Abstract
Intracerebral hemorrhage (ICH) is a major cause of morbidity and mortality in Japan. Seventeen Japanese institutions are participating in the Antihypertensive Treatment for Acute Cerebral Hemorrhage (ATACH) II Trial (ClinicalTrials.gov no. NCT01176565; UMIN 000006526). This phase III trial is designed to determine the therapeutic benefit of early intensive systolic blood pressure (BP) lowering for acute hypertension in ICH patients. This report explains the long run-up to reach the start of patient registration in ATACH II in Japan, including our preliminary study, a nationwide survey on antihypertensive treatment for acute ICH patients, a multicenter study for hyperacute BP lowering (the SAMURAI-ICH study), revision of the official Japanese label for intravenous nicardipine, and construction of the infrastructure for the trial.
Keywords: acute stroke, antihypertensive treatment, blood pressure, clinical trial, hypertension, intracerebral hemorrhage, nicardipine
Introduction
Asian ethnic origin is an important risk factor for intracerebral hemorrhage (ICH). A recent meta-analysis reported that the incidence of ICH per 100,000 person-years was 51.8% in Asian people as compared to 24.2% in Caucasians [1]. The high prevalence of small-artery cerebrovascular lesions in Asians may be responsible for the high prevalence of ICH. In Japan, acute ICH patients account for 17–30% of overall acute stroke patients [2]. Undoubtedly, ICH is a burden for the Japanese population that needs to be overcome, though the meta-analysis also provided some good news: the median case fatality at 1 month after ICH was lower in Japan than anywhere else in the world (16.7 vs. 42.3%) [1].
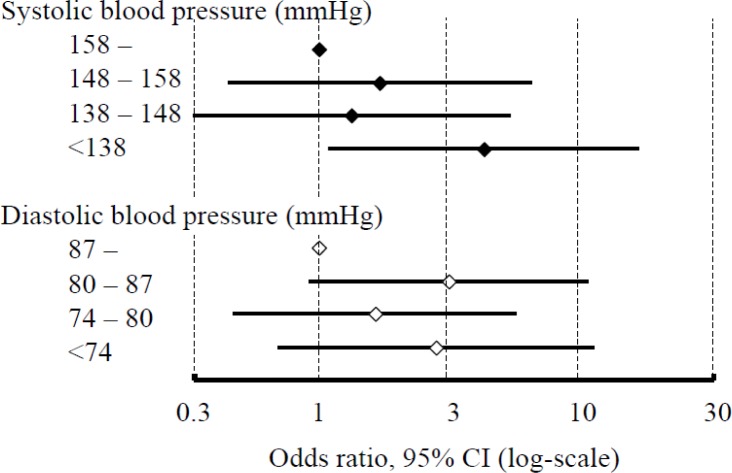
A problem in acute ICH management is the lack of a therapeutic strategy that brings dramatic symptomatic improvement like that seen with thrombolysis for ischemic stroke. Blood pressure (BP) lowering during the hyperacute stage may prevent hematoma expansion and improve outcomes after ICH. We reported an observational study involving 244 patients who were admitted to the National Cerebral and Cardiovascular Center, Osaka, or the National Hospital Organization Kyushu Medical Center, Fukuoka, within 24 h after ICH onset [3]. Lowering the systolic BP (SBP) to less than 138 mmHg during the initial 24 h immediately after identification of ICH on emergency computed tomography was predictive of independent activity corresponding to a modified Rankin Scale (mRS) score of 1 or less at 3 weeks (Figure 1). Although this result is promising, a prospective interventional trial is required to ascertain the clinical significance of the cutoff level (138 mmHg or roughly 140 mmHg) as an emergent antihypertensive goal.
Figure 1.
Average of blood pressure levels during the initial 24 h and an mRS of 1 or less at 3 weeks after intracerebral hemorrhage from a Japanese dual-center observational study.
Compared with patients with the average of systolic blood pressure at least 158 mmHg, patients with the level <138 mmHg more frequently had an mRS of 1 or less after multivariate adjustment (OR 4.36, 95% CI 1.10–17.22). The frequency did not differ among the patient quartiles based on diastolic blood pressure.
See Itabashi et al [3] for more information.
Palesch and Qureshi offered us the chance to be involved in such a trial by inviting us to join the NIH-funded trial, the Antihypertensive Treatment for Acute Cerebral Hemorrhage (ATACH) II [4], and they visited Tokyo for our first domestic meeting on this trial in October, 2008. However, to facilitate trial participation in Japan, some problems needed to be resolved, including reassessment of the official label for nicardipine, a trial drug. This is a report of the long run-up to reach the start of patient registration in ATACH II in Japan.
Nationwide survey of acute BP control in Japan
As the first step, it was necessary to ascertain the current status of antihypertensive treatment for acute ICH patients in Japan. Thus, a nationwide survey was conducted in 2008 [5]. Web questionnaires regarding acute ICH management and antihypertensive treatment strategies were sent to 1,424 hospitals, and 600 (42%) responded. Most respondents answered that the goal of lowering SBP was to reach a maximum of 140, 150, or 160 mmHg (82%). The results indicated that aggressive BP lowering was common in Japan as compared to the recommendations of domestic and Western guidelines. In addition, nicardipine was the major first choice of intravenous antihypertensive drug (57%) and the second choice (27%) in the survey. However, 26% of the respondents thought that nicardipine was inappropriate mainly due to contraindications included on the official Japanese label for this drug. According to the official label, nicardipine was contraindicated for ICH patients with a suspicion of ongoing intracranial bleeding, since the drug may enhance bleeding, and it was also contraindicated for acute stroke patients with elevated intracranial pressure, since the drug may accelerate intracranial pressure elevation.
SAMURAI-ICH study: a multicenter study of hyperacute antihypertensive therapy
The next step was to elucidate the safety and feasibility of SBP lowering to 160 mmHg or less in acute ICH using nicardipine, the standard strategy in most Japanese hospitals according to the Web survey. A prospective, multicenter study was conducted in Japan from July 2009 through July 2011 by the Stroke Acute Management with Urgent Risk-factor Assessment and Improvement (SAMURAI) study investigators; this multicenter group was funded by the Ministry of Health, Labour and Welfare (MHLW), Japan, and dealt with themes related to acute stroke management.
Most protocols for patient selection and nicardipine injection were similar to those of ATACH or ATACH II [4, 6]. Patients with supratentorial ICH within 3 h of onset, admission SBP ≥180 mmHg, Glasgow Coma Scale (GCS) score ≥5, and hematoma volume ≤60 ml were initially treated with intravenous nicardipine to maintain SBP between 120 and 160 mmHg with 24-h frequent BP monitoring. The primary endpoints were neurological deterioration within 72 h (GCS decrement ≥2 points or NIHSS increment ≥4 points) and serious adverse effects resulting in stopping intravenous nicardipine within 24 h. The secondary endpoints included hematoma expansion >33% at 24 h and an mRS score of 4 or more and death at 3 months. The endpoints were compared with predicted proportions based on the weighted average of previous studies.
A total of 211 patients were enrolled. The main results and a substudy on conjugate eye deviation in the cohort were described in our previous articles [7, 8]. Briefly, all the endpoints were close to or below the estimated level. Thus, SBP lowering to ≤160 mmHg using nicardipine appeared to be safe and feasible for Japanese ICH patients. The interim and final results of the study were presented at domestic and international conferences in 2011, including the 20th European Stroke Conference, where the necessity for the reassessment of the official label for nicardipine was stressed.
Revision of the official label for intravenous nicardipine
As far as we could determine, the contraindication of the use of nicardipine for patients with ongoing intracranial bleeding or high-intracranial pressure was suggested only by a few experimental or clinical studies reported a couple decades ago. The detailed situations related to the label were described in our previous article [5]. The nationwide survey clarified the prevalence of nicardipine administration to acute ICH patients without reports of any significant adverse events despite the contraindications. Based on the results of the survey and other considerations, a formal request for reassessment of the official label for nicardipine was submitted to the MHLW by the Japan Stroke Society, the Japan Neurosurgical Society, and the Japanese Society of Hypertension in October 2008. After several discussions, the MHLW finally ordered the pharmaceutical manufacturers of nicardipine to revise the label in June 2011, deleting the ICH-related contraindications.
Advance creation of the research network for ATACH II in Japan
After the above-mentioned steps, we formed a Japanese study group for ATACH II, which consists of 17 Japanese stroke institutes, involving the SAMURAI study investigators (Table 1). We recently introduced this network elsewhere in the Japanese language [2]. Briefly, we ensured independent relationships among clinical sites, data coordination, and financial management by managing the three parts independently by the Departments of Cerebrovascular Medicine (Toyoda) and Advanced Medical Technology Development (Yamamoto), the National Cerebral and Cardiovascular Center, and the Japan Cardiovascular Research Foundation (Yamaguchi), respectively. We registered the trial design in the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR) in Japan as trial number 000006526.
Table 1.
List of Japanese institutions participating in the ATACH-II trial.
| Institution | Department | Location | Responsible investigator |
|---|---|---|---|
| Clinical sites | |||
| National Cerebral and Cardiovascular Center | Cerebrovascular Medicine | Suita, Osaka | Kazunori Toyoda (Clinical PI) |
| Neurology | Kazuyuki Nagatsuka | ||
| Nakamura Memorial Hospital | Neurosurgery and Stroke Center | Sapporo, Hokkaido | Jyoji Nakagawara |
| Kohnan Hospital | Stroke Neurology | Sendai, Miyagi | Eisuke Furui |
| Kyorin University Hospital | Neurosurgery and Stroke Center | Mitaka, Tokyo | Yoshiaki Shiokawa |
| St. Marianna University Hospital | Neurology | Kawasaki, Kanagawa | Yasuhiro Hasegawa |
| NHO Nagoya Medical Center | Neurology | Nagoya, Aichi | Satoshi Okuda |
| Kobe City Medical Center General Hospital | Stroke Center | Kobe, Hyogo | Nobuyuki Sakai |
| Kawasaki Medical School Hospital | Stroke Medicine | Kurashiki, Okayama | Kazumi Kimura |
| NHO Kyushu Medical Center | Cerebrovascular Medicine | Fukuoka, Fukuoka | Yasushi Okada |
| Gifu University Hospital | Neurosurgery | Gifu, Gifu | Shin-Ichi Yoshimura |
| Tokyo Saiseikai Central Hospital | Neurology | Minato-ku, Tokyo | Haruhiko Hoshino |
| Toranomon Hospital | Neurology | Minato-ku, Tokyo | Yoshikazu Uesaka |
| NHO Kagoshima Medical Center | Neurology | Kagoshima, Kagoshima | Takahiro Nakashima |
| Keio University Hospital | Neurology | Shinjuku-ku, Tokyo | Yoshiaki Itoh |
| St. Marianna University Toyoko Hospital | Strokology | Kawasaki, Kanagawa | Toshihiro Ueda |
| Saiseikai Kumamoto Hospital | Neurosurgery | Kumamoto, Kumamoto | Tohru Nishi |
| Saiseikai Yokohamashi Tobu Hospital | Neurology | Yokohama, Kanagawa | Jun Gotoh |
| Data Coordination Unit (DCU) | |||
| National Cerebral and Cardiovascular Center | Advanced Medical Technology Development | Suita, Osaka | Haruko Yamamoto (DCU PI) |
| Management of subcontract | |||
| Japan Cardiovascular Research Foundation | Suita, Osaka | Takenori Yamaguchi | |
| Supervisory adviser | |||
| National Cerebral and Cardiovascular Center | Suita, Osaka | Kazuo Minematsu | |
NHO National Hospital Organization
A problem for the smooth start of this trial is the lack of experienced and well-funded support systems and human resources for investigator-initiated clinical trials in Japan [9], although they are gradually forming in other medical fields such as oncology [10]. Government-funded clinical trial support systems like those in the United States are necessary to enable us to plan and conduct clinical trials effectively and reliably, cooperating with other clinical research professionals including biostatisticians. This time, two projects are available for maintaining the trial infrastructure: a study funded by an Intramural Research Fund of the National Cerebral and Cardiovascular Center and another funded by a Health and Labor Sciences Research Grant of the MHLW. There is a substantial need to encourage Japanese stroke researchers to join in the international, investigator-initiated, multicenter trials to obtain universal clinical evidence that is also common to Japanese. We are learning much about how to support several stroke institutes in Japan academically and financially through the experience of preparing for ATACH II.
The ATACH-II trial could be the seminal research project for stroke researchers in Japan to demonstrate themselves as effective contributors to investigator-initiated, international clinical trials. The first Japanese patient was enrolled in ATACH-II on March 1, 2012.
Acknowledgments
This study is supported by the National Institutes of Health grant 1U01NS062091-01A2 and in part by an Intramural Research Fund (H23-4-3, PI: Toyoda) for Cardiovascular Diseases of National Cerebral and Cardiovascular Center and by a Grant-in-Aid (PI: Yamamoto) from the Ministry of Health, Labor and Welfare, Japan.
List of abbreviations
- ATACH
Antihypertensive Treatment for Acute Cerebral Hemorrhage
- BP
Blood pressure
- GCS
Glasgow Coma Scale
- ICH
Intracerebral hemorrhage
- MHLW
Ministry of Health, Labour and Welfare
- mRS
Modified Rankin Scale
- SAMURAI
Stroke Acute Management with Urgent Risk-factor Assessment and Improvement
- SBP
Systolic blood pressure
References
- 1.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9:167–76. doi: 10.1016/S1474-4422(09)70340-0. [DOI] [PubMed] [Google Scholar]
- 2.Sato S, Yamamoto H, Qureshi AI, et al. Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH)-II at Japan site: study design and advance construction of domestic research network. Rinsho Shinkeigaku. 2012;52 doi: 10.5692/clinicalneurol.52.642. (in press) [DOI] [PubMed] [Google Scholar]
- 3.Itabashi R, Toyoda K, Yasaka M, et al. The impact of hyperacute blood pressure lowering on the early clinical outcome following intracerebral hemorrhage. J Hypertens. 2008;26:2016–21. doi: 10.1097/HJH.0b013e32830b896d. [DOI] [PubMed] [Google Scholar]
- 4.Qureshi AI, Palesch YY. Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) II: design, methods, and rationale. Neurocrit Care. 2011;15:559–76. doi: 10.1007/s12028-011-9538-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koga M, Toyoda K, Naganuma M, et al. Nationwide survey of antihypertensive treatment for acute intracerebral hemorrhage in Japan. Hypertens Res. 2009;32:759–64. doi: 10.1038/hr.2009.93. [DOI] [PubMed] [Google Scholar]
- 6.Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) investigators Antihypertensive treatment of acute cerebral hemorrhage. Crit Care Med. 2010;38:637–48. doi: 10.1097/CCM.0b013e3181b9e1a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koga M, Toyoda K, Yamagami H, et al. Systolic blood pressure lowering to ≤160 mmHg using nicardipine in acute intracerebral hemorrhage: a prospective, multicenter, observational study (the SAMURAI-ICH study) J Hypertens. 2012 doi: 10.1097/HJH.0b013e328359311b. (in press) [DOI] [PubMed] [Google Scholar]
- 8.Sato S, Koga M, Yamagami H, et al. Conjugate eye deviation in acute intracerebral hemorrhage: SAMURAI-ICH study. Stroke. 2012 doi: 10.1161/STROKEAHA.112.666750. (in press) [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto H, Minematsu K. A system to enforce multicenter clinical trials with public funds: introduction of a neurological cooperative group in the United States. Rinsho Shinkeigaku. 2011;51:612–6. doi: 10.5692/clinicalneurol.51.612. [DOI] [PubMed] [Google Scholar]
- 10.Fukuda H. Development of cancer cooperative groups in Japan. Jpn J Clin Oncol. 2010;40:881–90. doi: 10.1093/jjco/hyq135. [DOI] [PubMed] [Google Scholar]