Abstract
The availability of internet connectivity and mobile application software used by low-power handheld devices makes smart phones of unique value in time-sensitive clinical trials. Trial-specific applications can be downloaded by investigators from various mobile software distribution platforms or web applications delivered over HTTP. The Antihypertensive Treatment in Acute Cerebral Hemorrhage (ATACH) II investigators in collaboration with MentorMate released the ATACH-II Patient Recruitment mobile application available on iPhone, Android, and Blackberry in 2011. The mobile application provides tools for pre-screening, assessment of eligibility, and randomization of patients. Since the release of ATACH-II mobile application, the CLEAR-IVH (Clot Lysis Evaluating Accelerated Resolution of Intraventricular Hemorrhage) trial investigators have also adopted such a mobile application. The video-conferencing capabilities of the most recent mobile devices open up additional opportunities to involve central coordinating centers in the recruitment process in real time.
Keywords: mobile application, intracerebral hemorrhage, randomized trial, patient recruitment
Introduction
To address important gaps in current knowledge, we conducted a pilot study funded by the National Institute of Neurological Disorders and Stroke (NINDS), Antihypertensive Treatment in Acute Cerebral Hemorrhage (ATACH I) trial [1] in 2004–2008 to determine the appropriate level of systolic blood pressure (SBP) reduction in patients with intracerebral hemorrhage (ICH). We subsequently initiated a multicenter, randomized phase III trial, the ATACH-II Trial [2], to definitively determine the efficacy of early, intensive systolic blood pressure (SBP) reduction using intravenous (IV) nicardipine initiated within 4.5 h of onset of ICH and continued for the next 24 h in subjects with spontaneous supratentorial ICH. One of the issues identified in ATACH I was the difficulty in accessing documentation that was necessary to determine eligibility of acute stroke patients for the trial and initiating the recruitment through web-based systems. There is additional data to highlight the unique challenges posed by trials recruiting acute stroke patients. In one review [3], multicenter randomized controlled trials (RCTs) comparing efficacy end points between two or more treatment groups and having >5 sites enrolling patients with cerebrovascular diseases between 1980 and 2008 were identified. Of the 51 trials included in the analysis, 33 (65%) recruited acute stroke patients that required time-sensitive availability of the research team and necessary documentation within the time period for recruitment. The proportion of low-recruiting sites, defined by sites recruiting <10 patients, was significantly higher in acute trials compared with non-acute or secondary prevention trials. Trials requiring assessment of stroke scales or imaging-based ascertainment were also associated with higher number of low-recruiting sites. While such findings are related to multifactorial issues, the issue regarding provision of necessary documentation and initiation of recruitment in a user-friendly time-sensitive manner was amenable to improvement by strategies that can be broadly applied.
In 1993, the International Business Machines Corporation introduced what could be considered the smart phone, Simon. Smart phones became widely available after 2000 when Apple developed a touch screen mobile phone. These phones are capable of advanced functionality because they contain software applications that can be run directly from the phone itself. The availability of Internet connectivity and mobile application software that can be used by low-power handheld devices appeared to make smart phones of unique value in time-sensitive clinical trials. Trial-specific applications could be downloaded by investigators from various mobile software distribution platforms or web applications delivered over HTTP. The ATACH-II investigators in collaboration with MentorMate (Minneapolis, MN) released the ATACH-II Patient Recruitment mobile application available on iPhone, Android, and Blackberry in 2011. The current report summarizes the features of this mobile application because of its value for other time sensitive clinical trials. Since the release of ATACH-II mobile application, the CLEAR-IVH (Clot Lysis Evaluating Accelerated Resolution of Intraventricular Hemorrhage) trial investigators have also adopted a similar mobile application.
An agile software real-time development methodology known as Scrum was used in the creation of the ATACH-II mobile application. This practice ensured swift, deliverable results that met the needs of the investigators in a flexible timeframe. To aid in the construction of the application, multiple “sprint” sessions─or quick, pre-defined bursts of programming from which working pieces of code are produced─were applied to help the ATACH-II investigators receive exactly what they needed in an interactive application. A comprehensive backlog of priorities was regularly revised to meet the ongoing scope of the project. The agile methodology allowed for routine adaptation to the various features of the application as they were presented. After storyboarding, a hybrid mobile application was proposed as the application architecture. This approach allows application updates to be made once for all platforms in future iterations.
This application was available on iPhones, Blackberries, and Android-based smart phones. Most ATACH-II investigators, and many study coordinators, carry one of these devices with them at all times after they have installed the ATACH-II application. The server was a standard HTTP server, and the website did not require implementation of Adobe Flash or third party JavaScript components. The mobile application stores static HTML pages directly on the respective devices. The application required an active Internet connection through WIFI or 3G/4G network to download the application; but after downloading, the user was be able to access the application regardless of Internet connectivity.
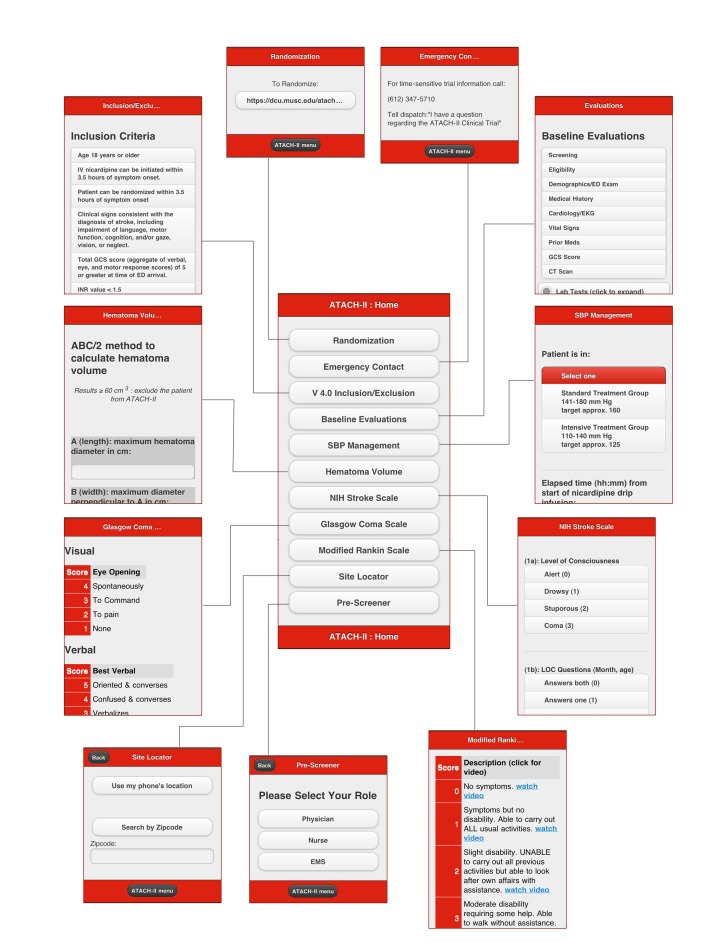
The initial version had three main parts. The first two parts provide step-by-step assessment information used to enroll a patient with ICH and providing additional information to increase the value of the application to market the assessment tools. These pages consisted of static HTML content and a combination of approximately 10–20 sections. The third main part was the inclusion of a calculator that provides users with an easy way to measure the volume of a hematoma because hematoma volume is required for assessment of eligibility. This version of ATACH-II mobile application (available for download at the Apple App Store, Google Play, and BlackBerry App World) consisted of the following features (see Figure 1):
Randomization─a link WebDCU optimized for mobile devices
Emergency Contact─24 h emergency phone number
Inclusion/Exclusion Criteria─a list of the inclusion/exclusion criteria
Baseline Evaluations─a list of the baseline evaluations including laboratory tests
SBP Management─an interactive nicardipine titration chart
Hematoma Volume Calculator─an interactive calculator
NIH Stroke Scale─displays the key elements of the National Institutes of Health Stroke Scale (NIHSS) score
Glasgow Coma Scale─displays the key elements of Glasgow Coma Scale (GCS) score
Modified Rankin Scale─displays the key elements of the modified Rankin Scale (mRS)
Figure 1.
The visual interface for various functions of the mobile application for ATACH-II.
The randomization interface requires a username (e-mail address) and password to log into the WebDCU at https://dcu.musc.edu/atach2. The webpage requires entering the site identification number, initial GCS score, and hematoma volume, and subsequently provides the assignment into standard SBP reduction or intensive SBP reduction group.
ATACH-II released an update to the existing mobile application in 2012 to accomplish the following purposes:
The ability to autonomously update clinical sites or contacts and have that information available in the mobile application. This feature allows storage of basic contact information for clinical sites and personnel. This content will be updated autonomously through a tool provided to an ATACH-II administrator.
A pre-screening tool allowing a preliminary assessment. The prescreening tool was developed for referring hospital staff, computed tomographic (CT) technicians, and nursing staff. This feature allows selection of a user type and having inclusion/exclusion criteria for each user type. Users have the ability to mark yes or no for each question, and if all questions are marked yes they will be brought to the site locator to find the nearest clinical trial center and contact information.
Site locator will allow users to search by zip code for a clinical site. This feature allows identification of nearest Joint Commission-certified stroke center and ATACH-II sites by zip codes. The feature also provides phone numbers of emergency department (ED) triage at these sites and ATACH-II 24 h contact.
Language translations for international users. This feature introduces functionality to allow viewing the mobile website in different languages. After this functionality is implemented, translated content for each language will be inserted.
In the near future, mobile applications will serve a much larger role in clinical trials, particularly those performed in the emergent setting. The next step will involve the integration of mobile devices into clinical trial management systems that will enable convenient access to clinical site status information and direct data entry into study databases. The video-conferencing capabilities of the most recent mobile devices open up additional opportunities to involve central coordinating centers in the recruitment process in real time and further develop innovative solutions to meet the logistical challenges of clinical trials.
Acknowledgments
The ATACH-II study is funded by National Institutes of Health grants U-01-NS062091 and R-01-NS061861 (medication is provided by EKR Therapeutics).
References
- 1.Antihypertensive Treatment of Acute Cerebral Hemorrhage Investigators Antihypertensive treatment of acute cerebral hemorrhage. Crit Care Med. 2010;38(2):637–48. doi: 10.1097/CCM.0b013e3181b9e1a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qureshi AI, Palesch YY. Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) II: design, methods, and rationale. Neurocrit Care. 2011;15(3):559–76. doi: 10.1007/s12028-011-9538-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qureshi AI, Tariq N, Vazquez G, Novitzke J, Suri MF, Lakshminarayan K, et al. Low patient enrollment sites in multicenter randomized clinical trials of cerebrovascular diseases: associated factors and impact on trial outcomes. J Stroke Cerebrovasc Dis. 21(2):131–42. doi: 10.1016/j.jstrokecerebrovasdis.2010.05.014. [DOI] [PubMed] [Google Scholar]