Abstract
Peripherally induced Treg (iTregs) are being recognized as a functional and physiologically relevant T cell compartment. Understanding the molecular basis of their development is a necessary step before the therapeutic potential of iTreg manipulation can be exploited. In this study, we report that the differentiation of primary human T cells to suppressor iTregs involves the relocation of key proximal TCR signaling elements to the highly active IL-2-Receptor (IL2-R) pathway. In addition to the recruitment of Lymphocyte-specific protein tyrosine kinase (Lck) to the IL2-R complex, we identified the dissociation of the voltage gated K+ channel Kv1.3 from the TCR pathway and its functional coupling to the IL2-R. The regulatory switch of Kv1.3 activity in iTregs may constitute an important contributing factor in the signaling rewiring associated with the development of peripheral human iTregs and sheds new light upon the reciprocal cross-talk between the TCR and IL2-R pathways.
Keywords: human Treg, Kv1.3 ion channel, T cell signaling, peripheral T cell differentiation, IL-2 Receptor pathway
INTRODUCTION
Recent evidence supports the major role of peripherally induced Regulatory T cells (iTregs) in controlling the immune response during inflammatory processes and against infectious agents [1–3]. The high degree of plasticity in the iTreg developmental program represents an additional challenge to the inherent difficulties associated with the study of human Tregs, such as insufficient number of cells, heterogeneous cell population and staggering differences between human and mouse models. These intrinsic limitations have prevented a comprehensive understanding of the differential signaling events that govern the development and function of Tregs. In the study presented here, we have taken advantage of a cell culture system that simultaneously generates functional populations of naïve, memory, effector T cells and iTregs from an original pool of primary human CD4+ T cells. With this unique platform, we wanted to gain a better understanding of the signaling events that contribute to the altered proximal TCR-mediated activation of iTregs. A comparative analysis among T cell subsets identified the uncoupling of voltage-gated Shaker family K+-channel Kv1.3 activity from TCR engagement in iTregs. Kv1.3 has been implicated in the activation, migration, adhesion and volume regulation of human T cells[4]. Our findings demonstrate that, in iTregs, Kv1.3 and the reportedly functionally linked Lymphocyte-specific protein tyrosine kinase (Lck) relocate to and participate in the signaling pathway triggered by the highly active IL2-Receptor (IL2-R) complex. We propose that the physical and functional redistribution of protein clusters, in addition of being instrumental to the altered TCR pathway in iTregs, may represent a common contributing mechanism to the signaling plasticity that governs T cell lineage differentiation.
RESULTS and DISCUSSION
Altered TCR signaling in iTregs
The physiological relevance of Tregs in the control of the immune response emphasizes the importance of understanding the molecular mechanisms underlying their development and function. In addition to naturally occurring, thymus-derived CD4+CD25highFoxP3+ cells (nTregs), a microenvironment enriched with IL2 and TGFβ can induce peripheral CD4+ cells to differentiate into suppressor T cells in vivo and ex vivo [1,2]. We have optimized culture conditions for CD4+ CD25− primary T cells to allow the induction and discrimination of iTregs, naïve, memory and effector T cells. Cells that differentiate to a Treg-like phenotype also exhibit potent suppression of conventional CD4+ T cell proliferation (Supporting Fig. A). As expected, iTregs generated in our conditions had already acquired specific Treg signaling trademarks. Notably, iTregs exhibited a low activation of AKT in response to TCR engagement. In contrast, ERK activation was remarkably effective (Fig. 1A), indicating that the blockage of the AKT pathway in iTregs did not occur as a result of a general failure of the TCR signaling machinery. Therefore, we propose that the differentiation to iTregs involves the rewiring of the signaling network associated with TCR–dependent events upstream of the AKT pathway.
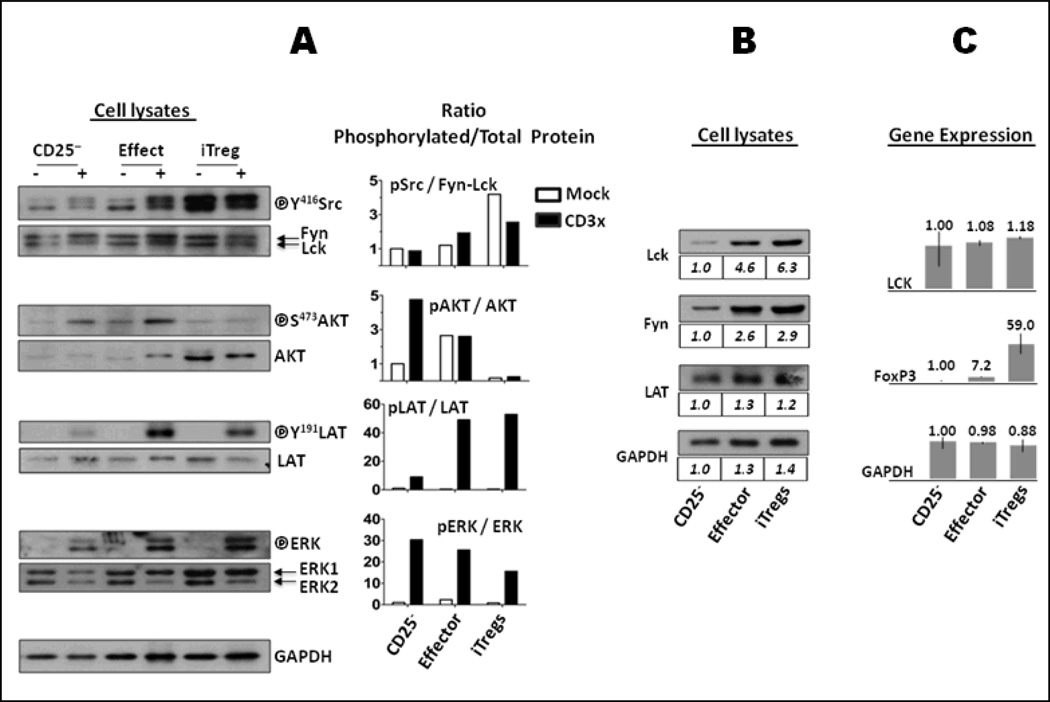
Figure 1. TCR-dependent signaling in iTregs.
(A). TCR response. Sorted CD4+CD25−, effector T cells and iTregs were rested for 2 hours on ice and activated (+) or not (−) by anti-CD3 and anti-mouse IgG crosslinking for 3 min. Lysates of 5×105 cells were analyzed by Western blot to detect activated (phosphorylated) forms and total protein expression of Lck/Fyn (anti-pY416-Src recognizes both pY394-Lck and pY417-Fyn), AKT, LAT and ERK. GAPDH expression demonstrated equivalent loading. Protein band densities were quantified by densitometry. Ratio of phosphorylated: total protein was normalized to that of mock-treated CD25− cells and depicted as mock-treated (empty bars) or CD3 crosslinked (black bars) cells. iTregs exhibited intense phosphorylation of Lck/Fyn in mock cells and impaired capacity to activate AKT upon TCR engagement. (B). Comparative expression of proteins. Lysates from 5×105 cells were analyzed by Western blot to detect the pattern of expression of proteins involved in early events of TCR signaling. Protein band densities were quantified and normalized to that of CD25− cells as indicated below each blot. No substantial differences were noted between effector and iTregs. (C) Regulation of gene expression. Transcription of the LCK gene is not differentially regulated in iTregs. FOXP3 (as a positive control) and GAPDH (housekeeping gene) were assessed as control. Gene expression was quantified from CD25−, effector T cells and iTregs. Briefly, total RNA was extracted from 5–7×106 sorted cells. cDNA was obtained by reverse transcription and used as a template in a microarray analysis with the Affymetrix GeneChip System (Human Genome U133 Plus 2.0 Array microchip). The results represent the comparative mean value of triplicates from three different donors relative to CD25− samples; bars represent the experimental range of the triplicates. Results were validated with Real Time-PCR (not shown).
Functional dissociation between Kv1.3 and TCR activation in iTregs
Previously, we demonstrated that, in contrast to conventional CD4+ and CD8+ T cells, the activation of the K+ channel Kv1.3 was refractory to TCR stimulation in human nTregs[5]. To explore whether iTregs displayed the same functional trait along with the acquisition of other regulatory/suppressor trademarks, we analyzed and compared the response of Kv1.3 to TCR engagement among different T cell subsets. Using an automated, high-throughput patch-clamp screening assay, we found a similar profile of weak Kv1.3 response to TCR stimulation in induced and natural Tregs (Fig 2A). Based on the observation that the mRNA and protein expression of Kv1.3 remained steady compared to effector cells (Fig. 2B), these findings suggest that the functional dissociation between TCR and Kv1.3 activity is likely due to the remodeling of the TCR signaling network during iTreg differentiation.
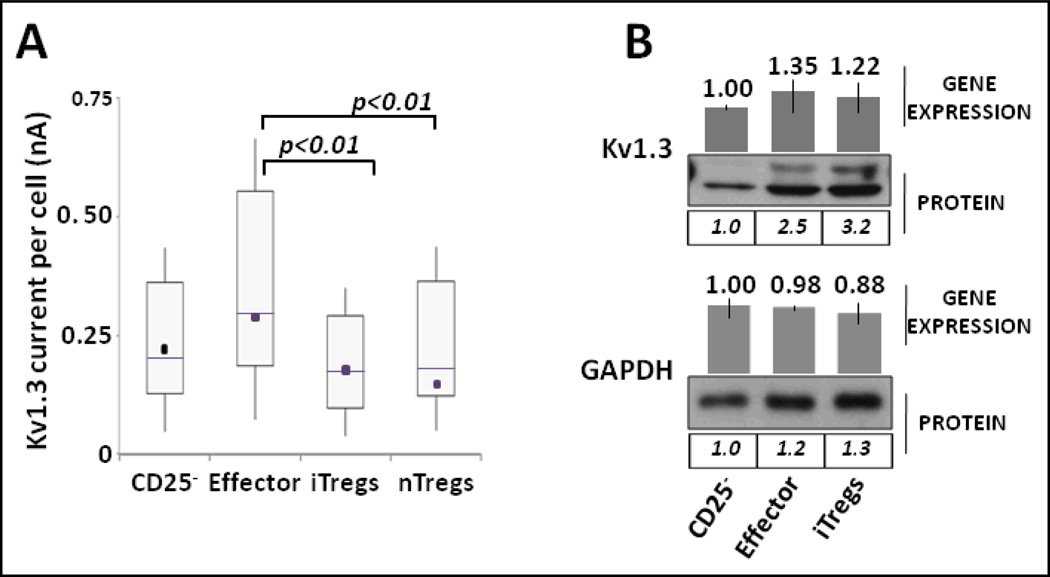
Figure 2. Kv1.3 in iTregs.
(A). Electrophysiology. CD4+CD25− T cells and nTregs isolated from same donors were cultured in iTreg medium (see Supporting information for details). After 6 days, CD4+CD25−, effector T cells and iTregs were sorted and maintained for 60 h in serum-free AIMV medium (Invitrogen) with or without 0.15 µg/mL of soluble anti-CD3 antibody. Kv1.3 current intensity in sorted cells and nTregs was measured with high-throughput patch-clamping screening (see Supporting Information and [5]). Experimental values represent the combined distribution of Kv1.3 measurements on individual cells isolated from three independent experiments. N=255 CD4+CD25− cells; N=186 effector cells; N= 88 natural Tregs; N=129 iTregs. Treg cells (“natural” or “induced”) did not increase Kv1.3 specific current intensity in response to TCR engagement. The distribution of Kv1.3 values are depicted in box plots with whiskers representing the 10th and 90th percentile of experimental values; boxes are limited by the 25th and the 75th percentiles; solid lines are the medians, and dark squares represent the mean values. A two-sample Kolmogorov-Smirnov test was used to compare paired distributions of Kv1.3 currents. (B). Expression. Sorted CD4+CD25−, effector T cells and iTregs were analyzed by Western blot to compare the expression of Kv1.3. The double banded pattern depicted in effector and iTreg Kv1.3 blots may likely correspond to different glycosylation forms of Kv1.3(22,23). Quantification of protein expression was normalized to the band density of CD25− cells. GAPDH levels demonstrate equal protein loading. Gene expression was determined as detailed in Fig. 1C.
Regulation of Lck activity by IL2-R pathway in iTregs
The association between the Src-family tyrosine kinase Lck and Kv1.3 is necessary for the initiation of the TCR-mediated signaling triggered during the formation of the immune synapse[7–9] and has been reported in other, non-TCR-dependent, T cell responses as well[10–12]. Therefore, we explored whether a differential Lck activation in iTregs may concur with the loss of control of Kv1.3 activity by TCR. In our experimental setting of comparative Western blot analysis depicted in Fig. 1A, iTreg cells exhibited the highest levels of active Lck in mock-treated samples under normal resting conditions (2 hours on ice), as indicated by the intense phosphorylation of Lck at Y394. In spite (or because) of this high basal activity, iTregs displayed a reduced capacity to increase Lck activation in response to TCR ligation. A phenotypic attribute of iTregs is the high expression of CD25, the IL2-Rα subunit that associates with the IL2-Rβ (CD122) and the common γ (CD132) chains to form the high affinity IL2-R complex. In conjunction with the requirement for a constant supply of IL2, the enhanced expression of the high affinity IL2-R complex underscores the critical role that the IL2/IL2-R pathway plays in iTreg development [13–14]. In this context, the fact that Lck exhibited a similarly enhanced activity in iTregs when the TCR signaling machinery was not triggered suggests that an alternative pathway might activate Lck. In order to assess the potential involvement of the highly active IL2-R, we performed co-immunoprecipitation assays of Lck from effector T cells and iTreg lysates. The results in Fig. 3A confirmed that the physical association between Lck and the IL2-Rβ subunit occurred only in iTregs. In addition, we generated evidence that Lck is activated upon IL2-R engagement in iTregs. Sorted iTreg cells rested for additional period of time (6–7 hours on ice) revealed the activation of TCR-dependent downstream events (as depicted in Fig. 3B by the phosphorylation of LAT), even though the actual level of activated Lck was essentially identical for TCR cross-linked and mock-activated cells. Conversely, a marked increase of Y394-phosphorylated Lck was detected in iTreg cells treated with IL2 that paralleled the IL2-R-induced phosphorylation of STAT5. In agreement with others[15,16], these findings support the direct involvement of Lck in the IL2-R signal transduction pathway of iTregs. Since we observed no differences in Lck expression between conventional T cells and iTregs (Figs. 1B and 1C), these results are consistent with the redistribution of Lck to the IL2-R cluster occurring during the development of iTregs.
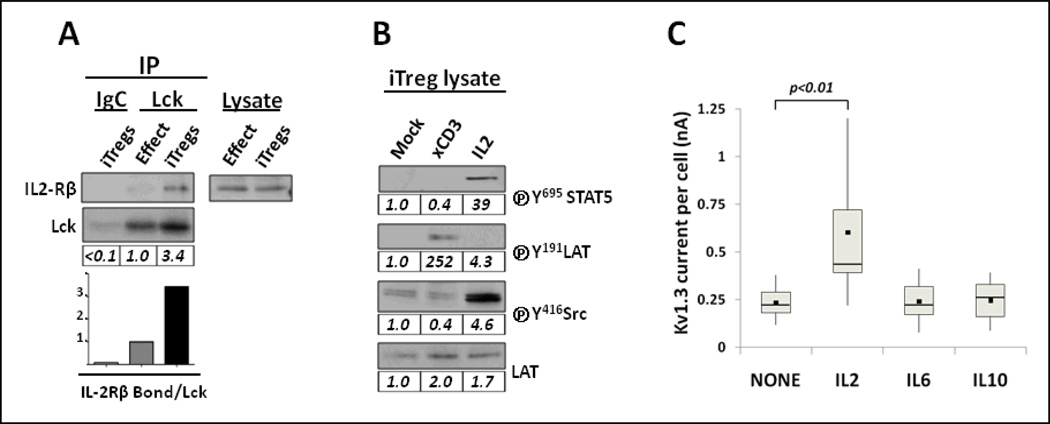
Figure 3. Lck and Kv1.3 respond to IL2 in iTregs.
(A). Physical association between Lck and IL2-R complex in iTregs. Lysates from 107 effector T cells and iTregs were incubated with Lck antibody-loaded agarose beads or Ig-isotype control (IgC). Cell extracts and immunoprecipitates were analyzed by SDS-PAGE and Western blots using CD122 (IL2-Rβ) and Lck antibodies. The results show that Lck co-immunoprecipitates with IL2-Rβ subunit in iTregs but not in effector cells. Lck blot verified equivalent yield of immunoprecipitate. Blotting total cell lysates with anti-CD122 demonstrated equal expression of IL2-Rβ in both cell types. Quantification of co-immunoprecipitates was determined by the relative ratio of IL2-Rβ bound to Lck and normalized at 1.0 in effector cells. (B). Functional association of Lck and IL2-R complex in iTregs. Sorted iTregs were rested for 6–7 hours on ice and mock-activated, anti-CD3 crosslinked or IL2 treated. Lysates of 5×105 cells were subjected to immunoblotting using the indicated antibodies. Phosphorylation of STAT5 and Lck were detected upon IL2 treatment; phosphorylation of LAT demonstrated an effective response to CD3 crosslink. LAT expression verified equal loading. Quantification of protein expression was normalized on the basis of mock-treated iTregs for each individual blot. (C). IL2 treatment supports Kv1.3 current in iTregs. Sorted iTregs were cultured for 60 h in AIMV with or without 5 ng/mL of IL2, IL6 or IL10. Depiction, measurement of Kv1.3 currents and statistical analysis were performed as described in Fig. 2. The results demonstrate that iTregs cultured with IL2 displayed the largest Kv1.3 currents compared to IL6 and IL10. Profiles of Kv1.3 currents represent the combined values of two independent experiments.
Regulation of Kv1.3 function by IL2-R pathway in iTregs
We next wanted to address the question of whether the relocalization and clustering of Lck to the IL2-R complex in iTregs encompassed the regulatory switch of Kv1.3 activity. The functional incidence of Kv1.3 spatial redistribution has been already documented in T cells[7–12]. In order to examine whether the TCR-dissociated Kv1.3 becomes functionally linked to the highly active IL2-R complex, we performed high-throughput patch clamp analysis with sorted iTregs incubated with IL2, IL6 or IL10. Fig.3C shows that, in contrast to the weak response observed upon TCR ligation, iTregs in culture with IL2 sustain significantly larger pick magnitude of Kv1.3 currents compared to cells cultured with IL6 or IL10. These results provide, to our knowledge, the first evidence of the functional integration of Kv1.3 into the IL2-R network in human T cells. Moreover, the linkage of Kv1.3 to the highly Lck-activating IL2-R coincides with the functional uncoupling from TCR control. The enzymatic activity of Lck is required to initiate the TCR signaling cascade that regulates T cell development, differentiation and activation[17]. However, despite extensive investigation, the precise mechanistic principles that orchestrate the earliest signaling events induced by Lck are still elusive. Recent reports propose that a pool of pre-activated-Lck gains access to the ITAM sequences within the CD3/TCRζ complex exposed by the conformational changes upon TCR engagement[18,19]. According to this model, rather than the activation of Lck, the critical priming step in the initiation of the TCR pathway would be the substrate accessibility (i.e. CD3 and TCRζ cytoplasmic tails) to the pool of available Lck. Haughn et al.[20] demonstrated that a very active, high-affinity IL2-R in T cells might cause the functional uncoupling of the TCR signaling machinery through diversion of the subcellular localization of Lck to the IL2-R multiprotein. Accordingly, the strong activation of Lck by the IL2-R pathway in iTregs may compromise the amount of Lck available in CD3/TCR complexes and therefore alter the TCR signaling cascade. In agreement with other reports[7–9] , our findings also suggest that Kv1.3 remains functionally linked with the pool of Lck susceptible to lateral mobility. Interestingly, the differentiation to iTregs occurs preferentially in cells that undergo strong proliferative expansion (not shown), which involves continuous TCR-mediated regulation of Kv1.3 function and requires a strong activation of the AKT pathway. During the transition to the differentiation stage and the acquisition of the suppressor phenotype, the formation of new protein complexes occurs concomitantly with the remodeling of functional signaling paths that eventually dissociate AKT and Kv1.3 activities from the TCR network. We provide evidence that uncoupling early TCR signaling elements occurs, in addition to other potential mechanisms[21], through the competitive cross-talk between TCR and IL2-R pathways in cells with high IL2-dependent activity. These findings support a dynamic model by which the redistribution of common, key signaling components (Lck-Kv1.3) may represent a rapid and efficient mechanism of adapting the cell signaling machinery to a new environmental context. The functional switch from antigen-dependent signaling in effector cells to cytokine-dependent responsiveness in iTregs is consistent with the physiological prevalence of the suppressor activity of iTregs upon antigen clearance.
Concluding remarks
Collectively, these results: (1) provide a novel mechanistic link between the remodeling of a signaling network and the acquisition of suppressor iTreg phenotype with the selective relocation of TCR-associated proximal components; (2) support the importance of the finely-tuned TCR/IL2-R cross-talk in the control of T cell fate decisions, and (3) underscore the differential role that K+ channels may play in specific T cell subpopulations and/or their functional responses.
MATERIALS AND METHODS
Reagents, cell isolation, culture conditions and suppression assay
are detailed in Supporting Information.
T cell activation and lysates
When required, cells were stimulated with anti-CD3 (0.5 µg/106 cells) and cross-linked with anti-mouse IgG (1.2 µg/106 cells) for 3 min. at 37�C or were incubated with IL2 (5 ng/mL) for 6–7 h. Cells were lysed in RadioImmunoPrecipitation Assay (RIPA) buffer or in Immunoprecipitation lysis buffer for Western blot analysis of whole cell lysates or immunoprecipitates, respectively (see buffer composition in Supporting Information).
Immunoprecipitation and Western blot
analysis were performed as indicated in Supporting Information.
Electrophysiology
We used the automated IonWorks HT high-throughput patch-clamping system (Essen Instruments, Ann Arbor, MI) as a screening platform to determine Kv1.3 current intensity profiles in different T cell subsets and culture conditions. We followed the protocols and conditions as detailed elsewhere[5] and summarized in Fig. 2.
Statistics
The Kruskal-Wallis ANOVA test was used to determine differences in peak currents among multiple groups. Statistical comparisons of current distributions between two samples were performed using the Kolmogorov-Smirnov test. Differences were considered significant when p values were <0.01.
Supplementary Material
Acknowledgments
The authors wish to thank Jennifer Strange and Greg Bauman for their assistance with Flow Cytometry analysis and sorting. This work was supported by NIH Grant Numbers R03AR052904-02 from the NIAMS and 2P20 RR020171 from the NCRR to F.M. and University of Kentucky startup funds to F.M.; D.J.E. acknowledges a fellowship from the Cellular Biotechnology Training Program (CBTP) from NIH and a Rackham Pre-Doctoral Fellowship from the University of Michigan.
Abbreviations used in this paper
- nTreg
natural Treg
- iTreg
induced Treg
- IL2-R
IL2-Receptor
- Lck
Lymphocyte-specific protein tyrosine kinase
- AKT
Protein Kinase B
- GITR
Glucocorticoid-Induced Tumor Necrosis Factor Receptor Related protein
- LAT
linker of activated T cells
References
- 1.Vukmanovic-Stejic M, Zhang Y, Cook JE, Fletcher JM, McQuaid A, Masters JE, Rustin MH, Taams LS, Beverley PC, Macallan DC, Akbar AN. Human CD4+ CD25hi Foxp3+ regulatory T cells are derived by rapid turnover of memory populations in vivo. J. Clin. Invest. 2006;116:2423–2433. doi: 10.1172/JCI28941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cools N, Ponsaerts P, Van Tendeloo VF, Berneman ZN. Regulatory T cells and human disease. Clin. Dev. Immunol. 2007;8:91–95. doi: 10.1155/2007/89195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weaver CT, Hatton RD. Interplay between the TH17 and Treg cell lineages: a (co-) evolutionary perspective. Nat. Rev. Immunol. 2009;9:883–889. doi: 10.1038/nri2660. [DOI] [PubMed] [Google Scholar]
- 4.Cahalan MD, Chandy KG. The functional network of ion channels in T lymphocytes. Immunol. Rev. 2009;231:59–87. doi: 10.1111/j.1600-065X.2009.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Estes DJ, Memarsadeghi S, Lundy SK, Marti F, Mikol DD, Fox DA, Mayer M. High-throughput profiling of ion channel activity in primary human lymphocytes. Anal. Chem. 2008;80:3728–3735. doi: 10.1021/ac800164v. [DOI] [PubMed] [Google Scholar]
- 6.Horwitz DA, Zheng SG, Wang J, Gray JD. Critical role of IL-2 and TGF-β in generation, function and stabilization of Foxp3(+)CD4(+) Treg. Eur. J. Immunol. 2008;38:912–915. doi: 10.1002/eji.200738109. [DOI] [PubMed] [Google Scholar]
- 7.Panyi G, Vámosi G, Bacsó Z, Bodnár A, Varga Z, Gáspár R, Mátyus L, Damjanovich S. Kv1.3 potassium channels are localized in the immunological synapse formed between cytotoxic and target cells. Proc. Natl. Acad. Sci. U. S. A. 2004;101:1285–1290. doi: 10.1073/pnas.0307421100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panyi G, Bagdány M, Bodnár A, Vámosi G, Szentesi G, Jenei A, Mátyus L, et al. Colocalization and nonrandom distribution of Kv1.3 potassium channels and CD3 molecules in the plasma membrane of human T lymphocytes. Proc. Natl. Acad. Sci. U. S. A. 2003;100:2592–2597. doi: 10.1073/pnas.0438057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicolaou SA, Szigligeti P, Neumeier L, Lee SM, Duncan HJ, Kant SK, Mongey AB, et al. Altered Dynamics of Kv1.3 Channel Compartmentalization in the Immunological Synapse in Systemic Lupus Erythematosus. J. Immunol. 2007;179:346–356. doi: 10.4049/jimmunol.179.1.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szigligeti P, Neumeier L, Duke E, Chougnet C, Takimoto K, Lee SM, Filipovich AH, Conforti L. Signalling during hypoxia in human T lymphocytes - critical role of the src protein tyrosine kinase p56Lck in the O2 sensitivity of Kv1.3 channels. J. Physiol. 2006;573:357–370. doi: 10.1113/jphysiol.2006.109967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levite M, Cahalon L, Peretz A, Hershkoviz R, Sobko A, Ariel A, Desai R, et al. Extracellular K+ and Opening of Voltage-Gated Potassium Channels Activate T Cell Integrin Function: Physical and Functional Association between Kv1.3 Channels and β1 Integrins. J. Exp. Med. 2000;191:1167–1176. doi: 10.1084/jem.191.7.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsushita Y, Ohya S, Suzuki Y, Itoda H, Kimura T, Yamamura H, Imaizumi Y. Inhibition of Kv1.3 potassium current by phosphoinositides and stromal-derived factor-1alpha in Jurkat T cells. Am. J. Physiol. Cell. Physiol. 2009;296:C1079–C1085. doi: 10.1152/ajpcell.00668.2008. [DOI] [PubMed] [Google Scholar]
- 13.Almeida AR, Legrand N, Papiernik M, Freitas AA. Homeostasis of peripheral CD4+ T cells: IL-2Rα and IL-2 shape a population of regulatory cells that controls CD4+ T cell numbers. J. Immunol. 2002;169:4850–4860. doi: 10.4049/jimmunol.169.9.4850. [DOI] [PubMed] [Google Scholar]
- 14.Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 Is Essential for TGF-beta to Convert Naive CD4+CD25− Cells to CD25+Foxp3+ Regulatory T Cells and for Expansion of These Cells. J. Immunol. 2007;178:2018–2027. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- 15.Hatakeyama M, Kono T, Kobayashi N, Kawahara A, Levin SD, Perlmutter RM, Taniguchi T. Interaction of the IL-2 receptor with the src-family kinase p56lck: identification of novel intermolecular association. Science. 1991;252:1523–1528. doi: 10.1126/science.2047859. [DOI] [PubMed] [Google Scholar]
- 16.Minami Y, Kono T, Yamada K, Kobayashi N, Kawahara A, Perlmutter RM, Taniguchi T. Association of p56lck with IL-2 receptor β chain is critical for the IL-2- induced activation of p56lck. EMBO (Eur. Mol. Biol. Organ) J. 1993;12:759–776. doi: 10.1002/j.1460-2075.1993.tb05710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salmond RJ, Filby A, Qureshi I, Caserta S, Zamoyska R. T-cell receptor proximal signaling via the Src-family kinases, Lck and Fyn, influences T-cell activation, differentiation, and tolerance. Immunol. Rev. 2009;228:9–22. doi: 10.1111/j.1600-065X.2008.00745.x. [DOI] [PubMed] [Google Scholar]
- 18.Nika K, Soldani C, Salek M, Paster W, Gray A, Etzensperger R, Fugger L, et al. Constitutively active Lck kinase in T cells drives antigen receptor signal transduction. Immunity. 2010;32:766–777. doi: 10.1016/j.immuni.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paster W, Paar C, Eckerstorf P, Jakober A, Drbal K, Schütz GJ, Sonnleitner A, Stockinger H. Genetically encoded Förster resonance energy transfer sensors for the conformation of the Src family kinase Lck. J. Immunol. 2009;182:2160–2167. doi: 10.4049/jimmunol.0802639. [DOI] [PubMed] [Google Scholar]
- 20.Haughn L, Leung B, Boise L, Veillette A, Thompson C, Julius M. Interleukin 2-mediated uncoupling of T cell receptor alpha/beta from CD3 signaling. J. Exp. Med. 1998;188:1575–1586. doi: 10.1084/jem.188.9.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bensinger SJ, Walsh PT, Zhang J, Carroll M, Parsons R, Rathmell JC, Thompson CB, et al. Distinct IL-2 receptor signaling pattern in CD4+CD25+ regulatory T cells. J. Immunol. 2004;172:5287–5296. doi: 10.4049/jimmunol.172.9.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spencer L, Sokolov Y, Li H, Takenaka B, Milici AJ, Aiyar J, Nguyen A, et al. Purification, visualization and biophysical characterization of Kv1.3 tetramers. J. Biol. Chem. 1997;272:2389–2395. doi: 10.1074/jbc.272.4.2389. [DOI] [PubMed] [Google Scholar]
- 23.Zhu J, Watanabe I, Gomez B, Thornhill WB. Determinants involved in Kv1 potassium channel folding in the endoplasmic reticulum, glycosylation in the Golgi, and cell surface expression. J. Biol. Chem. 2001;276 doi: 10.1074/jbc.M107399200. 39419–394127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.