The search for highly stereoselective olefin addition reactions has a long and continuing history, and cyclopropanation reactions of olefins using diazoacetates are core elements in this quest.[1] Catalytic methodologies, especially those built upon a platform of transition metals having chiral ligands, have stimulated developments that today offer diastereocontrol and enantioselectivities that approach the limits of stereoisomer detection.[2] Beginning with copper catalysis having chiral salen ligands[3] and progressing to chiral semicorrin[4] and bis-oxazoline[5] ligands, rapid advances were made in enantiocontroled intermolecular addition reactions of diazoacetates. Chiral dirhodium(II) carboxamidates fulfilled a role in intramolecular reactions[6] and, although results from many other transition metals and chiral ligands have been reported, none have surpassed the overall stereocontrol provided by copper and dirhodium catalysts until the recent emergence of cobalt(II) porphyrins.
One of the first successes in enantioselective intermolecular cyclopropanation reactions was a report by Nakamura and coworkers of a chiral cobalt(II) dioximato complex derived from camphor,[7] but difficulties in catalyst homogeneity with chiral dioximato ligands inhibited further activities. Subsequently, Katsuki,[8] Yamada,[9] and coworkers reported intriguing results in stereocontrolled cyclopropanation reactions with chiral cobalt(III) salen complexes;[8] however, at least one of the key ingredients for a breakthrough (yield, diastereoselectivity, or enantioselectivity) was missing with these catalysts. Whereas both copper and dirhodium catalysts promote additions to simple and conjugated olefins, but not unsaturated esters, nitriles, and ketones, cobalt catalysts showed activity even with unsaturated esters and nitriles. Taking cobalt catalysis one step further, Zhang and coworkers have combined cobalt(II) with chiral porphyrins to produce catalysts that exhibit unique reactivities and exceptional selectivities.[10] In this recent manifestation he has reported applications with α-nitro-diazoacetates to prepare Z-cyclopropanes in high yield with exceptional diastereo- and enantioselectivity. [11]
Inspired by the potential of vitamin B12 as a catalyst, aquocobalamin and some of its derivatives were demonstrated to be effective for cyclopropanation of styrene derivatives using ethyl diazoacetate but with modest diastereo- and enantioselectivities.[12] As with other catalytic systems, the challenge was to enhance both diastereocontrol and enantioselectivity in cyclopropanation reactions using commonly accessible diazoacetates, and this was first reported by Zhang in 2003 using modified tetraphenylporphyrins, the most successful being those with chiral cyclopropylcarboxamide attachments.[10] Prior to Zhang’s undertaking, Kodadek and coworkers had introduced chiral porphyrin ligands for rhodium catalyzed cyclopropanation reactions with diazoacetates and found low selectivities but high turnover numbers.[13] Convenience in porphyrin modification has been critical to building chiral tetraphenylporphyrins as effective ligands, and Zhang has accomplished this using palladium-catalyzed coupling processes of chiral amides on bromoporphyrin templates.
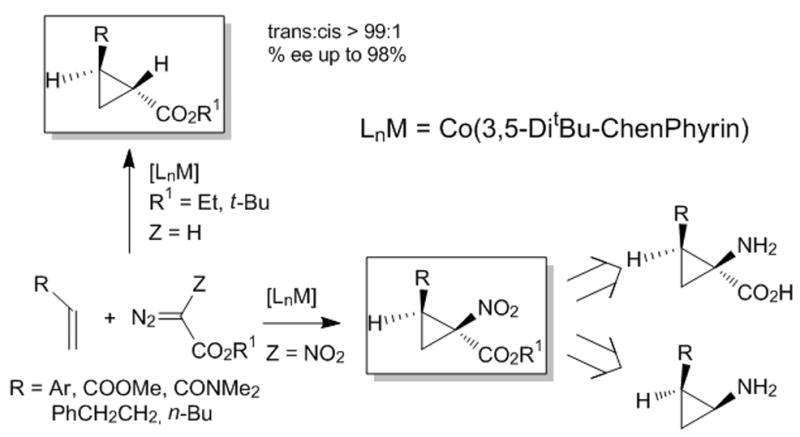
Beginning with the initial findings of exceptional diastereocontrol and enantioselectivity in reactions with styrene with Co(3,5-di-t-Bu-ChenPhyrin),[10b,d] Zhang reported that similarly highly stereoselective cyclopropanation could be achieved with p-tosyldiazomethane using Co(2,6-diMeO-ChenPyrin), and that these carbenoid addition reactions could be performed with the alkene as the limiting reagent.[10e] And following those with tosyldiazomethane, the latest application has taken nitrodiazoacetates[14] to the corresponding cyclopropanes11 that themselves afford convenient access to cyclopropyl amino acids and cyclopropyl amines. These transformations are performed stoichiometrically without high dilution and at room temperature, and diastereocontrol for the trans isomer (trans for R and COOR1) is complete when R1 = t-Bu. Relative to chiral copper and rhodium catalysts, the chiral porphyrin catalysts are unprecedented in their stereocontrol, their ability to effect cyclopropanation with stoichiometric or near stoichiometric amounts of alkenes avoiding carbene dimmer formation, and their catalytic reactivity. They are also unique in their ability to undergo addition to α,β-unsaturated carbonyl compounds and nitriles,[10d] and this characteristic distinguishes them from copper and rhodium catalysts that are generally understood to undergo electrophilic addition to alkenes and do not undergo catalytic cyclopropanation with electron-deficient alkenes. Further highly stereoselective applications to other diazo compounds are anticipated, and those with which high selectivities have not yet been achieved (diazomalonates, diazoacetoacetates, for example) may be realized with chiral cobalt porphyrin catalysts. The mechanism for addition will continue to be a topic of considerable interest.
While the principal breakthroughs with these catalysts have occurred in cyclopropanation reactions, catalytic aziridination reactions with sulfonyl and phosphoryl azides have also been reported.[15] Enantioselectivities are modest, but the application with azides rather than iodonium ylides is a promising direction. The ability of the porphyrin ring to serve as an electron sink, stabilizing transition metals in their reactions and moderating reactivities is abundantly evident in this research with chiral cobalt porphyrins.
Figure 1.
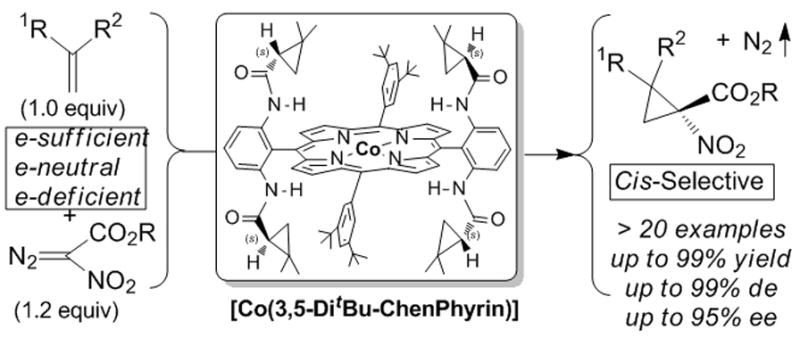
Figure 2.
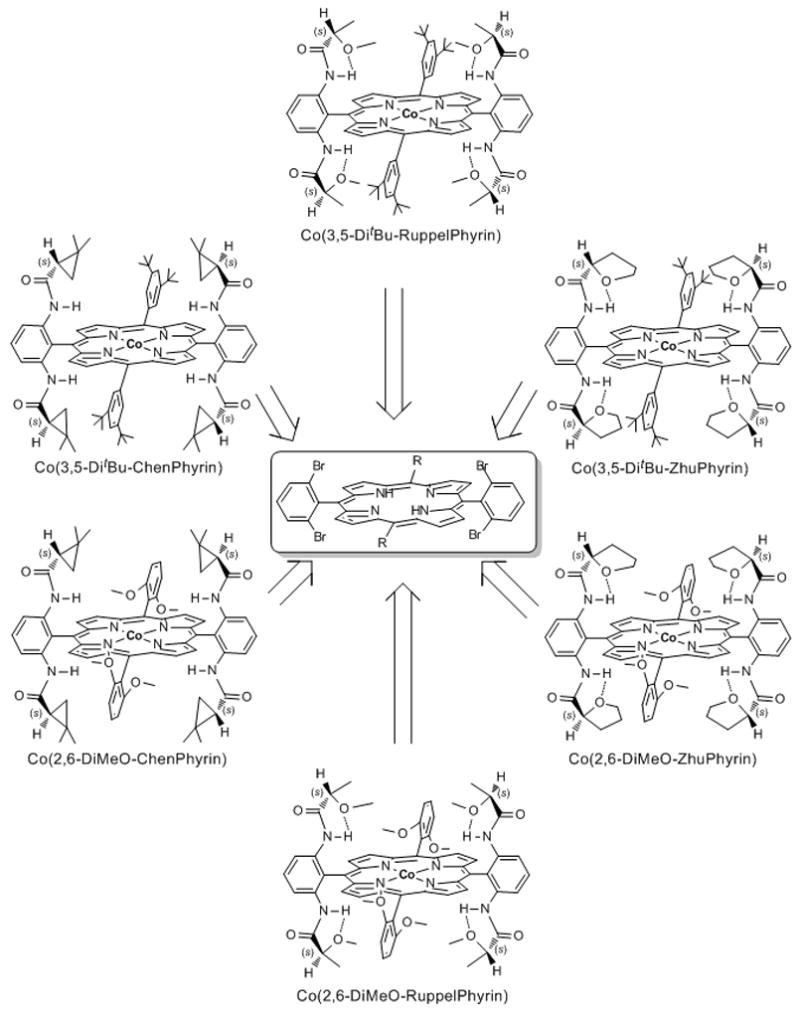
Figure 3.
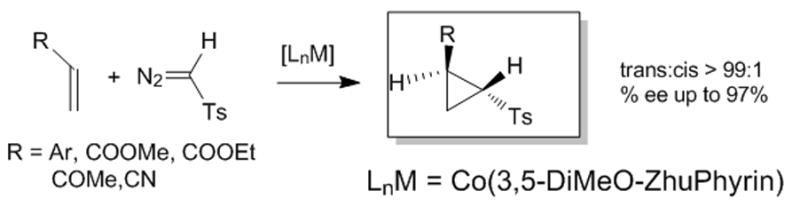
Figure 4.
Footnotes
I thank the national Science Foundation and the National Institutes of Health for their generous financial support.
References
- 1.Doyle MP, McKervey MA, Ye T. Modern Catalytic Methods for Organic Synthesis with Diazo Compounds. John Wiley & Sons, Inc; New York, NY: 1998. [Google Scholar]
- 2.a) Doyle MP. In: Modern Rhodium-Catalyzed Transformations. Evans PA, editor. Ch 15 Wiley-VCH; New York: 2005. [Google Scholar]; b) Davies HML, Walji AM. In: Modern Rhodium-Catalyzed Transformations. Evans PA, editor. Vol. 14 Wiley-VCH; New York: 2005. [Google Scholar]; c) Davies HML, Antoulinakis EG. Org React. 2001;57:1–326. [Google Scholar]
- 3.a) Nozaki H, Moriuti S, Takaya H, Noyori R. Tetrahedron Lett. 1966:5239–5244. [Google Scholar]; b) Aratani T. Pure Appl Chem. 1985;57:1839–1844. [Google Scholar]
- 4.Fritschi H, Leutenegger U, Pfaltz A. Agnew Chem. 1986;98:1028–1029. [Google Scholar]; Agnew Chem, Int Ed Engl. 1986;25:1005–1006. [Google Scholar]
- 5.a) Lowenthal RE, Abiko A, Masamune S. Tetrahedron Lett. 1990;31:6005. [Google Scholar]; b) Evans DA, Woerpel KA, Hinman MM, Faul MM. J Am Chem Soc. 1991;113:726–728. [Google Scholar]
- 6.a) Doyle MP, Pieters RJ, Martin SF, Austin RE, Oalmann CJ, Müller P. J Am Chem Soc. 1991;113:1423–1424. [Google Scholar]; b) Doyle MP, Austin RE, Bailey AS, Dwyer MP, Dyatkin AB, Kalinin AV, Kwan MMY, Liras S, Oalmann CJ, Pieters RJ, Protopopova MN, Raab CE, Roos GHP, Zhou QL, Martin SF. J Am Chem Soc. 1995;117:5763. [Google Scholar]
- 7.Nakamura A, Konishi A, Tatsuno Y, Otsuka S. J Am Chem Soc. 1978;100:3443–3448. [Google Scholar]
- 8.Niimi T, Uchida T, Irie R, Katsuki T. Adv Synth Catal. 2001;343:79–88. [Google Scholar]
- 9.Ikeno T, Sato M, Sekino H, Nishizuka A, Yamada T. Bull Chem Soc Jpn. 2001;74:2139–2150. [Google Scholar]
- 10.a) Huang L, Chen Y, Gao GY, Zhang XP. J Org Chem. 2003;68:8179–8184. doi: 10.1021/jo035088o. [DOI] [PubMed] [Google Scholar]; b) Chen Y, Fields KB, Zhang XP. J Am Chem Soc. 2004;126:14718–14719. doi: 10.1021/ja044889l. [DOI] [PubMed] [Google Scholar]; c) Chen Y, Zhang XP. J Org Chem. 2007;72:5931–5934. doi: 10.1021/jo070997p. [DOI] [PubMed] [Google Scholar]; d) Chen Y, Ruppel JV, Zhang XP. J Am Chem Soc. 2007;129:12074–12075. doi: 10.1021/ja074613o. [DOI] [PubMed] [Google Scholar]; e) Zhu S, Ruppel JV, Lu H, Wojtas L, Zhang XP. J Am Chem Soc. 2008;130:5042–5043. doi: 10.1021/ja7106838. [DOI] [PubMed] [Google Scholar]
- 11.Zhu S, Perman JA, Zhang XP. Angew Chem Int Ed. 2008;47:xxxx–yyyy. doi: 10.1002/anie.200803857. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Zhang XP. J Org Chem. 2004;69:2431–2435. doi: 10.1021/jo049870f. [DOI] [PubMed] [Google Scholar]
- 13.Maxwell JL, O’Malley S, Brown KC, Kodadek T. Organometal. 1992;11:645–652. and prior articles. [Google Scholar]
- 14.a) O’Bannon PE, Dailey WP. Tetrahedron. 1990;46:7341–7358. [Google Scholar]; b) Wurz RP, Charette AB. Org Lett. 2005;7:2313–2316. doi: 10.1021/ol050442l. [DOI] [PubMed] [Google Scholar]
- 15.a) Ruppel JV, Jones JE, Huff CA, Kamble RM, Chen Y, Zhang XP. Org Lett. 2008;10:1995–1998. doi: 10.1021/ol800588p. [DOI] [PubMed] [Google Scholar]; b) Jones JE, Rupple JV, Gao GY, Moore TE, Zhang XP. J Org Chem. 2008;73:7260–7265. doi: 10.1021/jo801151x. [DOI] [PubMed] [Google Scholar]


