Lignocellulosic biomass has recently received great interest as a renewable source of fuel and chemicals.[1] Among the three major components of non-edible lignocellulose (cellulose, hemicellulose, and lignin), extensive efforts have been made to convert cellulose to ethanol and other biofuels. In contrast, research on the conversion of lignin has been limited to its removal from biomass either to enhance the accessibility of chemicals and enzymes to other components of biomass or to prevent photo-yellowing of paper and pulp. Despite the fact that lignin corresponds up to 30% of the weight and 40% of the energy content of lignocellulosic biomass, few novel processes aimed at producing high value compounds have been reported. Recently, several reports inspired by the pulp bleaching process have been published regarding the mechanism and product distribution of enzymatic and chemical oxidation reactions.[2] Using dimeric lignin model compounds (e.g. 1) containing a β-O-4 linkage that represents the most common substructure in lignin,[3] aromatic aldehydes were obtained as the main products in low yield. Although these methods show promises for selective conversion of lignin, fundamentally new catalytic processes need to be developed to fully realize lignin's potential as a chemical feedstock. In addition, thorough understanding of the mechanism of these processes is necessary to successfully achieve high selectivity.
Aiming to develop a novel method to selectively convert lignin to highly functionalized aromatic compounds, we explored various homogeneous vanadium complexes for the conversion of 1 (Table 1).[4] Most of the vanadium catalysts tested yielded benzylic alcohol oxidation product 4 as the major product[5] in addition to small amounts of C–O bond cleavage products 2 and 3 (entries 2–7). In spite of the low yield, the formation of 2 distinguishes this reaction from previous reports: not only is 2 a novel product, but it is also a redox-neutral transformation. Excited by this new reactivity, we explored other vanadium catalysts and found that tridendate Schiff base ligands favor C–O bond cleavage over benzylic oxidation (entries 8–11). Higher selectivity for C–O bond cleavage was observed when ligands with larger bite angles were employed (entries 8 vs. 9 and 10 vs. 11).[6] The increased reactivity of catalyst 11 compared to 9 (entry 11 vs. 9) may be attributed to its tBu substituents, which enable intermediates from 11 to remain as catalytically active monomeric species instead of forming insoluble aggregates.[7] Thus, through subtle changes in the ligand structure, the reactivity of the vanadium(V)-oxo catalyst was tuned away from simple alcohol oxidation toward the cleavage of the β-O-4 carbon-oxygen bond.
Table 1.
Ligand effects on degradation of lignin model compound 1.
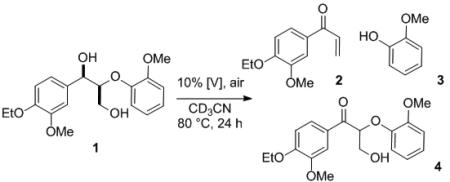
| |||||
|---|---|---|---|---|---|
| entry[a] | [V] | conversion (%) | 2 (%) | 3 (%) | 4 (%) |
| 1 | None | 0 | – | – | – |
| 2 | VOSO4·xH2O | 34 | 2 | 2 | 6 |
| 3 | VO(acac)2 | 79 | 13 | 22 | 31 |
| 4 | VO(Oi-Pr)3 | 82 | 5 | 11 | 45 |
| 5 | 5 | 86 | 6 | 6 | 59 |
| 6 | 6 | 66 | 13 | 14 | 41 |
| 7 | 7 | 55 | 3 | – | 37 |
| 8 | 8 | >95 | 61 | 45 | 27 |
| 9 | 9 | 86 | 70 | 62 | 8 |
| 10 | 10 | 95 | 65 | 50 | 18 |
| 11 | 11 | >95 | 82 | 57 | 7 |
By 1H NMR versus an internal standard.
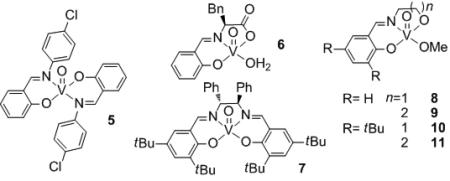
To understand the role of oxygen in the formally non-oxidative process, the reaction was performed under anaerobic conditions with catalyst 11. After 24 h, the same products were obtained as those under aerobic conditions, albeit with lower conversion, along with pale purple precipitate (Scheme 1). The collected precipitate exhibited the same analytical properties as independently prepared V(IV) complex 12.[8] The EPR spectrum of the dark purple reaction mixture under aerobic conditions with 11 after 30 minutes also indicated the presence of V(IV) species. The V(IV) complex 12 is insoluble in various organic solvents and stable under air in the solid state. However, a heterogeneous reaction mixture with 12 turns into a dark purple solution under aerobic reaction conditions and furnishes a similar result to that with 11: >95% conversion, 80% 2, 58% 3, and 1% 4 (Scheme 2). These results indicate that the V(V) catalyst is reduced to V(IV) species during the reaction, but oxygen is not essential for the catalyst turnover, although it does increase the reaction rate. The facile interconversion between 11 and 12 allows one to employ the high reactivity of a homogeneous catalyst during the reaction and then easily recover the vanadium catalyst as an insoluble complex after the reaction by simply controlling the reaction atmosphere.
Scheme 1.
Degradation of 1 under anaerobic conditions.
Scheme 2.
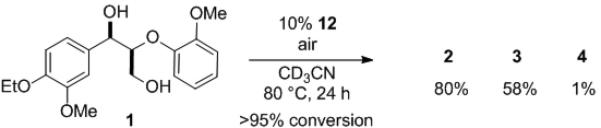
Recovery of the reactivity of V(IV) complex 12 under aerobic conditions.
To gain further insight on the mechanism of the non-oxidative C–O bond cleavage, we performed the reaction with various derivatives of 1 (Scheme 3). To test the possibility of oxidation of 1 to ketone 4 followed by reductive cleavage, 4 was subjected to the reaction conditions with or without benzylic alcohol 13. In both cases, 4 was recovered in high yield without any evidence of degradation to 2 or 3. The corresponding ketone 14 was obtained when 13 was added to reproduce the catalytic species formed after oxidation of 1 to 4. The lack of reactivity of 4 under the reaction conditions eliminates the possibility of a sequential oxidation and cleavage process of 1. Additionally, this experiment suggests that a V(III)–V(V) cycle,[9] which has been proposed for some vanadium-catalyzed aerobic alcohol oxidation,[4a,4c,4d] is likely not operational for this transformation. When the benzylic hydroxy group was replaced by a methoxy group (1a), the reaction proceeded with only 12% conversion, to afford conjugated aldehyde 15 as the product, indicating the importance of ligand exchange on 11 with the benzylic hydroxy group. In contrast, methyl ether 1b was converted to 2 and 3 with only slightly diminished yield and selectivity compared to 1. When the aryloxy group was absent (1c), only the corresponding ketone 16 was observed.
Scheme 3.
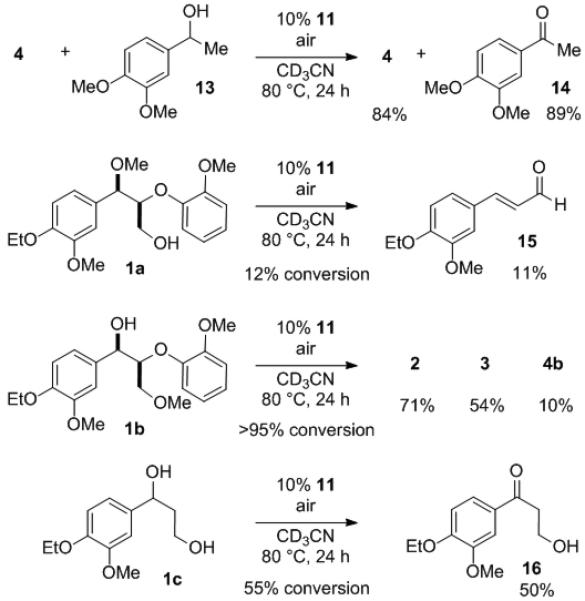
Reactivity study of analogues of 1.
Based on these data, we propose a one-electron process as the most plausible mechanism (Figure 1). After ligand exchange on the vanadium complex with the benzylic hydroxy group, the benzylic hydrogen is abstracted to generate the ketyl radical, which eliminates the aryloxy radical.[10] The elimination of the hydroxy group from the resulting enolate produces enone 2 and a vanadium(IV) complex (17a or 17b), which is re-oxidized to vanadium(V) by the aryloxy radical.[11] Degradation of lignin via a ketyl radical had been suggested by several groups as a mechanism responsible for photo-yellowing of paper.[12] However, this hypothesis was later disputed because the ketyl radical generated by various methods reacted readily with oxygen to produce the corresponding ketone, and only a small amount of fragmentation products was formed by secondary photolysis of this ketone.[13] The high yield and good selectivity observed for β-O-4 cleavage of vanadium-catalyzed reaction indicates a dramatic reactivity change of the ketyl radical by coordination to vanadium.
Figure 1.
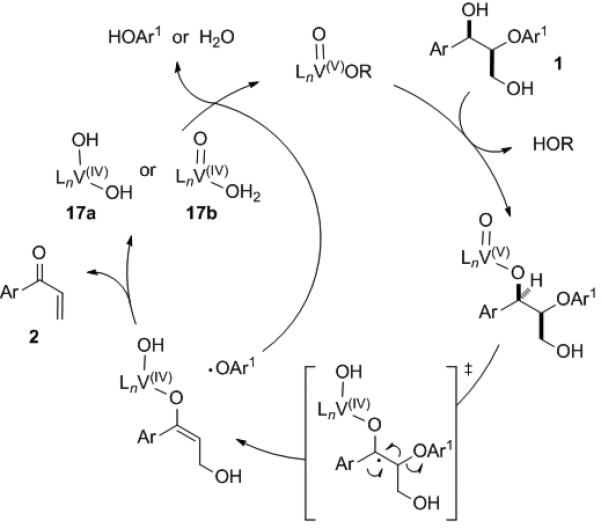
Plausible mechanism for vanadium-catalyzed non-oxidative cleavage of 1.
Formation of the insoluble V(IV) complex 12 and only a slightly lower turnover number under anaerobic conditions suggests that regeneration of a V(V) complex by the aryloxy radical competes with formation of an insoluble V(IV) aggregate. Molecular oxygen probably accelerates the reaction by increasing the effective concentration of catalytically active species by converting insoluble 12 to soluble V(V) species.
Although the vanadium-catalyzed non-oxidative degradation of 1 proceeds with high efficiency and selectivity in CH3CN, it is desirable to utilize solvents without nitrogen atoms for application to biofuel synthesis to prevent formation of NOx. When the reaction was performed in EtOAc on 1 mmol scale, the desired products were obtained with higher yields and selectivity than reactions run in CH3CN (Scheme 4).[14] Moreover, a more complex trimeric model compound 18[2f, 15] underwent clean C–O cleavage to provide three monomeric compounds, demonstrating the possibility of application of this reaction to more complex systems (Scheme 5).[16]
Scheme 4.
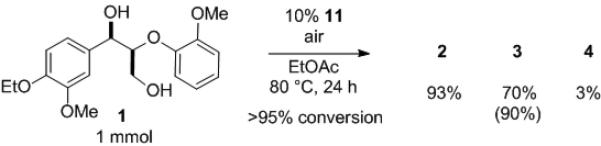
Conversion of 1 on 1 mmol scale in a solvent without nitrogen atoms.
Scheme 5.
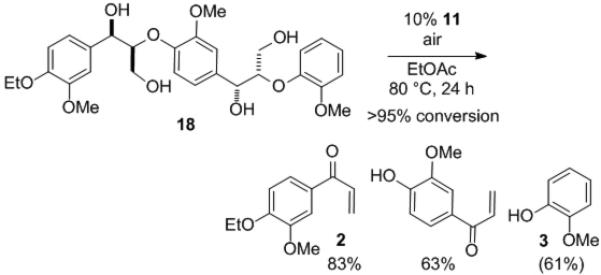
Conversion of trimeric lignin model compound 18.
In conclusion, we have demonstrated that changes in the ancilliary ligands can divert the reactivity of vanadium-oxo complexes from the typical alcohol oxidation to an unprecedented C-O bond cleavage reaction. This novel reactivity has been applied to a vanadium-catalyzed non-oxidative C–O bond cleavage reactions of dimeric lignin model compounds that produces aryl enones as unprecedented degradation products. This transformation is proposed to proceed via a ketyl radical generated by hydrogen atom transfer to a vanadium(V) complex. Oxygen is not essential for the reaction, although it increases the reaction rate. The novel reactivity of this transformation combined with good selectivity for a highly functionalized aryl enone demonstrates a great potential of lignin as a chemical feedstock. Further mechanistic studies and applications to biomass degradation are underway.
Footnotes
FDT gratefully acknowledges NIHGMS (R01 GM074774) and British Petroleum through the EBI for financial support of this work.
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.For recent reviews, see: Corma A, Iborra S, Velty A. Chem. Rev. 2007;107:2411. doi: 10.1021/cr050989d.Chheda JN, Huber GW, Dumesic JA. Angew. Chem. Int. Ed. 2007;46:7164. doi: 10.1002/anie.200604274.Huber GW, Corma A. Angew. Chem. Int. Ed. 2007;46:7184. doi: 10.1002/anie.200604504.Huber GW, Iborra S, Corma A. Chem. Rev. 2006;106:4044. doi: 10.1021/cr068360d.
- 2.a Sales FG, Maranhao LCA, Filho NML, Abreu CAM. Chem. Eng. Sci. 2007;62:5386. [Google Scholar]; b Kawai S, Kobayashi Y, Nakagawa M, Ohashi H. J. Wood. Sci. 2006;52:363. [Google Scholar]; c Crestini C, Caponi MC, Argyropoulos DS, Saladino R. Bioorg. Med. Chem. 2006;14:5292. doi: 10.1016/j.bmc.2006.03.046. [DOI] [PubMed] [Google Scholar]; d Bohlin C, Persson P, Gorton L, Lundquist K, Jeonsson LJ. J. Mol. Cat. B; Enzym. 2005;35:100. [Google Scholar]; e Crestini C, Pastorini A, Tagliatesta P. Eur. J. Inorg. Chem. 2004:4477. [Google Scholar]; f Baciocchi E, Fabbri C, Lanzalunga O. J. Org. Chem. 2003;68:9061. doi: 10.1021/jo035052w. [DOI] [PubMed] [Google Scholar]
- 3.Brunow G, Lundquist K, Gellersted G. In: Analytical Methods in Wood Chemistry, Pulping, and Paper-making. Sjöström E, Alén R, editors. Vol. 1. Springer-Verlag; Berlin: 1999. p. 77. [Google Scholar]
- 4.Typical experimental procedure: A 4 mL vial charged with 1 (17 mg, 0.05 mmol), catalyst (10 mol%), and CD3CN (0.5 mL) was closed with a screw cap and heated in an oil bath at 80 °C for 24 h. The reaction mixture was cooled to room temperature, and internal standard (2-timethylsilylethanol, 7.2 μL, 0.05 mmol) was added before 1H NMR analysis. ACS grade solvents were used as received, and no special precaution was taken to exclude trace amounts of water from the reaction mixture.
- 5.For recent examples of vanadium-catalyzed aerobic alcohol oxidation, see: Figiel PJ, Sobczak JM. J. Catal. 2009;263:167.Jiang N, Ragauskas AJ. J. Org. Chem. 2007;72:7030. doi: 10.1021/jo0707737.Pawar VD, Bettigeri S, Weng SS, Kao JQ, Chen CT. J. Am. Chem. Soc. 2006;128:6308. doi: 10.1021/ja060639o.Radosevich AT, Musich C, Toste FD. J. Am. Chem. Soc. 2005;127:1090. doi: 10.1021/ja0433424.Velusamy S, Punniyamurthy T. Org. Lett. 2004;6:217. doi: 10.1021/ol036166x.Maeda Y, Kakiuchi N, Matsumura S, Nishimura T, Kawamura T, Uemura S. J. Org. Chem. 2002;67:6718. doi: 10.1021/jo025918i.
- 6.For X-ray structure data of these compounds, see supporting information.
- 7.As the reaction progresses, the dark purple reaction mixture with catalyst 9 turns clear, and grey precipitate is formed. The reaction mixture with catalyst 11 stays as a dark purple solution. X-ray crystal structure data show that 9 has a dimer unit cell unlike 11 with a monomeric unit cell in solid state.
- 8.12 was prepared following a procedure for similar V(IV) complexes: Maurya MR, Kumar U, Manikandan P. Eur. J. Inorg. Chem. 2007:2303. See supporting information for details.
- 9.For a recent report on the evidence of reduction of V(V) to V(III) under anaerobic conditions, see: Hanson SK, Baker RT, Gordon JC, Scott BL, Sutton AD, Thorn DL. J. Am. Chem. Soc. 2009;131:428. doi: 10.1021/ja807522n.
-
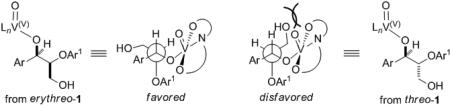
10.A concerted mechanism is proposed based on the slower reaction rate of threo-1. The rationalization of this rate difference is based on the required anti-periplanar alignment of hydrogen and aryloxy group for a concerted transition state as shown below.
- 11.Intermediates and thermodynamic study of hydrogen atom transfer reactions between vanadium oxo complexes and phenol derivatives have recently been reported: Waidmann CR, Zhou X, Tsai EA, Kaminsky W, Hrovat DA, Borden WT, Mayer JM. J. Am. Chem. Soc. 2009;131:4729. doi: 10.1021/ja808698x.
- 12.a Schmidt JA, Berinstain AB, De Rege F, Heitner C. Can. J. Chem. 1991;69:104. [Google Scholar]; b Scaiano JC, Netto-Ferreira JC, Wintgens V. J. Photochem. Photobiol. A: Chem. 1991;59:265. doi: 10.1039/b108116k. [DOI] [PubMed] [Google Scholar]; c Scaiano JC, Whittlesey MK, Berinstain AB, Malenfant PRM, Schuler RH. Chem. Mater. 1994;6:836. [Google Scholar]
- 13.a Huang Y, Pagé D, Wayner DDM, Mulder P. Can. J. Chem. 1995;73:2079. [Google Scholar]; b Ruggiero R, Machado AEH, Castellan A, Grelier S. J. Photochem. Photobiol. A: Chem. 1997;110:91. [Google Scholar]; c Fabbri C, Bietti M, Lanzalunga O. J. Org. Chem. 2005;70:2720. doi: 10.1021/jo047826u. [DOI] [PubMed] [Google Scholar]
- 14.1H NMR of the crude reaction mixture indicated ca. 90% yield of 3, but the isolated yield was significantly lower (70%) due to its high volatility.
- 15.Ciofi-Baffoni S, Banci L, Brandi A. J. Chem. Soc., Perkin Trans. 1998;1:3207. [Google Scholar]
- 16.When a mixture of 1 and cellobiose, a model compound for cellulose, was subjected to the standard reaction conditions as in Scheme 3, the reaction proceeded with the same efficiency and selectivity as without cellobiose (Table 1, entry 11): >95% conversion, 85% 2, 59% 3, and 7% 4. In addition, no conversion of cellobiose was observed.


