Abstract
The controlled patterning of nanomaterials presents a major challenge to the field of nanolithography because of differences in size, shape and solubility of these materials. Matrix-assisted dip-pen nanolithography and polymer pen lithography provide a solution to this problem by utilizing a polymeric matrix that encapsulates the nanomaterials and delivers them to surfaces with precise control of feature size.
Keywords: Dip-Pen Nanolithography, Polymer Pen Lithography, Nanomaterials, Poly(ethylene glycol)
The controlled patterning of nanomaterials (NMs), with differing size, shape, solubility, and diffusion constant, is a major challenge that has hindered the development of NM-containing devices [1, 2]. A wide variety of techniques including electron-beam lithography [3,4], nanoimprint [5], and scanning probe lithography [6,7] for positioning NMs with features on the 100 nm length scale are continuously being developed. However, these techniques rely on the surface modification [3,4,6,7] and involve the destructive delivery of energy to a surface in the form of electron [3,4], thermal [5], mechanical [6], and electrical energy[7]. These indirect approaches have a fundamental limitation with respect to the types and number of materials and surfaces that can be used with them. Scanning probe molecular printing methods [8] – specifically dip-pen nanolithography (DPN) [9–11] and polymer pen lithography (PPL) [12] – are promising techniques for patterning NMs because of their high resolution, compatibility with soft and hard matter, and near perfect pattern registration capabilities. NMs present a major set of obstacles for these techniques. Such structures, because of their large size and masses, compared to molecular systems, often do not transport extremely well from tip to surface [13,14]. Indeed, the use of NM inks with these techniques has experienced very modest success [15–17]. Herein we report a novel matrix-assisted approach for patterning NMs, which relies on the use of poly(ethylene glycol) (PEG) as a matrix to facilitate the transport of NMs with different sizes and solubilities to an underlying substrate. Specifically, nano and microscale features of Au nanoparticles (AuNPs), Fe3O4 magnetic nanoparticles (MNPs) and C60 have been generated on metal, semiconducting and insulating surfaces in the context of matrix assisted (MA) DPN and PPL experiments. Under similar deposition conditions, MA-DPN and MA-PPL, can produce nearly identical features of different NMs, and the utility of this approach is demonstrated by depositing C60 between electrodes to create a photoresponsive transistor. The controlled deposition of NMs is an important prerequisite for combinatorial library screening, probing NM structure-activity relationships, and forming functional hybrid NM-electronic devices.
Previous attempts were made to pattern AuNPs [15, 16] and Fe3O4 MNPs [17] on a variety of surfaces by conventional DPN methods, but the strong dependence of the techniques on substrate-surface interactions, tip inking, and transport through the meniscus resulted in inhomogenous features. Silver colloids were deposited by DPN in an unsuccessful attempt to make conductive traces with poor control over transport rate and feature size [18]. To circumvent solubility barriers to NM deposition, the Liu group prepared insoluble semiconductor materials on a surface by inking the tip with water-soluble precursor salts [19]. However, this route resulted in irregular feature sizes and could not be used for reliable patterning because formation of these semiconductor crystals relied on poorly understood crystal nucleation and growth. Template-directed DPN patterning of NMs, in which specific interaction between the NMs and the surface are utilized to form kinetic or thermodynamic structures, has been used to organize single virus particles [20], CNTs [21], Fe@C nanoparticles [22], and phase segregating polymers [23]. However, templating is inherently indirect and not ideal because either the surface, the NMs, or both must be modified prior to assembly. Patterns of different phospholipids have been formed by co-depositing the lipids during a DPN experiment within a carrier lipid present in excess, thereby serving as a carrier matrix; however lipids are not ideal as universal carriers for NMs because they do not deposit on hydrophilic and hydrophobic surfaces in the same fashion and because lipid patterns spread upon exposure to ambient moisture [23]. In this report, the patterning of NMs encapsulated within a polymer matrix followed by deposition of the polymer-NM composite ink in the context of DPN or PPL experiments results in uniform patterns of NMs with sub-micron feature sizes. Importantly, the rate of polymer transport as opposed to NM transport controls feature size during deposition, and the same experimental conditions result in nearly identical features for different materials, thereby minimizing optimization and inking protocols for each new NM deposited by this versatile strategy (Figure 1a). The polymer PEG was chosen as the matrix for these investigations because of its (1) solubility in a broad range of solvents, (2) chemical inertness, (3) facile transport by DPN [24, 25], and (4) supramolecular chemistry, which results in the encapsulation of the NMs within the PEG.
Figure 1.
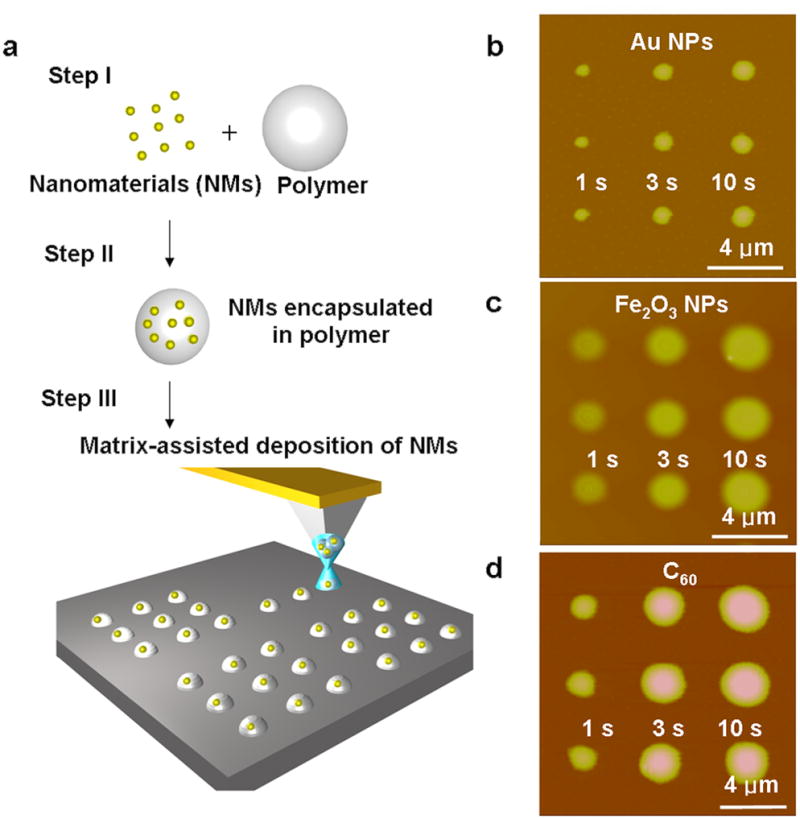
Illustration of matrix-assisted dip-pen nanolithography (MA-DPN) and arrays generated by MA-DPN. a, Scheme illustrating the process involved in patterning surfaces by MA-DPN. b, AFM topographical image of a pattern of three dots of PEG-AuNPs of different sizes on an Au surface created by intentionally varying the dwell time (1, 3 and 10 s) of the tip on the surface. c, AFM topographical image of a pattern of dot arrays of PEG-Fe3O4 MNP dots of different sizes on a Si/SiOx surface created by intentionally varying the dwell time (1, 3 and 10 s) of the tip on the surface. d, AFM topographical image of a pattern of dot arrays of PEG-C60 of different sizes on a Si/SiOx surface created by intentionally varying the dwell time (1, 3 and 10 s) of the tip on the surface.
The production of similar features of different NMs with varying solubilities and sizes by MA-DPN was demonstrated by the patterning of citrate-capped AuNPs, Fe3O4 MNPs and C60, which are soluble in water, ethanol and o-dichlorobenzene, respectively, and range in size from ~1 to 13 nm. The three NMs selected for this study are a representative sampling of the range of solubilities and sizes found in common NMs. To prepare the PEG-AuNP ink, PEG (2,000 g/mol, 5 mg/mL) was dissolved in an aqueous solution containing AuNPs (13 nm, 10 mg/mL). 12-pen tip arrays (NanoInk, Inc., USA) were dipped into the ink solution and dried under an N2 stream. To produce patterns by MA-DPN, the inked tips were brought into contact with an hexamethyldisilazane (HMDS) coated Si/SiOx surface, and the dwell times were intentionally varied (1, 3, 10 s) using an NScriptor (NanoInk, Inc., USA) at a humidity of 90%. The resulting increase in feature diameter with increasing dwell time (650, 900 and 1050 nm), an important characteristic of DPN [9], is maintained in MA-DPN (Figure 1b). By patterning the PEG-AuNP ink on a TEM grid and imaging by TEM, the presence of the AuNPs within these features was confirmed (Figure S1). To pattern Fe3O4 MNPs, tips were immersed in a 1:1 water/ethanol solution containing PEG (2,000 g/mol, 5 mg/mL) and the MNPs (10 nm, 10 mg/mL), and dried under a N2 stream. In a DPN experiment on an HMDS coated Si/SiOx surface, dots of different sizes were produced (1640, 1920 and 2250 nm) (Figure 1c) by varying dwell time (1, 3 and 10 s), and the presence of the Fe3O4 MNPs was confirmed by magnetic force microscopy (Figure S2). Finally, by immersing the tips in an o- dichlorobenzene solution containing PEG (2,000 g/mol) and C60 (10 mg/mL) and drying under N2, tips were inked with the PEG-C60 mixture. Again, features of different sizes (1160, 1910 and 2230 nm) were produced by varying the dwell time between the tip and an HMDS coated Si/SiOx surface surface (1, 3, 10 s) during a DPN experiment (Figure 1d). Importantly, the deposition of Fe3O4 MNPs and C60 demonstrates that MA-DPN forms homogenous and reproducible structures of NMs that would not otherwise move through the aqueous meniscus, and the dwell time of the tip on the surface can be varied to produce the same diameter features of different NMs. This is especially important if the technique is going to be used to generate combinatorial libraries from different NMs.
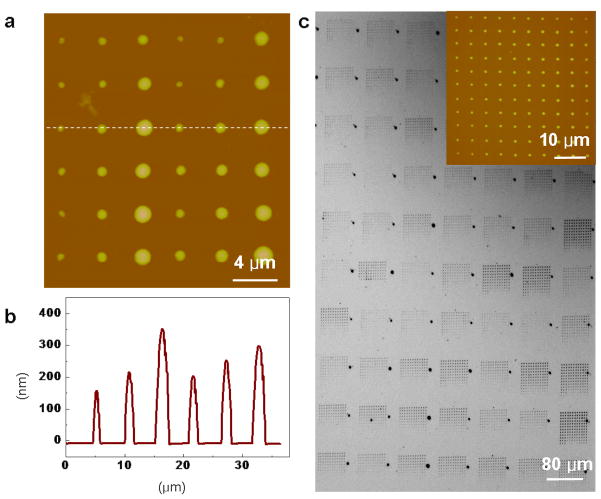
PPL is a scanning-probe molecular printing method that combines the pattern flexibility and registration afforded by DPN with the high-throughput of μCP [26], and matrix-assisted deposition of NMs was also adapted to PPL. As a proof-of-concept, a 1 cm2, 62,500 pen poly(dimethylsiloxane) (PDMS) polymer array with 80 μm separation between tips was inked with a PEG-Fe3O4 composite dissolved in 1:1 water/ethanol by spin coating (Ocean Nanotech, USA) at a rate of 2000 rpm for 3 min. Using an NScriptor platform at 90 % humidity, a 6×6 dot array of the composite was patterned on an HMDS coated Si/SiOx surface. The dwell times for the 130 nm, 200 nm and 350 nm diameter features were 1, 3 and 10 s, respectively, thereby demonstrating that feature diameter control with varying dwell time is maintained in MA-PPL (Figure 2a). In addition, height increases with dwell time as well (Figure 2b). As a demonstration of the registration, uniformity and throughput of MA-PPL, an array of approximately 9,000,000 dots (144 dots/pen) of 1 μm diameter with 5 μm spacing between dots was prepared in approximately 5 min (Figure 2c). The PEG-Fe3O4 ink was delivered onto an Au surface using a 1 cm2 polymer pen array (62,500 pens) by bringing the pen array into contact with the surface for 1 s for each dot. Feature diameter variation, which was measured by topographical AFM on a representative 144 dot sample generated by a single pen was 1.05 μm ± 4 %. Removal of the polymer matrix by exposure to oxygen plasma followed by washing with 1:1 water/ethanol resulted in selective removal of the PEG where the ink was patterned, leaving only the Fe3O4 MNPs (Figure S3).
Figure 2.
Arrays of dots created by matrix-assisted polymer pen nanolithography (MA-PPL). a, AFM topographical image of a 6×6 dot array of PEG-Fe3O4 MNP ink on a HMDS-coated Si/SiOx surface created by intentionally varying the dwell times of the polymer tips on the surface (1, 3 and 10 s). b, Height profile of one line of PEG-Fe3O4 MNPdots demonstrating control of feature size with varying dwell times. c, An optical microscopy image of a large- scale pattern of PEG-Fe3O4 MNP dots (~1 μm width, 1 s dwell time) created on a HMDS-coated Si/SiOx surface by a 62,500 pen array. The inset is an AFM topographical image of the 12×12 dot pattern of PEG-Fe3O4 ink written by a single polymer pen.
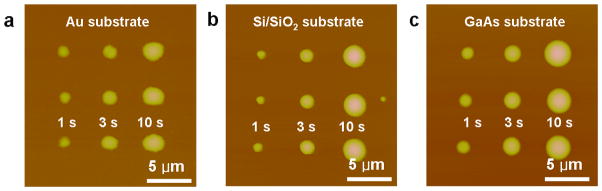
The ability to deposit NMs on different surfaces and produce similar features under nearly identical experimental conditions is an important challenge in nanolithography. As a proof-of-concept demonstration, the MA-PPL approach was utilized to deposit a PEG-Fe3O4 ink mixture onto a metallic Au surface (Figure 3a), a semiconducting GaAs surface (Figure 3b), an insulating Si/SiOx surface (Figure 3c), and an HMDS surface (Figure 2a). Dot arrays were prepared at high humidity (>90%) to create 3×3 matrices by intentionally varying dwell times (1, 3 and 10 s) of the pens on the surface (Figure 3 and Table S1). On any surface, the deviation of feature diameter at a given dwell time was less than 10% for a single pen, and the difference in feature height varied from 1–20%. However, the feature diameter for a given dwell time did vary between surfaces, for example, for a 1 s dwell time, the feature diameter varied from 0.90 μm on SiO2 to 1.20 μm on GaAs. Interestingly, while the aspect ratio of the features on different surfaces ranged from 0.086 (Au) to 0.185 (HMDS), the deviation in the aspect ratio for features deposited by MA-PPL was ≤5 % for any of the surfaces, indicating that surface polymer interactions have a significant effect on feature shape. While some variation of feature size over time is expected as a result of the loss or uptake of water by the PEG, patterns were shown to be stable for more than five days in ambient laboratory conditions (20–30% humidity).
Figure 3.
MA-PPL printed dot arrays on metallic, insulating, and semiconducting surfaces. a, A 3×3 dot array of PEG-Fe3O4 MNP ink patterned by MA-PPL on an Au substrate by intentionally varying the dwell time (1, 3 and 10s). b, A 3×3 dot array of PEG-Fe3O4 MNP ink patterned by MA-PPL on a Si/SiOx substrate by varying the dwell time (1, 3 and 10s). c, A 3a3 dot array of PEG-Fe3O4 MNP ink patterned by MA-PPL on a GaAs substrate by intentionally varying the dwell time (1, 3 and 10s).
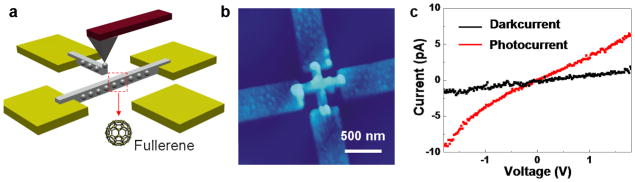
Finally, the ability to fabricate a functional nanodevice using MA-DPN was demonstrated by assembling a photoresponsive transistor device. Lines of C60-PEG ink deposited by MA-DPN bridged a 500 nm gap fabricated between 4 adjacent nanoelectrodes (Figures 4a), and sub-100 nm feature sizes have been attained using the C60-PEG composite ink (Figure S4). The AFM topographic image shows two intersecting contiguous lines across the gaps (Figure 4b). I–V measurements monitoring the output current of this device at voltages ranging from −1.8 to 1.8 V are shown in Figure 4c. The black line is the I–V response of the transistor measured in the dark, and the red trace represents the device response under white light from a 50 W tungsten-halogen lamp (USHIO, Inc., USA) (Figure 4c). The fivefold increase in current, a characteristic response of C60 devices to illumination [27, 28], shows that the devices containing the C60 within the MA-DPN generated patterns exhibit the typical and highly reproducible (6 different devices) increase in current upon exposure to light. Also, a scanning electron microscope (SEM) images of the patterns after removal of the PEG by O2 plasma shows features that are consistent with C60 thin films (Figure S5), confirming this method deposits the NM at sufficient concentration to form a contiguous channel in the device. It is worth noting that because of the precise spatial registration allowed by DPN, the C60 lines were deposited only between the electrodes.
Figure 4.
A photoresponsive transistor by MA-DPN. a, Illustration of the deposition of the C60-PEG ink between electrodes by MA-DPN. b, AFM topographical image of the photoresponsive transistor. The four electrodes are connected by two perpendicular lines of the C60-PEG ink. c, The I–V curve of the transistor in the dark (black trace) and under white light from a 50 W tungsten-halogen lamp (red trace).
In conclusion, we have developed a versatile matrix assisted direct patterning method for NMs that retains many of the advantages of DPN and PPL – namely arbitrary pattern design, sub-micron features with precise control over size, and nanometer registration between features – while overcoming the solubility and surface interactions that previously inhibited uniform pattern formation by DPN. By encapsulating the NMs within a matrix of the polymer PEG, patterns are formed on metal, semiconducting, insulating or organic surfaces in MA-DPN or MA-PPL experiments. Importantly, features are homogenous, and, while surface effects do alter the feature aspect ratios, features of the same diameter can be formed with different NMs on different surfaces. MA-DPN has been used to position fullerenes between electrodes to form a functional nanodevice, thereby demonstrating the utility of this method to overcome one of the major challenges in nanotechnology: the positioning of NMs within devices with nanoscale features. MA-DPN and MA-PPL are low-cost, high-resolution, high-throughput NM printing methods that will simplify greatly the positioning of NMs within electronic devices with broad implications in many fields.
Experimental Section
Materials
Poly(ethylene glycol) (PEG) (MW: 2,000 g/mol) and C60 were purchased from Sigma Aldrich (USA). 13 nm Au nanoparticles [29] and 5 nm Fe3O4 magnetic nanoparticles [30] were prepared in our labs following literature protocols. Si/SiOx (100) wafers with a 500 nm oxide coating layer and GaAs (100) wafers were purchased from Nova Materials Inc. (USA). Au substrates were prepared by thermal evaporation (BOC Edwards Auto306, UK) of an Au thin film (30 nm) on a Si/SiOx substrate cleaned by sonication in ethanol and pre-coated with a Ti adhesion layer (7 nm). AFM topographical and MFM images were recorded on a Veeco Bioscope equipped with a Nanoscope IIIA controller, and optical microscopy images were taken using a Zeiss Axiovert 200M (Carl Zeiss, Inc., Germany). DPN and PPL experiments were performed with a NScriptor nanolithography platform (NanoInk, Inc., USA) equipped with an environmental chamber with active humidity control driven by commercially available InkCAD DPN software (NanoInk, Inc., USA). High resolution magnetic probes for MFM (MESP-HR) were purchased from Veeco Probes (USA), and Pointprobe Plus Si SPM sensors (Nanosensors, Inc., USA) were used for topographical and non-contact imaging.
DPN Experiments
In a typical experiment, PEG (2,000 g/mol, 5 mg/mL) was dissolved in a solvent (10 mL). 50 μL of the NM solution (10 mg/mL) were added to the PEG solution and sonicated 1 min. 12 pen M-type tip arrays (NanoInk, Inc., USA) were dipped in the solution and dried under a flowing Nitrogen stream. DPN experiments were performed in an environmental chamber with active humitity control at a relative humidity ranging of 90% at a temperature of 25–29 °C. The patterns were generated using InkCAD software (NanoInk, Inc., USA) that could control dwell time and position of the tip arrays on the surface.
PPL Experiments
Polymer pen tip arrays with 90 μm spacing between tips were prepared as previously reported [12]. In a typical experiment, PEG (2,000 g/mol, 5 mg/mL) was dissolved in a solvent (10 mL). 50 μL of the NM solution (10 mg/mL) were added to the PEG solution and sonicated 1 min. The ink solution was added to the PDMS tip arrays by spin coating (1 mL, 2000 rpm, 3 min). Polymer pen lithography experiments were carried out on an NScriptor workstation as described previously [12] using InkCAD software (NanoInk, Inc., USA) with feedback turned off, and a relative humidity of <90% and temperature of 25–29 °C.
Fabrication of transistor device
A large Au electrode pad was fabricated by conventional photolithography on a Si/SiOx wafer with a 500 nm thermal oxide layer. The inner nanoelectrodes were patterned by electron beam lithography followed by thermal evaporation and resist liftoff, resulting in electrodes with a gap of 500 nm (Figure S4). The C60/PEG composite ink was deposited by MA-DPN at a scanning rate of 0.01 μm/s.
Supplementary Material
Footnotes
L. H., A. B. B. and W. S. contributed equally to this work. C.A.M. acknowledges AFOSR and DARPA-SPAWAR for support of this work. A.B.B. is grateful for an NIH Postdoctoral Fellowship.
Supporting Information is available on the WWW under http://www.small-journal.com or from the author.
Contributor Information
Prof. Ling Huang, School of Chemical and Biomedical Engineering, Nanyang Technological University, 70 Nanyang Drive, Singapore 637457 Singapore
Dr. Adam B. Braunschweig, Department of Chemistry, Department of Materials Science and Engineering, International Institute for Nanotechnology, Northwestern University, 2145 Sheridan Road, Evanston, Illinois 60208-3113 (USA), Fax: (+1) 847-467-5123
Wooyoung Shim, Department of Chemistry, Department of Materials Science and Engineering, International Institute for Nanotechnology, Northwestern University, 2145 Sheridan Road, Evanston, Illinois 60208-3113 (USA), Fax: (+1) 847-467-5123.
Dr. Lidong Qin, Department of Chemistry, Department of Materials Science and Engineering, International Institute for Nanotechnology, Northwestern University, 2145 Sheridan Road, Evanston, Illinois 60208-3113 (USA), Fax: (+1) 847-467-5123
Dr. Jong Kuk Lim, Department of Chemistry, Department of Materials Science and Engineering, International Institute for Nanotechnology, Northwestern University, 2145 Sheridan Road, Evanston, Illinois 60208-3113 (USA), Fax: (+1) 847-467-5123
Dr. Sarah J. Hurst, Department of Chemistry, Department of Materials Science and Engineering, International Institute for Nanotechnology, Northwestern University, 2145 Sheridan Road, Evanston, Illinois 60208-3113 (USA), Fax: (+1) 847-467-5123
Fengwei Huo, Department of Chemistry, Department of Materials Science and Engineering, International Institute for Nanotechnology, Northwestern University, 2145 Sheridan Road, Evanston, Illinois 60208-3113 (USA), Fax: (+1) 847-467-5123.
Dr. Can Xue, Department of Chemistry, Department of Materials Science and Engineering, International Institute for Nanotechnology, Northwestern University, 2145 Sheridan Road, Evanston, Illinois 60208-3113 (USA), Fax: (+1) 847-467-5123
Dr. Jae-Won Jang, Department of Chemistry, Department of Materials Science and Engineering, International Institute for Nanotechnology, Northwestern University, 2145 Sheridan Road, Evanston, Illinois 60208-3113 (USA), Fax: (+1) 847-467-5123
Prof. Chad A. Mirkin, Email: chadnano@northwestern.edu, Department of Chemistry, Department of Materials Science and Engineering, International Institute for Nanotechnology, Northwestern University, 2145 Sheridan Road, Evanston, Illinois 60208-3113 (USA), Fax: (+1) 847-467-5123.
References
- 1.Xia YN, Rogers JA, Paul KE, Whitesides GM. Chem Rev. 1999;99:1823. doi: 10.1021/cr980002q. [DOI] [PubMed] [Google Scholar]
- 2.Gates BD, Xu QB, Stewart M, Ryan D, Willson CG, Whitesides GM. Chem Rev. 2005;105:1171. doi: 10.1021/cr030076o. [DOI] [PubMed] [Google Scholar]
- 3.Coskun UC, et al. Appl Phys Lett. 2008;93:123101. [Google Scholar]
- 4.Mendes P, et al. Langmuir. 2004;20:3766. doi: 10.1021/la049803g. [DOI] [PubMed] [Google Scholar]
- 5.Ko S, et al. Nano Lett. 2007;7:1869. doi: 10.1021/nl070333v. [DOI] [PubMed] [Google Scholar]
- 6.Garno J, et al. Nano Lett. 2003;3:389. [Google Scholar]
- 7.Silva-Pinto E, et al. Langmuir. 2009;25:3356. doi: 10.1021/la900045f. [DOI] [PubMed] [Google Scholar]
- 8.Braunschweig AB, Huo FW, Mirkin CA. Nat Chem. 2009;1:353. doi: 10.1038/nchem.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piner RD, Zhu J, Xu F, Hong SH, Mirkin CA. Science. 1999;283:661. doi: 10.1126/science.283.5402.661. [DOI] [PubMed] [Google Scholar]
- 10.Salaita K, Wang YH, Mirkin CA. Nat Nano. 2007;2:145. doi: 10.1038/nnano.2007.39. [DOI] [PubMed] [Google Scholar]
- 11.Ginger DS, Zhang H, Mirkin CA. Angew Chem Int Ed. 2004;43:30. doi: 10.1002/anie.200300608. [DOI] [PubMed] [Google Scholar]
- 12.Huo FW, Zheng ZJ, Zheng GF, Giam LR, Zhang H, Mirkin CA. Science. 2008;321:1658. doi: 10.1126/science.1162193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang XF, Bullen DA, Zou J, Liu C, Mirkin CA. J Vac Sci Tech B. 2004;22:2563. [Google Scholar]
- 14.Bullen D, Chung SW, Wang XF, Zou J, Mirkin CA, Liu C. Appl Phys Lett. 2004;84:789. [Google Scholar]
- 15.Ben Ali M, Ondarcuhu T, Brust M, Joachim C. Langmuir. 2002;18:872. [Google Scholar]
- 16.Wang WCM, Stoltenberg RM, Liu SH, Bao ZN. ACS Nano. 2008;2:2135. doi: 10.1021/nn8005416. [DOI] [PubMed] [Google Scholar]
- 17.Gundiah G, John NS, Thomas PJ, Kulkarni GU, Rao CNR, Heun S. Appl Phys Lett. 2004;84:5341. [Google Scholar]
- 18.Wang HT, Nafday OA, Haaheim JR, Tevaarwerk E, Amro NA, Sanedrin RG, Chang CY, Ren F, Pearton SJ. Appl Phys Lett. 2008:93. [Google Scholar]
- 19.Ding L, Li Y, Chu HB, Li XM, Liu J. J Phys Chem B. 2005;109:22337. doi: 10.1021/jp053389r. [DOI] [PubMed] [Google Scholar]
- 20.Vega RA, Maspoch D, Salaita K, Mirkin CA. Angew Chem Int Ed. 2005;44:6013. doi: 10.1002/anie.200501978. [DOI] [PubMed] [Google Scholar]
- 21.Wang YH, Maspoch D, Zou SL, Schatz GC, Smalley RE, Mirkin CA. Proc Natl Acad Sci USA. 2006;103:2026. doi: 10.1073/pnas.0511022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang YH, Wei W, Maspoch D, Wu JS, Dravid VP, Mirkin CA. Nano Lett. 2008;8:3761. doi: 10.1021/nl8020768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sekula S, Fuchs J, Weg-Remers S, Nagel P, Schuppler S, Fragala J, Theilacker N, Franueb M, Wingren C, Ellmark P, Borrebaeck CAK, Mirkin CA, Fuchs H, Lenhert S. Small. 2008;4:1785. doi: 10.1002/smll.200800949. [DOI] [PubMed] [Google Scholar]
- 24.Zheng Z, Jang JW, Zheng G, Mirkin CA. Angew Chem Int Ed. 2008;47:9951. doi: 10.1002/anie.200803834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jang JW, Sanedrin RG, Senesi AJ, Zheng Z, Chen X, Hwang S, Mirkin CA. Small. doi: 10.1002/smll.200801837. [DOI] [PubMed] [Google Scholar]
- 26.Xia YN, Whitesides GM. Angew Chem Int Ed. 1998;37:551. doi: 10.1002/(SICI)1521-3773(19980316)37:5<550::AID-ANIE550>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 27.Hamed A, Sun YY, Tao YK, Meng RL, Hor PH. Phys Rev B. 1993;47:10873. doi: 10.1103/physrevb.47.10873. [DOI] [PubMed] [Google Scholar]
- 28.Park H, Park J, Lim AKL, Anderson EH, Alivisatos AP, McEuen PL. Nature. 2000;407:57. doi: 10.1038/35024031. [DOI] [PubMed] [Google Scholar]
- 29.Frens G. Nature-Phys Sci. 1973;241:20. [Google Scholar]
- 30.Stoeva SI, Huo FW, Lee JS, Mirkin CA. J Am Chem Soc. 2005;127:15362. doi: 10.1021/ja055056d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.