Abstract
Acrolein, an α,β-unsaturated aldehyde, is a ubiquitous pollutant that is also produced endogenously through lipid peroxidation. This compound is hundreds of times more reactive than other aldehydes such as 4-hydroxynonenal, is produced at much higher concentrations, and persists in solution for much longer than better known free radicals. It has been implicated in disease states known to involve chronic oxidative stress, particularly spinal cord injury and multiple sclerosis. Acrolein may overwhelm the anti-oxidative systems of any cell by depleting glutathione reserves, preventing glutathione regeneration, and inactivating protective enzymes. On the cellular level, acrolein exposure can cause membrane damage, mitochondrial dysfunction, and myelin disruption. Such pathologies can be exacerbated by increased concentrations or duration of exposure, and can occur in normal tissue incubated with injured spinal cord, showing that acrolein can act as a diffusive agent, spreading secondary injury. Several chemical species are capable of binding and inactivating acrolein. Hydralazine in particular can reduce acrolein concentrations and inhibit acrolein-mediated pathologies in vivo. Acrolein scavenging appears to be a novel effective treatment which is primed for rapid translation to the clinic.
Keywords: acrolein, spinal cord injury, multiple sclerosis, oxidative stress, secondary injury
1. Introduction
Contrary to popular belief, the most severe damage resulting from spinal cord injury (SCI) does not occur immediate following the physical wound. Rather, research has shown that mechanical trauma induces a cascade of biochemical reactions leading to a delayed secondary process that amplifies the effects of injury and spreads the damage throughout the cord. Oxidative stress, a hallmark of secondary injury, plays a critical role in mediating functional loss in SCI[1]. In fact, oxidative stress and free radical-mediated injuries have been associated with a great number of diseases, perhaps more than any other pathological factors [2]. This process has been implicated in pathologies associated with pollution, smoking, aging, trauma, and chronic neurodegenerative diseases [3]. The mechanisms of the generation and action of reactive oxygen species (ROS) and lipid peroxidation (LPO) have been an area of intense research aiming to prevent, slow down, or even reverse various disease processes. There is strong evidence to suggest that oxidative stress plays a critical role in the pathogenesis of spinal cord injury following trauma [4, 5]. In neurodegenerative diseases lacking obvious physical trauma, oxidative stress is considered one of the primary mechanisms leading to structural and functional deficits[6]. However, free radical scavengers have been largely unsuccessful at mitigating these CNS injuries and diseases. Therefore, improving understanding of the mechanisms of oxidative stress is of great importance.
Over the last decade, we and others have demonstrated that acrolein (2-propenal) plays an important role in oxidative stress and many neurological diseases and disorders [7–13]. This aldehyde is an environmental pollutant that can also be produced endogenously through lipid peroxidation [14]. Acrolein has been shown to be toxic to neural tissues [7, 8, 10, 11, 15], to catalyze production of ROS, and to possess a half-life orders of magnitude longer than conventional ROS [16]. Further, acrolein concentrations are elevated in animal models of SCI [17] and multiple sclerosis (MS) [13]. Thus, acrolein appears to be a key factor in generating oxidizing species and perpetuating oxidative stress. In this paper, we will review acrolein’s neurotoxicity and possible mechanisms of action. Further evidence will be presented to demonstrate that acrolein could be a novel therapeutic target to provide neuroprotection and enhance functional recovery. Animal experiments with these anti-acrolein therapies show them to be safe and effective new treatments that could be rapidly translated to clinical therapies for human patients.
2. The nature of acrolein
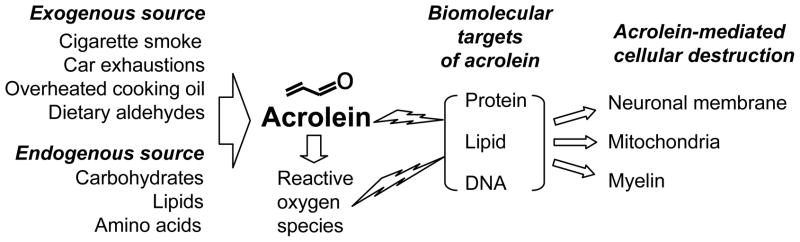
Acrolein is an α,β-unsaturated aldehyde with a variety of endogenous and exogenous sources (Fig. 1). Acrolein commonly occurs as an environmental pollutant released from numerous manufacturing processes, burning cigarettes [18, 19], exhaust from combustion engines [16, 20], and vapors of overheated cooking oil [21]. Acrolein is also generated endogenously through the oxidation of various compounds. Details of these free-radical mediated pathways that generate acrolein have been covered previously [16, 22–24], so this review will instead focus on the neural cytotoxicity of this chemical species and potential as a target for therapy.
Figure 1.
Acrolein originates from multiple exogenous and endogenous sources and is known to attack protein, lipids, and DNA. Such toxicity can disrupt neuronal membranes, mitochondria, and myelin
Acrolein is the strongest electrophile among the unsaturated aldehydes [8, 22, 25], and occurs at 40 times greater concentration than other α,β-unsaturated aldehydes such as 4-hydroxynonenal (HNE) [22]. It reacts readily with many biomolecules including proteins, DNA, and phospholipids [22, 23] (Fig. 1). Within proteins, acrolein binds to cysteine, histidine, and lysine residues [23], generating carbonyl derivatives [26] and encouraging protein oligomerization [27]. Acrolein can also react with the nucleophilic bases of DNA to form exocyclic adducts [22]. In addition, acrolein has been shown to be the most reactive product of LPO, consuming glutathione (GSH) 110–150 times faster than HNE or crotonaldehyde [16, 22, 23, 26, 28] and further stimulating the production of ROS. Therefore, as both a product of, and catalyst for lipid peroxidation [22], acrolein seems to induce a vicious cycle of oxidative stress, dramatically amplifying its effects.
Acrolein is active within biological systems much longer than the better studied ROS. For example, the half-life of acrolein is on the order of hours to days [16]. Hence, acrolein persists in the body for significantly longer than the transient ROS (λ ~= 10−12 sec) [22]. In addition, acrolein readily forms conjugates with proteins and GSH that remain highly reactive [27, 29–31] and likely have half lives much longer than free acrolein. In fact, trapping and sequestering acrolein-protein adducts prevented subsequent protein cross-linking and cytotoxicity, providing significantly more cytoprotection in cultured hepatocytes than scavenging free acrolein directly [27]. These observations demonstrate acrolein’s reactivity to crucial cell components, extended activity within cells, and persistent elevation in pathological conditions. It is therefore almost certain that acrolein plays a particularly damaging role in oxidative stress.
3. Vulnerability of the endogenous anti-oxidative system
The central nervous system has several methods of reducing oxidative stress, though these detoxification systems can be overwhelmed and are themselves vulnerable to inactivation by ROS. GSH, the cell’s primary mechanism for reducing unstable molecules binds and sequesters acrolein [3]. However, this important endogenous antioxidant may be overwhelmed as GSH becomes depleted, along with other factors known to reduce oxidative stress such as vitamin E and ascorbic acid [2]. In fact, due to acrolein’s great reactivity with thiols, GSH may itself be one of the primary targets of acrolein-mediated injury. GSH reacts with the third carbon of acrolein to produce GS-propionaldehyde [29], which is subsequently metabolized by both aldehyde dehydrogenase (ALDH) and alcohol dehydrogenase [32]. Specific forms of these enzymes are present at varying concentrations in different tissues: glial cells produce ALDH2 but not ALDH1, and neither is expressed in neurons[33]. This suggests that neurons may be particularly vulnerable to acrolein-mediated injury. Depletion of GSH may compromise reduction of hydrogen peroxide and lipid peroxides by GSH peroxidase, and these compounds may then react in the presence of iron to form free radicals that induce further LPO. Studies supporting this mechanism found GSH and N-acetylcysteine protected against acrolein-mediated injuries in vitro [34–37] and in vivo [35]. In fact, acrolein directly inactivates glutathione reductase [38], preventing GSH reduction and further impairing the cell’s capability to survive oxidative stress.
The reaction of GSH with acrolein is essential to the endogenous elimination of acrolein; however, this process depletes GSH reserves, limiting the body’s ability to handle additional oxidative stress. Additionally, this reaction produces a toxic intermediate that could further contribute to cellular injury. Furthermore, acrolein inactivates glutathione reducatase, a key antioxidative enzyme [38]. Intracellular GSH can suppress oxidative stress when acrolein concentrations are low, but can be overwhelmed by high levels of acrolein.
4. Acrolein toxicity in the CNS: in vitro and ex vivo evidence
Membrane damage
Cellular deterioration leading to functional deficits and cell death may be triggered by membrane damage [39–41]. This is one mechanism by which acrolein likely contributes to functional loss in neurological disease and injury where ROS have been implicated (Fig. 2C, D). Exposure to acrolein concentrations as low as 1 μM for 4 hours resulted in increased permeability of cell membranes to ethidium bromide (MW: 400 Da) in ex vivo spinal cord [10] (Fig. 1,2). As duration of exposure and/or acrolein concentration increased, larger molecules such as horseradish peroxidase (HRP, 44Kd) and lactate dehydrogenase (LDH, 144Kd) were able to pass through the plasmalemma [10].
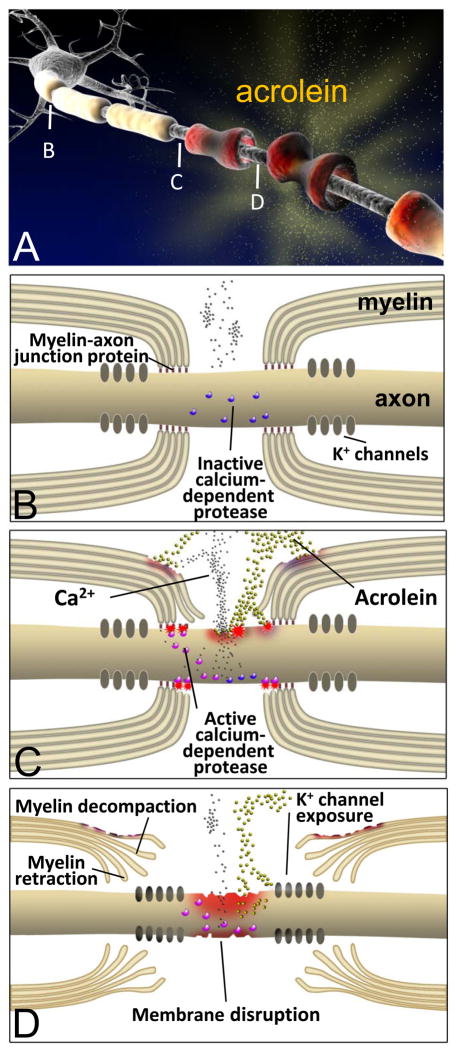
Figure 2.
Diagram (A) representing the steps in acrolein-mediated neural injury. For healthy nerves (B) proteins such as Caspr, contactin, Nfac, and spectrin comprise axoglial septate junctions that secure the myelin to the axon at the periphery of the nodes of Ranvier. These act to sequester voltage gated K+ channels to the internode region. Intact axonal membranes maintain an ionic balance with low concentrations of intracellular Ca2+, keeping intracellular proteases inactive. Exposure to acrolein results in damage to myelin, the myelin-axon junction, and the axonal membranes (B). Influx of calcium activates proteases, which may cause further damage to the paranodal axoglial junction. Ultimately, acrolein damages both axonal membranes and myelin structures (C). Myelin decompaction and retraction prevent saltatory conduction and allow K+ channels to spread into the node of Ranvier, furthering disrupting neuronal function.
There are important differences between membrane damage mediated by acrolein and that resulting from physical impact. First, the most severe plasma membrane leakage occurs immediately following gross trauma [42–45]. Membrane breaches due to primary injury in vitro reseal in a time dependent manner [42–45]. Conversely, micromolar levels of acrolein disrupt the plasma membrane in a progressive process: damage becomes evident following a delay ranging from minutes to hours [10, 11]. As acrolein accumulates and catalyzes additional oxidative stress, mounting membrane damage could eventually lead to cell lysis. The delayed and progressive nature of acrolein-mediated membrane damage demonstrates that this aldehyde may play an important role in secondary injury following mechanical trauma.
The finding that very low concentrations of acrolein can induce significant membrane damage to otherwise healthy tissue suggests that acrolein may be responsible for the delayed membrane damage to the tissue adjacent to the original injury site. This may help to explain the phenomenon of “diffuse axonal injury” first postulated by Povlishock and colleagues to characterize axonal deterioration outside of the impact zone [46–48]. We hypothesize that acrolein may act as a key factor in spreading secondary and diffuse membrane damage following physical trauma [49]. Consistent with this hypothesis, we detected a significant elevation of acrolein and concomitant membrane damage at a location more than 10 mm distant from the original compression site in a guinea pig SCI model [17, 49]. Furthermore, this membrane damage accompanied functional deficits such as the loss of compound action potential (CAP) conduction [11, 50]. Since plasmalemmal integrity is indispensible for action potential generation and conduction, it is predictable that acrolein-mediated membrane disruption is associated with functional abnormalities.
Acrolein is significantly more toxic than other aldehydes implicated in spinal cord trauma [51, 52]. Specifically, at a concentration of 200 μM, HNE did not cause significant HRP labeling when applied for 4 hours, while the same concentration of acrolein inflicted significant membrane disruption as early as 15 min. [10]. This is consistent with the notion that acrolein is 100 times more reactive than HNE and may play a more significant role in oxidative stress and cell death [22]. Further supporting the high sensitivity of membranes to acrolein, Hall and colleagues found that acrolein is significantly more toxic to mitochondria than HNE [53].
The mechanisms of acrolein-mediated membrane disruption have not been fully elucidated, though ROS likely play a role in this process. As discussed above, there is a significant increase of ROS, LPO, and protein carbonyl levels as well as depletion of endogenous GSH upon acrolein exposure [10, 15]. Acrolein-mediated membrane damage can be significantly attenuated by the application of antioxidants in vitro and ex vivo [10]. This suggests that much of acrolein-induced membrane damage is mediated by ROS. The remainder of acrolein’s detrimental effects can likely be attributed to other intermediate compounds or acrolein itself.
Mitochondrial dysfunction
Mitochondria produce energy that is critical for cell growth, function, and survival. Unfortunately, the same properties that allow them to efficiently generate ATP make mitochondria one of the primary producers of ROS and a target for pathological oxidation [54, 55]. Specifically, 1–2% of the electrons which flow into the mitochondrial respiratory chain catalyze the incomplete reduction of O2 to superoxide radical (O2•−) [56]. Generation of ROS increases significantly when the electron transport chain is compromised [54, 55].
Acrolein impairs mitochondrial respiratory function in heart, spinal cord, and brain tissues [15, 34, 53, 57]. Therefore, it is likely that acrolein exacerbates oxidative stress in part by inhibiting normal metabolism in injured neuronal tissue. Consistent with this hypothesis, we have found that acrolein stimulated significant ROS production in isolated brain mitochondria, while decreasing concentrations of antioxidants such as GSH [15]. As brain mitochondria do not express high levels of catalase [58, 59], the role of GSH in detoxifying hydrogen peroxide is critically important for this organelle and with neurons. As acrolein rapidly depletes intracellular GSH [23], increased mitochondrial oxidative stress likely plays an important roles in free radical-mediated damage in central neurons.
Deficient adenine nucleotide translocase (ANT) activity leads to inhibition of the mitochondrial electron transport function, which in turn could promote oxidative stress [60–62]. We noted that the impairment of ANT activity accompanies acrolein-induced oxidative stress and inhibition of electron transport[15]. Therefore, it is postulated that ANT inactivation is an important intermediate step contributing to overall ROS increases in acrolein toxicity. Since acrolein has been shown to have little effect on the activities of respiratory complexes I–V [34], aldehyde inhibition of ANT likely constitutes the major method of acrolein induced toxicity on the electron transport chain and therefore ROS increase in mitochondria.
Because ANT contains sulfhydryl moieties in its cysteine residues [63, 64], it is probable that acrolein directly binds and modifies ANT. Consistent with such hypothesis ANT is the single most abundant protein within the inner mitochondrial membrane [63], where acrolein likely accumulates. There have also been accumulated evidence that calcium overload and opening of a transmembrane pore in the inner mitochondrial membrane, two established mechanism through which mitochondria produce ROS[65–68], play only minor roles in acrolein-induced mitochondrial generation of ROS[15]. In addition, xanthine oxidase-dependent ROS generation contributes minimally to the overall level of oxidative stress induced by acrolein [15]. These data further highlight the importance of ANT inhibition in acrolein mediated-ROS production in mitochondria. Additionally, brain mitochondria are 10–20 times more sensitive to acrolein that those of heart muscle, suggesting that within the CNS, mitochondria may be more vulnerable to acrolein than in other organ systems [15, 34, 57, 69]
Myelin disruption
Myelin damage can lead to the loss of axonal conduction and paralysis in MS [70, 71] and SCI [72–76]. Similar histophatological changes are seen acutely when acrolein is incubated with isolated guinea pig spinal cord segments [77] (Fig. 2). Such myelin disruption can lead to failure of axonal conduction, which can be partially restored by potassium channel blockade [78–82]. In addition, it is reported that sodium channel blockade with phenytoin also protects spinal cord axons, enhances conduction, and improves functional motor recovery after contusion SCI [83]. This relationship supports our assertion that acrolein is capable of damaging both myelin and axonal membranes, and may represent an important factor in the pathogenesis of conditions involving damage to both neurons and glia (Fig. 2).
Upon acrolein exposure in ex vivo, specific structural abnormalities are found at the paranodal region which include the lengthening of the nodes of Ranvier and myelin splitting [77] (Fig. 2D). Morphometric analysis revealed that 12 hours of incubation with 200μM acrolein increased the average nodal length ratio (length of node / axonal diameter) up to 4 fold. Concomitantly, the index of paranodal myelin (a measurement of myelin splitting or decompaction) near the node of Ranvier was more than doubled [77]. Since no mechanical trauma was exerted during these experiments, it was concluded that the main contributor to the observed nodal lengthening was the retraction of the paranodal myelin towards the internode. These changes occur in part through calcium-dependent processes (Fig. 2C, D). Calcium is a key cofactor of several enzymes including proteases believed to be involved in demyelination, such as calpain [84]. Alternatively, paranodal myelin splitting appears to be calcium-independent since decreasing calcium concentration did not significantly alleviated acrolein-mediated myelin splitting [77]. A related observation is that degradation of myelin basic protein was observed at the paranodal region following acrolein incubation [77], suggesting that acrolein reacts directly with this key component of myelin, leading to delamination and myelin splitting [85–87] (Fig. 2D).
The mechanisms of myelin retraction and the involvement of calcium in this degenerating process are not fully understood. Axoglial paranodal junctions are known to be critical for maintaining the proper structure of the nodes of Ranvier [88] (Fig. 2B). This axon-glial interaction is generated and maintained by an axoglial septate junction involving several proteins, such as contactin [89], contactin associated protein (Caspr) [90], glial neurofascin 155 (NF155) [91], protein 4.1B [92, 93], spectrin and actin [94, 95]. Following acrolein exposure, the myelin sheath detaches from axonal Caspr, indicating the dissociation of paranodal protein complex and confirming the destruction of axoglial septate junctions [77]. Calpain, a calcium-dependent protease, can be activated as a result of acrolein incubation [96] and disrupts spectrin and protein 4.1 [88, 97]. Thus, calpain activation likely plays a role in interrupting the normal axoglial connection and producing structural abnormalities such as myelin retraction [97, 98] (Fig. 2C, D).
Acrolein induced demyelination affects neurons in several critical ways, including the exposure of voltage gated potassium channels (VGPC) (Fig. 2D). Such unmasking is known to cause ionic dysregulation and conduction failure [77–81]. Specifically, the majority of axonal VGPC in adult mammalian myelinated axons are located in the juxtaparanodal region beneath the myelin sheath, while sodium channels aggregate within the node of Ranvier [88] (Fig. 2B). Acrolein incubation results in exposure of potassium channels in the juxtaparanodal region [77] (Fig. 2D). Furthermore, Ampyra® (4-aminopyridine), a potassium channel blocker, has been shown to partially restore conduction in axons demyelinated by acrolein, supporting the notion that the exposure of potassium channels is a primary mechanism of conduction failure in the presence of acrolein [77–79, 99].
Acrolein-induced demyelination affects these channels in other ways. In a recent study by Shi and colleagues, extending duration of acrolein exposure from 6 hours to more than 10 hours resulted in redistribution of VGPCs to the node of Ranvier [77]. That was the first report that VGPC can be induced to expand across the node of Ranvier following acute incubation with an endogenous toxin such as acrolein. The mechanisms of VGPC diffusion or redistribution are not likely to be explained by retraction of myelin alone. Axoglial septate junctions may function as a barrier to the movement of VGPC [88, 100]. Therefore, acrolein and ROS damage to the anchoring protein complexes could remove this limitation and lead to aberrant movement and redistribution of VGPC (Fig. 2D). In such case, VGPC diffusion would be caused by the same mechanism as myelin damage. Due to the longer duration of acrolein exposure, this pathology may play an important role in the secondary phase of traumatic injuries or chronic degenerative diseases. In fact, myelin damage in chronic human MS is associated with abnormal distribution of Caspr, a protein normally restricted to the paranodal region [101].
As acrolein has been shown to damage neurons and cause severe myelin disruption [10, 11, 50, 77], it is likely that both pathologies contribute to the functional deficits in neurotrauma and neurodegenerative diseases (Fig. 2). Since the integrities of both myelin and axons are indispensible for neuronal function, therapies targeting only one of these pathologies are expected to only partially restore function. Indeed, blocking exposed potassium channels with 4-aminopyridine only partially restored CAP conductance after trauma and acrolein-mediated damage [40, 77]. The use of a combination therapy targeting myelin and axonal repair is expected to provide better treatment than either strategy used alone. Shi and colleagues have shown that polyethylene glycol, a membrane repair agent, can significantly enhance the effect of potassium channel blockade in ex vivo spinal cord injury, indicating a synergistic effect between these two agents[102].
5. Concentration, duration of exposure, and compounding factors in acrolein toxicity
As a ubiquitous pollutant and endogenous toxin, acrolein is normally present in serum at a concentration of 0.5 μM [103, 104]. Acrolein was observed to cause neuronal damage ex vivo at concentrations between 1 and 500 μM, a range known to produce various degrees of structural and functional damage in neurons [10, 11, 15, 37, 50, 96, 105] and myelin [13, 77]. Although the exact concentrations of acrolein in trauma or neurodegenerative diseases have not been fully quantified, μM-mM levels of acrolein are likely to occur in pathological tissues. For example, the concentration of acrolein is reported to reach 80 μM in the respiratory fluids of smokers [106] and 180 μM in the plasma of patients with renal failure [107]. Acrolein concentrations in the brains of Alzheimer’s disease patients have been found to increase four-fold from a range of 0.7–0.9 nmol/mg protein to 2.5–5 nmol/mg protein [7]. We previously calculated that 100–500 μM acrolein corresponds to 1.0–5.2 nmol/mg protein in spinal cord [50], which is similar to the concentrations in AD brains, and thus may be within the scope of MS and SCI victims as well.
Another factor in the pathophysiology of acrolein-mediated toxicity is the duration of exposure. Due to the limitations of in vitro and ex vivo experimentation, functional spinal cord tissues could only be incubated with acrolein for hours [10, 77]. However, acrolein levels are likely to remain elevated significantly longer in vivo. Luo et al. reported that acrolein production is increased for at least 7 days following spinal cord compression [17]. In chronic neurodegenerative diseases where the course of the ailment progresses for years, acrolein concentrations are likely to remain elevated for much longer periods. Additionally, other secondary outcomes of injury, such as ischemia [108–111], inflammation [70, 112, 113], and excitotoxicities [8, 114], can significantly exacerbate the formation of oxidative species and the severity of acrolein-mediated neuronal insult. Thus, the concentration threshold at which acrolein can inflict significant damage in vivo may actually be lower than the values used in reported studies (1–500 μM) due to differences in exposure time and potential coupling effects in vivo. From these findings, we conclude that in vitro and ex vivo studies support the notions that acrolein occurs at toxic concentrations in pathological conditions, and this elevation plays a critical role in mediating pathology in neurotrauma and neurodegenerative diseases.
Despite overwhelming evidence of acrolein toxicity in vitro, the concentrations of this endogenous toxin occurring in CNS disorders in vivo have not been fully established. Acrolein reacts rapidly, binding to lipids and proteins of varying chemistries and molecular masses, making accurate quantification of such a volatile small molecule technically challenging. Despite the uncertainty, we recently described a unique method to show that endogenous levels of acrolein are capable of producing tissue damage [115]. By injuring a segment of spinal cord, before incubating with uninjured spinal tissue in a closed media bath, the effects of diffused acrolein was effectively isolated from those of mechanical injury. It was shown that acrolein is capable of spreading from the primary site of trauma and injuring separate tissue that was not subject to trauma. This indicates that regardless of the concentration, endogenously produced acrolein is capable of causing significant neuronal damage. The broader impact of this study is the demonstration that secondary injury can produce significant injury distinct from the effects of mechanical trauma. Such a condition likely also mimics the chronic conditions present in neurodegenerative diseases.
The evidence of widespread elevation of acrolein following focal compression injury to spinal cord is clearly consistent with the role of acrolein as a diffusive factor in secondary injury [17, 77, 115]. The observed accumulation of lipid peroxidation products as long lasting pathological byproducts capable of spreading injury is consistent with several previous reports. Using rat spinal cord contusion models, Baldwin and colleagues observed a significant increase of HNE in a region two segments outside of the compression zone [51], as did Springer et al.[52]. In addition, LPO byproducts such as acrolein and HNE likely spread farther than conventional ROS while inflicting more tissue damage due to their stability in solution [16]. These findings corroborate our hypothesis that reactive aldehydes cause secondary oxidative stress that is not limited to the original impact zone, but is more widely distributed.
Consistent with such hypothesis, we have presented some evidence linking acrolein to diffusive membrane damage, mitochondrial dysfunction and neuronal degeneration. Notably, the diffusive elevation of acrolein spatially coincides with the range of membrane disruption [17, 49]. As mentioned above, acrolein is diffusively elevated 10 mm from the injury site following in vivo SCI for at least 24 hours [17]. In the same injury model, severe axonal membrane damage was observed, but was not apparent until several days post-injury [49]. Toklu et al. found myelin degradation at 7 days post-injury correlated with lipid peroxidation and DNA fragmentation [116]. These data are consistent with the hypothesis that diffusive elevation of acrolein precedes and leads to membrane disruption and subsequent cell death following in vivo spinal cord trauma. Similarly, diffusive mitochondrial dysfunction and LPO were also observed following initial impact in the same injury model [117, 118]. This evidence—coupled with the observations that acrolein can cause mitochondrial dysfunction, LPO and other secondary injury mechanisms—clearly suggests that this aldehyde plays an important role in the pathogenesis of diffusive secondary injury following spinal cord trauma.
6. Acrolein as a novel therapeutic target for spinal cord injury and multiple sclerosis
Acrolein scavengers offer a powerful tool to study the mechanisms of acrolein-mediated cytotoxicity and to evaluate the utility of anti-acrolein treatments for SCI and MS[13, 50, 105]. Most research in this area has examined an antihypertensive drug, Apresoline® (hydralazine), that has been shown to bind to and neutralize acrolein [31, 119, 120] and acrolein-protein adducts [27, 30, 121]. The molecular structure of hydralazine (Fig. 3) shows this species to be a strong nucleophile possessing a hydrazide functional group. This imparts properties similar to the nucleophilic centers of proteins and DNA: acrolein reacts with a hydrazide through a Michael-type addition [119]. Using 1H NMR, Burcham and colleagues have identified the primary reaction product of hydralazine and acrolein to be (1E)-acrylaldehyde phthalazin-1-yl hydrazone (E-APH), formed in a 1:1 reaction [31]. In a cell-free system, equimolar concentrations of hydralazine and acrolein resulted in nearly complete elimination of acrolein. Formation of E-APH greatly reduces the cellular toxicity of acrolein, allowing it to be safely excreted from the body [31].
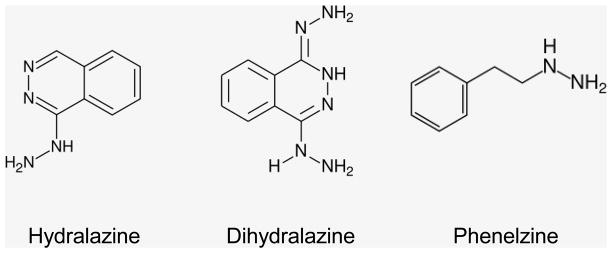
Figure 3.
Chemical structures of the acrolein scavengers: hydralazine, dihydralazine, and phenelzine
Repeated experiments have shown hydralazine to be effective at preventing or reducing the severity of pathologies associated with acrolein. Acrolein mediated cytotoxicity was reduced by incubation with this drug [105]. In ex vivo spinal cord, hydralazine mitigated acrolein-mediated oxidative stress, membrane damage, and loss of compound action potential conduction [50, 115]. Hydralazine also reduced accumulation of acrolein-protein adducts in ex vivo spinal cord compression, which correlated well with findings that hydralazine mitigated compression-mediated oxidative stress and membrane damage [50]. Previous studies report that hydralazine is not an efficient scavenger of superoxide, nor does it directly influence damaged membranes [50]. Therefore, hydralazine likely acts to reduce injuries through acrolein scavenging mechanisms. This evidence further substantiates the theory that acrolein accumulates at pathologic concentrations following compression injury ex vivo, and plays a key role in secondary injury mechanisms following traumatic SCI. Therefore, acrolein is a potential novel target of therapy to promote improved recovery following spinal cord injury.
Intraperitoneal (IP) injection of hydralazine can deliver safe and effective doses in vivo [122], reducing acrolein concentration in spinal cord and leading to enhanced functional recovery in EAE mouse [13]. Specifically, hydralazine at 1 mg/kg body mass, can successfully inhibit acrolein-mediated myelin damage and reduce behavioral deficits in EAE mice. Hydralazine directly induces vasodilation, likely by inhibiting calcium release within vascular smooth muscle and involves opening of vascular potassium channels [123–126]. Accordingly, hypotension might be expected as a potentially life threatening side effect if used for SCI patients in traumatic shock. However, these fears have not been borne out in animal studies. The blood pressure of injured animals was monitored and no serious hypotension was observed [13]. In fact, this dosage of hydralazine (1 mg/kg) is significantly lower than the suggested upper limit of safe dosing for human pediatric patients (7.5 mg/kg) [127]. Assuming the bioavailability of hydralazine through IP injection (in mice) was more than 90%, compared to about 40% through oral application [128], the systemic concentration hydralazine achieved in EAE mice is still less than a third that of pediatric human patients. Therefore it is likely that a dose effective at trapping acrolein can be reached without eliciting significant side effects.
In recent rat spinal cord contusion experiments, an even higher dose of hydralazine (5 mg/kg) effectively lowered acrolein concentrations and enhanced behavioral recovery without inducing hypotension (unpublished observations). Using a unique method of mass spectrometry, it has been shown that hydralazine concentration can reach an effective range of 10–30 μM in spinal cord tissues within hours of IP injection in rats [105, 122]. These findings further support the proposed use of hydralazine to effectively treat neurotrauma [50] and neurodegenerative diseases [13] by trapping acrolein within the CNS following a systemic application. It is noteworthy that hydralazine is a FDA-approved medication for hypertension[125, 126]. Considering the efficacy in trapping acrolein in vivo, the safe effective dosage, and the established FDA approval, hydralazine therapy is primed for rapid translation to clinical therapy.
In addition to hydralazine, several other drugs that have potential use in scavenging acrolein, but have not yet received extensive study. In general, these compounds possess aromatic rings and nucleophilic hydrazine groups. Dihydralazine is structurally and pharmacologically similar to hydralazine, but has two hydrazine groups (Fig. 3). Thus, it could potentially be up to twice as efficient at trapping acrolein. It was shown to be effective at trapping acrolein in a cell-free system and also protected against acrolein-induced LDH release in cultured hepatocytes [31]. Another potential acrolein scavenger, Nardil® (phenelzine, Fig. 3), is a monoamine oxidase inhibitor (MAOI) that also possesses a hydrazine group. Phenelzine has been shown to protect against acrolein induced LDH release in vitro, as well as ischemia-reperfusion injury in gerbils in vivo [129]. Phenelzine reacts with acrolein at the same 1:1 molecular ratio as hydralazine, so it is expected to be as effective at removing acrolein from solution. Since the half-life of phenelzine is 11.6 hours [130] as compared to 0.5–1 hour for hydralazine [131], it might provide additional neuroprotection. Though phenelzine is not a direct vasodilator, as an MAOI it can have a transient effect on blood pressure [132]. Following SCI, when hypotensive effects are particularly undesirable, phenelzine could be used as an alternative to hydralazine. However, the safety of phenelzine would need to be established through further testing.
7. Conclusion statement
There is strong evidence to indicate that acrolein plays a critical role in oxidative stress: its long half-life, ability to generate free radicals, potent cytotoxicity, and elevated concentrations in disease conditions. It also has synergistic effects with other common secondary injuries where oxidative stress is known to exist. Therefore, we postulate that acrolein is the primary factor in perpetuating oxidative stress. This would explain why years of research efforts targeting transient ROS using free radical scavengers have not yielded any effective treatment. Hence, acrolein constitutes a more logical target for effective therapeutic intervention to reduce oxidative stress.
It is well known that mammalian cells have a built-in antioxidant system to combat oxidative stress in most of the situations [3]. However, in the event of injury, acute or chronic, the endogenous antioxidant system is frequently overwhelmed by rapid production of free radicals [66, 133–137]. Acrolein could be responsible for stimulating the generation of free radicals while also depleting endogenous antioxidants such as GSH. Therefore, scavenging acrolein may impede the vicious cycle that perpetuates oxidative stress. The strong scientific rationale to trap acrolein to treat neurotrauma and diseases is supported by experiments that have shown existing acrolein scavengers to not only be effective for in vitro testing, but safe and practical during in vivo applications. Specifically, hydralazine serves as an excellent acrolein-scavenger that has prior FDA-approval, making it likely to be rapidly adopted for clinical usage. Because of the wide variety of pathological processes associated with acrolein, research into anti-acrolein therapies may have benefits beyond SCI and MS, and potentially could also be used to treat patients with other diseases associated with oxidative stress.
Acknowledgments
Authors acknowledge the financial support from National Institute of Health (1RO1EB007243-01) and the State of Indiana. We also thank Michel Schweinsberg for figure illustrations.
Abbreviations
- ALDH
aldehyde dehydrogenase
- AD
Alzheimer’s disease
- ANT
adenine nucleotide translocase
- CAP
compound action potential
- Caspr
contactin associated protein
- CNS
central nervous system
- EAE
experimental autoimmune encephalomyelitis
- E-APH
(1E)-acrylaldehyde phthalazin-1-yl hydrazone
- EDTA
Ethylenediaminetetraacetic acid
- GSH
glutathione
- HNE
4-hydroxynonenal
- HRP
horseradish peroxidase
- IP
Intraperitoneal
- LDH
lactate dehydrogenase
- LPO
lipid peroxidation
- MAOI
monoamine oxidase inhibitor
- MS
multiple sclerosis
- ROS
reactive oxygen species
- SCI
spinal cord injury
- VGPC
voltage gated potassium channels
Footnotes
Conflict of interests:
There is no conflict of interests for any of the authors.
References
- 1.Hall ED, Braughler JM. Central nervous system trauma and stroke II. Physiological and pharmacological evidence for involvement of oxygen radicals and lipid peroxidation. Free Radic Biol Med. 1989;6:303–313. doi: 10.1016/0891-5849(89)90057-9. [DOI] [PubMed] [Google Scholar]
- 2.Hall ED. Lipid peroxidation. Advances in Neurology. 1996;71:247–57. discussion 257–8. [PubMed] [Google Scholar]
- 3.Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. Oxford: Oxford University Press; 1999. [Google Scholar]
- 4.Hall ED, Yonkers PA, Andrus PK, Cox JW, et al. Biochemistry and pharmacology of lipid antioxidants in acute brain and spinal cord injury. J Neurotrauma. 1992;9:S425–42. [PubMed] [Google Scholar]
- 5.Hall ED. Free radicals and CNS injury. Critical Care Clinics. 1989;5:793–805. [PubMed] [Google Scholar]
- 6.Smith KJ, Kapoor R, Felts PA. Demyelination: the role of reactive oxygen and nitrogen species. Brain Pathol. 1999;9:69–92. doi: 10.1111/j.1750-3639.1999.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lovell MA, Xie C, Markesbery WR. Acrolein is increased in Alzheimer’s disease brain and is toxic to primary hippocampal cultures. Neurobiol Aging. 2001;22:187–94. doi: 10.1016/s0197-4580(00)00235-9. [DOI] [PubMed] [Google Scholar]
- 8.Lovell MA, Xie C, Markesbery WR. Acrolein, a product of lipid peroxidation, inhibits glucose and glutamate uptake in primary neuronal cultures. Free Radic Biol Med. 2000;29:714–20. doi: 10.1016/s0891-5849(00)00346-4. [DOI] [PubMed] [Google Scholar]
- 9.Luo J, Shi R. Acrolein disrupts neuronal membrane in isolated guinea pig spinal cord. Soc Neurosci Abst. 2002:28. [Google Scholar]
- 10.Luo J, Shi R. Acrolein induces axolemmal disruption, oxidative stress, and mitochondrial impairment in spinal cord tissue. Neurochem Int. 2004;44:475–486. doi: 10.1016/j.neuint.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Shi R, Luo J, Peasley MA. Acrolein inflicts axonal membrane disruption and conduction loss in isolated guinea pig spinal cord. Neuroscience. 2002;115:337–340. doi: 10.1016/s0306-4522(02)00457-8. [DOI] [PubMed] [Google Scholar]
- 12.Williams TI, Lynn BC, Markesbery WR, Lovell MA. Increased levels of 4-hydroxynonenal and acrolein, neurotoxic markers of lipid peroxidation, in the brain in Mild Cognitive Impairment and early Alzheimer’s disease. Neurobiol Aging. 2005 doi: 10.1016/j.neurobiolaging.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Leung G, Sun W, Zheng L, Brookes S, et al. Anti-acrolein treatment improves behavioral outcome and alleviates myelin damage in experimental autoimmune enchephalomyelitis mouse. Neuroscience. 2011;173:150–5. doi: 10.1016/j.neuroscience.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uchida K, Kanematsu M, Morimitsu Y, Osawa T, et al. Acrolein is a product of lipid peroxidation reaction. Formation of free acrolein and its conjugate with lysine residues in oxidized low density lipoproteins. J Biol Chem. 1998;273:16058–66. doi: 10.1074/jbc.273.26.16058. [DOI] [PubMed] [Google Scholar]
- 15.Luo J, Shi R. Acrolein induces oxidative stress in brain mitochondria. Neurochem Int. 2005;46:243–52. doi: 10.1016/j.neuint.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Ghilarducci DP, Tjeerdema RS. Fate and effects of acrolein. Rev Environ Contam Toxicol. 1995;144:95–146. doi: 10.1007/978-1-4612-2550-8_2. [DOI] [PubMed] [Google Scholar]
- 17.Luo J, Uchida K, Shi R. Accumulation of acrolein-protein adducts after traumatic spinal cord injury. Neurochem Res. 2005;30:291–5. doi: 10.1007/s11064-005-2602-7. [DOI] [PubMed] [Google Scholar]
- 18.Feng Z, Hu W, Hu Y, Tang MS. Acrolein is a major cigarette-related lung cancer agent: Preferential binding at p53 mutational hotspots and inhibition of DNA repair. Proc Natl Acad Sci U S A. 2006;103:15404–9. doi: 10.1073/pnas.0607031103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burcham PC, Raso A, Thompson CA. Toxicity of smoke extracts towards A549 lung cells: role of acrolein and suppression by carbonyl scavengers. Chem Biol Interact. 2010;183:416–24. doi: 10.1016/j.cbi.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Magnusson R, Nilsson C, Andersson B. Emissions of aldehydes and ketones from a two-stroke engine using ethanol and ethanol-blended gasoline as fuel. Environ Sci Technol. 2002;36:1656–64. doi: 10.1021/es010262g. [DOI] [PubMed] [Google Scholar]
- 21.Ho SS, Yu JZ, Chu KW, Yeung LL. Carbonyl emissions from commercial cooking sources in Hong Kong. J Air Waste Manag Assoc. 2006;56:1091–8. doi: 10.1080/10473289.2006.10464532. [DOI] [PubMed] [Google Scholar]
- 22.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol & Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 23.Kehrer JP, Biswal SS. The molecular effects of acrolein. Toxicol Sci. 2000;57:6–15. doi: 10.1093/toxsci/57.1.6. [DOI] [PubMed] [Google Scholar]
- 24.Stevens JF, Maier CS. Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol Nutr Food Res. 2008;52:7–25. doi: 10.1002/mnfr.200700412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dennis KJ, Shibamoto T. Gas chromatographic analysis of reactive carbonyl compounds formed from lipids upon UV-irradiation. Lipids. 1990;25:460–4. doi: 10.1007/BF02538089. [DOI] [PubMed] [Google Scholar]
- 26.Uchida K. Current status of acrolein as a lipid peroxidation product. Trends Cardiovasc Med. 1999;9:109–13. doi: 10.1016/s1050-1738(99)00016-x. [DOI] [PubMed] [Google Scholar]
- 27.Burcham PC, Pyke SM. Hydralazine inhibits rapid acrolein-induced protein oligomerization: role of aldehyde scavenging and adduct trapping in cross-link blocking and cytoprotection. Mol Pharmacol. 2006;69:1056–65. doi: 10.1124/mol.105.018168. [DOI] [PubMed] [Google Scholar]
- 28.Witz G. Biological interactions of alpha, beta-unsaturated aldehydes. Free Radic Biol Med. 1989;7:333–49. doi: 10.1016/0891-5849(89)90137-8. [DOI] [PubMed] [Google Scholar]
- 29.Adams JD, Jr, Klaidman LK. Acrolein-induced oxygen radical formation. Free Radic Biol Med. 1993;15:187–93. doi: 10.1016/0891-5849(93)90058-3. [DOI] [PubMed] [Google Scholar]
- 30.Burcham PC, Fontaine FR, Kaminskas LM, Petersen DR, et al. Protein adduct-trapping by hydrazinophthalazine drugs: mechanisms of cytoprotection against acrolein-mediated toxicity. Mol Pharmacol. 2004;65:655–64. doi: 10.1124/mol.65.3.655. [DOI] [PubMed] [Google Scholar]
- 31.Kaminskas LM, Pyke SM, Burcham PC. Reactivity of hydrazinophthalazine drugs with the lipid peroxidation products acrolein and crotonaldehyde. Org Biomol Chem. 2004;2:2578–84. doi: 10.1039/B408796H. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell DY, Petersen DR. Metabolism of the glutathione-acrolein adduct, S-(2-aldehydo-ethyl)glutathione, by rat liver alcohol and aldehyde dehydrogenase. J Pharmacol Exp Ther. 1989;251:193–8. [PubMed] [Google Scholar]
- 33.O’Brien PJ, Siraki AG, Shangari N. Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Crit Rev Toxicol. 2005;35:609–62. doi: 10.1080/10408440591002183. [DOI] [PubMed] [Google Scholar]
- 34.Picklo MJ, Montine TJ. Acrolein inhibits respiration in isolated brain mitochondria. Biochim Biophys Acta. 2001;1535:145–52. doi: 10.1016/s0925-4439(00)00093-4. [DOI] [PubMed] [Google Scholar]
- 35.Pocernich CB, Cardin AL, Racine CL, Lauderback CM, et al. Glutathione elevation and its protective role in acrolein-induced protein damage in synaptosomal membranes: relevance to brain lipid peroxidation in neurodegenerative disease. Neurochem Int. 2001;39:141–9. doi: 10.1016/s0197-0186(01)00012-2. [DOI] [PubMed] [Google Scholar]
- 36.Logan MP, Parker S, Shi R. Glutathione and ascorbic acid enhance recovery of Guinea pig spinal cord white matter following ischemia and acrolein exposure. Pathobiology. 2005;72:171–8. doi: 10.1159/000086786. [DOI] [PubMed] [Google Scholar]
- 37.Luo J, Robinson JP, Shi R. Acrolein-induced cell death in PC12 cells: role of mitochondria-mediated oxidative stress. Neurochem Int. 2005;47:449–57. doi: 10.1016/j.neuint.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Vander Jagt DL, Hunsaker LA, Vander Jagt TJ, Gomez MS, et al. Inactivation of glutathione reductase by 4-hydroxynonenal and other endogenous aldehydes. Biochem Pharmacol. 1997;53:1133–40. doi: 10.1016/s0006-2952(97)00090-7. [DOI] [PubMed] [Google Scholar]
- 39.Fitzpatrick MO, Maxwell WL, Graham DI. The role of the axolemma in the initiation of traumatically induced axonal injury [editorial] J Neurol Neurosurg Psychiatry. 1998;64:285–7. doi: 10.1136/jnnp.64.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi R, Blight AR. Compression injury of mammalian spinal cord in vitro and the dynamics of action potential conduction failure. J Neurophysiol. 1996;76:1572–1580. doi: 10.1152/jn.1996.76.3.1572. [DOI] [PubMed] [Google Scholar]
- 41.Shi R, Pryor JD. Pathological changes of isolated spinal cord axons in response to mechanical stretch. Neuroscience. 2002;110:765–777. doi: 10.1016/s0306-4522(01)00596-6. [DOI] [PubMed] [Google Scholar]
- 42.Luo J, Borgens RB, Shi R. Polyethylene glycol immediately repairs neuronal membranes and inhibits free radical production after acute spinal cord injury. J Neurochem. 2002;83:471–480. doi: 10.1046/j.1471-4159.2002.01160.x. [DOI] [PubMed] [Google Scholar]
- 43.Shi R, Asano T, Vining NC, Blight AR. Control of membrane sealing in injured mammalian spinal cord axons. J Neurophysiol. 2000;84:1763–1769. doi: 10.1152/jn.2000.84.4.1763. [DOI] [PubMed] [Google Scholar]
- 44.Shi R, Pryor JD. Temperature dependence of membrane sealing following transection in mammalian spinal cord axons. Neuroscince. 2000;98:157–166. doi: 10.1016/s0306-4522(00)00096-8. [DOI] [PubMed] [Google Scholar]
- 45.Shi R, Qiao X, Emerson N, Malcom A. Dimethylsulfoxide enhances CNS neuronal plasma membrane resealing after injury in low temperature or low calcium. J Neurocytol. 2001;30:829–839. doi: 10.1023/a:1019645505848. [DOI] [PubMed] [Google Scholar]
- 46.Povlishock JT, Erb DE, Astruc J. Axonal response to traumatic brain injury: reactive axonal change, deafferentation, and neuroplasticity. J Neurotrauma. 1992 [PubMed] [Google Scholar]
- 47.Povlishock JT. Traumatically induced axonal injury: pathogenesis and pathobiological implications. Brain Pathol. 1992;2:1–12. [PubMed] [Google Scholar]
- 48.Christman CW, Grady MS, Walker SA, Holloway KL, et al. Ultrastructural studies of diffuse axonal injury in humans. J Neurotrauma. 1994;11:173–86. doi: 10.1089/neu.1994.11.173. [DOI] [PubMed] [Google Scholar]
- 49.Shi R. The dynamics of axolemmal disruption in guinea pig spinal cord following compression. J Neurocytol. 2004;33:203–11. doi: 10.1023/b:neur.0000030695.76840.19. [DOI] [PubMed] [Google Scholar]
- 50.Hamann K, Nehrt G, Ouyang H, Duerstock B, et al. Hydralazine inhibits compression and acrolein-mediated injuries in ex vivo spinal cord. J Neurochem. 2008;104:708–18. doi: 10.1111/j.1471-4159.2007.05002.x. [DOI] [PubMed] [Google Scholar]
- 51.Baldwin SA, Broderick R, Osbourne D, Waeg G, et al. The presence of 4-hydroxynonenal/protein complex as an indicator of oxidative stress after experimental spinal cord contusion in a rat model. Journal of Neurosurgery. 1998;88:874–83. doi: 10.3171/jns.1998.88.5.0874. [DOI] [PubMed] [Google Scholar]
- 52.Springer JE, Azbill RD, Mark RJ, Begley JG, et al. 4-hydroxynonenal, a lipid peroxidation product, rapidly accumulates following traumatic spinal cord injury and inhibits glutamate uptake. J Neurochem. 1997;68:2469–76. doi: 10.1046/j.1471-4159.1997.68062469.x. [DOI] [PubMed] [Google Scholar]
- 53.Vaishnav RA, Singh IN, Miller DM, Hall ED. Lipid peroxidation-derived reactive aldehydes directly and differentially impair spinal cord and brain mitochondrial function. J Neurotrauma. 2010;27:1311–20. doi: 10.1089/neu.2009.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29:222–30. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 55.Lenaz G, Bovina C, D’Aurelio M, Fato R, et al. Role of mitochondria in oxidative stress and aging. Ann N Y Acad Sci. 2002;959:199–213. doi: 10.1111/j.1749-6632.2002.tb02094.x. [DOI] [PubMed] [Google Scholar]
- 56.Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–16. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Biagini RE, Toraason MA, Lynch DW, Winston GW. Inhibition of rat heart mitochondrial electron transport in vitro: implications for the cardiotoxic action of allylamine or its primary metabolite, acrolein. Toxicology. 1990;62:95–106. doi: 10.1016/0300-483x(90)90034-e. [DOI] [PubMed] [Google Scholar]
- 58.Mavelli I, Rigo A, Federico R, Ciriolo MR, et al. Superoxide dismutase, glutathione peroxidase and catalase in developing rat brain. Biochem J. 1982;204:535–40. doi: 10.1042/bj2040535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bai J, Cederbaum AI. Mitochondrial catalase and oxidative injury. Biol Signals Recept. 2001;10:189–99. doi: 10.1159/000046887. [DOI] [PubMed] [Google Scholar]
- 60.Esposito LA, Melov S, Panov A, Cottrell BA, et al. Mitochondrial disease in mouse results in increased oxidative stress. Proc Natl Acad Sci U S A. 1999;96:4820–5. doi: 10.1073/pnas.96.9.4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wallace DC. Mitochondrial diseases in man and mouse. Science. 1999;283:1482–8. doi: 10.1126/science.283.5407.1482. [DOI] [PubMed] [Google Scholar]
- 62.Wallace DC. Mouse models for mitochondrial disease. Am J Med Genet. 2001;106:71–93. doi: 10.1002/ajmg.1393. [DOI] [PubMed] [Google Scholar]
- 63.Klingenberg M, Nelson DR. Structure-function relationships of the ADP/ATP carrier. Biochim Biophys Acta. 1994;1187:241–4. doi: 10.1016/0005-2728(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 64.Fiore C, Trezeguet V, Le Saux A, Roux P, et al. The mitochondrial ADP/ATP carrier: structural, physiological and pathological aspects. Biochimie. 1998;80:137–50. doi: 10.1016/s0300-9084(98)80020-5. [DOI] [PubMed] [Google Scholar]
- 65.Dykens JA. Isolated cerebral and cerebellar mitochondria produce free radicals when exposed to elevated CA2+ and Na+: implications for neurodegeneration. J Neurochem. 1994;63:584–91. doi: 10.1046/j.1471-4159.1994.63020584.x. [DOI] [PubMed] [Google Scholar]
- 66.Lewen A, Matz P, Chan PH. Free radical pathways in CNS injury. J Neurotrauma. 2000;17:871–90. doi: 10.1089/neu.2000.17.871. [DOI] [PubMed] [Google Scholar]
- 67.Starkov AA, Polster BM, Fiskum G. Regulation of hydrogen peroxide production by brain mitochondria by calcium and Bax. J Neurochem. 2002;83:220–8. doi: 10.1046/j.1471-4159.2002.01153.x. [DOI] [PubMed] [Google Scholar]
- 68.Zamzami N, Kroemer G. The mitochondrion in apoptosis: how Pandora’s box opens. Nat Rev Mol Cell Biol. 2001;2:67–71. doi: 10.1038/35048073. [DOI] [PubMed] [Google Scholar]
- 69.Cohen G, Farooqui R, Kesler N. Parkinson disease: a new link between monoamine oxidase and mitochondrial electron flow. Proc Natl Acad Sci U S A. 1997;94:4890–4. doi: 10.1073/pnas.94.10.4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–17. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 71.Gold R, Linington C, Lassmann H. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain. 2006;129:1953–71. doi: 10.1093/brain/awl075. [DOI] [PubMed] [Google Scholar]
- 72.Totoiu MO, Keirstead HS. Spinal cord injury is accompanied by chronic progressive demyelination. J Comp Neurol. 2005;486:373–83. doi: 10.1002/cne.20517. [DOI] [PubMed] [Google Scholar]
- 73.Blight AR. Delayed demyelination and macrophage invasion: a candidate for secondary cell damage in spinal cord injury. Cent Nerv Syst Trauma. 1985;2:299–315. doi: 10.1089/cns.1985.2.299. [DOI] [PubMed] [Google Scholar]
- 74.Blight AR. Morphometric analysis of a model of spinal cord injury in guinea pigs, with behavioral evidence of delayed secondary pathology. J Neurol Sci. 1991;103:156–171. doi: 10.1016/0022-510x(91)90159-5. [DOI] [PubMed] [Google Scholar]
- 75.Blight AR. Remyelination, revascularization and recovery of function in experimental spinal cord injury. In: Seil FJ, editor. Advances in Neurology, Vol. 59. Neural Injury and Regeneration. Raven Press; New York: 1993. pp. 91–104. [PubMed] [Google Scholar]
- 76.Ouyang H, Sun W, Fu Y, Li J, et al. Compression induces acute demyelination and potassium channel exposure in spinal cord. J Neurotrauma. 2010;27:1109–20. doi: 10.1089/neu.2010.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shi Y, Sun W, McBride JJ, Cheng JX, et al. Acrolein induces myelin damage in mammalian spinal cord. J Neurochem. 2011 Feb 26; doi: 10.1111/j.1471-4159.2011.07226.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 78.Jensen JM, Shi R. Effects of 4-aminopyridine on stretched mammalian spinal cord: the role of potassium channels in axonal conduction. J Neurophysiol. 2003;90:2334–40. doi: 10.1152/jn.00868.2002. [DOI] [PubMed] [Google Scholar]
- 79.Shi R, Blight AR. Differential effects of low and high concentrations of 4-aminopyridine on axonal conduction in normal and injured spinal cord. Neuroscience. 1997;77:553–562. doi: 10.1016/s0306-4522(96)00477-0. [DOI] [PubMed] [Google Scholar]
- 80.Shi R, Kelly TM, Blight AR. Conduction block in acute and chronic spinal cord injury: Different dose-response characteristics for reversal by 4-Aminopyridine. Exp Neurology. 1997;148:495–501. doi: 10.1006/exnr.1997.6706. [DOI] [PubMed] [Google Scholar]
- 81.Sun W, Smith D, Fu Y, Cheng JX, et al. Novel potassium channel blocker, 4-AP-3-MeOH, inhibits fast potassium channels and restores axonal conduction in injured guinea pig spinal cord white matter. J Neurophysiol. 2010;103:469–78. doi: 10.1152/jn.00154.2009. [DOI] [PubMed] [Google Scholar]
- 82.Leung G, Sun W, Brookes S, Smith D, et al. Potassium channel blocker, 4-Aminopyridine-3-Methanol, restores axonal conduction in spinal cord of an animal model of multiple sclerosis. Exp Neurol. 2011;227:232–235. doi: 10.1016/j.expneurol.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hains BC, Saab CY, Lo AC, Waxman SG. Sodium channel blockade with phenytoin protects spinal cord axons, enhances axonal conduction, and improves functional motor recovery after contusion SCI. Exp Neurol. 2004;188:365–77. doi: 10.1016/j.expneurol.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 84.Shields DC, Tyor WR, Deibler GE, Hogan EL, et al. Increased calpain expression in activated glial and inflammatory cells in experimental allergic encephalomyelitis. Proc Natl Acad Sci U S A. 1998;95:5768–72. doi: 10.1073/pnas.95.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Boggs JM. Myelin basic protein: a multifunctional protein. Cell Mol Life Sci. 2006;63:1945–61. doi: 10.1007/s00018-006-6094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Readhead C, Takasashi N, Shine HD, Saavedra R, et al. Role of myelin basic protein in the formation of central nervous system myelin. Ann N Y Acad Sci. 1990;605:280–5. doi: 10.1111/j.1749-6632.1990.tb42401.x. [DOI] [PubMed] [Google Scholar]
- 87.Omlin FX, Webster HD, Palkovits CG, Cohen SR. Immunocytochemical localization of basic protein in major dense line regions of central and peripheral myelin. J Cell Biol. 1982;95:242–8. doi: 10.1083/jcb.95.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Poliak S, Peles E. The local differentiation of myelinated axons at nodes of Ranvier. Nat Rev Neurosci. 2003;4:968–80. doi: 10.1038/nrn1253. [DOI] [PubMed] [Google Scholar]
- 89.Rios JC, Melendez-Vasquez CV, Einheber S, Lustig M, et al. Contactin-associated protein (Caspr) and contactin form a complex that is targeted to the paranodal junctions during myelination. J Neurosci. 2000;20:8354–64. doi: 10.1523/JNEUROSCI.20-22-08354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Einheber S, Zanazzi G, Ching W, Scherer S, et al. The axonal membrane protein Caspr, a homologue of neurexin IV, is a component of the septate-like paranodal junctions that assemble during myelination. J Cell Biol. 1997;139:1495–506. doi: 10.1083/jcb.139.6.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tao-Cheng JH, Rosenbluth J. Axolemmal differentiation in myelinated fibers of rat peripheral nerves. Brain Res. 1983;285:251–63. doi: 10.1016/0165-3806(83)90023-8. [DOI] [PubMed] [Google Scholar]
- 92.Menegoz M, Gaspar P, Le Bert M, Galvez T, et al. Paranodin, a glycoprotein of neuronal paranodal membranes. Neuron. 1997;19:319–31. doi: 10.1016/s0896-6273(00)80942-3. [DOI] [PubMed] [Google Scholar]
- 93.Denisenko-Nehrbass N, Oguievetskaia K, Goutebroze L, Galvez T, et al. Protein 4. 1B associates with both Caspr/paranodin and Caspr2 at paranodes and juxtaparanodes of myelinated fibres. Eur J Neurosci. 2003;17:411–6. doi: 10.1046/j.1460-9568.2003.02441.x. [DOI] [PubMed] [Google Scholar]
- 94.Ogawa Y, Schafer DP, Horresh I, Bar V, et al. Spectrins and ankyrinB constitute a specialized paranodal cytoskeleton. J Neurosci. 2006;26:5230–9. doi: 10.1523/JNEUROSCI.0425-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kontrogianni-Konstantopoulos A, Frye CS, Benz EJ, Jr, Huang SC. The prototypical 4.1R-10-kDa domain the 4.1g-10-kDa paralog mediate fodrin-actin complex formation. J Biol Chem. 2001;276:20679–87. doi: 10.1074/jbc.M010581200. [DOI] [PubMed] [Google Scholar]
- 96.Liu-Snyder P, McNally H, Shi R, Borgens RB. Acrolein-mediated mechanisms of neuronal death. J Neurosci Res. 2006;84:209–18. doi: 10.1002/jnr.20863. [DOI] [PubMed] [Google Scholar]
- 97.Goll DE, Thompson VF, Li H, Wei W, et al. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 98.Wang KK. Calpain and caspase: can you tell the difference? Trends Neurosci. 2000;23:20–6. doi: 10.1016/s0166-2236(99)01479-4. [DOI] [PubMed] [Google Scholar]
- 99.Sherratt RM, Bostock H, Sears TA. Effects of 4-aminopyridine on normal and demyelinated mammalian nerve fibers. Nature. 1980;283:570–572. doi: 10.1038/283570a0. [DOI] [PubMed] [Google Scholar]
- 100.Dupree JL, Girault JA, Popko B. Axo-glial interactions regulate the localization of axonal paranodal proteins. J Cell Biol. 1999;147:1145–52. doi: 10.1083/jcb.147.6.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wolswijk G, Balesar R. Changes in the expression and localization of the paranodal protein Caspr on axons in chronic multiple sclerosis. Brain. 2003;126:1638–49. doi: 10.1093/brain/awg151. [DOI] [PubMed] [Google Scholar]
- 102.Shi R, Borgens RB. Acute repair of crushed guinea pig spinal cord by polyethylene glycol. J Neurophysiology. 1999;81:2406–14. doi: 10.1152/jn.1999.81.5.2406. [DOI] [PubMed] [Google Scholar]
- 103.Sakata K, Kashiwagi K, Sharmin S, Ueda S, et al. Acrolein produced from polyamines as one of the uraemic toxins. Biochem Soc Trans. 2003;31:371–4. doi: 10.1042/bst0310371. [DOI] [PubMed] [Google Scholar]
- 104.Togashi M, Urano Y, Kojima H, Terai T, et al. Sensitive detection of acrolein in serum using time-resolved luminescence. Org Lett. 2010;12:1704–7. doi: 10.1021/ol1002219. [DOI] [PubMed] [Google Scholar]
- 105.Liu-Snyder P, Borgens RB, Shi R. Hydralazine rescues PC12 cells from acrolein-mediated death. J Neurosci Res. 2006;84:219–27. doi: 10.1002/jnr.20862. [DOI] [PubMed] [Google Scholar]
- 106.Nardini M, Finkelstein EI, Reddy S, Valacchi G, et al. Acrolein-induced cytotoxicity in cultured human bronchial epithelial cells. Modulation by alpha-tocopherol and ascorbic acid. Toxicology. 2002;170:173–185. doi: 10.1016/s0300-483x(01)00540-6. [DOI] [PubMed] [Google Scholar]
- 107.Sakata K, Kashiwagi K, Sharmin S, Ueda S, et al. Increase in putrescine, amine oxidase, and acrolein in plasma of renal failure patients. Biochem Biophys Res Commun. 2003;305:143–9. doi: 10.1016/s0006-291x(03)00716-2. [DOI] [PubMed] [Google Scholar]
- 108.Hall ED, Wolf DL. Post-traumatic spinal cord ischemia: relationship to injury severity and physiological parameters. Cent Nerv Syst Trauma. 1987;4:15–25. doi: 10.1089/cns.1987.4.15. [DOI] [PubMed] [Google Scholar]
- 109.Sandler AN, Tator CH. Review of the effect of spinal cord trama on the vessels and blood flow in the spinal cord. J Neurosur. 1976;45:638–46. doi: 10.3171/jns.1976.45.6.0638. [DOI] [PubMed] [Google Scholar]
- 110.White BC, Sullivan JM, DeGracia DJ, O’Neil BJ, et al. Brain ischemia and reperfusion: molecular mechanisms of neuronal injury. J Neurol Sci. 2000;179:1–33. doi: 10.1016/s0022-510x(00)00386-5. [DOI] [PubMed] [Google Scholar]
- 111.Peasley MA, Shi R. Ischemic insult exacerbates acrolein-induced conduction loss and axonal membrane disruption in guinea pig spinal cord white matter. J Neurol Sci. 2003;216:23–32. doi: 10.1016/s0022-510x(03)00201-6. [DOI] [PubMed] [Google Scholar]
- 112.Facchinetti F, Amadei F, Geppetti P, Tarantini F, et al. Alpha, beta-unsaturated aldehydes in cigarette smoke release inflammatory mediators from human macrophages. Am J Respir Cell Mol Biol. 2007;37:617–23. doi: 10.1165/rcmb.2007-0130OC. [DOI] [PubMed] [Google Scholar]
- 113.Park YS, Taniguchi N. Acrolein induces inflammatory response underlying endothelial dysfunction: a risk factor for atherosclerosis. Ann N Y Acad Sci. 2008;1126:185–9. doi: 10.1196/annals.1433.034. [DOI] [PubMed] [Google Scholar]
- 114.Barger SW, Goodwin ME, Porter MM, Beggs ML. Glutamate release from activated microglia requires the oxidative burst and lipid peroxidation. J Neurochem. 2007;101:1205–13. doi: 10.1111/j.1471-4159.2007.04487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hamann K, Durkes A, Ouyang H, Uchida K, et al. Critical role of acrolein in secondary injury following ex vivo spinal cord trauma. J Neurochem. 2008;107:712–21. doi: 10.1111/j.1471-4159.2008.05622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Toklu HZ, Hakan T, Celik H, Biber N, et al. Neuroprotective effects of alpha-lipoic acid in experimental spinal cord injury in rats. J Spinal Cord Med. 2010;33:401–9. doi: 10.1080/10790268.2010.11689719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Luo J, Borgens R, Shi R. Polyethylene glycol improves function and reduces oxidative stress in synaptosomal preparations following spinal cord injury. J Neurotrauma. 2004;21:994–1007. doi: 10.1089/0897715041651097. [DOI] [PubMed] [Google Scholar]
- 118.Luo J, Shi R. Diffusive oxidative stress following acute spinal cord injury in guinea pigs and its inhibition by polyethylene glycol. Neurosci Lett. 2004;359:167–70. doi: 10.1016/j.neulet.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 119.Burcham PC, Kerr PG, Fontaine F. The antihypertensive hydralazine is an efficient scavenger of acrolein. Redox Rep. 2000;5:47–9. doi: 10.1179/rer.2000.5.1.47. [DOI] [PubMed] [Google Scholar]
- 120.Burcham PC, Kaminskas LM, Fontaine FR, Petersen DR, et al. Aldehyde-sequestering drugs: tools for studying protein damage by lipid peroxidation products. Toxicology. 2002;181–182:229–36. doi: 10.1016/s0300-483x(02)00287-1. [DOI] [PubMed] [Google Scholar]
- 121.Kaminskas LM, Pyke SM, Burcham PC. Strong protein adduct trapping accompanies abolition of acrolein-mediated hepatotoxicity by hydralazine in mice. J Pharmacol Exp Ther. 2004;310:1003–10. doi: 10.1124/jpet.104.067330. [DOI] [PubMed] [Google Scholar]
- 122.Wang H, Manicke NE, Yang Q, Zheng L, et al. Direct analysis of biological tissue by paper spray mass spectrometry. Anal Chem. 2011;83:1197–201. doi: 10.1021/ac103150a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gurney AM, Allam M. Inhibition of calcium release from the sarcoplasmic reticulum of rabbit aorta by hydralazine. Br J Pharmacol. 1995;114:238–44. doi: 10.1111/j.1476-5381.1995.tb14931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bang L, Nielsen-Kudsk JE, Gruhn N, Trautner S, et al. Hydralazine-induced vasodilation involves opening of high conductance Ca2+-activated K+ channels. Eur J Pharmacol. 1998;361:43–9. doi: 10.1016/s0014-2999(98)00701-8. [DOI] [PubMed] [Google Scholar]
- 125.Khan MA. Effect of hydralazine in hypertension. Br Med J. 1953;1:27–9. doi: 10.1136/bmj.1.4800.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pandit RB. Long term propranolol and hydralazine in hypertension. J Assoc Physicians India. 1984;32:199–202. [PubMed] [Google Scholar]
- 127.Physicians’ Desk Reference. 54. Montvale, NJ: Medical Economics Company; 2000. [Google Scholar]
- 128.Talseth T. Studies on hydralazine. III. Bioavailability of hydralazine in man. Eur J Clin Pharmacol. 1976;10:395–401. doi: 10.1007/BF00563075. [DOI] [PubMed] [Google Scholar]
- 129.Wood PL, Khan MA, Moskal JR, Todd KG, et al. Aldehyde load in ischemia-reperfusion brain injury: neuroprotection by neutralization of reactive aldehydes with phenelzine. Brain Res. 2006;1122:184–90. doi: 10.1016/j.brainres.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 130.Nardil. New York, NY: Pfizer; 2009. [Google Scholar]
- 131.Reece PA. Hydralazine and related compounds: chemistry, metabolism, and mode of action. Med Res Rev. 1981;1:73–96. doi: 10.1002/med.2610010105. [DOI] [PubMed] [Google Scholar]
- 132.Sheehan DV, Claycomb JB, Kouretas N. Monoamine oxidase inhibitors: prescription and patient management. Int J Psychiatry Med. 1980;10:99–121. doi: 10.2190/uvvy-h0n2-b7v1-mhw0. [DOI] [PubMed] [Google Scholar]
- 133.Halliwell B. Reactive oxygen species and the central nervous system. J Neurochem. 1992;59:1609–1623. doi: 10.1111/j.1471-4159.1992.tb10990.x. [DOI] [PubMed] [Google Scholar]
- 134.Halliwell B. Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment.PG - 685–716. Drugs Aging. 2001:18. doi: 10.2165/00002512-200118090-00004. [DOI] [PubMed] [Google Scholar]
- 135.Hall ED, Braughler JM. Free radicals in CNS injury. Research Publications - Association for Research in Nervous & Mental Disease. 1993;71:81–105. [PubMed] [Google Scholar]
- 136.Kontos HA, Povlishock JT. Oxygen radicals in brain injury. Cent Nerv Syst Trauma. 1986;3:257–63. doi: 10.1089/cns.1986.3.257. [DOI] [PubMed] [Google Scholar]
- 137.Povlishock JT, Kontos HA. The role of oxygen radicals in the pathobiology of traumatic brain injury. Human Cell. 1992;5:345–53. [PubMed] [Google Scholar]