Abstract
The Src-family tyrosine kinase Lyn negatively regulates BCR signaling and also myeloid cell activity. Mice deficient in Lyn have substantially decreased numbers of peripheral B cells, despite spontaneously producing IgG anti-DNA antibodies. We have examined the mechanism underlying the B cell depletion in these mice. Lyn-deficient B cells were out-competed by wild type B cells in mixed bone marrow chimeras at two steps, at the T1 to T2 transitional maturation stage in the spleen and again between the T2 or T3 stage and the mature follicular B cell population. Lyn-deficient T2 and follicular B cells expressed elevated levels of the pro-apoptotic factor Bim and deletion of Bim restored splenic B cells of Lyn-deficient mice to close to wild type numbers. Lyn-deficient T2 and later stage B cells also had changes in cell surface phenotype consistent with increased in vivo BCR signaling. Similarly, an increased proportion of T2 and follicular B cells had elevated basal intracellular free calcium levels. Taken together, these observations suggest that increased BCR signaling is responsible for increased death of weakly self-reactive Lyn-deficient B cells at the T2 stage and additionally as these cells mature to follicular B cells.
Keywords: Lyn, B cell tolerance, Bim, Akt
Introduction
Human autoimmune diseases are thought to result from environmental exposures encountered by a person with a genetic susceptibility. Genetic analysis of autoimmune susceptibility in humans and in autoimmunity prone strains of mice have identified a number of genetic loci contributing to disease in both species, although in all but a few cases the precise genes and molecular pathways responsible remain poorly characterized [1, 2]. Murine models have greatly enhanced the understanding of the molecular pathways that lead to organ-specific and systemic autoimmune disease, particularly through the examination of mice with loss of function alleles or enhanced function alleles of known genes. Defects in genes controlling T cell tolerance typically lead to organ-specific autoimmunity [3, 4] , whereas a variety of other genetic defects lead to systemic autoimmunity that resembles human systemic lupus erythematosus (SLE), including a duplication of TLR7 [5, 6] , an increased function allele of CD45 [7] , and loss of function alleles of negative regulators of B cell antigen receptor (BCR) signaling, including FcγRIIb, SHP-1 and Lyn [8–10].
Lyn is a Src-family tyrosine kinase, which, along with two other Src-like kinases, Blk and Fyn, is necessary for BCR signaling due to their role in phosphorylation of the ITAM motifs on Igα/Igβ (CD79a/CD79b). Tyrosine phosphorylated Igα/Igβ recruits Syk via the latter’s tandem SH2 domains and leads to downstream signaling events [11]. While this positive signaling function of Lyn is redundant with the functions of Fyn and Blk [12], biochemical and genetic evidence has demonstrated that Lyn has a non-redundant function to provide feedback inhibition of BCR signaling by phosphorylation of cell surface proteins containing immunoreceptor tyrosine-based inhibitory motifs (ITIMs). These ITIM-containing inhibitory receptors include the inhibitory Fc receptor for IgG, FcγRIIb, and a sialic acid-binding protein expressed on B cells, CD22 [10]. Phosphorylation of these ITIMs generates binding sites for the recruitment to the membrane of phosphatases that inhibit BCR signaling, including the SH2-domain-containing inositol phosphatase (SHIP-1) and the SH2-domain-containing tyrosine phosphatase (SHP-1). The net result of Lyn-deficiency is exaggerated signaling by the BCR, a phenotype that is moderate in immature B cells and highly pronounced in mature follicular B cells [13]. Lyn also functions to inhibit receptor signaling in myeloid cells , and recent studies demonstrate that hyperactivity of myeloid cells contributes importantly to autoimmunity in Lyn−/− mice [14]. Lyn-deficiency in B cells as well as in myeloid cells is required to get spontaneous production of IgG anti-DNA antibodies, a major hallmark of human SLE [14]. Interestingly, recent data indicate that genetic alterations in the Lyn-CD22-SHP-1 inhibitory pathway of B cells contribute to susceptibility to autoimmunity in humans as well as mice. For example, a polymorphism in the Lyn gene has been associated with decreased risk for development of SLE[15], and loss-of-function mutations in sialic acid acetylesterase (SIAE), an enzyme which generates the sialic acid ligand for CD22 and appears to enable the latter molecule’s inhibitory function, are enriched by nine-fold in individuals with a variety of autoimmune diseases, including SLE [16].
As BCR signaling contributes to central and peripheral B cell tolerance induction as well as to B cell activation, it is not clear why enhanced BCR signaling in Lyn−/− mice should lead to production of lupus-like autoantibodies. Previously, evidence has been presented suggesting that receptor editing in the bone marrow, a major central tolerance mechanism, is not decreased and may be slightly increased in Lyn−/− mice. In the experiments presented here, we have examined the effect of Lyn-deficiency on B cell development and survival in the spleen to better understand how loss of Lyn affects B cell peripheral tolerance mechanisms that are believed to restrain autoantibody production. We observed that Lyn-deficient B cells exhibit a substantial cell-intrinsic competitive disadvantage relative to wild type B cells at both the transitional T2 stage in the spleen and later at the T2 or T3 to follicular B cell transition. The decrease in the numbers of Lyn-deficient B cells in the spleen was found to be largely but not completely Bim-dependent, suggesting that Lyn−/− B cells have defects in survival rather than in developmental maturation per se. Moreover, Lyn-deficient B cells had increased levels of Bim and cell surface phenotypic changes consistent with enhanced in vivo BCR signaling. Thus, it appears that Lyn−/− self-reactive B cells have enhanced BCR signaling and greater clonal deletion of self-reactive clones as they mature in the spleen. The autoimmunity seen in Lyn-deficient mice may therefore result from a breakdown in tolerance of self-reactive anergic B cells rather than a failure to induce tolerance initially.
Results
Lyn-deficient mice exhibit a B cell-intrinsic decrease in the numbers of different splenic B cell subpopulations
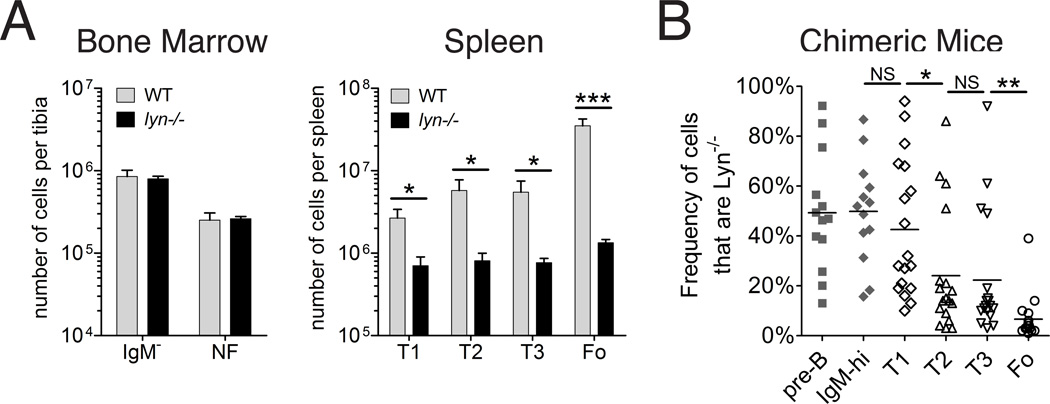
Previous studies have demonstrated that Lyn-deficient mice have reduced numbers of splenic immature and mature B cells[17–20]. To better understand the nature of this defect, we started by measuring the numbers of the different immature and mature B cells subpopulations found in the bone marrow and spleen. We found, in agreement with previous reports [18, 19, 21], that Lyn-deficient mice have a substantially reduced number of mature follicular B cells (Figure 1A). There were also significantly decreased numbers of all three of the transitional B cell types in the spleen (T1, T2, and T3)[19], but numbers of newly formed bone marrow B cells expressing IgM were similar in lyn−/− and wild-type mice (Figure 1A). Defects in the maturation and/or survival of Lyn-deficient B cells were clearly evident also in mixed bone marrow chimeras created with 75% Lyn−/− bone marrow and 25% wild type bone marrow (Figure 1B). In this circumstance, both Lyn-deficient and wild type B cells are present in the same mice with the same environmental influences, so any changes in the ratio of the two genotypes reflects a cell-intrinsic effect of the Lyn-deficiency directly on the B cells. In these mixed bone marrow chimeras, Lyn−/− B cells had a considerable competitive disadvantage at the T2 stage and again at the mature follicular B cell stage, representing an overall average 7-fold decrease in the frequency of Lyn−/− follicular B cells compared to what was seen at the pre-B cell stage in the bone marrow. These results demonstrate that Lyn-deficient B cells exhibit a cell-intrinsic defect in maturation and/or survival in the spleen.
FIGURE 1. Effect of Lyn-deficiency on different B cell subpopulations.
(A) Shown are the numbers of bone marrow IgM− pro and pre B cells (B220+, AA4+, CD23−, IgM−) and newly formed (NF) B cells (B220+, AA4+, CD23−, IgM++) per mouse tibia (left panel), and transitional (T1, T2 and T3) and mature follicular B cells in the spleen (right panel) of wild type and Lyn-deficient young adult mice, as determined by flow cytometry using AA4, CD23 and IgM levels to distinguish these populations. WT (gray bars) and Lyn−/− (black bars) mice. n=4 per group; similar results obtained from multiple other experiments; bars represent mean ± std dev; * indicates P<0.01 and *** indicates P<0.0001 (unpaired t-test). (B) Percentages of B cells that are Lyn−/− in different bone marrow (n=13) and splenic (n=18) populations from mice reconstituted with a mixture of 80% Lyn−/− CD45.1 bone marrow cells and 20% wild type (CD45.2) bone marrow cells. The two genotypes were distinguished with the allelic difference at CD45. CD45.1 was detected with biotinylated monoclonal antibody A20 and CD45.2 was detected with pacific blue-labeled monoclonal antibody 104. Data from individual mice are shown; the means are shown by horizontal bars; B cell populations described above from bone marrow (solid gray symbols) and spleen (open symbols); *indicates P<0.01, and ** indicates P<0.001 (paired t-test). This experiment was repeated once with similar results.
Elevated expression of Bim in Lyn-deficient B cells
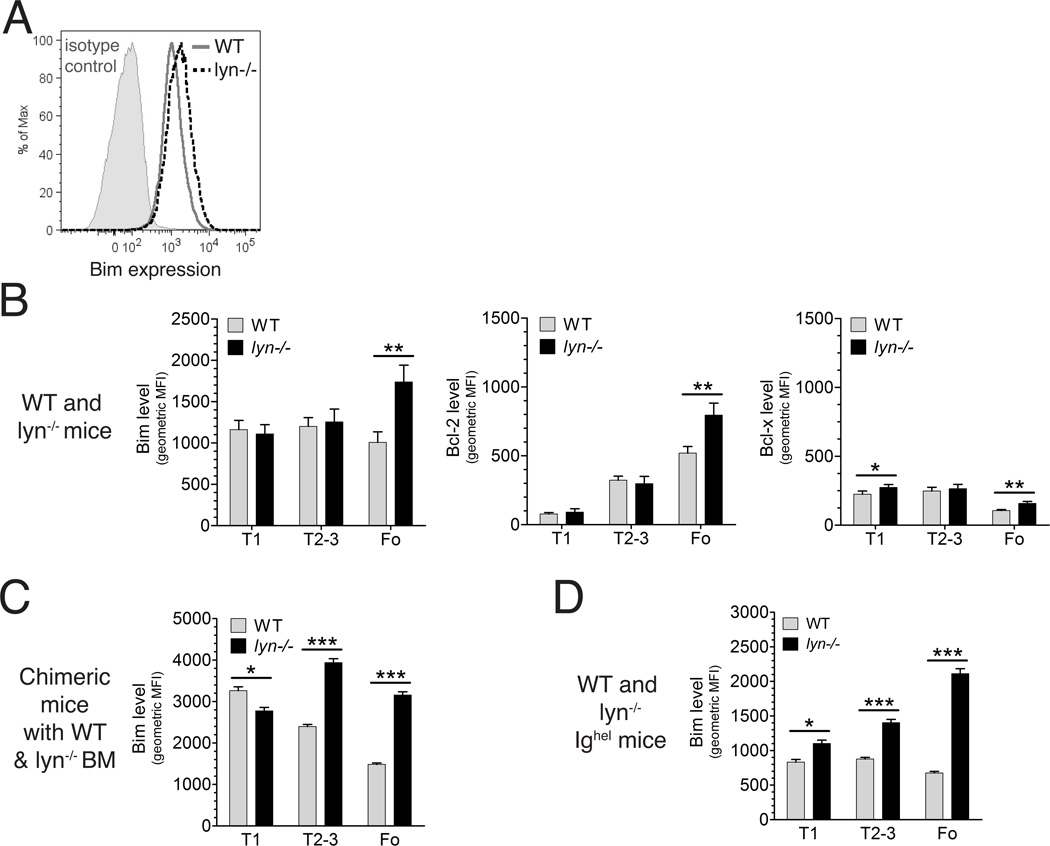
Recent studies indicate that over 50% of B cells developing in the bone marrow are initially self-reactive and that there are multiple checkpoints in the bone marrow and in the periphery that edit, delete, or anergize these self-reactive B cells [22] . Among these checkpoints are the antigen-induced clonal deletion and clonal anergy of self-reactive transitional T1 and T2 cells in the spleen [23, 24]. Death of self-reactive B cells in the spleen appears to be partially dependent on Bim, a BH3-only protein that antagonizes the pro-survival activity of Bcl-2 and related survival factors in the cell [25]. Therefore, we measured the level of Bim in splenic B cell populations by intracellular staining with a Bim-specific antibody. As shown in Figure 2, while intracellular Bim levels decreased somewhat as B cells matured in the spleen in wild type mice, Bim levels increased in splenic Lyn-deficient B cells as they matured, with the result that average Bim levels were elevated in Lyn−/− follicular B cells compared to their wild type counterparts (Figure 2B). We also examined Bim expression in mixed bone marrow chimeric mice , which would equalize any environmental contributions due to the myeloid cells hyperactivity of Lyn−/− mice [14]. In the mixed bone marrow chimeric mice, Bim was more strongly elevated in Lyn−/− B cells compared to their wild type counterparts at both the immature T2/T3 stages and at the mature follicular stage. Whereas the difference in Bim levels between wild type and Lyn-deficient follicular B cells was similar to what was seen in non-chimeric mice, the elevation seen in Lyn-deficient T2/T3 transitional B cells in chimeric mice was not present in the non-chimeric mice. The Lyn−/− mice are known to have elevated levels of BAFF, a major survival factor for B cells [14], and this may account for the environmental effect on Bim levels of T2/T3 B cells [26]. The increase in Bim levels resulting from Lyn-deficiency was also seen in MD4 IgHEL transgenic [27] T2+T3 and follicular B cells, and the degree of Bim elevation was similar to that seen in the mixed bone marrow chimeric mice (Figure 2C). The IgHEL transgenic BCR is not overtly self-reactive, but may have a low level of cross-reactivity to one or more self antigens, as suggested by previous studies in which the magnitude of BCR signaling was modulated by genetic mutations in regulators of signaling [28], including Lyn [20]. In any case, the elevated levels of Bim in Lyn-deficient B cells likely contributed to the selective disadvantages seen for the T2, T3, and mature follicular B cell subpopulations in the competitive reconstitution experiment described above [29, 30].
FIGURE 2. Altered levels of Bim, Bcl-2 and Bcl-X in Lyn-deficient B cells.
(A)Representative intracellular staining of Bim in total splenic B cells of a wild type (solid gray line) and a Lyn−/− (dashed black line) mouse, as well as staining with an isotype control (solid gray histogram) is shown. Geometric mean fluorescence of intracellular Bim (B–D), Bcl-2 (B) and Bcl-x (B) in different splenic B cell subpopulations from wild type (n=5) and Lyn−/− (n=5) mice (B), in wild type and Lyn−/− splenic B cell populations from mice reconstituted with a mixture of 75% Lyn−/− bone marrow cells and 25% wild type bone marrow cells (n=7 or 8 per group) (C) and in splenic B cell populations from IgHEL transgenic (n =5) and IgHEL transgenic Lyn−/− (n=4) mice (D). WT (gray bars) and Lyn−/− (black bars); bars represent mean ± std dev; * indicates P<0.01, ** indicates P<0.001, *** indicates P<0.0001 (unpaired t-test) The data in panels C and D were from single experiments with the indicated numbers of mice. The data in A and B were representative of several independent experiments. Note, data represented in panels B, C and D were obtained on different days and therefore MFI values from cannot be compared between different panels.
We also assessed the intracellular levels of two major pro-survival Bcl-2 family members, Bcl-2 and Bcl-X. Interestingly, the levels of Bcl-2 and to a lesser extent Bcl-X were increased somewhat in Lyn−/− follicular B cells (Figure 2B). As only viable cells were analyzed, higher levels of these factors may have been required to keep alive the Lyn-deficient B cells, which expressed elevated levels of Bim. Alternatively, the elevated BAFF levels of Lyn−/− mice may contribute to the increased level of Bcl-2 in the Lyn-deficient B cells. In agreement with the latter possible explanation, the level of Bcl-2 was increased in the Lyn−/− follicular B cells from mixed bone marrow chimeric mice, but the magnitude was less pronounced, despite greater elevation in Bim (data not shown).
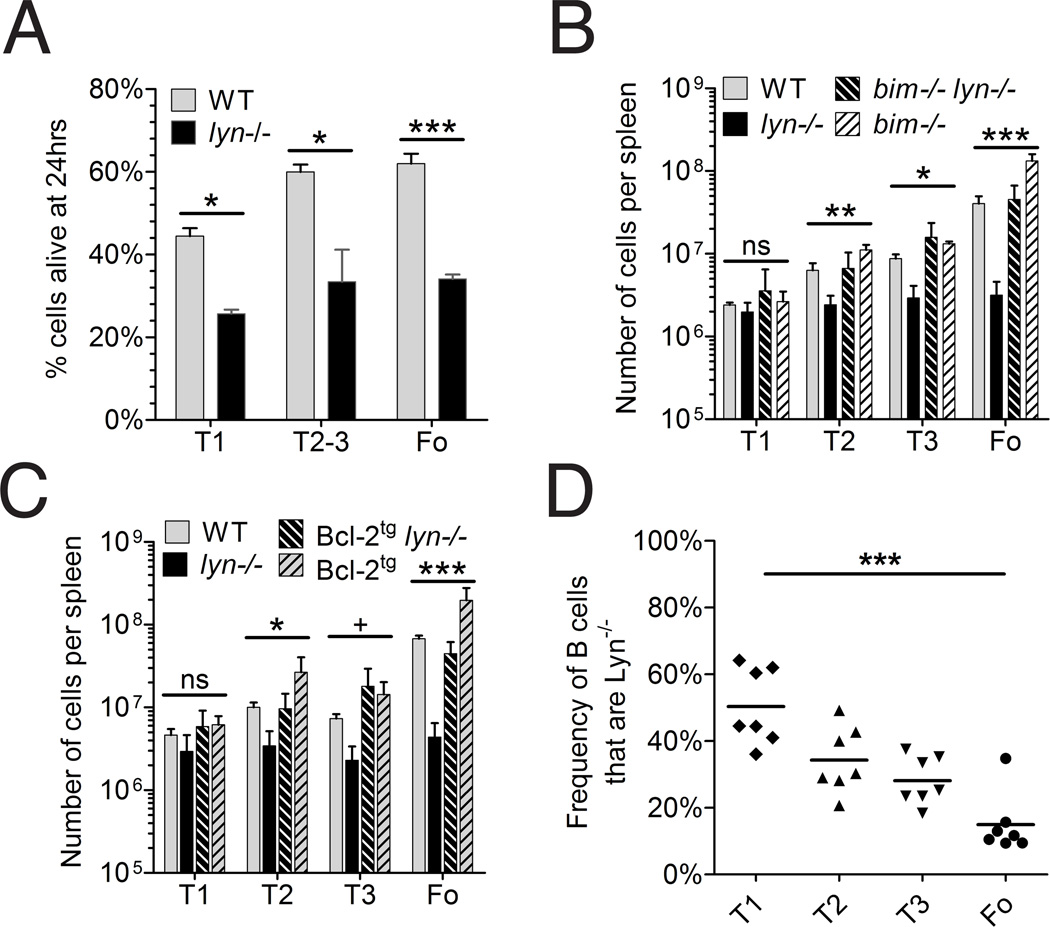
Because Lyn-deficient B cells competed poorly with wild-type B cells in vivo, and expressed elevated levels of Bim, we wondered whether Lyn-deficient B cells were more prone to cell death than wild-type B cells. To investigate this, we sorted populations of splenic B cells from lyn−/− and WT mice, and measured the frequency of surviving cells after 24 hours of culture in vitro. As shown in Figure 3A, Lyn-deficient transitional and follicular B cells were twice as likely to die after 24 hours in vitro compared to WT follicular B cells. These data support the possibility that the reduced numbers of follicular B cells seen in Lyn-deficient mice are a consequence of poorer survival of these cells due to increased Bim levels.
FIGURE 3. Depletion of splenic B cell subpopulations in Lyn-deficient mice is rescued by deletion of Bim or by transgenic expression of Bcl-2 in B cells.
(A) Survival in vitro of cultured unstimulated splenic B cell populations. Splenic B cell populations from wild type or Lyn-deficient mice were isolated by cell sorting and were cultured for 24 hr in 10% FCS-containing medium, after which the numbers of viable cells were counted by flow cytometry. WT (gray bars) and Lyn−/− (black bars); bars represent mean ± std dev of 3 or 4 replicates per group except for Lyn−/− T1 cells which had only 2 replicates; * P<0.01, *** P<0.0001 (unpaired t-test). (B) Numbers of T1, T2, T3 and follicular B cells from the spleens of wild type (solid gray bars, n=3), Lyn−/−(solid black bars, n=5), Bim−/− (hatched gray bars, n=5), and Bim−/− Lyn−/− (hatched black bars, n=6) mice. (C) Numbers of T1, T2, T3 and follicular B cells from the spleens of wild type (solid gray bars, n=4), Lyn−/− (solid black bars, n=5), Bcl-2 transgenic (hatched gray bars, n=3), and Bcl-2 transgenic Lyn−/− (hatched black bars, n=4) mice. Each experiment was repeated once with similar results. (D) Ratio of the numbers of CD45.2 Lyn−/− Bcl-2 transgenic and CD45.1/.2 Lyn+/+ Bcl-2 transgenic cells from splenic T1, T2, T3 and follicular (FO) B cells from 7 mixed bone marrow chimeric mice generated from a 50%/50% mixture of bone marrow cells from the two donor genotypes into lethally irradiated CD45.1 recipients. In this experiment, the recipient mice were CD45.1, the Lyn-expressing cells were CD45.1/D45.2 heterozygotes, and Lyn-deficient cells were CD45.2. CD45.1 and CD45.2 were distinguished as described in the legend to Figure 1B, except that the anti-CD45.2 reagent was labeled with flourescein. Shown are data from all of the chimeric mice generated with this mixture, all of which were generated at one time and analyzed subsequently at one time. For panels B, C, and D, the values among the four genotypes were statistically different (ANOVA) at the following levels: + <0.05, * <0.01, **<0.01, ***<0.001. For panel D, ANOVA indicated that the groups were statistically different and posthoc analysis indicated that T1 was different from FO at the level shown.
The frequency of mature follicular Lyn-deficient B cells is rescued by deletion of Bim or overexpression of Bcl-2
To test in a more direct fashion the role of Bim in controlling the survival of Lyn-deficient B cells, we bred the Lyn− allele to Bim-deficient mice [25]. Bim−/−Lyn−/− mice had splenic T2, T3 and follicular B cell populations of similar size to those of wild type mice (Figure 3B). Bim-deficient Lyn+/+ mice exhibited a two- to three-fold increase in the number of follicular B cells compared to wild-type mice, indicating that Bim plays a role in limiting the survival of wild type B cells as well as that of Lyn-deficient B cells. Nonetheless, Bim-deficiency substantially corrected the poor survival of Lyn-deficient T2, T3 and follicular B cells, providing direct support for the conclusion that elevated Bim expression in these cells was substantially responsible for their poor survivial. An almost identical result was obtained when we bred Lyn−/− mice to mice with a B cell-expressing Bcl-2 transgene [31] (Figure 3C). Whereas the Bim-deficiency affects all cells in the mice with this mutant allele, expression of the Bcl-2 transgene is restricted to B cells. Together these data reinforce the conclusion that the decreased numbers of T2, T3 and follicular B cells in Lyn−/− mice reflects increased cell death due at least in part to increased levels of Bim. It should be noted, however, Lyn-deficient mice still had a several-fold reduction in the number of follicular B cells compared to their wild type counterparts in the context of Bim-deficiency or Bcl-2 overexpression (Fig. 3B and C), suggesting that their survival was not entirely corrected by these alterations. To further explore this issue, we analyzed mixed bone marrow chimeras between Lyn−/− Bcl-2 transgenic and Lyn+/+ Bcl-2 transgenic donors. In the chimeric mice, Lyn-deficient follicular B cells still had a competitive disadvantage in the presence of Bcl-2 overexpression (Fig. 3D), although it was apparently less than observed in the experiments in which the Bcl-2 transgene was not present (Fig. 1B). As there was still a demonstrable competitive disadvantage to Lyn-deficient follicular B cells in the presence of overexpression of Bcl-2, it is likely that elevation in Bim was not completely responsible for the decreased numbers of follicular B cells in Lyn−/− mice.
Elevated activation marker expression and spontaneous BCR signaling in Lyn-deficient B cells
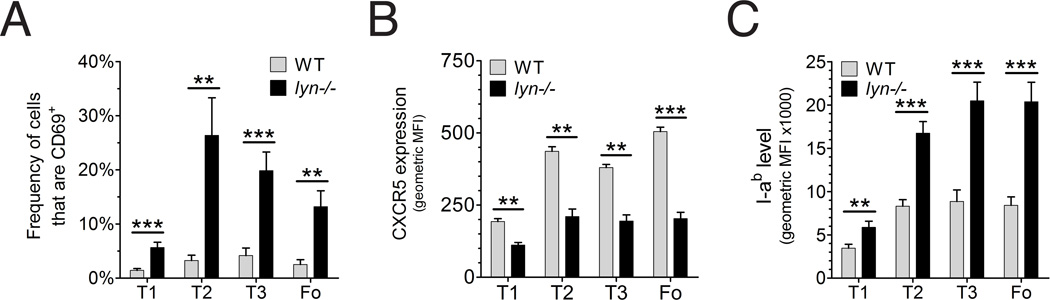
Mice expressing an Ig transgene that recognizes a soluble self antigen typically enter an anergic state that is characterized by low grade BCR signaling and attenuated responsiveness to further BCR stimulation [32]. Interestingly, these anergic B cells have elevated Bim levels and shortened in vivo survival [29, 30]. As described above, Lyn-deficient follicular B cells of diverse repertoire resemble these anergic cells in regard to elevated Bim expression and decreased survival. Therefore, we wanted to assess whether mature follicular Lyn-deficient B cells experience chronic low level BCR signaling in vivo similarly to anergic B cells. First we looked at changes in cell surface phenotype that might be indicative of continual low grade BCR signaling. Interestingly, Lyn−/− mice had a higher fraction of T2, T3, and follicular B cells expressing CD69 (Figure 4A), which is induced by BCR stimulation and interacts with receptors for sphingosine 1-phosphate to inhibit egress from lymph nodes[33]. Lyn-deficient splenic T2, T3 and follicular B cells also expressed lower levels of CXCR5 (Figure 4B), which is a highly sensitive response to BCR stimulation [34]. Decreased expression of CXCR5 contributes to the movement of antigen-stimulated follicular B cells from the follicle to the T-B boundary, where they can interact with antigen-stimulated helper T cells[35]. These B cell populations also had elevated expression of class II MHC molecules (Fig 4C), which is also consistent with in vivo BCR stimulation [20].
FIGURE 4. Increased fraction of activated B cells in Lyn-deficient mice.
(A) fraction of B cells from wild type (grey bars, n=4) or Lyn−/− (black bars, n=5) mice with elevated levels of CD69; ** P<0.001, *** P<0.0001 (unpaired t-test). (B) mean expression level of CXCR5 on different bone marrow and splenic B cell subpopulations from wild type (n=3) and Lyn−/− (n=3) mice. (C) median expression level of the MHC-II molecule I-Ab in wild type (n=4) and Lyn−/− (n=5) mice. Experiments were repeated at least once with similar results.
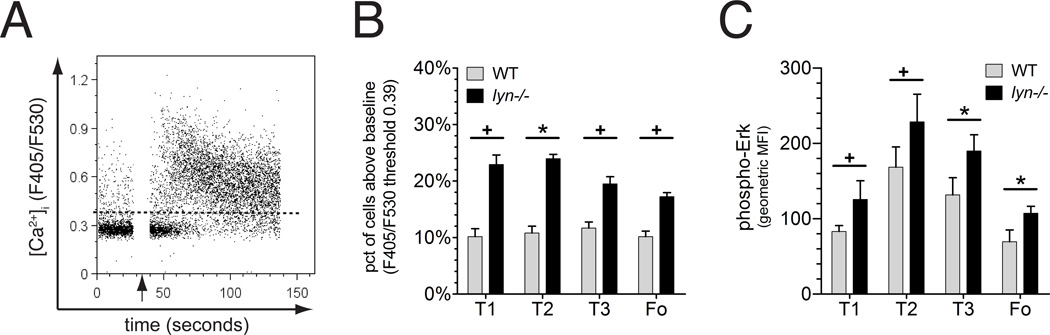
We next examined Lyn-deficient B cells ex vivo for their basal calcium levels and phospho-Erk levels. A larger subpopulation of Lyn-deficient transitional and mature follicular B cells exhibited elevated basal BCR signaling, as assessed by either parameter, than was seen in the corresponding wild type B cell subsets (Figure 5). The observation that basal Erk signaling was elevated in Lyn-deficient B cells is consistent with elevated cell surface expression of CD69, since previous studies have shown that CD69 is induced via the Ras-Erk MAP kinase signaling pathway[36]. Thus, elevated basal calcium and phospho-Erk signaling, increased expression of CD69 and downregulated CXCR5 expression in Lyn-deficient follicular B cells all are consistent with the conclusion that these cells experienced a higher level of autoantigen and/or tonic BCR signaling in vivo than did their wild type counterparts.
Figure 5. Elevated BCR signaling reactions in Lyn-deficient B cells.
(A) Representative example of intracellular free calcium measurement of wild type B cells responding to anti-IgM stimulation at the time indicated by the arrow. The dashed line represents the level of fluorescence below which were 90% of wild type B cells of that subpopulation prior to stimulation. (B) Comparison of the fraction of B cells with spontaneous ex vivo intracellular calcium levels above the 90% threshold line as described in panel A (note: the wild type values are all 10% by definition). (C) Mean levels of phospho-Erk intracellular staining of wild type or Lyn-deficient B cells of different subsets (geometric mean fluorescence intensity). Experiments were repeated at least once with similar results. Statistical analysis: the values between the two genotypes were statistically different (unpaired t-test) at the following levels: + <0.05, * <0.01.
Alterations in PIP3-Akt signaling in Lyn-deficient B cells
Recent studies have identified PIP3-Akt signaling as the major survival signal provided by tonic BCR signaling in resting follicular B cells [37]. Moreover, negative regulation of this pathway plays a key role in promoting clonal deletion[38] and anergy[39] of immature B cells in response to recognition of self antigens. Therefore, we examined the effect of Lyn-deficiency on Akt signaling in different B cell subpopulations. Unlike calcium signaling, a consistent increase in basal Akt signaling was not detected by examining intracellular staining for phosphorylation of Akt on S473, which is one of two phosphorylation sites that control Akt enzymatic activity [40], or by examining phosphorylation of the downstream target ribosomal protein S6, which reflects a regulatory event leading to enhanced translation of a subset of mRNAs [40](Table 1).
Table 1. Activation of the Akt signaling pathway by BCR stimulation in wild type and Lyn-deficient splenic B cells.
Values represent % positive cells, for data shown in Figure 6. Similar results were obtained in one additional independent experiment.
| % phospho-Akt+ | % phospho-S6+ | |||||||
|---|---|---|---|---|---|---|---|---|
| WT | Lyn−/− | WT | Lyn−/− | |||||
| sample | T1 | FO | T1 | FO | T1 | FO | T1 | FO |
| Unstim. | 4.6 | 4.2 | 6.2 | 7.8 | 5.2 | 14.6 | 8.1 | 13.8 |
| 5 µg/ml; 30 min | 35.4 | 39.5 | 48.9 | 37.6 | 53.0 | 36.1 | 45.7 | 42.0 |
| 50µg/ml; 30 min | 29.4 | 53.2 | 53.7 | 77.6 | 53.2 | 67.7 | 57.6 | 92.3 |
| 5µg/ml; 120 min | 14.5 | 66.8 | 42.1 | 34.4 | 11.7 | 63.2 | 28.5 | 26.4 |
| 50µg/ml; 120 min | 18.3 | 75.6 | 45.5 | 84.3 | 13.7 | 74.8 | 35.3 | 86.3 |
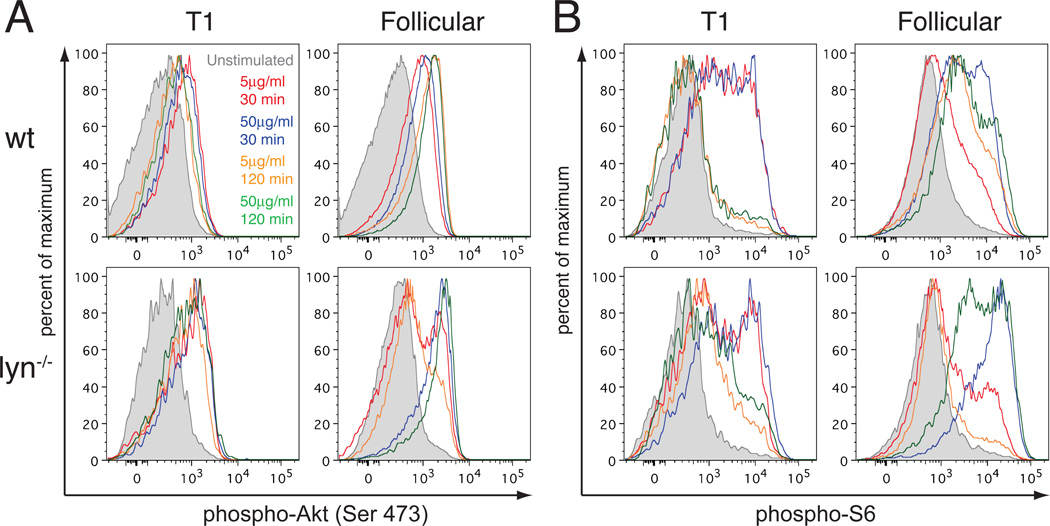
We also examined signaling by the PIP3-Akt pathway in response to either intermediate dose (5µg/ml) or high dose (50µg/ml) anti-IgM stimulation of the BCR at an early time when the response was strongly induced (30 min), and at a later time (120 min) to see how well the response was sustained. Interestingly, wild type B cells showed a substantial developmental change in the magnitude and kinetics of Akt activation via the BCR. Splenic transitional T1 B cells exhibited phospho-Akt and phospho-S6 responses that were robust at 30 min but were transient and not well sustained. In contrast, splenic follicular B cells had a robust and prolonged response, even at the dose of 5µg/ml anti-IgM (Figure 6). Analysis of combined T2 + T3 transitional B cell populations revealed a response that was intermediate between T1 and follicular B cells under each condition examined (data not shown). The dichotomy between the responses of T1 and follicular B cells was evident in Akt S473 phosphorylation, but was further enhanced with regard to the downstream phosphorylation of ribosomal protein S6.
Figure 6. Effects of Lyn-deficiency on Akt signaling by T1 and follicular B cell subsets.
Levels of phospho-S473 Akt (A) and of phospho-S6 (B) were determined in T1 and follicular B cells from wild type (upper panels) and Lyn−/− (lower panels) mice. Shown are levels of signaling-related phosphorylations for unstimulated B cells, B cells stimulated with 5 µg/ml anti-IgM for 30min (red curves) or 120min (orange curves) or with 50µg/ml anti-IgM for 30min (blue curves) or 120 min (green curves). Results are representative of 2–3 mice per genotype analyzed on 2 different occasions.
Lyn-deficient T1 cells had an phospho-Akt response to anti-IgM that was greater than that seen with wild type T1 cells at 30 min and was better sustained at 120 min. Again, these differences compared to wild type T1 cells were magnified in the phospho-S6 response. Both wild type and Lyn-deficient T1 cells exhibited robust ribosomal protein S6 phosphorylation at 30 min, which declined by 120 min, but this response was maintained at a much higher level in the Lyn-deficient T1 cells. The response of Lyn−/− follicular B cells to a high dose of anti-IgM was increased compared to their wild type counterparts and also was well sustained, as was seen in wild type follicular B cells. Surprisingly, however, the phospho-Akt response of Lyn-deficient follicular B cells to an intermediate dose of anti-IgM was not enhanced compared to wild type B cells, and moreover, the downstream phospho-ribosomal protein S6 response declined between 30 min and 120 min, in contrast to the sustained response seen in wild type follicular B cells (Fig. 6). We also examined these signaling responses to lower doses of anti-IgM. Wild type and Lyn−/− T1 cells responded positively to 2µg/ml anti-IgM and partially to 1µg/ml anti-IgM, whereas wild type follicular B cells responded poorly to these lower doses. Lyn-deficient follicular B cells had a positive response to 1µg/ml and 2µg/ml anti-IgM, in contrast to their wild type counterparts, but it was less than the response to 5µg/ml anti-IgM (data not shown). In summary, PIP3-Akt signaling in Lyn-deficient follicular B cells failed to exhibit elevated basal signaling, but did have an enhanced response to BCR stimulation at low doses of anti-IgM and also at high doses of anti-IgM in the case of phosphorylation of ribosomal protein S6.
Discussion
In the studies described here, we have extended earlier observations indicating that Lyn-deficient mice have a substantial decrease in the numbers of B lymphocytes in the peripheral lymphoid organs and have addressed the mechanisms for this decrease in numbers. We examined alterations in B cell development and survival specifically induced by Lyn-deficiency in these cells by using mixed bone marrow chimeras where wild type and Lyn-deficient B cells develop in the same environment. This approach normalizes any effects of environmental alterations such as elevation of BAFF due to B cell lymphopenia or elevation of cytokines produced by hyperactive Lyn-deficient myeloid cells[14]. Using this approach, we found that Lyn-deficient B cells had a competitive defect relative to wild type B cells at the T1 to T2 stage in the spleen and an additional competitive disadvantage at the transition to the mature follicular B cell stage. These defects appeared to reflect primarily a defect in survival rather than a block in developmental maturation, since Lyn-deficient B cells exhibited elevated levels of the pro-apoptotic regulator Bim, and deletion of Bim largely restored B cell numbers, as did B cell-specific overexpression of the pro-survival factor Bcl-2.
The competitive disadvantage of T2 transitional B cells from the spleen of Lyn−/− mice likely represents an enhanced clonal deletion response among the substantial fraction, probably over 50%, of developing B cells with endogenous Ig rearrangements that have self-reactivity [41]. In wild type mice, these B cells are restrained by receptor editing, clonal deletion, or clonal anergy[23]. Previous studies have observed somewhat elevated BCR crosslinking-induced calcium and Erk signaling responses in both T1 and T2 Lyn-deficient B cell populations [13], and this enhanced signaling could be responsible for inducing death of a greater number of self-reactive B cells than occurs in wild type mice. In support of this hypothesis, we observed that Lyn−/− mice had increased proportions of T1 and T2 cells with elevated basal intracellular calcium levels (Fig. 5) and increased proportions of T2 immature B cells expressing the activation marker CD69 (Fig. 4). Transitional splenic B cells, especially at the T2 and T3 stages, also expressed increased levels of class II MHC and decreased expression of CXCR5 (Fig. 4), two additional phenotypic changes that can be induced by BCR signaling. Together these observations indicate that Lyn−/− T2 cells were experiencing greater levels of in vivo BCR signaling than their wild type counterparts, which may explain the elevated levels of Bim seen in the T2 cells from mixed bone marrow chimeras (Fig. 2). Indeed, in previous work, in vivo exposure to soluble HEL of Lyn-deficient B cells expressing the MD4 IgHEL transgene was found to induce clonal deletion, rather than the clonal anergy seen in Lyn+/+ transgenic B cells, suggesting that at least some self-reactive Lyn−/− B cells have compromised survival [20] . These observations suggest that the modestly enhanced BCR signaling of Lyn-deficient T1 and T2 cells was sufficient to induce significantly greater fractions of weakly self-reactive cells to undergo clonal deletion, resulting in a competitive disadvantage at the T2 transitional stage in mixed bone marrow chimeric mice.
An additional competitive disadvantage of Lyn−/− B cells was seen in the decreased proportion of mature follicular B cells relative to the proportion of T2 immature B cells of the spleen (Fig. 1). Whereas signaling in T1 and T2 transitional B cells is modestly enhanced in Lyn-deficient mice, signaling in T3 and follicular B cells is greatly exaggerated [13]. A similar increase in BCR signaling occurs in mature B cells in cd22−/− mice[13]. These observations likely reflect a substantial increase in the role of the Lyn-CD22-SHP-1 feedback inhibitory pathway for limiting BCR signaling in T3 and follicular B cells compared to less mature B cell subpopulations. In addition to increased clonal deletion of self-reactive B cells at the T2 stage in Lyn−/− mice, there was a further compromise in the survival of B cells at the follicular stage. We hypothesize that the decreased survival at this stage resulted from elevated BCR signaling of weakly self-reactive B cells in the absence of normal attenuation of signaling by the Lyn-CD22-SHP-1 feedback inhibitory pathway. According to this hypothesis, self-reactive B cells in Lyn−/− mice would experience a substantial increase in BCR signaling as they progress from the T2 stage to the T3 and/or follicular B cell stages, in many cases inducing apoptosis during this transition.
The conclusion that Lyn−/− mice have decreased populations of T2, T3 and follicular B cells due to increased death of self-reactive B cells is consistent with several previous observations made with Ig transgenic mice. Cornall et al., observed that HEL expression causes an almost complete loss in mature Lyn-deficient IgHEL transgenic B cells, presumably reflecting clonal deletion instead of the clonal anergy that occurs predominantly in Lyn-expressing IgHEL transgenic B cells in HEL-expressing mice[20]. In addition, a less dramatic but still clearcut (five-fold) decrease in the numbers of mature IgHEL transgenic B cells were seen in Lyn−/− IgHEL transgenic mice lacking HEL[20] . Similarly, mice expressing the 3–3 Ig transgene in the absence of the known self antigen were decreased in number by approximately 7-fold in Lyn−/− mice[18]. It is striking that the effects of Lyn deficiency on survival of IgHEL and 3–83 Ig transgenic B cells in the absence of their nominal self-antigens were similar to the effect on the survival of diverse repertoire Lyn−/− B cells, as described here. A number of observations have suggested that maturation to the follicular B cell stage is promoted by weak interactions with endogenous self ligands [28, 42, 43], as is the case for T cells developing in the thymus. If this is correct, then B cells expressing these Ig transgenes in an otherwise wild type setting are normally receiving low level stimulation from their BCR due to interactions with endogenous low affinity ligands. Lyn-deficiency would increase the amount of BCR signaling generated in response to those endogenous ligands. Thus, the poor survival of Lyn−/− IgHEL and 3–83 Ig transgenic B cells may have the same cause as the poor survival of most Lyn−/− B cells of diverse repertoire. In support of this interpretation, the decrease in the number of 3–83 Ig transgenic B cells due to removal of Lyn was largely corrected by expression of a Bcl-2 transgene [18], as was seen with diverse repertoire B cells in the experiments described here. Interestingly, Bim-deficiency was able to rescue the poor survival of Lyn+/+ IgHEL B cells in the presence of soluble HEL, but not the clonal deletion of these cells in mice expressing membrane-bound HEL, a strong stimulus for that transgenic BCR [25]. Thus, Bim-deficiency can promote the survival of anergic B cells but cannot protect highly self-reactive B cells that undergo clonal deletion at high frequency. The failure of Bim-deficiency or Bcl-2 transgene expression to fully restore mature B cell numbers in Lyn−/− mice may reflect a fraction of strongly self-reactive B cells that are not rescued.
In contrast to what was seen in the HEL system, Lyn-deficiency did not decrease the frequency of λ+ anergic DNA-reactive B cells in mice expressing the IgH3H9 transgene[21]. B cells of this specificity retained their anergic phenotype with little obvious change in Lyn−/− vs. Lyn+/+ backgrounds. Why the IgHEL and IgH3H9 λ specificities behave so differently in response to deletion of Lyn is not at all clear and deserves further study, although it may be relevant in the latter case that the self antigen, endogenous DNA, has the potential to stimulate B cell TLR9 as well as the BCR.
The results presented here provide novel insights into how Lyn-deficiency in B cells affects peripheral tolerance mechanisms in the spleen. Survival of diverse repertoire Lyn−/− B cells was significantly compromised as they matured in the spleen, and the surviving cells exhibited several hallmarks of enhanced in vivo BCR signaling, suggestive of enhanced BCR signaling in response to weakly bound self antigens. Thus, the peripheral tolerance mechanisms of clonal deletion and clonal anergy were not compromised and even were enhanced, as expected from analysis of the in vitro BCR signaling properties of Lyn−/− B cells, but surprising in light of the fact that these mice spontaneously produce anti-nuclear antibodies of the types produced in the human disease SLE [10]. The self-reactive antibodies produced in Lyn−/− mice primarily recognize nuclear components that contain or are complexed with ligands for TLR9 or TLR7 [44], so TLR stimulation may play a key role in breaking B cell tolerance in these mice. In agreement with this hypothesis, Lyn−/− Myd88−/− mice fail to make autoantibodies [45] . Thus, although Lyn-deficiency does not induce defects in the initial tolerization of self-reactive B cells by receptor editing, clonal deletion or clonal anergy, it may affect the ability to maintain tolerance of anergic B cells in the face of TLR stimulation of B cells and/or an inflammatory milieu generated by Lyn-deficient myeloid cells [14].
Materials and Methods
Mice
Mice aged 7–12 weeks were used for most experiments. Lyn−/− mice were used as described [17], and backcrossed at least fifteen generations onto C57BL/6 background. MD4 transgenic IgHEL mice and Eμ-Bcl-2.22 mice [31] backcrossed at least 10 generations onto C57BL/6 were obtained from J. Cyster (University of California, San Francisco). 129-Bcl2l11tm1.1 Ast/J (Bim−/−) mice [46] were obtained from A. Abbas (University of California, San Francisco). Bone marrow chimeric mice were generated by lethally irradiating 8–12 week old C57BL/6 mice and reconstituted them with mixtures of bone marrow cells from donors of the same sex and marked with allelic forms of CD45. Recipients were analyzed 8–12 weeks after bone marrow transplantation. All animals were housed in a specific pathogen-free facility at UCSF according to University and National Institutes of Health (NIH) guidelines. Animal use was approved by the UCSF Institutional Animal Care and Use Committee.
Antibodies, Immunofluorescence Analysis, B cell purification and Cell Sorting
Fluorophore-conjugated antibodies directed against the following molecules were used: B220 (RA3-6B2), CD23 (B3B4), IgM (II/41), CD16/CD32 (2.4G2), Bcl-2 (3F11), and CXCR5 (2G8) all from BD Biosciences; CD24 (M1/69), CD45.1 (A20), CD45.2 (104) from BioLegend; CD93 (AA4.1) from eBioscience; Bcl-x (7B2.5) from Southern Biotech, IgM (goat polyclonal F(ab’) monomer, μ-chain specific) (Jackson Immuoresearch). B cell subsets were defined as described [13, 19]. Polyclonal antibody to Bim/BOD (Stressgen) was obtained from the A. Abbas (UCSF). Cells were analyzed on an LSR II or FACSCalibur (both from BD Pharmingen). To detect Bcl-2, Bcl-x, and Bim, cells were fixed and stained in Perm/Wash buffer from BD Biosciences.
Lymph node B cell were purified by negative selection using CD43 microbeads (Miltenyi Biotech) according to the manufacturer’s protocol and passage through an autoMACS Separator (Miltenyi Biotech). For cell sorting, splenocytes or BM cells were stained for CD23, CD93, and IgM F(ab') monomer for 30 min and either B220 or a non-B cell cocktail (antibodies to CD4, CD8, CD11b, Gr1, NK1.1, Ter119) in HBSS, 1% fetal calf serum (FCS), 0.5% BSA. Cells were then sorted on a MoFlo sorter (Dako-Cytomation). Dead cells were excluded by propidium iodide (PI)(BioChemika) uptake. All FACS data were analyzed with FlowJo v. 6.4.1 (TreeStar software).
Analysis of BCR signaling reactions
Calcium elevation and Erk phosphorylation were measured as described previously[13]. For intracellular measurement of phospho-Akt and phospho- ribosomal protein S6, 5×106 splenocytes were warmed to 37°C for 30 min in Iscoves medium supplemented with 1% BSA and 20 mM Hepes. During the final 10 min of warming, the cells were labeled with anti-IgM Fab FITC (Jackson). Cells were then stimulated with 0, 5 or 50 µg/ml goat anti-mouse IgM F(ab’)2 (Jackson) for 30 or 120 minutes. Cells were fixed with 4% paraformaldehyde for 10 minutes (Electron Microscopy Sciences) and permeabilized with ice-cold 100% methanol (Electron Microscopy Sciences). Cells were split into two samples and labeled with rabbit anti-mouse antibodies to phospho-Akt (Ser473) or phospho-S6 ribosomal protein (Ser235/236) (Cell Signaling Technology) for 1 hour. Cells were then washed and labeled with donkey anti-rabbit IgG APC (Jackson Immunoresearch) as well as antibodies to B220 (PE-Cy7), CD23 (PE), and CD24 (Pacific Blue). Cells were analyzed by flow cytometry on an LSR II (BD Biosciences).
Cell Death Assays
Sorted splenic B cells from mice of each genotype were suspended at a concentration of 2×106 cells/ml in RPMI 1640 medium with 10% FCS, 20mM HEPES, 2mM glutamine, and 1mM sodium pyruvate in 96-well tissue culture plates (Corning) at 37°C, 5% CO2. The percentage of viable and dead cells was determined after 24 hours of culture by staining cells with propidium iodide (PI) and analyzing cells by FACS for PI uptake and small size by FSC. The accuracy of this method was confirmed by Annexin V staining (data not shown).
Statistical Analyses
Unpaired two-tailed t-test were performed with Prism v.5.0 (GraphPad software).
Acknowledgements
We thank Dr. Abul Abbas (UCSF) for help with Bim intracellular staining, and Drs. J. Cyster and A. Abbas (UCSF) for providing several mouse stains. This work was supported by U.S. National Institutes of Health Grants AI-20038 and AI-52249 and by NIH training grant T32AI-007334.
Abbreviations
- ITIM
immunoreceptor tyrosine-based inhibitory motif
- SHIP-1
SH2-domain-containing inositol phosphatase
- SHP-1
the SH2-domain-containing tyrosine phosphatase
- SLE
systemic lupus erythematosus
Footnotes
Conflict of Interest
The authors declare no financial or commercial conflict of interest.
References
- 1.Xavier RJ, Rioux JD. Genome-wide association studies: a new window into immune-mediated diseases. Nat Rev Immunol. 2008;8:631–643. doi: 10.1038/nri2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanta H, Mohan C. Three checkpoints in lupus development: central tolerance in adaptive immunity, peripheral amplification by innate immunity and end-organ inflammation. Genes Immun. 2009;10:390–396. doi: 10.1038/gene.2009.6. [DOI] [PubMed] [Google Scholar]
- 3.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 4.Sakaguchi N, Takahashi T, Hata H, Nomura T, Tagami T, Yamazaki S, Sakihama T, Matsutani T, Negishi I, Nakatsuru S, Sakaguchi S. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature. 2003;426:454–460. doi: 10.1038/nature02119. [DOI] [PubMed] [Google Scholar]
- 5.Subramanian S, Tus K, Li QZ, Wang A, Tian XH, Zhou J, Liang C, Bartov G, McDaniel LD, Zhou XJ, Schultz RA, Wakeland EK. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc Natl Acad Sci U S A. 2006;103:9970–9975. doi: 10.1073/pnas.0603912103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pisitkun P, Deane JA, Difilippantonio MJ, Tarasenko T, Satterthwaite AB, Bolland S. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312:1669–1672. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- 7.Majeti R, Xu Z, Parslow TG, Olson JL, Daikh DI, Killeen N, Weiss A. An inactivating point mutation in the inhibitory wedge of CD45 causes lymphoproliferation and autoimmunity. Cell. 2000;103:1059–1070. doi: 10.1016/s0092-8674(00)00209-9. [DOI] [PubMed] [Google Scholar]
- 8.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 9.Pao LI, Lam KP, Henderson JM, Kutok JL, Alimzhanov M, Nitschke L, Thomas ML, Neel BG, Rajewsky K. B cell-specific deletion of protein-tyrosine phosphatase Shp1 promotes B-1a cell development and causes systemic autoimmunity. Immunity. 2007;27:35–48. doi: 10.1016/j.immuni.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 10.Xu Y, Harder KW, Huntington ND, Hibbs ML, Tarlinton DM. Lyn tyrosine kinase: accentuating the positive and the negative. Immunity. 2005;22:9–18. doi: 10.1016/j.immuni.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Kurosaki T, Hikida M. Tyrosine kinases and their substrates in B lymphocytes. Immunol. Rev. 2009;228:132–148. doi: 10.1111/j.1600-065X.2008.00748.x. [DOI] [PubMed] [Google Scholar]
- 12.Saijo K, Schmedt C, Su IH, Karasuyama H, Lowell CA, Reth M, Adachi T, Patke A, Santana A, Tarakhovsky A. Essential role of Src-family protein tyrosine kinases in NF-kappaB activation during B cell development. Nat Immunol. 2003;4:274–279. doi: 10.1038/ni893. [DOI] [PubMed] [Google Scholar]
- 13.Gross AJ, Lyandres JR, Panigrahi AK, Prak ET, DeFranco AL. Developmental acquisition of the Lyn-CD22-SHP-1 inhibitory pathway promotes B cell tolerance. J Immunol. 2009;182:5382–5392. doi: 10.4049/jimmunol.0803941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scapini P, Hu Y, Chu CL, Migone TS, Defranco AL, Cassatella MA, Lowell CA. Myeloid cells, BAFF, and IFN-gamma establish an inflammatory loop that exacerbates autoimmunity in Lyn-deficient mice. J Exp Med. 2010;207:1757–1773. doi: 10.1084/jem.20100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harley IT, Kaufman KM, Langefeld CD, Harley JB, Kelly JA. Genetic susceptibility to SLE: new insights from fine mapping and genome-wide association studies. Nat Rev Genet. 2009;10:285–290. doi: 10.1038/nrg2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Surolia I, Pirnie SP, Chellappa V, Taylor KN, Cariappa A, Moya J, Liu H, Bell DW, Driscoll DR, Diederichs S, Haider K, Netravali I, Le S, Elia R, Dow E, Lee A, Freudenberg J, De Jager PL, Chretien Y, Varki A, Macdonald ME, Gillis T, Behrens TW, Bloch D, Collier D, Korzenik J, Podolsky DK, Hafler D, Murali M, Sands B, Stone JH, Gregersen PK, Pillai S. Functionally defective germline variants of sialic acid acetylesterase in autoimmunity. Nature. 2010;466:243–247. doi: 10.1038/nature09115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan VW, Meng F, Soriano P, DeFranco AL, Lowell CA. Characterization of the B lymphocyte populations in Lyn-deficient mice and the role of Lyn in signal initiation and down-regulation. Immunity. 1997;7:69–81. doi: 10.1016/s1074-7613(00)80511-7. [DOI] [PubMed] [Google Scholar]
- 18.Meade J, Fernandez C, Turner M. The tyrosine kinase Lyn is required for B cell development beyond the T1 stage in the spleen: rescue by over-expression of Bcl-2. Eur J Immunol. 2002;32:1029–1034. doi: 10.1002/1521-4141(200204)32:4<1029::AID-IMMU1029>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 19.Allman D, Lindsley RC, DeMuth W, Rudd K, Shinton SA, Hardy RR. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J. Immunol. 2001;167:6834–6840. doi: 10.4049/jimmunol.167.12.6834. [DOI] [PubMed] [Google Scholar]
- 20.Cornall RJ, Cyster JG, Hibbs ML, Dunn AR, Otipoby KL, Clark EA, Goodnow CC. Polygenic autoimmune traits: Lyn, CD22, and SHP-1 are limiting elements of a biochemical pathway regulating BCR signaling and selection. Immunity. 1998;8:497–508. doi: 10.1016/s1074-7613(00)80554-3. [DOI] [PubMed] [Google Scholar]
- 21.Seo S, Buckler J, Erikson J. Novel roles for Lyn in B cell migration and lipopolysaccharide responsiveness revealed using anti-double-stranded DNA Ig transgenic mice. J Immunol. 2001;166:3710–3716. doi: 10.4049/jimmunol.166.6.3710. [DOI] [PubMed] [Google Scholar]
- 22.Nemazee D. Receptor editing in lymphocyte development and central tolerance. Nat Rev Immunol. 2006;6:728–740. doi: 10.1038/nri1939. [DOI] [PubMed] [Google Scholar]
- 23.Tussiwand R, Bosco N, Ceredig R, Rolink AG. Tolerance checkpoints in B-cell development: Johnny B good. Eur J Immunol. 2009;39:2317–2324. doi: 10.1002/eji.200939633. [DOI] [PubMed] [Google Scholar]
- 24.von Boehmer H, Melchers F. Checkpoints in lymphocyte development and autoimmune disease. Nat Immunol. 2010;11:14–20. doi: 10.1038/ni.1794. [DOI] [PubMed] [Google Scholar]
- 25.Enders A, Bouillet P, Puthalakath H, Xu Y, Tarlinton DM, Strasser A. Loss of the pro-apoptotic BH3-only Bcl-2 family member Bim inhibits BCR stimulation-induced apoptosis and deletion of autoreactive B cells. J Exp Med. 2003;198:1119–1126. doi: 10.1084/jem.20030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Craxton A, Draves KE, Gruppi A, Clark EA. BAFF regulates B cell survival by downregulating the BH3-only family member Bim via the ERK pathway. J Exp Med. 2005;202:1363–1374. doi: 10.1084/jem.20051283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodnow CC. Transgenic mice and analysis of B-cell tolerance. Annu Rev Immunol. 1992;10:489–518. doi: 10.1146/annurev.iy.10.040192.002421. [DOI] [PubMed] [Google Scholar]
- 28.Cyster JG, Healy JI, Kishihara K, Mak TW, Thomas ML, Goodnow CC. Regulation of B-lymphocyte negative and positive selection by tyrosine phosphatase CD45. Nature. 1996;381:325–328. doi: 10.1038/381325a0. [DOI] [PubMed] [Google Scholar]
- 29.Oliver PM, Vass T, Kappler J, Marrack P. Loss of the proapoptotic protein, Bim, breaks B cell anergy. J Exp Med. 2006;203:731–741. doi: 10.1084/jem.20051407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lesley R, Xu Y, Kalled SL, Hess DM, Schwab SR, Shu HB, Cyster JG. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity. 2004;20:441–453. doi: 10.1016/s1074-7613(04)00079-2. [DOI] [PubMed] [Google Scholar]
- 31.Strasser A, Harris AW, Bath ML, Cory S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature. 1990;348:331–333. doi: 10.1038/348331a0. [DOI] [PubMed] [Google Scholar]
- 32.Goodnow CC, Sprent J, Fazekas de St Groth B, Vinuesa CG. Cellular and genetic mechanisms of self tolerance and autoimmunity. Nature. 2005;435:590–597. doi: 10.1038/nature03724. [DOI] [PubMed] [Google Scholar]
- 33.Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 34.Ekland EH, Forster R, Lipp M, Cyster JG. Requirements for follicular exclusion and competitive elimination of autoantigen-binding B cells. J. Immunol. 2004;172:4700–4708. doi: 10.4049/jimmunol.172.8.4700. [DOI] [PubMed] [Google Scholar]
- 35.Okada T, Cyster JG. B cell migration and interactions in the early phase of antibody responses. Curr Opin Immunol. 2006;18:278–285. doi: 10.1016/j.coi.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 36.Villalba M, Hernandez J, Deckert M, Tanaka Y, Altman A. Vav modulation of the Ras/MEK/ERK signaling pathway plays a role in NFAT activation and CD69 up-regulation. Eur J Immunol. 2000;30:1587–1596. doi: 10.1002/1521-4141(200006)30:6<1587::AID-IMMU1587>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 37.Srinivasan L, Sasaki Y, Calado DP, Zhang B, Paik JH, DePinho RA, Kutok JL, Kearney JF, Otipoby KL, Rajewsky K. PI3 kinase signals BCR-dependent mature B cell survival. Cell. 2009;139:573–586. doi: 10.1016/j.cell.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng S, Hsia CY, Feng B, Liou ML, Fang X, Pandolfi PP, Liou HC. BCR-mediated apoptosis associated with negative selection of immature B cells is selectively dependent on Pten. Cell Res. 2009;19:196–207. doi: 10.1038/cr.2008.284. [DOI] [PubMed] [Google Scholar]
- 39.Browne CD, Del Nagro CJ, Cato MH, Dengler HS, Rickert RC. Suppression of phosphatidylinositol 3,4,5-trisphosphate production is a key determinant of B cell anergy. Immunity. 2009;31:749–760. doi: 10.1016/j.immuni.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shlomchik MJ. Sites and stages of autoreactive B cell activation and regulation. Immunity. 2008:18–28. doi: 10.1016/j.immuni.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 42.Allman DM, Ferguson SE, Lentz VM, Cancro MP. Peripheral B cell maturation. II. Heat-stable antigen(hi) splenic B cells are an immature developmental intermediate in the production of long-lived marrow-derived B cells. J Immunol. 1993;151:4431–4444. [PubMed] [Google Scholar]
- 43.Allman D, Pillai S. Peripheral B cell subsets. Curr Opin Immunol. 2008;20:149–157. doi: 10.1016/j.coi.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marshak-Rothstein A, Rifkin IR. Immunologically active autoantigens: the role of toll-like receptors in the development of chronic inflammatory disease. Annu Rev Immunol. 2007;25:419–441. doi: 10.1146/annurev.immunol.22.012703.104514. [DOI] [PubMed] [Google Scholar]
- 45.Silver KL, Crockford TL, Bouriez-Jones T, Milling S, Lambe T, Cornall RJ. MyD88-dependent autoimmune disease in Lyn-deficient mice. Eur J Immunol. 2007;37:2734–2743. doi: 10.1002/eji.200737293. [DOI] [PubMed] [Google Scholar]
- 46.Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Kontgen F, Adams JM, Strasser A. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]