Abstract
The marine-derived α-galactosylceramide is an exogenous ligand for natural killer T cells and leads to the secretion of both T help 1 (Th1) and Th2 cytokines. The relationship between the sugar moiety structure and invariant natural killer T (iNKT) cell stimulation ability has not been fully understood. With the series α-galactosylceramide analogues varied on C3′ and C4′ position, subjected to a murine system, we discovered that the 3′ hydroxyl is very crucial in maintaining the molecule’s immunogenicity. Any modification on this position will lead to the losing of activity. We also found that the C4′ position is not so sensitive and can tolerate some small modifications on it. Moreover, the C4′ substituted analogues induced biased Th2 cytokines release was observed.
INTRODUCTION
The marine-derived α-galactosylceramide (α-GalCer) was originally found to possess the ability to stimulate the secretion of different types of cytokines from natural killer T (iNKT) cells.[1] Specifically, a major histocompatibility complex (MHC) I-like protein named CD1d presents α-GalCer on the surface of antigen presenting cells (APC), enabling recognition by the T cell receptor (TCR) of iNKT cells.[2] Such recognition then triggers release of a large amount of cytokines in a relatively short time by iNKT cells. These cytokines include the inflammatory T help 1 (Th1) cytokines (e.g. IFN-γ and IL-2) and immunomodulatory Th2 cytokines (e.g. IL-4 and IL-10), which can regulate both the innate and adaptive immune responses. iNKT cells thus serve as the bridge between these two types of immune responses. Given these properties, α-GalCer has been extensively applied and studied in preclinical and clinical trials for treating various types of diseases such as cancer,[3] type I diabetes,[4] hepatitis,[5] and Pseudomonas aeruginosa infection.[6] There are promising results showing α-GalCer can suppress and/or reject tumor growth in a mice model.[7]
α-GalCer differs from the mammalian glycolipids by its distinguished α-linkage between the sugar moiety and lipid moiety, whereas the mammalian glycolipids’ anomeric linkage is β configuration.[8] This unique structure enables the acyl chain and phytosphingosine chain of α-GalCer to bind in the two deep pockets of CD1d while anchoring the sugar moiety above the surface of the protein in a position suitable for recognition by the iNKT TCR.[9] Such presentation by CD1d is stabilized by the 3-hydroxyl on phytosphingosine and the O-glycosidic linkage, which form H-bonding interactions with CD1d. Furthermore, the 2′ hydroxyl of galactose displays its importance by forming another H-bond with CD1d (mouse Asp153, human Asp151). These interactions stabilize the glycolipid/CD1d complex and orientate the sugar moiety in the correct position for recognition.[9a] In regards to this recognition, the ternary TCR/glycolipid/CD1d crystal structure revealed an additional crucial role of the 2′ hydroxyl group of α-GalCer, interaction with TCR by formation of H-bond with the Gly96α on the CDR3α loop.[9d] The crystal structural analysis is consistent with experiments in which substituting the 2′ equatorial hydroxyl group with fluoride, hydrogen, acetamide,[10] or an axial hydroxyl (mannosylceramide)[1a] resulted in total abolishment of ativity. The 6′ hydroxyl, however, does not form any H-bond with either CD1d or TCR,[9d] and was shown to be able to tolerate some modifications without affecting the glycolipid antigenicity.[1a,11] Nonetheless, few conclusions have been reached for the 3′ and 4′ hydroxyls except some initial results that attaching additional sugar moieties on these positions leads to elimination of activity.[11a,12] The ternary crystal structure does illustrate, however, that the 3′ and 4′ hydroxyls also make H-bonds with invariant TCR α-chain residues Ser3α and Phe29α.[9d] Yet, these H-bonds may not be as crucial as that of the 2′ hydroxyl since the α-glucosylceramide (α-GlcCer), which has an equatorial hydroxyl instead of axial hydroxyl as in α-GalCer, can still stimulate iNKT cells, albeit to a lesser extent.[1a]
RESULTS AND DISCUSSION
Based on these observations, we hypothesized that the 4′ hydroxyl group is not as essential as the 2′ hydroxyl group, although it contributes to the interaction with TCR, and some rational modifications can be carried out on it to change the profiles of the resulting derivatives. The role of the 3′ hydroxyl group is ambiguous, however, thus dictating the need for a structure-activity relationship study to clarify its importance. In this study, we accordingly utilized a variety of analogues of α-GalCer with varying substitutions at the C3′ or C4′ position of the galactose ring in order to elucidate the roles of these two hydroxyls in iNKT cell stimulation.
In a variety of C3′ analogues, the hydroxyl group was first replaced with the non H-bonding azido group to give 3′-azido-α-GalCer 2 (Figure 1). The azido group was then reduced to amino group, leading to an ionically charged analogue 3′-amino-α-GalCer 3, which was hypothesized to potentially form a salt bridge with the TCR loops. The amino group was further converted to an acetamide group, yielding 3′-NAc-α-GalCer 4, which should possess different preferences in H-bond formation compared to α-GalCer 1. For the C4′ analogues, the acetamide group was also exploited to replace the hydroxyl in the analogue 4′-NAc-α-GalCer 5. In addition, a methyl group was introduced onto the 4′ hydroxyl so that the resulting 4′-MeO-α-GalCer 6 would only serve as H-bond acceptor, whereas a hydroxyl group could act as both a donor and acceptor. In order to probe the importance of distance between the 4′ hydroxyl and TCR, 4′-ethanol-α-GalCer 7 was also synthesized by elongating the hydroxyl with two additional carbons. Finally, the 3′ and 4′ deoxy analogues 8 and 9 were also used as control compounds.[13]
Figure 1.

Structures of α-GalCer 1 and its analogues with different substations on the C3′ or C4′ positions.
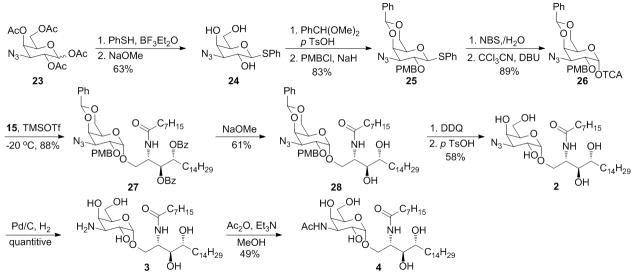
To synthesize the 4′-N-substituted analog 5, the corresponding donor 19 was prepared from the 4,6-benzylidene protected glucosyl derivative 16. Selective reduction of the benzylidene in acid condition resulted in the 4-hydroxyl galactose 17. The newly freed hydroxyl was subjected to trifluoromethylsulfonic anhydride to form the triflic ester. Then it was reacted with sodium azide in DMF to introduce the azido group in axial position as the stereo structure of galactose. The thiophenol was removed and converted to the Schmidt’s trichloroacetimide donor to increase the ratio of formation α-isomer product in the following glycosylation reaction. The glycolipid 20 was obtained in good yield when TMSOTf was used as catalyst. We wanted to firstly reduce the azido group by Staudinger method using triphenylphosphine and water as reagents, and then protect the amino group as acetylamide. But the reaction only gave the triphenylphosphosimine which actually was an intermediate of the reduction. Increasing the reaction temperature up to 80°C didn’t decompose the intermediate. It’s intriguing that when the two benzoyl groups in the phytosphingosine were firstly hydrolyzed and then did the same Staudinger reduction, the reaction went very smoothly and gave the amino product in excellent yield. A selective acetylation was performed in methanol to convert the amine group to amide 22. Then the compound 22 was subjected to hydrogenation using palladium hydroxide as catalyst under 40 psi H2 to give the 4′-NAc-GalCer 5.
The preparation of 3′-N-substituted α-GalCer analogs 2, 3 and 4 took the similar protocol as 5. The 3-azido-galactose derivative 23 was synthesized from the diacetone-D-glucose in 8 steps.[14] After it was converted to compound 24 using the standard method, the C4 and C6 hydroxyls were protected with benzylidene and C2 with p-methoxylbenzyl, both protecting groups can be removed in acidic conditions or oxidation without affecting the azido group. After all the protecting groups being assembled, the thiophenol group was removed and also converted to trichloroacetimide donor. The glycosidation catalyzed by TMSOTf went smoothly and gave the product in 88% yield. The 3′-azido analogue 2 was afforded by saponification of the benzoyl groups on ceramide, and removal of the PMB and benzylidene by oxidation with DDQ and toluenesulfonic acid respectively. Reduction of the azido by hydrogenation gave the 3′-amino analogue 3. Finally, selective acetylation of amine group in methanol by acetic anhydride generated the 3′-NAc analogue 4.
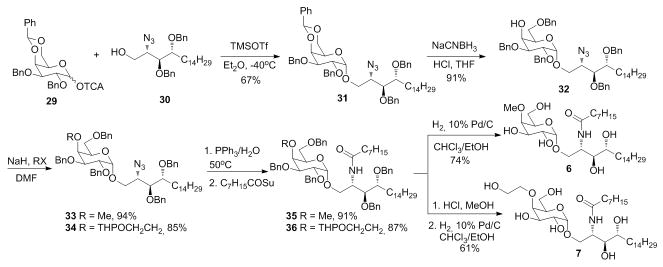
The 4′-O-substutited analogues 6 and 7 were synthesized using a different method where the azido-lipid 30 was subjected to glycosidation instead of ceramide 15. After glycosidation, the benzylidene was region-selectively reduced in acidic condition by NaCNBH3. The methyl or O-THP protected ethanol groups were introduced by alkylation under the assistance of NaH to give 33 and 34. Reduction the azido group and amidation of the newly formed amino with octanoic NHS ester afforded the protected glycolipid. The product 6 was obtained by hydrogenation, while the 7 by first treatment with HCl to remove THP and then hydrogenation.
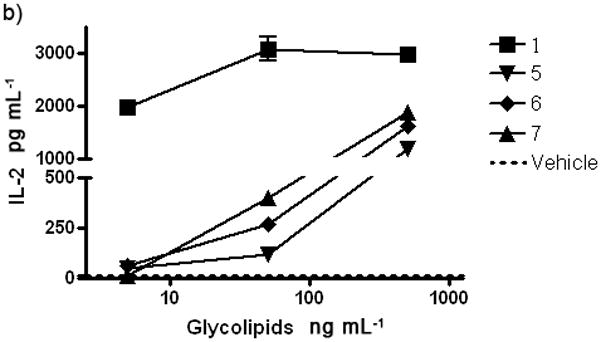
The α-GalCer analogues were first assayed using iNKT hybridoma cells (DN3A4-1.2 hybridoma which expresses Vα14Jα281/Vβ8.2Jβ2.1 TCR).[15] CD1d expressing A20/CD1d cells were applied to the iNKT hybridomas in order to present glycolipids to these cells. The IL-2 cytokine released by stimulated iNKT hybridomas was measured so as to evaluate the immunogenic strength of the presented analogues. As shown in Figure 2a, all of the C3′ analogues exhibited a complete loss of iNKT cell stimulation and subsequent IL-2 cytokine release. Given that such a loss of activity corresponds to elimination of a hydroxyl group, one can conclude that the H-bond between the 3′ hydroxyl and the iNKT TCR and/or CD1d is very important in maintaining complex stability. Although the acetamide group of analogue 4 may actually form an H-bond with the protein, the type of H-bond is still different from that of the hydroxyl group, thus explaining why activation of iNKT cells by this analogue is not apparent. Unlike the C3′ analogues, most of the C4′ analogues can in fact stimulate the iNKT cells, albeit in a relatively weak manner (Figure 2b). The activity of methyl substituted 6 indicates that the C4′ position as H-bond donor is not required for binding with TCR. Analogue 7, which possesses a hydroxyl group at the terminus of the ethanol substituent, exhibited activity that was nearly equivalent to that of 6, indicating that this group did not facilitate cytokine release. It’s worthy to mention the fact that the 4′-deoxy analogue 9 still possess measurable iNKT cell stimulation, although it is much weaker than α-GalCer 1. Such a result is quite surprising given that the α-dihexosylceramides such as α-lactosylceramide[12] and Galα1,4GalαCer[11a], which have an additional sugar cap on the C4′ position, can not stimulate iNKT cells unless the sugar cap is hydrolyzed by a glycosidase. A plausible explanation is that the 4′ hydroxyl contributes to some extent in the binding with iNKT TCR, but is not as crucial as the 2′ and 3′ hydroxyls. Nevertheless, the very bulky sugar cap on the C4′ position will block the TCR, inhibiting access to the glycolipid/CD1d complex, thus resulting in the loss of activity. It was also observed that the 4′-NAc-α-GalCer 5 showed no activity, in contrast to the other C4′ analogues.
Figure 2.


After stimulation by α-GalCer analogue pulsed A20/CD1d cells, DN3A4-1.2 hybridoma cells released IL-2 cytokine. The glycolipid analogues were applied at gradient concentrations, DMSO was used as vehicle. a) IL-2 released after C3′ analogue stimulation. b) IL-2 released after C4′ analogue stimulation. Experiments were done three times.
Given that the processing procedure in APC cells may have some effect on glycolipid/CD1d complex recognition, the coat-plate-assay was applied to verify the above results. In this assay, glycolipid was directly presented to iNKT hybridoma cells by surface-bound purified CD1d protein to avoid the processing inside the CD1d expressing cells. N3A4-1.2 and DN3A4-1.4 hybridomas were used as iNKT cells in the assays. All of the C4′ modified α-GalCer analogues showed similar activity spectra to those in the A20/CD1d-hybridoma assay with the exception of the 4′-α-NAc-GalCer 5 (Figure 3a & 3b). While it was mentioned above that 5 did not display any activity in the AC20/CD1d cell assay, it exhibited noticeable immunogenicity in this case. We hypothesize that such a discrepancy may arise from hydrolysis of the 4′-acetamide group in 4′-NAc-GalCer 5 by amide hydrolase(s) inside the lysosome of the AC20/CD1d cell, thus generating a NH3+ group and leading to a loss of activity. Additional experiments are currently in progress to verify this hypothesis.
Figure 3.

The hybridoma cells released IL-2 cytokine after being stimulated by surface-bound CD1d presented C4′ analogues. DMSO was used as vehicle. Two iNKT hybridoma cell lines were applied here, a) DN3A4-1.2 and b) DN3A4-1.4. Gradient concentrations of glycolipid analogues were applied for loading onto CD1d protein. Experiments were done three times.
The C4′ analogues were additionally subjected to an assay using a splenocyte mixture as a pseudo in vivo model. Specifically, the C57BL/6 mouse spleens were ground into single cell suspensions and stimulated by the α-GalCer analogues. Through the amount of IFN-γ and IL-4 released by the stimulated splenocyte cells, the Th1/Th2 cytokine profile of these analogues was generated. Remarkably, the C4′-substituted α-GalCer analogues were found to induce a Th2 biased iNKT cell response (Figure 4). Although the total amount of the IFN-γ is still larger than the IL-4 when NKT cells were stimulated analogs 5, 6, 7 and 9, the quantity of IFN-γ released by NKT cells decreased more than IL-4 when these analogs were administrated, compare to the original amount of these cytokines stimulated by α-GalCer 1. So, the new analogs significantly changed the relative ratio between released Th1 and Th2 cytokines. Such bias in the iNKT cell response has been previously observed in experiments involving truncation of the length of the phytosphingosine chain (e.g. OCH)[16] or the metabolically stable α-C-GalCer[17]. And most recently, a series of 6′-derivatised α-GalCer analogues also can induce the Th1 biased iNKT cell response. The observation that C4′-substituted analogues can change the profile of iNKT cells stimulation towards Th2 cytokines is thus quite intriguing, as such immunomodulatory Th2 cytokines can be utilized to ameliorate autoimmune diseases. One possible explanation for this shift in the cytokine profile may be the stability of the TCR/glycolipid/CD1d complex, as it occurred with OCH and others. However, there is a difference between human and murine NKT cells response[18], human NKT cells will be performed in the future to evaluate this biased cytokine releasing profile.
Figure 4.
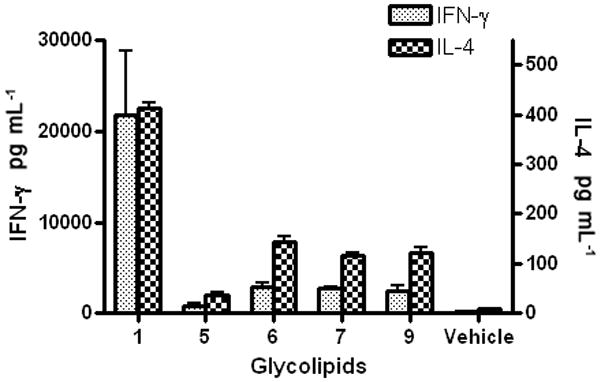
The splenocyte mixture was stimulated by C4′ analogues, after which the released cytokines (IFN-γ and IL-4) were quantified by ELISA. Compared to α-GalCer 1, the C4′ analogues (5, 6, 7, 9) stimulated more Th2 (IL-4) cytokines than Th1 (IFN-γ) cytokines. DMSO was used as vehicle. Experiments were done three times.
CONCLUSION
In summary, we investigated a series of α-GalCer analogues with different substitutions on the C3′ and C4′ positions of the galactose ring to evaluate the roles of the 3′ and 4′ hydroxyls in iNKT cell stimulation. Assay results indicated that the 3′ hydroxyl is a very critical feature of the TCR/glycolipid/CD1d ternary complex, and elimination of this hydroxyl group results in a loss of activity. In contrast, the C4′ position can tolerate some modifications without a dramatic decrease in immunogenicity. Moreover, it was observed that the C4′-subsittuted analogues can change the iNKT cell response profile toward a biased Th2 cytokine release.
Experimental Section
1. General information
All solvents were dried with solvent-purification system from Innovative Technology, Inc. All reagents were obtained from commercial sources and used without further purification. Analytical TLC was carried out on silica gel 60 F254 aluminum-backed plates (E. Merck). The 230–400 mesh size of the same absorbent was utilized for all chromatographic purifications. 1H and 13C NMR spectra were recorded at the indicated field strengths. The high-resolution mass spectra were collected at The Ohio State University Campus Chemical Instrumentation Center.
(2 S,3S,4R)-2-Octanoylamino-octadecan-1,3,4-triol (12) 21
A suspension of ester 10 (1.38 g, 5.71 mmol), phytosphingosine 11 (1.51 g, 4.76 mmol) and Et3N (2.4 mL, 17.1 mmol) in THF (50 mL) was stirred at 50 °C for 14 h. After cooled to room temperature, the solvent was removed in vacuo. The residue was dissolved in hot ethyl acetate (10 mL). The precipitate was collected by centrifuge (3000 rpm, 30 min) to give 1.84 g of product in 89% yield. 1H NMR (400 MHz, Py-d5) δ 8.39 (d, J = 10.6 Hz, 1H), 5.01–5.07 (m, 2H), 4.41–4.49 (m, 2H), 4.34 (d, J = 7.6 Hz, 1H), 4.22–4.27 (m, 1H), 2.38–2.43 (m, 2H), 2.17–2.24 (m, 1H), 1.86–1.97 (m, 2H), 1.74–1.81 (m, 2H), 1.63–1.69 (m, 1H), 1.09–1.36 (m, 28H), 0.94 (t, J = 8.9 Hz, 1H), 0.85 (t, J = 8.1 Hz, 3H), 0.79 (t, J = 6.6 Hz, 3H); 13C NMR (100 MHz, Py-d5) δ 174.2, 77.6, 73.9, 63.0, 54.6, 47.5, 37.7, 34.9, 33.0, 32.8, 31.2, 31.0, 30.9, 30.87, 30.81, 30.52, 30.50, 30.3, 27.5, 27.3, 26.9, 23.8, 23.7, 15.2, 15.1, 13.1.
(2S,3S,4R)-1-Triphenylmethyl-2-octanoylamino-octadecan-1,3,4-triol (13)
To a solution of triol 12 (1.84 g, 4.15 mmol) in Pyridine (40 mL) were added TrtCl (5.78 g, 20.7 mmol) and DMAP (100 mg, 0.82 mmol) and stirred at 50 °C overnight. The solution was worked up by adding H2O (1 ml) and stirred for 1 h. The solution was concentrated under vacuum. The residue was dissolved by EtOAc and washed by water. The organic layer was dried over anhydrous Na2SO4. The concentrated residue was purified by column chromatography with hexane/ethyl acetate (4:1) to give 2.62 g of product in 92% yield as colorless oil. 1H NMR (400 MHz, CDCl3) δ 7.42–7.44 (m, 6H), 7.31–7.35 (m, 6H), 7.25–7.28 (m, 3H), 6.06 (d, J = 8.4 Hz, 1H), 4.25–4.30 (m, 1H), 3.58–3.60 (m, 1H), 3.51–3.54 (m, 2H), 3.35–3.42 (m, 2H), 3.15 (d, J = 8.4 Hz, 1H), 2.28 (d, J = 7.6 Hz, 1H), 2.17 (t, J = 7.6 Hz, 2H), 1.71 (b, 2H), 1.60–1.66 (m, 2H), 1.44–1.46 (m, 2H), 1.25–1.32 (m, 28H), 0.90 (t, J = 6.4 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ 173.2, 143.2, 128.5, 128.1, 127.4, 87.7, 75.6, 73.2, 65.9, 63.0, 50.4, 36.9, 33.3, 31.9, 31.7, 29.7, 29.7, 29.6, 29.4, 29.3, 29.0, 25.8, 25.7, 22.7, 22.6, 14.1, 14.1; HRMS calcd for C45H67NO4Na ([M + Na]+) 708.4962, found 708.4969.
(2S,3S,4R)-1-Triphenylmethyl-3,4-di-O-benzoyl-2-octanoylamino-octadecan-1,3,4-triol (14)
To a solution of compound 13 (2.8 g, 4.08 mmol) in Pyridine (40 mL) were added BzCl (2.8 ml, 24.5 mmol) and DMAP (49 mg, 0.4 mmol) and stirred at 50 °C for 8 h. The solution was concentrated under reduced pressure. The residue was partitioned between ethyl acetate and water. The organic layer was separated and washed with cooled 1N HCl, saturated aqueous NaHCO3 and brine. After dried over anhydrous Na2SO4, it was purified by column chromatography with hexane/ethyl acetate (15:1) to give 3.14 g of product in 86% yield as colorless oil. 1H NMR (400 MHz, CDCl3) δ 7.98 (d, J = 8.0 Hz, 2H), 7.90 (d, J = 7.6 Hz, 2H), 7.55–7.62 (m, 2H), 7.42 (dd, J = 14.8, 7.2 Hz, 4H), 7.31–7.33 (m, 6H), 7.19–7.7.17 (m, 9H), 6.02 (d, J = 9.6 Hz, 1H), 5.81 (dd, J = 8.8, 2.4 Hz, 1H), 5.36–5.38 (m, 1H), 4.57–4.64 (m, 1H), 3.32–3.35 (m, 2H), 2.16–2.23 (m, 2H), 1.86–1.91 (m, 2H), 1.62–1.68 (m, 2H), 1.23–1.40 (m, 32H), 0.88–0.911 (m, 6H); 13C NMR (100 MHz, CDCl3) δ 172.8, 166.4, 165.1, 143.3, 133.07, 132.9, 130.2, 129.9, 129.79, 129.76, 128.6, 128.41, 128.36, 127.8, 127.0, 86.8, 74.2, 72.8, 61.7, 48.6, 36.9, 31.9, 31.7, 29.68, 29.66, 29.62, 29.57, 29.52, 29.36, 29.34, 291, 25.80, 25.71, 25.69, 22.70,22.66, 14.13, 14.09; HRMS calcd for C59H75NO6Na ([M + Na]+) 916.5487, found 916.5466.
(2 S,3S,4R)-3,4-Di-O-benzoyl-2-octanoylamino-octadecan-1,3,4-triol (15)
To a solution of 14 (1.8 g, 2.0 mmol) in CH2Cl2 and MeOH (2:1, 20 mL) were added p-toluenesulfonic acid monohydrate (400 mg, 2.1 mmol) and stirred for 3 h. The solution was quenched by addition Et3N (0.2 ml). The solution was concentrated under vacuum. The concentrated residue was purified by column chromatography with hexane/ethyl acetate (5:1) to give 1.2 g of product in 92% yield as colorless oil. 1H NMR (500 MHz, CDCl3) δ 8.04 (d, J = 1.0 Hz, 2H), 7.95 (d, J = 1.0 Hz, 2H), 7.63 (t, J = 7.5 Hz, 1H), 7.49–7.54 (m, 3H), 7.38 (d, J = 7.5 Hz, 2H), 6.50 (d, J = 9.5 Hz, 1H), 5.46 (dd, J = 9.5, 2.5 Hz, 1H), 5.38–5.40 (m, 1H), 4.38–4.43 (m, 1H), 3.66 (dd, J = 12.0, 2.0 Hz, 2H), 2.95 (br, 1H), 2.29 (d, J = 7.5 Hz, 2H), 2.02–2.04 (m, 2H), 1.68–1.72 (m, 2H), 1.24–1.46 (m, 32H), 0.88 (J = 7.0 Hz, 6H); 13C NMR (125 MHz, CDCl3) δ 173.3, 167.0, 166.4, 133.8, 133.1, 130.0, 129.9, 129.7, 129.2, 128.7, 128.4, 86.8, 74.0, 73.7, 61.6, 50.0, 36.9, 31.9, 31.7, 29.70, 29.68, 29.65, 29.59, 29.57, 29.40, 29.36, 29.3, 29.1, 28.5, 25.84, 25.75, 22.69, 22.63, 14.11, 14.07; HRMS calcd for C40H61NO6Na ([M + Na]+) 674.4391, found 674.4407.
Phenyl 1-thio-2,3,6-tri-O-benzyl-α-D-glucopyranoside (17) 22
To a mixture of Phenyl 1-thio-2,3-di-O-benzyl-4,6-O-benzyliden-α-D-glucopyranoside 16 23 (2.53 g, 4.6 mmol), NaCNBH3 (3.63 g, 57.8 mmol) and 4 Å molecular sieves (3 g) in dry THF (60 mL) was added dropwise a solution of HCl-Et2O under nitrogen atmosphere at room temperature. The reaction mixture was stirred for 10 min, and then filtered off through Celite. The filtrate was washed sequentially with saturated NaHCO3 aqueous and brine. After dried over anhydrous Na2SO4, it was purified by flash chromatography (hexane/ethyl acetate 3:1) to give 2.1 g of product in 86% yield. 1H NMR (400 MHz, CDCl3) δ 7.43–7.26 (m, 20 H), 4.93 (d, J = 11.0 Hz, 1H), 4.92 (d, J = 11.2 Hz, 1H), 4.80 (d, J = 11.4 Hz, 1H), 4.76 (d, J = 9.8 Hz, 1H), 4.71 (d, J = 9.4 Hz, 1H), 4.62 (d, J = 11.8 Hz, 1H), 4.58 (d, J = 11.8 Hz, 1H), 3.83–3.78 (m, 2H), 3.68 (t, J = 8.8 Hz, 1H), 3.56–3.49 (m, 3H).
Phenyl 1-thio-4-deoxy-4-azido-2,3,6-tri-O-benzyl-α-D-galactopyranoside (18)
To a solution of glucoside 17 (1.83 g, 3.37 mmol) and anhydrous pyridine (0.44 mL, 5.39 mmol) in dry CH2Cl2 (25 mL) was added Tf2O (0.85 mL, 5.06 mmol) slowly. After being stirred for 30 min, the reaction mixture was diluted with CH2Cl2, washed with water, saturated NaHCO3 aqueous and brine. Dried over anhydrous Na2SO4, the solution was concentrated and dissolved with dry DMF (30 mL). NaN3 (0.88 g, 13.5 mmol) was added and the resulting suspension was stirred for 20 h at room temperature. The solvent was removed in vacuo and the residue was partitioned between ethyl acetate and water. The organic layer was separated and dried over anhydrous Na2SO4. The residue was purified by flash chromatography (hexane/ethyl acetate 6:1) to give 1.41 g of product in 74% yield. 1H NMR (400 MHz, CDCl3) δ 7.57 (m, 2H), 7.42-7.28 (m, 18H), 4.83 (d, J = 10.2 Hz, 1H), 4.79 (d, J = 11.5 Hz, 1H), 4.77 (d, J = 10.2 Hz, 1H), 4.74 (d, J = 11.5 Hz, 1H), 4.61 (d, J = 8.6 Hz, 1H), 4.58 (d, J = 12.4 Hz, 1H), 4.55 (d, J = 12.4 Hz, 1H), 4.07 (d, J = 1.8 Hz, 1H), 3.79-3.73 (m, 2H), 3.71-3.63 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 138.1, 137.7, 137.5, 133.6, 132.0, 128.9, 128.6, 128.5, 128.4, 128.3, 128.0, 127.96, 127.9, 127.88, 127.5, 88.1, 82.8, 77.2, 75.9, 75.6, 73.7, 72.8, 68.7, 60.0; HRMS calcd for C33H33N3O4SNa ([M + Na]+) 590.2084, found 590.2076.
4-Deoxy-4-azido-2,3,6-tri-O-benzyl-α-D-galactopyranosyl-trichloroacetimide (19)
To a solution of thio-compound 18 (1.30 g, 2.3 mmol) in acetone and water (9:1, 20 mL) cooled at 0 °C was added NBS (0.90 g, 5.0 mmol). The resulting mixture was stirred for 30 min and the reaction was quenched by addition of saturated NaHCO3 aqueous. The organic solvent was removed under reduced pressure. The aqueous was extracted with ethyl acetate. After dried over anhydrous Na2SO4, it was purified by flash chromatography.
The above galactose derivative was dissolved in dry CH2Cl2 (10 mL), CCl3CN (2.3 mL, 23 mmol) and DBU (0.18 mL, 1.2 mmol) were added. The resulting mixture was stirred for 2h. The solvent was removed in vacuo. The residue was purified by chromatography (hexane/ethyl acetate, 5:1) to give 1.12 g of product in 79% yield for two steps. 1H NMR (400 MHz, CDCl3) δ 8.59 (s, 1H), 7.42-7.29 (m, 15H), 6.46 (d, J = 3.3 Hz, 1H), 4.85 (d, J = 11.7 Hz, 1H), 4.81 (d, J = 11.7 Hz, 1H), 4.78 (d, J = 11.7 Hz, 1H), 4.75 (d, J = 11.7 Hz, 1H), 4.58 (d, J = 11.7 Hz, 1H), 4.54 (d, J = 11.7 Hz, 1H), 4.22 (t, J = 7.4 Hz, 1H), 4.16 (m, 1H), 4.13 (dd, J = 9.6, 3.3 Hz, 1H), 4.07 (dd, J = 9.6, 3.4 Hz, 1H), 3.67-3.59 (m, 2H); HRMS calcd for C29H29Cl3N4O5Na ([M + Na]+) 641.1096, found 641.1105.
4-Deoxy-4-azido-2,3,6-tri-O-benzyl-α-D-galactopyranosyl-(1,1)-(2S,3S,4R)-2-octanoylamino -3,4-di-O-benzoyl-octadecan-1,3,4-triol (20)
A suspension of trichloroacetimide donor19 (0.87 g, 1.40 mmol), ceramide acceptor 15 (0.61 g, 0.94 mmol) and molecular sieves (1.0 g) in Et2O and THF (5:1, 12 mL) was stirred at room temperature for 30 min. Then the mixture was cooled at −25 °C and TMSOTf (20 μL, 0.09 mmol) was added by syringe. The resulting mixture was continued stirring for 2h. The molecular sieves were filtered through Celite pad. The filtrate was diluted with ethyl acetate and washed with saturated aqueous NaHCO3 and brine, dried over anhydrous Na2SO4. The product was purified by flash chromatography (hexane/ethyl acetate/dichloromethane 12:1:1) to give 0.70 g of product in 67% yield. 1H NMR (400 MHz, CDCl3) δ 8.06 (dd, J = 8.4, 1.3 Hz, 2H), 7.97 (dd, J = 8.4, 1.3 Hz, 2H), 7.63 (tt, J = 7.5, 1.2 Hz, 1H), 7.55 (tt, J = 7.4, 1.1 Hz, 1H), 7.50-7.20 (m, 19H), 6.77 (d, J = 9.4 Hz, 1H), 5.70 (dd, J = 9.4, 2.8 Hz, 1H), 5.42 (m, 1H), 4.73 (d, J = 11.5 Hz, 1H), 4.70 (d, J = 11.5 Hz, 1H), 4.69 (d, J = 3.7 Hz, 1H), 4.65 (d, J = 12.1 Hz, 1H), 4.64 (m, 2H), 4.59 (m, 1H), 4.53 (d, J = 12.1 Hz, 1H), 4.13 (t, J = 6.5 Hz, 1H), 3.98 (m, 1H), 3.96 (dd, J = 9.3, 3.6 Hz, 1H), 3.91 (dd, J = 11.8, 3.4 Hz, 1H), 3.81 (dd, J = 9.3, 3.7 Hz, 1H), 3.66 (dd, J = 11.6, 2.7 Hz, 1H), 3.57 (dd, J = 9.4, 6.6 Hz, 1H), 3.51 (dd, J = 9.3, 6.5 Hz, 1H), 2.19 (d, J = 7.4 Hz, 2H), 1.92 (m, 2H), 1.66 (m, 2H), 1.36-1.22 (m, 32H), 0.91 (t, J = 6.5 Hz, 3H), 0.90 (t, J = 6.7 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 173.1, 166.1, 165.3, 138.1, 138.0, 137.6, 133.3, 133.0, 130.1, 129.8, 129.78, 128.6, 128.5, 128.4, 128.35, 128.0, 127.9, 127.85, 127.8, 127.7, 99.8, 77.4, 77.3, 76.4, 73.8, 73.6, 73.5, 73.1, 72.4, 69.8, 69.0, 68.1, 61.3, 48.6, 36.7, 31.9, 31.8, 29.7, 29.68, 29.6, 29.59, 29.55, 29.4, 29.37, 29.3, 29.1, 28.5, 25.7, 22.7, 22.67, 14.1, 14.11; HRMS calcd for C67H88N4O10Na ([M + Na]+) 1131.6393, found 1131.6385.
4-Deoxy-4-azido-2,3,6-tri-O-benzyl-α-D-galactopyranosyl-(1,1)-(2S,3S,4R)-2-octanoylamino -octadecan-1,3,4-triol (21)
To a solution of protected glycoceramide 20 (400 mg, 0.36 mmol) in dry MeOH (4 mL) was added a freshly prepared NaOMe (19 mg, 0.36 mmol). The resulting mixture was stirred at 50 °C for 10 h. The reaction was neutralized by addition of Dowex ion-exchange resin and filtered. The filtrate was concentrated in vacuo and purified by flash chromatography (hexane/ethyl acetate, 2:1) to give 0.31 g of product in 93% yield. 1H NMR (400 MHz, CDCl3) δ 7.35-7.28 (m, 15H), 6.40 (d, J = 8.4 Hz, 1H), 4.89 (d, J = 11.5 Hz, 1H), 4.81 (d, J = 3.6 Hz, 1H), 4.78 (d, J = 11.5 Hz, 1H), 4.76 (d, J = 11.5 Hz, 1H), 4.67 (d, J = 11.5 Hz, 1H), 4.59 (d, J = 11.6 Hz, 1H), 4.52 (d, J = 11.6 Hz, 1), 4.23 (m, 1H), 4.08 (dd, J = 3.4, 1.1 Hz, 1H), 4.00-3.93 (m, 2H), 3.89-3.84 (m, 2H), 3.75 (m, 1H), 3.61-3.54 (m, 2H), 3.48 (m, 1H), 2.28 (m, 1H), 2.16 (m, 2H), 1.63 (m, 2H), 1.61 (m, 1H), 1.32-1.24 (m, 32H), 0.92 (t, J = 6.5 Hz, 3H), 0.91 (t, J = 6.6 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 173.2, 137.6, 137.58, 137.4, 128.6, 128.2, 128.1, 128.0, 127.98, 127.8, 99.0, 78.2, 77.3, 76.2, 75.6, 74.5, 73.8, 73.3, 72.7, 70.0, 68.7, 67.7, 60.7, 49.4, 36.8, 33.4, 32.0, 31.7, 29.7, 29.4, 29.3, 29.1, 25.9, 25.7, 22.7, 22.66, 14.2, 14.1; HRMS calcd for C53H80N4O8Na ([M + Na]+) 923.5868, found 923.5852.
4-Deoxy-4-acetamido-2,3,6-tri-O-benzyl-α-D-galactopyranosyl-(1,1)-(2S,3S,4R)-2-octanoyla mino-octadecan-1,3,4-triol (22)
A solution of azidogalactosylceramide 21 (82 mg, 0.091 mmol) and PPh3 (48 mg, 0.18 mmol) in benzene (2 mL) containing trace amount of water was heated to 60 °C for 5 h. After cooling to room temperature, the solvent was removed in vacuo, and the residue was directly used for next steps without further purification.
The above residue was dissolved in anhydrous MeOH (2 mL) and Ac2O (12 μL, 0.13 mmol) was added. The resulting mixture was stirred for 8 h at room temperature and then the solvent was concentrated. The residue was purified by silica gel chromatography (hexane/ethyl acetate, 1:2) to give 70 mg of product in 84% yield. 1H NMR (400 MHz, CDCl3) δ 7.48-7.29 (m, 15H), 6.45 (d, J = 8.3 Hz, 1H), 5.56 (d, J = 9.9 Hz, 1H), 4.92 (d, J = 11.6 Hz, 1H), 4.87 (d, J = 4.0 Hz, 1H), 4.86 (d, J = 10.7 Hz, 1H), 4.83 (m, 1H), 4.65 (d, J = 11.6 Hz, 1H), 4.55 (d, J = 11.9 Hz, 1H), 4.51 (d, J = 10.9 Hz, 1H), 4.28 (d, J = 11.8 Hz, 1H), 4.22 (m, 1H), 4.13 (t, J = 5.1 Hz, 1H), 3.95-3.92 (m, 3H), 3.56-3.47 (m, 5H), 2.14 (m, 2H), 2.01 (s, 3H), 1.60 (m, 3H), 1.48 (m, 1H), 1.33-1.22 (m, 32H), 0.91 (t, J = 5.7 Hz, 3H), 0.90 (t, J = 6.2 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 173.3, 170.3, 137.9, 137.8, 137.5, 128.5, 128.49, 128.4, 128.2, 128.1, 127.9, 127.7, 98.9, 83.7, 77.2, 76.2, 75.4, 74.4, 73.7, 73.3, 71.4, 70.1, 69.3, 68.5, 49.7, 47.7, 36.7, 33.3, 31.9, 31.7, 29.7, 29.67, 29.4, 29.3, 29.0, 25.9, 25.7, 23.5, 22.7, 22.6, 14.1, 14.08; HRMS calcd for C55H84N2O9Na ([M + Na]+) 939.6069, found 939.6081.
4-Deoxy-4-acetamido-α-D-galactopyranosyl-(1,1)-(2S,3S,4R)-2-octanoylamino-octadecan-1, 3,4-triol (5)
A suspension of tribenzylGalCer 22 (20 mg, 0.022 mmol) and 20 % Pd(OH)2 on carbon (10 mg) in EtOH (1 mL) and CHCl3 (0.25 mL) was stirred overnight under 1atm H2 overnight. The catalyst was filtered through a Celite pad and the filtrate was concentrated. The residue was purified by silica gel chromatography (CHCl3/MeOH, 5:1) to afford 9 mg of product in 63% yield. 1H NMR (500 MHz, pyridine-d5) δ 8.71 (d, J = 9.5 Hz, 1H), 8.47 (d, J =8.6 Hz, 1H), 5.39 (d, J = 3.9 Hz, 1H), 5.17 (m, 1H), 5.08 (m, 1H), 4.61-4.53 (m, 3H), 4.42 (dd, J = 10.2, 4.0 Hz, 1H), 4.32-4.25 (m, 3H), 4.09 (dd, J = 11.5, 6.6 Hz, 1H), 4.03 (dd, J = 11.2, 6.6 Hz, 1H), 2.39 (td, J = 7.5, 2.5 Hz, 2H), 2.23 (m, 1H), 2.06 (s, 3H), 1.86 (m, 2H), 1.75 (m, 2H), 1.63 (m, 1H), 1.38-1.10 (m, 32H), 0.84 (t, J = 6.5 Hz, 3H), 0.78 (t, J = 6.6 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ; HRMS calcd for C34H66N2O9Na ([M + Na]+) 669.4661, found 699.4685.
Phenyl 3-deoxy-3-azido-1-thio-β-D-galactopyranoside (24) 24
The peracetyl protected 3-azido galactose 23 (4.0 g, 10.7 mmol) was dissolved in CH2Cl2 (80 mL) and 4 Å MS (1 g) and thiophenol (1.65 mL, 16.1 mmol) was added. The mixture was stirred at r.t. for 0.5 h and then cooled to 0 °C. After that, boron trifluoride ether solution (2.0 mL, 16.1 mmol) was injected into the solution. The solution was slowly warmed to room temperature and stirred for 48h. The reaction was neutralized by NaHCO3 and washed with water and brine. The organic layer was dried by Na2SO4 and the residue was chromatographied by hexane/EtOAc (5/1, v/v) to give compound (3.5 g, 77 %) as yellowish solid. 1H NMR (400 MHz, CDCl3) δ 2.05 (s, 3H), 2.14 (s, 3H), 2.17 (s, 3H), 3.63–3.66 (m, 1H), 3.90 (t, J = 6.4, 1H), 4.12 (d, J = 6.4 Hz, 2H), 4.70 (dd, J = 9.9, 1.3 Hz, 1H), 5.18–5.24 (m, 1H), 5.18–5.44–5.45 (m, 1H), 7.31–7.33 (m, 3H), 7.50–7.53 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 20.59, 20.66, 20.86, 61.85, 62.89, 67.81, 68.34, 86.91, 128.17, 128.91, 132.52, 169.37, 169.95, 170.37.
To a solution of the above compound (2.70 g, 6.38 mmol) in MeOH (20 mL) were added NaOMe (10.3 mg, 0.19 mmol). The reaction mixture was stirred at room temperature for 5 h. Neutralize the solution by Amberlyst 15 cation exchange resin and filter the resin. The solution was concentrated under reduced pressure and then purified by column chromatography (EtOAc/Hexane 3:2) to give white solid 15 (82.3 %). 1H NMR (500 MHz, CD3OD) δ 7.52 (d, J = 7.5 Hz, 2H), 7.28-7.20 (m, 3H), 3.93 (d, J = 2.7 Hz, 1H), 3.79 (t, 1H), 3.72-3.63 (m, 2H), 3.55 (t, J = 6.0 Hz, 1H), 3.35 (dd, J = 9.9, 3.0 Hz, 1H); 13C NMR (125 MHz, CD3OD) δ 174.2, 134.4, 130.9, 128.5, 126.8, 89.5, 79.5, 68.1, 67.7, 61.1.
Phenyl 1-thio-2-O-p-methoxybenzyl-3-deoxy-3-azido-4,6-O-benzylidene-β-D-galacto-pyranoside (25) 25
To a solution of azido compound 24 (1.13 g, 3.80 mmol) in DMF (20 mL) were added benzaldehyde dimethylacetal (0.87 mL, 5.70 mmol) and p-toluenesulfonic acid (36 mg, 0.19 mmol) and stirred at 60 °C for 2h. The solution was worked up by adding Et3N (0.25 mL) and stirred for 10 min. The solution was concentrated under vacuum. The residue was dissolved by DMF (20 mL) and added NaH (228 mg, 5.70 mmol). The solution was stirred at room temperature for 0.5 h and then added p-methoxybenzyl chloride. The reaction is continued overnight. The concentrated residue was purified by column chromatography with hexane/dichloromethane (1:3) to give 16 in 83.3% yields as white solid. 1H NMR (500 MHz, CDCl3) δ 7.75 (d, J = 7.5 Hz, 2H), 7.55 (m, 2H), 7.45-7.40 (m, 5H), 7.29-7.23 (m, 3H), 6.92 (d, J = 8.5 Hz, 2H), 5.59 (s, 1H), 4.82 (d, J = 9.6 Hz, 1H), 4.67 (d, J = 9.4 Hz, 1H), 4.59 (d, J = 9.6 Hz, 1H), 4.39 (d, J = 12.3 Hz, 1H), 4.23 (d, J = 3.2 Hz, 1H), 4.04 (d, J = 12.3 Hz, 1H), 3.96 (t, J = 9.6 Hz, 1H), 3.83 (s, 3H), 3.57 (dd, J = 9.7, 3.2 Hz, 1H), 3.45 (s, 1H); 13C NMR (125 MHz, CDCl3) δ 159.6, 137.5, 132.8, 132.3, 130.4, 129.7, 129.2, 129.1, 128.3, 127.6, 126.4, 113.9, 101.2, 86.9, 75.0, 74.7, 74.4, 70.1, 69.3, 65.0, 55.3.
2-O-p-methoxybenzyl-3-deoxyl-3-azido-4,6-O-benzylidene-1-O-α-D-glactopyranosyl-tricholoroacetimidate (26)
To a solution of thiophenyl 25 (0.20 g, 0.48 mmol) in CH2Cl2 (10 mL) were added DBU (36 μL, 0.24 mmol) and trichloroacetonitrile (0.39 mL, 3.87 mmol) and stirred at room temperature for 2 h. The solution was concentrated under vacuum. The concentrated residue was purified by column chromatography with hexane/ethyl acetate (4:1) to give 17 in 89% yields as white solid. 1H NMR (500 MHz, CDCl3) δ 8.67 (s, 1H), 7.54 (dd, J = 7.9, 1.7 Hz, 2H), 7.43-7.39 (m, 3H), 7.29 (d, J = 8.4 Hz, 2H), 6.88 (d, J = 8.6 Hz, 2H), 6.68 (d, J = 3.1 Hz, 1H), 5.62 (s, 1H), 4.68 (dd, J = 20.0, 11.0 Hz, 2H), 4.37 (d, J = 3.0 Hz, 1H), 4.34 (dd, J = 12.6, 0.8 Hz, 1H), 4.29 (dd, J = 10.6, 3.2 Hz, 1H), 4.09 (dd, J = 12.6, 1.3 Hz, 1H), 3.97 (dd, J = 10.7, 3.2 Hz, 1H), 3.83 (s, 1H), 3.82 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 161.0, 159.5, 137.2, 129.4, 129.1, 128.3, 126.1, 113.9, 101.0, 94.3, 91.2, 73.1, 72.5, 69.0, 64.9, 58.9, 55.3; HRMS calculated for C23H23Cl3N4O6Na ([M + Na]+) 579.0575, found 579.0612.
2-O-p-methoxybenzyl-3-deoxy-3-azido-4,6-O-benzylidene-1-O-α-D-galactopyranosyl-(1,1)-(2S,3S,4R)-3,4-di-O-benzoyl-2-octanoylamino-octadecan-1,3,4-triol (27)
To a solution of trichloroacetimide donor 26 (0.24 g, 0.43 mmol) in Ether/THF (2:1, 6 mL) were added ceramide acceptor 15 (0.19 g, 0.29 mmol) and 4 Å molecular sieves (2g) and stirred for 0.5h. The solution was cooled to −20 °C and stirred for 15 min. Then, TMSOTf (15 μL, 0.086 mmol) was injected and the solution was stirred at −20 °C for 2 h. The solution was concentrated under vacuum. The concentrated residue was purified by column chromatography with hexane/ethyl acetate (4:1) to give 27 in 88% yields as white solid. 1H NMR (500 MHz, CDCl3) δ 8.05 (m, 2H), 7.96 (m, 2H), 7.63 (m, 1H), 7.56 (m, 1H), 7.52-7.48 (m, 4H), 7.43-7.40 (m, 2H), 7.39-7.35 (m, 3H), 7.24 (d, J = 8.7 Hz, 2H), 6.79 (d, J = 9.6 Hz, 1H), 6.73 (d, J = 8.7 Hz, 2H), 5.67 (dd, J = 9.4, 2.9 Hz, 1H), 5.56 (s, 1H), 5.29 (m, 1H), 4.90 (d, J = 3.3 Hz, 1H), 4.63 (d, J = 11.0 Hz, 1H), 4.60-4.55 (m, 2H), 4.27 (dd, J = 12.8, 1.3 Hz, 1H), 4.23 (d, J = 3.2 Hz, 1H), 4.07-4.02 (m, 2H), 3.80-3.75 (m, 6H), 3.68 (dd, J = 11.6, 3.1 Hz, 1H), 2.23 (t, J = 7.6 Hz, 2H), 1.90 (dd, J = 13.5, 6.6 Hz, 2H), 1.68-1.64 (m, 2H), 1.38-1.40 (m, 1H), 1.33-1.21 (m, 34H), 0.90-0.87 (m, 6H); 13C NMR (125 MHz, CDCl3) δ 173.2, 166.4, 165.5, 159.5, 137.3, 133.6, 133.1, 130.0, 129.8, 129.7, 129.5, 129.4, 129.0, 128.7, 128.4, 128.2, 126.1, 113.9, 100.9, 98.9, 75.5, 74.2, 74.0, 72.7, 72.3, 69.3, 68.9, 63.3, 58.7, 55.2, 48.6, 36.9, 31.9, 31.8, 29.70, 29.69, 29.66, 29.63, 29.58, 29.55, 29.4, 29.30, 29.29, 29.1, 28.2, 25.8, 25.7, 22.70, 22.66, 14.14, 14.11; ESI-MS calculated for C61H82N4O11Na ([M + Na]+) 1069.6, found 1069.5.
2-O-p-Methoxybenzyl-3-deoxy-3-azido-4,6-O-benzylidene-1-O-α-D-galactopyranosyl-(1,1)-(2S,3S,4R)-2-octanoylamino-octadecan-1,3,4-triol (28)
To a solution of compound 27 (0.27 g, 0.248 mmol) in MeOH (20 mL) were added sodium methoxide (0.67 mg, 0.0124 mmol) and stirred at 60 °C overnight. The solution was cooled to room temperature and concentrated under vacuum. The concentrated residue was purified by column chromatography with isopropanol/dichloromethane (1:50 V/V) to give product in 61% yields as white solid. 1H NMR (500 MHz, CDCl3) δ 7.50 (m, 2H), 7.38-7.34 (m, 3H), 7.31(m, 2H), 6.89 (m, 2H), 6.29 (d, J = 8.1 Hz, 1H), 5.57 (s, 1H), 5.00 (d, J = 3.5 Hz, 1H), 4.75 (d, J = 11.0 Hz, 1H), 4.59 (d, J = 11.1 Hz, 1H), 4.28 (d, J = 2.9 Hz, 1H), 4.25-4.22 (m, 2H), 4.07-4.03 (m, 2H), 3.98 (dd, J = 10.4, 3.5 Hz, 1H), 3.83-3.75 (m, 4H), 3.62 (br, 1H), 3.49-3.46 (m, 3H), 2.15 (t, J = 7.4 Hz, 2H), 2.09 (d, J = 5.4 Hz, 1H), 1.61-1.58 (m, 3H), 1.49-1.43 (m, 2H), 1.47-1.26 (m, 35H), 0.90-0.87 (m, 6H); 13C NMR (125 MHz, CDCl3) δ 173.2, 159.7, 137.2, 129.9, 129.1, 129.0, 128.2, 126.1, 114.1, 101.0, 98.3, 75.8, 75.5, 73.8, 73.4, 73.36, 69.5, 69.2, 62.9, 59.5, 55.3, 49.8, 36.8, 33.4, 31.9, 31.7, 29.7, 29.67, 29.4, 29.3, 29.1, 25.8, 25.79, 25.7, 22.7, 22.6, 14.11, 14.08; HRMS calculated for C47H74N4O9Na ([M + Na]+) 861.5348, found 861.5359.
3-Deoxy-3-azido-1-O-α-D-galactopyranosyl-(1,1)-(2S,3S,4R)-2-octanoylamino-octadecan-1, 3,4-triol (2)
To a solution of 28 (80 mg, 0.095 mmol) in CH2Cl2 (10 mL) with trace amount of water was added 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (40 mg, 0.143 mmol) and stirred at room temperature overnight. The solution was concentrated in vacuo. Flash chromatographic purification (isopropanol/chloroform, 3:100) give a compound in 76% yield as white solid. This compound (60 mg, 0.084 mmol) in MeOH (8 mL) was added p-toluene sulfonic acid (16 mg, 0.084 mmol) and stirred overnight. The solution was concentrated and purified by flash chromatography (MeOH/CHCl3, 6%) to give colorless oil (58 % yield for two steps). 1H NMR (500 MHz, pyridine-d5) δ 8.66 (d, J = 8.6 Hz, 1H), 7.43 (br, 1H), 7.27 (d, J = 5.7 Hz, 1H), 6.69 (br, 1H), 6.52 (d, J = 6.5 Hz, 1H), 6.16 (br, 1H), 5.52 (d, J = 3.6 Hz, 1H), 5.26 (m, 1H), 4.81 (m, 1H), 4.68 (dd, J = 10.5, 4.4 Hz, 1H), 4.51 (br, 1H), 4.46-4.41 (m, 2H), 4.33 (m, 3H), 4.28 (m, 1H), 3.95 (dd, J = 10.6, 2.7 Hz, 1H), 2.43 (t, J = 7.7 Hz, 2H), 2.28 (m, 1H), 1.92-1.86 (m, 2H), 1.81-1.75 (m, 2H), 1.69 (m, 1H), 1.43-1.10 (m, 35H), 0.84 (t, J = 5.9 Hz, 3H), 0.78 (t, J = 5.9 Hz, 3H); 13C NMR (125 MHz, pyridine-d5) δ 173.2, 100.8, 76.4, 72.6, 72.4, 69.4, 68.6, 67.5, 63.8, 61.9, 51.5, 36.5, 34.0, 31.9, 31.7, 30.1, 29.9, 29.8, 29.7, 29.41, 29.39, 29.2, 26.3, 26.1, 22.7, 22.6, 14.1, 14.0; HRMS calculated for C32H62N4O8Na ([M + Na]+) 653.4460, found 653.4465.
3-Deoxy-3-amino-O-α-D-galactopyranosyl-(1,1)-(2S,3S,4R)-2-octanoylamino-octadecan-1,3, 4-triol (3)
To a solution of 2 (20 mg, 0.032 mmol) in EtOH/CHCl3 (2:1, 6 mL) was added Pd/C (6 mg) and stirred under H2 (atomospher) at room temperature for 8h. The solution was concentrated in vacuo to give compound 3 as colorless solid (quantitive). 1H NMR (500 MHz, pyridine-d5) δ 8.90 (d, J = 8.6 Hz, 1H), 5.52 (br, 1H), 5.16 (m, 1H), 5.10 (br, 1H), 4.73 (d, J = 10.0 Hz, 1H), 4.58 (dd, J = 10.4, 3.7 Hz, 1H), 4.46 (t, J = 5.9 Hz, 1H), 4.43-4.33 (m, 4H), 4.28-4.22 (m, 2H), 2.51 (t, J = 7.2 Hz, 2H), 2.24 (m, 1H), 1.92-1.86 (m, 2H), 1.82-1.75 (m, 2H), 1.60 (m, 1H), 1.43-1.09 (m, 34H), 0.83 (t, J = 5.5 Hz, 3H), 0.76 (t, J = 5.6 Hz, 3H); 13C NMR (125 MHz, pyridine-d5) δ 174.9, 101.6, 77.4, 73.8, 73.4, 70.1, 68.5, 68.0, 62.8, 55.5, 53.0, 38.1, 35.1, 33.4, 33.2, 31.7, 31.5, 31.34, 31.32, 31.28, 31.2, 30.93, 30.89, 30.7, 27.8, 27.7, 24.2, 24.1, 15.6, 15.5; HRMS calculated for C32H64N2O8Na ([M + Na]+) 627.4560, found 627.4553.
3-Deoxy-3-acetamido-O-α-D-galactopyranosyl-(1,1)-(2S,3S,4R)-2-octanoylamino-octadecan -1,3,4-triol (4)
To a solution of amino 3 (10 mg, 0.017 mmol) in EtOH/CHCl3 (2:1, 2 ml) was added acetyl anhydride (7 μl, 0.066 mmol) and Et3N (10 μl). The solution was stirred at room temperature for 1h then worked up by adding one drop of concentrated ammonium hydroxide. The concentrated residue (22 mg) was purified by column chromatography with CHCl3/MeOH (8:1) to give 4 in 49.3% yields as a white solid. 1H NMR (500 MHz, pyridine-d5) δ 8.78 (d, J = 3.0 Hz, 1H), 8.56 (d, J = 8.5 Hz, 1H), 5.49 (d, J = 3.5 Hz, 1H), 4.60-4.54 (m, 3H), 4.47-4.41 (m, 2H), 4.37-4.34 (m, 1H), 4.22-4.32 (m, 3H), 2.45-2.40 (m, 2H), 2.21 (m, 1H), 2.03 (s, 3H), 1.91-1.85 (m, 2H), 1.79-1.73 (m, 2H), 1.65 (m, 1H), 1.27-1.10 (m, 36H), 0.84 (t, J = 7.0 Hz, 3H), 0.78 (t, J = 7.0 Hz, 3H); 13C NMR (125 MHz, pyridine-d5) δ 173.4, 170.7, 100.9, 76.0, 72.6, 72.3, 69.1, 68.4, 67.9, 62.3, 52.9, 51.7, 36.6, 33.7, 31.9, 31.7, 30.1, 29.9, 29.80, 29.76, 29.7, 29.4, 29.37, 29.1, 26.3, 26.1, 23.0, 22.7, 22.6, 14.0, 13.1; HRMS calculated for C34H66N2O9Na ([M + Na]+) 669.4666, found 669.4670.
2,3-Di-O-benzyl-4,6-O-benzyliden-α-D-galactopyranosyl-(1,1)-(2S,3S,4R)-2-azido-3,4-di-O-benzyl-octadecan-1,3,4-triol (31)
Powered 4Å molecule sieves (1.0 g) were added to a stirred solution of benzylidene protected trichloroacetimide donor 29 (192 mg, 0.32 mmol) and azido lipid 30 (170 mg, 0.32 mmol) in fresh dried CH2Cl2 (4 mL). After 30 min, the mixture was cooled to −40 °C, TMSOTf (6 μL, 0.03 mmol) was added by syringe and the resulting mixture was stirred for 2h. The reaction was quenched by addition of Et3N (0.1 mL), and the mixture was filtered through Celite pad. The filtered was concentrated and purified by column chromatography with hexane/ethyl acetate (8:1) to give 205 mg of product in 67% yield. 1H NMR (500 MHz, CDCl3) δ 7.56-7.24 (m, 25H), 5.48 (s, 1H), 5.00 (d, J = 3.4 Hz, 1H), 4.89 (d, J = 11.8 Hz, 1H), 4.84 (d, J = 12.4 Hz, 1H), 4.77 (d, J = 12.4 Hz, 1H), 4.70 (d, J = 12.1 Hz, 1H), 4.69 (d, J = 11.3 Hz, 1H), 4.64 (d, J = 11.6 Hz, 1H), 4.61 (d, J = 11.8 Hz, 1H), 4.53 (d, J = 11.6 Hz, 1H), 4.19 (d, J = 3.2 Hz, 1H), 4.13 (dd, J = 10.3, 3.4 Hz, 1H), 4.10 (d, J = 11.5 Hz, 1H), 4.06 (dd, J = 10.4, 3.3 Hz, 1H), 4.03 (d, J = 7.9 Hz, 1H), 3.91 (d, J = 12.0 Hz, 1H), 3.78-3.72 (m, 3H), 3.66 (m, 1H), 3.60 (br, 1H), 1.70 (m, 1H), 1.57 (m, 1H), 1.44 (m, 1H), 1.34-1.25 (m, 23H), 0.92 (t, J = 6.9 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 138.8, 138.4, 138.1, 137.9, 128.9, 128.4, 128.38, 128.3, 128.2, 128.1, 127.9, 127.8, 127.78, 127.73, 127.70, 127.6, 127.5, 127.48, 126.4, 101.1, 99.2, 79.5, 79.0, 75.9, 75.5, 74.7, 73.8, 73.5, 72.1, 72.0, 69.4, 68.5, 63.0, 61.7, 31.9, 30.1, 29.8, 29.7, 29.68, 29.66, 29.6, 29.4, 25.5, 22.7, 14.1; HRMS calcd for C59H75N3O8Na ([M + Na]+) 976.5446, found 976.5467.
2,3,6-Tri-O-benzyl-α-D-galactopyranosyl-(1,1)-(2S,3S,4R)-2-azido-3,4-di-O-benzyl-octadeca n-1,3,4-triol (32)
The compound 31 (190 mg, 0.20 mmol) was concentrated twice from toluene and then dissolved in fresh dried THF (5 mL) containing 4Å molecule sieves (0.50 g). After stirring for 20 min, NaCNBH3 (125 mg, 2.0 mmol) was added, followed by a crystal of methyl orange. A solution of hydrochloride in ethyl ether (2 M) was added dropwise until a pink color persists. After 90 min, an additional portion of the hydrochloride in ethyl ether solution (0.5 mL) was added and the reaction mixture was continued stirring for 2.5 h. The reaction mixture was filtered into a separatory funnel containing a 1:1 mixture of CH2Cl2 and water. The organic layer was separated and the aqueous was extracted with additional CH2Cl2 (2 × 5 mL). The combined organic layers were washed successively with saturated aqueous NaHCO3 and brine. After dried over anhydrous Na2SO4, filtered and concentrated, the residue was purified by column chromatography with hexane/ethyl acetate (5:1) to give 174 mg of product as colorless oil in 91% yield. 1H NMR (500 MHz, CDCl3) δ 7.42-7.25 (m, 25H), 4.94 (d, J = 2.8 Hz, 1H), 4.82 (d, J = 11.7 Hz, 1H), 4.81 (d, J = 12.0 Hz, 1H), 4.75 (d, J = 11.7 Hz, 1H), 4.71 (d, J = 12.4 Hz, 1H), 4.70 (d, J = 11.5 Hz, 1H), 4.67 (d, J = 11.4 Hz, 1H), 4.61 (d, J = 11.6 Hz, 1H), 4.59 (d, J = 11.9 Hz, 1H), 4.55 (d, J = 12.2 Hz, 1H), 4.53 (d, J = 11.6 Hz, 1H), 4.10 (m, 1H), 4.08 (dd, J = 10.3, 1.9 Hz, 1H), 3.97 (t, J = 5.9 Hz, 1H), 3.95 (m, 2H), 3.80-3.75 (m, 3H), 3.72 (m, 1H), 3.68-3.64 (m, 2H), 2.67 (br, 1H), 1.70 (m, 1H), 1.58 (m, 1H), 1.44 (m, 1H), 1.36-1.25 (m, 23H), 0.93 (t, J = 6.7 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 138.6, 138.5, 138.2, 138.16, 138.0, 128.5, 128.4, 128.37, 128.35, 128.3, 127.9, 127.8, 127.7, 127.68, 127.6, 98.4, 79.4, 79.1, 77.5, 75.7, 73.8, 73.6, 73.2, 72,6, 72.0, 69.6, 68.9, 68.1, 68.1, 62.0, 32.0, 30.0, 29.8, 29.74, 29.72, 29.70, 29.68, 29.65, 29.4, 25.4, 22.7, 14.2; HRMS calcd for C59H77N3O8Na ([M + Na]+) 978.5603, found 978.5589.
2,3,6-Tri-O-benzyl-4-O-methyl-α-D-galactopyranosyl-(1,1)-(2S,3S,4R)-2-azido-3,4-di-O-ben zyl-octadecan-1,3,4-triol (33)
To a solution of the hydroxyl compound 32 (85 mg, 0.089 mmol) in dry DMF (2 mL) was added 60% NaH (5 mg, 0.12 mmol). The resulting suspension was stirred for 30 min. MeI (8 μL, 0.12 mmol) was added by syringe, and the reaction mixture was continued stirring for 8 h. DMF was removed in vacuo. The residue was dissolved in water (5 mL) and extracted with CH2Cl2 (3 × 5 mL). The combined organic layers were dried over anhydrous Na2SO4, filtered and concentrated. The residue was purified by column chromatography with hexane/ethyl acetate (9:1) to give 81 mg of product as colorless oil in 94% yield. 1H NMR (500 MHz, CDCl3) δ 7.43-7.24 (m, 25H, 5×Ph), 4.91 (d, J = 2.9 Hz, 1H, H-1′), 4.84 (d, J = 12.3, 2H, PhCH2), 4.77 (d, J = 11.8 Hz, 1H, PhCH2), 4.70 (d, J = 11.6 Hz, 2H, PhCH2), 4.65 (d, J = 11.4 Hz, 1H, PhCH2), 4.61 (d, J = 11.6 Hz, 1H, PhCH2), 4.57 (d, J = 11.8 Hz, 1H, PhCH2), 4.51 (d, J = 11.5 Hz, 1H, PhCH2), 4.50 (d, J = 11.8 Hz, 1H, PhCH2), 4.04 (d, J = 8.6 Hz, 1H), 3.99 (m, 3H), 3.77-3.72 (m, 4H), 3.66 (dd, J = 9.2, 7.5, 1H), 3.64 (m, 1H), 3.59 (s, 3H), 3.54 (dd, J = 9.2, 5.9 Hz, 1H), 1.70 (m, 1H), 1.58 (m, 1H), 1.44 (m, 1H), 1.36-1.25 (m, 23H), 0.93 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 138.8, 138.77, 138.5, 138.2, 138.0, 128.4, 128.35, 128.3, 127.9, 127.8, 127.7, 127.69, 127.67, 127.64, 127.6, 127.5, 127.4, 98.7, 79.4, 79.1, 78.6, 76.6, 73.7, 73.6, 73.4, 73.0, 72.1, 69.5, 68.6, 68.5, 62.0, 61.4, 32.0, 30.0, 29.8, 29.74, 29.71, 29.70, 29.67, 29.6, 29.4, 25.4, 22.7, 14.1; HRMS calcd for C60H79N3O8Na ([M + Na]+) 992.5759, found 992.5782.
2,3,6-Tri-O-benzyl-4-O-methyl-α-D-galactopyranosyl-(1,1)-(2S,3S,4R)-2-octanoylamino-3,4-di-O-benzyl-octadecan-1,3,4-triol (35)
A solution of the azido compound 33 (70 mg, 0.072 mmol) and PPh3 (37 mg, 0.14 mmol) in benzene (5 ml) and stoichiometric amount of water was stirred at 50 °C for 12 h. The solvent was removed in vacuo, and the residue was co-evaporated twice with benzene to remove the remaining trace amount of water. Then the residue was re-dissolved in anhydrous THF (4 mL) and the activated ester 1 (26 mg, 0.11 mmol) was added. The reaction was stirred at room temperature overnight. The solvent was removed in vacuo and the residue was purified by column chromatography with hexane/ethyl acetate (5:1) to give 72 mg of product in 91% yield. 1H NMR (500 MHz, CDCl3) δ 7.40-7.23 (m, 25H, 5×Ph), 6.06 (d, J = 8.5 Hz, 1H, CONH), 4.85 (d, J = 3.7 Hz, 1H, H-1′), 4.82 (d, J = 11.7 Hz, 1H, PhCH2), 4.80 (d, J = 11.7 Hz, 1H, PhCH2), 4.78 (d, J = 11.1 Hz, 1H, PhCH2), 4.76 (d, J = 11.4 Hz, 1H, PhCH2), 4.64 (d, J = 11.7 Hz, 1H, PhCH2), 4.61 (d, J = 11.4 Hz, 1H, PhCH2), 4.58 (d, J = 10.9 Hz, 1H, PhCH2), 4.54 (d, J = 11.5 Hz, 1H, PhCH2), 4.49 (d, J = 11.9 Hz, 1H, PhCH2), 4.46 (d, J = 11.6 Hz, 1H, PhCH2), 4.21 (m, 1H), 3.99 (dd, J = 10.9, 5.6 Hz, 1H), 3.95 (m, 2H), 3.88 (t, J = 2.4 Hz, 1H), 3.86 (dd, J = 5.4, 2.9 Hz, 1H), 3.78 (dd, J = 10.9, 4.1 Hz, 1H), 3.71 (d, J = 2.0 Hz, 1H), 3.63 (dd, J = 9.2, 7.0 Hz, 1H), 3.57 (s, 3H), 3.55 (dd, J = 9.3, 3.0 Hz, 1H), 3.52 (m, 1H), 1.96 (m, 2H), 1.66 (m, 3H), 1.49 (m, 3H), 1.35-1.21 (m, 30H), 0.91 (t, J = 6.9 Hz, 3H), 0.89 (t, J = 7.2 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 172.9, 138.7, 138.68, 138.6, 138.5, 137.7, 128.4, 128.38, 128.36, 128.34, 128.3, 127.9, 127.84, 127.83, 127.8, 127.7, 127.6, 127.54, 127.52, 99.5, 80.1, 78.8, 78.7, 77.2, 73.64, 73.60, 73.5, 72.8, 71.8, 69.8, 69.1, 68.8, 61.4, 50.4, 36.7, 31.9, 31.8, 29.9, 29.86, 29.73, 29.68, 29.4, 29.3, 29.1, 26.1, 25.7, 22.7, 22.6, 14.11, 14.08; HRMS calcd for C68H95NO9Na ([M + Na]+) 1092.6899, found 1092.6911.
4-O-Methyl-α-D-galactopyranosyl-(1,1)-(2S,3S,4R)-2-octanoylamino-octadecan-1,3,4-triol (6)
A suspension of compound 35 (32 mg, 0.030 mmol) and Pd(OH)2 (10 mg) in a mixture of EtOH and CHCl3 (4:1, 2.5 mL) was stirred under 1 atm H2 for 2 h. The catalyst was filtered through Celite pad, and the filtrate was concentrated and purified by column chromatography (CHCl3/MeOH, 6:1) to give 14 mg product in 74% yield. 1H NMR (500 MHz, pyridine-d5) δ 8.40 (d, J = 8.7 Hz, 1H), 6.42 (br, 2H), 6.04 (br, 1H), 5.49 (d, J = 3.8 Hz, 1H), 5.20 (m, 1H), 4.96 (br, 1H), 4.60 (dd, J = 10.9, 5.3 Hz, 1H), 4.52 (dd, J = 10.0, 3.8 Hz, 1H), 4.44 (t, J = 6.5 Hz, 1H), 4.41 (dd, J = 10.1, 3.1 Hz, 1H), 4.31 (dd, J = 10.9, 5.0 Hz, 1H), 4.26 (m, 2H), 4.23 (dd, J = 10.5, 6.5 Hz, 1H), 4.18 (dd, J = 10.5, 6.6 Hz, 1H), 4.00 (d, J = 3.0 Hz, 1H), 3.08 (s, 3H), 2.38 (t, J = 7.2 Hz, 2H), 2.24 (m, 1H), 1.86 (m, 2H), 1.73 (m, 2H), 1.64 (m, 1H), 1.38 (m, 2H), 1.30-1.08 (m, 28H), 0.85 (t, J = 6.7 Hz, 3H), 0.78 (t, J = 6.8 Hz, 3H); 13C NMR (125 MHz, pyridine-d5) δ 173.0, 101.2, 80.6, 76.4, 72.9, 72.2, 71.8, 70.3, 68.4, 61.4, 51.1, 36.5, 34.1, 31.9, 31.7, 30.1, 29.9, 29.78, 29.75, 29.67, 29.4, 29.1, 26.2, 26.1, 22.7, 22.6, 14.04, 13.98; HRMS calcd for C33H65NO9Na ([M + Na]+) 642.4552, found 642.4546.
2,3,6-Tri-O-benzyl-4-O-(tetrahydro-2H-pyran-2-yloxyethyl)-α-D-galactopyranosyl-(1,1)-(2S,3S,4R)-2-azido-3,4-di-O-benzyl-octadecan-1,3,4-triol (34)
To a solution of the hydroxyl compound 32 (50 mg, 0.052 mmol) in dry DMF (2 mL) was added 60% NaH (3 mg, 0.073 mmol). The resulting suspension was stirred for 30 min. THPOCH2CH2Cl (11 uL, 0.073 mmol) was added by syringe, and the reaction mixture was continued stirring for 8 h. DMF was removed in vacuo. The residue was dissolved in water (5 mL) and extracted with CH2Cl2 (3 × 5 mL). The combined organic layers were dried over anhydrous Na2SO4, filtered and concentrated. The residue was purified by column chromatography with hexane/ethyl acetate (9:1) to give 48 mg of product as colorless oil in 85% yield. 1H NMR (500 MHz, CDCl3) δ 7.39-7.22 (m, 25H), 4.92 (d, J = 3.5 Hz, 0.5H), 4.91 (d, J = 3.5 Hz, 0.5H), 4.81 (d, J = 11.9 Hz, 2H), 4.75 (d, J = 11.8 Hz, 1H), 4.69-4.48 (m, 8H), 4.12 (m, 1H), 4.04-3.94 (m, 4H), 3.90-3.79 (m, 3H), 3.76-3.70 (m, 5H), 3.63-3.54 (m, 3H), 3.47 (m, 1H), 1.82 (m, 1H), 1.68 (m, 2H), 1.61-1.53 (m, 5H), 1.50 (m, 2H), 1.42 (m, 1H), 1.34-1.23 (m, 22H), 0.91 (t, J = 6.8 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 138.84, 138.78, 138.5, 138.3, 138.2, 128.5, 128.44, 128.39, 128.34, 128.30, 128.24, 128.19, 127.93, 127.87, 127.73, 127.70, 127.66, 127.61, 127.57, 127.53, 127.46, 127.4, 98.9, 98.74, 98.70, 98.5, 79.4, 79.0, 78.69, 78.67, 76.6, 76.5, 76.48, 76.1, 73.7, 73.5, 73.3, 73.25, 73.0, 72.9, 72.6, 72.3, 72.0, 69.9, 69.8, 69.1, 69.0, 68.5, 67.2, 66.6, 62.3, 62.0, 61.9, 32.0, 30.7, 30.6, 30.0, 29.8, 29.73, 29.69, 29.67, 29.65, 29.4, 25.51, 25.49, 25.4, 22.7, 19.6, 19.4, 14.1; HRMS calcd for C66H89N3O10Na ([M + Na]+) 1106.6440, found 1106.6429.
2,3,6-Tri-O-benzyl-4-O-(tetrahydro-2H-pyran-2-yloxyethyl)-α-D-galactopyranosyl-(1,1)-(2S,3S,4R)-2-octanoylamino-3,4-di-O-benzyl-octadecan-1,3,4-triol (36)
A solution of the azido compound 34 (48 mg, 0.044 mmol) and PPh3 (23 mg, 0.088 mmol) in benzene (5 ml) and stoichiometric amount of water was stirred at 50 °C for 12 h. The solvent was removed in vacuo, and the residue was co-evaporated twice with benzene to remove the remaining trace amount of water. Then the residue was re-dissolved in anhydrous THF (4 mL) and the activated ester 1 (16 mg, 0.066 mmol) was added. The reaction was stirred at room temperature overnight. The solvent was removed in vacuo and the residue was purified by column chromatography with hexane/ethyl acetate (5:1) to give 46 mg of product in 87% yield. 1H NMR (500 MHz, CDCl3) δ 7.39-7.22 (m, 25H), 6.19 (d, J = 8.6 Hz, 0.5H), 6.15 (d, J = 8.6 Hz, 0.5H), 4.88 (d, J = 3.7 Hz, 0.5 H), 4.87 (d, J = 3.7 Hz, 0.5H), 4.82-4.74 (m, 4H), 4.66-4.50 (m, 7H), 4.50 (d, J = 11.7 Hz, 1H), 4.19 (m, 1H), 4.14-3.94 (m, 4H), 3.90-3.69 (m, 9H), 3.64-3.50 (m, 3H), 3.46 (m, 1H), 2.36 (t, J = 7.5 Hz, 2H), 1.97 (m, 2H), 1.80 (m, 1H), 1.70-1.61 (m, 5H), 1.58-1.45 (m, 7H), 1.38-1.21 (m, 25H), 0.91 (t, J = 6.7 Hz, 3H), 0.89 (t, J = 7.1 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ; HRMS calcd for C74H105NO11Na ([M + Na]+) 1206.7580, found 1206.7601.
4-O-(2-Hydroxylethyl)-α-D-galactopyranosyl-(1,1)-(2S,3S,4R)-2-octanoylamino-octadecan-1,3,4-triol (7)
To a solution of the compound 36 (40 mg, 0.034 mmol) in a mixture of CH2Cl2/MeOH (2:1, 3 mL) was added p-TsOH (10 mg, 0.053 mmol). The resulting mixture was stirred for 2h. The reaction was quenched by addition of Et3N (100 μL). The solvent was removed in vacuo, and the residue was purified by column chromatography (hexane/ethyl acetate, 3:1) to give 32 mg of product.
A suspension of the above product (32 mg, 0.030 mmol) and Pd(OH)2 (10 mg) in a mixture of EtOH and CHCl3 (4:1, 2.5 mL) was stirred under 1 atm H2 for 3 h. When TLC showed that reaction completed, the catalyst was filtered through Celite pad, and the filtrate was concentrated and purified by column chromatography (CHCl3/MeOH, 6:1) to give 14 mg of product in 61% yield for two steps. 1H NMR (500 MHz, pyridine-d5) δ 8.41 (d, J = 8.7 Hz, 1H), 7.96 (br, 1H), 6.48 (br, 2H), 6.05 (br, 1H), 5.50 (d, J = 3.7 Hz, 1H), 5.21 (m, 1H), 4.95 (m, 1H), 4.60 (dd, J = 10.8, 5.3 Hz, 1H), 4.55 (dd, J = 10.0, 3.8 Hz, 1H), 4.44 (t, J = 6.7 Hz, 1H), 4.42 (dd, J = 9.6, 3.2 Hz, 1H), 4.32-4.17 (m, 8H), 3.95 (t, J = 4.6 Hz, 2H), 2.38 (t, J = 7.5 Hz, 2H), 2.24 (m, 1H), 1.86 (m, 2H), 1.74 (m, 2H), 1.63 (m, 1H), 1.37 (m, 2H), 1.29-1.08 (m, 28H), 0.85 (t, J = 6.9 Hz, 3H), 0.78 (t, J = 6.9 Hz, 3H); 13C NMR (125 MHz, pyridine-d5) δ 173.0, 101.2, 79.6, 76.4, 75.7, 72.6, 72.2, 71.6, 70.3, 68.3, 62.2, 61.2, 51.1, 36.5, 34.1, 31.9, 31.7, 30.1, 29.9, 29.8, 29.76, 29.69, 29.4, 29.1, 26.2, 26.1, 22.7, 22.6, 14.1, 14.0; HRMS calcd for C34H67NO10Na ([M + Na]+) 672.4657, found 672.4682.
NKT hybridoma stimulation assay
The synthetic glycolipids were dissolved in DMSO as 1.0 mg mL−1 and then diluted with medium to the indicated concentration. A population of 100,000 CD1d-transfected A20/CD1cells were pulsed by the glycolipids at concentration of 1000 ng mL−1, 100 ng mL−1, 10 ng mL−1 and 1 ng mL−1 in total volume of 200 μL, and then incubated overnight. After washed with medium, the pulsed A20/CD1 cells were mixed with 50,000 DN3A4-1.2 hybridoma cells and co-cultured for 24 hrs in total volume of 200 μL. After another 24 hours, the supernatant was collected. The released IL-2 in the culture supernatant was measured by Sandwich ELISA assay. Data is representative of 3 independent experiments.
For the ELISA, purified rat-anti-mouse IL-2 antibody (eBioscience) was diluted 1:200 in pH 7.4 PBS buffer and 100 μL of diluted antibody was applied to each well and incubate overnight at 37 °C. After blocking by 10% BSA in PBS, the 100μL 1:4 diluted supernatant from in vitro stimulation was applied to each ELISA well. After 2 hour incubation, the wells were washed by PBST (PBS with 0.2% v/v Tween-20) and the biotin-labeled second antibody (eBioscience) was applied. Finally, the HRP-conjugated-streptavidin and its substrate were used to develop and the absorbance of each well was measured (FlexStation 3 MicroPlate Reader, Molecular Devices). The concentration of IL-2 was calculated according to the standard wells added on each plate using SoftMax Pro (Molecular Devices).
Coat plate hybridoma assay
The assay was carried out according to previous publish protocol.[19] First, for each well, 0.5 μg mice CD1d protein in 100 μL pH 7.4 PBS was used to coat the surface. Glycolipid antigens with gradient concentrations (1000 ng mL−1, 100 ng mL−1, 10 ng mL−1 and 1 ng mL−1 in total volume of 100 μL) will be incubated in the coated wells for 24 hours. After being washed, 1×105 iNKT hybridoma cells, which have been well described,[20] will be cultured in the treated micro-wells for 18 hours. The IL-2 in the supernatant was collected and the IL-2 concentration was measured by ELISA as described above.
Splenocytes assay
Spleens from 6 week C57BL/6 mice were grinded down to single cell suspension. For each well, 1×106 cells were cultured with glycolipid antigen at concentration of 1000 ng mL−1 in 200 μL total volume. The mixtures were culture for 72 hours at 37 °C before the supernatants were collected. The IFN-γ and IL-4 concentration in the supernatants was measured by ELISA, as described above.
Scheme 1.
Preparation of 4′-NAc α-GalCer analogue 5.
Scheme 2.
Synthesis of 3′-substituted analogues 2, 3 and 4.
Scheme 3.
Preparation of 4′-O-substituted analogues 6 and 7.
Acknowledgments
P. G. Wang acknowledges National Cancer Institute (R01 CA118208), NSF (CHE-0616892), Bill & Melinda Gates Foundation (51946) for financial support.
Contributor Information
Prof. Dr. Chengfeng Xia, Email: xiachengfeng@mail.kib.ac.cn.
Prof. Dr. Peng G. Wang, Email: wang.892@osu.edu.
References
- 1.a) Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. Science. 1997;278:1626. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]; b) Kronenberg M. Annu Rev Immunol. 2005;23:877. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 2.Parekh VV, Wilson MT, Van Kaer L. Crit Rev Immunol. 2005;25:183. doi: 10.1615/critrevimmunol.v25.i3.20. [DOI] [PubMed] [Google Scholar]; b) Bendelac A, Rivera MN, Park SH, Roark JH. Annu Rev Immunol. 1997;15:535. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 3.a) Nakagawa R, Serizawa I, Motoki K, Sato M, Ueno H, Iijima R, Nakamura H, Shimosaka A, Koezuka Y. Oncol Res. 2000;12:51. doi: 10.3727/096504001108747521. [DOI] [PubMed] [Google Scholar]; b) Fuji N, Ueda Y, Fujiwara H, Toh T, Yoshimura T, Yamagishi H. Clin Cancer Res. 2000;6:3380. [PubMed] [Google Scholar]; c) Morita M, Motoki K, Akimoto K, Natori T, Sakai T, Sawa E, Yamaji K, Koezuka Y, Kobayashi E, Fukushima H. J Med Chem. 1995;38:2176. doi: 10.1021/jm00012a018. [DOI] [PubMed] [Google Scholar]; d) Kobayashi E, Motoki K, Uchida T, Fukushima H, Koezuka Y. Oncol Res. 1995;7:529. [PubMed] [Google Scholar]
- 4.a) Forestier C, Takaki T, Molano A, Im JS, Baine I, Jerud ES, Illarionov P, Ndonye R, Howell AR, Santamaria P, Besra GS, DiLorenzo TP, Porcelli SA. J Immunol. 2007;178:1415. doi: 10.4049/jimmunol.178.3.1415. [DOI] [PubMed] [Google Scholar]; b) Chen YG, Choisy-Rossi CM, Holl TM, Chapman HD, Besra GS, Porcelli SA, Shaffer DJ, Roopenian D, Wilson SB, Serreze DV. J Immunol. 2005;174:1196. doi: 10.4049/jimmunol.174.3.1196. [DOI] [PubMed] [Google Scholar]; c) Naumov YN, Bahjat KS, Gausling R, Abraham R, Exley MA, Koezuka Y, Balk SB, Strominger JL, Clare-Salzer M, Wilson SB. Proc Natl Acad Sci USA. 2001;98:13838. doi: 10.1073/pnas.251531798. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Hong S, Wilson MT, Serizawa I, Wu I, Singh N, Naidenko OV, Miura T, Haba T, Scherer DC, Wei J, Kronenberg M, Koezuka Y, Van Kaer L. Nature Med. 2001;7:1052. doi: 10.1038/nm0901-1052. [DOI] [PubMed] [Google Scholar]
- 5.Kakimi K, Guidotti LG, Koezuka Y, Chisari FV. J Exp Med. 2000;192:921. doi: 10.1084/jem.192.7.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.a) Nieuwenhuis EES, Matsumoto T, Exley M, Schleipman RA, Glickman J, Bailey DT, Corazza N, Colgan SP, Onderdonk AB, Blumberg RS. Nature Med. 2002;8:588. doi: 10.1038/nm0602-588. [DOI] [PubMed] [Google Scholar]; b) Hazlett LD, Li Q, Liu J, McClellan S, Du W, Barrett RP. J Immunol. 2007;179:1138. doi: 10.4049/jimmunol.179.2.1138. [DOI] [PubMed] [Google Scholar]
- 7.a) Tahir SM, Cheng O, Shaulov A, Koezuka Y, Bubley GJ, Wilson SB, Balk SP, Exley MA. J Immunol. 2001;167:4046. doi: 10.4049/jimmunol.167.7.4046. [DOI] [PubMed] [Google Scholar]; b) Dhodapkar MV, Geller MD, Chang DH, Shimizu K, Fujii S, Dhodapkar KM, Krasovsky J. J Exp Med. 2003;197:1667. doi: 10.1084/jem.20021650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Natori T, Koezuka Y, Higa T. Tetrahedron Lett. 1993;34:5591. [Google Scholar]
- 9.a) Koch M, Stronge VS, Shepherd D, Gadola SD, Mathew B, Ritter G, Fersht AR, Besra GS, Schmidt RR, Jones EY, Cerundolo V. Nature Immunol. 2005;6:819. doi: 10.1038/ni1225. [DOI] [PubMed] [Google Scholar]; b) Wu D, Zajonc DM, Fujio M, Sullivan BA, Kinjo Y, Kronenberg M, Wilson IA, Wong CH. Proc Natl Acad Sci U S A. 2006;103:3972. doi: 10.1073/pnas.0600285103. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Zajonc Dirk M, Cantu C, 3rd, Mattner D, Zhou, Savage Paul B, Bendelac A, Wilson Ian A, Teyton L. J Nature Immunol. 2005;6:810. doi: 10.1038/ni1224. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Borg NA, Wun KS, Kjer-Nielsen L, Wilce MCJ, Pellicci DG, Koh R, Besra GS, Bharadwaj M, Godfrey DI, McCluskey J, Rossjohn J. Nature. 2007;448:44. doi: 10.1038/nature05907. [DOI] [PubMed] [Google Scholar]
- 10.Wu D, Xing GW, Poles MA, Horowitz A, Kinjo Y, Sullivan B, Bodmer-Narkevitch V, Plettenburg O, Kronenberg M, Tsuji M, Ho DD, Wong CH. Proc Natl Acad Sci USA. 2005;102:1351. doi: 10.1073/pnas.0408696102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.a) Prigozy TI, Naidenko O, Qasba P, Elewaut D, Brossay L, Khurana A, Natori T, Koezuka Y, Kulkarni A, Kronenberg M. Science. 2001;291:664. doi: 10.1126/science.291.5504.664. [DOI] [PubMed] [Google Scholar]; b) Zhou XT, Forestier C, Goff RD, Li C, Teyton L, Bendelac A, Savage PB. Org Lett. 2002;4:1267. doi: 10.1021/ol025565+. [DOI] [PubMed] [Google Scholar]
- 12.Zhang W, Zheng X, Xia C, Perali RS, Yao Q, Liu Y, Zheng P, Wang PG. Chembiochem. 2008;9:1423. doi: 10.1002/cbic.200700625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wun KS, Borg NA, Kjer-Nielsen L, Beddoe T, Koh R, Richardson SK, Thakur M, Howell AR, Scott-Browne JP, Gapin L, Godfrey DI, McCluskey J, Rossjohn J. J Exp Med. 2008;205:939. doi: 10.1084/jem.20072141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elhalabi J, Rice KG. Nucleosides, Nucleotides Nucleic Acids. 2004;23:195. doi: 10.1081/ncn-120027828. [DOI] [PubMed] [Google Scholar]
- 15.a) Burdin N, Brossay L, Koezuka Y, Smiley ST, Grusby MJ, Gui M, Taniguchi M, Hayakawa K, Kronenberg M. J Immunol. 1998;161:3271. [PubMed] [Google Scholar]; b) Brossay L, Tangri S, Bix M, Cardell S, Locksley R, Kronenberg M. J Immunol. 1998;160:3681. [PubMed] [Google Scholar]
- 16.Miyamoto K, Miyake S, Yamamura T. Nature. 2001;413:531. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 17.Yang G, Schmieg J, Tusji M, Franck RW. Angew Chem. 2004;116:3906. doi: 10.1002/anie.200454215. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2004;43:3818. [Google Scholar]
- 18.a) Brigl M, Van den Elzen P, Chen X, Meyers JH, Wu D, Wong CH, Reddington F, Illarianov PA, Besra GS, Brenner MB, Gumperz JE. J Immunol. 2006;176:3625. doi: 10.4049/jimmunol.176.6.3625. [DOI] [PubMed] [Google Scholar]; b) Spada FM, Koezuka Y, Porcelli SA. J Exp Med. 1998;188:1529. doi: 10.1084/jem.188.8.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinjo Y, Tupin E, Wu D, Fujio M, Garcia-Navarro R, Benhnia MREI, Zajonc DM, Ben-Menachem G, Ainge GD, Painter GF, Khurana A, Hoebe K, Behar SM, Beutler B, Wilson IA, Tsuji M, Sellati TJ, Wong CH, Kronenberg M. Nature Immunol. 2006;7:978. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- 20.a) Sidobre S, Naidenko OV, Sim BC, Gascoigne NRJ, Garcia KC, Kronenberg M. J Immunol. 2002;169:1340. doi: 10.4049/jimmunol.169.3.1340. [DOI] [PubMed] [Google Scholar]; b) Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, Tsuji M, Kawahara K, Wong CH, Kronenberg M. Nature. 2005;434:520. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 21.Mormeneo D, Casas J, Llebaria A, Delgado A. Org Biomol Chem. 2007;5:3769. doi: 10.1039/b709421c. [DOI] [PubMed] [Google Scholar]
- 22.Nicolaou KC, Randall JL, Furst GT. J Am Chem Soc. 1985;107:5556. [Google Scholar]
- 23.Li Z, Gildersleeve JC. J Am Chem Soc. 2006;128:11612. doi: 10.1021/ja063247q. [DOI] [PubMed] [Google Scholar]
- 24.Greenberg WA, Priestley ES, Sears PS, Alper PB, Rosenbohm C, Hendrix M, Hung SC, Wong CH. J Am Chem Soc. 1999;121:6527. [Google Scholar]
- 25.Wang J, Li J, Chen H-N, Chang H, Tanifum CT, Liu H-H, Czyryca PG, Chang Ch-WT. J Med Chem. 2005;48:6271. doi: 10.1021/jm050368c. [DOI] [PubMed] [Google Scholar]