Weak hydrogen bonds recently have arisen as a topic of interest in supramolecular chemistry.[1] Among the various interactions being studied, the C-H···anion hydrogen bonds have drawn considerable attention. Positively-charged groups, such as imidazolium cations, provide strong C-H donors and have been used extensively in the design of numerous anionophore architectures.[2] There is increasing evidence, however, that even charge-neutral C-H donors may be strong enough to be exploited effectively in anion recognition chemistry. Such interactions, which involve both aliphatic and aryl C-H groups, have been inferred from gas phase studies,[3] deduced from NMR spectroscopic studies via, e.g., chemical shifts changes,[4-7] and observed in solid state structures.[4,5,8] A recent review of anion-arene adducts notes that C-H···anion hydrogen bonding, rather than interaction with the π-system, is by far the most prevalent bonding motif observed in the solid state.[9] These experimental observations are supported by theoretical analyses.[10,11] For instance, Hay and co-workers have calculated that benzene C-H···anion hydrogen bonds are significant,[11a] being roughly half the strength of typical neutral N-H···anion hydrogen bonds. In a subsequent theoretical report, it was noted that the aryl C-H···Cl binding energies in the gas phase can be tuned from –8 to –16 kcal/mol by altering the para substitution from NH2 to NO2.[11b] Although there are a couple examples where complementary aryl C-H anion interactions have been deliberately incorporated into the design of anion receptors,[7,12] to the best of our knowledge, no efforts have been made to date to test theoretical predictions via the synthesis and experimental study of a matched series of anion receptors. We now report efforts along these lines.
In the context of ongoing efforts to develop the chemistry of calixpyrroles (e.g., 1, Figure 1), we recently reported the synthesis of the strapped calixpyrrole 2.[13] In this case, a single crystal X-ray diffraction study revealed the presence of a short C-H···Cl– contact in the solid state.[4] This evidence for hydrogen bonding was later confirmed in solution via NMR spectroscopic means. However, because it provided only a single datum point, it was not possible to assess the extent to which this C-H···Cl– interaction contributed to the overall chloride anion binding process. In an effort to address this issue, we have now synthesized the corresponding pyrrole- and furan-strapped congeners 3 and 4, where 3 allows a comparison of NH vs. CH hydrogen bonding and 4, which lacks a donor, provides a “negative control”. The chloride anion binding properties of this series were then analyzed in the solid state, in acetonitrile and dimethylsulfoxide solution, and via theoretical analyses. The results obtained provide support for the notion that C-H···Cl– interactions are significant, at least within the well-defined[14] anion recognition environment provided by this series of diametrically strapped calixpyrroles.
Figure 1.
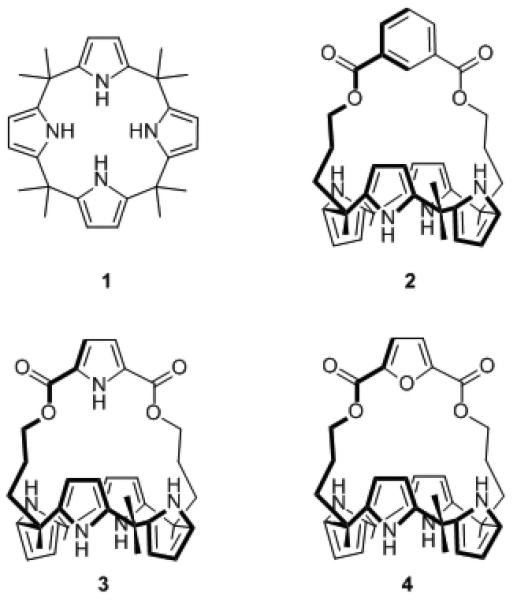
meso-Octamethylcalix[4]pyrrole 1 and strapped calix[4]pyrroles 2-4.
The synthesis of the pyrrole strapped calix[4]pyrrole 3 involved the key intermediate, pyrrole-2,5-dicarboxylic acid 5a, which was prepared from the diester 6.[15] This ester was then converted to the bis-acid chloride 7a by heating at reflux in thionyl chloride, followed by simple distillation and rigorous drying under high vacuum. Reaction of 7a with 2.0 molar equivalents of 5-methyl-5-(3-hydroxypropyl)dipyrromethane 8[13a] in the presence of pyridine yielded the bis-dipyrromethane 9a. Finally, acid-catalyzed condensation of 9a in the presence of excess acetone afforded the desired pyrrole strapped calix[4]pyrrole 3 in 15% yield (Scheme 1).
Scheme 1.
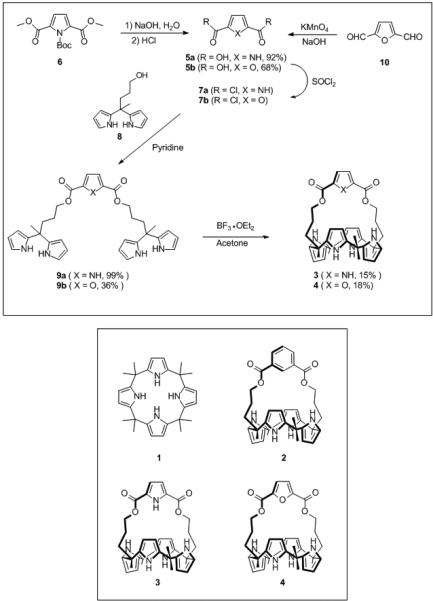
Synthesis of the pyrrole strapped calix[4]pyrrole 3 and analogous furan system 4.
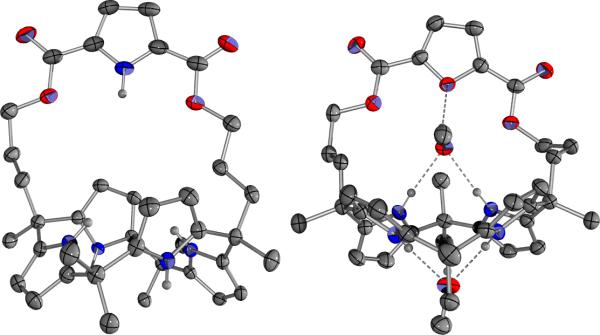
The analogous furan strapped calix[4]pyrrole 4 was synthesized via furan-2,5-dicarboxylic acid 5b. This latter precursor was readily prepared from furan by formylation with n-BuLi and DMF followed by acidic work-up; this gave the 2,5-diformyl furan 10, which was then subjected to oxidation with KMnO4. In analogy to above, the resulting dicarboxylic acid 5b was then converted to the corresponding acid chloride 7b using thionyl chloride, reacted with 8 to give the bis-dipyrromethane 9b, and then condensed with acetone in the presence of BF3-OEt2 to afford the desired furan strapped calix[4]pyrrole 4 in 18% yield (Scheme 1). Both new compounds, 3 and 4, were characterized by standard spectroscopic means, as well as by X-ray diffraction analysis (cf. Figure 2). Interestingly, compound 3 adopts a 1,3-alternate conformation in its anion-free form, while compound 4 (containing two molecules of methanol) adopts a 1,2-alternate conformation, at least in the solid state.
Figure 2.
Single crystal X-ray diffraction structures of the anion-free forms of receptors 3 (left) and 4·2MeOH (right).[16]
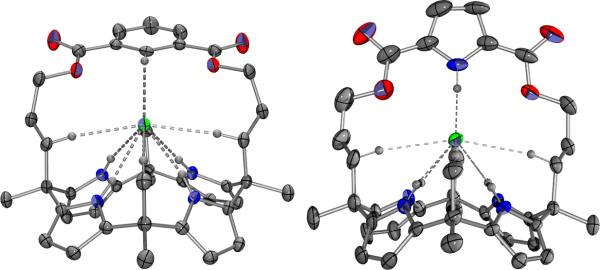
Previously, we reported the X-ray crystal structure of 2·Cl–.[4] Evaluation of this structure reveals three types of receptor-anion contact in the solid state, namely ones involving the four pyrrolic NH protons, the CH of the phenyl strap, and aliphatic CH contacts with two methylene substituents and two methyl substituents (cf. Figure 3). In the present study, diffraction-grade crystals of 3·Cl– were obtained via slow diffusion of methanol into a dichloromethane solution of 3 containing an excess of tetrabutylammonium chloride (TBACl). The resulting X-ray diffraction structure revealed that the calix[4]pyrrole subunit exists in the expected cone conformation and that the chloride anion exhibits hydrogen bonding contacts analogous to those observed in 2·Cl–. The average calixpyrrole N-H···Cl– distances are comparable in both complexes, being 2.407 Å and 2.425 Å for 3 and 2, respectively. Similarly, the average aliphatic C-H···Cl– distances are comparable in both complexes, methylene 2.578 Å and 2.467 Å and methyl 2.945 Å and 2.979 Å for 3 and 2, respectively. However, there is a large difference in the distance involving the apical strap-derived contact, with the pyrrole strap providing a closer N-H···Cl– contact by 0.63 Å than the corresponding phenyl CH interaction. The dihedral angles around the 2,5-substituted pyrrole and the 1,5-substituted phenyl moieties in the straps also differ, i.e. the phenyl subunit is tilted more than the pyrrole, presumably to compensate the shorter net linker distance. At present, no experimental structural information is available for the corresponding chloride anion complex of the congeneric furan-based system, 4.
Figure 3.
Single crystal X-ray diffraction structures of the chloride anion complexes of the strapped calix[4]pyrroles 2 and 3.[16] In both cases, NH- and CH-anion contacts of ≤ 3 Å are shown. The structure of 2•Cl– was originally published in reference #4.
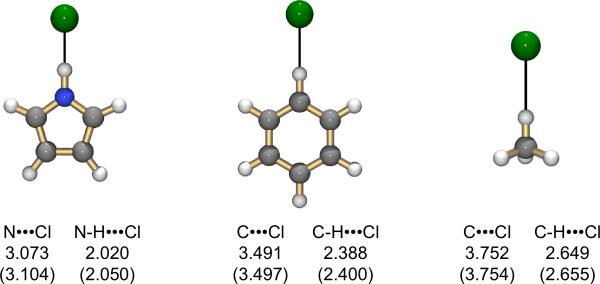
Electronic structure calculations were performed to assess the relative strengths of the three types of hydrogen bonding interactions observed above in the absence of complicating effects arising from steric constraints imposed by the ligand architecture and environmental factors. As seen in Table 1, electronic binding energies, ΔE, for complexes of Cl– with simple donors pyrrole, benzene, and methane are fully consistent with experimentally measured gas phase ΔH values. The expected trend in hydrogen bond strengths is obtained with pyrrole > benzene > methane.[11a] Moreover, these results confirm that the C-H···Cl contacts observed in the crystal structures of 2·Cl– and 3·Cl– are attractive in nature and make a significant contribution to the overall binding. In all cases geometries for these 1:1 complexes, Figure 4, reveal closer contacts than those observed in the strapped calixpyrrole macrocycles. This effect is predominantly due to the fact that a single anion-molecule interaction polarizes and redistributes the anion charge differently than multiple anion-molecule interactions, leading to stronger interactions and shorter distances.
Table 1.
Comparison of calculated electronic binding energies, ΔE (kcal/mol),[a] with experimental gas phase ΔH (kcal/mol) values for Cl– complexes with simple hydrogen bond donors.
| Donor | ΔE (DFT)[b] | ΔE (MP2)[c] | Δ H |
|---|---|---|---|
| pyrrole | –23.09 | –22.50 | –18.8[3b] |
| benzene | –8.32 | –8.42 | –8.6 to –10.5[3b, 10a, 17] |
| methane | –3.06 | –3.36 | –3.6[18] |
ΔE = E(complex) – E(chloride) – E(donor). Electronic structure calculations were performed with NWChem.[19]
B3LYP/DZVP2
MP2/aug-cc-pVDZ
Figure 4.
Optimized geometries for Cl– complexes with pyrrole, benzene, and methane. Distances are given below each structure at the MP2/aug-cc-pVDZ and B3LYP/DZVP2 (in parentheses) levels of theory. Cartesian coordinates and absolute energies for all optimizations are included in the Supporting Information.
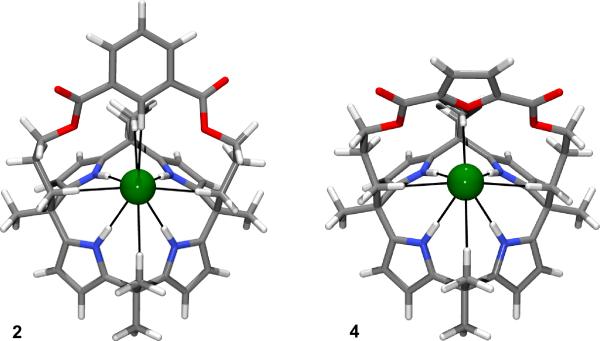
To gain further theoretical insight into the contribution of the C-H···Cl and N-H···Cl contacts provided by the straps in 2 and 3, the less expensive B3LYP/DZVP2 level of theory was used to optimize the geometry of the chloride-bound structures for strapped calixpyrroles 2 and 3, as well as for the furan analogue 4 (Figure 5). Starting from the crystallographic coordinates, 2 and 3 were optimized under gas phase conditions. This yielded geometries that were little changed from the solid state having comparable average calixpyrrole N-H···Cl– distances in both complexes of 2.398 Å and 2.311 Å for 3 and 2, respectively. Similarly, the average aliphatic C-H···Cl distances are comparable in both complexes (i.e., methylene 2.640 Å and 2.563 Å and methyl 2.933 Å and 3.019 Å for 3 and 2, respectively). Optimization using starting coordinates derived by replacing the phenyl group in 3 with furan, yielded a putative geometry for 4·Cl–. This complex shows the same hydrogen bonding motifs and distances as seen in 2 and 3, with the exception that the furan moiety fails to provide an additional hydrogen bond donor. To obtain a measure of the chloride affinity offered by each of the host configurations, single point energies were calculated after removal of the chloride anion. Subtracting the energy of Cl– and the host binding configuration from that of the complex yields the following ΔE values: 3, –67.64 kcal/mol < 2, –63.46 kcal/mol < 4, –58.25 kcal/mol. Consistent with the calculations on the simple prototype donors, these binding energies predict that the pyrrole strapped system, 3, should prove to be a better receptor than the corresponding phenyl species, 2, and that the latter system should prove to be a better receptor than the furan strapped calix[4]pyrrole, 4.[20]
Figure 5.
Optimized B3LYP/DZVP2 geometries of the chloride anion complex of the phenyl strapped calix[4]pyrrole 2 and the furan strapped calix[4]pyrrole 4. A corresponding view for the pyrrole strapped system 3, as well as Cartesian coordinates and absolute energies for all three species, are included in the supporting information.
In light of the above prediction, attempts were made to study the anion binding properties of the pyrrole strapped calix[4]pyrrole 3 using proton NMR spectroscopy. Unfortunately, even in DMSO-d6, which was expected to favor a rapid equilibrium, sets of peaks consistent with slow association/dissociation kinetics (i.e., slow exchange) were observed. Nonetheless, these spectroscopic studies provided insights into the mode of binding and the stoichiometry. For instance, in the absence of anions two singlets were observed for the pyrrolic NH's, namely one at 11.58 ppm (pyrrole on the strap) and another at 9.43 ppm (calix[4]pyrrole). However, upon the addition of less than 1.0 equivalent TBACl two singlets appeared at 12.87 ppm and 10.92 ppm. The beta-pyrrolic protons were also shifted upfield upon chloride anion binding as expected (see Supporting Information). Complete appearance of these new signals, with a corresponding disappearance of the original signals, was observed after the addition of 1.0 equivalent of chloride anion. These observations are consistent with a strong 1:1 binding motif and the inclusion of chloride in the cavity of the pyrrole strapped calix[4]pyrrole 3. With the furan strapped calix[4]pyrrole 4, slow association/dissociation kinetics were also observed in the case of TBACl. The pyrrolic NH's were shifted downfield from 9.40 ppm to 11.47 ppm. Additionally, the beta protons on the furan subunit were shifted upfield, along with the calix[4]pyrrole beta pyrrolic protons, as in the case of 2 and 3.
Given that the system was undergoing slow exchange relative to the NMR time scale, we chose ITC as the method to quantify the interaction of chloride (as the TBA salt) with calixpyrroles 1-4 (Table 2). Although we have already reported the chloride anion binding affinity for the phenyl strapped calixpyrrole 2, measured in acetonitrile at 30 °C,[13a] the titrations have been repeated at 25 °C to allow for a direct intracomparison between calixpyrroles 1-4 under identical conditions. The lowering of the temperature by 5 degrees afforded a slightly higher affinity constant for the interaction of 2 with TBACl (Ka = 2.2 × 106 vs. 1.4 × 106 M–1; estimated errors < 10%). As expected, the introduction of an additional NH hydrogen bond donor into the strap enhanced the chloride affinity by an order of magnitude relative to 2 (Ka = 2.2 × 106 and 1.8 × 107 M–1, for 2 and 3, respectively; see Table 2). The interaction of 3 with chloride has both a more favorable enthalpy and entropy, explaining the higher affinity observed for this system than for 2. Conversely, when compared to 2, the enthalpy of the furan analogue 4 drops by 15 percent, and the observed Ka = 1.9 × 105 M–1 represents an order of magnitude decrease.
Previously we had shown an order of magnitude increase in the chloride affinity of calix[4]pyrrole 1 by adding a CH donor to the calixpyrrole scaffold (to produce receptor 2). Now, by tuning the substituent on the strap we have been able to span a wider range of binding affinities. Specifically, we have found that replacing the CH hydrogen bond donor of the phenyl strapped system by a pyrrolic NH donor, increases the chloride anion affinity by an additional order of magnitude. Conversely, the furan equivalent 4, a “negative control”, displayed a lower chloride anion affinity relative to the phenyl- and pyrrole-strapped receptors 2 and 3 by ca. 1 and 2 orders of magnitude, respectively. As with the theoretical analysis,[20] other factors obscure/prevent/preclude the quantitative assignment of the aryl C-H···anion bond strength from these solution data. Nevertheless, it is important to stress that the present experimental findings are fully consistent with theory. They thus provide support for the proposal that C-H···anion interactions can be exploited to good effect in the design of anion receptors and that such not-so-weak hydrogen bonds may have a role to play in terms of explaining biological anion recognition processes.
At present, we are working to generalize these findings by extending them to other receptor systems and by investigating the extent to which the choice of strapping entity can affect the binding of other anionic guests. The results of these efforts will be reported in due course.
Table 2.
Thermodynamic data for the interaction of calixpyrroles 1-4 with chloride.[a]
| Host | T Δ S | Δ H | Δ G | Ka (M–1) | Ka/K4 [b] |
|---|---|---|---|---|---|
| 1[c] | –2.91 | –10.16 | –7.29 | 2.2 × 105 | 1.2 |
| 2 | –1.90 | –10.54 | –8.64 | 2.2 × 106 | 12 |
| 3 | –1.44 | –11.34 | –9.90 | 1.8 × 107 | 95 |
| 4 | –1.67 | –8.87 | –7.20 | 1.9 × 105 | 1 |
Units of TΔS, ΔH, and ΔG are (kcal·mol–1); titrations were run at 25 °C in acetonitrile, and chloride was used in the form of its tetrabutylammonium salt.
Ka/K4 is the affinity enhancement ratio relative to the furan-strapped system 4.
From reference #14b.
Footnotes
This work was supported by the NIH (grant GM 58908 to J.L.S.), Korea Research Foundation Grant funded by the Korean Government (MOEHRD)(KRF-2006-214-C00047 to D.-W.Y.), Korea Science and Engineering Foundation (grant no. R01-2006-000-10001-0 to C.-H.L.), and B.P.H. acknowledges support from the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences, U.S. Department of Energy (DOE) under contract number DE-AC05-00OR22725 with Oak Ridge National Laboratory (managed by UT-Battelle, LLC).
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
Contributor Information
Dae-Wi Yoon, Dept. of Chemistry and Biochemistry The University of Texas at Austin Austin, TX 78712, USA.
Dustin E. Gross, Dept. of Chemistry and Biochemistry The University of Texas at Austin Austin, TX 78712, USA
Vincent M. Lynch, Dept. of Chemistry and Biochemistry The University of Texas at Austin Austin, TX 78712, USA
Jonathan L. Sessler, Dept. of Chemistry and Biochemistry The University of Texas at Austin Austin, TX 78712, USA Fax: (+1) 512-471-7550 sessler@mail.utexas.edu
Benjamin P. Hay, Chemical Sciences Division Oak Ridge National Laboratory Oak Ridge, TN 37830-6119, USA
Chang-Hee Lee, Dept. of Chemistry and Vascular System Research Center Kangwon National University Chun-Chon 200-701, Korea.
References
- 1.Nishio M. Encyclopedia of Supramolecular Chemistry. 2004:1576–1585. [Google Scholar]
- 2.a Sato K, Arai S, Yamagishi T. Tetrahedron Lett. 1999;40:5219–5222. [Google Scholar]; b Alcalde E, Alvarez-Rua C, Garcia-Granda S, Garcia-Rodriguez E, Mesquida N, Perez-Garcia L. Chem. Commun. 1999:295–296. [Google Scholar]; c Ihm H, Yun S, Kim HG, Kim JK, Kim KS. Org. Lett. 2002;4:2897–2900. doi: 10.1021/ol026373h. [DOI] [PubMed] [Google Scholar]; d Kim SK, Kang BG, Koh HS, Yoon YJ, Jung SJ, Jeong B, Lee K-D, Yoon J. Org. Lett. 2004;6:4655–4658. doi: 10.1021/ol048285y. [DOI] [PubMed] [Google Scholar]; e Chellappan K, Singh NJ, Hwang I-C, Lee JW, Kim KS. Angew. Chem. 2005;117:2959–2963. doi: 10.1002/anie.200500119. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2005;44:2899–2903. doi: 10.1002/anie.200500119. [DOI] [PubMed] [Google Scholar]; f Wong WWH, Vickers MS, Cowley AR, Paul RL, Beer PD. Org. Biomol. Chem. 2005;3:4201–4208. doi: 10.1039/b510068b. [DOI] [PubMed] [Google Scholar]; g In S, Cho SJ, Kang J. Supramol. Chem. 2005;17:443–446. [Google Scholar]; h Yoon J, Kim SK, Singh NJ, Kim KW. Chem. Soc. Rev. 2006;35:355–360. doi: 10.1039/b513733k. [DOI] [PubMed] [Google Scholar]; i Amendola V, Boiocchi M, Colasson B, Fabbrizzi L, Rodriguez Douton M-J, Ugozzoli F. Angew. Chem. 2006;118:7074–7078. doi: 10.1002/anie.200602598. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2006;45:6920–6924. doi: 10.1002/anie.200602598. [DOI] [PubMed] [Google Scholar]; j Fahlbusch T, Frank M, Schatz J, Schmaderer H. Eur. J. Org. Chem. 2006:1899–1903. [Google Scholar]; k Belcher WJ, Fabre M, Farhan T, Steed JW. Org. Biomol. Chem. 2006;4:781–786. doi: 10.1039/b516027h. [DOI] [PubMed] [Google Scholar]; l Sato K, Sadamitsu Y, Arai S, Yamagishi T. Tet. Lett. 2007;48:1493–1496. [Google Scholar]
- 3.a French MA, Ikuta S, Kebarle P. Can. J. Chem. 1982;60:1907–1918. [Google Scholar]; b Larson JW, McMahon TB. J. Am. Chem. Soc. 1984;106:517–521. [Google Scholar]; c Alberti M, Castro A, Lagana A, Moix M, Pirani F, Cappelletti D. Eur. Phys. J. D. 2006;185:185–191. [Google Scholar]; d Schneider H, Vogelhuber KM, Schinte F, Weber JM. J. Am. Chem. Soc. 2007;129:13022–13026. doi: 10.1021/ja073028k. [DOI] [PubMed] [Google Scholar]; e Zhu SS, Staats H, Brandhorst K, Grunnenberg J, Gruppi F, Dalcanale E, Lutzen A, Rissanen K, Schalley CA. Angew. Chem. 2008;120:800–804. doi: 10.1002/anie.200703451. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2008;47:788–792. doi: 10.1002/anie.200703451. [DOI] [PubMed] [Google Scholar]
- 4.Lee C-H, Na H-K, Yoon D-W, Won D-H, Cho W-S, Lynch VM, Shevchuk SV, Sessler JL. J. Am. Chem. Soc. 2003;125:7301–7306. doi: 10.1021/ja029175u. [DOI] [PubMed] [Google Scholar]
- 5.a Kang SO, VanderVelde D, Powell D, Bowman-James K. J. Am. Chem. Soc. 2004;126:12272–12273. doi: 10.1021/ja046078n. [DOI] [PubMed] [Google Scholar]; b Vega IED, Gale PA, Light ME, Loeb SJ. Chem. Commun. 2005:4913–4915. doi: 10.1039/b510506d. [DOI] [PubMed] [Google Scholar]; c Maeda H, Kusunose Y. Chem.—Eur. J. 2005;11:5661–5666. doi: 10.1002/chem.200500627. [DOI] [PubMed] [Google Scholar]; d Zielinski T, Kedziorek M, Jurczak J. Chem.—Eur. J. 2008;14:838–846. doi: 10.1002/chem.200700592. [DOI] [PubMed] [Google Scholar]
- 6.a Chen Q-Y, Chen C-F. Tetrahedron Lett. 2004;45:6493. [Google Scholar]; b Kwon JY, Jang YJ, Kim SK, Lee KH, Kim JS, Yoon J. J. Org. Chem. 2004;69:5155–5157. doi: 10.1021/jo049281+. [DOI] [PubMed] [Google Scholar]; c Fujimoto C, Kusunose Y, Maeda H. J. Org. Chem. 2006;71:2389–2394. doi: 10.1021/jo052511f. [DOI] [PubMed] [Google Scholar]; d Nishiyabu R, Palacios MA, Dehaen W, Anzenbacher P., Jr. J. Am. Chem. Soc. 2006;128:11496–11504. doi: 10.1021/ja0622150. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Flood AH. Angew. Chem. 2008;120:2689–2692. [Google Scholar]; Angew. Chem. Int. Ed. 2008;47:2649–2652. doi: 10.1002/anie.200704717. [DOI] [PubMed] [Google Scholar]
- 8.a Ilioudis CA, Tocher DA, Steed JW. J. Am. Chem. Soc. 2004;126:12395–12402. doi: 10.1021/ja047070g. [DOI] [PubMed] [Google Scholar]; b Chmielewski MJ, Charon M, Jurczak J. Org. Lett. 2004;6:3501–3504. doi: 10.1021/ol048661e. [DOI] [PubMed] [Google Scholar]
- 9.Hay BP, Bryantsev VS. Chem. Commun. 2008 doi: 10.1039/b800055g. DOI:10.1039/b800055g. [DOI] [PubMed] [Google Scholar]
- 10.a Hiraoka K, Mizuse S, Yamabe S. Chem. Phys. Lett. 1988;147:174–178. [Google Scholar]; b Loh ZM, Wilson RL, Wild DA, Bieske EJ, Zehnacker A. J. Chem. Phys. 2003;119:9559–9567. [Google Scholar]; c Ortuno JA, Exposito R, Sanchez-Pedreno C, Albero MI, Espinosa A. Anal. Chim. Acta. 2004;525:231–237. [Google Scholar]; d Botschwina P, Oswald R, Dyczmons V. Int. J. Mass Spec. 2007;267:308–314. [Google Scholar]
- 11.a Bryantsev VS, Hay BP. J. Am. Chem. Soc. 2005;127:8282–8283. doi: 10.1021/ja0518272. [DOI] [PubMed] [Google Scholar]; b Bryantsev VS, Hay BP. Org. Lett. 2005;7:5031–5034. doi: 10.1021/ol0520119. [DOI] [PubMed] [Google Scholar]; c Berryman OB, Bryantsev VS, Stay DP, Johnson DW, Hay BP. J. Am. Chem. Soc. 2007;129:48–58. doi: 10.1021/ja063460m. [DOI] [PubMed] [Google Scholar]
- 12.Zuo C-S, Quan J-M, Wu Y-D. Org. Lett. 2007;9:4219–4222. doi: 10.1021/ol701740p. [DOI] [PubMed] [Google Scholar]
- 13.a Yoon D-W, Hwang H, Lee C-H. Angew. Chem. 2002;114:1835–1837. [Google Scholar]; Angew. Chem. Int. Ed. 2002;41:1757–1759. doi: 10.1002/1521-3773(20020517)41:10<1757::aid-anie1757>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]; b Lee C-H, Miyaji H, Yoon D-W, Sessler JL. Chem. Commun. 2008:24–34. doi: 10.1039/b713183f. [DOI] [PubMed] [Google Scholar]
- 14.a Gale PA, Sessler JL, Kral V, Lynch V. J. Am. Chem. Soc. 1996;118:5140–5141. [Google Scholar]; b Sessler JL, Gross DE, Cho W-S, Lynch VM, Schmidtchen FP, Bates GW, Light ME, Gale PA. J. Am. Chem. Soc. 2006;128:12281–12288. doi: 10.1021/ja064012h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donohoe TJ, Headley CE, Cousins RPC, Cowley A. Org. Lett. 2003;5:999–1002. doi: 10.1021/ol027504h. [DOI] [PubMed] [Google Scholar]
- 16.The crystal data and experimental details for the structural refinement of 3, 3·Cl, and 4 are provided in the Supporting Information. The CCDC numbers are 682547 3, 682548 3·Cl, and 682549 4, which contain the crystallographic data. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 17.a Sunner J, Nishizawa K, Kebarle P. J. Phys. Chem. 1981;85:1814–1820. [Google Scholar]; b Paul GJC, Kebarle P. J. Am. Chem. Soc. 1991;113:1148–1154. [Google Scholar]
- 18.Hiraoka K, Mizuno T, Iino T, Eguchi D, Yamabe S. J. Phys. Chem. A. 2001;105:4887–4893. [Google Scholar]
- 19.Bylaska EJ, et al. NWChem, A Computational Chemistry Package for Parallel Computers, Version 5.0. Pacific Northwest National Laboratory; Richland, Washington 99352-0999, USA: 2006. See supporting information for the full citation.
- 20.Whereas this analysis provides a quantitative measure of the chloride affinity offered by each binding configuration, we recognize that it does not account for other contributions to the overall binding affinity, such as conformational reorganization that may occur prior to binding or the influence of solvation.