Abstract
The detection of recombinant human growth hormone (rhGH) is difficult due to its short half-life; therefore, novel and robust biomarkers of rhGH abuse are needed. In this study, serum samples derived from subjects treated with rhGH in a randomized, double blind, placebo-controlled crossover study were analyzed by 2-DE coupled with MS. Eight healthy male subjects aged 23.2 ± 0.6 yr were injected with rhGH (2 mg/day) or saline for 7 days with serum samples drawn at days 0, 3, and 8. Protein intensities were quantified and analyzed for differences between rhGH versus placebo treatments. Protein that showed significant changes were identified and confirmed by Western blotting. These included specific isoforms of alpha-1 antitrypsin and transthyretin that increased; and inter-alpha-trypsin inhibitor heavy chain H4, apolipoprotein A-1 and hemoglobin beta chain that decreased. These proteins represent novel biomarkers of short-term rhGH exposure and may lead to a new method for detecting rhGH doping.
Keywords: 2-DE, biomarker, doping, growth hormone
1 Introduction
Recombinant human growth hormone (rhGH) is widely abused by athletes and is on the prohibited substance list by World Anti-Doping Agency (http://www.wada-ama.org/), although it remains controversial whether rhGH administration improves athletic performance [1, 2]. A relatively large dose of rhGH is used by athletes, e.g. 10–25 IU (or 3.3–8.3 mg)/day [3], which is much higher than the therapeutic dose (0.6–1.5 IU/day) used for adult GH deficiency [4].
It is difficult to detect rhGH doping because rhGH is identical to endogenous hGH and hGH has a relatively short plasma half life of about 15 – 20 min [5]. To date, there are two approaches to detect rhGH doping in blood samples- the isoform approach and the biomarker approach. hGH is composed of several forms including the most abundant 22 kDa isoform, a 20 kDa isoform and several others of varying sizes [6]. When rhGH (22 kDa isoform) is injected, a change in the ratio of 22 kDa isoform relative to the total GH concentration will happen and this is the basis for the current method in use [7]. The limitation of this approach is that the ‘window’ for detection is only about 36 hours [7]. Using this technique, no positive rhGH doping results were detected in the 2004 Athens and 2006 Turin Olympic Games. However, recently (2010) the first rhGH abuse case was identified using this method [8].
A second approach, GH-dependent biomarkers, is promising because proteins with longer half lives than GH are used. Two international studies entitled ‘The GH-2000 Project’ and ‘The GH-2004 Project’ were undertaken and confirmed insulin-like growth factor-1 (IGF-1) and procollagen type III (P-III-P) as two markers of hGH in serum [9, 10]. However, IGF- 1 and P-III-P display relatively large inter- and intra-individual variations. For example, serum samples obtained over a two- to three-week period from 1103 elite athletes have shown a within-subject coefficient of variation of nearly 21% for IGF-1 and 15% for the P-III-P [11]. Thus, other serum biomarkers for GH action would be valuable. In this regard, it is generally agreed that a combination of multiple biomarkers rather than a single marker will generate more robust and reliable results.
In this study, we used a serum proteomic approach, i.e., 2-DE coupled with MS, to identify and quantify novel biomarkers of high dose exogenous GH exposure.
2 Materials and methods
2.1 Subjects and design
Serum samples were obtained from 8 healthy young male volunteers (23.2 ± 0.6 yr old) that underwent placebo and rhGH injections in a randomized cross-over design. The study was conducted at the Good Clinical Practice Unit of Aarhus University Hospital, Denmark. Data related to substrate metabolism derived from this study have previously been published [12]. Each subject underwent two periods of 8 days in a randomized, double-blind, placebo-controlled manner: (Period I) placebo injections, (Period II) daily rhGH treatment (Norditropin SimplexX; Novo Nordisk, Copenhagen, Denmark; 2 mg sc at 10 PM, last injection on day 7). There was a 1–3 week washout period between the two study periods. Compliance was evaluated by returned vials. Serum samples, obtained at days 0, 3 and 8 for both study periods were shipped to Ohio University, USA and stored at −80 °C until proteomic analysis.
All subjects were given and signed a written informed consent before participating in the study, which was approved by the Central Denmark Region Committees on Biomedical Research Ethics (200401184) in adherence to the declaration of Helsinki. The protocol was also approved by the Ohio University Institutional Review Board.
2.2 Total serum protein concentration measurement
Serum protein concentration was measured by the Bradford method [13] using protein assay reagents from Bio-Rad (Hercules, CA).
2.3 2-DE
The method for 2-DE was described previously [14–20]. For each sample, 750 ug of serum proteins were treated for 2 h at room temperature with 8M urea, 1.8M thiourea, 4% CHAPS, 5mM reducing agent tributylphosphine, and a protease inhibitor cocktail (50X) containing 2 mM AEBSF, 1 uM Phosphoramidon, 0.2 uM aprotinin, 1 uM leupeptin, 130 uM bestatin, 10 uM pepstatin A and 14 uM E-64 (Sigma-Aldrich, Inc., St. Louis, MO). Then 15mM iodoacetamide was added for alkylation of reduced sulfur side chains. The sample was loaded onto a 17cm IPG strip with a linear pI range of 3–10 (Bio-Rad, Hercules, CA). After actively rehydration(50V) for 12 hours at 20 °C using a Protean IEF cell (Bio-Rad), the strips were subjected to first dimensional IEF at 10000V for 60000V-Hr, followed by incubation of the strips in a buffer containing 2% (w/v) SDS, 0.5M Tris/HCl (pH 6.8), 20% (v/v) glycerol for 25min. The middle section of the strip (pI 5–8) possessing the majority of proteins [21] was cut out and subjected to second dimension SDS-PAGE at a current of 25mA/gel for 250 V-Hr. Afterwards the gels were fixed overnight in a buffer containing 40% ethanol and 2% acetic acid followed by washing in 2% acetic acid. The gels were then stained with SYPRO Orange (1:5000) (Molecular Probes, Eugene, OR) for 2 h before gel images were captured using a laser-scanner Pharos FX plus (Bio-Rad) with an excitation wavelength of 488nm and an emission wavelength of 604nm.
2.4 Protein quantification and statistical analysis
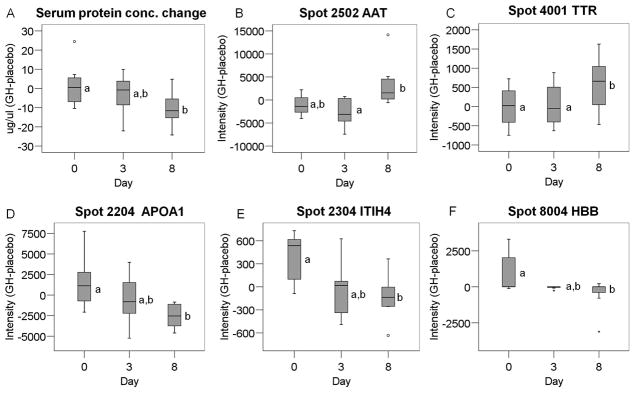
Protein spots were matched across all images using PDQuest (Bio-Rad) software and manually checked and corrected. For quantification, the intensity of each protein spot was determined based on the fluorescence signal strength, and then normalized by the total density of each image using PDQuest software. The results were then exported and analyzed using SPSS version 14·0 software (Chicago, IL). Blood samples were repeatly collected from the same eight subjects, For each protein spot, a corrected value was obtained by subtracting the spot intensity of the placebo phase from that of the rhGH treatment phases as shown in the y axis of Figure 1 [intensity (GH-placebo)]. This corrected intensity value was then subjected to one-way repeated measures ANOVA to evaluate the effect of time (day 0, 3, and 8) followed by the Tukey Test, specific for repeated measures, using software termed SigmaPlot 11.0 (San Jose, CA) with p<0.05 as a significance cutoff value. Data for total serum protein concentration were analyzed with the same statistical approach. All data were presented as box-and-whisker plot graphs.
Figure 1.
Protein level changes by rhGH treatment. A, total serum protein concentration difference between GH and placebo (GH-placebo). B–F, adjusted intensities of AAT (B), TTR (C), APOA1 (D), ITIH4 (E) and HBB (F). One-way repeated measures ANOVA followed by Tukey Test revealed a significant effect of time (p < 0.05). Different letters (a and b) denote a significant difference (p<0.05). Data are presented in box- and-whisker plots, where the box represents the interquartile range (25–75th percentile). The horizontal line in the box represents the median and the whiskers protruding from the box indicate the 5 and 95% percentile. Small circles represent outliers beyond the 1.5 x interquartile range and asterisks represent outliers beyond the 3 x interquartile range.
2.5 MS and MS/MS analysis (Performed at Protea Biosciences, Inc.)
Proteins of interest were excised manually from the SDS-PAGE gels and shipped to Protea Biosciences, Inc. (Morgantown, WV) for MS and MS/MS analyses using MALDI-TOF and MALDI-TOF-TOF. The methods were described in details previously [14–20]. Gel plugs were treated with acetonitrile and 50mM ammonium bicarbonate, then reduced and alkylated with 250mM DTT (60min / 55°C) and 650mM iodoacetamide (60min / room temperature / in the dark). Digestion was performed with 500ng trypsin in 50mM ammonium bicarbonate buffer overnight. Peptides were extracted using 5% formic acid in 50% acetonitrile (dehydration), followed by rehydration with 50mM ammonium bicarbonate. For each extraction step, the solution was aspirated, collected, and collated. Three extraction cycles (dehydration and rehydration) were performed per sample. The recovered peptides were lyophilized, reconstituted in 10mM acetic acid, and re-lyophilized to yield a purified protein digest.
After in-gel digestion by trypsin, proteins were analyzed by ABI 4800 MALDI TOF-TOF analyzer. A C18 ProteaTip was washed and then equilibrated in a 0.1% TFA / 50% acetonitrile solution followed by a 0.1% TFA / 2% acetonitrile solution. The remaining reconstituted protein digest solution in an auto sampler vial (~65% of sample) was loaded onto the C18 ProteaTip by aspirating and expelling the sample 5–10 times. The bound sample was washed twice with the 0.1% TFA / 2% acetonitrile solution by aspirating and expelling 20μL of the wash solution 5–10 times. The sample was spotted directly onto a MALDI target that was pre-spotted with 0.6μL MALDI matrix CHCA using 1μL of an elution solution (0.1% TFA / 90% acetonitrile).
For mass calibration, a plate containing a mixture of six standard peptides including des-Arg-1-Bradykinin (904.5 Da), Angiotensin (1296.7 Da), Glu-1-Fibrinopeptide B (1570.7 Da), ACTH (1–17, 2093.1 Da), ACTH (18–39, 2465.2 Da), and ACTH (7–38, 3657.9 Da) was used. In the MSMS mode, calibration was performed by analysis of the Glu-1-Fibrinopeptide B (1570.7 Da) ionization fragments.
The MALDI MS parameters used for analyses were: MS acquisition in reflector mode; positive ion mode; mass range = mass/charge (m/z) = 850 – 4000; 400 laser shots per spectrum; minimum signal/noise (S/N) = 10 for MS acquisition; 15 strongest precursors chosen for MS/MS; minimum S/N = 30 for MS/MS precursors; MALDI spot interrogated until at least 4 peaks in the MS/MS spectra achieved a S/N = 70.
2.6 Manual search in protein database for identification
MS and MS/MS data were manually submitted to MASCOT at http://www.matrixscience.com/ for protein identification. For MS data, the searching criteria were as follows: Swiss-Prot as the database; human as the species; trypsin digestion; maximum one missed cleavage; fixed carbamidomethylation of Cys; variable modifications of oxidation-M (methionine), phosphorylation (Serine, Threonine, Tyrosine), acetylation (Lysine); monoisotopic; and 50 ppm of peptide mass or parent tolerance. For MS/MS ion search, in addition to the above conditions, a peptide charge of +1, a fragment mass tolerance of 0.5 Da and instrument MALDI-TOF-TOF were used [16–20].
2.7 Western blotting
A subset of human serum proteins were subjected to 1-D and 2-D Western blotting using a standard protocol as described previously [18, 20]. For 1-D Western blotting, 50 ug of serum was loaded in each well and for 2-D Western blotting, 750 ug serum proteins were treated as for 2-DE (described above), then subjected to the blotting procedure. Primary antisera (Santa Cruz Biotechnology Inc., Santa Cruz, CA) including rabbit anti-human alpha-1 antitrypsin (AAT), goat anti-human inter-alpha-trypsin inhibitor heavy chain H4 (ITIH4), or rabbit anti-human transthyretin (TTR) were incubated with the membrane (1:1000 dilution) at 4 °C overnight. Following washing, the membrane was incubated in horseradish peroxidase (HRP)-linked secondary antibodies (1:5000) including donkey anti-goat (Santa Cruz Biotechnology), donkey anti-mouse (Santa Cruz Biotechnology) or goat anti-rabbit (Millipore, Temecula, CA) for 2 h at room temperature. The membranes were then exposed to Pierce® ECL Western blotting substrate (Thermo Scientific, Rockford, IL) for 1 min; then exposed to HyBlot CL™ autoradiography film (Denville Scientific Inc., Metuchen, NJ) for 0.5–2 min depending on the signal strength.
3 Results
3.1 Serum protein concentration
Adjusted total serum protein concentration showed a significant change by rhGH treatment (p<0.05). While concentration differences between rhGH and placebo treatments were negligible at days 0 and 3, they were significantly reduced at day 8 (Fig. 1A).
3.2 Serum protein levels which were significantly altered by rhGH treatment
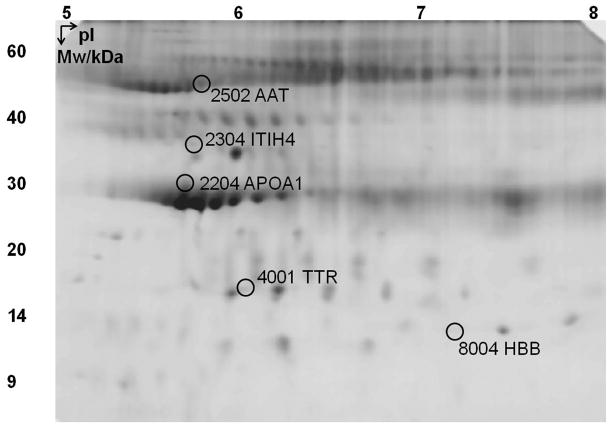
A total of 94 protein spots were separated by 2-DE and subsequently analyzed. After adjusting protein intensity (intensity of GH – phase intensity of placebo phase to control for baseline levels), five spots showed significant differences as a function of time (day 0, day 3, and day 8; p<0.05), indicated by circles in Fig. 2. General information concerning these proteins, including MS, MS/MS scores, molecular weight (Mw) and pIs are listed in Table S1 and Figs S1–S5 in the on-line supporting information. Among these, AAT (spot 2502, Fig. 1B) and TTR (spot 4001, Fig. 1C) did not change from day 0 to day 3 but increased at day 8. Apolipoprotein A-1 (APOA1, spot 2204, Fig. 1D), ITIH4 (spot 2304, Fig. 1E) and hemoglobin beta (HBB, spot 8004, Fig.1F) showed a significant decrease at day 8. The corrected intensity values of these proteins are listed in Table S2 in the supplementary information.
Figure 2.
A representative image of 2-DE gel. Spots in circles were significantly altered by rhGH treatment (p<0.05). Each spot was assigned a unique number by PDQuest, for example, spot 2502 corresponds to AAT. AAT: alpha-1 antitrypsin; ITIH4: inter-alpha-trypsin inhibitor heavy chain H4; APOA1: apolipoprotein A-1; TTR: transthyretin; HBB: hemoglobin beta chain.
3.3 Western blotting confirmation
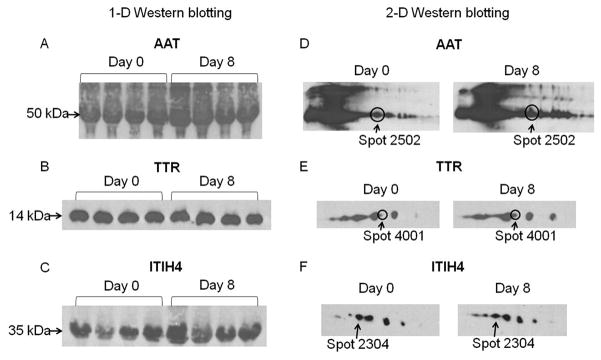
AAT, TTR and ITIH4 were confirmed by Western blotting using samples from day 0 and day 8 of rhGH treatment. The total levels of these proteins were not different between day 0 and day 8, as shown by 1-D Western blotting (Fig. 3A–C). However, each of these proteins was resolved as multiple spots on a 2-D gel, which we have called ‘isoforms’. The specific isoforms identified by 2-DE showed the same trend of change with rhGH treatment when analyzed by 2-D Western blotting (Fig. 3D–F). In the train of spots recognized as AAT, one corresponding to spot 2502 appeared more intense at day 8 compared to day 0 (Fig. 3D). Similarly, among the seven TTR isoforms, one corresponding to spot 4001 was increased in intensity at day 8 compared to day 0 (Fig. 3E). Finally, among the seven isoforms of ITIH4, one corresponding to spot 2304 appeared less intense at day 8 than day 0 (Fig. 3F).
Figure 3.
Western blotting of AAT, TTR and ITIH4. A–C, 1-D Western blotting of AAT, TTR and ITIH4. Serum samples from the same four subjects at day 0 and day 8 of GH treatment phase were loaded and immunoblotted with antibodies against AAT, TTR and ITIH4. D–F, 2-D Western blotting of AAT, TTR and ITIH4. The isoform corresponding to the one identified by 2-DE was circled and/or indicated by an arrow.
4 Discussion
In order to discover novel biomarkers of GH action in humans, we employed a proteomic approach to analyze serum proteins in healthy young male volunteers that underwent a cross-over treatment with rhGH and placebo. A previous study has shown these subjects to have significantly elevated levels of serum IGF-1 after rhGH treatment [12] validating the efficacy of GH action.
IGF-1 is a well-known biomarker induced by GH [22]. In this regard, ~75–80 % of serum IGF-1 is derived from liver [23], a target of GH action. The liver also has relatively high levels of GH receptors. Additionally, many serum proteins are secreted by liver. We hypothesize that the levels of liver derived serum proteins would change upon rhGH administration since the liver is one of the main targets of GH action. Indeed, we have found four liver-derived proteins (AAT, TTR, ITIH4 and APOA1) to be altered by rhGH treatment.
Upon rhGH administration, the total serum protein concentration decreased significantly after 8 days of treatment (Fig. 1), which is compatible with the well known fluid retaining effect of GH [24]. GH promotes fluid retention by increasing both extracellular water and plasma volume [25] via stimulation of renin-angiotensin-aldosterone system, which is central to fluid homeostasis. To correct for this GH dependent protein concentration decrease, we loaded equal amounts of protein when performing 2-DE.
Since albumin isoforms often result in a ‘smear’ in the region above 50 kDa when analyzed by 2-DE [26], we used a relatively high concentration (15%) acrylamide gel to resolve proteins smaller than 50 kDa. The limitation of this approach was that resolution of proteins greater than 50 kDa was lost, resulting in a decrease in total identifiable proteins. However, this approach did allow for a rapid and reproducible screening system to identify relatively small protein biomarkers from a ‘subset’ of serum proteins.
The protein spots identified as AAT, TTR, ITIH4, APOA1 and HBB in this study have also been reported in the SWISS 2DPAGE database (http://ca.expasy.org/ch2d/) and are known to exist in human serum as multiple isoforms (differing in Mw and/or pI). These isoforms are likely the result of PTMs that alter the protein size and/or charge. Each protein described in this study showed only one isoform to be significantly altered by rhGH treatment. The chemical identification of the PTMs was (and is) difficult as we have tried the multiple variable PTM algorithm in MASCOT and failed to detect any modified tryptic peptide fragments In addition, we have also used the commercial software termed PEAKS 5.2 (Bioinformatics Solutions, Inc.), and could not identify any PTMs. Since only the 15 most prominent precursor peptides from the MS spectrum are selected for MS/MS, it is possible that peptides with the actual PTMs were not selected for MS/MS; therefore, no PTMs were detected. In future studies, PTM identification will continue to be addressed.
We also performed 2-D Western blotting, which confirmed the identification of AAT, ITIH4 and TTR, and observed their relative levels of expression from day 0 to day 8 during rhGH treatment. The changes of the isoforms identified by 2-DE were consistent with those shown by 2-D Western blotting. The constant levels of the remaining isoforms served as an internal control in 2-D Western blotting. Since only one out of multiple isoforms (identified by 2-D Western blotting) was observed to change for all of these proteins, it is unlikely that the total levels would be different. Indeed we found no difference in total levels of AAT, TTR and ITIH4 by 1-D Western blotting. This demonstrates that a specific isoform, rather than total level of a given protein, responds to short-term rhGH.
We found one isoform of AAT significantly increased at day 8 with rhGH treatment. AAT is a serine protease inhibitor in plasma. It is also an acute phase protein that is secreted in large amounts by the liver in response to acute infection or injury [27]. AAT expression in the liver responds to GH in both rats and humans [28, 29], and GH-deficiency is often associated with deficiency in AAT [30]. The one isoform of AAT that significantly increased at day 8 with rhGH treatment is consistent with these findings.
One isoform of APOA1 was significantly down-regulated at day 8 of rhGH treatment. This result is consistent with a previous 2-DE based study which found another isoform of APOA1 being decreased by short-term treatment of a GH releasing hormone analogue [16]. However, a third isoform of APOA1 identified by 2-DE was found to be decreased after surgical treatment of acromegalic patients (from abnormally high to normal GH activity) [20]. The discrepancy may be a result of chronic high levels of GH in a neoplastic disease state (acromegaly) versus short-term exogenous rhGH exposure in healthy humans. However, the fact that different isoforms of APOA1 are changing in different ‘states’ of GH action may have future implications when considering APOA1 as a common biomarker of GH excess.
In the present study, we found one isoform of HBB to be decreased by rhGH treatment. HBB and hemoglobin α-chain (HBA) are subunits of hemoglobin, the major protein in red blood cells. GH deficient children have lower levels of hemoglobin, which is increased by rhGH treatment [31]. Two different HBB isoforms by 2-DE have been shown to be up-regulated by increased GH activity [16, 20]. Hemoglobin α-chain is also shown to be increased in response to rhGH using SELDI-TOF MS technique [32]. The isoform of HBB that decreased following rhGH treatment is a different one compared to those found in other studies [16, 20]; thus, the combined results suggest isoform-specific HBB changes induced by GH action.
In conclusion, we found that high dose rhGH administration significantly reduced total serum protein concentrations and up- or down-regulated specific isoforms of five serum proteins. These protein isoforms may serve as potential biomarkers of rhGH treatment including when rhGH is mis-used and abused. Certainly, more studies are required including both genders in larger populations treated with rhGH for a variety of time intervals before these biomarkers can be tested in an athletic doping scenario.
Supplementary Material
Acknowledgments
We thank Dr. Brian McCarthy for statistical advice and Britt Christensen and Dr. Lucila Sackmann-Sala for additional statistical assistance. This work was supported in part by a grant from World Anti-Doping Agency to JOJ and JJK. JJK also is supported by the State of Ohio’s Eminent Scholar’s Program that includes a gift by Milton and Lawrence Goll and by the following grants: National Institute of Health (NIH) R15DK075436, NIH R01AG019899, and 1P01AG031736–01A1.
Abbreviations
- APOA1
apolipoprotein A-1
- AAT
alpha-1 antitrypsin
- HBB
hemoglobin beta chain
- IGF-1
insulin-like growth factor-1
- ITIH4
inter-alpha-trypsin inhibitor heavy chain H4
- P-III-P
procollagen type III
- rhGH
recombinant human growth hormone
- TTR
transthyretin
Footnotes
Conflict of interest statement
JOJ receives lecture fees from Ipsen and research grants from Ipsen and Pfizer. The other authors declare no conflict of interest.
References
- 1.Rubeck KZ, Bertelsen S, Vestergaard P, Jorgensen JO. Impact of GH substitution on exercise capacity and muscle strength in GH-deficient adults: a meta-analysis of blinded, placebo-controlled trials. Clin Endocrinol (Oxf) 2009;71:860–866. doi: 10.1111/j.1365-2265.2009.03592.x. [DOI] [PubMed] [Google Scholar]
- 2.Meinhardt U, Nelson AE, Hansen JL, Birzniece V, et al. The Effects of Growth Hormone on Body Composition and Physical Performance in Recreational Athletes. Ann Intern Med. 2010;152:568–577. doi: 10.7326/0003-4819-152-9-201005040-00007. [DOI] [PubMed] [Google Scholar]
- 3.Saugy M, Robinson N, Saudan C, Baume N, et al. Human growth hormone doping in sport. Br J Sports Med. 2006;40(Suppl 1):i35–i39. doi: 10.1136/bjsm.2006.027573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ho KKY. Consensus guidelines for the diagnosis and treatment of adults with GH deficiency II: a statement of the GH Research Society in association with the European Society for Pediatric Endocrinology, Lawson Wilkins Society, European Society of Endocrinology, Japan Endocrine Society, and Endocrine Society of Australia. Eur J Endocrinol. 2007;157:695–700. doi: 10.1530/EJE-07-0631. [DOI] [PubMed] [Google Scholar]
- 5.Holl RW, Schwarz U, Schauwecker P, Benz R, et al. Diurnal variation in the elimination rate of human growth hormone (GH): the half-life of serum GH is prolonged in the evening, and affected by the source of the hormone, as well as by body size and serum estradiol. J Clin Endocrinol Metab. 1993;77:216–220. doi: 10.1210/jcem.77.1.8325945. [DOI] [PubMed] [Google Scholar]
- 6.Baumann GP. Growth hormone isoforms. Growth Horm IGF Res. 2009;19:333–340. doi: 10.1016/j.ghir.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Wu Z, Bidlingmaier M, Dall R, Strasburger CJ. Detection of doping with human growth hormone. Lancet. 1999;353:895. doi: 10.1016/S0140-6736(99)00775-8. [DOI] [PubMed] [Google Scholar]
- 8.Travis J. Growth Hormone Test Finally Nabs First Doper. Science. 2010;327:1185. doi: 10.1126/science.327.5970.1185. [DOI] [PubMed] [Google Scholar]
- 9.Dall R, Longobardi S, Ehrnborg C, Keay N, et al. The effect of four weeks of supraphysiological growth hormone administration on the insulin-like growth factor axis in women and men. GH-2000 Study Group. The Journal of clinical endocrinology and metabolism. 2000;85:4193–4200. doi: 10.1210/jcem.85.11.6964. [DOI] [PubMed] [Google Scholar]
- 10.Longobardi S, Keay N, Ehrnborg C, Cittadini A, et al. Growth hormone (GH) effects on bone and collagen turnover in healthy adults and its potential as a marker of GH abuse in sports: a double blind, placebo-controlled study. The GH-2000 Study Group. J Clin Endocrinol Metab. 2000;85:1505–1512. doi: 10.1210/jcem.85.4.6551. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen TV, Nelson AE, Howe CJ, Seibel MJ, et al. Within-subject variability and analytic imprecision of insulinlike growth factor axis and collagen markers: implications for clinical diagnosis and doping tests. Clin Chem. 2008;54:1268–1276. doi: 10.1373/clinchem.2008.105726. [DOI] [PubMed] [Google Scholar]
- 12.Krag MB, Gormsen LC, Guo Z, Christiansen JS, et al. Growth hormone-induced insulin resistance is associated with increased intramyocellular triglyceride content but unaltered VLDL-triglyceride kinetics. Am J Physiol Endocrinol Metab. 2007;292:E920–927. doi: 10.1152/ajpendo.00374.2006. [DOI] [PubMed] [Google Scholar]
- 13.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 14.List EO, Berryman DE, Palmer AJ, Qiu L, et al. Analysis of mouse skin reveals proteins that are altered in a diet-induced diabetic state: a new method for detection of type 2 diabetes. Proteomics. 2007;7:1140–1149. doi: 10.1002/pmic.200600641. [DOI] [PubMed] [Google Scholar]
- 15.Qiu L, List EO, Kopchick JJ. Differentially expressed proteins in the pancreas of diet-induced diabetic mice. Mol Cell Proteomics. 2005;4:1311–1318. doi: 10.1074/mcp.M500016-MCP200. [DOI] [PubMed] [Google Scholar]
- 16.Sackmann-Sala L, Ding J, Frohman LA, Kopchick JJ. Activation of the GH/IGF-1 axis by CJC-1295, a long-acting GHRH analog, results in serum protein profile changes in normal adult subjects. Growth Horm IGF Res. 2009;19:471–477. doi: 10.1016/j.ghir.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okada S, List EO, Sankaran S, Kopchick JJ. Plasma Protein Biomarkers Correlated with the Development of Diet-Induced Type 2 Diabetes in Mice. Clin Proteomics. 2010;6:6–17. doi: 10.1007/s12014-009-9040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding J, Kopchick JJ. Plasma biomarkers of mouse aging. Age (Dordr) 2010 doi: 10.1007/s11357-010-9179-z. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christensen B, Sackmann-Sala L, Cruz-Topete D, Jorgensen JO, et al. Novel serum biomarkers for erythropoietin use in humans: a proteomic approach. J Appl Physiol. 2011;110:149–156. doi: 10.1152/japplphysiol.00665.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cruz-Topete D, Christensen B, Sackmann-Sala L, Okada S, et al. Serum proteome changes in acromegalic patients following transsphenoidal surgery: novel biomarkers of disease activity. Eur J Endocrinol. 2011;164:157–167. doi: 10.1530/EJE-10-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.List EO, Berryman DE, Palmer AJ, Gosney E, et al. Application of bioinformatics and scalable computing to perform proteomic analysis of stomach tissue from diabetic mice. Scalable Computing: Practice and Experience. 2007;8:173–183. [Google Scholar]
- 22.Daughaday WH, Rotwein P. Insulin-like growth factors I and II. Peptide, messenger ribonucleic acid and gene structures, serum, and tissue concentrations. Endocr Rev. 1989;10:68–91. doi: 10.1210/edrv-10-1-68. [DOI] [PubMed] [Google Scholar]
- 23.Yakar S, Liu JL, Fernandez AM, Wu Y, et al. Liver-specific igf-1 gene deletion leads to muscle insulin insensitivity. Diabetes. 2001;50:1110. doi: 10.2337/diabetes.50.5.1110. [DOI] [PubMed] [Google Scholar]
- 24.Moller J, Frandsen E, Fisker S, Jorgensen JO, Christiansen JS. Decreased plasma and extracellular volume in growth hormone deficient adults and the acute and prolonged effects of GH administration: a controlled experimental study. Clin Endocrinol (Oxf) 1996;44:533–539. doi: 10.1046/j.1365-2265.1996.728550.x. [DOI] [PubMed] [Google Scholar]
- 25.Moller J. Effects of growth hormone on fluid homeostasis. Clinical and experimental aspects. Growth Horm IGF Res. 2003;13:55–74. doi: 10.1016/s1096-6374(03)00011-x. [DOI] [PubMed] [Google Scholar]
- 26.Ding J, List EO, Okada S, Kopchick JJ. Perspective: Proteomic approach to detect biomarkers of human growth hormone. Growth Horm IGF Res. 2009;19:399–407. doi: 10.1016/j.ghir.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weeke B, Jarnum S. Serum concentration of 19 serum proteins in Crohn’s disease and ulcerative colitis. Gut. 1971;12:297–302. doi: 10.1136/gut.12.4.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwarzenberg SJ, Sharp HL, Berry SA, Manthei RD, Seelig S. Hormonal regulation of serum alpha 1-antitrypsin and hepatic alpha 1-antitrypsin mRNA in rats. Biochem Biophys Res Commun. 1987;147:936–941. doi: 10.1016/s0006-291x(87)80160-2. [DOI] [PubMed] [Google Scholar]
- 29.Schwarzenberg SJ, Sharp HL, Freier EF, Seelig S. Response of serine antiproteases to growth hormone therapy in growth hormone deficient children. Horm Res. 1989;31:221–225. doi: 10.1159/000181120. [DOI] [PubMed] [Google Scholar]
- 30.Schydlower M, Waxman SH, Patterson PH. Coexistence of deficiency in alpha1 antitrypsin and in growth hormone. N Engl J Med. 1979;300:366. doi: 10.1056/NEJM197902153000715. [DOI] [PubMed] [Google Scholar]
- 31.Eugster EA, Fisch M, Walvoord EC, DiMeglio LA, Pescovitz OH. Low hemoglobin levels in children with in idiopathic growth hormone deficiency. Endocrine. 2002;18:135–136. doi: 10.1385/ENDO:18:2:135. [DOI] [PubMed] [Google Scholar]
- 32.Chung L, Clifford D, Buckley M, Baxter RC. Novel biomarkers of human growth hormone action from serum proteomic profiling using protein chip mass spectrometry. J Clin Endocrinol Metab. 2006;91:671–677. doi: 10.1210/jc.2005-1137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.