039/b604484k. [DOI] [PubMed] [Google Scholar]; f) Kang SS, Berkshire BM, Xue Z, Gupta M, Layode C, May PA, Mayer MF. J. Am. Chem. Soc. 2008;130:15246–15247. doi: 10.1021/ja806122r. [DOI] [PubMed] [Google Scholar]; g) Yang H, McLaughlin CK, Aldaye FA, Hamblin GD, Rys AZ, Rouiller I, Sleiman HF. Nature Chem. 2009;1:390–396. doi: 10.1038/nchem.290. [DOI] [PubMed] [Google Scholar]
- 6.Blanco MJ, Chambron JC, Heitz V, Sauvage J-P. Org. Lett. 2000;2:3051–3054. doi: 10.1021/ol006137b. [DOI] [PubMed] [Google Scholar]
- 7.Dietrich-Buchecker CO, Sauvage J-P. Tetrahedron Lett. 1983;24:5091–5094. [Google Scholar]
- 8.Single crystal data for [1·Cu]·PF6: C187H164Cl2Cu3F18N12O30P3, red crystals, Mr = 3755.73, crystal size 0.15 × 0.15 × 0.10 mm, triclinic, space group P1̅, a = 20.5328(8) Å, b = 22.1408(8) Å, c = 24.8729(9) Å, α = 91.721(2), β = 101.310(2), γ = 117.2740(10)°, V = 9761.5(6) Å3, Z = 2, ρcalcd = 1.278 g cm−3, T = 173(2) K, R1 (F2 > 2σF2) = 0.1532, wR2 = 0.4433. CCDC 852836 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 9.Dietrich-Buchecker CO, Sauvage J-P, Kern J-M. J. Am. Chem. Soc. 1984;106:3043–3045. [Google Scholar]
- 10.Single crystal data for MOF-1040 after SQUEEZE: C654Cu12N48O126Zn16, Mr = 12351.42, crystal size 0.2 × 0.2 × 0.2 mm, monoclinic, space group P21/c, a = 19.650, b = 37.980, c = 28.820 Å, β = 94.57°, V = 21440.2 Å3, Dcalcd = 0.957 g cm−3, T = 100 K, λ = 0.92017 Å, Z = 1, R1-[I > 2σ(I)] = 0.1930, wR2 = 0.4418, GOF = 1.818. CCDC 846359 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 11.O'Keeffe M, Peskov MA, Ramsden SJ, Yaghi OM. Acc. Chem. Res. 2008;41:1782–1789. doi: 10.1021/ar800124u. [DOI] [PubMed] [Google Scholar]
- 12.Generally speaking, copper(II) complexes are strongly distorted in a four-coordinate tetrahedral envinronment in contrast with their copper(I) analogues. In addition, the Cu(II)−N bonds in 1,10-phenanthroline (and 2,2′-bipyridine) complexes are significantly shorther than the same bonds in the corresponding monovalent complexes (G. Murphy, C. O'Sullivan, B. Murphy and B. Hathaway, Inorg. Chem., 1998, 37, 240–248). As a consequence, oxidation of copper(I) to copper(II) is very likely to trigger a shrinking and flattening of the entire metal coordination sphere.
- 13.a) Baumann F, Livoreil A, Kaim W, Sauvage J-P. Chem. Commun. 1997:35–36. [Google Scholar]; b) Livoreil A, Sauvage JP, Armaroli N, Balzani V, Flamigni L, Ventura B. J. Am. Chem. Soc. 1997;119:12114–12124. doi: 10.1021/ja9720826. [DOI] [PubMed] [Google Scholar]
- 14.a) Dietrich-Buchecker CO, Sauvage J-P. Chem. Rev. 1987;87:795–810. [Google Scholar]; b) Chambron JC, Heitz V, Sauvage J-P. J. Am. Chem. Soc. 1993;115:12378–12384. [Google Scholar]; c) Armaroli N, Balzani V, Collin J-P, Gavina P, Sauvage J-P, Ventura B. J. Am. Chem. Soc. 1999;121:4397–4408. [Google Scholar]
- 15.All our attempts to date to increase the degree of demetalation from MOF-1040 have been unsuccessful. Prolonged demetalation reaction times and high-temperatures result in the partial degradation of the framework. We hypothesize, however, that the three-fold interpenetrated structure of the framework provides additional stabilization to the copper complexes and renders them inaccessible. We believe that decreasing the interpenetration of framework could increase the demetalation ratio.
- 16.Olson MA, Botros YY, Stoddart JF. Pure Appl. Chem. 2010;82:1569–1574. [Google Scholar]
- 17.Forgan RS, Sauvage J-P, Stoddart JF. Chem. Rev. 2011;111:5434–5464. doi: 10.1021/cr200034u. [DOI] [PubMed] [Google Scholar]
- 18.Ockwig NW, Delgado-Friedrichs O, O'Keeffe M, Yaghi OM. Acc. Chem. Res. 2005;38:176–182. doi: 10.1021/ar020022l. [DOI] [PubMed] [Google Scholar]