Bacteria can grow into surface-associated communities termed biofilms that are pervasive virtually everywhere on earth.[1] This mode of growth poses a significant obstacle to the successful treatment of infectious disease, with an estimated 80% of human infections in the biofilm state.[2] Biofilms are particularly problematic to clear due to their encasement in a protective and impermeable extracellular matrix,[3] which render biofilm-associated bacteria resistant to both host immune responses and standard antibiotic agents. Indeed, treatment with ~10-1000-fold higher doses of antibiotic is often required for biofilm clearance relative to planktonic bacteria.[2] Biofilm growth by the Gram-negative bacterium Pseudomonas aeruginosa has attracted particular attention, as biofilms of this pathogen are the origin of the fatal chronic lung infections in most cystic fibrosis patients.[4] P. aeruginosa biofilm infections also plague burn victims, AIDS patients, and are endemic on the medical implants and devices universal in healthcare today.[5] As such, the development of new methods to attenuate bacterial biofilm growth is of significant importance and represents a major research area.[6] Small molecules capable of inhibiting the growth of or removing (i.e., dispersing) preformed biofilms would be extremely useful to combat bacterial infection and in a range of other applications in industry, agriculture, and the environment.[7] Molecules of this class, however, remain rare.[5-8] Here, we report our discovery of a chemical approach for the inhibition and dispersion of P. aeruginosa biofilms based on 2-aminobenzimidazoles.
Biofilm growth only occurs after a critical bacterial cell density is achieved, and in many bacteria is under the direct control of the cell-cell signaling pathway termed quorum sensing (QS).[6-7, 9] Notably, P. aeruginosa mutants lacking a functional QS system are unable to grow into mature biofilms and are largely avirulent.[10] Our group and others have previously shown that non-native analogs of natural N-acylated L-homoserine lactone (AHL) QS signals can strongly modulate QS in Gram-negative bacteria,[11] and several of these AHLs also attenuate biofilm growth in P. aeruginosa.[8d] One challenge to the application of AHLs as biofilm or QS inhibitors, however, is the hydrolytic instability of the lactone head group.[12] Hydrolyzed AHLs are biologically inactive, and therefore additional measures (e.g., multiple dosing, controlled delivery, etc.) are required for sustained activity of AHLs.[13] Furthermore, and of particular relevance to biofilms, our early AHL-derived biofilm inhibitors failed to disperse preformed biofilms, similar to most antibiotics.[8d] In this context, we currently seek to identify alternative, hydrolytically stable molecular scaffolds for QS and/or biofilm modulation with enhanced activities.
A few natural products have been shown to inhibit bacterial QS or biofilm growth. The halogenated furanones from the macroalga Delisea pulchra have seen the most intensive study in this regard.[8b] Three other notable examples include bromoageliferin, 3-indolylacetonitrile, and resveratrol (Figure 1). Bromoageliferin displays anti-biofilm activity in the Gram-negative bacterium Rhodospirillum salexigens, and recent elegant studies by Melander and co-workers have revealed simplified analogs of this marine natural product with anti-biofilm activities (most notably, 2-aminoimidazole (2-AI) derivatives in Gram-negative bacteria[8a] and 5-amido or 5-imido 2-aminobenzimidazole (2-ABI) derivatives in Gram-positive bacteria).[14] The plant auxin 3-indolylacetonitrile was found to inhibit the formation of P. aeruginosa biofilms via a QS-dependent mechanism,[15] and the phytoalexin resveratrol[16] and related stilbene derivatives[17] have recently been shown to inhibit the LuxR-type QS receptors in Gram-negative bacteria. We reasoned that combining structural attributes of these three compounds into a simple molecular scaffold could reveal molecules with heightened anti-biofilm and/or QS properties.
Figure 1.
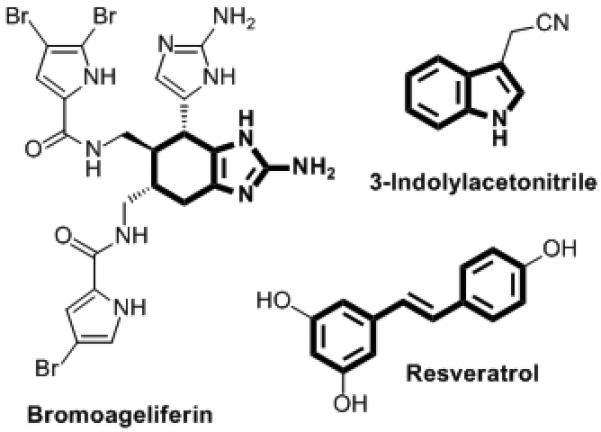
Selected natural products with anti-biofilm or QS activities. Substructures of interest to this work are shown in bold.
To explore this hypothesis, we designed and synthesized a small set of stilbenes containing 2-AI or indole moieties (Scheme 1). The 2-ABI-stilbene derivative 1 was synthesized by initial formation of the stilbene framework via the Heck reaction, and then a tin (II) dichloride reduction to form the diamine intermediate. Condensation with cyanogen bromide afforded stilbene 1. We generated 2-AI-stilbene 2 in good yield via the palladium-catalyzed regioselective arylation of imidazo[1,2-a]pyrimidine[18] followed by hydrazine-mediated pyrimidine ring cleavage.[19] Indole-stilbenes 4a-b were synthesized via Heck reactions according to published methods.[20]
Scheme 1.
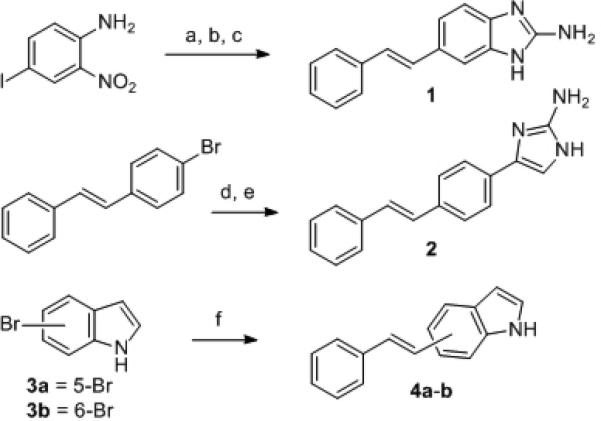
Reaction conditions for the synthesis of stilbene derivatives: (a) Styrene, Pd(OAc)2, CH3CN, DIPEA, 80 °C, 76%; (b) SnCl2·2H2O, EtOAc, 80 °C; (c) CNBr, MeOH:H2O 1:1, 50 °C, 91% (over two steps); (d) Imidazo[1,2-a]pyrimidine hydrobromide, Pd(OAc)2, PPh3, Cs2CO3, 1,4-dioxane, 100 °C, 82%; (e) 20% N2H4/EtOH, 105 °C, 84%; (f) Styrene, Pd(OAc)2, P(o-tolyl)3, NEt3, 100 °C, 73-76%. DIPEA = N,N-diisopropylethylamine.
We next tested the ability of stilbenes 1, 2, and 4a-b to inhibit biofilm formation in a wild-type P. aeruginosa strain (PAO1) at 500 μM using standard static biofilm growth assays. Biofilms were grown in a modified M9 minimal media in 96-well microtiter plates, and crystal violet staining of the surface-associated biomass was used to quantify biofilm growth at 12 and 24 h (see Supp. Info.) This preliminary screen and subsequent dose-response analyses revealed that 1 and 2 were able to inhibit biofilm growth at 24 h in P. aeruginosa by 56% and 48%, respectively, at 100 μM. Neither indole derivative (4a-b) showed appreciable anti-biofilm activity.
We selected 2-ABI-stilbene 1 as our most promising lead compound, and sought to identify potential structural motifs within 1 that were responsible for the observed anti-biofilm activity in order to further improve its inhibitory properties. We dissected stilbene 1 into five simple sub-structures (5-9, outlined in Figure 2), and tested each of these compounds in analogous P. aeruginosa biofilm assays. Removing the amino-group or 2-AI unit, affording 5 and 6, led to complete loss of inhibitory activity. However, removal of the styrene moiety (to yield 2-ABI 7) revealed a sub-structure that exhibited greater activity than lead compound 1, almost completely inhibiting biofilm formation at 24 h (94% inhibition, IC50 = 47 μM).[21],[22] The aryl component of 2-ABI (7) was essential for anti-biofilm activity, as neither 2-AI (8) nor guanidine (9) displayed significant inhibitory activity in P. aeruginosa.
Figure 2.
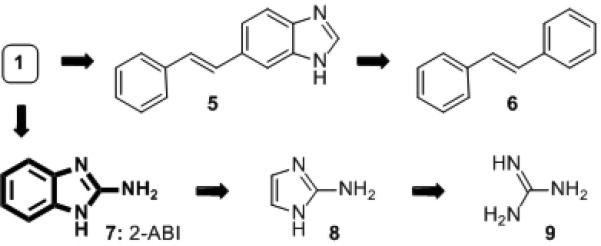
Compounds studied (5–9) to dissect the structural features necessary for the activity of initial lead stilbene 1.
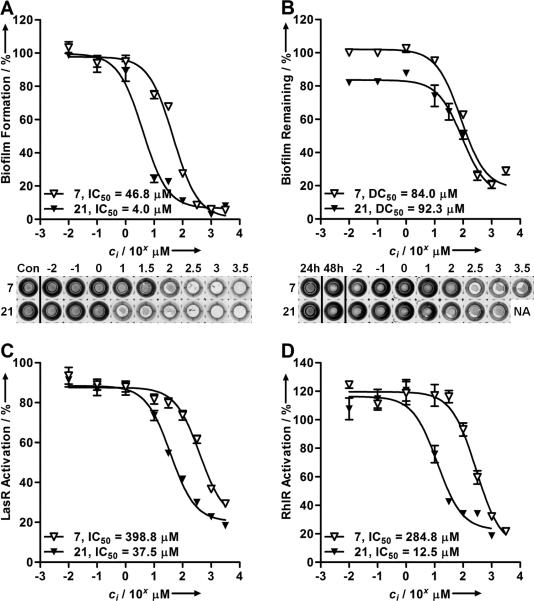
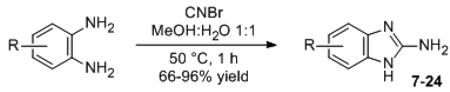
Intrigued by this result, we synthesized a small library of 2-ABI derivatives to further probe the activity of this compound class in P. aeruginosa. Fifteen 2-ABIs (10-24) were readily generated in one-step via condensation of various functionalized o-diaminobenzenes with cyanogen bromide (Table 1).[23] We examined the biofilm inhibitory activities of these compounds in P. aeruginosa, and identified several 2-ABI derivatives capable of inhibiting biofilm growth by ~90%, but with improved potencies (i.e., lower IC50 values) relative to 7. An increase in potency was achieved either by halide (10-13) or methyl substitutions (19-21) on the 2-ABI aryl group. In turn, aryl-substitutions containing hydrogen bond donors or acceptors led to either a total loss (16) or significant reductions in biofilm inhibitory activity (Table 1). The most potent biofilm inhibitor identified overall was the 5,6-dimethyl 2-ABI (21, IC50 = 4.0 μM), which was ~10-fold more active than the parent compound 7. Amongst the few biofilm inhibitors for which IC50 data has been reported,[8a, 8c, 8e, 8g] 21 constitutes one of the most active P. aeruginosa biofilm inhibitors known. Dose-response curves and images of crystal violet-stained biofilms in the presence of 7 and 21 are shown in Figure 3A.
Table 1.
P. aeruginosa PAO1 biofilm inhibition data for 2-ABI derivatives.
| ||
|---|---|---|
| Compound | R | IC50 (μM)[a] |
| 7 | H | 47 |
| 10 | 5-I | 20 |
| 11 | 5-Br | 22 |
| 12 | 5-Cl | 28 |
| 13 | 5-F | 35 |
| 14 | 5-COPh | ND |
| 15 | 5-CO2Me | 140 |
| 16 | 5-CO2H | ND |
| 17 | 5-CN | 180 |
| 18 | 5-NO2 | 63 |
| 19 | 4-Me | 39 |
| 20 | 5-Me | 25 |
| 21 | 5,6-Me | 4.0 |
| 22 | 5-OMe | 80 |
| 23 | 5-NH2 | ND |
| 24 | Fused 5,6-Ph[b] | 48 |
IC50 values were only obtained for 2-ABI derivatives exhibiting >60% biofilm inhibition after 24 h; ND = not determined. See Supp. Info. for 95% confidence intervals for IC50 values.
Full name: 2- amino-1H-naphtho[2,3-d]imidazole.
Figure 3.
Dose-response curves and images of crystal violet biofilm inhibitory (A), and dispersion (B) assays for 7 and 21 in P. aeruginosa (PAO1). NA = not available. Dose-response curves for 7 and 21 in P. aeruginosa PAO1/plasI-LVAgfp (C), and PAO1/pRhI-LVAgfp (D) QS reporter strains.
Compounds capable of not only inhibiting biofilm growth, but also dispersing preformed biofilms, are of particular value for a range of clinical and other applications. We thus tested the ability of compounds 7 and 21 to disperse 24 h-old P. aeruginosa biofilms using the crystal violet staining assay (Figure 3B). Biofilms were allowed to develop in the absence of compound for 24 h, after which non-biofilm material was removed by washing with buffer and fresh media with compound was added. Biofilm was quantified after an additional 24 h, and the amount of dispersed biofilm was determined via comparison of the amount of biofilm at 48 h in the presence of compound versus the amount of biofilm at 24 h in the absence of compound. We found that 2-ABIs 7 and 21 were capable of strongly dispersing P. aeruginosa biofilms (~80%), with half maximal dispersion (DC50) values of 84 μM and 92 μM, respectively (Figure 3B).
Little is known about the actual mechanisms of action of most small molecule biofilm inhibitors. As such, we sought to investigate the mechanism by which the 2-ABI scaffold elicits its biofilm inhibitory and dispersive activity in P. aeruginosa. Planktonic growth curve analyses (under conditions identical to biofilm growth) demonstrated that the observed activities were not a result of a bactericidal mechanism. Melander and co-workers have shown that 2-ABI derivatives bearing 5-amido substituents inhibit and disperse biofilms through a zinc-dependent mechanism, albeit in Gram-positive as opposed to Gram-negative bacteria (see above).[14] We screened a wide range of metals, including zinc, in a dose-dependent manner for mitigating effects on the biofilm inhibitory activity of 7 in P. aeruginosa, but observed no change in activity.
As introduced above, the role of QS in biofilm formation is well documented,[6-8, 9] and therefore we next evaluated the abilities of 7 and 21 to inhibit QS in P. aeruginosa. For this purpose, we prepared two wild-type P. aeruginosa QS reporter strains that contain the plasmids plasI-LVAgfp and prhlI-LVAgfp (see Supp. Info.) These strains report the activity of two intracellular QS receptors in P. aeruginosa (LasR and RhlR) by the production of green fluorescent protein (gfp), allowing for LasR and RhlR activities, and thus QS levels, to be quantified by fluorescence. We observed a significant reduction in both LasR and RhlR activities in the presence of 7 and 21 at 1–10× their IC50 values for biofilm inhibition (Figures 3C–D). Studies of related 2-ABI derivatives have shown that this class of molecules is bacterial cell permeable, allowing us to surmise that 7 and 21 could act on the Las and Rhl systems intracellularly.[24] Further, we utilized a P. aeruginosa strain that constitutively-expresses genomic gfp to demonstrate that 7 and 21 do not simply affect global protein synthesis (see Supp. Info.) Additional experiments are required to elucidate the precise targets of 7 and 21 that result in QS disruption. Nevertheless, these preliminary findings suggest that biofilm modulation by 2-ABI derivatives could be occurring, at least in part, through interference with the P. aeruginosa Las and Rhl QS circuits.
In summary, we have identified 2-ABI derivatives as potent anti-biofilm agents in P. aeruginosa. We uncovered this compound class through the study of hybrid compounds derived from the structures of three natural products with known biofilm and QS inhibitory activities, and the subsequent structure-activity analyses of simplified derivatives. This discovery is significant, as several of these 2-ABI derivatives are among the most active P. aeruginosa biofilm modulators to be reported. Moreover, these compounds are capable of both inhibiting the growth of and dispersing preformed biofilms. Our results are surprising in light of previous data on related 2-ABI derivatives that indicated they were inactive in P. aeruginosa,[14] and support the continued study of this structurally simple, chemically robust compound class in Gram-negative bacteria.[25] Lastly, our studies indicate that 2-ABIs 7 and 21 are also capable of QS inhibition in P. aeruginosa, suggesting a possible mechanism for biofilm inhibition. A link between 2-ABI-type anti-biofilm agents and QS, to our knowledge, is previously undocumented. Ongoing work in our laboratory is directed towards the study of additional 2-ABI derivatives, as well as assessing their biofilm inhibitory activities across an expanded set of bacterial species.
Experimental Section
Full details of compound synthesis, compound characterization data, and biological protocols and assay data can be found in the Supporting Information.
Footnotes
Financial support for this work was provided by the NIH (AI063326), ONR (N00014-07-1-0255), Greater Milwaukee Foundation Shaw Scientist Program, Burroughs Welcome Fund, and Johnson & Johnson. A.S.B. was funded in part by an NIH Chemistry-Biology Interface Training Grant (NIGMS T32 GM008505). We gratefully acknowledge Prof. Barbara Iglewski and Prof. Søren Molin for donations of bacterial strains, and J. P. Gerdt for technical assistance.
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.Hall-Stoodley L, Costerton JW, Stoodley P. Nat. Rev. Microbiol. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 2.Davies D. Nat. Rev. Drug Disc. 2003;2:114–122. doi: 10.1038/nrd1008. [DOI] [PubMed] [Google Scholar]
- 3.Flemming HC, Wingender J. Nat. Rev. Microbiol. 2010;8:623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- 4.Costerton JW, Stewart PS, Greenberg EP. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 5.Smith KM, Bu YG, Suga H. Chem. Biol. 2003;10:81–89. doi: 10.1016/s1074-5521(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 6.Musk DJ, Hergenrother PJ. Curr. Med. Chem. 2006;13:2163–2177. doi: 10.2174/092986706777935212. [DOI] [PubMed] [Google Scholar]
- 7.Sintim HO, Smith JA, Wang J, Nakayama S, Yan L. Future Med. Chem. 2010;2:1005–1035. doi: 10.4155/fmc.10.185. [DOI] [PubMed] [Google Scholar]
- 8.a Richards JJ, Melander C. Anti-Infective Agents Med. Chem. 2009;8:295–314. [Google Scholar]; b Hentzer M, Riedel K, Rasmussen TB, Heydorn A, Andersen JB, Parsek MR, Rice SA, Eberl L, Molin S, Hoiby N, Kjelleberg S, Givskov M. Microbiology. 2002;148:87–102. doi: 10.1099/00221287-148-1-87. [DOI] [PubMed] [Google Scholar]; c Musk DJ, Banko DA, Hergenrother PJ. Chem. Biol. 2005;12:789–796. doi: 10.1016/j.chembiol.2005.05.007. [DOI] [PubMed] [Google Scholar]; d Geske GD, Wezeman RJ, Siegel AP, Blackwell HE. J. Am. Chem. Soc. 2005;127:12762–12763. doi: 10.1021/ja0530321. [DOI] [PubMed] [Google Scholar]; e Junker LM, Clardy J. Antimicrob. Agents Chemother. 2007;51:3582–3590. doi: 10.1128/AAC.00506-07. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Kim C, Kim J, Park HY, Park HJ, Lee JH, Kim CK, Yoon J. Appl. Microbiol. Biotechnol. 2008;80:37–47. doi: 10.1007/s00253-008-1474-6. [DOI] [PubMed] [Google Scholar]; g Sambanthamoorthy K, Gokhale AA, Lao WW, Parashar V, Neiditch MB, Semmelhack MF, Lee I, Waters CM. Antimicrob. Agents Chemother. 2011;55:4369–4378. doi: 10.1128/AAC.00583-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, Greenberg EP. Science. 1998;280:295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 10.a Tang HB, DiMango E, Bryan R, Gambello M, Iglewski BH, Goldberg JB, Prince A. Infect. Immun. 1996;64:37–43. doi: 10.1128/iai.64.1.37-43.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Rumbaugh KP, Griswold JA, Hamood AN. Microbes Infect. 2000;2:1721–1731. doi: 10.1016/s1286-4579(00)01327-7. [DOI] [PubMed] [Google Scholar]
- 11.Geske GD, O'Neill JC, Blackwell HE. Chem. Soc. Rev. 2008;37:1432–1447. doi: 10.1039/b703021p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glansdorp FG, Thomas GL, Lee JJK, Dutton JM, Salmond GPC, Welch M, Spring DR. Org. Biomol. Chem. 2004;2:3329–3336. doi: 10.1039/B412802H. [DOI] [PubMed] [Google Scholar]
- 13.a Breitbach AS, Broderick AH, Jewell CM, Gunasekaran S, Lin Q, Lynn DM, Blackwell HE. Chem. Commun. 2011;47:370–372. doi: 10.1039/c0cc02316g. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Palmer AG, Streng E, Blackwell HE. ACS Chem. Biol. 2011;6:1348–1356. doi: 10.1021/cb200298g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rogers SA, Huigens RW, 3rd, Melander C. J. Am. Chem. Soc. 2009;131:9868–9869. doi: 10.1021/ja9024676. [DOI] [PubMed] [Google Scholar]
- 15.Lee J-H, Cho MH, Lee J. Environ. Microbiol. 2011;13:62–73. doi: 10.1111/j.1462-2920.2010.02308.x. [DOI] [PubMed] [Google Scholar]
- 16.Fulghesu L, Giallorenzo C, Savoia D. J. Chemotherapy. 2007;19:388–391. doi: 10.1179/joc.2007.19.4.388. [DOI] [PubMed] [Google Scholar]
- 17.Frei R, Blackwell HE. Chem.-Eur. J. 2010;16:2692–2695. doi: 10.1002/chem.200903445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li WJ, Nelson DP, Jensen MS, Hoerrner RS, Javadi GJ, Cai D, Larsen RD. Org. Lett. 2003;5:4835–4837. doi: 10.1021/ol035878k. [DOI] [PubMed] [Google Scholar]
- 19.Ermolat'ev DS, Van der Eycken EV. J. Org. Chem. 2008;73:6691–6697. doi: 10.1021/jo8008758. [DOI] [PubMed] [Google Scholar]
- 20.Yang JS, Liau KL, Li CY, Chen MY. J. Am. Chem. Soc. 2007;129:13183–13192. doi: 10.1021/ja0741022. [DOI] [PubMed] [Google Scholar]
- 21.2-thiobenzimidazole (2-TBI) derivatives have been reported to display anti-biofilm activity in P. aeruginosa (see ref. 8g). However, 2-TBI and 5-OMe-2-TBI were markedly less active than the 2-ABI derivatives in our biofilm assay.
- 22.Several 2-ABI derivatives were recently claimed to be P. aeruginosa biofilm inhibitors in a patent application. No biological data was provided in support of this claim. See: Eur. Pat. Appl. 2010;(A1) WO 2010144686.
- 23.Valdez J, Cedillo R, Hernandez-Campos A, Yepez L, Hernandez-Luis F, Navarrete-Vazquez G, Tapia A, Cortes R, Hernandez M, Castillo R. Bioorg. Med. Chem. Lett. 2002;12:2221–2224. doi: 10.1016/s0960-894x(02)00346-3. [DOI] [PubMed] [Google Scholar]
- 24.a Grossman TH, Mani N, Gross CH, Parsons JD, Hanzelka B, Muh U, Mullin S, Liao YS, Grillot AL, Stamos D, Charifson PS. Antimicrob. Agents Chemother. 2006;50:1228–1237. doi: 10.1128/AAC.50.4.1228-1237.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Coldham NG, Webber M, Woodward MJ, Piddock LJV. J. Antimicrob. Chemother. 2010;65:1655–1663. doi: 10.1093/jac/dkq169. [DOI] [PubMed] [Google Scholar]
- 25.In the course of these studies, 2-ABI derivative 18 was shown to be a biofilm inhibitor in Gram-positive bacteria. See: Liu C, Worthington RJ, Melander C, Wu H. Antimicrob. Agents Chemother. 2011;55:2679–2687. doi: 10.1128/AAC.01496-10.