Table 2.
Dinuclear zinc-catalyzed direct aldol addition reaction optimization.
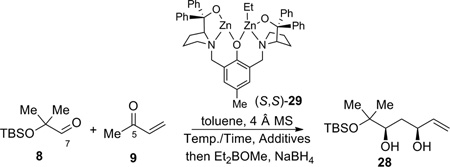 | ||||||
|---|---|---|---|---|---|---|
| Entry | mol% cat. | Temp./Time | Additives | Yielda | eeb | drc |
| 1 | 10 | −15 °C/7h | 5 equiv i-PrOH | 20% | 93% | 24:1 |
| 2 | 10 | 4 °C/7h | 5 equiv i-PrOH | 25% | 97% | 22:1 |
| 3 | 10 | 25 °C/7h | 5 equiv i-PrOH | 41% | 97% | 18:1 |
| 4 | 10 | 25 °C/9h | 5 equiv i-PrOH | 25% | 96% | 38:1 |
| 5 | 10 | 25 °C/24h | 5 equiv i-PrOH | 25% | 98% | 26:1 |
| 6 | 10 | 50 °C/9h | 5 equiv i-PrOH | 0% | -- | -- |
| 7 | 10 | 4 °C/7h | 5 equiv i-PrOH, 0.6% AcOH 3% Hydroquinone |
45% | 98% | 35:1 |
| 8 | 10 | 4 °C/7h |
5 equiv i-PrOH, 1.6% AcOH 3% Hydroquinone |
52% | 97% | 31:1 |
| 9 | 5 | 4 °C/7h | 5 equiv i-PrOH, 1.6% AcOH 3% Hydroquinone |
50% | 90% | 25:1 |
| 10 | 10 | 25 °C/7h | 5 equiv i-PrOH, 1.6% AcOH 3% Hydroquinone |
32% | 98% | 17:1 |
| 11 | 10 | 25 °C/4h | 5 equiv i-PrOH, 1.6% AcOH 3% Hydroquinone |
40% | 92% | 50:1 |
| 12 | 10 | 4 °C/20h | 5 equiv i-PrOH, 2.6% AcOH 3% Hydroquinone |
trace | -- | -- |
| 13 | 10 | 4 °C/7h | 16 mol% methyl β-hydroxypropionate |
16% | 84% | 25:1 |
Isolated yield.
ee was determined by chiral HPLC
dr was determined by 1H NMR.
