Abstract
Palladium-catalyzed amination of aryl halides has undergone rapid development in the last 12 years. This has been largely driven by implementation of new classes of ligands. Biaryl phosphines have proven to provide especially active catalysts in this context. This review discusses the applications that these catalysts have found in C-N cross-coupling in heterocycle synthesis, pharmaceuticals, materials science and natural product synthesis.
Keywords: C-N coupling, palladium, phosphine ligands, homogeneous catalysis, heterocycles
Introduction
The development of phosphine ligands for transition metal-catalyzed reactions has had a huge impact on catalytic processes in chemistry. The steric and electronic properties of phosphines may be varied readily and independently. This permits fine tuning of the coordinated species allowing the desired properties of the complex at different steps of a catalytic cycle to be enhanced. In 1998 we introduced a new class of air-stable[1] phosphine ligands based on the dialkylbiaryl phosphine backbone.[2] These phosphines have been used as ligands for gold,[3-11] silver,[12] rhodium,[13, 14] ruthenium[15-17] and copper[18] where they have been shown to impart improvements in reactivity and catalyst stability. It is in reactions catalyzed by palladium, however, that they have had by far the greatest impact including the Sonogashira,[19] Negishi,[20] Hiyama,[21-23] Kumada[24] and Suzuki[25-30] cross-coupling reactions, Heck reaction,[31-33] enolate arylation[34-37] and allylation,[38] reductive cyclization[39] and etherification,[40-43] silylation,[44] borylation,[45-47] cyanation,[48, 49] methylation,[50] direct arylation[51] and dehalogenation[52] of aryl halides. This review, however, focuses on the use of dialkylbiaryl phosphine ligands in palladium-catalyzed amination reactions of aryl halides and pseudo-halides.[53, 54] Palladium-catalyzed amination has rapidly become an important tool in organic synthesis, pharmaceutical and materials science.[55-69] Progress in this reaction have been driven largely by the implementation of new classes of ligands. Initial studies made use of tri-o-tolylphosphine.[70-72] Later the scope was improved by the application of chelating ligands, especially BINAP[73] and dppf.[74] Subsequent research using these and other ligand classes, notably monodentate phosphines including trialkylphosphines,[75-77] dialkylarylphosphines,[78] QPhos,[79] chelating phosphines including Josiphos-type ligands[80] and Xantphos[81] and other ligand classes such as Verkade's phosphatranes,[82] secondary phosphine oxides[83] and N-heterocyclic carbenes[84] have served to widen the substrate scope and resulted in milder reaction conditions.
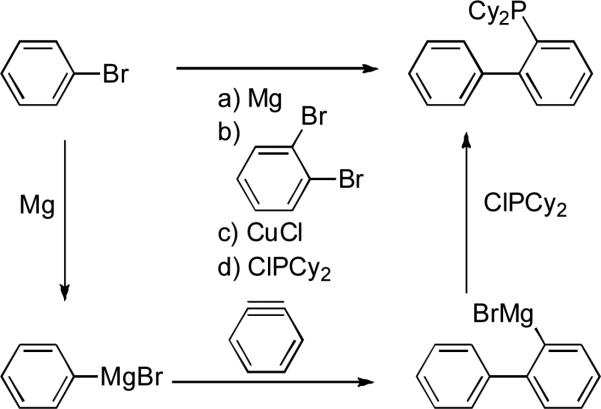
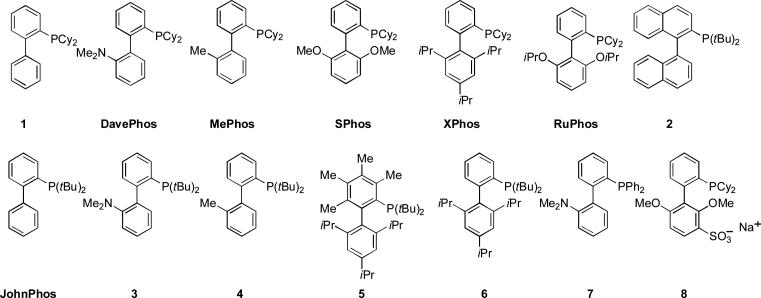
Synthesis of the dialkylbiaryl phosphine ligands is straightforward and completed in a single synthetic operation. An aryl halide is treated with excess magnesium to form the Grignard reagent. A 1,2-dihaloarene is then added, which reacts with more magnesium metal to form benzyne in situ. The aryl magnesium halide then adds to the benzyne and the resulting Grignard reagent is treated with a dialkylchlorophosphine in the presence of a catalytic quantity of copper(I) chloride to generate the ligand (Scheme 1).[85, 86] As a result of their ease of synthesis and utility a significant number of this class are now commercially available (Figure 1) and their large scale synthesis has been investigated by Rhodia Pharma Solutions and Lanxess[87, 88] and now at Shasun and Saltigo. The utility of these ligands has resulted in the introduction of structurally related variants by others[89-105] and has spawned innovative alternative synthetic routes by Diels-Alder reaction[96, 106] and formal rhodium-catalyzed [2+2+2] cycloaddition.[107, 108]
Scheme 1.
Synthesis of biaryl phosphines.
Figure 1.
Representative commercially available biaryl phosphine ligands.
Structural Features of the Dialkylbiaryl Phosphine Ligands
The use of dialkylbiaryl phosphine ligands often allows reactions to proceed with short reaction times, low catalyst loadings and under mild reaction conditions and a number of studies have been directed towards finding the origin of these effects to further optimize catalyst design. The mechanism of the palladium-catalyzed amination has been the subject of intensive study with numerous ligand systems (Scheme 2).[109-116] In the case of the dialkylbiaryl phosphines the catalytically active species is believed to be the monoligated, highly reactive L1Pd(0) complex which exists in equilibrium with the L2Pd(0) species.[117] The considerable steric bulk and strong electron donor ability of the dialkylbiaryl phosphine ligands presumably serves to encourage the formation of this active species which allows oxidative addition to occur under mild conditions even with unactivated aryl chlorides. It has been shown using reaction calorimetry that the size of the substituents on the lower ring have a significant effect on the rate of aryl halide amination.[118] For example, the metal:ligand ratio has a marked effect on the initial reaction rate.
Scheme 2.
Proposed catalytic cycle.
In the case of ligand 9 (figure 2) the rate of reaction was much lower when a M:L ratio of 1:4 was used, while with bulkier ligand XPhos little dependency on the metal:ligand ratio was found. This was taken to suggest that the larger lower ring substituents on XPhos had a role in promoting the formation of the reactive L1Pd(0) species. Another interesting feature which came to light in this study, was that for ligands with only one lower ring substituent the reaction rate increases with turnover number, while for 2’,6’-disubstituted ligands it remains essentially constant. It was later demonstrated through deuterium labelling studies that, in the case of 2’-monosubstituted dialkylbiaryl ligands the Pd(II) precatalysts have a tendency to form palladacycles 10 (Figure 3).[119] This effect would serve to reduce the rate of catalyst activation as such palladacycles are only slowly converted into the active catalyst.[120]
Figure 2.

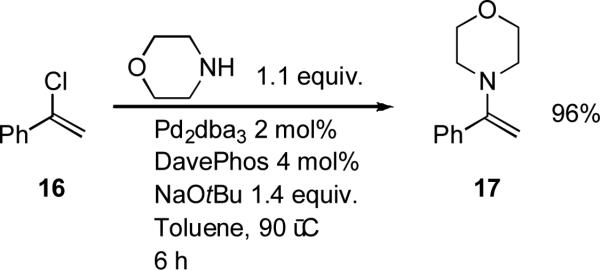
Structure of ligand 9.
Figure 3.
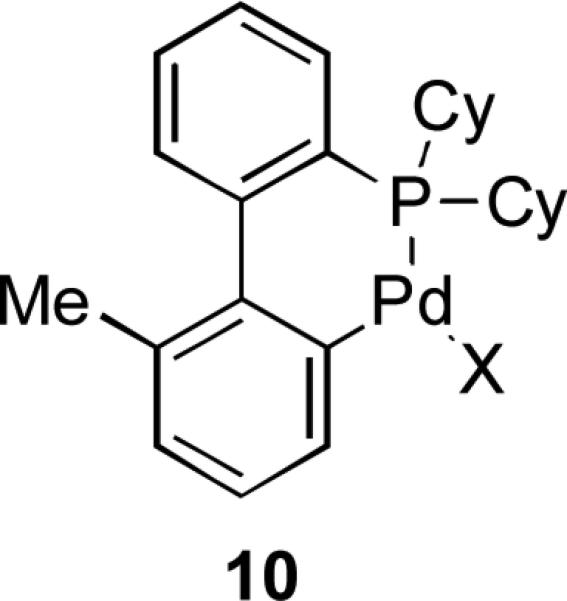
Palladacycle structure.
Another characteristic of the dialkylbiaryl phosphine ligands which is believed to promote catalyst stability and increase electron density at the metal center is the possibility of Pd-arene interactions between the metal atom and the lower ring of the ligand.[121] Such interactions have been observed in a number of palladium-biaryl phosphine X-ray crystal structures[27-29, 122-125] and even in an oxidative addition complex with methyl triflate.[126] Obtaining experimental information on the importance of these interactions in the reactive, catalytically active species has proven more difficult.[127] These species have been examined by DFT methods, however, which has served to underline the importance of the Pdarene interactions in intermediates in the catalytic cycle (Figure 4).[128, 129]
Figure 4.

Palladium-arene interaction.
These studies have also indicated that the lower ring promotes the reductive elimination step. The palladium-arene interaction stabilizes the palladium-amido intermediate and reduces the transition state energy when the palladium is proximal to the lower ring (relative to the corresponding species with palladium distal to the lower aromatic ring). A further advantage of reductive elimination from the proximal complex is that directly following reductive elimination the stable L1Pd(0) complex is regenerated to reenter the catalytic cycle.
Synthesis of Imines, Enamines and Enamides
The metal-mediated synthesis of enamines and imines from vinyl halides and pseudohalides has received much attention in recent years and has seen application in the synthesis of heterocycles and natural products.[130, 131] The first amination of vinyl halides was documented by Voskoboynikov and Beletskaya using azoles and phenothiazines as nucleophiles.[132] The best system required lithium azoles and JohnPhos as ligand for palladium. Chelating ligands were less effective, but tri-t-butylphosphine resulted in a marginally less efficient catalytic system and so due to its lower cost was used to explore the substrate scope of the reaction. Importantly the process was stereospecific for cis- and trans-β-bromostyrenes. Subsequently Movassaghi has extended this reaction type to the readily available vinyl triflates by using XPhos as ligand (Scheme 3).[133] Tribasic potassium phosphate was the most useful base and rigorous drying was essential in order to minimize the formation of ynoate and β-ketoester byproducts.
Scheme 3.
Movassaghi's coupling of a vinyl triflate with 3-cyanoindole.
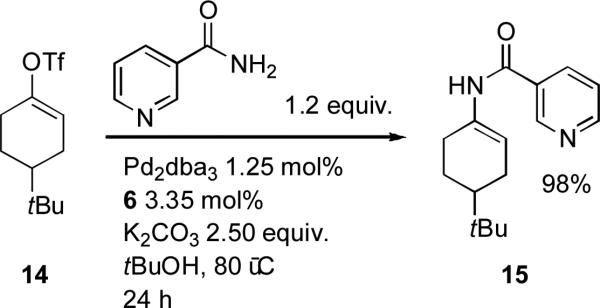
In order to couple vinyl triflates with cyclic aliphatic amines Willis found that dialkylbiaryl phosphine ligands such as 1 could be employed, but that with the weaker base cesium carbonate the chelating ligand BINAP gave superior results.[134] Chemists at Merck have shown that to couple vinyl triflates[135] and vinyl tosylates[136] with an activating substituent at the β position with amides the best systems are provided by the chelating ligands Xantphos and dipf (1,1′-bis(di-iso-propylphosphino)ferrocene) respectively. When Willis came to examine the coupling of unactivated vinyl triflates and tosylates, however, ligand 6 proved to be optimal (Scheme 4).[137]
Scheme 4.
Willis’ coupling of amides with vinyl triflates.
For the amination of vinyl bromides with alkyl and aromatic amines Barluenga showed that BINAP was a highly effective ligand.[138, 139] In order to utilize vinyl chlorides as substrates, however, DavePhos was required (Scheme 5).[140]
Scheme 5.
Barluenga's coupling of vinyl chlorides with amines.
Similarly for 1-halo-1,3-butadienes the same workers found that XPhos provided the most useful results (Scheme 6).[141]
Scheme 6.
Barluenga's coupling of 1-halo-1,3-butadienes with amines.
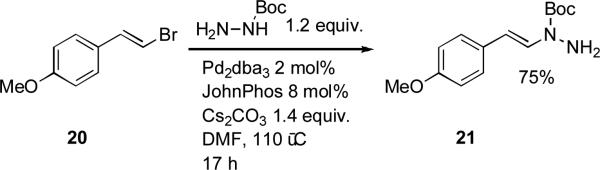
Furthermore, in order to prepare N-alkenyl hydrazines JohnPhos was the best ligand (Scheme 7).[142] They discovered that the reaction was very sensitive to the choice of base and solvent and that it was highly selective for coupling at the N-Boc-substituted nitrogen. They attribute these observations to deprotonation of the N-Boc nitrogen occurring prior to palladium coordination.
Scheme 7.
Barluenga's coupling of vinyl bromides with hydrazines.
In order to couple N-silylimines with vinyl bromides the best results were obtained with DavePhos, though BINAP showed a superior activity for aryl bromides (Scheme 8).[143]
Scheme 8.
Barluenga's coupling of vinyl bromides with trialkylsilylamines.
Applications in the Construction of Heterocycles
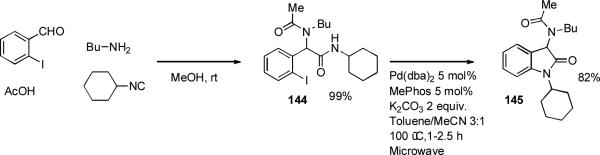
Catalysts based on palladium dialkylbiaryl phosphine complexes have seen use in several novel heterocycle syntheses.[144-148] The indole nucleus appears in many important compounds and a number of palladium-based methods have been disclosed for its synthesis and functionalization.[149] The introduction of highly reactive dialkylbiaryl phosphine palladium complexes, however, has allowed some significant new protocols to be developed.
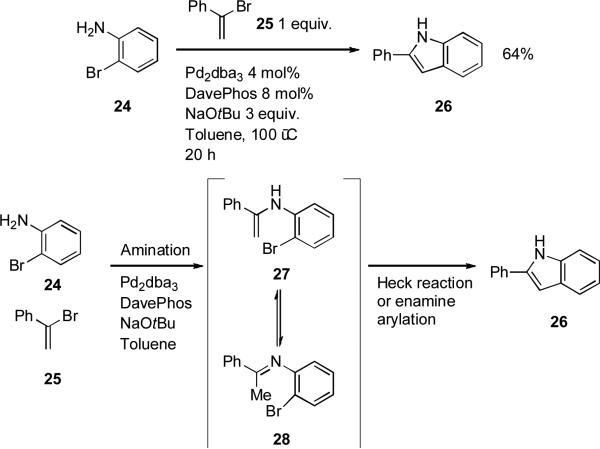
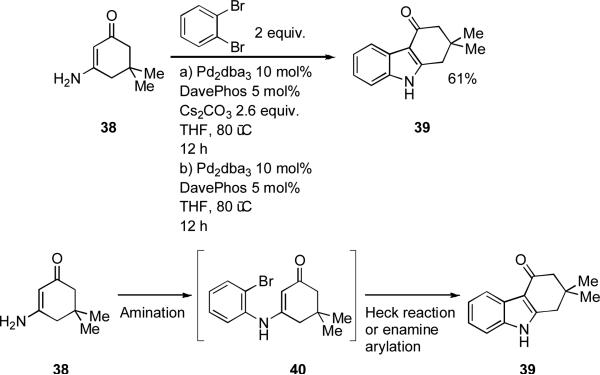
Barluenga[150] has exploited the large difference in reactivity of 2-aminoaryl halides and vinyl halides towards palladium-catalyzed amination in order to bring about a one-pot synthesis of indoles from o-haloanilines and vinyl bromides. It was suggested that ring closure occurs via a Heck process. A reasonable alternative would involve attack of the enamine 27 at the palladium(II) center, followed by reductive elimination (Scheme 9). A range of different ligands can bring about the initial amination of the vinyl bromide, but a bulky dialkylbiaryl phosphine is necesssary for the subsequent cyclization to occur. This process bears a strong mechanistic similarity to the Merck indole synthesis[151] and Zhu's synthesis of tryptophan derivatives from 2-haloanilies and aldehydes under palladium catalysis with XPhos as ligand.[152]
Scheme 9.
Barluenga's synthesis of indoles from vinyl bromides and 2-haloanilines.
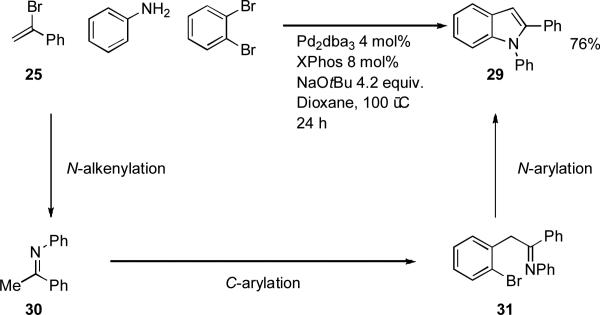
In a related procedure it has been shown that N-aryl indoles may also be synthesized from o-dihaloarenes and imines in the presence of a palladium source and XPhos (Scheme 10).[153] The required imines can be made in situ by the coupling of amines with vinyl bromides. The striking feature of this reaction is once again the selectivity of the process, initial coupling of the amine occurs exclusively with the vinyl halide due to its greater reactivity.
Scheme 10.
Barluenga's synthesis of indoles from vinyl bromides, 1,2-dihaloarenes and anilines.
In an alternative route to the indole core Willis has made use of 2-(2-haloalkenyl)-aryl halides as coupling partners for primary amines (Scheme 11).[154] Several different dialkylbiaryl phosphine ligands were serviceable in these reactions, the exact choice depending on the substrate, although for primary alkylamines the chelating DPEPhos was more effective. The geometry of the alkenyl halide was unimportant, presumably because the intermediate enamine formed in the reaction is able to undergo isomerization.
Scheme 11.
Willis’ synthesis of indoles from 2-(2-haloalkenyl)-aryl halides and anilines.
Lautens prepared various substituted indoles by palladium-catalyzed tandem amination/Heck[155] and amination/Suzuki processes[156-158] of ortho-gem-dihalovinylanilines (Scheme 12). Of particular note is the ability to synthesize azaindoles by this method, a group of compounds of significant interest in pharmaceutical research.
Scheme 12.
Lautens’ tandem amination/Suzuki heterocycle synthesis.
Cacchi has employed an alternative path to indoles taking advantage of the cyclization of o-alkynyltrifluoroacetanilides in the presence of aryl chlorides (Scheme 13).[159] The use of dialkylbiaryl phosphine ligands allowed aryl chlorides to be reactive. The authors proposed that the mechanism of the reaction involves initial oxidative addition of a palladium(0) species to the aryl halide to form a σ-arylpalladium(II) complex. This then forms a π-complex with the alkyne, which is thus activated towards intramolecular nucleophilic attack by the aniline nitrogen. The resulting σ-aryl palladium species then undergoes reductive elimination to generate a carbon-carbon bond and furnish the final product. Other ligands such as an N-heterocyclic carbene and tri-t-butylphosphine proved much less effective.
Scheme 13.
Cacchi's synthesis of indoles by cyclization of o-alkynyltrifluoroacetanilides in the presence of aryl chlorides.
Workers at Merck have developed an approach to 2,3-disubstituted indoles based on a palladium-catalyzed C-N coupling followed by an intramolecular Heck reaction or enamine arylation between a vinylogous amide and 1,2-dibromobenzene (Scheme 14).[151] In order for the proposed second step to occur it was necessary to add a second equivalent of palladium and ligand. Other ligands such as BINAP, tri-t-butylphosphine and a JosiPhos-type ligand were ineffective.
Scheme 14.
Merck synthesis of indoles from vinylogous amides and 1,2-dihaloarenes.
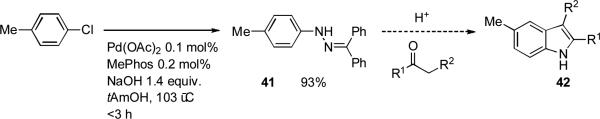
The preparation of indoles is often achieved by the Fischer indole synthesis in which an N-aryl hydrazone undergoes acid-catalyzed or thermal sigmatropic rearrangement to yield an indole after elimination of ammonia.[160] In 1998 Buchwald showed that N-aryl benzophenone hydrazones could be made by palladium-catalyzed amination of aryl bromides using the chelating phosphines BINAP or Xantphos and that these intermediates could participate in Fischer indolization (Scheme 15).[161, 162] This process has subsequently been extended to aryl chlorides by the application of dialkylbiaryl phosphine ligands[163] and has been studied in detail on a larger scale by workers at Rhodia.[164, 165]
Scheme 15.
Synthesis of indoles by arylation of benzophenone hydrazones followed by cyclization.
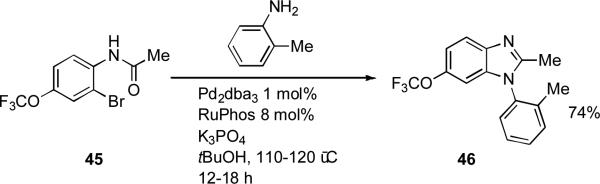
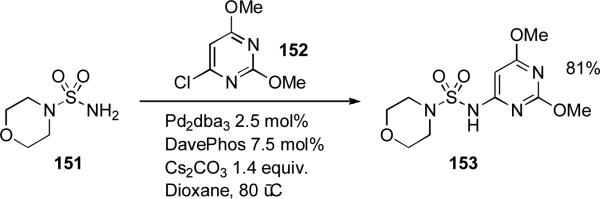
Analogously, chemists at Boehringer Ingelheim have demonstrated that heterocyclic aryl halides such as 2-chloropyrazine and 2-bromopyrimidine can be coupled with hydrazones through the use of dialkylbiaryl phosphine ligands.[166] The intermediate heteroaryl hydrazones were then converted into heteroaryl pyrazoles (Scheme 16).
Scheme 16.
Boehringer Ingelheim heterocycle synthesis by arylation of benzophenone hydrazones.
Buchwald has demonstrated that N-aryl benzimidazoles may be constructed by the coupling of anilines with o-haloacetanilides, cyclization occurring under the reaction conditions (Scheme 17).[167] This is a valuable method as it allows benzimidazoles to be synthesized in regioisomerically pure form, which can be difficult to access otherwise.
Scheme 17.
Buchwald's synthesis of benzimidazoles from o-haloacetanilides and anilines.
In 2003 Nozaki introduced the palladium-catalyzed double amination of primary amines with 2,2’-dihalobiphenyls as a route to carbazoles.[168, 169] It was subsequently shown by Chida that significantly improved yields of product with alkyl amines could be obtained by using dialkylbiaryl phosphine ligands.[170] In particular, for the coupling of the glucopyranosylamine derivative 47, it was necessary to use ligand 2 in order to obtain reasonable yields of product (Scheme 18). This is a challenging reaction due to the limited stability of the amine substrate.
Scheme 18.
Nozaki's synthesis of carbazoles from 2,2’-dihalobiphenyls and amines.
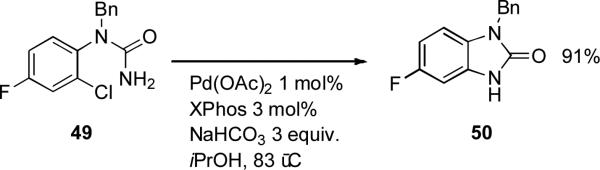
Workers at Merck have employed XPhos as ligand in the palladium-catalyzed cyclization of ureas to synthesize benzoimidazolones (Scheme 19).[171] The dialkylbiarylphosphine catalyst facilitated the utilization of chloroaniline substrates, although 2-chloropyridines could be activated by the chelating ligands Xantphos or dppb.
Scheme 19.
Merck synthesis benzoimidazolones by cyclization of ureas.
Applications in Pharmaceutical Synthesis
Palladium-catalyzed amination has had a large impact in the manufacture of pharmaceuticals[172, 173] and it is perhaps in this area that dialkylbiaryl phosphine ligands have seen the most use.
Scientists from GlaxoSmithKline utilized a palladium catalyst using the supporting ligand JohnPhos for the coupling of cyclopentylamine with an 8-chloroimidazopyridine 51 in the synthesis of novel imidazo[1,2-a]pyridines which have demonstrated potent activity against the herpes virus (Scheme 20).[174] They noted that the reaction did not proceed in the absence of catalyst and that with other ligands such as BINAP significantly lower yields of product were obtained.
Scheme 20.
GlaxoSmithKline synthesis of antiherpes agents.
Chemists at Merck have applied a palladium-catalysed cross-coupling of aryl iodides with aromatic amines in the synthesis of a series of substituted tetrazoles for SAR studies relating to the design of an antagonist for the metabotropic glutamate subtype 5 receptor (Scheme 21).[175]
Scheme 21.
Merck synthesis of mGlu5 receptor antagonists.
At Johnson & Johnson[176] 1 has been used as the ligand in the low yield synthesis of PDE 5 inhibitors for the treatment of erectile dysfunction (Scheme 22).
Scheme 22.
Johnson & Johnson synthesis of PDE5 inhibitors.
The preparation of caspase-3 inhibitors on solid phase was achieved at Merck Frosst by utilizing JohnPhos as ligand (Scheme 23).[177] This is the first example of the coupling of bromopyrazinones. The use of a mild base such as cesium carbonate was crucial; stronger bases gave only products of ester hydrolysis.
Scheme 23.
Merck Frosst synthesis of caspase-3 inhibitors.
Vince at the University of Minnesota employed XPhos as ligand in a key step of the synthesis of HIV integrase inhibitors (Scheme 24).[178] The coupling of picolinamide was found to be extremely challenging with a careful choice of conditions required in order to obtain any of the desired product. Protocols with the chelating ligand dppf gave none of the product. The choice of palladium source and base were also key to ensuring that the desired reaction occurred.
Scheme 24.
Vince's synthesis of HIV integrase inhibitors.
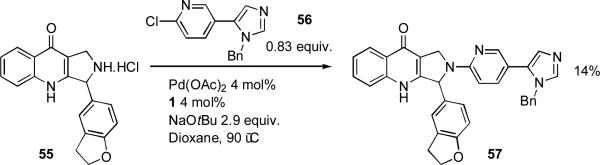
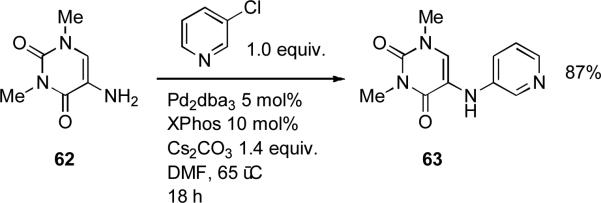
At the University of Hokkaido, XPhos was exploited by Kobayashi as ligand in the coupling of 3-chloropyridine with 5-amino-1,3-dimethyluracil 62 on route to hybrids of caffeine and eudistomin D as modulators of adenosine receptor activity (Scheme 25).[179]
Scheme 25.
Kobayashi's synthesis of hybrids of caffeine and eudistomin D.
During efforts to define the SAR of 2, 6-methano-3-benzazocines by Wentland at the Rensselaer Polytechnic Institute[180]a coupling between triphenylsilylamide and the aryl triflate 64 was performed, with subsequent protiolysis of the amine to yield the aniline (Scheme 26).
Scheme 26.
Wentland's synthesis of benzazocine analogues.
In a related series, Begtrup used benzophenone imine as ammonia surrogate (Scheme 27).[181] Interestingly a range of chelating phosphines proved unsuccessful in this system. It was also found that a large excess of base was necessary to effect a successful reaction and that the addition of a second batch of base to the reaction after 75 minutes was necessary in order to reach full conversion.
Scheme 27.
Begtrup's use of benzophenone imine in the synthesis of 2-fluoronorapomorphine.
Chemists at Novartis used a palladium-catalyzed amination in the synthesis of selective estrogen receptor modulators with potential for treatment of postmenopausal osteoporosis (Scheme 28).[182] BINAP gave unsatisfactorily low yields but DavePhos and SIPr carbene produced product in acceptable yields.
Scheme 28.
Novartis synthesis of estrogen receptor modulators.
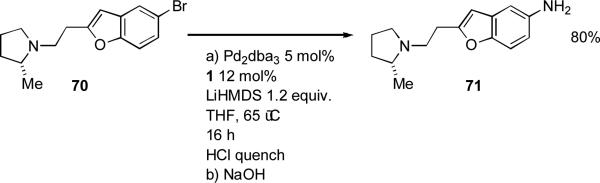
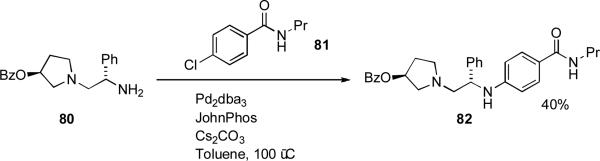
In the syntheis of histamine H3 receptor antagonists at Abbott, LiHMDS was coupled with aryl bromide 70 making use of 1 as ligand (Scheme 29).[183] Subsequent deprotection allowed a more efficient access to the aniline 71 than a alternative route which involved reduction of an aromatic nitro group.
Scheme 29.
Abbott synthesis of histamine receptor H3 receptor antagonists.
Synthesis of estrogen receptor ligands at Merck involved the coupling of aryl triflate 72 with Cbz-protected piperazine (Scheme 30). This is a challenging substrate because of the risk of epimerizing the benzylic chiral centers but this was not reported to be a problem under the conditions of the reaction mediated by the weak base potassium phosphate.[184]
Scheme 30.
Merck synthesis of estrogen receptor ligands.
In the construction of KDR kinase inhibitors, which have potential as cancer therapeutic agents, Lautens employed a palladium-catalyzed tandem amination/Suzuki coupling as a key step (Scheme 31).[185] This was significantly more efficient than synthesis of these targets by other published routes.
Scheme 31.
Lautens's synthesis of KDR kinase inhibitors.
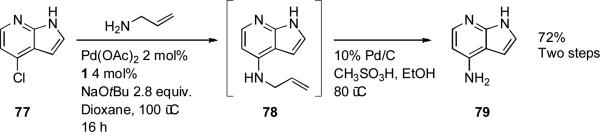
Workers at Bristol-Myers Squibb described a palladium-catalyzed amination in the synthesis of substituted 7-aza-indoles (Scheme 32).[186] The reaction was carried out on a 20 g scale. The presence of the free NH group of the azaindole 77 was not problematic, consistent with other results.[187]
Scheme 32.
Bristol-Myers Squibb synthesis of substituted 7-azaindoles.
Workers at Pfizer used JohnPhos as ligand in one approach to the κ-opiod receptor agonist CJ-15,161 drug candidate which has applications as a potential non-addictive analgesic (Scheme 33).[188, 189] Although the coupling could also be performed on the corresponding aryl bromide using BINAP, JohnPhos allowed the corresponding chloride to be employed.
Scheme 33.
Pfizer synthesis of a κ-opiod receptor agonist.
Pfizer chemists have also reported a palladium-catalyzed synthesis of N-aryloxazolidinones from aryl chlorides (Scheme 34).[190] Dialkylbiaryl phosphine ligands generally gave a much faster reaction than processes with chelating ligands such as BINAP, Xantphos, DPEPhos or dppf, although the reaction is only efficient with electron-poor aryl chlorides.
Scheme 34.
Pfizer synthesis of N-aryloxazolidinones.
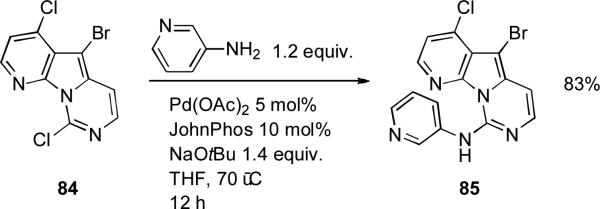
In studies on the derivatization of the variolin core (a heterocyclic family of natural products isolated from an Antarctic sponge) Burgos and Vaquero in Madrid used JohnPhos as ligand in coupling of the aryl chloride substrate 84 with a range of amines; attempts to do the reaction by direct nucleophilic substitution in the absence of a catalyst were unsuccessful (Scheme 35).[191] These couplings are challenging due to the large number of heteroatoms and the selectivity issues between the 3 halogens present in the heteroaromatic core.
Scheme 35.
Burgos’ and Vaquero's synthesis of variolin derivatives.
Tanatani at the University of Tokyo utilized a palladium-catalyzed C-N coupling process during the synthesis of a nonsteroidal/non-anilide type androgen antagonist (Scheme 36).[192] JohnPhos (and in some cases BINAP) was useful for the transformation of a wide range of aryl halides.
Scheme 36.
Tanatani's synthesis of a non-steroidal/non-anilide type androgen antagonist.
Scientists at Athersys performed a C-N coupling in their synthesis of a series of noscapine analogues with improved antimitotic action (Scheme 37).[193] They found that in order to avoid epimerization of the phthalide center it was essential to employ a weak base. They explored the use of K2CO3, Cs2CO3 and KOH but these were all reported to give poor conversion. They also experimented with other ammonia equivalents such as benzophenone imine, LiHMDS and t-butyl carbamate yet all gave low conversion. Eventually it was discovered that the desired reaction of benzylamine could be effected by barium hydroxide as base in a biphasic solvent system of toluene and water, although α,α,α-trifluorotoluene and water proved more practical on large scale due to problems of foaming with other solvents. Noteworthy is the use of air-stable palladacycle 89 which was introduced by Buchwald.[194] This was found to be highly efficient, giving complete reaction in only 15 minutes on 5 gram scale. It is tempting to speculate that the reason for the alacrity of this process is that it occurs by a sequence involving aminolysis of the lactone, intramolecular lactam formation and lactam alcoholysis.
Scheme 37.
Athersys synthesis of noscapine analogues.
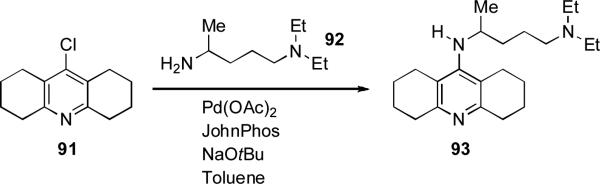
At Merck JohnPhos was applied as ligand for the coupling of primary amine 92 with octahydrochloroacridine 91 during the synthesis of analogues of ligands for voltage-gated calcium channels as potential anticonvulsants (Scheme 38).[195]
Scheme 38.
Merck synthesis calcium channel ligands
GlaxoSmithKline scientists used a bisannulation process in a new approach to the potent CRF1 antagonist GW808990 (Scheme 39).[196] The chelating ligand DPEPhos was also found to be capable of mediating the reaction, but in significantly lower yield than observed with MePhos.
Scheme 39.
GlaxoSmithKline syntheis of CRF1 receptor antagonists.
At Bristol-Myers Squibb researchers used JohnPhos as ligand in the coupling of purines with thiazoles in the synthesis of a library phosphodiesterase 7 inhibitors (Scheme 40).[197] The authors state that a wide range of conditions were examined but that dialkylbiaryl phosphine ligands were most beneficial in terms of yield and reproducibility (during earlier studies on the solid phase TolBINAP was found to provide optimal results).
Scheme 40.
Bristol-Myers Squibb synthesis of phosphodiesterase 7 inhibitors.
At Wyeth, studies have been made towards the development of cytosolic phospholipase A2α inhibitors which have promise in the treatment of multiple indications including asthma, arthritis and pain. During the course of the SAR study which eventually led to the compound Ecopladib, a C-N coupling with JohnPhos was employed (Scheme 41).[198] It is notable that the presence of multiple acidic hydrogens in the aryl halide substrate did not interfere with the coupling which had previously been successfully applied in the transformation of simpler members of the series.[199]
Scheme 41.
Wyeth synthesis of Ecopladib.
Augustyns at the University of Antwerp utilized the coupling of a primary amine and bromobenzene during the course of work on the synthesis of selective dipeptidyl peptidase II inhibitors which have potential for the treatment of a range of diseases (Scheme 42).[200]
Scheme 42.
Augustyns’ synthesis of dipeptidyl peptidase II inhibitors.
For the synthesis of radiolabelled compounds the reaction time for a cross-coupling is an important factor when dealing with compounds labelled with isotopes with a short half-life. During studies aimed at the synthesis of 18F-labelled σ2-receptor ligands for positron emission tomography studies Wüst employed a coupling between indole 103 and 4-[18F]fluoroiodobenzene (Scheme 43).[201] Because of the short half-life of 18F (t1/2 = 109.6 min) reaction times have to be short. Copper-mediated arylation proved too slow, as did a palladium-Xantphos system. When DavePhos was used as ligand,[202] however, a satisfactory radiochemical yield of product could be obtained with a reaction time of just 20 min.
Scheme 43.
Wüst's synthesis of radiolabelled ligands.
In 2005 Buchwald made use of XPhos as ligand in the synthesis DAPH analogues which have potential for the treatment of Alzheimer's disease (Scheme 44).[203] A palladium-catalyzed coupling between a 4,5-dihalophthalimide and anilines resulted in a more efficient route to these compounds which was also more amenable to diversification.
Scheme 44.
Buchwald's synthesis of DAPH analogues.
Scientists at 4SC investigated aromatic amination reactions on a range of substrates (Scheme 45).[204] JohnPhos as ligand was the most versatile, but dppf, BINAP and Xantphos were most successful for particular substrate classes. The JohnPhos system was practical in synthesizing a library of compounds in a parrallel synthesizer.
Scheme 45.
4SC study of aromatic amination.
Applications in Natural Product Synthesis
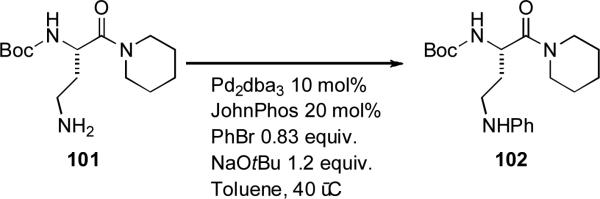
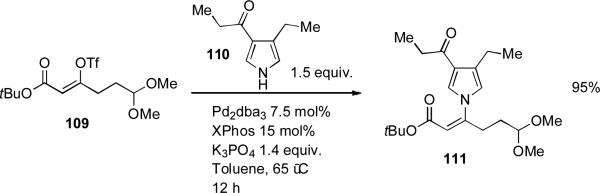
Dialkylbiaryl phosphine-based catalyst systems have also seen application in the synthesis of natural products. Movassaghi exploited a coupling between a functionalized pyrrole 110 and vinyl triflate 109 on a 7 gram scale on route to a synthesis of tricyclic myrmicarin alkaloids (Scheme 46). It was noted that attempts to perform a copper-based coupling on these substrates were unsuccessful.[205]
Scheme 46.
Movassaghi's pyrrole couplings.
Kerr[206] carried out an intramolecular amination of aryl triflate 112 with a Pd2dba3/JohnPhos catalyst combination to form a dihydropyrrolo[3,2-e]indole as part of a synthetic studies on the synthesis of the CC-1065 CPI subunit, such natural products have been studied intensively for their antitumor properties (Scheme 47).
Scheme 47.
Kerr's studies on the CC-1065 CPI subunit.
Hosokawa and Tatsuka[207] brought about the coupling of a functionalized aryl bromide and dimethylamine during the synthesis of the actinomycete natural product trichostatin (Scheme 48). This is an interesting transformation as examples of palladium-catalyzed arylation of dimethylamine are rare.
Scheme 48.
Hosokowa and Tatsuka's synthesis of trichostatin.
Bringmann[208] utilized the coupling of t-butylcarbamate with aryl bromide 116 in the course of synthesizing the N,C-napthylisoquinoline alkaloid anchisheynine (Scheme 49). This synthesis allowed the separation of the two atropoisomeric products and assignment of their absolute configurations.
Scheme 49.
Bringmann's synthesis of anchisheynine.
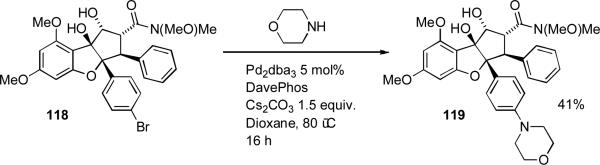
During synthesis of analogues of the insecticidal natural product rocaglamide, workers at Novartis employed a palladium-catalyzed amination using DavePhos as ligand between morpholine and the complex aryl bromide 118 (Scheme 50).[209]
Scheme 50.
Novartis synthesis of rocaglamide analogues.
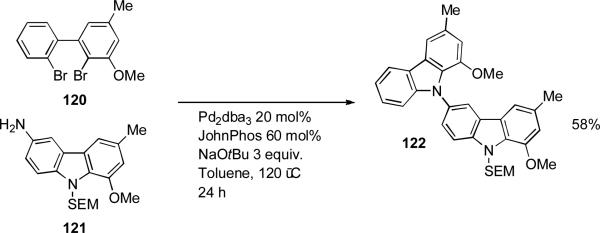
In the synthesis of the carbazole natural product murrastifoline A Chida brought about the double N-arylation[168, 169] of primary amine 121 with 2,2’-dihalobiphenyl 120 as a key step (Scheme 51).[170]
Scheme 51.
Chida's synthesis of murrastifoline A
During construction of a library of natural product-like diketopiperazines for biological screening Porco and Panek at Boston University used ligand 1 in coupling morpholine with the complex aryl bromide 123 (Scheme 52).[210]
Scheme 52.
Porco and Panek's synthesis of a library of diketopiperazines.
In 2000 Buchwald and Seeberger introduced halobenzyl ethers as protecting groups for hydroxyl groups in organic synthesis.[211] Such ethers can undergo amination in the presence of a suitable combination of palladium and ligand. The resulting arylamines can then be removed by brief exposure to acid or oxidant. These protecting groups, in particular the PBB (p-bromobenzyl) group have seen application in the synthesis of saccharides. Crich illustrated the utility of this method in the synthesis of mannosylerythritol lipid A, an unusual biosurfactant produced by Candida antartica.[212] The PBB groups were selectively removed from the intermediate 125 in the presence of a benzylidene acetal, allowing subsequent installation of the key alkyl ester groups at the 2 and 3 positions (Scheme 53).
Scheme 53.
Crich's synthesis of mannosylerythritol lipid A.
Seeberger also used this protecting group in the synthesis of branched high-mannose oligosaccharides from the HIV-1 viral surface envelope glycoprotein gp120 which are important in the binding of the virus to human CD4 lymphocyte receptors (Scheme 54).[213] Here the PBB group could be removed in the presence of two benzyl ethers, allowing subsequent glycosylation of the free hydroxyl group.
Scheme 54.
Seeberger's synthesis of gp120 oligosaccharide.
Applications in Modification of Biomolecules
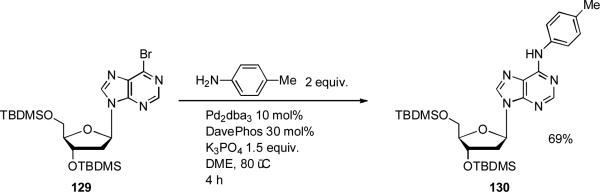
Another important area of application of dialkylbiaryl phosphine ligands in C-N coupling has been in the chemical modification of 2’-deoxynucleosides.[214, 215] Such N6-modified adenine and N2-modified guanine derivatives have a range of interesting biological activities and have also been implicated in the alterations to DNA leading to mutagenesis. Lakshman was able to synthesize N6-aryl 2’-deoxyadenosine analogues using DavePhos in combination with K3PO4 as base (Scheme 55).[216]
Scheme 55.
Lakshman's modification 2’-deoxyadenosine derivatives.
Similar conditions were of merit in the synthesis of N2-modified guanine derivatives.[217] The method was subsequently extended to nucleoside arylsulfonates.[218] This represented an important advance as O6-arylsulfonate nucleosides of purine are readily available. The same ligand system has also been employed in the synthesis of C8 nucleoside adducts (Scheme 56).[219] Such compounds are of significance because of their carcinogenicity and their occurrence in cooked meats. The authors found that although the dialkylbiaryl phosphine ligand system was useful, BINAP could effect similar results under the same conditions.
Scheme 56.
Rizzo's C8 modified nucleosides.
Hocek took advantage of a palladium-catalyzed amination in the synthesis of 6-substituted pyridin-2-yl and pyridin-3-yl C-nucleosides (Scheme 57).[220, 221] The choice of ligand seemed to depend strongly on the substrates with JohnPhos, ligand 1, JosiPhos and tri-t-butylphosphine being preferred for different combinations of amines and halides.
Scheme 57.
Hocek's synthesis of pyridin-3-yl C-nucleosides.
Process Development
The palladium-catalyzed hydrazone arylation utilizing dialkylbiaryl phosphine ligands has been extensively investigated on large scale by Rhodia Pharma Solutions and Lanxess.[87, 88, 164, 165] These workers followed the progress of the reaction by both calorimetry and in situ Raman spectroscopy. In the coupling of the model system of 4-bromotoluene with benzophenone hydrazone the structure of the ligand was crucial (Scheme 58). The nature of the base and solvent was also important in determining the outcome of the reaction and controlling the formation of unwanted by-products. For example, sodium t-butoxide gave much higher yields than potassium t-butoxide and the choice of solvent had a significant influence on the formation of the by-product diphenylmethane. The low catalyst loadings are of particular note.
Scheme 58.
Rhodia study of benzophenone hydrazone arylation.
The palladium-catalyzed amination of aryl halides with dialkylbiaryl phosphine ligands has also been performed on process scale. Chemists at Pfizer reported such a reaction on a 3 kg scale during the manufacture of the cholesteryl ester transfer protein inhibitor Torcetrapib which can lower blood cholesterol levels (Scheme 59).[222] A number of conditions were examined, but the combination of DavePhos and cesium carbonate as base proved best. The reaction temperature was important, if the temperature was raised above 80 °C slight erosion of enantiomeric purity was observed.
Scheme 59.
Pfizer synthesis of Torcetrapib.
Development of Reaction Conditions
Microwave heating in organic synthesis is becoming an increasingly important area.[223] Maes has shown that temperature-controlled microwave heating allows the reaction time of palladium-catalyzed aryl chloride amination reactions with dialkylbiaryl phosphine ligands to be reduced to just 10 minutes with yields comparable to those obtained with conventional heating (Scheme 60).[224, 225] The same authors were later able to increase the scale of these reactions in an appropriate microwave reactor.[226]
Scheme 60.
Maes’ development of microwave conditions.
A modified version of these conditions was subsequently employed in an intramolecular reaction by Rozman in the synthesis of chlorinated phenothiazines as models for dioxin-like compounds (Scheme 61).[227] Copper-mediated Ullmann-type conditions usually used for these cyclizations were unsatisfactory in this case due to decomposition of the product under the reaction conditions.
Scheme 61.
Rozman's synthesis of phenothiazines under microwave conditions.
Subsequent studies by Skjaerbaek in the course of synthesizing p38 MAP kinase inhibitors have illustrated that under microwave irradiation XPhos can be an even more effective ligand and that the process can be extended to aryl triflates as electrophiles.[228] Heo has demonstrated that halopyridines can be substrates for amination under similar conditions on route to amino-substituted 2-pyridones.[229, 230]
Buchwald has shown that soluble, organic amine bases DBU and MTBD can be advantageous for the palladium-catalyzed amination of aryl nonaflates under microwave heating (Scheme 62).[231] They suggested that this had the benefit of rendering the reactions homogeneous which has advantages for heat transfer and stirring. As is often observed with microwave irradiation, reaction times were significantly reduced compared to thermal conditions.[232]
Scheme 62.
Buchwald's amination of aryl nonaflates under microwave conditions.
Intramolecular amidation was used by Turner in a palladium-catalyzed synthesis of oxindoles (Scheme 63).[233] In this approach an amide was initially generated from from an amine and 2-haloarylacetic acid under solvent-free conditions with microwave heating. The crude product was then subjected to a palladium-XPhos catalyst system in order to effect the cyclization. These conditions led to a significant decrease in reaction time to 30 minutes. Previously under thermal conditions and with chelating ligands reaction times of 20-48 hours had been reported for such palladium-catalyzed intramolecular amidations;[234] with XPhos under thermal conditions reaction times of 3 hours have been reported for such oxindole formation by van den Hoogenband.[235, 236] Microwave heating was also employed by Zhu in an Ugi 4-component reaction/intramolecular amidation sequence to synthesize oxindoles for diversity-orientated synthesis (Scheme 64).[237]
Scheme 63.
Turner's synthesis of oxindoles by cyclization.
Scheme 64.
Zhu's synthesis of oxindoles by Ugi 4 component coupling.
Microwave heating has been applied by scientists at Solvay in an improved route to 3-aminoestrone, a key intermediate in the synthesis of biologically active steroids (Scheme 65).[238] Although the reaction could also be performed in similar yield under thermal conditions, reaction times were longer (15 h compared to 10 min) and much higher palladium (10 mol% compared to 1 mol%) and ligand (10 mol% compared to 5 mol%) loadings were required.
Scheme 65.
Solvay 3-aminoestrone synthesis.
At GlaxoSmithKline, microwave heating has been found useful in the synthesis of N-aryl sulfonamides from aryl chlorides and sulfonamides (Scheme 66).[239] A range of ligands were suitable for the transformation including a monodentate N-heterocyclic carbene and chelating phosphines but the dialkylbiaryl phosphine DavePhos gave the most consistent results under these conditions. Lower conversion was observed under thermal conditions even after prolonged heating.
Scheme 66.
GlaxoSmithKline sulfonamide synthesis under microwave conditions.
In a related study at AstraZeneca N-heteroaryl sulfamides were prepared under thermal conditions by using either XPhos, DavePhos or Xantphos, depending on the choice of substrate (although the reaction was limited to heteroaryl chlorides and activated aryl bromides) (Scheme 67).[240]
Scheme 67.
AstraZeneca synthesis of N-aryl sulfamides.
Procter showed that microwave heating promoted the amination of oxindole aryl bromides bearing a fluorous tag which could later undergo traceless removal (Scheme 68).[241]
Scheme 68.
Procter's synthesis of fluorous tagged heterocycles.
Microwave irradiation was used by workers at GlaxoSmithKline in a key coupling step in the synthesis of analogues of CRF1 receptor antagonists for the treatment of stress-related disorders (Scheme 69).[242] It was found that by using microwave irradiation and MePhos as ligand reaction times were minimized and yields maximized.
Scheme 69.
GlaxoSmithKline synthesis of CRF1 receptor antagonists.
In a recent example from Amgen microwave heating was also used in the palladium-catalyzed amination of a protected imidazole in the synthesis of vanilloid receptor antagonists which have potential use an analgesics (Scheme 70).[243]
Scheme 70.
Amgen synthesis of vanilloid receptor antagonists.
Another area of reaction development has been in the application of supercritical carbon dioxide (scCO2) as solvent for the palladium-catalyzed amination of aryl halides using biaryl phosphine ligands (Scheme 71).[244, 245] Holmes showed that for successful reaction to occur in this medium it is necessary to protect the amine with a trimethylsilyl group. The authors hypothesize that this is necessary to prevent carbamate formation from occurring. A range of aryl bromides and chlorides could be coupled using a variety of different dialkylbiaryl phosphine ligands.
Scheme 71.
Holmes’ amination of aryl halides in supercritical carbon dioxide.
At Rhodia efforts have been made to apply palladium-catalyzed reaction in continuous microreactors.[246] In the model reaction of 4-bromotoluene and piperidine a ligand system composed of Pd(OAc)2 and DavePhos was highly efficient, at 110 °C with residence times around 10 min. 100% conversion of the aryl halide was observed with >99% selectivity.
Solid-Supported Catalysts
Phosphine-functionalized polymers have become increasingly common in organic synthesis.[247, 248] Such immobilized catalysts are of interest because of the possibility of catalyst recovery and the minimization of metal-impurities in the product.[249] In 2001 Buchwald reported Merrifield resin-supported dialkylphosphinobiphenyl ligands for amination and Suzuki cross-coupling (Scheme 72).[250] This represented the first example of aryl chloride amination with solid-supported ligands.
Scheme 72.
Buchwald's application of solid-supported of a dialkylbiaryl phosphine in aryl chloride amination.
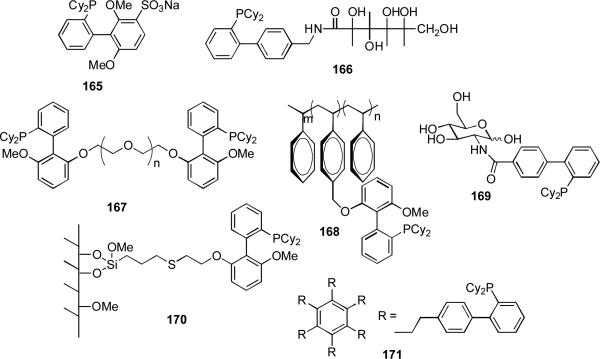
García has prepared a dialkylbiarylphosphine ligand on high surface area silica 170 but this material was found to be inactive in amination and Suzuki reactions. The same author has also prepared SPhos anchored on soluble supports such as non-cross-linked polystyrene 168 and polyethylene glycol 167. These complexes were active in amination reactions of aryl chlorides.[251] The PEG-SPhos ligand 167 could be recovered at the end of the reaction by precipitation and retained activity through four runs. A similar ligand has been employed by Plenio[252] and by Framery and Sinou[253] (with a PEG-polystyrene copolymer) for biphasic Suzuki reactions. Similarly a water-soluble dialkylbiaryl phosphine has been prepared by Miyaura[89] 166 and by Framery and Sinou[254] 169 by attachment of a carbohydrate side-chain and by Buchwald by sulfonation (Figure 6).[255] Another approach pioneered by Heuzé and Astruc has been to use dendrimer phosphine derivatives 171 which may be recovered at the end of the reaction by precipitation.[256]
Figure 6.
Solid-supported and solubilized biaryl dialkylphosphine ligands.
An alternative way of rendering these reactions heterogeneous is to employ polymer-incarcerated palladium. Kobayashi showed that amination of aryl halides could be performed using a range of dialkylbiaryl phosphine ligands with this type of palladium source with minimal palladium leaching if the solvent were chosen correctly (Scheme 73).[257]
Scheme 73.
Kobayashi's use of polymer-incarcerated palladium with dialkylbiaryl phosphine ligands.
Chemists at Elan Pharmaceuticals have shown that unmodified JohnPhos may be recovered from palladium-catalyzed reactions by a catch and release approach with cross-linked polystyrene bound sulfonic acid or Silicycle® sulfonic acid functionalized silica gel in a variety of solvents.[258] The phosphine could subsequently be released in pure form from the solid support by treatment with a solution of ammonia in methanol
Substituted quinazolines can be synthesised by making use of Wang resin-supported substrates (Scheme 74).[259] For this class of substrate aryl bromides could be coupled successfully using BINAP as ligand, but aryl chlorides required JohnPhos or tri-t-butylphosphine. Interestingly the latter ligand proved more effective for secondary amines, but for primary amines JohnPhos was better because of the lower amount of diarylation.
Scheme 74.
Amination of solid supported heteroaryl chlorides.
Applications in Synthesis of Ligands and Sensors
The palladium-catalyzed amination of aryl halides with palladium-dialkylbiaryl phosphine systems has also seen application in the synthesis of ligands for other metals.
The development of methods for the palladium-catalyzed arylation of macrocyclic amines[260-262] has served to significantly improve the synthesis of this class of ionophore compared to more traditional synthetic routes.[263] Stang used DavePhos as ligand in the palladium-catalyzed arylation of 175 in the synthesis of precursors for the assembly of supramolecules by coordination-driven self-assembly (Scheme 75).[264]
Scheme 75.
Stang's synthesis of precursors for self-assembling supramolecules.
Bolm employed similar conditions in the arylation of 1,4,7-triazacyclononanes which can act as chelating ligands in bioorganic chemistry (Scheme 76).[265] These latter authors found that chelating phosphine ligands were ineffective.
Scheme 76.
Bolm's synthesis of triazacyclononane derivatives.
Lippard has used this method in the synthesis of fluorescent sensors for zinc ions, once again the dialkylbiaryl phosphine DavePhos was the ligand of choice (Scheme 77).[266]
Scheme 77.
Lippard's synthesis of fluorescent zinc sensors.
The same author brought about a palladium-catalyzed amination in one approach to a rhodamine fluorophore (Scheme 78).[267] The synthetic route allowed access to isomerically pure material, such dyes are of interest due to their biocompatibility and high brightness and quantum yields.
Scheme 78.
Lippard's synthesis of substituted rhodamine fluorophores.
Johansson enlisted a palladium-catalyzed amination with SPhos as ligand in the synthesis of terpyridine ligands with the ultimate goal of synthesizing ruthenium(II) complexes (Scheme 79).[268] The author succeeded in coupling a range of amines with 4’-chloro-tpy 183 all in high yield, an important result considering the strong metal-coordinating ability of this heteroaryl chloride.
Scheme 79.
Johansson's synthesis of ruthenium(II) ligands.
In 2006 Schrock performed a palladium-catalysed amination of BINAM on route to ligands for Group IV metals which were being synthesised as potential alkene polymerization catalysts (Scheme 80).[269] This reaction provided over 13 g of purified product.
Scheme 80.
Schrock's synthesis of alkene polymerization catalysts.
Applications in Materials Science
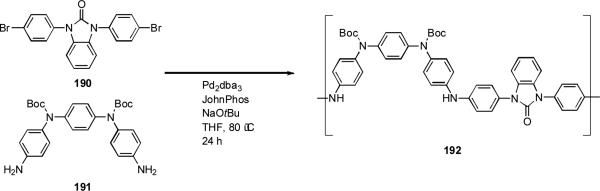
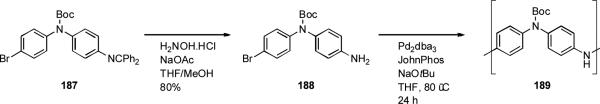
The palladium-catalyzed amination reaction of aryl halides also lends itself to polymerization processes for the formation of polyaniline (PANI). p-Polyaniline has tunable electrical conductivity and high environmental stability. Buchwald has demonstrated that dialkylbiarylphosphine ligands allow high molecular weight, protected polyaniline 189 to be synthesized from a suitable aryl bromide precursor (Scheme 80).[270] This protected polymer is readily processed as it is soluble in organic solvents, in this way thin films may be fabricated.
In a similar way, Meyer generated copolymers of ortho- and para-substituted polyaniline 192 (Scheme 82).[271] This material exhibited comparable conductivity and oxidation properties to polyaniline.
Scheme 82.
Meyer's synthesis of a polyaniline copolymer.
Nozaki has exploited the palladium-catalyzed double N-arylation of primary amines to synthesize ladder-type π-conjugated heteroacenes which show potential as organic semiconductors in field-effect transistors (Scheme 83).[272] The dialkylbiaryl phosphine ligand RuPhos was found essential to obtain good yields of product.
Scheme 83.
Nozaki's synthesis of heteroacenes.
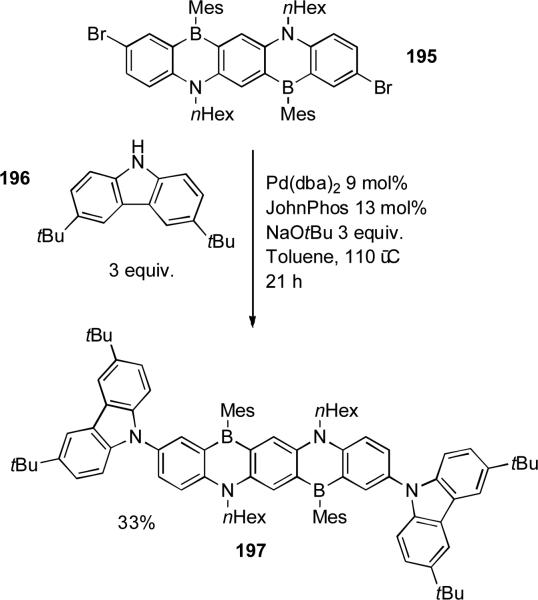
Kawashima carried out a palladium-catalyzed amination to functionalize ladder-type azaborines, such aminated compounds exhibited enhanced photo-luminescence (Scheme 84). Hexylamine and diphenylamine could be coupled with this aryl bromide by using a Pd/BINAP system, but JohnPhos was required to effect the reaction of carbazole 196.[273]
Scheme 84.
Kawashima's synthesis of azaborines.
Triarylamines have been the subject of continuing research because of their optoelectronic properties.[274, 275] Numerous methods have been disclosed for their synthesis by palladium-catalyzed arylation of diarylamines. It has been demonstrated by Buchwald that under suitable conditions with dialkylbiaryl phosphine ligands it is possible to synthesize these molecules from anilines and an aryl chloride and an aryl bromide by exploiting the difference in reactivity of the two halides.[276] Subsequently it was discovered that the selective arylation of ammonia[277] with three different aryl halides could be achieved by sequential addition.[278] In this way the important triarylamine TPD (N,N’-bis(3-methylphenyl)-N,N’-diphenylbenzidine) 198 could be made in a one pot procedure from three aryl halides and ammonia (Scheme 85).
Scheme 85.
Synthesis of TPD by selective arylation of ammonia.
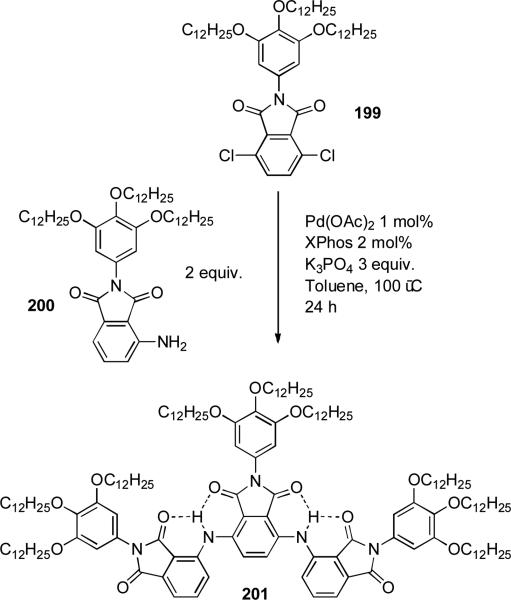
Meijer showed that a palladium-catalyzed amination is successful in the synthesis of poly(aminophthalimide) dimers and trimers (Scheme 86).[279] These authors noted that the choice of base and palladium source was essential in obtaining an optimal yield of product and that aryl chlorides gave higher yields of the desired oligomers than either aryl bromides or iodides as coupling partners.
Scheme 86.
Meijer's synthesis of poly(aminophthalimide) oligomers.
Burgess disclosed a palladium-catalyzed amination with JohnPhos in the synthesis of energy transfer systems based on squaraines (Scheme 87).[280]
Scheme 87.
Burgess's synthesis of squaraine-based energy transfer systems.
Yang used a palladium-catalyzed arylation of an indole during the synthesis of aminostilbenes for studies on torsional motion during photoinduced intramolecular charge transfer (Scheme 88).[281]
Scheme 88.
Yang's synthesis of aminostilbenes for studies on charge transfer.
The palladium-catalyzed amination of 2,2’-dibromo-9,9’-spirobifluorene 207 with LiHMDS using 1 as ligand has been reported by Lützen in the synthesis of derivatives of 9,9’-spirobifluorene for resolution with a view to applications in, for example, macromolecular chemistry (Scheme 89).[282]
Scheme 89.
Lützen's synthesis of derivatives of 9,9’-spirobifluorenes.
Conclusions and Outlook
Dialkylbiaryl phosphine ligands have seen a wide-range of practical applications in palladium-catalyzed aryl and vinyl halide amination reactions. Other ligand systems can provide highly active catalysts, especially for the amination of aryl bromides but none has seen so many applications in the use of the more economically attractive aryl chlorides. A noticeable trend in this field is increasing use of microwave heating due to the resulting short reaction times. Challenges remain, in particular in the design of ligands which allow low catalyst loadings with substrates bearing multiple heteroatoms. A more detailed understanding of the subtle effects imparted by different ligand substituents will be important as at present the choice of dialkylbiaryl phosphine used for a particular reaction is still often empirical. It is hoped that this review inspires further uses of amination reactions using these ligands and spurs further developments in ligand design.
Figure 5.
Important structural features of dialkylbiaryl phosphines.
Scheme 81.
Buchwald's synthesis of polyaniline.
Acknowledgments
We thank the National Institutes of Health for supporting this work. We thank Merck, Amgen and Boehringer Ingelheim for additional unrestricted support. D.S.S. acknowledges the Royal Commission for the Exhibition of 1851 for a Research Fellowship.
Biography
 Stephen L. Buchwald is currently the Camille Dreyfus Professor of Chemistry at the Massachusetts Institute of Technology (MIT). He has received numerous awards including the American Chemical Society award in Organometallic Chemistry (2000) and for Creative Work in Synthetic Organic Chemistry (2006), the Bristol-Myers Squibb Award for Distinguished Achievement in Organic Synthesis (2005) and the Siegfried medal (2006).
Stephen L. Buchwald is currently the Camille Dreyfus Professor of Chemistry at the Massachusetts Institute of Technology (MIT). He has received numerous awards including the American Chemical Society award in Organometallic Chemistry (2000) and for Creative Work in Synthetic Organic Chemistry (2006), the Bristol-Myers Squibb Award for Distinguished Achievement in Organic Synthesis (2005) and the Siegfried medal (2006).
 David S. Surry completed his undergraduate and PhD studies at the University of Cambridge, UK. During his undergraduate years he worked in the laboratory of Prof. I. Fleming and performed his PhD under the supervision of Dr. D. R. Spring. He is currently a Royal Commission for the Exhibition of 1851 Research Fellow with Prof. S. L. Buchwald.
David S. Surry completed his undergraduate and PhD studies at the University of Cambridge, UK. During his undergraduate years he worked in the laboratory of Prof. I. Fleming and performed his PhD under the supervision of Dr. D. R. Spring. He is currently a Royal Commission for the Exhibition of 1851 Research Fellow with Prof. S. L. Buchwald.
Footnotes
Support has been provided by the Royal Commission for the Exhibition of 1851 (Research Fellowship to D. R. S.)
References
- 1.Barder TE, Buchwald SL. J. Am. Chem. Soc. 2007;129:5096. doi: 10.1021/ja0683180. [DOI] [PubMed] [Google Scholar]
- 2.Old DW, Wolfe JP, Buchwald SL. J. Am. Chem. Soc. 1998;120:9722. [Google Scholar]
- 3.Ferrer C, Amijs CHM, Echavarren AM. Chem. Eur. J. 2007;13:1358. doi: 10.1002/chem.200601324. [DOI] [PubMed] [Google Scholar]
- 4.Ferrer C, Echavarren AM. Angew. Chem. 2006;118:1123. doi: 10.1002/anie.200503484. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2006;45:1105. [Google Scholar]
- 5.Herrero-Gomez E, Nieto-Oberhuber C, Lopez S, Benet-Buchholz J, Echavarren AM. Angew. Chem. 2006;118:5581. doi: 10.1002/anie.200601688. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2006;45:5455. [Google Scholar]
- 6.Jimenez-Nunez E, Claverie CK, Nieto-Oberhuber C, Echavarren AM. Angew. Chem. 2006;118:5578. doi: 10.1002/anie.200601575. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2006;45:5452. [Google Scholar]
- 7.Nieto-Oberhuber C, Lopez S, Echavarren AM. J. Am. Chem. Soc. 2005;127:6178. doi: 10.1021/ja042257t. [DOI] [PubMed] [Google Scholar]
- 8.Nieto-Oberhuber C, Lopez S, Munoz MP, Jimenez-Nunez E, Bunuel E, Cardenas DJ, Echavarren AM. Chem. Eur. J. 2006;12:1694. doi: 10.1002/chem.200501089. [DOI] [PubMed] [Google Scholar]
- 9.Partyka DV, Zeller M, Hunter AD, Gray TG. Angew. Chem. 2006;118:8368. doi: 10.1002/anie.200603350. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2006;45:8188. [Google Scholar]
- 10.Cabello N, Rodriguez C, Echavarren AM. Synlett. 2007:1753. [Google Scholar]
- 11.Han XQ, Widenhoefer RA. Angew. Chem. 2006;118:1779. doi: 10.1002/anie.200600052. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2006;45:1747. [Google Scholar]
- 12.Porcel S, Echavarren AM. Angew. Chem. 2007;119:2726. doi: 10.1002/anie.200605041. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2007;46:2672. [Google Scholar]
- 13.Dhondi PK, Chisholm JD. Org. Lett. 2006;8:67. doi: 10.1021/ol0525260. [DOI] [PubMed] [Google Scholar]
- 14.Dhondi PK, Carberry P, Choi LB, Chishohm JD. J. Org. Chem. 2007;72:9590. doi: 10.1021/jo701643h. [DOI] [PubMed] [Google Scholar]
- 15.Faller JW, D'Alliessi DG. Organometallics. 2003;22:2749. [Google Scholar]
- 16.Movassaghi M, Hill MD. J. Am. Chem. Soc. 2006;128:14254. doi: 10.1021/ja066405m. [DOI] [PubMed] [Google Scholar]
- 17.Faller JW, Fontaine PP. Organometallics. 2005;24:4132. [Google Scholar]
- 18.Haider J, Kunz K, Scholz U. Adv. Synth. Catal. 2004;346:717. [Google Scholar]
- 19.Gelman D, Buchwald SL. Angew. Chem. 2003;115:6175. doi: 10.1002/anie.200353015. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2003;42:5993. [Google Scholar]
- 20.Milne JE, Buchwald SL. J. Am. Chem. Soc. 2004;126:13028. doi: 10.1021/ja0474493. [DOI] [PubMed] [Google Scholar]
- 21.Sahoo AK, Oda T, Nakao Y, Hiyama T. Adv. Synth. Catal. 2004;346:1715. [Google Scholar]
- 22.Denmark SE, Butler CR. Org. Lett. 2006;8:63. doi: 10.1021/ol052517r. [DOI] [PubMed] [Google Scholar]
- 23.Mowery ME, DeShong P. Org. Lett. 1999;1:2137. doi: 10.1021/ol991186d. [DOI] [PubMed] [Google Scholar]
- 24.Martin R, Buchwald SL. J. Am. Chem. Soc. 2007;129:3844. doi: 10.1021/ja070830d. [DOI] [PubMed] [Google Scholar]
- 25.Billingsley K, Buchwald SL. J. Am. Chem. Soc. 2007;129:3358. doi: 10.1021/ja068577p. [DOI] [PubMed] [Google Scholar]
- 26.Billingsley KL, Anderson KW, Buchwald SL. Angew. Chem. 2006;118:3564. doi: 10.1002/anie.200600493. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2006;45:3484. [Google Scholar]
- 27.Barder TE, Walker SD, Martinelli JR, Buchwald SL. J. Am. Chem. Soc. 2005;127:4685. doi: 10.1021/ja042491j. [DOI] [PubMed] [Google Scholar]
- 28.Walker SD, Barder TE, Martinelli JR, Buchwald SL. Angew. Chem. 2004;116:1907. doi: 10.1002/anie.200353615. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2004;43:1871. [Google Scholar]
- 29.Yin JJ, Rainka MP, Zhang XX, Buchwald SL. J. Am. Chem. Soc. 2002;124:1162. doi: 10.1021/ja017082r. [DOI] [PubMed] [Google Scholar]
- 30.Wolfe JP, Singer RA, Yang BH, Buchwald SL. J. Am. Chem. Soc. 1999;121:9550. [Google Scholar]
- 31.Okano K, Tokuyama H, Fukuyama T. J. Am. Chem. Soc. 2006;128:7136. doi: 10.1021/ja0619455. [DOI] [PubMed] [Google Scholar]
- 32.Ebran JP, Hansen AL, Gogsig TM, Skrydstrup T. J. Am. Chem. Soc. 2007;129:6931. doi: 10.1021/ja070321b. [DOI] [PubMed] [Google Scholar]
- 33.Cameron M, Foster BS, Lynch JE, Shi YJ, Dolling UH. Org. Process Res. Dev. 2006;10:398. [Google Scholar]
- 34.Nguyen HN, Huang XH, Buchwald SL. J. Am. Chem. Soc. 2003;125:11818. doi: 10.1021/ja036947t. [DOI] [PubMed] [Google Scholar]
- 35.Vogl EM, Buchwald SL. J. Org. Chem. 2002;67:106. doi: 10.1021/jo010953v. [DOI] [PubMed] [Google Scholar]
- 36.Fox JM, Huang XH, Chieffi A, Buchwald SL. J. Am. Chem. Soc. 2000;122:1360. [Google Scholar]
- 37.Martin R, Buchwald SL. Angew. Chem. 2007;119:7374. doi: 10.1002/anie.200703009. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2007;46:7236. [Google Scholar]
- 38.Patil NT, Song D, Yamamoto Y. Euro. J. Org. Chem. 2006:4211. [Google Scholar]
- 39.Gray BL, Schreiber SL. J. Combi. Chem. 2007;9:1028. doi: 10.1021/cc7001028. [DOI] [PubMed] [Google Scholar]
- 40.Anderson KW, Ikawa T, Tundel RE, Buchwald SL. J. Am. Chem. Soc. 2006;128:10694. doi: 10.1021/ja0639719. [DOI] [PubMed] [Google Scholar]
- 41.Burgos CH, Barder TE, Huang XH, Buchwald SL. Angew. Chem. 2006;118:4427. doi: 10.1002/anie.200601253. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2006;45:4321. [Google Scholar]
- 42.Vorogushin AV, Huang XH, Buchwald SL. J. Am. Chem. Soc. 2005;127:8146. doi: 10.1021/ja050471r. [DOI] [PubMed] [Google Scholar]
- 43.Torraca KE, Huang XH, Parrish CA, Buchwald SL. J. Am. Chem. Soc. 2001;123:10770. doi: 10.1021/ja016863p. [DOI] [PubMed] [Google Scholar]
- 44.McNeill E, Barder TE, Buchwald SL. Org. Lett. 2007;9:3785. doi: 10.1021/ol701518f. [DOI] [PubMed] [Google Scholar]
- 45.Billingsley KL, Barder TE, Buchwald SL. Angew. Chem. 2007;119:5455. doi: 10.1002/anie.200701551. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2007;46:5359. [Google Scholar]
- 46.Stadlwieser JF, Dambaur ME. Helv. Chim. Acta. 2006;89:936. [Google Scholar]
- 47.Goossen LJ, Ferwanah ARS. Synlett. 2000:1801. [Google Scholar]
- 48.Chobanian HR, Fors BP, Lin LS. Tetrahedron Lett. 2006;47:3303. [Google Scholar]
- 49.Littke A, Soumeillant M, Kaltenbach RF, Cherney RJ, Tarby CM, Kiau S. Org. Lett. 2007;9:1711. doi: 10.1021/ol070372d. [DOI] [PubMed] [Google Scholar]
- 50.Cooper T, Novak A, Humphreys LD, Walker MD, Woodward S. Adv. Synth. Catal. 2006;348:686. [Google Scholar]
- 51.Lafrance M, Fagnou K. J. Am. Chem. Soc. 2006;128:16496. doi: 10.1021/ja067144j. [DOI] [PubMed] [Google Scholar]
- 52.Logan ME, Oinen ME. Organometallics. 2006;25:1052. [Google Scholar]
- 53.Ney JE, Wolfe JP. J. Am. Chem. Soc. 2005;127:8644. doi: 10.1021/ja0430346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peng JS, Jiang DH, Lin WQ, Chen YW. Org. Biomol. Chem. 2007;5:1391. doi: 10.1039/b701509g. [DOI] [PubMed] [Google Scholar]
- 55.Kienle M, Dubbaka SR, Brade K, Knochel P. Euro. J. Org. Chem. 2007:4166. [Google Scholar]
- 56.Hartwig JF. Angew. Chem. 1998;110:2154. [Google Scholar]; Angew. Chem. Int. Ed. 1998;37:2047. [Google Scholar]
- 57.Wolfe JP, Wagaw S, Marcoux JF, Buchwald SL. Acc. Chem. Res. 1998;31:805. [Google Scholar]
- 58.Schlummer B, Scholz U. Adv. Synth. Catal. 2004;346:1599. [Google Scholar]
- 59.Prim D, Campagne JM, Joseph D, Andrioletti B. Tetrahedron. 2002;58:2041. [Google Scholar]
- 60.Yang BH, Buchwald SL. J. Organomet. Chem. 1999;576:125. [Google Scholar]
- 61.Hartwig JF. Handbook of Organopalladium Chemistry for Organic Synthesis. Wiley-Interscience; New York: 2002. p. 1051. [Google Scholar]
- 62.Hartwig JF. In: Modern Arene Chemistry. Didier A, editor. Wiley-VCH; Weinheim: 2002. p. 107. [Google Scholar]
- 63.Hartwig JF. In: Comprehensive Coordination Chemistry II. Ward MD, editor. Vol. 9. Elsevier; Amsterdam: 2003. p. 369. [Google Scholar]
- 64.Hartwig JF. In: Modern Amination Methods. Ricci A, editor. Wiley-VCH; Weinheim: 2000. p. 195. [Google Scholar]
- 65.Beletskaya IP, Averin AD. Pure Appl. Chem. 2004;76:1605. [Google Scholar]
- 66.Hartwig JF, Shekar S, Shen Q, Barrios-Landeros F. In: The Chemistry of Anilines. Rappoport Z, editor. Wiley; New York: 2007. p. 455. [Google Scholar]
- 67.Janey JM. In: Name Reactions for Functional Group Transformations. Li JJ, Corey EJ, editors. John Wiley & Sons; Hoboken, NJ: 2007. p. 564. [Google Scholar]
- 68.Buchwald SL, Jiang L. In: Metal-Catalyzed Cross-Coupling Reactions. Meijere A, Diederich F, editors. Wiley-VCH; Weinheim: 2004. p. 699. [Google Scholar]
- 69.Muci AR, Buchwald SL. Top. Curr. Chem. 2002;219:131. [Google Scholar]
- 70.Louie J, Hartwig JF. Tetrahedron Lett. 1995;36:3609. [Google Scholar]
- 71.Guram AS, Rennels RA, Buchwald SL. Angew. Chem. 1995;107:1456. [Google Scholar]; Angew. Chem. Int. Ed. 1995;34:1348. [Google Scholar]
- 72.Wolfe JP, Buchwald SL. J. Org. Chem. 1996;61:1133. [Google Scholar]
- 73.Wolfe JP, Wagaw S, Buchwald SL. J. Am. Chem. Soc. 1996;118:7215. [Google Scholar]
- 74.Driver MS, Hartwig JF. J. Am. Chem. Soc. 1996;118:7217. [Google Scholar]
- 75.Ehrentraut A, Zapf A, Beller M. J. Mol. Catal. A: Chem. 2002;182:515. [Google Scholar]
- 76.Hill LL, Moore LR, Huang RC, Craciun R, Vincent AJ, Dixon DA, Chou J, Woltermann CJ, Shaughnessy KH. J. Org. Chem. 2006;71:5117. doi: 10.1021/jo060303x. [DOI] [PubMed] [Google Scholar]
- 77.Nishiyama M, Yamamoto T, Koie Y. Tetrahedron Lett. 1998;39:617. [Google Scholar]
- 78.Bei XH, Uno T, Norris J, Turner HW, Weinberg WH, Guram AS, Petersen JL. Organometallics. 1999;18:1840. [Google Scholar]
- 79.Kataoka N, Shelby Q, Stambuli JP, Hartwig JF. J. Org. Chem. 2002;67:5553. doi: 10.1021/jo025732j. [DOI] [PubMed] [Google Scholar]
- 80.Hamann BC, Hartwig JF. J. Am. Chem. Soc. 1998;120:7369. [Google Scholar]
- 81.Guari Y, van Es DS, Reek JNH, Kamer PCJ, van Leeuwen P. Tetrahedron Lett. 1999;40:3789. [Google Scholar]
- 82.Urgaonkar S, Xu JH, Verkade JG. J. Org. Chem. 2003;68:8416. doi: 10.1021/jo034994y. [DOI] [PubMed] [Google Scholar]
- 83.Ackermann L. Synthesis. 2006:1557. [Google Scholar]
- 84.Kantchev EAB, O'Brien CJ, Organ MG. Angew. Chem. 2007;119:2824. doi: 10.1002/anie.200601663. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2007;46:2768. [Google Scholar]
- 85.Tomori H, Fox JM, Buchwald SL. J. Org. Chem. 2000;65:5334. doi: 10.1021/jo000691h. [DOI] [PubMed] [Google Scholar]
- 86.Kaye S, Fox JM, Hicks FA, Buchwald SL. Adv. Synth. Catal. 2001;343:789. [Google Scholar]
- 87.Buchwald SL, Mauger C, Mignani G, Scholz U. Adv. Synth. Catal. 2006;348:23. [Google Scholar]
- 88.Mauger CC, Mignani GA. Org. Process Res. Dev. 2004;8:1065. [Google Scholar]
- 89.Nishimura M, Ueda M, Miyaura N. Tetrahedron. 2002;58:5779. [Google Scholar]
- 90.Singer RA, Caron S, McDermott RE, Arpin P, Do NM. Synthesis. 2003:1727. [Google Scholar]
- 91.Singer RA, Dore ML, Sieser JE, Berliner MA. Tetrahedron Lett. 2006;47:3727. [Google Scholar]
- 92.Rataboul F, Zapf A, Jackstell R, Harkal S, Riermeier T, Monsees A, Dingerdissen U, Beller M. Chem. Eur. J. 2004;10:2983. doi: 10.1002/chem.200306026. [DOI] [PubMed] [Google Scholar]
- 93.Iwasawa T, Komano T, Tajima A, Tokunaga M, Obora Y, Fujihara T, Tsuji Y. Organometallics. 2006;25:4665. [Google Scholar]
- 94.Dai Q, Gao WZ, Liu D, Kapes LM, Zhang XM. J. Org. Chem. 2006;71:3928. doi: 10.1021/jo060321e. [DOI] [PubMed] [Google Scholar]
- 95.Xie XM, Zhang TY, Zhang ZG. J. Org. Chem. 2006;71:6522. doi: 10.1021/jo060945k. [DOI] [PubMed] [Google Scholar]
- 96.Ashburn BO, Carter RG. Angew. Chem. 2006;118:6889. doi: 10.1002/anie.200602683. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2006;45:6737. [Google Scholar]
- 97.So CM, Lau CP, Kwong FY. Org. Lett. 2007;9:2795. doi: 10.1021/ol070898y. [DOI] [PubMed] [Google Scholar]
- 98.Schwarz N, Tillack A, Alex K, Sayyed IA, Jackstell R, Beller M. Tetrahedron Lett. 2007;48:2897. [Google Scholar]
- 99.Roca FX, Richards CJ. Chem. Commun. 2003:3002. doi: 10.1039/b310526a. [DOI] [PubMed] [Google Scholar]
- 100.Demchuk OM, Yoruk B, Blackburn T, Snieckus V. Synlett. 2006:2908. [Google Scholar]
- 101.Jensen JF, Johannsen M. Org. Lett. 2003;5:3025. doi: 10.1021/ol034943n. [DOI] [PubMed] [Google Scholar]
- 102.Baillie C, Xiao JL. Tetrahedron. 2004;60:4159. [Google Scholar]
- 103.Ishikawa S, Manabe K. Chem. Lett. 2007;36:1302. [Google Scholar]
- 104.Ishikawa S, Manabe K. Chem. Lett. 2007;36:1304. [Google Scholar]
- 105.Ishikawa S, Manabe K. Org. Lett. 2007;9:5593. doi: 10.1021/ol702646s. [DOI] [PubMed] [Google Scholar]
- 106.Ashburn BO, Carter RG, Zakharov LN. J. Am. Chem. Soc. 2007;129:9109. doi: 10.1021/ja071163r. [DOI] [PubMed] [Google Scholar]
- 107.Nishida G, Noguchi K, Hirano M, Tanaka K. Angew. Chem. 2007;119:4025. doi: 10.1002/anie.200700064. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2007;46:3951. [Google Scholar]
- 108.Kondoh A, Yorimitsu H, Oshima K. J. Am. Chem. Soc. 2007;129:6996. doi: 10.1021/ja071622o. [DOI] [PubMed] [Google Scholar]
- 109.Shekhar S, Ryberg P, Hartwig JF, Mathew JS, Blackmond DG, Strieter ER, Buchwald SL. J. Am. Chem. Soc. 2006;128:3584. doi: 10.1021/ja045533c. [DOI] [PubMed] [Google Scholar]
- 110.Hartwig JF. Acc. Chem. Res. 1998;31:852. [Google Scholar]
- 111.Hartwig JF. Inorg. Chem. 2007;46:1936. doi: 10.1021/ic061926w. [DOI] [PubMed] [Google Scholar]
- 112.Hartwig JF. Synlett. 2006:1283. [Google Scholar]
- 113.Hartwig JF. Synlett. 1997:329. [Google Scholar]
- 114.Jordan RB. Organometallics. 2007;26:4763. [Google Scholar]
- 115.Ikawa T, Barder TE, Biscoe MR, Buchwald SL. J. Am. Chem. Soc. 2007;129:13001. doi: 10.1021/ja0717414. [DOI] [PubMed] [Google Scholar]
- 116.Christensen H, Kiil S, Dam-Johansen K, Nielsen O, Sommer MB. Org. Process Res. Dev. 2006;10:762. [Google Scholar]
- 117.Christmann U, Vilar R. Angew. Chem. 2005;115:370. doi: 10.1002/anie.200461189. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2005;44:366. [Google Scholar]
- 118.Strieter ER, Blackmond DG, Buchwald SL. J. Am. Chem. Soc. 2003;125:13978. doi: 10.1021/ja037932y. [DOI] [PubMed] [Google Scholar]
- 119.Strieter ER, Buchwald SL. Angew. Chem. 2006;118:939. doi: 10.1002/anie.200502927. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2006;45:925. [Google Scholar]
- 120.Dupont J, Consorti CS, Spencer J. Chem. Rev. 2005;105:2527. doi: 10.1021/cr030681r. [DOI] [PubMed] [Google Scholar]
- 121.Espinet P, Albeniz AC. In: Comprehensive Organometallic Chemistry III. Canty A, editor. Vol. 8. Elsevier; Oxford: 2007. p. 315. [Google Scholar]
- 122.Christmann U, Vilar R, White AJP, Williams DJ. Chem. Commun. 2004:1294. doi: 10.1039/b402283a. [DOI] [PubMed] [Google Scholar]
- 123.Christmann U, Pantazis DA, Benet-Buchholz J, McGrady JE, Maseras F, Vilar R. J. Am. Chem. Soc. 2006;128:6376. doi: 10.1021/ja057825z. [DOI] [PubMed] [Google Scholar]
- 124.Barder TE. J. Am. Chem. Soc. 2006;128:898. doi: 10.1021/ja0558995. [DOI] [PubMed] [Google Scholar]
- 125.Reid SM, Boyle RC, Mague JT, Fink MJ. J. Am. Chem. Soc. 2003;125:7816. doi: 10.1021/ja0361493. [DOI] [PubMed] [Google Scholar]
- 126.Yamashita M, Takamiya I, Jin K, Nozaki K. J. Organomet. Chem. 2006;691:3189. [Google Scholar]
- 127.Phan NTS, Van Der Sluys M, Jones CW. Adv. Synth. Catal. 2006;348:609. [Google Scholar]
- 128.Barder TE, Biscoe MR, Buchwald SL. Organometallics. 2007;26:2183. [Google Scholar]
- 129.Barder TE, Buchwald SL. J. Am. Chem. Soc. 2007;129:12003. doi: 10.1021/ja073747z. [DOI] [PubMed] [Google Scholar]
- 130.Dehli JR, Legros J, Bolm C. Chem. Commun. 2005:973. doi: 10.1039/b415954c. [DOI] [PubMed] [Google Scholar]
- 131.Barluenga J, Valdes C. Chem. Commun. 2005:4891. doi: 10.1039/b509311b. [DOI] [PubMed] [Google Scholar]
- 132.Lebedev AY, Izmer VV, Kazyul'kin DN, Beletskaya IP, Voskoboynikov AZ. Org. Lett. 2002;4:623. doi: 10.1021/ol0172370. [DOI] [PubMed] [Google Scholar]
- 133.Movassaghi M, Ondrus AE. J. Org. Chem. 2005;70:8638. doi: 10.1021/jo051450i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Willis MC, Brace GN. Tetrahedron Lett. 2002;43:9085. [Google Scholar]
- 135.Wallace DJ, Klauber DJ, Chen CY, Volante RP. Org. Lett. 2003;5:4749. doi: 10.1021/ol035959g. [DOI] [PubMed] [Google Scholar]
- 136.Klapars A, Campos KR, Chen CY, Volante RP. Org. Lett. 2005;7:1185. doi: 10.1021/ol050117y. [DOI] [PubMed] [Google Scholar]
- 137.Willis MC, Brace GN, Holmes IP. Synthesis. 2005:3229. [Google Scholar]
- 138.Barluenga J, Fernandez MA, Aznar F, Valdes C. Chem. Commun. 2002:2362. doi: 10.1039/b207524e. [DOI] [PubMed] [Google Scholar]
- 139.Barluenga J, Fernandez MA, Aznar F, Valdes C. Chem. Eur. J. 2004;10:494. doi: 10.1002/chem.200305406. [DOI] [PubMed] [Google Scholar]
- 140.Barluenga J, Fernandez MA, Aznar F, Valdes C. Chem. Commun. 2004:1400. doi: 10.1039/b403655g. [DOI] [PubMed] [Google Scholar]
- 141.Barluenga J, Aznar F, Moriel P, Valdes C. Adv. Synth. Catal. 2004;346:1697. [Google Scholar]
- 142.Barluenga J, Moriel P, Aznar F, Valdes C. Org. Lett. 2007;9:275. doi: 10.1021/ol062726r. [DOI] [PubMed] [Google Scholar]
- 143.Barluenga J, Aznar F, Valdes C. Angew. Chem. 2004;116:347. doi: 10.1002/anie.200352808. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2004;43:343. [Google Scholar]
- 144.Wolfe JP, Thomas JS. Curr. Org. Chem. 2005;9:625. [Google Scholar]
- 145.Nakamura I, Yamamoto Y. Chem. Rev. 2004;104:2127. doi: 10.1021/cr020095i. [DOI] [PubMed] [Google Scholar]
- 146.Zeni G, Larock RC. Chem. Rev. 2006;106:4644. doi: 10.1021/cr0683966. [DOI] [PubMed] [Google Scholar]
- 147.Li JJ, Gribble GW. Palladium in heterocyclic chemistry : a guide for the synthetic chemist. 2nd ed. Elsevier; Amsterdam ; Boston: 2007. [Google Scholar]
- 148.D'Souza DM, Muller TJJ. Chem. Soc. Rev. 2007;36:1095. doi: 10.1039/b608235c. [DOI] [PubMed] [Google Scholar]
- 149.Cacchi S, Fabrizi G, Goggiamani A, Licandro E, Maiorana S, Perdicchia D. Org. Lett. 2005;7:1497. doi: 10.1021/ol050130i. [DOI] [PubMed] [Google Scholar]
- 150.Barluenga J, Fernandez MA, Aznar F, Valdes C. Chem. Eur. J. 2005;11:2276. doi: 10.1002/chem.200401274. [DOI] [PubMed] [Google Scholar]
- 151.Edmondson SD, Mastracchio A, Parmee ER. Org. Lett. 2000;2:1109. doi: 10.1021/ol000031z. [DOI] [PubMed] [Google Scholar]
- 152.Jia YX, Zhu JP. J. Org. Chem. 2006;71:7826. doi: 10.1021/jo061471s. [DOI] [PubMed] [Google Scholar]
- 153.Barluenga J, Jimenez-Aquino A, Valdes C, Aznar F. Angew. Chem. 2007;119:1551. doi: 10.1002/anie.200604407. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2007;46:1529. [Google Scholar]
- 154.Willis MC, Brace GN, Findlay TJK, Holmes IP. Adv. Synth. Catal. 2006;348:851. [Google Scholar]
- 155.Fayol A, Fang YQ, Lautens M. Org. Lett. 2006;8:4203. doi: 10.1021/ol061374l. [DOI] [PubMed] [Google Scholar]
- 156.Fang YQ, Lautens M. Org. Lett. 2005;7:3549. doi: 10.1021/ol051286l. [DOI] [PubMed] [Google Scholar]
- 157.Fang YQ, Yuen J, Lautens M. J. Org. Chem. 2007;72:5152. doi: 10.1021/jo070460b. [DOI] [PubMed] [Google Scholar]
- 158.Fang YQ, Lautens M. J. Org. Chem. 2008;73:538. doi: 10.1021/jo701987r. [DOI] [PubMed] [Google Scholar]
- 159.Cacchi S, Fabrizi G, Goggiamani A. Adv. Synth. Catal. 2006;348:1301. [Google Scholar]
- 160.Robinson B. The Fischer indole synthesis. Wiley; Chichester; New York: 1982. [Google Scholar]
- 161.Wagaw S, Yang BH, Buchwald SL. J. Am. Chem. Soc. 1998;120:6621. [Google Scholar]
- 162.Gao GYY, Chen Y, Zhang XP. J. Org. Chem. 2003;68:6215. doi: 10.1021/jo034576t. [DOI] [PubMed] [Google Scholar]
- 163.Wolfe JP, Tomori H, Sadighi JP, Yin JJ, Buchwald SL. J. Org. Chem. 2000;65:1158. doi: 10.1021/jo991699y. [DOI] [PubMed] [Google Scholar]
- 164.Mauger C, Mignani G. Adv. Synth. Catal. 2005;347:773. [Google Scholar]
- 165.Mauger C, Mignani G. Synth. Commun. 2006;36:1123. [Google Scholar]
- 166.Haddad N, Salvagno A, Busacca C. Tetrahedron Lett. 2004;45:5935. [Google Scholar]
- 167.Zheng N, Anderson KW, Huang XH, Nguyen HN, Buchwald SL. Angew. Chem. 2007;119:7653. doi: 10.1002/anie.200702542. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2007;46:7509. [Google Scholar]
- 168.Zheng N, Anderson KW, Huang XH, Nguyen HN, Buchwald SL. Angew. Chem. 2007;119:7653. doi: 10.1002/anie.200702542. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2007;46:7509. [Google Scholar]
- 169.Kuwahara A, Nakano K, Nozaki K. J. Org. Chem. 2005;70:413. doi: 10.1021/jo048472+. [DOI] [PubMed] [Google Scholar]
- 170.Kitawaki T, Hayashi Y, Ueno A, Chida N. Tetrahedron. 2006;62:6792. [Google Scholar]
- 171.McLaughlin M, Palucki M, Davies IW. Org. Lett. 2006;8:3311. doi: 10.1021/ol061233j. [DOI] [PubMed] [Google Scholar]
- 172.Carey JS, Laffan D, Thomson C, Williams MT. Org. Biomol. Chem. 2006;4:2337. doi: 10.1039/b602413k. [DOI] [PubMed] [Google Scholar]
- 173.King AO, Yasuda N. Top. Organomet. Chem. 2004;6:205. [Google Scholar]
- 174.Gudmundsson KS, Johns BA. Org. Lett. 2003;5:1369. doi: 10.1021/ol0343616. [DOI] [PubMed] [Google Scholar]
- 175.Huang DH, Poon SF, Chapman DF, Chung J, Cramer M, Reger TS, Roppe JR, Tehrani L, Cosford NDP, Smith ND. Bioorg. Med. Chem. Lett. 2004;14:5473. doi: 10.1016/j.bmcl.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 176.Jiang WQ, Guan JH, Macielag MJ, Zhang SY, Qiu YH, Kraft P, Bhattacharjee S, John TM, Haynes-Johnson D, Lundeen S, Sui ZH. J. Med. Chem. 2005;48:2126. doi: 10.1021/jm0401098. [DOI] [PubMed] [Google Scholar]
- 177.Isabel E, Aspiotis R, Black WC, Colucci J, Fortin R, Giroux A, Grimm EL, Han YX, Mellon C, Nicholson DW, Rasper DM, Renaud J, Roy S, Tam J, Tawa P, Vaillancourt JP, Xanthoudakis S, Zamboni RJ. Bioorg. Med. Chem. Lett. 2007;17:1671. doi: 10.1016/j.bmcl.2006.12.110. [DOI] [PubMed] [Google Scholar]
- 178.Li XN, Vince R. Bioorg. Med. Chem. 2006;14:5742. doi: 10.1016/j.bmc.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 179.Ohshita K, Ishiyama H, Oyanagi K, Nakata H, Kobayashi J. Bioorg. Med. Chem. 2007;15:3235. doi: 10.1016/j.bmc.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 180.Wentland MP, Sun XF, Ye YC, Lou RL, Bidlack JM. Bioorg. Med. Chem. Lett. 2003;13:1911. doi: 10.1016/s0960-894x(03)00295-6. [DOI] [PubMed] [Google Scholar]
- 181.Sondergaard K, Kristensen JL, Gillings N, Begtrup M. Euro. J. Org. Chem. 2005:4428. [Google Scholar]
- 182.Renaud J, Bischoff SF, Buhl T, Floersheim P, Fournier B, Geiser M, Halleux C, Kallen J, Keller H, Ramage P. J. Med. Chem. 2005;48:364. doi: 10.1021/jm040858p. [DOI] [PubMed] [Google Scholar]
- 183.Sun MH, Zhao C, Gfesser GA, Thiffault C, Miller TR, Marsh K, Wetter J, Curtis M, Faghih R, Esbenshade TA, Hancock AA, Cowart M. J. Med. Chem. 2005;48:6482. doi: 10.1021/jm0504398. [DOI] [PubMed] [Google Scholar]
- 184.Tan Q, Birzin ET, Chan W, Yang YT, Pai LY, Hayes EC, DaSilva CA, DiNinno F, Rohrer SP, Schaeffer JM, Hammond ML. Bioorg. Med. Chem. Lett. 2004;14:3747. doi: 10.1016/j.bmcl.2004.04.100. [DOI] [PubMed] [Google Scholar]
- 185.Fang YQ, Karisch R, Lautens M. J. Org. Chem. 2007;72:1341. doi: 10.1021/jo062228w. [DOI] [PubMed] [Google Scholar]
- 186.Thibault C, L'Heureux A, Bhide RS, Ruel R. Org. Lett. 2003;5:5023. doi: 10.1021/ol036030z. [DOI] [PubMed] [Google Scholar]
- 187.Huang XH, Anderson KW, Zim D, Jiang L, Klapars A, Buchwald SL. J. Am. Chem. Soc. 2003;125:6653. doi: 10.1021/ja035483w. [DOI] [PubMed] [Google Scholar]
- 188.Ghosh A, Sieser JE, Caron S, Watson TJN. Chem. Commun. 2002:1644. doi: 10.1039/b204844b. [DOI] [PubMed] [Google Scholar]
- 189.Andresen BM, Caron S, Couturier M, DeVries KM, Do NM, Dupont-Gaudet K, Ghosh A, Girardin M, Hawkins JM, Makowski TM, Riou M, Sieser JE, Tucker JL, Vanderplas BC, Watson TJN. Chimia. 2006;60:554. [Google Scholar]
- 190.Ghosh A, Sieser JE, Riou M, Cai WL, Rivera-Ruiz L. Org. Lett. 2003;5:2207. doi: 10.1021/ol034428p. [DOI] [PubMed] [Google Scholar]
- 191.Baeza A, Burgos C, Alvarez-Builla J, Vaquero JJ. Tetrahedron Lett. 2007;48:2597. [Google Scholar]
- 192.Wakabayashi K, Miyachi H, Hashimoto Y, Tanatani A. Bioorg. Med. Chem. 2005;13:2837. doi: 10.1016/j.bmc.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 193.Anderson JT, Ting AE, Boozer S, Brunden KR, Crumrine C, Danzig J, Dent T, Faga L, Harrington JJ, Hodnick WF, Murphy SM, Pawlowski G, Perry R, Raber A, Rundlett SE, Stricker-Krongrad A, Wang JM, Bennani YL. J. Med. Chem. 2005;48:7096. doi: 10.1021/jm050674q. [DOI] [PubMed] [Google Scholar]
- 194.Zim D, Buchwald SL. Org. Lett. 2003;5:2413. doi: 10.1021/ol034561h. [DOI] [PubMed] [Google Scholar]
- 195.Lim JW, Stock N, Pracitto R, Boueres JK, Munoz B, Chaudhary A, Santini AM, Orr K, Schaffhauser H, Bezverkov RE, Aiyar J, Venkatraman S. Bioorg. Med. Chem. Lett. 2004;14:1913. doi: 10.1016/j.bmcl.2004.01.087. [DOI] [PubMed] [Google Scholar]
- 196.Popkin ME, Bellingham RK, Hayes JF. Synlett. 2006:2716. [Google Scholar]
- 197.Pitts WJ, Vaccaro W, Huynh T, Leftheris K, Roberge JY, Barbosa J, Guo JQ, Brown B, Watson A, Donaldson K, Starling GC, Kiener PA, Poss MA, Dodd JH, Barrish JC. Bioorg. Med. Chem. Lett. 2004;14:2955. doi: 10.1016/j.bmcl.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 198.Lee KL, Foley MA, Chen LR, Behnke ML, Lovering FE, Kirincich SJ, Wang WH, Shim J, Tam S, Shen MWH, Khor SP, Xu X, Goodwin DG, Ramarao MK, Nickerson-Nutter C, Donahue F, Ku MS, Clark JD, McKew JC. J. Med. Chem. 2007;50:1380. doi: 10.1021/jm061131z. [DOI] [PubMed] [Google Scholar]
- 199.McKew JC, Foley MA, Thakker P, Behnke ML, Lovering FE, Sum FW, Tam S, Wu K, Shen MWH, Zhang W, Gonzalez M, Liu SH, Mahadevan A, Sard H, Khor SP, Clark JD. J. Med. Chem. 2006;49:135. doi: 10.1021/jm0507882. [DOI] [PubMed] [Google Scholar]
- 200.Senten K, Van der Veken P, De Meester I, Lambeir AM, Scharpe S, Haemers A, Augustyns K. J. Med. Chem. 2004;47:2906. doi: 10.1021/jm031122f. [DOI] [PubMed] [Google Scholar]
- 201.Wust FR, Kniess T. Journal of Labelled Compounds & Radiopharmaceuticals. 2005;48:31. doi: 10.1002/jlcr.3708. [DOI] [PubMed] [Google Scholar]
- 202.Ali MH, Buchwald SL. J. Org. Chem. 2001;66:2560. doi: 10.1021/jo0008486. [DOI] [PubMed] [Google Scholar]
- 203.Hennessy EJ, Buchwald SL. J. Org. Chem. 2005;70:7371. doi: 10.1021/jo051096o. [DOI] [PubMed] [Google Scholar]
- 204.Tasler S, Mies J, Langa M. Adv. Synth. Catal. 2007;349:2286. [Google Scholar]
- 205.Movassaghi M, Ondrus AE. Org. Lett. 2005;7:4423. doi: 10.1021/ol051629f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ganton MD, Kerr MA. J. Org. Chem. 2007;72:574. doi: 10.1021/jo062064j. [DOI] [PubMed] [Google Scholar]
- 207.Hosokawa S, Ogura T, Togashi H, Tatsuta K. Tetrahedron Lett. 2005;46:333. [Google Scholar]
- 208.Bringmann G, Guider T, Reichert M, Meyer F. Org. Lett. 2006;8:1037. doi: 10.1021/ol052946p. [DOI] [PubMed] [Google Scholar]
- 209.Dobler MR, Bruce I, Cederbaum F, Cooke NG, Diorazio LJ, Hall RG, Irving E. Tetrahedron Lett. 2001;42:8281. [Google Scholar]
- 210.Dandapani S, Lan P, Beeler AB, Beischel S, Abbas A, Roth BL, Porco JA, Panek JS. J. Org. Chem. 2006;71:8934. doi: 10.1021/jo061758p. [DOI] [PubMed] [Google Scholar]
- 211.Plante OJ, Buchwald SL, Seeberger PH. J. Am. Chem. Soc. 2000;122:7148. [Google Scholar]
- 212.Crich D, de la Mora MA, Cruz R. Tetrahedron. 2002;58:35. [Google Scholar]
- 213.Ratner DM, Plante OJ, Seeberger PH. Euro. J. Org. Chem. 2002:826. [Google Scholar]
- 214.Lakshman MK. J. Organomet. Chem. 2002;653:234. [Google Scholar]
- 215.Lakshman MK. Curr. Org. Synth. 2005;2:83. [Google Scholar]
- 216.Lakshman MK, Keeler JC, Hilmer JH, Martin JQ. J. Am. Chem. Soc. 1999;121:6090. doi: 10.1021/ja0107172. [DOI] [PubMed] [Google Scholar]
- 217.Lakshman MK, Hilmer JH, Martin JQ, Keeler JC, Dinh YQV, Ngassa FN, Russon LM. J. Am. Chem. Soc. 2001;123:7779. doi: 10.1021/ja0107172. [DOI] [PubMed] [Google Scholar]
- 218.Gunda P, Russon LM, Lakshman MK. Angew. Chem. 2005;117:1178. doi: 10.1002/anie.200460782. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2005;44:1154. [Google Scholar]
- 219.Wang ZW, Rizzo CJ. Org. Lett. 2001;3:565. doi: 10.1021/ol006968h. [DOI] [PubMed] [Google Scholar]
- 220.Urban M, Pohl R, Klepetarova B, Hocek M. J. Org. Chem. 2006;71:7322. doi: 10.1021/jo061080d. [DOI] [PubMed] [Google Scholar]
- 221.Joubert N, Pohl R, Klepetarova B, Hocek M. J. Org. Chem. 2007;72:6797. doi: 10.1021/jo0709504. [DOI] [PubMed] [Google Scholar]
- 222.Damon DB, Dugger RW, Hubbs SE, Scott JM, Scott RW. Org. Process Res. Dev. 2006;10:472. [Google Scholar]
- 223.Nilsson P, Olofsson K, Larhed M. Top. Curr. Chem. 2006;266:103. [Google Scholar]
- 224.Maes BUW, Loones KTJ, Lemiere GLF, Dommisse RA. Synlett. 2003:1822. [Google Scholar]
- 225.Maes BUW, Loones KTJ, Hostyn S, Diels G, Rombouts G. Tetrahedron. 2004;60:11559. [Google Scholar]
- 226.Loones KTJ, Maes BUW, Rombouts G, Hostyn S, Diels G. Tetrahedron. 2005;61:10338. [Google Scholar]
- 227.Fried KW, Schneider CM, Schramm KW, Datta A, Chahbane N, Corsten C, Powell DR, Lenoir D, Kettrup A, Terranova P, Georg GI, Rozman KK. Chemmedchem. 2007;2:890. doi: 10.1002/cmdc.200700005. [DOI] [PubMed] [Google Scholar]
- 228.Jensen TA, Liang XF, Tanner D, Skjaerbaek N. J. Org. Chem. 2004;69:4936. doi: 10.1021/jo049572i. [DOI] [PubMed] [Google Scholar]
- 229.Heo JN, Song YS, Kim BT. Tetrahedron Lett. 2005;46:4621. [Google Scholar]
- 230.Kim YH, Kim YJ, Chang SY, Kim BT, Heo JN. Bull. Korean. Chem. Soc. 2007;28:777. [Google Scholar]
- 231.Tundel RE, Anderson KW, Buchwald SL. J. Org. Chem. 2006;71:430. doi: 10.1021/jo052131u. [DOI] [PubMed] [Google Scholar]
- 232.Anderson KW, Mendez-Perez M, Priego J, Buchwald SL. J. Org. Chem. 2003;68:9563. doi: 10.1021/jo034962a. [DOI] [PubMed] [Google Scholar]
- 233.Poondra RR, Turner NJ. Org. Lett. 2005;7:863. doi: 10.1021/ol0473804. [DOI] [PubMed] [Google Scholar]
- 234.Yang BH, Buchwald SL. Org. Lett. 1999;1:35. doi: 10.1021/ol9905351. [DOI] [PubMed] [Google Scholar]
- 235.van den Hoogenband A, den Hartog JAJ, Lange JHM, Terpstra JW. Tetrahedron Lett. 2004;45:8535. [Google Scholar]
- 236.van den Hoogenband A, Lange JHM, Iwema-Bakker WI, den Hartog JAJ, van Schaik J, Feenstra RW, Terpstra JW. Tetrahedron Lett. 2006;47:4361. [Google Scholar]
- 237.Bonnaterre F, Bois-Choussy M, Zhu JP. Org. Lett. 2006;8:4351. doi: 10.1021/ol061755z. [DOI] [PubMed] [Google Scholar]
- 238.Schon U, Messinger J, Buchholz M, Reinecker U, Thole H, Prabhu MKS, Konda A. Tetrahedron Lett. 2005;46:7111. [Google Scholar]
- 239.Burton G, Cao P, Li G, Rivero R. Org. Lett. 2003;5:4373. doi: 10.1021/ol035655u. [DOI] [PubMed] [Google Scholar]
- 240.Alcaraz L, Bennion C, Morris J, Meghani P, Thom SM. Org. Lett. 2004;6:2705. doi: 10.1021/ol049091l. [DOI] [PubMed] [Google Scholar]
- 241.McAllister LA, McCormick RA, James KM, Brand S, Willetts N, Procter DJ. Chem. Eur. J. 2007;13:1032. doi: 10.1002/chem.200601429. [DOI] [PubMed] [Google Scholar]
- 242.Gentile G, Di Fabio R, Pavone F, Sabbatini FM, St-Denis Y, Zampori MG, Vitulli G, Worby A. Bioorg. Med. Chem. Lett. 2007;17:5218. doi: 10.1016/j.bmcl.2007.06.077. [DOI] [PubMed] [Google Scholar]
- 243.Gore VK, Ma VV, Tamir R, Gavva NR, Treanor JJS, Norman MH. Bioorg. Med. Chem. Lett. 2007;17:5825. doi: 10.1016/j.bmcl.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 244.Smith CJ, Early TR, Holmes AB, Shute RE. Chem. Commun. 2004:1976. doi: 10.1039/b406868h. [DOI] [PubMed] [Google Scholar]
- 245.Smith CJ, Tsang MWS, Holmes AB, Danheiser RL, Tester JW. Org. Biomol. Chem. 2005;3:3767. doi: 10.1039/b509345g. [DOI] [PubMed] [Google Scholar]
- 246.Mauger C, Buisine O, Caravieilhes S, Mignani G. J. Organomet. Chem. 2005;690:3627. [Google Scholar]
- 247.Guino M, Hii KKM. Chem. Soc. Rev. 2007;36:608. doi: 10.1039/b603851b. [DOI] [PubMed] [Google Scholar]
- 248.Leadbeater NE, Marco M. Chem. Rev. 2002;102:3217. doi: 10.1021/cr010361c. [DOI] [PubMed] [Google Scholar]
- 249.End N, Schoning K-W. Top. Curr. Chem. 2004;242:241. doi: 10.1007/b96878. [DOI] [PubMed] [Google Scholar]
- 250.Parrish CA, Buchwald SL. J. Org. Chem. 2001;66:3820. doi: 10.1021/jo010025w. [DOI] [PubMed] [Google Scholar]
- 251.Leyva A, Garcia H, Corma A. Tetrahedron. 2007;63:7097. [Google Scholar]
- 252.an der Heiden M, Plenio H. Chem. Eur. J. 2004;10:1789. doi: 10.1002/chem.200305562. [DOI] [PubMed] [Google Scholar]
- 253.Glegola K, Framery E, Pietrusiewicz KM, Sinou D. Adv. Synth. Catal. 2006;348:1728. [Google Scholar]
- 254.Konovets A, Penciu A, Framery E, Percina N, Goux-Henry C, Sinou D. Tetrahedron Lett. 2005;46:3205. [Google Scholar]
- 255.Anderson KW, Buchwald SL. Angew. Chem. 2005;117:6329. doi: 10.1002/anie.200502017. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2005;44:6173. [Google Scholar]
- 256.Lemo J, Heuze K, Astruc D. Chem. Commun. 2007:4351. doi: 10.1039/b710289e. [DOI] [PubMed] [Google Scholar]
- 257.Nishio R, Wessely S, Sugiura M, Kobayashi S. J. Combi. Chem. 2006;8:459. doi: 10.1021/cc060011+. [DOI] [PubMed] [Google Scholar]
- 258.Marugg JL, Neitzel ML, Tucker J. Tetrahedron Lett. 2003;44:7537. [Google Scholar]
- 259.Weber C, Demeter A, Szendrei GI, Greiner I. Tetrahedron Lett. 2003;44:7533. [Google Scholar]
- 260.Zhang XX, Buchwald SL. J. Org. Chem. 2000;65:8027. doi: 10.1021/jo005577d. [DOI] [PubMed] [Google Scholar]
- 261.Subat M, Konig B. Synthesis. 2001:1818. [Google Scholar]
- 262.Sazonov PK, Artamkina GA, Beletskaya IP. Russian J. Org. Chem. 2006;42:438. [Google Scholar]
- 263.Krakowiak KE, Bradshaw JS, Zameckakrakowiak DJ. Chem. Rev. 1989;89:929. [Google Scholar]
- 264.Chi KW, Addicott C, Stang PJ. J. Org. Chem. 2004;69:2910. doi: 10.1021/jo049883t. [DOI] [PubMed] [Google Scholar]
- 265.Nakanishi M, Bolm C. Adv. Synth. Catal. 2006;348:1823. [Google Scholar]
- 266.Burdette SC, Lippard SJ. Inorg. Chem. 2002;41:6816. doi: 10.1021/ic026048q. [DOI] [PubMed] [Google Scholar]
- 267.Woodroofe CC, Lim MH, Bu WM, Lippard SJ. Tetrahedron. 2005;61:3097. [Google Scholar]
- 268.Johansson O. Synthesis. 2006:2585. [Google Scholar]
- 269.Tonzetich ZJ, Schrock RR. Polyhedron. 2006;25:469. [Google Scholar]
- 270.Zhang XX, Sadighi JP, Mackewitz TW, Buchwald SL. J. Am. Chem. Soc. 2000;122:7606. [Google Scholar]
- 271.Ward RE, Meyer TY. Macromolecules. 2003;36:4368. [Google Scholar]
- 272.Kawaguchi K, Nakano K, Nozaki K. J. Org. Chem. 2007;72:5119. doi: 10.1021/jo070427p. [DOI] [PubMed] [Google Scholar]
- 273.Agou T, Kobayashi J, Kawashima T. Chem. Commun. 2007:3204. doi: 10.1039/b706418g. [DOI] [PubMed] [Google Scholar]
- 274.Shirota Y. J. Mater. Chem. 2000;10:1. [Google Scholar]
- 275.Shirota Y. J. Mater. Chem. 2005;15:75. [Google Scholar]
- 276.Harris MC, Buchwald SL. J. Org. Chem. 2000;65:5327. doi: 10.1021/jo000674s. [DOI] [PubMed] [Google Scholar]
- 277.Shen QL, Hartwig JF. J. Am. Chem. Soc. 2006;128:10028. doi: 10.1021/ja064005t. [DOI] [PubMed] [Google Scholar]
- 278.Surry DS, Buchwald SL. J. Am. Chem. Soc. 2007;129:10354. doi: 10.1021/ja074681a. [DOI] [PubMed] [Google Scholar]
- 279.Katayama H, de Greef TFA, Kooijman H, Spek AL, Vekemans JAJM, Meijer EW. Tetrahedron. 2007;63:6642. [Google Scholar]
- 280.Jiao GS, Loudet A, Lee HB, Kalinin S, Johansson LBA, Burgess K. Tetrahedron. 2003;59:3109. [Google Scholar]
- 281.Yang JS, Liau KL, Li CY, Chen MY. J. Am. Chem. Soc. 2007;129:13183. doi: 10.1021/ja0741022. [DOI] [PubMed] [Google Scholar]
- 282.Thiemann F, Piehler T, Haase D, Saak W, Lutzen A. Euro. J. Org. Chem. 2005:1991. [Google Scholar]