The site-specific tagging of proteins with organic fluorophores[2] has been proven to be a powerful method in structural and functional studies of a wide range of biological systems.[3-5] The most challenging aspect of this approach is the regiospecific incorporation of a suitable fluorophore on or nearby a rationally selected amino acid residue within a protein chain. The demonstration that pyrrolysine (1, Figure 1), the 22nd genetically-encoded amino acid, can be incorporated into recombinant proteins in response to the UAG codon,[6,7] prompted us to search for similarly incorporable analogs of 1 harboring reactive functionalities suitable for anchoring small organic fluorophores. We previously reported the synthesis of the THF-containing lysine derivative 2 that reads through the UAG codon,[8] as does its close structural analog 3.[9] The latter compound, thanks to the presence of the terminal alkyne functionality, enables site-specific post-translational modification of the resulting protein with azide-based fluorophores via the CuI-catalyzed azide–alkyne cycloaddition reaction (CuAAC). Our recent work has gone beyond the field of protein click chemistry resulting in the synthesis and application of the cysteine derivative 4 for protein ubiquitination via native chemical ligation.[10]
Figure 1.

Pyrrolysine (1) and analogues 2-8.
The main obstacle in the widespread use of 3 stems from its inaccessibility and high cost associated with the length of its synthesis (16 steps from ascorbic acid).[9] The guiding principle in the design of both 2 and 3 was the desire to maintain the steric and electronic similarity between 1 and its analogues, as it was believed to be a prerequisite for a successful pyrrolysine mimic. An earlier report by Polycarpo et al.[1] on simple pyrrolysine analogs had seemed to reinforce this notion. In particular, lysine derivatives bearing an acyclic acyl substituent at N-6 had been reported not to read through the UAG codon. However, a more recent work by Yanagisawa et al.[11] revealed that some acyclic analogs can also serve as viable pyrrolysine substitutes. Moreover, it was also reported that even N6-acetyllysine, when supplied at high concentration[12] or in the presence of a PylS (pyrrolysyl-tRNA synthetase) mutant,[13] can be charged onto PylT (tRNAPyl) or even incorporated into recombinant proteins.
In light of these more recent findings, we decided to carry out systematic studies to establish which structural and electronic features associated with 1-3 are necessary to be present in an effective clickable pyrrolysine mimic. Our hope was to find an inexpensive and readily available alternative to the THF analog 3. Notably, while our studies were ongoing, Nguyen et al.,[14] building on the structural studies by Polycarpo et al.,[1] have reported on a simple carbamate-based pyrrolysine analog for protein click chemistry. In our work, we focused on identifying alternative structural motifs beneficial for effective mimicking of 1. With this in mind, we carried out readthrough experiments with a series of analogs 5-8, i.e., derivatives of lysine acylated at N-6 with various pent-4-ynoic acids 9-12 (Scheme 1). The requisite compounds 5-8 were prepared by coupling (BOP/NMM) Boc-Lys-OtBu (13)[9] with the corresponding acids 9-12 to give amides 14-17 in excellent yield (>90%) in each case. Their subsequent treatment with neat TFA provided the desired pyrrolysine analogs 5-8 as TFA salts. Because the racemic acids 10-12 were used as substrates, the preliminary screening tests were performed with analogs 6-8 as ~1:1 diastereomeric mixtures.
Scheme 1.

Synthesis of pyrrolysine analogs 5-8: a) Boc-Lys-OtBu (13), BOP, NMM, CH2Cl2, room temperature, 14-24 h, 91-95%; b) TFA, room temperature, 1 h, ~100%. BOP: (benzotriazol-1-yloxy)tris(dimethylamino)phosphonium hexa-fluorophosphate, NMM: N-methylmorpholine, TFA: trifluoroacetic acid.
The site-specific incorporation efficiency of analogs 5-8 in response to the UAG codon was then measured with our modified fluorescence protein assay. The use of the green fluorescent protein (GFP) as a reporter to monitor stop-codon readthrough of non-natural amino acids has been previously described.[15,16] We further improved on this methodology by substituting GFP with a red fluorescent protein, mCherry, to reduce background fluorescence associated with the growth medium. Moreover, being able to cultivate the host cells in 24-well plates facilitates handling of multiple samples and minimizes random disparities between them. Finally, measuring the fluorescence intensity of the cell culture directly in the plates avoids labor-intensive electrophoresis and western blotting.
In the current study, the Lys55 codon of mCherry was site-specifically mutated to UAG and inserted into pPylST (PylS–PylT plasmid) bearing PylS and PylT genes from Methanosarcina mazei.[9] Escherichia coli strain BL21(DE3) transformed with this plasmid was then grown in the Terrific Broth medium (pH 7.4) supplied with a suitable pyrrolysine analog (2 mm) to be examined. The results of these experiments revealed significant differences between compounds 5-8 in terms of readthrough efficiency (Figure 2). The most simplified analog 5, bearing neither a ring nor an additional heteroatom at the lysine N-6 acyl substituent, failed to promote any detectable mCherry production, as did its hydroxyl congener 6. A small level of UAG readthrough was observed for the methoxy analog 7, although the compound is ~30 times less efficient than the original clickable analog 3. In contrast, the NH2-bearing analog 8 turned out to be a much better substrate, with its readthrough efficiency exceeding that of 3 by ~70%. Similar results were observed when the supplied analog concentration was varied from 0.5 mm to 20 mm (Figure S2). The only notable difference is that at higher concentrations 8 matches even 2 in terms of the level of incorporation. Importantly, the site-specificity of incorporation for 8 was confirmed by tandem mass spectrometry (Figure S3 and Tables S1, S2).
Figure 2.

Readthrough efficiency of pyrrolysine analogs 2-3 and 5-8 at 2 mm (pH 7.4). (a) A top-down view of the fluorescent image of the 24-well plate. The top row is the controls without supplying any pyrrolysine analogs. (b) Normalized readthrough efficiency (relative to that of 3) based on the fluorescence measurements. Error bars denote standard deviations calculated from three independent runs.
These preliminary screening experiments indicated that dipeptide 8, N6-(2-propargylglycyl)lysine (Pra-ε-Lys), is by far the best substrate in terms of the readthrough efficiency among the four new compounds tested. Because analogs 6-8 were prepared and screened as equimolar mixtures of two diastereomers, we carried out further experiments with isomerically pure samples of 8 prepared from commercially available homochiral propargylglycines (see the Supporting Information).[17] The readthrough efficiency of (R)-8 (d-Pra-ε-Lys), (S)-8 (l-Pra-ε-Lys), and their equimolar mixture 8 was measured at various concentrations and the results showed that (R)-8 is incorporated much more efficiently than (S)-8 (Figure 3). For instance, at 2 mm, (R)-8 incorporates ~35 times more readily than its isomeric counterpart (S)-8. Interestingly, it is apparent from the data collected that (R)-8 is markedly more efficient on its own than in the presence of (S)-8. One possible explanation is that the significantly less readily incorporable isomer (S)-8 serves nonetheless as an effective competitive inhibitor of incorporation of (R)-8.
Figure 3.
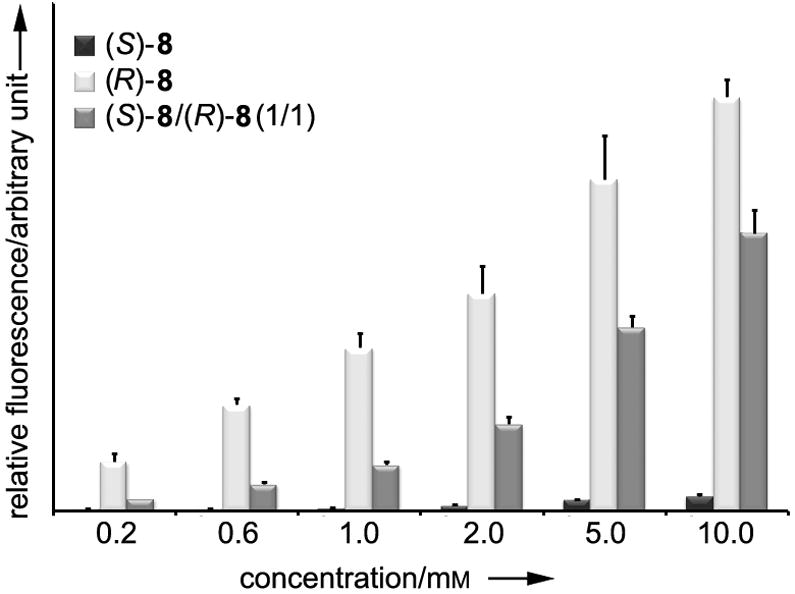
Concentration-dependent readthrough efficiency of pyrrolysine analogs (R)-8, (S)-8, and their equimolar mixture 8 at pH 8.5. Error bars indicate standard deviations calculated from three independent runs.
The observation that the d-Pra derivative (R)-8 exhibits a much higher readthrough efficiency than its l-Pra counterpart (S)-8 is notable. It was previously established by Polycarpo et al.[1] and Li et al.[8] that in amide-type analogs possessing cyclic acyl substituents at the lysine N-6 a heteroatom needs to be placed within the ring at a position strictly corresponding to that of the imine nitrogen in 1 (carbamate-type analogs do not require an additional heteroatom).[11] Therefore, while 2 and (R)-18 (Figure 4) are competent pyrrolysine mimics, (S)-18, (S)-19, and (R)-19 are not.[1,8]
Figure 4.

Potential pyrrolysine analogs studied by Polycarpo et al. (18)[1] and Li et al. (19).[8]
Having identified (R)-8 as a strong outperformer of 3 in both the ease of synthesis and incorporation efficiency, we then focused on optimizing the expression conditions by adjusting pH of the E. coli medium. Our working rationale for this study was based on the previous structural analysis of M. mazei PylS bound to adenylated 1 and pyrophosphate which suggested that a conserved tyrosine (Tyr384) on a flexible loop moves into the substrate-binding pocket to form, via its hydroxyl group, a hydrogen bond with the imine nitrogen of 1.[18] This interaction might be important for pyrrolysine recognition and subsequent charging onto PylT. We speculated that, given its analogous position, the amine in (R)-8 could participate in a similar hydrogen bond.[19] While the imine group of 1 would be fully deprotonated in standard E. coli media (pH 7.4), the amino group of (R)-8 would be chiefly protonated which would hinder its ability to serve as a hydrogen-bond acceptor. However, at slightly higher pH, the amine should be deprotonated and, consequently, the readthrough efficiency of (R)-8 would be expected to increase. Indeed, when 2 mm (R)-8 was used, the production of full-length mCherry was higher at more basic pH of the growth medium (Figure 5). The total amount of the produced protein peaks at pH 8.5, with a yield five times greater than that at pH 6.5.
Figure 5.

pH-dependent readthrough efficiency of 2 mm (R)-8. Fluorescence intensity of cell culture grown in media at various pH was normalized to that recorded at pH 6.5. Error bars indicate standard deviations calculated from three independent runs.
Although these data appeared to support our initial hypothesis, the results of other studies did not. Specifically, readthrough efficiency experiments with other pyrrolysine analogs, particularly 2 and 3, yielded similar pH-dependent profiles (Figure S4). These latter findings suggest that the hydrogen-bonding interaction which we invoked in our initial hypothesis (vide supra) is presumably not the major contributor to the observed pH effect. Instead, it is most likely that the α-amino group of the lysine residue, which is an invariant structural feature in all the pyrrolysine analogs tested, is responsible for the observed pH effect. Another possibility is that the intracellular concentration of the pyrrolysine analogs is also pH dependent consistent with the fact that this parameter is also known to affect transport and metabolism of amino acids in E. coli resulting in changes to their cellular concentration.[20] Finally, the ability of PylS to recognize its substrates could be influenced by pH via changes in the protonation state of key residues such as Tyr384 (the hydrogen-bond donor in our original hypothesis). Ultimately, whatever the actual reason, higher pH levels appear to be favorable for readthrough regardless of the structural and electronic properties of our pyrrolysine analogs.
Following the demonstration that (R)-8 can be efficiently incorporated into mCherry, we expanded our studies to determine whether the developed protocol could be applied to other proteins. Therefore, calmodulin (CaM),[21] a small 17-kDa model protein widely used in biochemical and structural studies, was chosen as the second target for the incorporation of (R)-8. Rattus norvegicus calmodulin cDNA bearing a UAG codon at position 34 was subcloned into pPylST and expressed in the host E. coli strain BL21(DE3) in the presence of 2 mm (R)-8 (Figure 6). About 40 mg of (R)-8-CaM can be isolated from 1 l of the culture – a considerably higher yield than that reported for any other clickable pyrrolysine mimic.[11,14,22]
Figure 6.

a) Incorporation of (R)-8 into CaM and subsequent labeling with azidocoumarin 21 by CuAAC click reaction. b) Following the click reaction, the protein bands on the same SDS-PAGE gel were visualized first by UV excitation (right panel) and then by coomassie blue staining (left panel).
To facilitate our protein taggability studies, we first prepared 20 (Scheme 2), the click product between our most effective pyrrolysine mimic (R)-8 and azidocoumarin 21.[23] Thus, the CuAAC reaction between (R)-17 and 21 in the presence of catalytic amounts of CuSO4, tristriazole ligand 22,[24,25] and sodium ascorbate gave the coupling product 23 in high yield (84%). Deprotection with TFA furnished 20 as its TFA salt. Subsequent spectroscopic studies revealed that when azidocoumarin 21 is CuAAC-coupled with either (R)-8 or an (R)-8-containing protein, two major spectrometric changes are expected to be observed, i.e., the red shift of absorbance from 350 nm to 396 nm and a concomitant significant increase in the fluorescence emission intensity at 470 nm (Figure S5).[26] Armed with this knowledge, we set about to test the taggability of the purified (R)-8-CaM with azidocoumarin 21 under the standard CuAAC conditions. By continuously measuring either absorbance or fluorescence of the reaction mixture, we were able to determine that the coupling between 21 and (R)-8-CaM was nearly completed within 1 h (Figure S5). To confirm the successful labeling of the protein, (R)-8-CaM before and after labeling was characterized by SDS-PAGE gel electrophoresis and UV imaging (Figure 6).
Scheme 2.

Synthesis of click product 20: a) sodium ascorbate (40 mol%), 22 (10 mol%), CuSO4 (10 mol%), tBuOH, H2O, 84%; b) TFA, room temperature, 2 h, ~100%.
Coumarin is a fluorophore whose spectral properties are strongly dependent on the environment it resides in.[27] It therefore serves as a useful dye for probing protein dynamics. CaM is known to undergo dramatic conformational changes in response to the concomitant presence of CaII and a CaM-binding protein. With our long-term goal of developing a CaM-based CaII sensor in mind, a highly efficient CaM-binding peptide, M13,[28] was genetically fused to the C-terminus of (R)-8-CaM. After labeling the resulting (R)-8-CaM M13 with azidocoumarin 21, we measured the fluorescence of this protein as a function of the CaII concentration – with its rise from 0 to 1.0 × 10−4 m, the fluorescence due to the coumarin tag decreases by 45% (Figure 7). Thus, the (R)-8-CaM M13 can be used as a tool to detect and quantitatively measure CaII within the concentration range of 10−9–10−4 m.
Figure 7.

Fluorescence intensity of coumarin-labeled (R)-8-CaM–M13 and 20 as a function of the CaII concentration. Data normalized to the fluorescence intensity in the absence of CaII. Error bars indicate standard deviations calculated from three independent runs.
In summary, through systematic studies, we have identified a structurally simple and easily accessible taggable pyrrolysine analog (R)-8 (d-Pra-ε-Lys) and demonstrated its ability to be readily incorporated into two selected proteins in response to the UAG codon. The ability of both (R)-8 and (R)-4[10] to be read through the UAG codon in the presence of PylST suggests the likely possibility that lysines acylated at N-6 with d-amino acids could constitute a general set of compounds amenable to translational incorporation by the pyrrolysine machinery. We have also developed a highly convenient UAG codon readthrough assay that should be indispensable in a search for new pyrrolysine analogs.
Acknowledgments
This research was supported by an American Heart Association Great Rivers Affiliate Predoctoral Fellowship (0815449D) to X.L. and National Institutes of Health grant (GM061796) to M.K.C. supplemented by American Recovery and Reinvestment Act (ARRA) funds. We also thank the staff of the CCIC Mass Spectrometry and Proteomics Facility at OSU for assistance with protein analysis.
Footnotes
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/asia.200xxxxxx.
References
- 1.Polycarpo CR, Herring S, Berube A, Wood JL, Soll D, Ambrogelly A. FEBS Lett. 2006;580:6695–6700. doi: 10.1016/j.febslet.2006.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonçalves MS. Chem Rev. 2009;109:190–212. doi: 10.1021/cr0783840. [DOI] [PubMed] [Google Scholar]
- 3.Giepmans BN, Adams SR, Ellisman MH, Tsien RY. Science. 2006;312:217–224. doi: 10.1126/science.1124618. [DOI] [PubMed] [Google Scholar]
- 4.Chen I, Ting AY. Curr Opin Biotechnol. 2005;16:35–40. doi: 10.1016/j.copbio.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Foley TL, Burkart MD. Curr Opin Chem Biol. 2007;11:12–19. doi: 10.1016/j.cbpa.2006.11.036. [DOI] [PubMed] [Google Scholar]
- 6.Srinivasan G, James CM, Krzycki JA. Science. 2002;296:1459–1462. doi: 10.1126/science.1069588. [DOI] [PubMed] [Google Scholar]
- 7.Hao B, Gong W, Ferguson TK, James CM, Krzycki JA, Chan MK. Science. 2002;296:1462–1466. doi: 10.1126/science.1069556. [DOI] [PubMed] [Google Scholar]
- 8.Li WT, Mahapatra A, Longstaff DG, Bechtel J, Zhao G, Kang PT, Chan MK, Krzycki JA. J Mol Biol. 2009;385:1156–1164. doi: 10.1016/j.jmb.2008.11.032. [DOI] [PubMed] [Google Scholar]
- 9.Fekner T, Li X, Lee MM, Chan MK. Angew Chem. 2009;121:1661–1663. [Google Scholar]; Angew Chem Int Ed Engl. 2009;48:1633–1635. doi: 10.1002/anie.200805420. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Fekner T, Ottesen JJ, Chan MK. Angew Chem. 2009;121:9348–9351. doi: 10.1002/anie.200904472. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed Engl. 2009;48:9184–9187. doi: 10.1002/anie.200904472. [DOI] [PubMed] [Google Scholar]
- 11.Yanagisawa T, Ishii R, Fukunaga R, Kobayashi T, Sakamoto K, Yokoyama S. Chem Biol. 2008;15:1187–1197. doi: 10.1016/j.chembiol.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi T, Yanagisawa T, Sakamoto K, Yokoyama S. J Mol Biol. 2009;385:1352–1360. doi: 10.1016/j.jmb.2008.11.059. [DOI] [PubMed] [Google Scholar]
- 13.Neumann H, Peak-Chew SY, Chin JW. Nat Chem Biol. 2008;4:232–234. doi: 10.1038/nchembio.73. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen DP, Lusic H, Neumann H, Kapadnis PB, Deiters A, Chin JW. J Am Chem Soc. 2009;131:8720–8721. doi: 10.1021/ja900553w. [DOI] [PubMed] [Google Scholar]
- 15.Liu W, Brock A, Chen S, Schultz PG. Nat Methods. 2007;4:239–244. doi: 10.1038/nmeth1016. [DOI] [PubMed] [Google Scholar]
- 16.Wang W, Takimoto JK, Louie GV, Baiga TJ, Noel JP, Lee KF, Slesinger PA, Wang L. Nat Neurosci. 2007;10:1063–1072. doi: 10.1038/nn1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chenault HK, Dahmer J, Whitesides GM. J Am Chem Soc. 2009;131:8720–8721. [Google Scholar]
- 18.Kavran JM, Gundllapalli S, O’Donoghue P, Englert M, Soll D, Steitz TA. Proc Natl Acad Sci U S A. 2007;104:11268–11273. doi: 10.1073/pnas.0704769104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yanagisawa T, Ishii R, Fukunaga R, Kobayashi T, Sakamoto K, Yokoyama S. J Mol Biol. 2008;378:634–652. doi: 10.1016/j.jmb.2008.02.045. [DOI] [PubMed] [Google Scholar]
- 20.Stancik LM, Stancik DM, Schmidt B, Barnhart DM, Yoncheva YN, Slonczewski JL. J Bacteriol. 2002;184:4246–4258. doi: 10.1128/JB.184.15.4246-4258.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manalan AS, Klee CB. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;18:227–278. [PubMed] [Google Scholar]
- 22.Deiters A, Schultz PG. Bioorg Med Chem Lett. 2005;15:1521–1524. doi: 10.1016/j.bmcl.2004.12.065. [DOI] [PubMed] [Google Scholar]
- 23.Sivakumar K, Xie F, Cash BM, Long S, Barnhill HN, Wang Q. Org Lett. 2004;6:4603–4606. doi: 10.1021/ol047955x. [DOI] [PubMed] [Google Scholar]
- 24.van Kasteren SI, Kramer HB, Jensen HH, Campbell SJ, Kirkpatrick J, Oldham NJ, Anthony DC, Davis BG. Nature. 2007;446:1105–1109. doi: 10.1038/nature05757. [DOI] [PubMed] [Google Scholar]
- 25.Kramer HB, Chalker JM, Davis BG. Nat Protoc. doi: 10.1038/nprot.2007.462. [DOI] [Google Scholar]
- 26.Chittepu P, Sirivolu VR, Seela F. Bioorg Med Chem. 2008;16:8427–8439. doi: 10.1016/j.bmc.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 27.Jones G, Jackson WR, Choi CY, Bergmark WR. J Phys Chem. 1985;89:294–300. [Google Scholar]
- 28.Ikura M, Clore GM, Gronenborn AM, Zhu G, Klee CB, Bax A. Science. 1992;256:632–638. doi: 10.1126/science.1585175. [DOI] [PubMed] [Google Scholar]


