Summary
Tapasin is disulfide linked to ERp57 within the peptide loading complex. In cell-free assays, a soluble variant of the tapasin-ERp57 dimer recruits MHC class I molecules and promotes peptide binding to them while soluble tapasin alone does not. Here we show that within cells, tapasin conjugation with ERp57 is as critical as its integration into the membrane for efficient MHC class I assembly, surface expression, and antigen presentation to CD8-positive T cells. Elimination of both of these properties severely compromises tapasin function, in keeping with predictions from in vitro studies.
Keywords: antigen processing, quality control, peptide loading complex
Introduction
Stable peptide binding to MHC class I–β2-microglobulin (β2m) dimers is facilitated by the peptide loading complex (PLC), which is composed of the Transporter associated with Antigen Processing (TAP), tapasin, ERp57, calreticulin (CRT), MHC class I heavy chain (HC) and β2m (reviewed in [1]). Association with peptide is essential for the stability and transport of MHC class I molecules, and tapasin plays a key role in their optimal assembly and peptide loading [2, 3]. It physically links newly-synthesized MHC class I/β2m dimers to TAP, favoring their exposure to translocated peptides [4, 5], and increases TAP steady-state levels, enhancing peptide transport into the ER [2]. Within the PLC, tapasin forms a stable heterodimer with ERp57 [6, 7], which is a member of the protein disulfide isomerase family with a four domain structure (abb′a′) containing thioredoxin motifs (CXXC) in the a and a′ domains. These ensure correct disulfide bond formation specifically in glycoproteins because ERp57 interacts with the ER chaperones calnexin and CRT, which bind to monoglucosylated N-linked glycans transiently present on newly-synthesized glycoproteins [8, 9].
Tapasin and ERp57 are covalently connected by a disulfide bond between Cys 95 of tapasin and Cys 57 of ERp57, which is in the a domain active site. The recent structural characterization of the heterodimer indicates that it is stabilized by non-covalent tapasin interactions with both the a and a′ domains [10]. Mutagenesis of the active site cysteine residues and structural considerations indicate that enzymatic activity of ERp57 plays no role in facilitating peptide binding [10, 11]. The most likely function for ERp57 in the PLC is that it enhances MHC class I recruitment by providing a site of interaction for CRT associated with the MHC class I N-linked glycan [1, 10].
Certain alleles, for example HLA-B27 and B*4405, are relatively well expressed on the cell surface in the absence of tapasin. However, the peptide repertoire is affected and class I turnover at the cell surface is increased [12]. This is because tapasin not only facilitates peptide binding but also ‘edits’ the repertoire to maximize peptide binding affinity [13]. Recently these functions were analyzed in vitro, using recombinant soluble tapasin species lacking the transmembrane domain. Wearsch et al. showed that a soluble tapasin/ERp57 dimer could bind to MHC class I molecules together with CRT, promote peptide loading, and function as a peptide editor [14]. Chen and Bouvier [15] attached leucine zipper peptides to the C-termini of tapasin and MHC class I to stabilize their interaction. Forcing the MHC class I/tapasin interaction in this way facilitated peptide loading. In both cases, interactions between MHC class I and soluble ERp57-free tapasin were almost undetectable.
The in vitro analyses used soluble tapasin species, making comparison with cellular experiments difficult. Expression of soluble tapasin in .220.B8 cells, which associates with MHC class I but not with TAP, restored some surface expression of HLA-B8 [2]. Expression of tapasin mutated at Cys 95, which does not recruit ERp57 to the PLC, also allowed some MHC class I expression on the cell surface [6]. To determine whether the importance of tapasin/ERp57 heterodimer formation suggested by the in vitro studies was true in the environment of the ER, we compared the properties of MHC class I molecules assembled in the presence of soluble and membrane associated tapasin capable or incapable of recruiting ERp57.
Results and Discussion
Recruitment of PLC components by tapasin mutants lacking membrane and/or ERp57 association
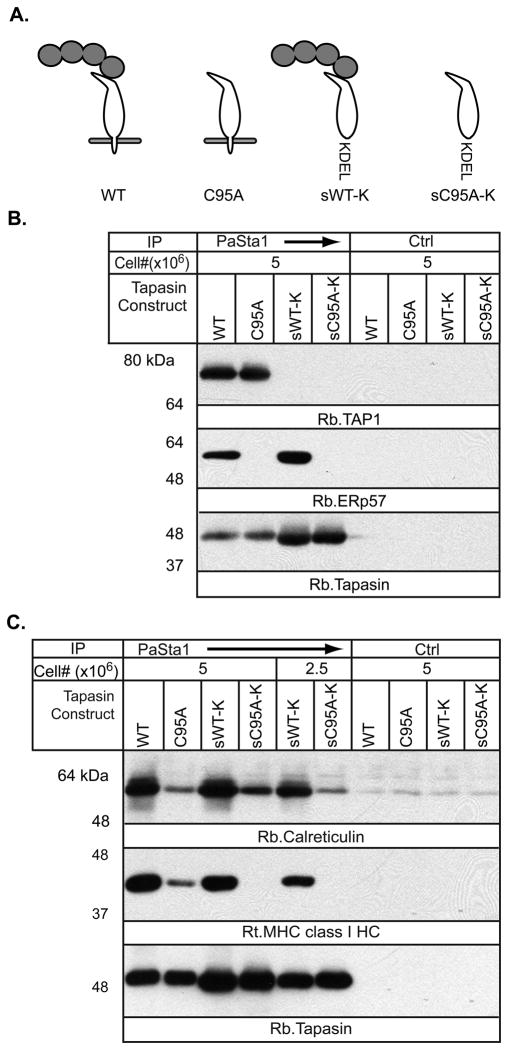
We constructed a series of tapasin variants (Figure 1A) encoding full-length tapasin (WT), C95A tapasin (cysteine to alanine substitution at position 95), or their soluble counterparts (sWT-K, sC95A-K). ER retention of the soluble constructs was assured by addition of a C-terminal KDEL motif. The constructs were stably expressed in the tapasin-negative cell line .220.B*4402 using a bicistronic EGFP-retroviral expression vector. We reasoned that the soluble conjugate used in vitro by Wearsch and Cresswell [14] is analogous to soluble, WT tapasin (sWT-K). The soluble leucine zipper-modified tapasin used by Chen and Bouvier[15] is analogous to full-length C95A tapasin (C95A), in that both provide the correct orientation relative to the MHC class I molecule, one by the leucine zipper interaction and the other by integration into the membrane, while both lack ERp57 association. The sC95A-K construct is analogous to soluble ERp57-free tapasin.
Figure 1.
A) Schematic of the tapasin constructs used (white), with or without associated endogenous ERp57 (gray). B) PLC formation is impaired in cells expressing tapasin constructs lacking ERp57 and/or membrane association. The indicated numbers of .220.B*4402 cells expressing WT, C95A, sWT-K, or sC95A-K tapasin were treated with MMTS before digitonin solubilisation and immunoprecipitation with anti-tapasin mAb PaSta-1 or the control anti-HLA-DP mAb B7/21. After SDS-PAGE and transfer, membranes were probed for TAP1, ERp57 and tapasin. Results are representative of two independent experiments. C) ERp57 association with tapasin contributes more to the recruitment of MHC class I to the PLC than membrane association. 220.B*4402 cells expressing WT, C95A, sWT-K, or sC95A-K tapasin were treated with MMTS before digitonin solubilization and immunoprecipitation with anti-tapasin mAb PaSta-1 or the control mAb B7/21. After SDS-PAGE and transfer, membranes were probed for CRT, MHC class I HC, and tapasin. The results are representative of two independent experiments.
Tapasin was immunoprecipitated from MMTS-treated digitonin lysates of cells expressing the constructs, and TAP1, ERp57, MHC class I and CRT association was evaluated by western blotting (Figure 1 B and C). The amounts of both soluble versions of tapasin were higher than the transmembrane versions, probably because of the destabilizing effect of charged residues in the tapasin transmembrane domain normally masked by TAP association [16]. As expected, the soluble species failed to bind TAP, while both C95A mutants did not bind ERp57 (Figure 1B). Confirming previous data [17], the lack of ERp57 association with C95A tapasin led to a decrease in MHC class I and CRT association. The decrease was greater than that observed by eliminating the transmembrane domain (Figure 1C). Loss of both ERp57 binding and membrane integration in the sC95A-K mutant reduced its association with MHC class I molecules to an undetectable level (Figure 1C). Surprisingly, despite the absence of detectable MHC class I binding to sC95A-K, CRT association remained similar to that of full length C95A tapasin, suggesting CRT may interact with sC95A-K tapasin directly. The single N-linked glycan of WT tapasin is not substantially mono-glucosylated [18], but without ERp57 soluble tapasin may be improperly folded and a substrate for UDP-glucose glycoprotein transferase (UGT), which glucosylates unfolded glycoproteins [19, 20]. Cys 95, which recruits ERp57 to mammalian tapasin, is not present in chicken tapasin, so significant misfolding of the full-length C95A mutant may be unlikely. Additionally, the steady state levels of the two soluble tapasin species are similar, indicating that lack of ERp57 association is not sufficiently destabilizing to cause degradation by the ER-associated degradation pathway (ERAD).
The tapasin variants and MHC class I expression
We next examined the ability of the tapasin mutants to support surface HLA-B*4402 expression (Figure 2A). C95A and sWT-K tapasin restored expression to approximately half the level obtained with WT tapasin, measured by reactivity with the class I mAb w6/32 (left panel). Maturation of HLA-B*4402 in the presence of soluble human tapasin occurs at similar rate to that of WT tapasin [13, 21], suggesting that in the presence of soluble tapasin, HLA complexes successfully exit the ER. Therefore, in our system, low level of expression of HLA-B*4402 in the presence of soluble tapasin, may result from dissociation of HLA molecules in a post-ER compartment [17] or at the cell surface [13].
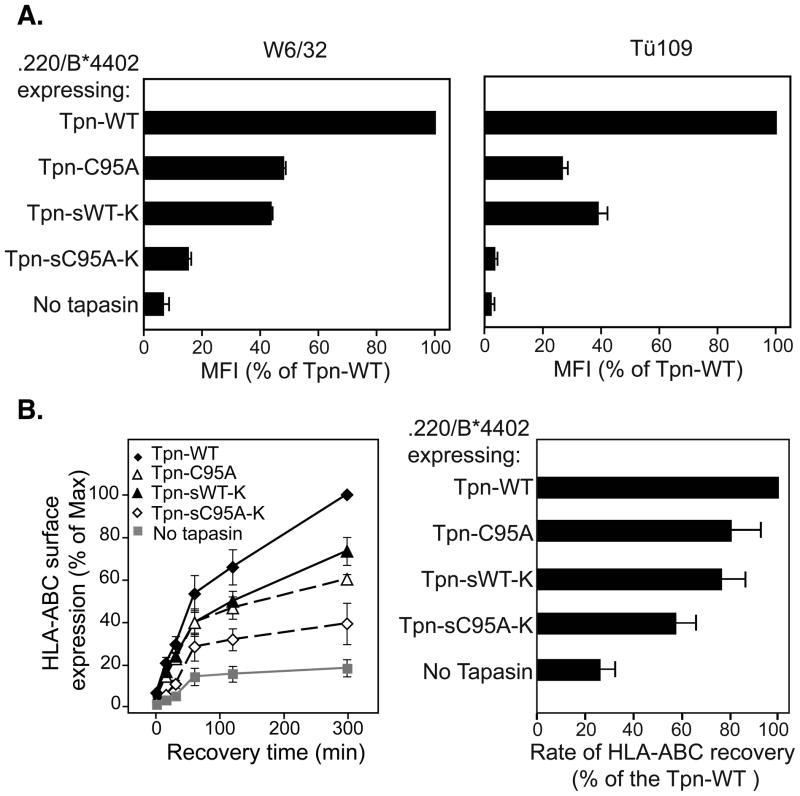
Figure 2.
A) Expression of MHC class I epitopes is synergistically affected by the lack of tapasin membrane association and ERp57 recruitment. Mean fluorescence intensities (MFI) of w6/32 (left panel) and Tü109 (right panel) are shown, and represent the mean (+/−SEM) of 2 experiments. B) The rate of surface arrival of HLA-ABC in cells lacking the conjugate or tapasin membrane association is slower. The recovery of w6/32-reactive HLA was measured after acid treatment to denature pre-existing surface HLA-peptide complexes (left panel). The rate of recovery of surface HLA-ABC (right panel) was estimated from slope of the curve fitted in the linear range of the data, and represents the mean (+/−SEM) of 4 independent experiments.
Expression of the HLA-Bw4 epitope of HLA-B*4402 assayed using the Tü109 mAb was more profoundly affected by the C95A mutation. Binding of Tü109 was previously reported to be influenced by the nature of the peptide cargo associated with HLA-B*5101 and HLA-B*4402 [22, 23]. Lower binding of Tü109 antibody to C95A expressing cells might therefore reflect the inability of the ERp57-free tapasin to effectively edit the peptide repertoire [14]. The loss of the transmembrane domain and ERp57 association (sC95A-K) dramatically reduced MHC class I expression. HLA-Bw4 mAb binding was virtually the same as in cells lacking tapasin, i.e. less than 5% of that seen with WT tapasin (Figure 2A, right panel). This is consistent with the in vitro studies of soluble tapasin and the soluble tapasin/ERp57 conjugate, where the latter was vastly superior in mediating peptide loading [14].
To assess kinetically the impact of the tapasin variants on peptide loading, cells were acid washed to denature surface MHC class I-β2m dimers and the rate of arrival of new molecules at the cell surface was monitored by flow cytometry (Figure 2B). The total MHC class I recovery, and the rate of recovery, was highest in cells expressing WT tapasin. In the absence of tapasin, the rate of recovery was almost 80% less, while in cells expressing sWT-K tapasin, it was reduced by approximately 20%. Introduction of the C95A mutation into full-length tapasin also reduced the recovery rate by 20%, while for soluble tapasin the C95A mutation decreased the recovery rate by 40%. Accumulation of HLA-B*4402 molecules at the cell surface over time appeared to be lower after loss of TAP/membrane association and/or ERp57 interaction. Expression levels are likely to reflect the assembly efficiency of HLA-B*4402 molecules in combination with their stability at the cell surface, which generally correlates with the quality of the associated peptide repertoire.
The tapasin variants and MHC class I-β2m dimer stability
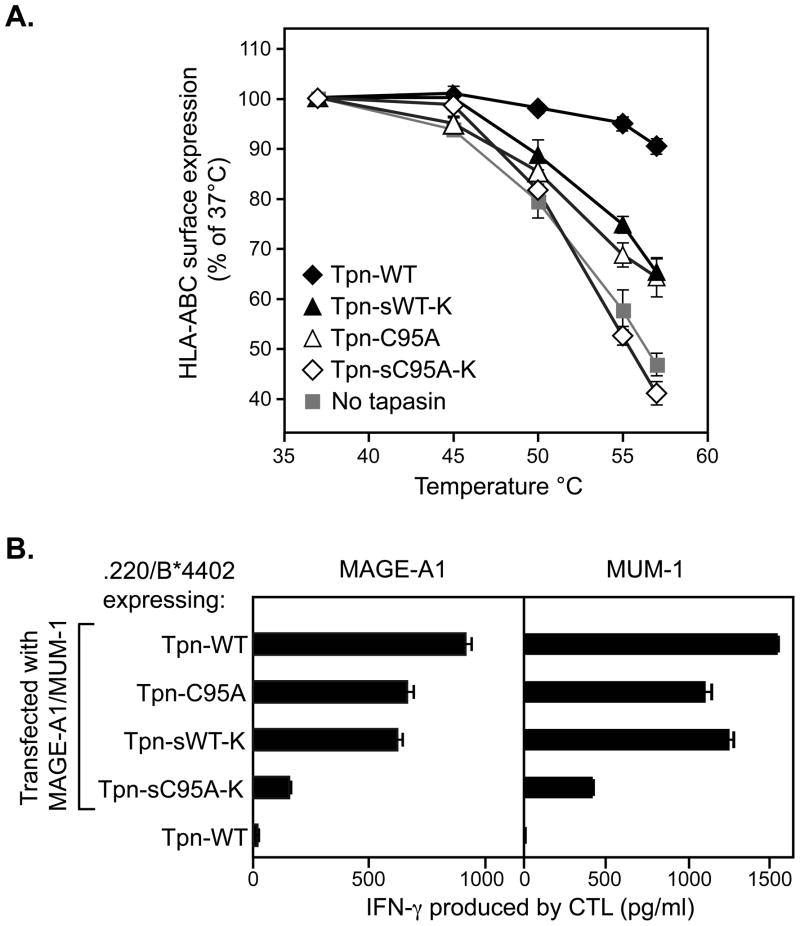
As a surrogate measure of the affinity of their peptide repertoire [6, 12, 13], we examined the thermostability of HLA molecules in .220.B*4402 cells expressing the tapasin variants (Figure 3A). After heating the cells at temperatures ranging from 37°C to 57°C, the w6/32 mAb was used to quantitate residual surface MHC class I-β2m dimers [24]. We observed that lack of tapasin membrane association (sWT-K) or of recruitment of ERp57 into the PLC (C95A) led to similar reductions in thermostability. This suggests that the quality of the peptide repertoire is affected similarly by the loss of ERp57/tapasin interaction and the lack of TAP/membrane association. A more drastic reduction in thermostability, interpreted as a severe reduction in the affinity of the peptide repertoire, was found after loss of both these interactions in cells expressing sC95A-K tapasin.
Figure 3.
The peptide repertoire is synergistically affected by the lack of tapasin membrane association and ERp57 recruitment. A) HLA-B*4402 in cells expressing tapasin lacking membrane and/or ERp57 association has decreased thermostability. After heating the cells at the indicated temperatures, surface HLA-ABC was quantitated using the mAb w6/32 on the live-gated population. The means of four experiments are shown (+/− SEM). B) Antigen presentation is impaired in cells expressing tapasin lacking membrane and/or ERp57 association. 220 cells expressing HLA-B*4402 and WT or mutant tapasins were transfected with constructs encoding MUM-1 or MAGE-A1 antigenic peptides and cultured with the CTL recognizing the corresponding HLA-B*4402/peptide complex. After 20 hrs, IFN-γ was measured in the supernatants. Mean values of four replicates are shown (+/−SEM).
Roles of ERp57 recruitment and membrane association of tapasin in CTL recognition
The effectiveness of the antigen processing machinery is ideally assessed by sensitivity to CTL. We used two HLA-B*4402-restricted T cell clones recognizing peptides derived from the proteins MUM-1 [25] and MAGE-A1 (in preparation). Cells expressing the tapasin mutants were transiently transfected with constructs encoding MUM-1 or MAGE-A1 and tested for recognition by the relevant CTLs (Figure 3B). For both CTLs, recognition was reduced by 10 to 20% in cells expressing either C95A-FL or sWT-K. However, in line with the biochemical observations, loss of ERp57 recruitment and membrane association reduced CTL activation by 70 to 80%.
Concluding Remarks
Tapasin is necessary for efficient assembly and high affinity peptide binding to MHC class I molecules. It achieves this by directly associating with MHC class I, predominantly through a likely interaction with the α2 helix [10], and by increasing TAP levels [2]. Physical coupling of tapasin and ERp57 also plays a major role in tapasin function. This may result from the interaction of ERp57 with CRT, which in turn recruits MHC class I molecules bearing monoglucosylated N-linked glycans to the PLC. However, recently it has been suggested that the ERp57-CRT interaction is not essential for MHC class I assembly [26], indicating that this issue is not entirely resolved. Nevertheless, the experiments reported here agree well with in vitro studies using recombinant proteins[14, 15]: in the environment of the ER both the ERp57 interaction as well as the correct orientation and TAP association provided by the transmembrane domain are required for optimal tapasin function, while loss of both renders tapasin ineffective in facilitating peptide loading and T cell recognition.
Material and Methods
Cell lines and antibodies
721.220 cells transfected with HLA-B*4402 [6] expressing full length or soluble WT and C95A tapasin bicistronically with EGFP were generated by retroviral spinfection [27]. Transduced cells were isolated by sorting for EGFP-positive cells. CTL clones LB33-CTL 159/5 and LB1801-CTL 461/G4.2 recognizing peptides presented by HLA-B*4402 and derived respectively from MUM-1 and MAGE-A1 tumor antigens ([25], in preparation) were maintained in Iscove Modified Dulbecco Medium (IMDM, Invitrogen, Carlsbad, CA, USA) supplemented with 10% human serum, 50U/ml rIL-2 (Chiron, Emeryville, CA, USA), 200μM 1-methyl tryptophan (Sigma, St. Louis, MO, USA), L-arginine (116 mg/ml), L-asparagine (36 mg/ml), L-glutamine (216 mg/ml), penicillin (100U/ml), and streptomycin (100μg/ml). mAbs used were: 3B10.7 (anti-MHC class I HC [6]), 148.3 (anti-TAP1[28]), w6/32(anti-HLA-A,B,C [6]), Tü109 (anti-HLA-Bw4 [23]), PaSta-1 (anti-tapasin [6]). Rabbit antisera against TAP-1 (R.RING4C), CRT (PA3-900, ABR-affinity Bioreagents), ERp57 (R.ERp57-C) and tapasin (R.gp48N, R.SinE) were used as in [11]).
Plasmids
Bicistronic plasmids encoding WT or C95A tapasin and EGFP were prepared by ligating tapasin DNA fragments into PBMN-IRES-EGFP [6]. DNA fragments encoding the tapasin C-terminus truncated at Asp-392 and supplemented with a KDEL ER retrieval motif were ligated into digested WT and C95A constructs. pDsRed-N1 was generated by replacing the EGFP coding sequence of pEGFP-N1 with the DsRed monomer coding sequence of the vector pDsRed (Clontech, Mountain View, CA, USA). DNA encoding 35-amino acids including the MUM-1 peptide recognized by LB33-CTL 159/5 was fused to the C-terminus of DsRed in pDsRed-N1 following a short linker. The MAGE-A1 cDNA encoding the peptide recognized by LB1801-CTL 461/G4.2 was cloned in pcDNA1. All constructs were confirmed by sequencing.
Immunoprecipitation and Western Blotting
Cells were incubated with 10mM methyl methioninethiosulfonate (MMTS, Pierce, Rockford, IL, USA) in PBS and extracted in 1% digitonin as described [11]. Post-nuclear supernatants were incubated for 1 hour at 4°C with the appropriate mAb coupled to Biogel A15M beads. After washing, precipitated material was eluted in 2X reducing SDS-PAGE sample buffer at 95°C for 5 min and resolved by SDS-PAGE. After transfer membranes were blocked, probed, washed and proteins detected as described [11].
Flow cytometry
Cells were incubated at 4°C with w6/32 tissue culture supernatant or a 1:100 dilution of Tü109 ascites for 30 min, washed, and stained with goat anti-mouse Ig serum coupled to allophycocyanin (APC) (Invitrogen) for 30 min. For the thermostability experiments, cells were incubated for 10 min in PBS containing 0.1% sodium azide, then heated at various temperatures for 10 min. Cells were transferred to 4°C and stained with w6/32 directly conjugated to Alexa-647 (Invitrogen). To assess recovery after acid treatment, surface HLA complexes were denatured for 90 sec in 50 mM sodium citrate buffer, pH 3.0, and neutralized using 3 volumes of 150mM Na2HPO4, pH 10.5. After washing and incubation at 37°C cells were collected and kept on ice until staining with w6/32. Data were acquired with a FACScalibur (Becton Dickinson, Franklin Lakes, NJ, USA)
T cell assays
Two × 106 721.220B*4402 cells expressing the tapasin mutants were nucleofected using Amaxa kit R, program V01 (Lonza, Walkersville, MD, USA) with 4μg of pDsRed-N1 with or without 1 μg pDsRed-MUM-1. Transfection efficiency was assessed by flow cytometry using the dsRed signal. For the MAGE-A1 CTL recognition, transfection was with 0.5 μg pcDNA1-MAGE-A1. After 4 hrs, 104 nucleofected cells were incubated with 5 × 103 CTL in 150 μl of culture medium supplemented with rIL2 25U/ml. After 20 hrs co-culture, supernatants were collected and IFN-γ was measured by ELISA (Invitrogen).
Acknowledgments
We thank Nancy Dometios for aiding in the preparation of this manuscript, and Dr. Pamela Wearsch for reagents and helpful discussions. We are grateful to Pr. Pierre Coulie for providing us with the CTL lines, Thérèse Aerts for her precious technical assistance, and Pr. Van den Eynde for a careful review of the manuscript. Support for this work was provided by the Howard Hughes Medical Institute (PC), the NIH/National Institute of General Medical Sciences, Medical Scientists Training Grant-GM07205 (DP), Marie Curie Outgoing International Fellowship (OIF) from the European Union and the Fonds National de la Recherche Scientifique (F.N.R.S), Belgium (NV).
Footnotes
Conflict of interest statement
The authors declare no financial or commercial conflict of interest
References
- 1.Peaper DR, Cresswell P. Regulation of MHC class I assembly and peptide binding. Annu Rev Cell Dev Biol. 2008;24:343–368. doi: 10.1146/annurev.cellbio.24.110707.175347. [DOI] [PubMed] [Google Scholar]
- 2.Lehner PJ, Surman MJ, Cresswell P. Soluble tapasin restores MHC class I expression and function in the tapasin-negative cell line. 220. Immunity. 1998;8:221–231. doi: 10.1016/s1074-7613(00)80474-4. [DOI] [PubMed] [Google Scholar]
- 3.Garbi N, Tan P, Diehl AD, Chambers BJ, Ljunggren HG, Momburg F, Hammerling GJ. Impaired immune responses and altered peptide repertoire in tapasin-deficient mice. Nat Immunol. 2000;1:234–238. doi: 10.1038/79775. [DOI] [PubMed] [Google Scholar]
- 4.Ortmann B, Copeman J, Lehner PJ, Sadasivan B, Herberg JA, Grandea AG, Riddell SR, Tampe R, Spies T, Trowsdale J, Cresswell P. A critical role for tapasin in the assembly and function of multimeric MHC class I-TAP complexes. Science. 1997;277:1306–1309. doi: 10.1126/science.277.5330.1306. [DOI] [PubMed] [Google Scholar]
- 5.Garbi N, Tiwari N, Momburg F, Hammerling GJ. A major role for tapasin as a stabilizer of the TAP peptide transporter and consequences for MHC class I expression. Eur J Immunol. 2003;33:264–273. doi: 10.1002/immu.200390029. [DOI] [PubMed] [Google Scholar]
- 6.Dick TP, Bangia N, Peaper DR, Cresswell P. Disulfide bond isomerization and the assembly of MHC class I-peptide complexes. Immunity. 2002;16:87–98. doi: 10.1016/s1074-7613(02)00263-7. [DOI] [PubMed] [Google Scholar]
- 7.Peaper DR, Wearsch PA, Cresswell P. Tapasin and ERp57 form a stable disulfide-linked dimer within the MHC class I peptide-loading complex. Embo J. 2005;24:3613–3623. doi: 10.1038/sj.emboj.7600814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silvennoinen L, Myllyharju J, Ruoppolo M, Orru S, Caterino M, Kivirikko KI, Koivunen P. Identification and characterization of structural domains of human ERp57: association with calreticulin requires several domains. J Biol Chem. 2004;279:13607–13615. doi: 10.1074/jbc.M313054200. [DOI] [PubMed] [Google Scholar]
- 9.Frickel EM, Riek R, Jelesarov I, Helenius A, Wuthrich K, Ellgaard L. TROSY-NMR reveals interaction between ERp57 and the tip of the calreticulin P-domain. Proc Natl Acad Sci U S A. 2002;99:1954–1959. doi: 10.1073/pnas.042699099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong G, Wearsch PA, Peaper DR, Cresswell P, Reinisch KM. Insights into MHC class I peptide loading from the structure of the tapasin-ERp57 thiol oxidoreductase heterodimer. Immunity. 2009;30:21–32. doi: 10.1016/j.immuni.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peaper DR, Cresswell P. The redox activity of ERp57 is not essential for its functions in MHC class I peptide loading. Proc Natl Acad Sci U S A. 2008;105:10477–10482. doi: 10.1073/pnas.0805044105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Purcell AW, Gorman JJ, Garcia-Peydro M, Paradela A, Burrows SR, Talbo GH, Laham N, Peh CA, Reynolds EC, Lopez De Castro JA, McCluskey J. Quantitative and qualitative influences of tapasin on the class I peptide repertoire. J Immunol. 2001;166:1016–1027. doi: 10.4049/jimmunol.166.2.1016. [DOI] [PubMed] [Google Scholar]
- 13.Williams AP, Peh CA, Purcell AW, McCluskey J, Elliott T. Optimization of the MHC class I peptide cargo is dependent on tapasin. Immunity. 2002;16:509–520. doi: 10.1016/s1074-7613(02)00304-7. [DOI] [PubMed] [Google Scholar]
- 14.Wearsch PA, Cresswell P. Selective loading of high-affinity peptides onto major histocompatibility complex class I molecules by the tapasin-ERp57 heterodimer. Nat Immunol. 2007;8:873–881. doi: 10.1038/ni1485. [DOI] [PubMed] [Google Scholar]
- 15.Chen M, Bouvier M. Analysis of interactions in a tapasin/class I complex provides a mechanism for peptide selection. Embo J. 2007;26:1681–1690. doi: 10.1038/sj.emboj.7601624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petersen JL, Hickman-Miller HD, McIlhaney MM, Vargas SE, Purcell AW, Hildebrand WH, Solheim JC. A charged amino acid residue in the transmembrane/cytoplasmic region of tapasin influences MHC class I assembly and maturation. J Immunol. 2005;174:962–969. doi: 10.4049/jimmunol.174.2.962. [DOI] [PubMed] [Google Scholar]
- 17.Garbi N, Tanaka S, Momburg F, Hammerling GJ. Impaired assembly of the major histocompatibility complex class I peptide-loading complex in mice deficient in the oxidoreductase ERp57. Nat Immunol. 2006;7:93–102. doi: 10.1038/ni1288. [DOI] [PubMed] [Google Scholar]
- 18.Radcliffe CM, Diedrich G, Harvey DJ, Dwek RA, Cresswell P, Rudd PM. Identification of specific glycoforms of major histocompatibility complex class I heavy chains suggests that class I peptide loading is an adaptation of the quality control pathway involving calreticulin and ERp57. J Biol Chem. 2002;277:46415–46423. doi: 10.1074/jbc.M202466200. [DOI] [PubMed] [Google Scholar]
- 19.Trombetta SE, Bosch M, Parodi AJ. Glucosylation of glycoproteins by mammalian, plant, fungal, and trypanosomatid protozoa microsomal membranes. Biochemistry. 1989;28:8108–8116. doi: 10.1021/bi00446a022. [DOI] [PubMed] [Google Scholar]
- 20.Sousa MC, Ferrero-Garcia MA, Parodi AJ. Recognition of the oligosaccharide and protein moieties of glycoproteins by the UDP-Glc:glycoprotein glucosyltransferase. Biochemistry. 1992;31:97–105. doi: 10.1021/bi00116a015. [DOI] [PubMed] [Google Scholar]
- 21.Zernich D, Purcell AW, Macdonald WA, Kjer-Nielsen L, Ely LK, Laham N, Crockford T, Mifsud NA, Bharadwaj M, Chang L, Tait BD, Holdsworth R, Brooks AG, Bottomley SP, Beddoe T, Peh CA, Rossjohn J, McCluskey J. Natural HLA class I polymorphism controls the pathway of antigen presentation and susceptibility to viral evasion. J Exp Med. 2004;200:13–24. doi: 10.1084/jem.20031680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takamiya Y, Sakaguchi T, Miwa K, Takiguchi M. Role of HLA-B*5101 binding nonamer peptides in formation of the HLA-Bw4 public epitope. Int Immunol. 1996;8:1027–1034. doi: 10.1093/intimm/8.7.1027. [DOI] [PubMed] [Google Scholar]
- 23.Tan P, Kropshofer H, Mandelboim O, Bulbuc N, Hammerling GJ, Momburg F. Recruitment of MHC class I molecules by tapasin into the transporter associated with antigen processing-associated complex is essential for optimal peptide loading. J Immunol. 2002;168:1950–1960. doi: 10.4049/jimmunol.168.4.1950. [DOI] [PubMed] [Google Scholar]
- 24.Leonhardt RM, Keusekotten K, Bekpen C, Knittler MR. Critical role for the tapasin-docking site of TAP2 in the functional integrity of the MHC class I-peptide-loading complex. J Immunol. 2005;175:5104–5114. doi: 10.4049/jimmunol.175.8.5104. [DOI] [PubMed] [Google Scholar]
- 25.Coulie PG, Lehmann F, Lethe B, Herman J, Lurquin C, Andrawiss M, Boon T. A mutated intron sequence codes for an antigenic peptide recognized by cytolytic T lymphocytes on a human melanoma. Proc Natl Acad Sci U S A. 1995;92:7976–7980. doi: 10.1073/pnas.92.17.7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Kozlov G, Pocanschi CL, Brockmeier U, Ireland BS, Maattanen P, Howe C, Elliott T, Gehring K, Williams DB. ERp57 does not require interactions with calnexin and calreticulin to promote assembly of class I histocompatibility molecules and it enhances peptide loading independently of its redox activity. J Biol Chem. 2009 doi: 10.1074/jbc.M808356200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lackman RL, Cresswell P. Exposure of the promonocytic cell line THP-1 to Escherichia coli induces IFN-gamma-inducible lysosomal thiol reductase expression by inflammatory cytokines. J Immunol. 2006;177:4833–4840. doi: 10.4049/jimmunol.177.7.4833. [DOI] [PubMed] [Google Scholar]
- 28.Meyer TH, van Endert PM, Uebel S, Ehring B, Tampe R. Functional expression and purification of the ABC transporter complex associated with antigen processing (TAP) in insect cells. FEBS Lett. 1994;351:443–447. doi: 10.1016/0014-5793(94)00908-2. [DOI] [PubMed] [Google Scholar]