Abstract
A NO-delivery platform has been fabricated from polydimethylsiloxane (PDMS) and Pluorinc® F127 gel that contains the light-sensitive NO donor, [Mn(PaPy3)(NO)]ClO4. The material was assembled layer-by-layer. First, a thin PDMS membrane was cast. It was then layered with cold 25% (wt/v) Pluronic® F127 gel mixed with [Mn(PaPy3)(NO)]ClO4. Finally, it was covered with a thick layer (nearly impermeable to NO) of PDMS to allow release of NO only from the thinner side upon exposure to light. Light-induced NO release from this layered material has been confirmed via NO-specific electrode and by a modified soft Griess-agar assay. Incorporation of ~8 mg/g of [Mn(PaPy3)(NO)]ClO4 in the Pluronic gel layer affords a material that drastically reduces the microbial loads of Acinetobacter baumannii and Pseudomonas aeruginosa via the antibiotic effects of the photoreleased NO. Application of this flexible layered NO-donating composite as bandage material has been proposed.
Keywords: Antibacterial, Drug delivery, Infection, Nitric oxide, Pluronic gel, Polydimethylsiloxane
Introduction
Although there are over 2,000 bandages on the market, persistent wounds remain a serious problem, and improved remedies are required [1]. Diabetic and pressure ulcers in lower extremities are most common. The number of such cases is also increasing as more people are diagnosed with diabetes and as a greater proportion of the population advances to old age. Wound-healing dressings must prevent further trauma, maintain a moist environment, allow oxygen to reach the wound, and abate bacterial contamination while absorbing wound exudates [1]. Although antiseptic treatment of wounds has diminished mortality due to infection at injured sites, these wounds may serve as portals to colonizing organisms [2]. An added challenge is the emergence of multidrug-resistant and pandrug-resistant Gram-negative bacteria [3] [4]. The protective outer membrane acts as an effective barrier to most antibiotics, and bacteria may also express efflux pumps or antibiotic-degrading enzymes [4]. These problematic strains of bacteria include Acinetobacter baumannii, Escherichia coli, Pseudomonas aeruginosa, and Klebsiella pneumoniae [3] [4]. New remedies are clearly needed to treat such infections; the number of new antibiotics approved by the FDA has however been declining [5].
One promising approach is to use nitric oxide (NO) to mimic and enhance innate immunity [6]. Nitric oxide is produced by macrophages during the initial stages of the inflammatory response. Its antibiotic effects arise from inhibition of cellular respiration and DNA damage. NO also interacts with superoxide (O2−) to form peroxynitrite (ONOO−), a strong oxidant that initiates lipid peroxidation. Exogenous NO was shown to cure an infected, nonhealing wound in a clinical case study [7]. Although this treatment worked, the patient was effectively immobilized by the equipment used to formulate, administer, and trap excess NO gas. An alternative to administering NO gas is to use an NO donor.
Research in our group has investigated the application of metal nitrosyls (NO complexes of transition metals) as NO donors to biological targets. The nitrosyls derived from the Fe [8], Ru [9], and Mn [10] complexes of the deprotonated ligand N,N-bis(2-pyridylmethyl)amine-N-ethyl-2-pyridine-2-carboxamide (PaPy3−) are particularly promising NO donors because they release NO upon illumination with low intensity light. The nitrosyl, [Mn(PaPy3)(NO)]ClO4 (1) was selected for this investigation since it is stable in aqueous media and releases NO when exposed to visible light (Fig. 1). The dose and duration of NO administration by this complex can be easily controlled by light [10]. The biological utility of NO photoreleased from 1 has been demonstrated in numerous in vitro experiments. For example, the catalytic activity of the cysteine protease papain could be inhibited (via S-nitrosyltion of Cys25) by 1 under the control of light [11]. Similarly, light-dependent vasorelaxation of rat aorta rings was achieved (via NO-mediated activation of soluble guanylate cyclase) with 1 in tissue bath experiments [12]. The rapid photorelease of NO from 1 also permitted observation of NO-binding to cytochrome c oxidase and myoglobin at microsecond timescales not achievable by stopped-flow techniques [13]. Recently, we have embedded 1 in a prototype wound-healing dressing composed of polyurethane coated poly(2-hydroxyethyl methacrylate) hydrogels and examined its light-stimulated antibiotic effects [14]. We have also fabricated a fiber optic NO delivery prototype from [Mn(PaPy3)(NO)]ClO4-doped sol-gel (coated in polyurethane) for the treatment of bacterial infection [15]. When placed in intimate contact with liquid cultures of P. aeruginosa, E. coli, Staphylococcus aureus, and methicillin-resistant S. aureus and illuminated via the optical fiber, photoreleased NO alone diminished the colony forming ability of all cultures. Incorporation of the NO-releasing complex into polymeric matrices in both cases was intended to avoid toxicity, if any, associated with the heavy metal atoms [16] [17]. In the present work, we have extended the scope of this strategy and report an improved flexible layered NO-releasing patch which may serve as a light-controlled dermal NO delivery platform for the treatment of infection.
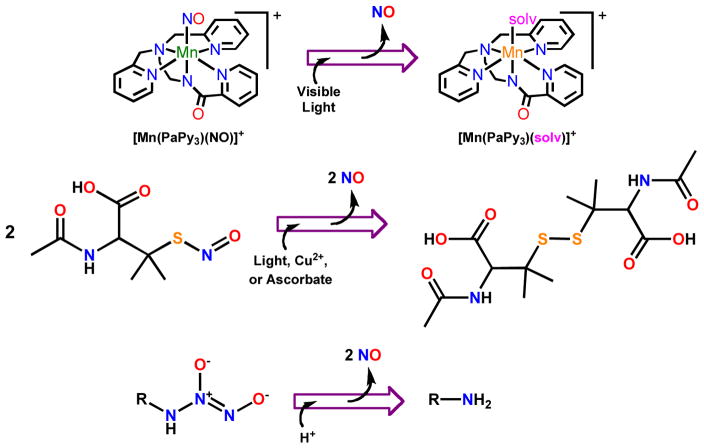
Fig. 1.
Nitric oxide donors and reactions that release NO. Top: Following light-induced Mn-NO bond cleavage in [Mn(PaPy3)(NO)]ClO4 (1), a solvent (solv, e.g. water) molecule fills the coordination site vacated by NO. Center: Three triggers, namely, Cu2+, ascorbate, or light can stimulate NO release from S-nitrosothiols like S-nitroso-N-acetylpenicillamine. Bottom: Upon hydration, N-diazeniumdiolates release two equivalents of NO.
There have been several reports on NO-releasing materials and their potential utility in medical applications in recent years [18–20]. Examples of layered materials are however quite limited. Meyerhoff and coworkers have reported a tri-layer film based on S-nitrosothiols [21] [22] that releases NO in processes mediated by light, copper ions, or ascorbate (Fig. 1). The S-NO moiety was first introduced into functionalized fumed silica particles and the particles were finally sandwiched between two layers of silicone rubber. Analogous films were prepared substituting polyurethane for the silicone rubber. The hydrophilic polyurethane films released NO to solutions containing either copper, ascorbate, or upon illumination. Schoenfisch and coworkers have coated PDMS implants with NO-releasing xerogels (containing N-diazeniumdiolates) to improve integration of implantable devices [23]. This class of NO donor liberates two equivalents of NO upon hydration (Fig. 1). When rats received these implants, reduced foreign body response and improved vascularization were observed.
The layered material described in this report makes use of PDMS and Pluronic® F127 gel. In any strategy of using light to trigger NO-delivery, the materials employed in the fabrication of the material must be optically transparent and highly NO permeable. Both PDMS and Pluronic® F127 gel fit these criteria. Additionally, the biocompatibility of silicone rubbers such as PDMS is established for drug delivery implants, notably Norplant® [24]. The durability and flexibility of PDMS lend strength to the NO-releasing material while the Pluronic® F127 filler acts as a reservoir for the water-soluble nitrosyl 1. While Pluronic® F127 can contact skin directly [25], it has been selected as the inside layer material principally for structural strength of the patches at body temperature (37 °C). The high water content (75%) of the Pluronic® F127 gel filler (necessary for rapid NO photorelease) requires the outer PDMS layers to afford a robust NO-delivery platform. In the following sections, the assembly of the NO-releasing layered material and the antibiotic effects of NO released from patches derived from it are described in detail.
Results and Discussion
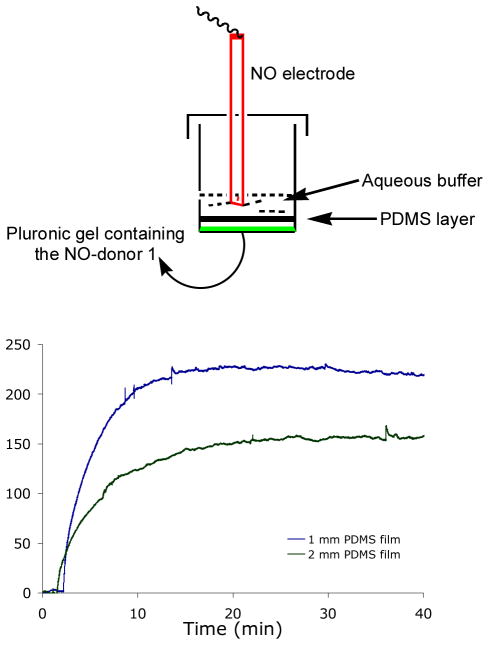
Layer by layer fabrication of transparent and biocompatible microfluidic devices from PDMS is well documented [26] [27]. This facile approach was applied to make the macroscale NO-releasing patch in the present work. First, the diffusion of NO through PDMS membranes of various thicknesses was investigated. A batch of 0.3 g of 20% Pluronic gel containing 5 mg of 1 was placed at the bottom of a 2 ml vial and carefully covered with cured PDMS discs of various thicknesses (Fig. 2). Following addition of 300 μl of water on the top of the double layer, the tip of an NO-sensitive electrode was dipped into it and the whole assembly was illuminated with white light. In line with literature results [28], the concentration of NO that accumulates on the external side of the membrane demonstrated that the NO concentration increases with decreasing PDMS thickness (Fig. 2). For example, the steady state concentration of NO peaked around 230 nM when the PDMS film was 1 mm thick, and the peak NO concentration dropped to approximately 150 nM when the thickness of the PDMS film was doubled to 2 mm.
Fig. 2.
The concentration of NO released to 300 μl of water, separated from 0.3 g samples of Pluronic® F127 gel containing 5 mg/g [Mn(PaPy3)(NO)]ClO4 (1) by 1 and 2 mm PDMS films. The NO flux increased from 0 nM to steady state peak concentrations (~230 nM and ~150 nM, respectively) when illuminated with visible light (100 mW/cm2).
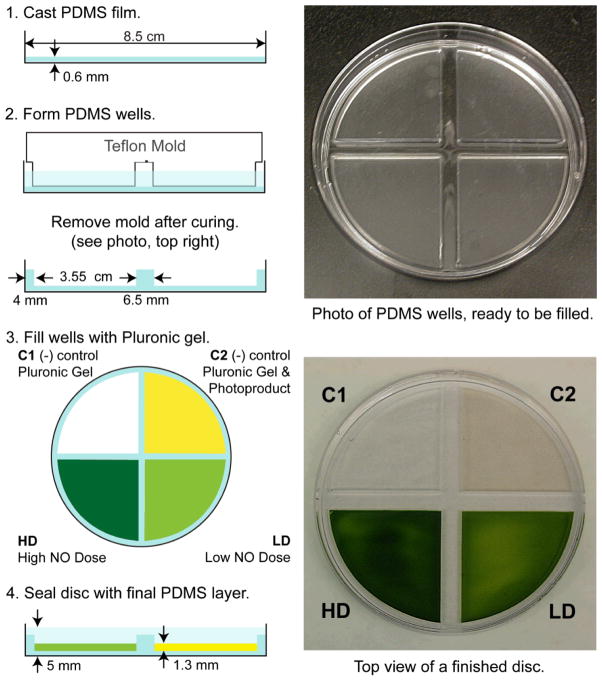
Quantities of PDMS between 0.5 and 3 g of elastomer, in half-gram increments, were cast into films (circular patches, dia 8.5 cm) on Petri dishes (used as the mold) and tested for structural integrity. Films made from 0.5 and 1 g tore easily upon removal from the Petri dish while sturdy films were obtained from 1.5 up to 3 g of the elastomer. Films made of 1.5 g of elastomer were selected for the layered patch fabrication to minimize the thickness (0.6 mm) of the films obtained (Fig. 3, Step 1).
Fig. 3.
Left: Fabrication scheme for the assembly of the layered NO-releasing patches. Step 1, a 0.6 mm PDMS membrane is cast in a culture dish. Step 2, PDMS wells cast from 5 g of elastomer using a Teflon mold. Following curing, empty PDMS wells are pictured top, right. Step 3, the four quadrants are filled with cold 25% (w/w) Pluronic® F127 gel formulated for four experimental conditions. Top, left quadrant C1 contains Pluronic® F127 gel only. Top, right quadrant C2 contains 4 mg/g of 1 in Pluronic® F127 gel that has been exposed to light for 2 h, so all NO has been released. Bottom, right quadrant LD contains a low dose of 4 mg/g of 1 in Pluronic® F127 gel. Bottom, left quadrant HD contains a high dose of 8 mg/g of 1 in Pluronic® F127 gel. Step 4, once the gels warmed to room temperature and solidified, a thick layer of PDMS was layered over the Pluronic® F127 gel. Following curing, a finished sample is pictured bottom, right.
Once the thin membrane was cast, another 5 g of PDMS was layered over the membrane and a Teflon mold was inserted to form the four wells (Fig. 3, Step 2). With the appropriate mold, any number and shape of wells can be cast. Following curing, the Teflon mold was removed, revealing the empty quadrants, having a depth of 0.13 cm and spacings of 0.65 cm (0.25 inch) between each well. Each well was filled with 2 g (~1.3 ml) of cold, liquid Pluronic® F127 gel (Fig. 3, Step 3) of different formulation. As shown in Fig. 3, the quadrant containing Pluronic® F127 gel only (C1) is transparent and clear. The transparent, yellow quadrant (C2) contains 4 mg/g of [Mn(PaPy3)(NO)]ClO4 (1) in Pluronic® F127 gel that was exposed to light for 2 h to release all of the NO from the complex prior to loading. The light green quadrant (LD) contains a low dose (4 mg/g) of NO-releasing compound 1 in the Pluronic® F127 gel. The quadrant loaded with the high NO dose (HD, 8 mg/g of 1 appears dark green. Upon warming to room temperature, the gel formed a rigid semi-solid that could support 18.5 g of PDMS elastomer, which was cured at room temperature for three days to avoid thermal decomposition of the manganese nitrosyl (Fig. 3, Step 4). The final layer of PDMS was cast thickly to ensure complete sealing of the Pluronic® F127 gel within the wells and to serve as a barrier to NO diffusion from the top of the patch quadrants.
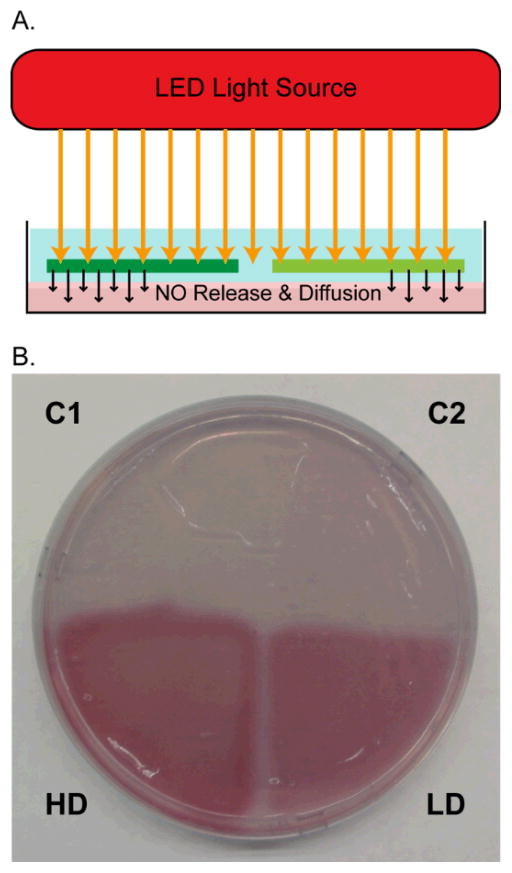
The ability of NO delivery of these LD and HD layered patches was first confirmed by performing the Griess assay in agar. The Griess assay generates a pink azo dye when the reagents react with nitrite, the oxidation product of NO. These reagents were mixed in agar that was cast to match the conditions used in antibiotic susceptibility tests, so the distribution of photoreleased NO could be visualized. A circular patch with four quadrants was placed on top of the prepared agar with the thin membrane in contact with the agar surface (Fig. 4). The patch was then illuminated (10 mW/cm2) through the thick side of the PDMS for one h. Following exposure to light, the patch was removed from the agar, and the agar surface was photographed (Fig. 4). The appearance of bright pink wedges under the regions LD and HD indicates that mM concentrations of NO accumulated in the agar lying below. The agar that received the high NO dose (Fig. 3C bottom left) appears larger and has a brighter pink color than the agar that received the low NO dose (Fig. 3C bottom right). This confirms that high and low concentrations of NO penetrated the agar surface, and the clean borders around each wedge show that NO diffusion from LD and HD was area-restricted. Accordingly, the absence of bright pink in the agar that was placed under C1 and C2 indicates that NO was not released from these quadrants and did not crossover from LD and HD. This experiment was replicated, placing the thick PDMS layer in contact with the agar and no formation of the pink azo dye was observed in the Griess agar following illumination. This demonstrates that, during one h of exposure to light, NO does not cross the thick PDMS layer, and so NO diffusion occurs only from the desired (thinner) side of the patch.
Fig. 4.
A. Illumination scheme to test NO-delivery from a patch to an agar plate that contains Griess reagents. B. Photograph of agar containing Griess reagents following contact with a disk and 1 h of illumination. Pink color appears in areas exposed to NO (LD and HD).
To test the antibiotic efficacy of the photoreleased NO, the circular patches were employed in a modified soft agar assay. To mimic an infected wound environment, trypticase soy agar plates were inoculated with a thin layer (6 ml) of bacteria suspended in a soft brine agar [29]. Preparations containing 0.5% agar broke or deformed when the layered patch was placed on the surface, so the percent agar was increased to 0.8%. At 0.8% agar, the bacteria mixed easily in the agar at 45 °C, and upon cooling the firm agar layer supported the circular patches without deforming. The patches were placed over the soft agar (containing bacterial colonies) so that the thin PDMS side was closest to the agar. The plates were gently tapped to remove air bubbles, ensuring good contact between the PDMS and bacteria inoculated agar. Following illumination for 1 h, the patches were removed, and the plates were incubated at 37 °C for 12 h to allow surviving bacteria to establish colonies.
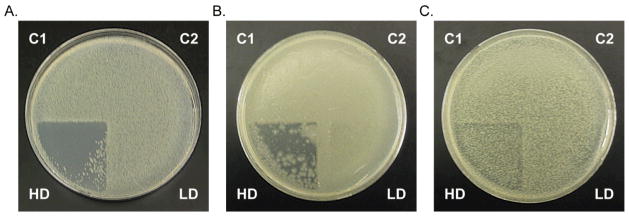
All the bacteria studied, namely, A. baumannii, P. aeruginosa, E. coli, and S. aureus grew vigorously under both of the negative control conditions. Thus, neither light alone, nor further illumination of the photoproduct (in quadrant C2) gives rise to any species that kills bacteria in the absence of NO. The low NO dose was partially lethal to the bacteria studied. However, the high dose of NO was very effective at killing the Gram-negative bacteria A. baumannii and reduced the load of P. aeruginosa to a significant extent. In case of A. baumannii, the few remaining colonies pictured in quadrants HD (Fig. 5) were located on the surface of the soft agar. The presence of these colonies is likely due to capillary forces drawing the bacteria from other quadrants while the patch was removed from the agar surface, before incubation. Application of sterile gauze over the agar surface, before the patch is placed, may prevent movement of this surface liquid when the patch is removed, and may prevent such contamination of the HD quadrant. This result suggests that NO may be an effective treatment against emerging drug-resistant strains of acinetobacteria [30–32]. Such organisms are commonly identified in the battle wounds of soldiers fighting in the Middle East, fortunately the pathogenicity of these isolates is low [32]. However, in hospital settings, A. baumannii infections can greatly complicate treatment of compromised patients [32], in some cases leading to amputation of infected limbs [33]. The antibiotic resistance in A. baumannii arises from efficient activation of resistance genes that code for: enzymes (β-lactamases, acetyltransferases, nucleotidyltransferases, and phosphotransferases), efflux pumps, outer membrane proteins, and ribosomal protection [32]. Together these resistance mechanisms protect against all classes of conventional antibiotics. NO therapy, which provides a distinct oxidative assault [6], may serve as an alternative treatment.
Fig. 5.
Photographs that show bacterial death as a result of NO exposure. A. Cultures of A. baumannii grew vigorously when exposed to control (C1 and C2) conditions. The low dose of NO (LD) had no effect, however the high dose of NO resulted in bacterial clearing from quadrant HD. B. Likewise, P. aeruginosa was also susceptible to the high NO dose (HD). C. Moderate attenuation of the E. coli population was achieved with the high NO dose (HD).
NO treatment only slightly attenuated the growth of E. coli (Fig. 5) and the Gram-positive bacteria S. aureus (result not shown). These latter results can be attributed to the NO-resistance mechanisms of these species. E. coli acquires protection against NO primarily through production of flavohemoglobin Hmp or flavorubredoxin NorV [34]. Under aerobic conditions flavohemoglobin Hmp converts NO to nitrite or NO−, both less toxic species. In addition to these mechanisms of NO resistance, S. aureus possesses an NO-inducible lactate dehydrogenase that allows the bacteria to replicate under nitrosative stress [35]. Although greater bacterial killing was not achieved by increasing the concentration of 1 to 20 mg/g in the Pluronic® F127 layer, alternative dosing strategies may prove to be effective against these strains, as has been reported by other groups [17].
Collectively the results demonstrate that the layered NO delivery platform provides a convenient means for controlled delivery of potentially therapeutic NO. These flexible patches release NO in response to low intensity (10 mW/cm2) white light and exhibit antibiotic effects after only 1 h of illumination. This strategy avoids bulky gas-handling equipment and decreases the duration of treatment to 1 h, from 8 h applied in the clinical study [7]. Additionally, using light to activate the manganese nitrosyl 1 locked within the Pluronic® F127 layer may be advantageous to mixing solutions of acid and nitrite as in another NO delivery scheme [17].
Conclusions
The prototype reported herein is an effective test structure for investigation of the biological effects of NO and may serve as a dermal NO delivery system. The antibiotic effects of NO delivered by the flexible patches against threatening Gram-negative pathogens A. baumannii and P. aeruginosa are promising preliminary results towards the treatment of these problematic microorganisms. These NO-delivering patches were fabricated in four simple steps using layer-by-layer PDMS molding. The PDMS layers ensure complete sealing of the NO-donating nitrosyl and its photoproduct(s). Thus, no side effects from the manganese compounds are expected at the infected site. Antibiotic concentrations of NO can be liberated in just 1 h of illumination, a significant decrease in the exposure time required in similar reports. We anticipate that flexible patches like this could be used as bandage material in chronic infections of antibiotic-resistant strains of bacteria, a common occurrence in hospitals at present.
Experimental Part
1. General
SYLGARD® 184 was purchased from K.R. Anderson, Inc. Griess Reagent Kit was obtained from Biotium. [Mn(PaPy3)(NO)]ClO4 (1) was synthesized as previously described [10]. NO release measurements were recorded using an NO-sensitive electrode (inNO-T NO-measuring system, Harvard Apparatus). The optical densities of bacterial cultures were monitored using a UV-Visible 1601 Shimadzu spectrometer.
2. Preparation of NO-releasing PDMS discs
The SYLGARD® 184 base (12 g) and curing agent (1.2 g) were mixed in a 10:1 ratio, in accordance with the manufacturer’s directions. A transfer pipette was used to deposit 1.5 g of this mixture into the center of each of eight polystyrene Petri dishes (100 mm). The elastomer was then spun at 1000 rpm for 8 sec to coat the bottom of the dish and placed under vacuum in a dessicator for 1 h to remove dissolved gasses. Following degassing, the films were cured in an 80°C oven for 1 h. Each dish was then filled with 5 g more base/curing agent mixture and degassed for 1 h under vacuum. Following degassing, the mixture spread to the edges of each dish. A Teflon mold was then placed in each Petri dish, and cured for 1 h at 80 °C. The poly(dimethylsiloxane) (PDMS) and molds were cooled and the Teflon molds removed, giving a PDMS disc with four wedge shaped wells. Each well was filled with 2 g of cold Pluronic® F127 gel mixture: one well with Pluronic® F127 gel only (negative control), the second well with 2 g Pluronic® F127 gel containing 4 mg of 1 that was exposed to light for 2 h to release NO (negative control), the third well with Pluronic® F127 gel containing 4 mg of 1 (low NO dose), and the last well with Pluronic® F127 gel containing 8 mg of 1 (high NO dose). This was best achieved by cooling the Pluronic® F127 gel (25% polymer in sterile filtered, MilliQ purified water) to 4 °C, at which point the semisolid liquefied. The liquid was then pipetted into each well and allowed to warm to room temperature at which point the Pluronic® F127 gel thickened to a semisolid. An additional 18.5 g of base/curing agent mixture that had been degassed for 1 h was then poured over the filled wells to seal-in the Pluronic® F127 gels. Following 3 days of curing at room temperature, the plastic Petri dishes were sniped with lab shears and peeled away from the PDMS-Pluronic® F127 circular patches.
3. Electrochemical measurement of NO concentrations
The concentration of photoreleased NO was measured electrochemically. Samples were prepared by depositing 0.3 g of Pluronic® F127 gel (25% in sterile filtered, MilliQ purified water) containing either 5 or 10 mg/g of 1 in 2 ml mass spectrometry vials. A layer of 0.1 g or 0.2 g of prepolymer was then layered over the solidified gel. The samples were left in the dark to cure at room temperature for three days. To measure the concentration of NO released, 300 μl of water was placed over the PDMS surface. A NO-sensitive electrode was suspended in the water and the layer was stirred with a microstirbar. The samples were illuminated with a 100 mW/cm2 white light for 1 h and the NO concentration was monitored. The data was normalized and plotted in Excel.
4. Visualization of NO diffusion in soft agar by the Griess reaction
A thick layer of agar was cast by dispensing a 20 ml aliquot of agar containing Griess reagents into a 100 mm culture dish. The stock solution was prepared by adding 1 ml of Griess reagents to 30 ml of a hot solution of MilliQ purified water and 0.45 g agar. A thinner layer of soft agar (6 ml, 0.8% agar to which 200 μl of Griess reagents had been added) was then layered on top of the thick layer of firm agar. The circular patch was then placed over the soft agar and the dish was firmly tapped on the counter to release air bubbles. The system was inverted and illuminated through the thick layer of PDMS for 1 h. Following illumination, the patch was carefully removed and the resulting pink color from photoreleased NO was photographed.
5. Soft Agar Assay
The procedure used by Benjamin [29] was adapted for this work. At 45°C, a 20μl aliquot of bacteria in log phase growth (OD = 0.7–1) was suspended in 20 ml of brine agar (0.8% agar, 1% NaCl). TSB agar (20 ml) plates were seeded with 6 ml of bacteria in warm brine agar. After the inoculated agar firmed-up, a patch was placed on the bacteria. Air bubbles were gently removed by pushing on top of the disc. The system was illuminated with the thick layer of PDMS closest to the light source for 1 h (100 mW/cm2). The disk was then removed, taking care to leave the soft agar intact and the plate was incubated for 12 h at 37°C (results apparent after 4 h).
Acknowledgments
Financial support from the NSF grant CHE-0957251 is gratefully acknowledged. GMH was supported by the NIH IMSD grant GM58903.
References
- 1.Hess CT. Skin & wound care. Lippincott Williams & Wilkins; Ambler: 2008. [Google Scholar]
- 2.D’Avignon LC, Hogan BK, Murray CK, Loo FL, Hospenthal DR, Cancio LC, Kim SH, Renz EM, Barillo D, Holcomb JB, Wade CE, Wolf SE. Burns. 2010;36:773. doi: 10.1016/j.burns.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Falagas ME, Bliziotis IA, Kasiakou SK, Samonis G, Athanassopoulou P, Michalopoulos A. BMC Infect Dis. 2005;5:24. doi: 10.1186/1471-2334-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fishbach MA, Walsh CT. Science. 2009;325:1089. doi: 10.1126/science.1176667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taubes G. Science. 2008;321:356. doi: 10.1126/science.321.5887.356. [DOI] [PubMed] [Google Scholar]
- 6.Fang FC. Nat Rev Microbiol. 2004;16:2373. doi: 10.1038/nrmicro1004. [DOI] [PubMed] [Google Scholar]
- 7.Miller CC, Miller MK, Ghaffari A, Kunimoto B. J Cutan Med Surg. 2004;8:233. doi: 10.1007/s10227-004-0106-8. [DOI] [PubMed] [Google Scholar]
- 8.a) Patra AK, Rowland JM, Marlin DS, Bill E, Olmstead MM, Mascharak PK. Inorg Chem. 2003;42:6812. doi: 10.1021/ic0301627. [DOI] [PubMed] [Google Scholar]; b) Patra AK, Afshar RK, Olmstead MM, Mascharak PK. Angew Chem Int Ed. 2002;41:2512. doi: 10.1002/1521-3773(20020715)41:14<2512::AID-ANIE2512>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 9.Patra AK, Mascharak PK. Inorg Chem. 2003;42:7363. doi: 10.1021/ic030110h. [DOI] [PubMed] [Google Scholar]
- 10.a) Merkle AC, Fry NL, Mascharak PK, Lehnert N. Inorg Chem. 2011;50:12192. doi: 10.1021/ic201967f. [DOI] [PubMed] [Google Scholar]; b) Eroy-Reveles AA, Leung Y, Beavers CM, Olmstead MM, Mascharak PK. J Am Chem Soc. 2008;130:4447. doi: 10.1021/ja710265j. [DOI] [PubMed] [Google Scholar]; c) Ghosh K, Eroy-Reveles AA, Avila B, Holman TR, Olmstead MM, Mascharak PK. Inorg Chem. 2004;43:2988. doi: 10.1021/ic030331n. [DOI] [PubMed] [Google Scholar]
- 11.Afshar RK, Patra AK, Mascharak PK. J Inorg Biochem. 2005;99:1458. doi: 10.1016/j.jinorgbio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Madhani M, Patra AK, Miller TW, Eroy-Reveles AA, Hobbs A, Fukuto JM, Mascharak PK. J Med Chem. 2006;49:7325. doi: 10.1021/jm0604629. [DOI] [PubMed] [Google Scholar]
- 13.Szundi I, Rose M, Sen I, Eroy-Reveles AA, Mascharak P, Einarsdottir O. Photochem Photobiol. 2006;82:1377. doi: 10.1562/2006-07-25-rc-984. [DOI] [PubMed] [Google Scholar]
- 14.Halpenny GM, Steinhardt RC, Okialda KA, Mascharak PK. J Mat Sci: Med Sci. 2009;20:2353. doi: 10.1007/s10856-009-3795-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halpenny GM, Mascharak PK. ACS Med Chem Lett. 2010;1:180. doi: 10.1021/ml1000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luckey TD, Venugopal B, Hutcheson D. In: Heavy metal toxicity, safety, and hormology. Thieme G, editor. Stuttgart, and Academic Press; New York: 1975. [Google Scholar]
- 17.Fricker SP. Dalton Trans. 2007;43:4903. doi: 10.1039/b705551j. [DOI] [PubMed] [Google Scholar]
- 18.Frost MC, Reynolds MM, Meyerhoff ME. Biomaterials. 2005;26:1685. doi: 10.1016/j.biomaterials.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Eroy-Reveles AA, Mascharak PK. Future Med Chem. 2009;1:1497. doi: 10.4155/fmc.09.111. [DOI] [PubMed] [Google Scholar]
- 20.Halpenny GM, Maschcarak PK. Anti-Infect Agents Med Chem. 2010;9:187. [Google Scholar]
- 21.Frost MC, Meyerhoff ME. J Am Chem Soc. 2004;126:1348. doi: 10.1021/ja039466i. [DOI] [PubMed] [Google Scholar]
- 22.Frost MC, Meyerhoff ME. J Biomed Mater Res. 2005;72A:409. doi: 10.1002/jbm.a.30275. [DOI] [PubMed] [Google Scholar]
- 23.Hetrick EM, Prichard HL, Klitzman B, Schoenfisch MH. Biomaterials. 2007;28:4571. doi: 10.1016/j.biomaterials.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shoupe D. Am J Obstet Gynecol. 1989;160:1286. doi: 10.1016/s0002-9378(89)80014-6. [DOI] [PubMed] [Google Scholar]
- 25.Shishido SM, Seabra AB, Loh W, de Oliveira MG. Biomaterials. 2003;24:3543. doi: 10.1016/s0142-9612(03)00153-4. [DOI] [PubMed] [Google Scholar]
- 26.McDonald JC, Whitesides GM. Acc Chem Res. 2002;35:491. doi: 10.1021/ar010110q. [DOI] [PubMed] [Google Scholar]
- 27.Malta A, Fleischman AJ, Roy S. Biomed Microdevices. 2005;7:281. doi: 10.1007/s10544-005-6070-2. [DOI] [PubMed] [Google Scholar]
- 28.Zacharia IG, Deen WM. Ann Biomed Eng. 2005;33:214. doi: 10.1007/s10439-005-8980-9. [DOI] [PubMed] [Google Scholar]
- 29.Hardwick JBJ, Tucker AT, Wilks M, Johnston A, Benjamin NA. Clin Sci. 2001;100:395. [PubMed] [Google Scholar]
- 30.Perez F, Hujer AM, Hujer KM, Decker BK, Rather PN. Antimicrob Agents Ch. 2007;51:3471. doi: 10.1128/AAC.01464-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dijkshoorn L, Nemec A, Seifert H. Nat Rev Microbiol. 2007;5:939. doi: 10.1038/nrmicro1789. [DOI] [PubMed] [Google Scholar]
- 32.Peleg AY, Seifert H, Paterson DL. Clin Microbiol Rev. 2008;21:538. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson EN, Burns TC, Hayda RA, Hospenthal DR, Murray CK. Clin Infect Dis. 2007;45:409. doi: 10.1086/520029. [DOI] [PubMed] [Google Scholar]
- 34.a) Gardner AM, Gardner PR. J Biol Chem. 2002;277:8172. doi: 10.1074/jbc.M110471200. [DOI] [PubMed] [Google Scholar]; b) Gardner PR, Gardner AM, Martin LA, Salzman AL. Proc Natl Acad Sci USA. 1998;95:10378. doi: 10.1073/pnas.95.18.10378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richardson AR, Libby AJ, Fang FC. Science. 2008;319:1672. doi: 10.1126/science.1155207. [DOI] [PubMed] [Google Scholar]